A fusion between a central isoprenoid pathway enzyme and phytoene synthase improves substrate conversion efficiency and redirects metabolites into carotenoid biosynthesis.
Abstract
Geranylgeranyl diphosphate (GGPP), a prenyl diphosphate synthesized by GGPP synthase (GGPS), represents a metabolic hub for the synthesis of key isoprenoids, such as chlorophylls, tocopherols, phylloquinone, gibberellins, and carotenoids. Protein-protein interactions and the amphipathic nature of GGPP suggest metabolite channeling and/or competition for GGPP among enzymes that function in independent branches of the isoprenoid pathway. To investigate substrate conversion efficiency between the plastid-localized GGPS isoform GGPS11 and phytoene synthase (PSY), the first enzyme of the carotenoid pathway, we used recombinant enzymes and determined their in vitro properties. Efficient phytoene biosynthesis via PSY strictly depended on simultaneous GGPP supply via GGPS11. In contrast, PSY could not access freely diffusible GGPP or time-displaced GGPP supply via GGPS11, presumably due to liposomal sequestration. To optimize phytoene biosynthesis, we applied a synthetic biology approach and constructed a chimeric GGPS11-PSY metabolon (PYGG). PYGG converted GGPP to phytoene almost quantitatively in vitro and did not show the GGPP leakage typical of the individual enzymes. PYGG expression in Arabidopsis resulted in orange-colored cotyledons, which are not observed if PSY or GGPS11 are overexpressed individually. This suggests insufficient GGPP substrate availability for chlorophyll biosynthesis achieved through GGPP flux redirection to carotenogenesis. Similarly, carotenoid levels in PYGG-expressing callus exceeded that in PSY- or GGPS11-overexpression lines. The PYGG chimeric protein may assist in provitamin A biofortification of edible plant parts. Moreover, other GGPS fusions may be used to redirect metabolic flux into the synthesis of other isoprenoids of nutritional and industrial interest.
Isoprenoids comprise a large group of plant metabolites with divergent functions, ranging from photosynthesis to plant growth regulation and interaction with the environment. Carotenoids are a class of isoprenoids that contribute to light harvesting, provide protection against excess light via nonphotochemical quenching, and color flowers and fruits to attract pollinators and to facilitate seed dispersal. Carotenoids are also cleaved to form phytohormones like abscisic acid and strigolactones (Cazzonelli, 2011; Giuliano, 2014). In contrast to plants, most animals, including humans, are incapable of synthesizing carotenoids and rely either on a regular dietary intake of β-carotene and other provitamin A carotenoids or the consumption of retinoids from animal sources for maintaining visual function, providing antioxidative protection, ensuring developmental processes, and preventing disease (von Lintig, 2012; Johnson, 2014).
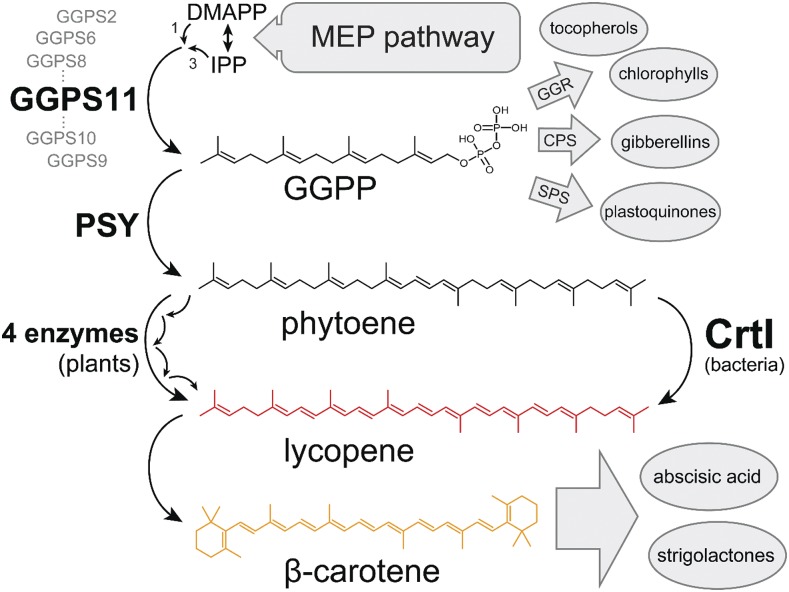
Plant carotenoid biosynthesis takes place in plastids and is characterized by a dramatic change in the solubility of the metabolites involved (Moise et al., 2014). Isopentenyl diphosphate (IPP), emerging from the plastid-localized methylerythritol phosphate (MEP) pathway (Fig. 1), represents the water-soluble building block common to all isoprenoids. One molecule of its isomer, namely dimethyl allyl diphosphate (DMAPP), is required as the starter molecule, and three molecules of IPP are condensed by the enzyme GGPP synthase (GGPS, Kloer et al., 2006) to form the strongly amphipathic C20 geranylgeranyl diphosphate (GGPP). Subsequently, phytoene synthase (PSY) catalyzes the symmetric condensation of two molecules of GGPP, which eliminates the diphosphates and generates the highly lipophilic hydrocarbon phytoene, the first committed step in the carotenoid pathway. This colorless compound is desaturated and isomerized via four enzymes into the red-colored lycopene, the main pigment of tomatoes. Lycopene is cyclized by two different cyclases into α- and β-carotene, the major carotene components of carrot roots for example (Giuliano et al., 2002; Maass et al., 2009; Arango et al., 2014). The predominant leaf carotenoids lutein and violaxanthin represent hydroxylated and epoxidated derivatives of α- and β-carotene, respectively.
Figure 1.
Carotenoid biosynthesis and branching pathways. The methylerythritol phosphate pathway (MEP) produces IPP and its isomer DMAPP which is condensed into geranylgeranyl diphosphate (GGPP) in plastids by GGPP synthase (GGPS). GGPS11 is the most abundant among the six plastid-localized GGPS isoforms. GGPP is metabolized into plastoquinones via solanesyl-diphosphate synthase (SPS), into gibberellins via ent-copalyl diphosphate synthase (CPS), and into phythyl-diphosphate via geranylgeranyl reductase (GGR), which represents a precursor for chlorophyll and tocopherols. Phytoene synthase (PSY) is the first enzyme of the carotenoid pathway producing colorless phytoene, which is subsequently converted into red-colored lycopene and orange-colored β-carotene. Lycopene synthesis requiring four enzymes in plants is carried out by the enzyme CrtI in bacteria. In this work, we employed a translational fusion between GGPS11 and PSY in order to direct isoprenoid pathway flux into carotenoid biosynthesis.
In most cases, PSY controls the entire pathway flux as the rate-limiting enzyme and thus, increased PSY protein levels achieved either through overexpression or by providing enhanced PSY stability increase the total carotenoid content in plant tissues (Fraser et al., 2002; Maass et al., 2009; Farré et al., 2010; Welsch et al., 2010, 2018; Zhou et al., 2015; Álvarez et al., 2016; Chayut et al., 2016). In biotechnological approaches, PSY overexpression is sometimes combined with the coexpression of the bacterial desaturase CrtI, which functionally replaces the four plant enzymes required for carotene desaturation toward lycopene. When the downstream pathway enzymes are sufficiently active, β-carotene and xanthophylls can be formed (Beyer et al., 2002; Paine et al., 2005; Schaub et al., 2005; Zhu et al., 2008).
However, in addition to carotenoid biosynthesis, the intermediate GGPP acts as the precursor for several essential isoprenoid biosynthetic pathways. In chloroplasts, a large fraction is reduced by GGPP reductase to phytyl diphosphate for phylloquinone and chlorophyll biosynthesis, the latter via chlorophyll synthase (CHLS) catalysis. Subsequently, upon degradation of chlorophylls, the phytyl residue can be remobilized for tocopherol synthesis (Tanaka et al., 1999; Vom Dorp et al., 2015; Almeida et al., 2016). Furthermore, GGPP provides the lipophilic chain for plastoquinone after being converted into solanesyl diphosphate by solanesyl diphosphate synthase (SPS, Hirooka et al., 2005; Hedden and Thomas, 2012). Cyclization of GGPP by ent-copalyl diphosphate synthase followed by dephosphorylation and further ring modification catalyzed by ent-kaurene synthase liberates this precursor from the plastid to undergo numerous cytosolic oxygenation reactions toward biologically active gibberellins (Prisic and Peters, 2007).
GGPSs are encoded by a gene family in Arabidopsis (Beck et al., 2013). Whereas some GGPS isoforms are localized in mitochondria, ER and cytoplasm, at least seven of them reside in plastids (Coman et al., 2014; Ruiz-Sola et al., 2016b). Recently, several plastid-localized isoforms (GGPS6, -7, -9, and -10) were found to predominantly synthesize the C25 prenyl diphosphate geranyl farnesyl diphosphate (GFDP) but only minor amounts of GGPP in vitro which raised question on the exact biochemical function of individual members of this family in planta (Nagel et al., 2015). The isoform GGPS11 is the most abundant one in almost all plant tissues, whereas the remaining five isoforms are preferentially expressed in roots (GGPS6, GGPS7) or weakly expressed in flowers or seeds (Beck et al., 2013). Interaction studies by coimmunoprecipitation, bimolecular fluorescence complementation (BiFC), and split-ubiquitin assays revealed that GGPS11 interacted with enzymes of the major off-branching pathways for chlorophyll, plastoquinone, and carotenoid biosynthesis (Ruiz-Sola et al., 2016b). Interestingly, a cytoplasmic alternative splicing product of GGPS11 encoding a truncated but functional enzyme is essential for embryo development (Ruiz-Sola et al., 2016a). Therefore, GGPS11 is considered an essential enzyme for the synthesis of these GGPP-derived compounds and downstream enzymes are thought to compete for the GGPP substrate. For instance, constitutive overexpression of PSY in tomato plants is reported to frequently result in dwarfism caused by reduced gibberellins levels (Fray et al., 1995).
Because of its high amphipathicity, GGPP is unlikely to diffuse in monodisperse form. This suggests a channeled distribution of GGPP into the different branching pathways allowing its directed conversion by subsequent enzymes avoiding GGPP “leakage”. Metabolite channels can thus be envisioned as (maybe transient) multienzyme complexes that are hardly directly experimentally accessible. Substrate channeling has been implicated in the synthesis of various plant natural products, e.g. cyanogenic glycosides, phenylpropanoids, alkaloids, and flavonoids (Burbulis and Winkel-Shirley, 1999; Jørgensen et al., 2005; Bassard et al., 2012; Laursen et al., 2016).
Some studies are in support of enzyme complexes. For instance, gel permeation chromatography studies suggested that PSY forms high molecular mass complexes containing GGPS and IPP/DMAPP isomerase (Maudinas et al., 1977; Camara, 1993; Fraser et al., 2000). Similarly, high-molecular mass membrane complexes containing carotene desaturases and cyclases (Bonk et al., 1997; Lopez et al., 2008) as well as protein-protein interactions observed for carotene hydroxylases (Quinlan et al., 2012) have been interpreted in terms of a structural basis for metabolite channeling. However, although the interaction between GGPS11 and PSY has recently been shown by yeast two-hybrid protein interaction studies and BiFC, direct biochemical evidence for substrate channeling is missing (Ruiz-Sola et al., 2016b).
In the present work, we approached this channeling conundrum using recombinant GGPS11 and PSY. We identified a strict requirement for de-novo synthesized GGPP to effectively carry out phytoene synthesis which is in favor of protein-protein interaction. Since this interaction could not be shown by classical biochemical methods, we pressure-tested the idea by bringing GGPS11 and PSY together artificially using a synthetic biology approach, i.e. by translationally fusing the two enzymes. The characterization of this “mini-metabolon” in vitro and in planta revealed improved substrate directionality into carotenogenesis. This also suggests that enzyme fusions at central pathway hubs like GGPS are capable of directing metabolite biosynthesis in a targeted manner.
RESULTS
Interactions between GGPS Isoenzymes and PSY
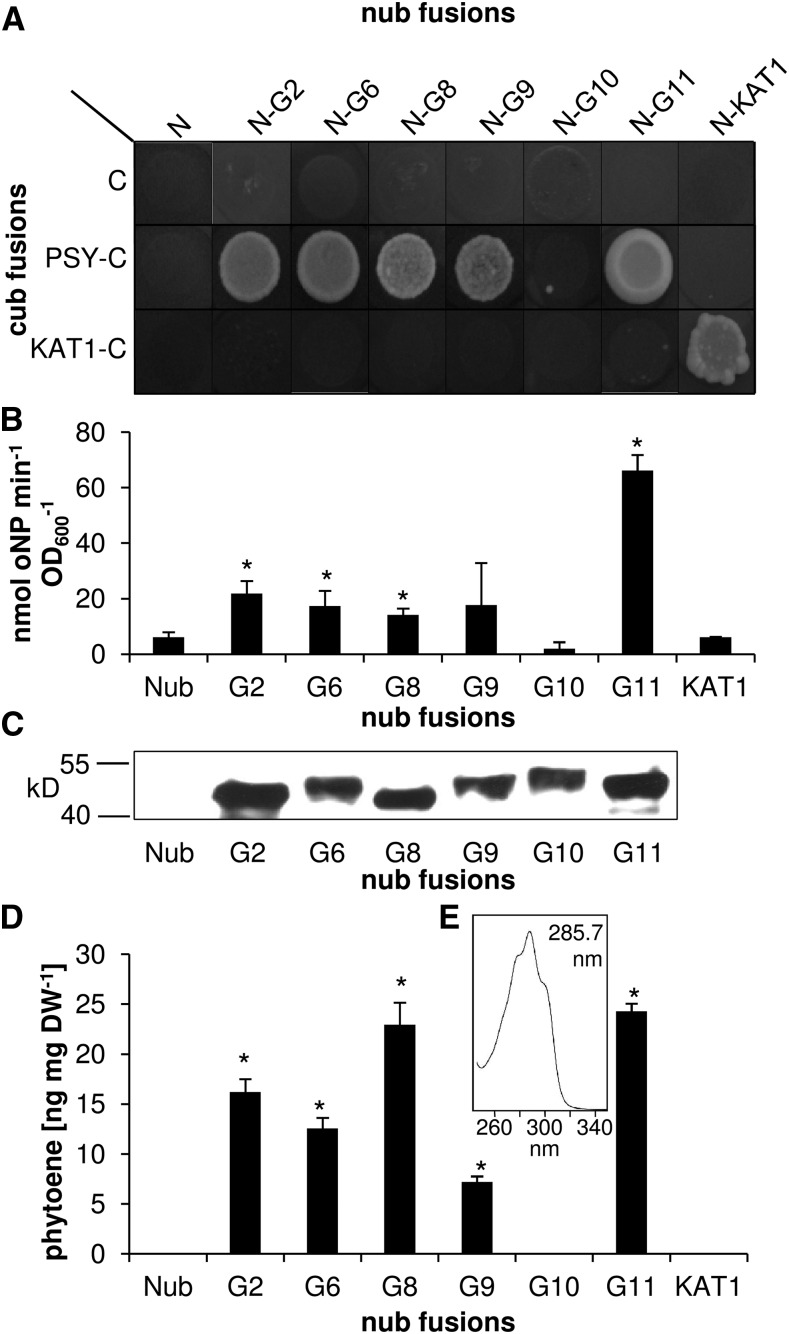
The role of GGPS11 serving several isoprenoid pathways including carotenogenesis was scrutinized by investigating whether other plastid-localized GGPS isoforms show differences in their interaction with Arabidopsis PSY (PSY). We included GGPS2, GGPS6, GGPS8, GGPS9, GGPS10, and GGPS11 for interaction assays using the split ubiquitin system (Fig. 2). GGPS7 was omitted as it shares 95% identity to GGPS6 and both enzymes diverged recently through tandem duplication, suggesting largely redundant functions (Coman et al., 2014). The split ubiquitin assays showed that, in addition to GGPS11, the isoenzymes GGPS2, GGPS6, GGPS8, and GGPS9 interacted with PSY and the resulting yeast cells accumulated phytoene. The only exception was GGPS10, which neither showed interaction nor formed phytoene, although its identity as a GGPS has been reported (Beck et al., 2013). Corroborating previous data, the interaction strength and phytoene levels were highest for the combination of PSY and GGPS11 (Beck et al., 2013; Ruiz-Sola et al., 2016b). However, phytoene levels were similarly high in GGPS8/PSY coexpressing yeast cells, although their interaction strength was about 70% lower compared to that of the GGPS11/PSY combination.
Figure 2.
Analysis of interaction between Arabidopsis GGPS isoforms with PSY. Yeast strains expressing Nub (N) or N-terminal Nub fusions with Arabidopsis GGPS isoforms 2, 6, 8, 9, 10, and 11 were combined with yeast strains expressing Cub only (C) or C-terminal Cub fusion with Arabidopsis PSY, respectively. Corresponding Nub/Cub fusions with the Arabidopsis kation channel protein KAT1 were used as negative controls whereas dimerization of Nub-KAT1/KAT1-Cub served as a positive control. A, Interaction assay. Growth on selective medium, supplemented with 150 µM Met after 2 d incubation. B, β-Gal activity determined by ONPG assays of yeast strains coexpressing combinations with PSY-Cub. C, Nub-GGPS protein levels in PSY-Cub combinations. Nub-GGPS proteins carried an N-terminal 3-HA tag and were detected using a 3-HA antibody. D, Phytoene amounts in PSY-Cub combinations, quantified by HPLC. E, Absorption spectrum of phytoene determined in yeast strains coexpressing PSY-Cub and GGPS11. Results in B and D are mean +/− SEM of three biological replicates. * Significant difference to that in Nub control (P > 0.05).
Individual Kinetic Investigation of Recombinant Arabidopsis GGPS11 and PSY
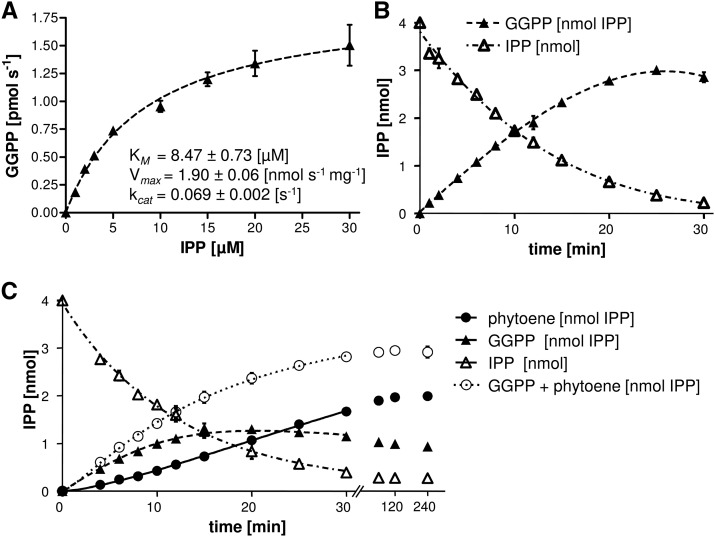
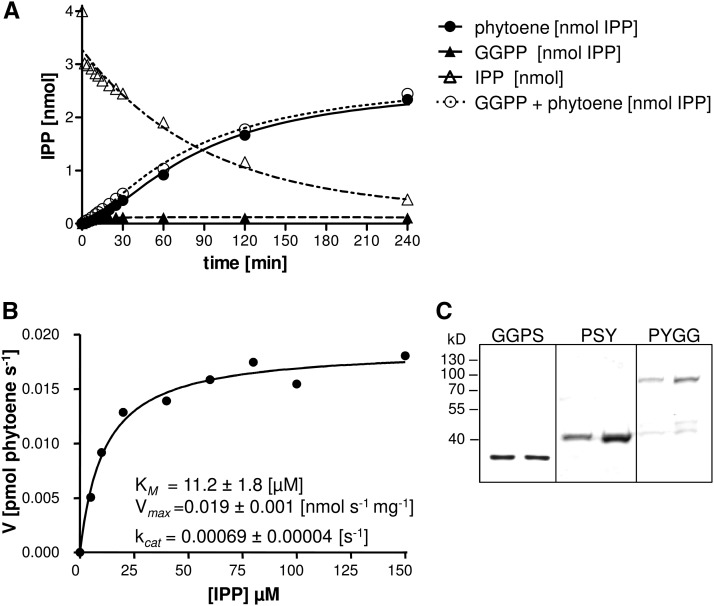
The physical interaction between GGPS and PSY might be required for efficient phytoene synthesis. We therefore investigated the properties of GGPS11 and PSY individually to compare them with those in coupled assays containing both enzymes. GGPS11, truncated by its transit peptide sequence, was expressed as an N-terminally His6-tagged protein in E. coli and purified. Enzyme assays were performed in a biphasic system including phosphatidyl cholin/Tween 80 micelles with a constant 20 µm DMAPP and varying [14C]IPP concentrations (1–30 µm) using isotope-dilution with unlabeled IPP. The [14C]GGPP formed was quantified by scintillation counting after extractive separation from unincorporated IPP (Fig. 3). For Arabidopsis GGPS11, fitting GGPP formation from IPP and DMAPP with the Michaelis-Menten equation allowed the determination of an apparent KM = 8.47 ± 0.73 µm IPP and a Vmax = 1.9 ± 0.06 nmol GGPP s−1 mg−1, corresponding to kcat = 0.069 ± 0.002 s−1. These values are comparable to those determined for the GGPS11 homolog from Sinapis alba (Kloer et al., 2006), with Vmax = 1.8 nmol GGPP s−1 mg−1, but with lower KM = 4.4 µm IPP (kcat = 0.065 s−1). Under standard assay conditions, 1 µg (138 nm) of Arabidopsis GGPS11 was capable of converting 4 nmol of IPP at saturating DMAPP amounts (4 nmol) into GGPP within 30 min of incubation time (Fig. 3).
Figure 3.
Enzyme properties of Arabidopsis GGPS11 and PSY. A, Dependence of GGPS11 activity on substrate IPP concentrations. DMAPP was supplied with constant 20 µm whereas IPP concentrations varied between 1 and 30 µm. Incubation time was 2 min. Data (R2 = 1.0) were fitted with the Michaelis-Menten equation using the GraphPad Prism software (for equations, see Methods). B, Time-course experiment of GGPP formation by GGPS11 under standard assay conditions. C, Time-course experiment of GGPP and phytoene formation under standard assay conditions with equimolar amounts of GGPS11 and PSY. Round open circles represent the sum of GGPP and phytoene. Product concentrations are expressed in IPP equivalents in order to facilitate direct comparison. Note that phytoene synthesis stops almost completely after 30 min although GGPP is still available. Standard assay conditions were 20 µm DMAPP and 20 µm IPP with 138 nm enzyme concentrations. Partial GGPP adhesion on plastic surfaces during sample incubation and transfer explains apparent incongruence of substrate and product amounts. Data are mean ± SEM of three replicate experiments.
In order to determine kinetic parameters of PSY, the PSY cDNA was truncated, removing the sequence encoding the transit peptide, and expressed as an N-terminal His6-tagged fusion protein. The protein was purified after chaotropic refolding, as reported elsewhere (Welsch et al., 2010). The substrate GGPP was synthesized upfront by carrying out a GGPS11 assay under standard conditions (20 µm DMAPP/20 µm IPP), providing 4 µM [14C]GGPP as PSY substrate. However, upon subsequent addition of recombinant PSY (1.22 µg, corresponding to 138 nm), [14C]phytoene was synthesized very slowly. Only 3% of the [14C]GGPP substrate was converted after a 2 h incubation (see Supplemental Table S1). In line with this inaccessibility of presynthesized GGPP, the conversion of externally added [3H]GGPP in the range of 0.5–4 µm was lesser still with only 0.1% conversion into [3H]phytoene after an incubation time of 2 h. Thus, the kinetic parameters for PSY could not be determined with presynthesized GGPP.
Phytoene Synthesis in a Coupled System with Recombinant GGPS11 and PSY
Alternatively, the simultaneous presence of PSY and GGPS11 was tested as a means of improving phytoene formation. These coupled assays contained the same protein and substrate concentrations as in the individual assays described above. In stark contrast to the situation with presynthesized GGPP, the coupled system carried about 60% of the IPP substrate into phytoene within 2 h (Fig. 3). After a short lag phase, the formation of phytoene proceeded almost linearly within the initial 30 min, it then slowed down and fully ceased after 1 h. In addition to phytoene, 30% of the IPP substrate was converted into the intermediate GGPP within 20 min. Then, GGPP amounts slowly decreased to 25% of substrate IPP as the conversion into phytoene exceeded GGPP formation. In this time-course experiment, the sum of both products (GGPP and phytoene) matched quantitatively with GGPP formation in an individual GGPS11 assay (Fig. 3). Incubations with half of the initial IPP concentrations (10 µm IPP with 20 µm DMAPP) preceded with similar product proportions of 60% phytoene and 30% GGPP (Supplemental Fig. S1). In contrast to assays with 20 µm IPP, the increase of GGPP ceased earlier, already after about 10 min and remained constant thereafter. Readdition of PSY after the synthesis of phytoene ceased was incapable of reinitiating phytoene synthesis from accumulated GGPP. This excludes progressive PSY inactivation by denaturation which is frequently observed as an artifact with highly purified proteins (e.g. Koschmieder et al., 2017).
Thus, the phytoene synthesis rate of recombinant PSY increased dramatically when GGPP was supplied by an actively synthesizing GGPS11, while preformed GGPP was hardly accessible to PSY. However, even in coupled assays, GGPP accumulated in addition to phytoene. Thus, GGPP remained unmetabolized suggesting a similar PSY inaccessibility as that seen for preformed or externally added GGPP.
The amphipathic character of GGPP led us to assume that GGPP sequestration into the micelles of the biphasic system used might cause its inaccessibility. We investigated the partition of GGPP into the lipid phase using phosphatidyl choline liposomes (Bozzuto and Molinari, 2015). In line with the above assumption, [14C]GGPP was almost quantitatively recovered in liposomal fractions obtained by ultracentrifugation after synthesis from DMAPP/[14C]IPP by GGPS11 (Supplemental Fig. S2). Thus, rapid sequestration of GGPP into membranes and the inability of this substrate to reach the active site of PSY once membrane-bound might explain why GGPP, once liberated and diffusing, is not efficiently used for phytoene synthesis.
Altered IPP Conversion Efficiency through Direct Protein Fusion
The necessity for immediate GGPP conversion by PSY, in the simplest explanation, calls for close proximity of GGPS11 and PSY to enable substrate channeling. Consequently, the availability of GGPS11 in a correct stoichiometric ratio with PSY might limit the carotenoid biosynthetic flux. A feasible approach to overcome these potential restrictions is to directly fuse GGPS11 with PSY, thereby eliminating potential variables required for the recruitment of GGPS11 by PSY and also potentially eliminating competition from other GGPP-utilizing pathways.
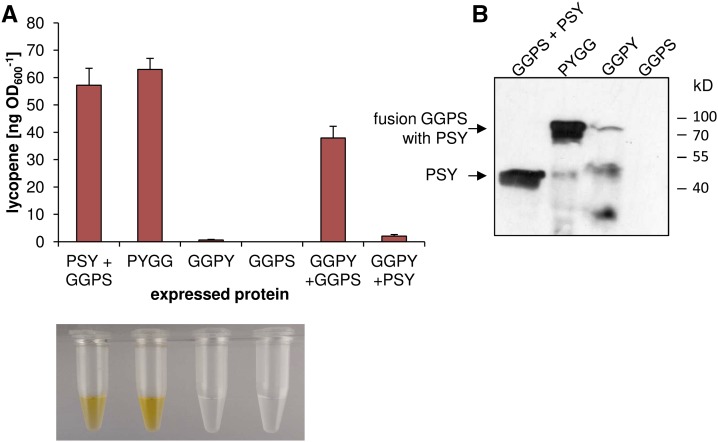
Using the in vivo activity assay in E. coli (Welsch et al., 2000), we evaluated whether an N-terminal or C-terminal translational fusion of GGPS11 and PSY generates a functional enzyme. The additional coexpression of the bacterial carotene desaturase CrtI converting the colorless phytoene into red-colored lycopene provided a photometrically accessible readout for the combined GGPS-PSY activity. No enzymatic activity was observed when GGPS was fused to the N terminus of PSY (GGPS11-PSY, GGPY; Fig. 4). However, activity was recovered by the additional coexpression of individual GGPS11 enzyme, indicating that GGPS11 activity in GGPY was impeded. In contrast, GGPS11 fused to the C terminus of PSY was enzymatically active (Fig. 4). We therefore focused on this fusion variant, which was denoted PYGG (for PSY-GGPS11).
Figure 4.
Enzymatic activity of GGPS11, PSY, and translational fusion proteins in E. coli. GGPS11 (GGPS), PSY, and fusion proteins GGPS11-PSY (GGPY) and PSY-GGPS11 (PYGG) in various combinations were coexpressed with CrtI in E. coli cells. A, Lycopene amounts; acetone extracts are shown below. B, Immunoblot with 60 µg bacterial lysates using anti-PSY antibodies. GGPS11 alone was included as a negative control. Data are means ± SEM of three replicate experiments.
Kinetic Investigation of the PYGG Fusion
Whereas recombinant GGPS11 could be recovered as soluble enzyme from E. coli with the expression system used (Welsch et al., 2000; Kloer et al., 2006), PYGG behaved like PSY in that it was found largely insoluble in inclusion bodies (Supplemental Fig. S3). Accordingly, His6-PYGG was purified with the same chaotropic refolding protocol developed for His6-PSY (see “Material and Methods”). This yielded the monodisperse PYGG fusion protein with the expected molecular mass of 79.6 kD, composed of 35.7 kD for GGPS11 and 44.2 kD for PSY (Fig. 5).
Figure 5.
Standard enzyme assay with PSY-GGPS fusion protein. A, Time-course experiment of phytoene and GGPP formation and IPP consumption under standard enzyme assay conditions with PSY-GGPS11 fusion protein (PYGG) and 20 µm DMAPP/20 µm IPP. Round open circles represent the sum of GGPP and phytoene. Product concentrations are expressed in IPP equivalents in order to facilitate direct comparison. Note that phytoene synthesis continues with almost unchanged velocity for about 2 h. B, Substrate dependancy of recombinant PYGG. IPP was provided with 5, 10, 20, 40, 60, 80, 100, and 150 µm, incubation time was 10 min. Data (R2 = 0.98) were fitted with the Michaelis-Menten equation using the GraphPad Prism software (for equations, see Methods). Results in A and B are means of three replicate experiments. C, Coomassie-stained SDS-PAGE of recombinant purified proteins of His6-GGPS11 (35.7 kD), His6-PSY (44.2 kD), and the fusion protein His6-PYGG (79.6 kD).
Enzyme assays were run with same protein concentrations as previously used under standard concentrations (20 µm DMAPP/20 µm IPP; 138 nm PYGG). As the PYGG fusion protein provided an equimolar ratio of the GGPS11 and PSY moieties, the results were directly compared with the coupled enzyme assays. Phytoene synthesis proceeded almost linearly for 2 h, but with an approximate 4-fold lower velocity than that in coupled assays, and thereafter continued with further reduced reaction velocity (Fig. 5). However, it is interesting to note that in contrast to the coupled assay, [14C]IPP was almost quantitatively converted into [14C]phytoene after 4 h. Only trace amounts of [14C]GGPP “leakage” was observed and this did not increase with continued reaction time (Fig. 5). This supports efficient GGPP channeling from GGPS11 to PSY in the PYGG protein fusion (Bera et al., 2000). Kinetic constants for PYGG were determined by fitting phytoene formation from IPP and DMAPP with the Michaelis-Menten equation and revealed an apparent KM = 11.2 ± 1.8 µm IPP and a Vmax = 0.019 ± 0.001 nmol phytoene s−1 mg−1 (kcat = 0.00069 ± 0.00004 s−1; Fig. 5).
Determination of Single Enzyme Activities in the Fusion Protein
Despite the strongly increased proportion of phytoene formed upon enzyme fusion, the concomitantly reduced phytoene synthesis rate may indicate that the individual kinetics of the GGPS11 and/or PSY moieties might also be affected. To decipher these possibilities, we reciprocally inactivated one or the other of the two enzymes in PYGG by introducing loss-of-function mutations. We then complemented the mutant PYGG versions by adding the respective wild-type enzyme. If this restored phytoene formation even beyond the levels formed with wild-type PYGG, the enzyme moiety impaired in the fusion protein would be identified.
To test the activity of the GGPS11 fusion moiety, PSY loss-of-function mutants were generated by introducing single-amino acid changes known to inactivate squalene synthase, which shares structural and catalytic similarities with PSY (Gu et al., 1998). The two independent PSY mutant versions were in the Asp-rich motif, namely PSY D313E and PSY D317E. The mutant fusion proteins were named pyGG1 and pyGG2, respectively. To test whether PSY activity was impaired in PYGG, GGPS11 mutants were designed based on amino acid substitutions known to strongly reduce farnesyl diphosphate synthase activity (Marrero et al., 1992; Joly and Edwards, 1993). In one mutant, two Asp residues in the second Asp-rich motif (SARM) were substituted by Lys, yielding GGPS11 D297E-D298E. In the other mutant, two Arg residues closely adjacent to the first Asp-rich motif (FARM) were replaced by Lys, yielding GGPS11 R166K-R167K. These mutant fusion proteins were named PYgg1 and PYgg2, respectively.
Prior to introducing these mutations into PYGG, we confirmed their inactivating effect through heterologous expression of individually mutated PSY and GGPS11 enzymes in our E. coli in vivo assay system coexpressing CrtI. In agreement with their intended function, not even trace amounts of lycopene were observed with any of the mutants whereas positive control combinations with wild-type enzymes accumulated up to 32 ng lycopene OD600−1 (Supplemental Fig. S4).
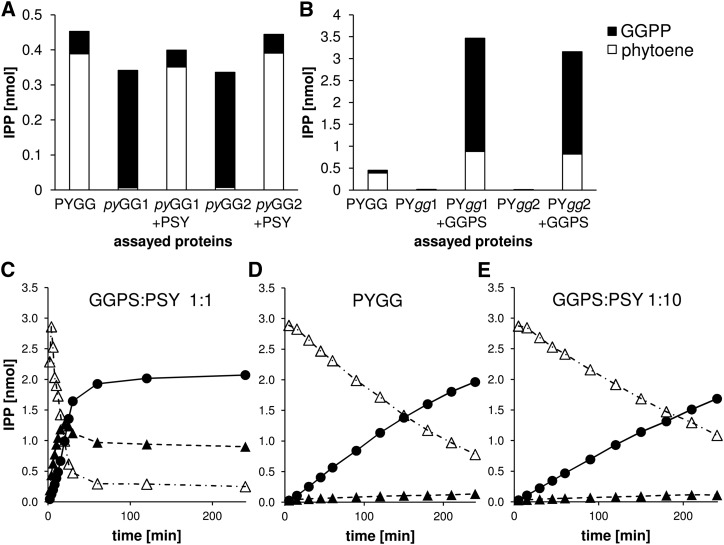
The fusion protein mutants were purified and incubated under standard assay conditions, either individually or supplemented with wild-type recombinant PSY or GGPS11. As expected, both pyGG mutants were incapable of synthesizing phytoene and accumulated GGPP exclusively (Fig. 6). Addition of equimolar amounts of recombinant wild-type PSY completely rescued phytoene synthesis, but the levels were identical to those obtained with the wild-type PYGG fusion protein. This indicates that enzymatic properties of the PSY enzyme moiety remained largely unaffected through the fusion with GGPS11.
Figure 6.
Complementation of mutated chimeric PSY-GGPS proteins. A and B, Recombinant PYGG was incubated with 20 µm DMAPP/20 µm IPP for 1 h, then amounts of GGPP and phytoene were determined and expressed in nmol IPP equivalents. A, Assay of PYGG fusion protein and versions with impaired PSY (pyGG1 and pyGG2) complemented with wild-type PSY (+PSY). B, Assay of PYGG fusion protein and versions with impaired GGPS11 (PYgg1 and PYgg2) complemented with wild-type GGPS11 (+GGPS). C to E, Standard enzyme assay with equimolar concentrations of GGPS11 and PSY (C), PYGG (D) and GGPS11 and PSY in a molar ratio of 1:10 (E). All assays were performed with 138 nm PSY and PYGG and 20 µm DMAPP/20 µm IPP. Data are means of three replicate experiments.
As expected, both PYgg mutants were enzymatically inactive and did not produce even trace amounts of GGPP (Fig. 6). Upon addition of recombinant wild-type GGPS11, slightly higher phytoene amounts compared to the wild-type PYGG control were produced. However, the levels of free GGPP increased dramatically to about 4-fold the amounts of phytoene. This indicated that the activity of GGPS11 in wild-type PYGG was strongly reduced upon the N-terminal fusion with PSY.
Emulating PYGG Kinetics with Adjusted Stoichiometry of Individual Enzymes
In conclusion, the reduced activity of the GGPS11 moiety in PYGG accounts for the reduced velocity of phytoene synthesis. However, this circumstance might also contribute to the improved efficiency, i.e., the strongly reduced GGPP leakage upon phytoene formation by the fusion protein. Accordingly, the coupled system with individual enzymes would be expected to behave similarly when the GGPP synthesis rate is reduced, e.g. through an altered GGPS11:PSY ratio. Based on the previously determined Michaelis-Menten parameters, we performed in silico assays of GGPS11 and PSY, which relied on the discrete solving of the Michaelis-Menten equations. The use of these computer simulations allowed us to examine the consequences of varying GGPS11 concentrations at constant PSY concentrations on phytoene formation in the coupled system. Using this approach, a GGPS11:PSY molar ratio of approximately 1:10 was predicted to match the phytoene formation velocity determined for PYGG, thus much lower than the 1:1 ratio used in the previous coupled assays (Supplemental Fig. S5). Confirmatory, coupled enzyme assays with individual enzymes provided in a 1:10 ratio resulted in reaction kinetics identical to those observed with PYGG (Fig. 6). In both cases, IPP conversion efficiency into phytoene was maximal with only trace amounts of accumulated GGPP.
Expression of the Chimeric Protein in Arabidopsis
The data from mutagenesis, the simulation results, and their verification by coupled assays indicate that substrate channeling may not be the underlying cause of the PYGG characteristics. Rather, considerably reduced GGPP leakage might be the result of altered kinetics of the fused two-enzyme cascade. However, adjusting the conditions of the coupled assay to meet the characteristics of PYGG does not rule out channeling. Logistically, it is not straightforward to test for true channeling with GGPS and PSY. Substrate competition experiments (with GGPP and IPP) that are classic in the investigation of metabolite channels (Møller and Conn, 1980) are not applicable here because of the inaccessibility of GGPP upon membrane or micellar sequestration (see above).
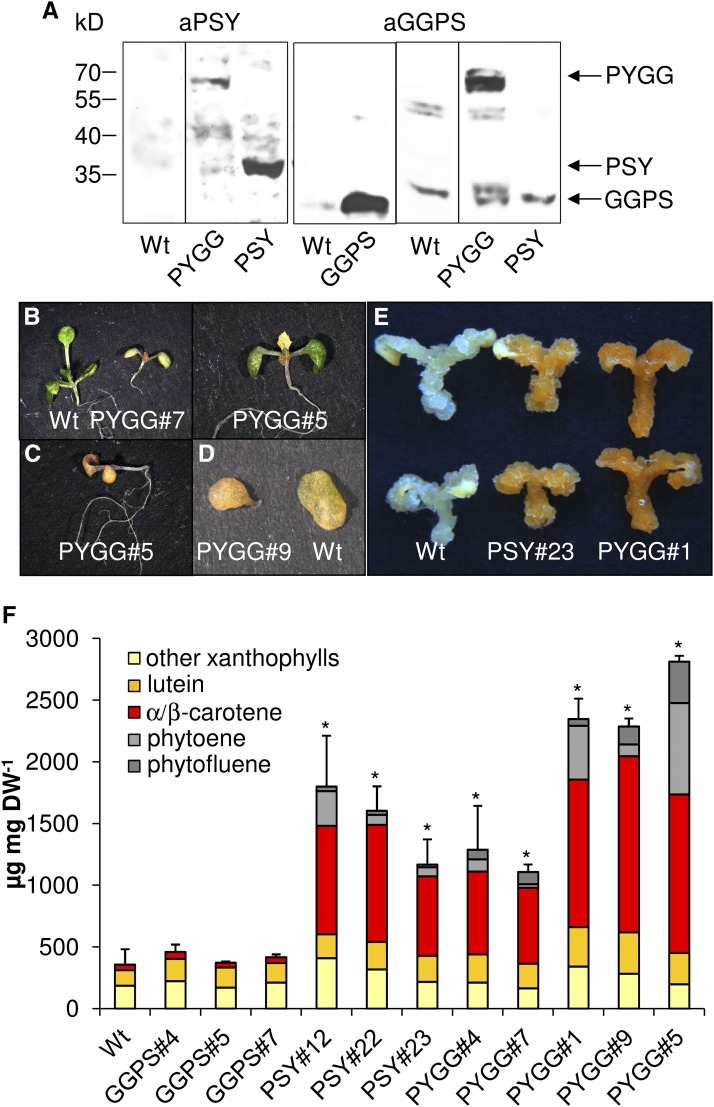
Assuming that PYGG is, in fact, capable of improving the metabolite flux into carotenogenesis through substrate channeling, the fusion was expressed in planta. This was guided by the expectation that, compared to individual enzyme overexpression, the competition for GGPP from other branches of the isoprenoid pathway may be altered. PYGG was expressed under control of the constitutive CaMV-35S promoter in Arabidopsis. For control lines, we also overexpressed GGPS11 individually under control of the nos promoter and included lines constitutively overexpressing PSY as published (Maass et al., 2009). In contrast to the recombinant proteins used so far in vitro, GGPS11 and PSY carried their native N-terminal transit peptides and PYGG was translationally fused with the PSY transit peptide. Efficient transit peptide processing was confirmed by immunoblot analysis using callus protein extracts (see Fig. 7) from one selected GGPS11, PYGG, and PSY-overexpressing line each. PYGG was detected with its correct molecular mass, calculated as 63.5 kD after plastid import.
Figure 7.
Carotenoid amounts in PYGG-overexpressing calli. Arabidopsis GGPS11, PSY, and the fusion protein PYGG were constitutively overexpressed in Arabidopsis. Callus was developed from seeds for 5 d in the light, followed by two weeks etiolation on callus-inducing medium. A, Detection of GGPS11, PSY, and PYGG in calli. Callus protein extracts from the wild type and one line overexpressing GGPS11 (GGPS), PSY, and PYGG were probed with anti-PSY antibodies (aPSY) and anti-GGPS11 (aGGPS) antibodies, respectively. Arrows indicate band positions corresponding to PYGG, PSY, and GGPS11. B to D, Phenotype of wild-type and PYGG-expressing seedlings grown for 7 d under long-day conditions (B, left and right), dark-grown seedlings (C), and detached primary leaves of dark-grown seedlings (D). E and F, Representative images (E) and carotenoid contents (F) of calli. Results are means ± SEM of three biological replicates. Asterisk indicates significant difference compared to that in the wild type (Student’s t test, P < 0.05).
From a total of 12 PYGG-expressing lines, viable homozygous lines could be generated only from five lines which did not show phenotypic deviation from the wild type. In contrast, all other lines exhibited about 25% of seedlings with orange cotyledons and/or primary leaves that did not continue to grow on soil and thus did not set seeds (Fig. 7). Upon continued growth on MS medium, leaves turned only slightly green with large white/transparent areas and showed extreme dwarfism. The remaining seedlings from these lines grew without phenotypic deviations from the wild type (Supplemental Fig. S6). Homozygous GGPS11-overexpressing lines were generated and showed no differences to Wt seedlings, as already observed in Nagel et al. (2015). Increased GGPS11 protein levels were confirmed by immunoblot analysis (Fig. 7).
In order to determine carotenoid pathway flux in nongreen cells, we employed callus tissue generated from germinating seedlings. This system was established recently for routine analysis of carotenoid pathway fluxes (Schaub et al., 2018). Calli from lines overexpressing GGPS11 showed no increase in carotenoid content whereas those overexpressing PSY increased total carotenoid content to 1800 µg mg DW−1 in the strongest lines. Interestingly, carotenoids in PYGG-expressing callus of lines from which homozygous plants could not be obtained showed a further increase in carotenoid levels by almost 50% to up to 3000 µg mg DW−1 (Fig. 7).
DISCUSSION
PSY Interacts with Most Plastid-Localized GGPS
GGPSs constitute a small gene family with twelve members in Arabidopsis, of which at least seven are plastid-localized (Beck et al., 2013; Coman et al., 2014). The reason for this redundancy remains unclear. Only GGPS11 knock-out lines are lethal whereas mutant lines of almost all other plastid-targeted GGPS isoenzymes do not show aberrant phenotypes (Ruiz-Sola et al., 2016b). Interestingly, GGPS6, -7, -9, and -10 were unable to rescue GGPS11 knock-out lines following their overexpression (Nagel et al., 2015). Moreover, GGPS11 has the highest expression level in most Arabidopsis tissues, especially in photosynthetic tissues, whereas expression of other GGPS isoforms dominate in roots, seeds, and flower organs (Beck et al., 2013). Thus, GGPS11 is considered as the essential enzyme for the major GGPP-consuming plastid-localized pathways, including carotenogenesis, and it is assumed that there is functional specialization for the remaining GGPS isoforms.
It is therefore somewhat surprising that PSY interacted not only with GGPS11 (Ruiz-Sola et al., 2016b), but with all other plastid-localized GGPS isoforms (GGPS2, -6, -8, and -9), except GGPS10 (Fig. 2). Moreover, again with GGPS10 as an exception, all GGPS isoforms included were capable of providing substrate GGPP for phytoene synthesis via PSY following coexpression in yeast cells.
The incapability of GGPS10 to functionally interact with PSY following coexpression in yeast conflicts with the observed phytoene synthesis following its coexpression with the bacterial phytoene synthase CrtB in bacteria (Beck et al., 2013). However, CrtB is active even with a GGPS isoform that does not colocalize with PSY in plastids e.g. with ER-localized GGPS3 (Beck et al., 2013).
This suggests that plant and bacterial phytoene synthases might differ in their modes of accepting substrate GGPP. Whereas under the conditions used, PSY activity strictly depends on actively synthesized GGPP supply in vitro, as demonstrated here, and PSY is incapable of metabolizing free or liposome-bound GGPP, recombinant CrtB was shown to efficiently convert substrate GGPP into phytoene, independent of an active GGPS (Iwata-Reuyl et al., 2003). Assuming that this property was present in phytoene synthases of early photosynthetic prokaryotes, its loss in plant phytoene synthases might be correlated with the diversification of GGPP-derived metabolites and the need to more tightly coordinate carotenogenesis with other pathways. This might be facilitated by the establishment of functional dependencies in the form of protein-protein interactions.
The capability of GGPS2, -6, -8, -9, and -11 to synthesize GGPP, which is concluded from this study and the work of Beck et al. (2013), conflicts with results from in vitro approaches with recombinant enzymes (Nagel et al., 2015). This revealed the C25 prenyl diphosphate geranylfarnesyl diphosphate (GFDP) as the predominant product for GGPS6 and -9, whereas GGPP was found only in trace amounts. Only GGPS2 and -11 were found to exclusively synthesize GGPP whereas GGPS8 did not show any enzymatic activity in these investigations. However, the fact that phytoene was formed for those GGPSs previously characterized in vitro as (mainly) GFDP-synthesizing enzymes doubtlessly shows that GGPP is indeed produced. Moreover, GGPP is synthesized in amounts which are high compared with those synthesized by GGPS11 (Fig. 2; Beck et al., 2013). This discrepancy to the in vitro situation might suggest that some GGPS isoforms are capable of altering their product pattern. This might be caused by different conditions of the system (in vitro versus in vivo when expressed in yeast or bacterial cells), but more likely depends on GGPP turnover by the activity of subsequent enzymes. Additional biochemical investigations with combinations of GGPS isoforms and downstream enzymes are required to answer these questions.
GGPS11-PSY Interaction Facilitates Efficient Substrate Metabolization
Recombinant PSY was almost inactive when preformed GGPP was used as substrate, either when added from solution or provided in a presynthesized form by GGPS11. In contrast, the simultaneous presence of active GGPP-synthesizing GGPS11 allowed phytoene formation at high rates (Fig. 3). This observation suggests the existence of microdomains between GGPS11 and PSY allowing GGPP to reach high local concentrations and to be metabolized into phytoene before it escapes from these microdomains (Lee et al., 2012). Moreover, GGPP incorporates into liposomal membranes, which renders it inaccessible for phytoene synthesis. Thus, the spacial proximity of GGPS11 and PSY is a precondition for efficient phytoene synthesis. This is ensured by protein-protein interaction, as shown by the split-ubiquitin system as well as through BiFC (Fig. 2; Ruiz-Sola et al., 2016b). Enzyme fusion between GGPS11 and PSY may well ensure the close proximity between GGPS and PSY, thereby allowing efficient GGPP metabolization.
The advantage of efficient substrate conversion in microdomains is not restricted to amphipathic compounds and is also observed, for instance, for soluble metabolites like cAMP (between adenyl cyclases and phosphodiesterases) and ATP (between creatine kinases and myosin ATPase; Selivanov et al., 2007; Baillie, 2009). Considering the amphipathic properties of GGPP it appears possible that these observations are not restricted to the GGPS11/PSY metabolon, but may as well apply to other branched pathways in planta. Although recombinant oat CHLS accepts free GGPP for chlorophyllide esterification and Arabidopsis SPS2 accepts free GGPP for prenylquinone synthesis, CHLS enzyme activities are weak and data on comparative assays done in the presence of active GGPS are not available (Schmid et al., 2001; Hirooka et al., 2003, 2005). Thus, regulated composition and stability of GGPS-containing metabolons might be essential to distribute GGPP into different metabolite avenues. Interestingly, Zhou et al. (2017) recently identified a GGPS-recruiting protein in rice (OsGRP) that efficiently competes with stromal rice GGPS1 dimerization and recruits it into large thylakoid membrane protein complexes including GGPP reductase, CHLS, as well as other proteins involved in chlorophyll biosynthesis. They found that OsGRP increases GGPP synthesis activity and specificity, evidently functioning as a complex organizer to support efficient substrate channeling toward chlorophyll biosynthesis.
Protein Complexes in the Carotenoid Pathway
Several observations support substrate channeling from IPP to phytoene in the carotenoid pathway. For instance, native plastid extracts from tomato fruits, daffodils flowers, and Capsicum fruits were shown to synthesize phytoene directly from IPP (Porter and Spurgeon, 1981; Lützow and Beyer, 1988; Fraser et al., 2000). Interestingly, intermediates did not accumulate nor were they accepted efficiently as substrates when added to the assay. This indicates that the entry of diffusible metabolites is largely prevented which is in agreement with our findings.
In contrast, the accessibility of lipophilic substrates in vitro to carotenogenic enzymes downstream to phytoene synthesis appears completely different. Both recombinant phytoene desaturase (PDS), β-carotene cyclase as well as carotenoid isomerase were enzymatically very active when lipophilic substrates were supplied in liposomes (Isaacson et al., 2004; Yu and Beyer, 2012; Gemmecker et al., 2015; Brausemann et al., 2017; Koschmieder et al., 2017). It was shown that PDS and ζ-carotene desaturase interacts monotopically with membranes to access their substrates (Koschmieder et al., 2017). Mathematical modeling implied that substrate channeling occurred between subunits of PDS homotetramers, whereas no kinetic information is available to date to show whether a channeling relation also exists between the different membrane-bound enzymes. These different properties compared with the clear dependency of PSY on an active GGPS11 may reflect different substrate solubilities: the highly amphipathic character of GGPP may necessitate a “special treatment”, whereas this is not the case with the uniformity of the lipophilic downstream metabolites. It may as well reflect the pronounced pathway branching point at the GGPP stage of the pathway.
The Advantage of the Translational Fusion between GGPS and PSY
The translational fusion between GGPS11 and PSY did not show accelerated phytoene formation. However, it exhibited improved substrate conversion efficiency by strongly reducing GGPP leakage, which generates a proportion of this intermediate that represents a dead end. One reason is the reduced GGPS11 activity (Fig. 5). This leads to a more balanced rate of GGPP formation for PSY to cope with, which is the rate-limiting step in this two enzyme cascade. It appears confirmatory, at first glance, that GGPP accumulation can be abolished by reducing GGPS11 amounts. Thus, one might argue whether reduced GGPS activity or improved channeling—or both—contribute to the improved efficiency observed. The only feasible way of testing a contribution of channeling is to test whether phytoene synthesis by PYGG is independent from competitive interactions with other GGPP-consuming reactions in vivo occurring at the GGPS11 hub (Ruiz-Sola et al., 2016b).
In fact, carotenogenesis responded differently to PYGG expression compared to that following the overexpression of individual GGPS11 and PSY. Higher levels of GGPS11 did not increase carotenoids and chlorophylls in leaves or carotenoids in the nongreen Arabidopsis callus system (Fig. 7). This may not be surprising assuming that specific GGPS11-containing metabolons control GGPP distribution, which would in turn be affected by stoichiometrically determined relative enzyme abundances of branching pathways or regulated complex formation. However, overexpression of PSY is known to increase carotenoid levels, but only in nongreen tissues (Fraser et al., 2007; Maass et al., 2009; Welsch et al., 2010; Bai et al., 2011). In green tissues, PSY overexpression has little effect on carotenoid accumulation just like the overexpression of GGPS. This is partially due to compensation by enhanced cleavage of xanthophylls (Lätari et al., 2015) but can as well reflect the GGPS/PSY protein stoichiometry. It remains to be investigated whether simultaneous overexpression of both GGPS11 and PSY is capable of increasing the abundance of active complexes and carotenoid amounts also in green tissues. These restrictions regarding complex formation, which might be different for green and nongreen systems, are overridden by PYGG-expression which results in increased carotenoid amounts in nongreen tissues as well as an approximate doubling of carotenoids in Arabidopsis callus, as compared with that in the strongest PSY-overexpressing lines.
The novelty in the PYGG-expression experiment resides in the fact that, for the first time after years of overexpressing individual or combined individual carotenoid genes in Arabidopsis, green leaves (cotyledons and primary leaves) show an orange phenotype (Fig. 7). This confirms, first, that the fusion protein is stable in plants and not subjected to nonspecific or targeted protein degradation and, second, the fusion is capable of increasing the IPP conversion efficiency specifically toward the synthesis of carotenoids. Moreover, we find a most evident impairment with chlorophyll formation. In light of the channeling discussion raised, the simplest explanation may be that the CHLS-branching point is in fact GGPP depleted and that, consequently, the fusion provides a directed and channeled metabolic flux toward carotenogenesis.
Other Natural and Synthetic Chimeric Enzymes
Bifunctional enzymes are widespread in various plants and sometimes exist even in parallel to their individual counterparts in related species (Hagel and Facchini, 2017). For instance, the epimerization of reticuline in the benzylisoquinoline alkaloid pathway occurs via two individual enzymes in field poppy whereas a single protein combining both enzymes separated by a short spacer region catalyzes the identical reaction in opium poppy. Moreover, natural enzyme fusions are also known from the carotenoid pathway, e.g. a bifunctional enzyme in the fungus Mucor circinelloides carries phytoene synthase and lycopene cyclase activity, which are encoded by separate genes in other organisms (Velayos et al., 2000). Bifunctional lycopene cyclases are known from the cyanobacterium Prochlorococcus marinus and the prasinophyte algae Ostreococcus lucimarinus generating β,β-, β,ε-, or ε,ε-carotene in various proportions (Stickforth et al., 2003; Blatt et al., 2015). One further example with remarkable similarity to the GGPS11-PSY fusion is known from the precursor biosynthesis for fungal fusicoccins, which involve GGPP as substrate. The corresponding chimeric enzyme includes a GGPS as well as a terpene cyclase domain (Toyomasu et al., 2007).
Moreover, several synthetic fusions of enzymes involved in isoprenoid biosynthesis revealed similarly improved efficiency as observed for PYGG. For instance, a fusion between farnesyl diphosphate synthase (FPS) and aristolochene synthase revealed more efficient substrate to product conversion compared with single enzyme reactions in vitro (Brodelius et al., 2002). Expression of a chimeric enzyme composed of amorpha-4,11-diene synthase (ADS), the rate-limiting enzyme of artemisinin biosynthesis, and FPS revealed high product levels, which were explained by higher local concentration of FPP at ADS than in the single enzyme situation that might enable ADS to operate at substrate saturation (Han et al., 2016). For further investigation, we propose the exploitation of PYGG in other nongreen plant tissues, such as tomato fruit, to show whether this synthetic bifunctionality might boost the pathway beyond the overexpression of PSY alone. This potential is already indicated by the strongly increased carotenoid formation in the callus system (Fig. 7). In light of the positive findings with other engineered enzyme fusions, e.g. combining xylanase and laccase activity (Ribeiro et al., 2011) or combining 4-coumarate CoA-ligase and stilbene synthase (Zhang et al., 2006), we find this a potentially valuable synthetic avenue to be pursued, which would also provide sufficient amounts of biological material required for in-depth biochemical analyses.
MATERIALS AND METHODS
Yeast Split Ubiquitin Assay
The split-ubiquitin system was used as described (Obrdlik et al., 2004; Welsch et al., 2018). ORFs were truncated by the length of transit peptides predicted by ChloroP (Emanuelsson et al., 1999; see Supplemental Table S2). GGPS11 cDNAs were cloned into pNXgate in THY.AP4 and mated with PSY-Cub, present in THY.AP5 (Cub) and the resulting diploid cells were cultured in synthetic complete medium lacking Leu and Trp. Interaction growth tests were performed on synthetic minimal agar, supplemented with 150 µM Met to reduce background activation of reporter genes. For β-galactosidase assays and phytoene extraction, yeast strains were grown overnight in synthetic complete medium supplemented with adenine and His at 28°C in order to eliminate growth differences caused by different interaction strengths. β-Galactosidase activity was determined with ortho-nitrophenyl-β-galactoside (ONPG) in biological triplicates as described (Chayut et al., 2016) and expressed relative to cell density measured at 600 nm.
For phytoene extraction, yeast cells from 200 mL of culture were pelleted and lyophilized. Four mL of ethanol containing 2 Sudan IV (Sigma-Aldrich) as internal standard was added. For saponification, 120 µL KOH (1 mg mL−1) was added and samples were incubated at 85°C for 5 min. After cooling on ice, 6 mL 1% NaCl (w/v) and 2 mL of petroleum ether:diethyl ether (2:1, v/v) was added. Samples were mixed and centrifuged. The petroleum ether:diethyl ether step was repeated, and the combined epiphases were dried and dissolved in 30 µL of chloroform. Ten microliters of each sample was injected into the HPLC system equipped with a C30 column (YMC Europe) using a gradient system (Hoa et al., 2003). Phytoene peaks were normalized relative to the internal standard and quantified according to Schaub et al. (2005).
Protein extracts from yeast cells were prepared according to Wang et al. (2004). Cells were lysed in 50 µL of 1.85 m NaOH per 3 A600 units and incubated on ice for 10 min. An equal volume of 50% (w/v) trichloroacetic acid was added, and proteins were recovered by centrifugation for 5 min. The pellet was suspended in 50 µL of SDS sample buffer containing 8 m urea and combined with 20 µL of 1 m Tris and incubated for 1.5 h at 37°C. Proteins were separated by SDS-PAGE, then blotted onto PVDF membrane. Immunodetection was performed using anti-HA antibodies (Sigma).
Protein Purification and Analysis
For recombinant His6-PSY expression, the PSY cDNA devoid of its sequence encoding the transit peptide was subcloned into the vector pCOLD1, revealing pCOLD1-PSY (Welsch et al., 2010). For pCOLD1-PYGG, the NcoI/PstI fragment from pRSF-PYGG (see below) was subcloned into pCOLD1, and digested similarly. Purification of His6-PSY and His6-PYGG was performed as described in Welsch et al. (2010). Aliquots of recombinant proteins were frozen in liquid nitrogen and stored at -80°C until use.
The recombinant His6-GGPS11 was created using the cDNA of GGPP11 from Arabidopsis (base 238 to 1189, accession no AK227130) was inserted into vector pETDuetTM-1 (Novagen) creating pET-GGPS11 that was transformed into E. coli BL21 (Novagen). Cells were grown at 37°C until an OD600 of 0.5 and expression was induced with 1 mm isopropylthiogalactoside (IPTG) for 4 h. Cells were centrifuged (10 000 g/10 min), resuspended in 10 mL buffer A (20 mm Tris/HCl, pH 8.0, 100 mm NaCl, 10 mm MgCl2, 10% [v/v] glycerol), disrupted with a French Press (Amicon), and centrifuged (10 000 g, 10 min). Subsequently, 600 µL TALON Co2+ resin (Clontech), equilibrated in buffer A, was added to the supernatant. After 30 min incubation on ice, the suspension was loaded onto an empty 5-mL TALON column (Clontech), then retained TALON resin was washed with 20 mL buffer A and His6-GGPS11 was eluted with 5 mL buffer A supplemented with 100 mm imidazol. Protein concentration was determined with Bradford assay (Bio-Rad) and the protein was stored at -20°C.
Enzyme Assays
In vitro enzyme assays were performed in a final volume of 200 µL. Activity of recombinant PSY depends on detergents (Iwata-Reuyl et al., 2003), which did not affect GGPS activity; thus the same buffer was used for all recombinant enzymes/combinations. All assay components except substrates were premixed in enzyme reaction buffer (100 mm Tris/HCl, pH 7.6; 0.08% [v/v] Tween 80; 20% [v/v] glycerol, 2 mm MnCl2; 10 mm MgCl2; 1 mm TCEP; 600 mm NaCl; 62.5 ng µL−1 phosphatidylcholin liposomes). For individual PSY assays [3H]GGPP (50 Ci mmol−1; in ethanol:NH4OH:H2O [6:3:1]; American Radiolabeled Chemicals) and for coupled and PYGG assays the substrate mixture (20 µm DMAPP, 16.25 µm IPP [isoprenoids.com] and 3.75 µm [14C]IPP [50 mCi mmol−1; in ethanol:NH4OH:H2O [6:3:1]; American Radiolabeled Chemicals]) was added to start the reaction, respectively. For standard assays, enzymes were used at 138 nm each. Reactions were stopped by adding 50 µL 160 mm EDTA. For extraction, 200 µL BuOH was added, mixed, and centrifuged (21 000 g, 5 min). The BuOH hyperphase containing [14C]GGPP and [14C]phytoene ([14C]IPP remained in the hypophase) was transferred into a new tube and the extraction was repeated with 100 µL BuOH. Combined BuOH extracts were mixed with 300 µL of 1 m MgCl2 in MeOH, inverted, centrifuged, 300 µL heptane was added, centrifuged, and the heptane hyperphase (containing [14C]phytoene; [14C]GGPP remained in the hypophase) was transferred into a new tube. The extraction was repeated with 200 µL of heptane. All fractions were measured by scintillation counting (Tri-Carb 2900 TR, Perkin-Elmer) in 6-mL scintillation cocktail (Rotiszint eco plus, Roth).
For GGPP sequestration assays, 20 µM DMAPP/[14C]IPP were converted into [14C]GGPP using 10 µg GGPS11 overnight in enzyme reaction buffer devoid of detergents and liposomes. 62.5 nmol µL−1 liposomes were added for 5 and 30 min, samples were centrifuged at 100 000 g for 1 h, pellets and supernatant were differentially extracted, and amounts of [14C]GGPP were determined by scintillation counting.
For GGPP distribution measurements, enzyme reaction buffer was prepared like above without Tween 80. Four pmol [3H]GGPP and 4 nmol GGPP (isoprenoids.com) were added, incubated for 5 and 30 min and centrifuged for 1 h at 100 000 g. The supernatant was transferred into a new tube and the liposome pellet was resuspended in 200 µL H2O. Supernatant and resuspended liposomes were measured by scintillation counting.
Protein Co-Expression in E. coli
pRSF-PSY and pACYC-GGPS11 vectors contained corresponding cDNAs devoid of sequences encoding transit peptides in the plasmids pRSFDuet and pACYCDuet, respectively. pRSF-GGPS-PSY and pRSF-PSY-GGPS were made by overlap extension PCR with the GGPS11 cDNA (amplified from pACYC-GGPS11) and the PSY cDNA (amplified from pRSF-PSY) and combined by Gibson assembly in NcoI-digested pRSFDuet (Gibson et al., 2009). A 20 amino acid linker containing alternating Gly and Ser residues (Chen et al., 2013) was introduced between the two proteins.
Mutations were introduced in GGPS11 and PSY by overlap extension PCR (primers see Supplemental Table S2) from plasmids pET-GGPS11 (R166K-R167K; D297E-D298E) and pCOLD1-PSY (D313E; D317E), respectively. pCOLD1-PYGG vectors containing mutated GGPS11 versions were made from corresponding pET vectors exchanging a XhoI/Bsu36I fragment with pCOLD1-PYGG. pCOLD1-PYGG vectors containing mutated PSY versions were made from corresponding pCOLD1 vectors by subcloning amplified PCR fragments containing introduced EcoRI/SmaI sites.
For lycopene assays, pRSFDuet containing separate PSY and GGPS11 ORFs was combined with pCDF-CrtI and used as positive control. pRSF-GGPS-PSY and pRSF-PSY-GGPS were combined with pCDF-CrtI. pCOLD1-PSY and mutants thereof were combined with pACYC-GGPS and pCDF-CrtI while pET-GGPS11 and mutants thereof were combined with pRSF-PSY and pCDF-CrtI.
For lycopene extraction, E. coli pellets were resuspended in 300 µL acetone, centrifuged (4 000 g, 5 min), and the supernatant transferred into a new tube. The pellet was re-extracted two times with 300 µL acetone each. Combined acetone supernatants were evaporated in a speed vac and resuspended in petrol ether. Lycopene was determined photometrically using ε474nm = 185 230 L mol−1 cm−1.
Generation of Transgenic Arabidopsis Lines and Growth Conditions
For PYGG-expressing Arabidopsis lines, the GGPS11 cDNA was amplified by PCR thereby providing an in-frame 5′ extension encoding a 20-amino acid Gly/Ser linker (Chen et al., 2013). The ORF was fused with the PSY ORF by overlap extension PCR and subcloned into pCAMBIA1390-35Spro (Álvarez et al., 2016), revealing pCAMBIA1390-35S::PYGG. For GGPS11-overexpressing lines, the GGPS11 ORF was subcloned into pCAMBIA1390-nospro. Arabidopsis (eco-type Wassilewskija) was transformed by vacuum infiltration (Bechtold and Pelletier, 1998). Homozygous T2 progenies were identified by the segregation pattern of the corresponding T3 progenies on Murashige and Skoog plates containing antibiotics. Plant and seed-derived callus growth and carotenoid analysis were performed as described (Maass et al., 2009).
Bioinformatics
The kinetic constants were evaluated by nonlinear least squares fitting of the data to the Michaelis-Menten equation using the software GraphPad Prism (v7.01): V = (Vmax*[S])/(KM+[S]).
In silico reactions of Michaelis-Menten protein kinetics were realized in Python, where the Michalis-Menten equations were solved via numerical integration, using the odeint function provided by the scipy.integrate module from the SciPy library (https://www.scipy.org/). The following equations were used:
Plotting of the graphs was performed using the matplotlib library (https://matplotlib.org/). The versions of SciPy and matplotlib used correspond to the versions which are bundled into the Anaconda distribution (v.5.0.1) for Python 3.6 (https://www.anaconda.com/).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtGGPS2 (At2G18620), AtGGPS6 (At3G14530), AtGGPS8 (At3g20160), AtGGPS9 (At3G29430), AtGGPS10 (At3G32040), AtGGPS11 (At4G36810), AtPSY (At5g17230).
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1: Time-course experiment for coupled enzyme assay with Arabidopsis GGPS11 and PSY
Supplemental Figure S2: Liposomal recovery of GGPP
Supplemental Figure S3: Expression of PSY and PSY-GGPS fusion protein in E. coli
Supplemental Figure S4: Activity of GGPS11 and PSY mutants in E. coli
Supplemental Figure S5: In silico-emulation of PYGG reaction kinetics
Supplemental Figure S6: Carotenoid and chlorophyll levels in seedlings
Supplemental Table S1: Conversion of GGPP into phytoene by recombinant PSY
Acknowledgments
We wish to thank Prof. Paul Fraser (University of London, UK) for critical reading of the manuscript. We thank Carmen Schubert (University of Freiburg) for her skillful technical assistance. We acknowledge the ABRC (Arabidopsis Biological Resource Center) and S.P. Dinesh-Kumar and M. Snyder for providing Arabidopsis GGPS cDNA clones.
Footnotes
This work was supported by the HarvestPlus research consortium (grant 2014H6320.FRE) to R.W.
Articles can be viewed without a subscription.
References
- Almeida J, Azevedo M da S, Spicher L, Glauser G, vom Dorp K, Guyer L, del Valle Carranza A, Asis R, de Souza AP, Buckeridge M, Demarco D, Bres C, et al. (2016) Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J Exp Bot 67: 919–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez D, Voß B, Maass D, Wüst F, Schaub P, Beyer P, Welsch R (2016) Carotenogenesis Is Regulated by 5'UTR-Mediated Translation of Phytoene Synthase Splice Variants. Plant Physiol 172: 2314–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango J, Jourdan M, Geoffriau E, Beyer P, Welsch R (2014) Carotene Hydroxylase Activity Determines the Levels of Both α-Carotene and Total Carotenoids in Orange Carrots. Plant Cell 26: 2223–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C, Twyman RM, Farré G, Sanahuja G, Christou P, Capell T, Zhu C (2011) A golden era—pro-vitamin A enhancement in diverse crops. In Vitro Cell.Dev.Biol.-Plant 47: 1–17 10.1007/s11627-011-9363-6 [DOI] [Google Scholar]
- Baillie GS. (2009) Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276: 1790–1799 [DOI] [PubMed] [Google Scholar]
- Bassard J-E, Richert L, Geerinck J, Renault H, Duval F, Ullmann P, Schmitt M, Meyer E, Mutterer J, Boerjan W, De Jaeger G, Mely Y, et al. (2012) Protein-protein and protein-membrane associations in the lignin pathway. Plant Cell 24: 4465–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Beck G, Coman D, Herren E, Ruiz-Sola MA, Rodríguez-Concepción M, Gruissem W, Vranová E (2013) Characterization of the GGPP synthase gene family in Arabidopsis thaliana. Plant Mol Biol 82: 393–416 [DOI] [PubMed] [Google Scholar]
- Bera AK, Smith JL, Zalkin H (2000) Dual role for the glutamine phosphoribosylpyrophosphate amidotransferase ammonia channel. Interdomain signaling and intermediate channeling. J Biol Chem 275: 7975–7979 [DOI] [PubMed] [Google Scholar]
- Beyer P, Al-Babili S, Ye X, Lucca P, Schaub P, Welsch R, Potrykus I (2002) Golden Rice: introducing the beta-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. J Nutr 132: 506S–510S [DOI] [PubMed] [Google Scholar]
- Blatt A, Bauch ME, Pörschke Y, Lohr M (2015) A lycopene β-cyclase/lycopene ε-cyclase/light-harvesting complex-fusion protein from the green alga Ostreococcus lucimarinus can be modified to produce α-carotene and β-carotene at different ratios. Plant J 82: 582–595 [DOI] [PubMed] [Google Scholar]
- Bonk M, Hoffmann B, Von Lintig J, Schledz M, Al-Babili S, Hobeika E, Kleinig H, Beyer P (1997) Chloroplast import of four carotenoid biosynthetic enzymes in vitro reveals differential fates prior to membrane binding and oligomeric assembly. Eur J Biochem 247: 942–950 [DOI] [PubMed] [Google Scholar]
- Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J Nanomedicine 10: 975–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brausemann A, Gemmecker S, Koschmieder J, Ghisla S, Beyer P, Einsle O (2017) Structure of Phytoene Desaturase Provides Insights into Herbicide Binding and Reaction Mechanisms Involved in Carotene Desaturation. Structure 25: 1222–1232.e3 [DOI] [PubMed] [Google Scholar]
- Brodelius M, Lundgren A, Mercke P, Brodelius PE (2002) Fusion of farnesyldiphosphate synthase and epi-aristolochene synthase, a sesquiterpene cyclase involved in capsidiol biosynthesis in Nicotiana tabacum. Eur J Biochem 269: 3570–3577 [DOI] [PubMed] [Google Scholar]
- Burbulis IE, Winkel-Shirley B (1999) Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc Natl Acad Sci USA 96: 12929–12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara B. (1993) Carotenoids Part B: Metabolism, Genetics, and Biosynthesis. Methods Enzymol [Google Scholar]
- Cazzonelli C. (2011) Goldacre Review: Carotenoids in nature: insights from plants and beyond. Funct Plant Biol 38: 833–847 [DOI] [PubMed] [Google Scholar]
- Chayut N, Yuan H, Ohali S, Meir A, Saar U, Tzuri G, Zheng Y, Mazourek M, Gepstein S, Zhou X, et al. (2016) Distinct Mechanisms of the ORANGE Protein in Controlling Carotenoid Flux. Plant Physiol pp.01256.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zaro JL, Shen W-C (2013) Fusion protein linkers: Property, design and functionality. Adv Drug Deliv Rev 65: 1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman D, Altenhoff A, Zoller S, Gruissem W, Vranová E (2014) Distinct evolutionary strategies in the GGPPS family from plants. Front Plant Sci 5: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré G, Sanahuja G, Naqvi S, Bai C, Capell T, Zhu C, Christou P (2010) Travel advice on the road to carotenoids in plants. Plant Sci 179: 28–48 [Google Scholar]
- Fraser PD, Schuch W, Bramley PM (2000) Phytoene synthase from tomato (Lycopersicon esculentum) chloroplasts--partial purification and biochemical properties. Planta 211: 361–369 [DOI] [PubMed] [Google Scholar]
- Fraser PD, Römer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM (2002) Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci USA 99: 1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PD, Enfissi EMA, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM (2007) Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 19: 3194–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D (1995) Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J 8: 693–701 [Google Scholar]
- Gemmecker S, Schaub P, Koschmieder J, Brausemann A, Drepper F, Rodriguez-Franco M, Ghisla S, Warscheid B, Einsle O, Beyer P (2015) Phytoene Desaturase from Oryza sativa: Oligomeric Assembly, Membrane Association and Preliminary 3D-Analysis. PLoS One 10: e0131717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA III, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6: 343–345 [DOI] [PubMed] [Google Scholar]
- Giuliano G. (2014) Plant carotenoids: genomics meets multi-gene engineering. Curr Opin Plant Biol 19: 111–117 [DOI] [PubMed] [Google Scholar]
- Giuliano G, Giliberto L, Rosati C (2002) Carotenoid isomerase: a tale of light and isomers. Trends Plant Sci 7: 427–429 [DOI] [PubMed] [Google Scholar]
- Gu P, Ishii Y, Spencer TA, Shechter I (1998) Function-structure studies and identification of three enzyme domains involved in the catalytic activity in rat hepatic squalene synthase. J Biol Chem 273: 12515–12525 [DOI] [PubMed] [Google Scholar]
- Hagel JM, Facchini PJ (2017) Tying the knot: occurrence and possible significance of gene fusions in plant metabolism and beyond. J Exp Bot 68: 4029–4043 [DOI] [PubMed] [Google Scholar]
- Han J, Wang H, Kanagarajan S, Hao M, Lundgren A, Brodelius PE (2016) Promoting Artemisinin Biosynthesis in Artemisia annua Plants by Substrate Channeling. Mol Plant 9: 946–948 [DOI] [PubMed] [Google Scholar]
- Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444: 11–25 [DOI] [PubMed] [Google Scholar]
- Hirooka K, Bamba T, Fukusaki E, Kobayashi A (2003) Cloning and kinetic characterization of Arabidopsis thaliana solanesyl diphosphate synthase. Biochem J 370: 679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka K, Izumi Y, An C-I, Nakazawa Y, Fukusaki E, Kobayashi A (2005) Functional analysis of two solanesyl diphosphate synthases from Arabidopsis thaliana. Biosci Biotechnol Biochem 69: 592–601 [DOI] [PubMed] [Google Scholar]
- Hoa TTC, Al-Babili S, Schaub P, Potrykus I, Beyer P, Schaub P, Beyer P (2003) Golden Indica and Japonica rice lines amenable to deregulation. Plant Physiol 133: 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson T, Ohad I, Beyer P, Hirschberg J (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol 136: 4246–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata-Reuyl D, Math SK, Desai SB, Poulter CD (2003) Bacterial phytoene synthase: molecular cloning, expression, and characterization of Erwinia herbicola phytoene synthase. Biochemistry 42: 3359–3365 [DOI] [PubMed] [Google Scholar]
- Johnson EJ. (2014) Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 72: 605–612 [DOI] [PubMed] [Google Scholar]
- Joly A, Edwards PA (1993) Effect of site-directed mutagenesis of conserved aspartate and arginine residues upon farnesyl diphosphate synthase activity. J Biol Chem 268: 26983–26989 [PubMed] [Google Scholar]
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005) Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8: 280–291 [DOI] [PubMed] [Google Scholar]
- Kloer DP, Welsch R, Beyer P, Schulz GE (2006) Structure and reaction geometry of geranylgeranyl diphosphate synthase from Sinapis alba. Biochemistry 45: 15197–15204 [DOI] [PubMed] [Google Scholar]
- Koschmieder J, Fehling-Kaschek M, Schaub P, Ghisla S, Brausemann A, Timmer J, Beyer P (2017) Plant-type phytoene desaturase: Functional evaluation of structural implications. PLoS One 12: e0187628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lätari K, Wüst F, Hübner M, Schaub P, Beisel KG, Matsubara S, Beyer P, Welsch R (2015) Tissue-Specific Apocarotenoid Glycosylation Contributes to Carotenoid Homeostasis in Arabidopsis Leaves. Plant Physiol 168: 1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen T, Borch J, Knudsen C, Bavishi K, Torta F, Martens HJ, Silvestro D, Hatzakis NS, Wenk MR, Dafforn TR, Olsen CE, Motawia MS, et al. (2016) Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354: 890–893 [DOI] [PubMed] [Google Scholar]
- Lee H, DeLoache WC, Dueber JE (2012) Spatial organization of enzymes for metabolic engineering. Metab Eng 14: 242–251 [DOI] [PubMed] [Google Scholar]
- Lopez AB, Yang Y, Thannhauser TW, Li L (2008) Phytoene desaturase is present in a large protein complex in the plastid membrane. Physiol Plant 133: 190–198 [DOI] [PubMed] [Google Scholar]
- Lützow M, Beyer P (1988) The isopentenyl-diphosphate Δ-isomerase and its relation to the phytoene synthase complex in daffodil chromoplasts. Biochim Biophys Acta - Lipids Lipid Metab 959: 118–126 [Google Scholar]
- Maass D, Arango J, Wüst F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS One 4: e6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero PF, Poulter CD, Edwards PA (1992) Effects of site-directed mutagenesis of the highly conserved aspartate residues in domain II of farnesyl diphosphate synthase activity. J Biol Chem 267: 21873–21878 [PubMed] [Google Scholar]
- Maudinas B, Bucholtz ML, Papastephanou C, Katiyar SS, Briedis AV, Porter JW (1977) The partial purification and properties of a phytoene synthesizing enzyme system. Arch Biochem Biophys 180: 354–362 [DOI] [PubMed] [Google Scholar]
- Moise AR, Al-Babili S, Wurtzel ET (2014) Mechanistic aspects of carotenoid biosynthesis. Chem Rev 114: 164–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller BL, Conn EE (1980) The biosynthesis of cyanogenic glucosides in higher plants. Channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (linn) Moench. J Biol Chem 255: 3049–3056 [PubMed] [Google Scholar]
- Nagel R, Bernholz C, Vranová E, Košuth J, Bergau N, Ludwig S, Wessjohann L, Gershenzon J, Tissier A, Schmidt A (2015) Arabidopsis thaliana isoprenyl diphosphate synthases produce the C25 intermediate geranylfarnesyl diphosphate. Plant J 84: 847–859 [DOI] [PubMed] [Google Scholar]
- Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, Ellerbrok H, Kamuzinzi R, Ledent V, Blaudez D, Sanders D, Revuelta JL, et al. (2004) K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA 101: 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 23: 482–487 [DOI] [PubMed] [Google Scholar]
- Porter JW, Spurgeon SL (1981) Biosynthesis of isoprenoid compounds. Wiley, New York [Google Scholar]
- Prisic S, Peters RJ (2007) Synergistic substrate inhibition of ent-copalyl diphosphate synthase: a potential feed-forward inhibition mechanism limiting gibberellin metabolism. Plant Physiol 144: 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RF, Shumskaya M, Bradbury LMT, Beltrán J, Ma C, Kennelly EJ, Wurtzel ET (2012) Synergistic interactions between carotene ring hydroxylases drive lutein formation in plant carotenoid biosynthesis. Plant Physiol 160: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LF, Furtado GP, Lourenzoni MR, Costa-Filho AJ, Santos CR, Nogueira SCP, Betini JA, Polizeli M de LTM, Murakami MT, Ward RJ (2011) Engineering bifunctional laccase-xylanase chimeras for improved catalytic performance. J Biol Chem 286: 43026–43038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Sola MÁ, Barja MV, Manzano D, Llorente B, Schipper B, Beekwilder J, Rodriguez-Concepcion M (2016a) A single gene encodes two differentially targeted geranylgeranyl diphosphate synthase isoforms. Plant Physiol pp.01392.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Sola MÁ, Coman D, Beck G, Barja MV, Colinas M, Graf A, Welsch R, Rütimann P, Bühlmann P, Bigler L, Gruissem W, Rodríguez-Concepción M, et al. (2016b) Arabidopsis GERANYLGERANYL DIPHOSPHATE SYNTHASE 11 is a hub isozyme required for the production of most photosynthesis-related isoprenoids. New Phytol 209: 252–264 [DOI] [PubMed] [Google Scholar]
- Schaub P, Al-Babili S, Drake R, Beyer P (2005) Why is golden rice golden (yellow) instead of red? Plant Physiol 138: 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub P, Rodriguez-Franco M, Cazzonelli CI, Álvarez D, Wüst F, Welsch R (2018) Establishment of an Arabidopsis callus system to study the interrelations of biosynthesis, degradation and accumulation of carotenoids. PLoS One 13: e0192158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid HC, Oster U, Kögel J, Lenz S, Rüdiger W (2001) Cloning and characterisation of chlorophyll synthase from Avena sativa. Biol Chem 382: 903–911 [DOI] [PubMed] [Google Scholar]
- Selivanov VA, Krause S, Roca J, Cascante M (2007) Modeling of spatial metabolite distributions in the cardiac sarcomere. Biophys J 92: 3492–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickforth P, Steiger S, Hess WR, Sandmann G (2003) A novel type of lycopene ε-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch Microbiol 179: 409–415 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Oster U, Kruse E, Rudiger W, Grimm B (1999) Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol 120: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Tsukahara M, Kaneko A, Niida R, Mitsuhashi W, Dairi T, Kato N, Sassa T (2007) Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi. Proc Natl Acad Sci USA 104: 3084–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos A, Eslava AP, Iturriaga EA (2000) A bifunctional enzyme with lycopene cyclase and phytoene synthase activities is encoded by the carRP gene of Mucor circinelloides. Eur J Biochem 267: 5509–5519 [DOI] [PubMed] [Google Scholar]
- Vom Dorp K, Hölzl G, Plohmann C, Eisenhut M, Abraham M, Weber APM, Hanson AD, Dörmann P (2015) Remobilization of Phytol from Chlorophyll Degradation Is Essential for Tocopherol Synthesis and Growth of Arabidopsis. Plant Cell 27: 2846–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J. (2012) Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr 96: 1234S–1244S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Pelletier J, Massaad MJ, Herscovics A, Shore GC (2004) The yeast split-ubiquitin membrane protein two-hybrid screen identifies BAP31 as a regulator of the turnover of endoplasmic reticulum-associated protein tyrosine phosphatase-like B. Mol Cell Biol 24: 2767–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J (2000) Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211: 846–854 [DOI] [PubMed] [Google Scholar]
- Welsch R, Arango J, Bär C, Salazar B, Al-Babili S, Beltrán J, Chavarriaga P, Ceballos H, Tohme J, Beyer P (2010) Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 22: 3348–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R, Zhou X, Yuan H, Álvarez D, Sun T, Schlossarek D, Yang Y, Shen G, Zhang H, Rodriguez-Concepcion M, Thannhauser TW, Li L (2018) Clp protease and OR directly control the proteostasis of phytoene synthase, the crucial enzyme for carotenoid biosynthesis in Arabidopsis. Mol Plant 11: 149–162 [DOI] [PubMed] [Google Scholar]
- Yu Q, Beyer P (2012) Reaction specificities of the ε-ionone-forming lycopene cyclase from rice (Oryza sativa) elucidated in vitro. FEBS Lett 586: 3415–3420 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li S-Z, Li J, Pan X, Cahoon RE, Jaworski JG, Wang X, Jez JM, Chen F, Yu O (2006) Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and Mammalian cells. J Am Chem Soc 128: 13030–13031 [DOI] [PubMed] [Google Scholar]
- Zhou F, Wang C-Y, Gutensohn M, Jiang L, Zhang P, Zhang D, Dudareva N, Lu S (2017) A recruiting protein of geranylgeranyl diphosphate synthase controls metabolic flux toward chlorophyll biosynthesis in rice. Proc Natl Acad Sci USA 114: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Welsch R, Yang Y, Álvarez D, Riediger M, Yuan H, Fish T, Liu J, Thannhauser TW, Li L (2015) Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc Natl Acad Sci USA 112: 3558–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Naqvi S, Breitenbach J, Sandmann G, Christou P, Capell T (2008) Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc Natl Acad Sci USA 105: 18232–18237 [DOI] [PMC free article] [PubMed] [Google Scholar]