Abstract
Human are exposed to ionizing radiation from natural and artificial sources, which consequently poses a possible risk to human health. However, accumulating evidence indicates that the biological effects of low-dose radiation (LDR) are different from those of high-dose radiation (HDR). Low-dose radiation–induced hormesis has been extensively observed in different biological systems, including immunological and hematopoietic systems. Adaptive responses in response to LDR that can induce cellular resistance to genotoxic effects from subsequent exposure to HDR have also been described and researched. Bystander effects, another type of biological effect induced by LDR, have been shown to widely occur in many cell types. Furthermore, the influence of LDR-induced biological effects on certain diseases, such as cancer and diabetes, has also attracted the interest of researchers. Many studies have suggested that LDR has the potential antitumor and antidiabetic complications effects. In addition, the researches on whether LDR could induce stochastic effects were also debated. Studies on the biological effects of LDR in China started in 1970s and considerable progress has been made since. In the present article, we provide an overview of the research progress on the biological effects of LDR in China.
Keywords: low-dose radiation, biological effects, research progress, China
Introduction
Currently, ionizing radiation from natural and artificial sources is ubiquitous in our daily life.1–3 Abnormal exposure to ionizing radiation, such as that experienced by individuals involved in nuclear mishaps, astronauts, and some medical professionals, can cause side effects.4 In addition, radiation therapy, one of the most important therapeutic strategies for treating malignancies, can also injure the normal cells and tissues surrounding the tumors.5
Moderate and high doses of radiation induce DNA strand breaks and impair immune function, which may result in apoptosis and transformation into cancer cells.6,7 Extensive epidemiological studies on atomic bomb survivors suggested that the incidence of solid cancers is significantly related to radiation exposure. Currently, the linear no-threshold (LNT) hypothesis, which assumes risk increases linearly with increasing radiation dose, is applied to assess the radiation-associated risk in many countries.8 However, the LNT hypothesis emphasizes the DNA damage caused by ionizing radiation and ignores the defensive and adaptive reactions in the body often activated by low-dose radiation (LDR).9 Therefore, many reports suggest that using the LNT hypothesis to assess the risk from LDR is unwise. While the effects of LDR are still controversial, many reports have shown that LDR, unlike high-dose radiation (HDR), can be beneficial to living organisms.3,10,11
Low-dose radiation is defined as a dose of radiation below which it is not possible to detect adverse health effects and has been set previously as less than 200 mGy for low linear energy transfer (LET) radiation or 50 mGy for high LET radiation by the UN Scientific Committee on Atomic Radiation.12,13 Recently, LDR was considered to be less than 100 mGy of low LET radiation (Nuclear Regulatory Commission, 2006) in the seventh report in a series of publications from the US National Academy concerning radiation health effects called the biological effects of ionizing radiation VII. Studies on the biological effects of LDR began in the 1970s. In 1982, Luckey was the first to conclude that LDR benefited animal growth, development, health, and longevity and termed these effects “radiation hormesis” in his monograph Hormesis with Ionizing Radiation.10 In 1984, Olivieri et al documented that the cultured human lymphocytes can acquire the resistance to chromosomal aberrations induced by subsequently HDR when preexposed to LDR and termed these effects an adaptive response (AR).14 More recently, the “bystander effect” of LDR was defined as exposure of a cell population to LDR causing in significant cytotoxic and genotoxic effects in nonirradiated cells in the population.15 Over the past several decades, the biological effects of LDR, such as the hormesis, AR, and bystander effect, have been the focus of many investigators.
In China, early studies on LDR aimed to identify indicators of damage and evidence that could be used for diagnosing “chronic radiation injury.” However, in a population health survey with high-background levels of natural radiation in Yangjiang, Guangdong Province, in the late 1970s, Liu observed an increase in the reactivity of T lymphocytes in the peripheral blood of a population exposed to a dose of radiation equivalent to more than 3 times the population in the control area.16 Later, it was found the DNA damage repair capacity of these T lymphocytes was increased and there was significantly more unscheduled DNA synthesis than in the control group.16 These 2 population-based observations prompted Chinese researchers to consider that LDR may have different biological effects than HDR. Subsequently, Luckey’s monograph Hormesis with Ionizing Radiation and Olivieri’s paper about the AR induced by LDR that was published in Science were introduced in China and Chinese researchers developed a new understanding and shifted research on the biological effects of LDR. Initially, Chinese researchers mainly focused on the hormesis, AR, and bystander effects of LDR. Since then, research on the mechanisms of the biological effects of LDR has developed with the further development and application of new technologies in molecular and cellular biology. In addition, the biological effects of LDR on germ cells, tumor cells, and cancers have attracted the attention of Chinese researchers. In this review, we summarize the research advances made on the biological effects of LDR in China.
Low-Dose Radiation Hormesis
Hormesis is a dose–response phenomenon that occurs in a wide spectrum of organisms in response to different environmental agents.3 Radiation hormesis is characterized by LDR stimulation and HDR inhibition of certain biological parameters.3 Over the past several decades, increasing evidence has indicated that LDR-induced hormesis was extensively observed in different biological systems, including immunological and hematopoietic systems.3,7,17 Here, we review the developments on LDR hormesis in China.
Low-Dose Radiation Hormesis of the Immune System
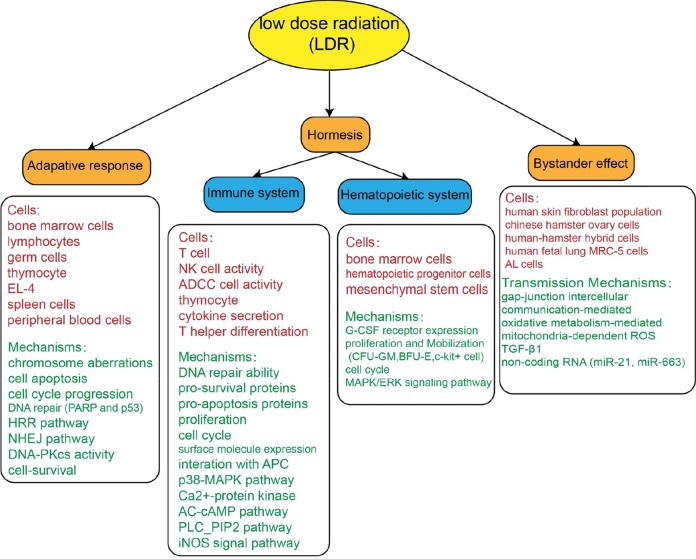
The immune system is one of the most important defenses against environmental insults and is strongly affected by ionizing radiation.18 In China, LDR hormesis was firstly observed in Chinese people exposed to high-background radiation at a low-dose rate of 1.96 mSv/y in Yangjiang, Guangdong Province.16 In this population, the reactivity and DNA repair ability of T cells were significantly higher than in people in surrounding low-background radiation areas. Therefore, some Chinese researchers began to concentrate on LDR hormesis of the immune system (Figure 1).
Figure 1.
Research on hormesis, adaptive responses, and bystander effects from low-dose radiation (LDR) by Chinese scholars.
First, the dose–response relationship of ionizing radiation with immunological parameters following exposure, particularly LDR, was analyzed. Liu observed that the lymphocytes and related functions displayed a J- or inverted J-shaped dose–response curve, which is not consistent with LNT, in a model where Kunming mice were exposed to X-rays whole-body irradiation (WBI); specifically, 25, 50, 75, 100, 200, 500, 1000, 2000, 4000, and 6000 mGy and a sham-irradiated control were used.19 Notably, this dose–response curve is still applicable when evaluating natural killer (NK) cell activity and antibody-dependent cell-mediated cytotoxicity activity at different doses, but more doses on the higher end are needed in order to reveal the suppressive effect.19,20
Second, LDR enhancement of the immune response has been demonstrated, especially for the adaptive immunity. Liu et al reported a significant reduction in the rate of thymocyte apoptosis to below control levels when doses within 0.2 Gy were given as WBI to male Kunming mice and in vitro irradiation of EL4 cells.21,22 In their study, the messenger RNA (mRNA) and protein expression levels of prosurvival molecules, such as Bcl-2 and Bcl-xl, and the ratio of prosurvival and proapoptotic molecules, such as Bcl-2/Bax and Bcl-xl/Bad, were significantly increased. Correspondingly, the mRNA and protein expression levels of proapoptotic molecules, such as p53, Bax, Bad, FasL, and Gadd45, were significantly decreased. Some studies have reported that LDR may also stimulate thymocytes through promoting thymocyte proliferation and cell-cycle progression.23,24 When Kunming mice were exposed to LDR through WBI (75 mGy), the total number of thymocytes, proportion of cells in S phase and thymocytes proliferation in response to ConA stimulation were increased. Liu et al demonstrated that LDR may also shift cytokine secretion and T-helper differentiation. When Kunming mice were exposed to whole-body LDR (75 mGy), the mRNA and protein levels of interleukin 10 (IL-10) were both suppressed while IL-12 expression was simultaneously stimulated, which may contribute to a shift in the immune response in favor of Th1 differentiation.25,26 They suggested that the effect of LDR on T-lymphocyte surface molecule expression and interactions with antigen-presenting cells may be the main reason for this alternation in cytokine secretion and Th-cell differentiation. In addition, the biological effects of LDR on NK cells, which are an important part of innate immunity, have also been investigated. Yang et al found NK cells expansion and cytotoxicity were significantly augmented by LDR. Interferon-γ and tumor necrosis factor α (TNF-α) levels in the supernatants of the cultured NK cells were markedly increased after LDR exposure. These findings also indicate LDR induces the expansion and activation of NK cells possibly through the p38-MAPK pathway.7
Third, the molecular mechanisms underlying the biological effects of LDR on the immune system have also been extensively studied by the Chinese researchers. Liu et al demonstrated the phospholipase C-phosphatidylinositol biphosphate signaling pathway (PLC-IP2) and the G-protein–adenylate cyclase-cyclic adenosine monophosphate (AC-cAMP) signaling pathway may be involved in the activation of thymocytes in response to WBI with LDR.27 When Kunming mice were exposed to whole-body LDR, Ca2+ mobilization, protein kinase C activation, and PLC-IP2 signaling pathway in T lymphocytes were increased, unlike when exposed to HDR. Furthermore, G-protein–AC-cAMP pathways signaling molecules were downregulated in response to LDR. They also suggested that alternations in these signaling pathways may achieve functional activation by activating transcription factors associated with cytokine secretion, cell proliferations, and cell-cycle progression. Besides, cell signal transmission in different immune cells may be one of the molecular mechanisms of the effect of LDR. For example, Yang et al suggested the p38-MAPK pathway is involved in the activation and expansion of NK cells in response to LDR.7 The results of the above Chinese studies demonstrate that LDR enhances the immune response by augmenting the proliferation-reactive response and suppressing the apoptosis-reactive response of immune cells, altering immune cell populations, and cytokine release, through complex signal transduction pathways.
Low-Dose Radiation Hormesis of the Hematopoietic Systems
In China, studies on LDR-induced hormesis first focused on the immune system. However, recently, many reports have been published indicating LDR may induce hormesis of the hematopoietic system. Depression of hematopoietic function often takes place in patients undergoing radiotherapy and/or chemotherapy, due to the high sensitivity of the hematopoietic system to these therapies.28–31 Activation of the immune system by LDR prompted us to consider whether LDR can activate the hematopoietic system. Therefore, many researchers began focusing on the LDR hormesis of the hematological system. Here, we review the research by Chinese researchers on the biological effects and the mechanisms of LDR on the hematopoietic system (Figure 1).
In 1993, Zhang detected the granulocyte colony-stimulating factor receptor expression on the surface of bone marrow cells (BMCs) was increased when Kunming mice were exposed to LDR, which resulted in a stimulating effect by LDR on hematopoietic cells proliferation.32 Low-dose radiation hormesis of the hematopoietic system was also observed in in vitro blood samples.33 Wang and Cai observed the obvious proliferative effects on hematopoietic progenitor cells that play an important role in the maintenance and development of the hematopoietic system, when Kunming mice were exposed to whole-body LDR through X-rays.34 Li et al reported bone marrow HPC proliferation (colony-forming unit granulocyte-macrophage and burst-forming unit erythroid formation) and mobilization were significantly stimulated when male Kunming mice were exposed to LDR (especially 75 mGy).31 Based on LDR hormesis of the hematopoietic systems, especially HPCs, Zhang et al investigated the effects of LDR-induced hormesis effect on hematopoietic reconstitution.17 When exposed to LDR (60 and 80 mGy) in vitro, BMCs underwent significant proliferation. In irradiated recipient BALB/C mice receiving these preexposed BMCs, there are consistently more white blood cells, bone marrow mononuclear cells, and CFUs in the recipient spleens than in the control. These results suggest LDR-induced hormesis may facilitate hematopoietic reconstitution in recipient mice. In addition, mesenchymal stem cells (MSCs), an important component of the hematopoietic system, have captured increased attention from researchers.35,36 Liang et al showed proliferation of rat MSCs in vitro significantly increases following exposure to LDR at 50 and 75 mGy, where 75 mGy is the most stimulating. There is also a significant increase in the proportion of MSCs in S-phase cells in response to LDR. The result also suggests that activation of the MAPK/ERK signaling pathway may have contributed to the MSCs proliferation.37 The results gathered by Chinese researchers suggest that LDR hormesis on the hematopoietic system occurs through promotion of proliferation of HPCs and MSCs.
Low-Dose Radiation–Induced ARs
Adaptive responses are potential adaptations of the living body to the external environment and are a widespread phenomenon in the living nature.38 Recently, many efforts have been made to prove LDR can induce ARs.12,39 An LDR-induced AR is a phenomenon in which pre-exposure of cells to LDR (inductive dose, D1) renders cells more resistant to damage from subsequent HDR (challenge dose, D2) or other toxic agents.14 In this section, we focus on the efforts and achievements made by Chinese researchers on AR and LDR-associated mechanisms of induction, especially for immune and hematopoietic systems (Figure 1).
As early as 1990, Liu et al found that WBI of C57BL/6 mice with LDR in the range of 2 to 100 mGy induces an AR in BMCs in the form of a reduction in chromosomal aberrations following subsequent exposure to HDR (650 mGy).40 Cai et al showed cross-induction of ARs occurs between ionizing radiation and chemical agents, including mitomycin C and H2O2, both in vitro in human lymphocytes and in vivo mouse BMCs and germ cells.41 Gong et al observed that when male Kunming mice were irradiated with LDR (D1, 75 mGy) 3 to 12 hours before exposure to HDR (D2, 1.5 Gy), the ARs of cell apoptosis and cell-cycle progression could be induced in thymocytes cultured for 4 and 20 hours after WBI with D2, which suggests there is a time-dependent effect for LDR-induced ARs in the form of mouse thymocyte apoptosis and cell-cycle progression.42
Several hypotheses have been considered for the mechanisms underlying LDR-induced AR. Many studies have suggested that LDR may minimize damage caused by subsequent HDR by enhancing DNA repair ability, antioxidant activity, production of protective proteins, and cell survival.43–45 Ionizing radiation-induced DNA double-strand breaks (DSBs) are a severe threat to cell survival. Many previous studies have demonstrated LDR-induced ARs may be mainly related to enhancement of DNA repair.46–48 Cheng et al found AR was induced in the form of cell apoptosis and cell-cycle progression in EL-4 cells preexposed to LDR (D1, 75 mGy) before being exposed to HDR (D2, 1.0, 1.5, and 2.0 Gy). The authors also demonstrated that poly-(ADP ribose) polymerase and p53, which might be crucial mediators of DNA repair, might play important roles in LDR-induced AR.38 Yu et al found that the protective role of LDR in reducing HDR-induced cell killing might depend on promotion of nonhomologous end joining through stimulation of DNA-protein kinase cytalytic subunit activity.49 Yu et al demonstrated LDR can induce an apoptosis-based AR in mouse spleen cells. When Kunming mice were irradiated with LDR (D1, 25, 50, 75, and 100 mGy) 6 hours before exposure to HDR (D2, 1.5 Gy), expression of caspase-3 and the apoptosis-related protein Bcl-2 increased, and proapoptotic protein Bax expression decreased, leading to a decrease in spleen cell apoptosis compared to the D2 group.50 All of the above studies documented that pre-exposure of cells to LDR in vivo and in vitro enhances DNA repair activity and reduces activity related to DNA damage-associated apoptosis.
However, a few studies have addressed the issue of LDR-induced cell proliferation and cell survival ARs to subsequent HDR-induced cytotoxicity. Wang and Cai suggested LDR could induce a cell survival AR to subsequent HDR in BMCs. When Kunming mice were irradiated with 0.5 Gy X-rays as an inductive dose (D1) before exposure to HDR (D2, 6 Gy), an AR to the D2-induced cytotoxic effect, termed the cell survival AR, was observed in both peripheral blood cells and BMCs.34 In summary, Chinese researchers have done a great deal of work in the field of LDR-induced ARs. However, further studies are required to delineate both the phenomenological features and mechanisms underlying LDR-induced ARs.
Bystander Effects of LDR
Radiation-induced bystander effects (RIBEs) were originally termed to describe the nontargeted effects exhibited by nonirradiated cells upon receiving signals from irradiated cells through diffusion of soluble molecules into the medium or cellular gap junctional intercellular communication.51,52 The RIBE was firstly discovered by Nagasawa and Little in an in vitro study, which revealed an induced frequency of sister chromatid exchanges of 20% to 40% of Chinese hamster ovary (CHO) cells when only 0.1% to 1% of the nuclei of the cells were exposed to a low dose of α particles.15 Compared to the bystander effects induced by HDR, the RIBE is very weak.53,54 However, the RIBE of LDR over the past 2 decades has received increasing attention.54–58 In this section, therefore, the progress made on LDR-associated RIBEs by Chinese researchers is reviewed and also illustrated in Figure 1.
During initial studies on RIBEs of LDR, scientists mostly focused on providing the evidence for the existence of the RIBEs by measuring DNA damages in bystander cells after exposure to LDR. Through in situ γ-H2AX immunofluorescence, Hu et al discovered the existence of the RIBE by finding more DSBs in bystander cells in a full confluent human skin fibroblasts than in cells subjects to a low dose of α particles.59 Following this, detection of the DNA damage sensor p53-binding protein 1 (53BP1) foci, which colocalized with γ-H2AX, was also be used as a method to measure DNA damage to show the existence of RIBEs in response to LDR. Han et al found a significant increase in 53BP1 foci formation in proliferating bystander CHO cells when they were cocultured with cells irradiated with α particles.60
Based on advancements of experimental techniques, various research groups began to study the earliest time point for the induction of RIBE by LDR. He et al found increased γ-H2AX foci formation in irradiated cells and nonirradiated cells could be visualized 2 minutes after radiation that peaked after 30 minutes.61 In addition to DSBs, Chen et al observed that conditioned medium harvested at 10 minutes postirradiation from 10 mGy irradiated human–hamster hybrid cells could induce reactive oxygen species (ROS) production, CD59-gene loci mutations, and delayed cell death in the bystander cells.62
The possible mechanisms underlying RIBEs may include the transmission of soluble factors generated by irradiated cells to nonirradiated cells, the gap junctional intercellular communication, and the ROS-based transmission. For instance, Hu et al found when AG1522 normal human diploid skin fibroblasts cells were pretreated with either lindane (a gap junctional communication inhibitor) or dimethyl sulphoxide (DMSO; a free radical scavenger), the fraction of DSBs-positive cells was reduced in nonirradiated cell population, suggesting gap junctional intercellular communication and ROS might play important roles in the induction of RIBEs.61 Regarding the possible role of ROS generated from irradiated cells in the induction of RIBEs, Chen et al provided further finding that mitochondria-dependent generation of ROS seems required.62,63 Chen et al showed that RIBE in the nonirradiated AL human–hamster hybrid cells was induced with the conditioned medium collected from donor cells irradiated by LDR only in the cells contain normal mitochondrial function and not in the cells with the deletion of mitochondrial DNA. This may be due to the fact that ROS production was increased only in irradiated cells with normal mitochondrial DNA but not in the cells without mitochondrial DNA. These results demonstrate that mitochondria-dependent ROS might be very important in RIBEs.62
The transmitted soluble factors generated by irradiated cells to nonirradiated cells may include NO, O2 − and TGF-β1. The study by Han et al showed that DSBs formation using 53BP1 immunofluorescence staining and proliferation using flow cytometry were increased in bystander CHO cells cocultured with LDR-irradiated CHO cells. These RIBEs were reduced when c-PTIO (a scavenger of NO), DMSO (a scavenger of ROS), or anti-TGF-β1 was added to the cultures collected from LDR-irradiated cells.60 These results are consistent with studies performed in different systems.64,65
In addition, recent studies implied that small noncoding RNAs, particularly microRNAs, are possible mediators of RIBEs. Xu et al found miR-21 was significantly upregulated in both directly irradiated and bystander human fetal lung Medical Research Council cell line 5 (MRC-5) fibroblasts cells and RIBE-like response can be induced in nonirradiated MRC-5 cells by transfecting miR-21. These data indicate miR-21 is involved in RIBEs.66 Hu et al also suggested miR-663 participates in regulation of biological effects in both directly irradiated and bystander nonirradiated human cervical cancer cells (HeLa) via targeting of TGF-β1.67
Biological Effects of LDR on Germ Cells
For the past 3 decades, the biological effects of LDR, such as hormesis, ARs, and bystander effects, in somatic cells have attracted the interest of many investigators. The LDR can stimulate cell proliferation and prevent HDR-induced inhibition of cell proliferation in lymphocytes, splenocytes, and hematopoietic cells under both in vitro and in vivo conditions.34,68,69 However, in terms of apoptotic cell death, there is controversy on the effects of LDR. Some researchers have found decreased apoptosis of HDR-exposed cells after pretreatment with LDR,21,70 whereas others have observed increased apoptosis.71,72 These discrepancies may be due to differences in doses and rates of LDR and cells types.70–73
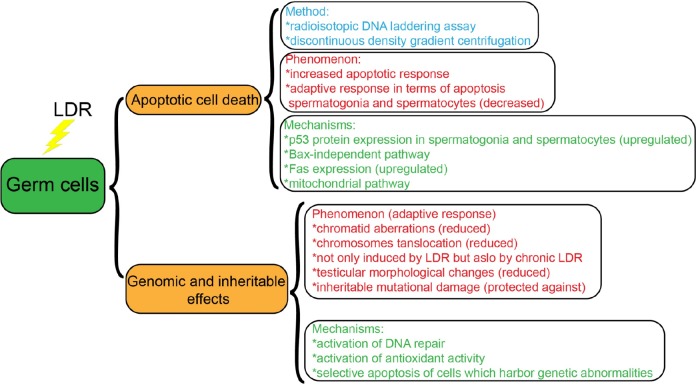
The testes are among the most radiosensitive organs. Low-dose radiation mostly leads to apoptotic death of male germ cells, while HDR mainly induces necrotic death.12,74 Over the past 20 years, the biological effects of LDR on germ cells, especially apoptosis, have been the focus of intense research.12 In this section, we summarize the work of Chinese researchers studying LDR-induced biological effects on germ cells (Figure 2).
Figure 2.
Research on the biological effects of low-dose radiation (LDR) on germ cells by Chinese scholars.
Low-Dose Radiation–Induced Apoptotic Germ Cell Death
As mentioned above, apoptotic cell death is a major manifestation of the biological effects of LDR on germ cells. Therefore, methods for detecting apoptotic germ cell death are needed. Currently, there are several well-established assays for apoptosis, including morphologic methods, quantitative DNA fragmentation assays, and flow cytometry measurement assays, used for male germ cells. The DNA-laddering assay, which is a well-established quantitative assay for apoptotic cell death, was optimized by Chinese researchers Cai et al to be a radioisotopic DNA-laddering assay and has been used to study apoptotic cell death of testicular cells. The radioisotopic DNA-laddering assay is more sensitive at detecting apoptotic cells within testicular tissue samples.75 Subsequently, the invention of flow cytometry permitted investigators to quantify the percentage of germ cells undergoing apoptosis. However, due to inherent differences in DNA content in male germ cells at different stages, apoptotic testicular cells cannot be identified by flow cytometry without first isolating and separating the different types of germ cells. Liu et al first used discontinuous density gradient centrifugation to separate the different types of germ cells. This method, which minimizes the distribution alternation of different cells types, promotes flow cytometry for detection of apoptotic cells in mouse testes exposed to LDR.76
Benefiting from the development of apoptotic cell death detection methods, some studies have focused on the apoptotic response of male germ cells induced by LDR in China. In 2006, Liu et al characterized the effect of LDR on apoptosis at doses of X-rays ranging from 25 to 200 mGy on germ cells of Kunming mice after irradiation, where the maximal effect was observed at 75 mGy, using multiple apoptotic cell death detection methods. It was found that germ cells exhibit increased apoptosis in response to LDR compared to somatic cells, which exhibit decreased apoptosis in response to the same doses of LDR.76 However, the differential responses to LDR of somatic and germ cells cannot be explained based on current studies. Recently, many studies have been performed on the LDR-associated ARs in somatic cells. When LDR induction of ARs in male germ cells was investigated based on apoptosis, Liu et al found the number of apoptotic spermatogonia and spermatocytes significantly decreased when the Kunming mice were preexposed to 75 mGy 6 hours before being irradiated with HDR (1, 2, or 3 Gy). Low-dose radiation did not induce the same AR in spermatids and spermatozoa.76
As summarized above, there is an apoptotic cell death response due to LDR in germ cells. Therefore, the mechanisms of apoptotic cell death due to LDR in germ cells have attracted wide interest from Chinese researchers. The molecule p53 has been reported to play a critical role in radiation-induced apoptotic cell death in testes.77,78 Liu et al observed a significant upregulation in p53 protein expression in spermatogonia and spermatocytes of Kunming mice exposed to LDR in the form of X-rays at 25 to 75 mGy but not 200 mGy. This result suggests that LDR-induced apoptotic cell death in the testes is likely p53 dependent within a low-dose range of LDR.76 Bcl-2, as an antiapoptotic molecule, and Bax, as a proapoptotic molecule, have been reported to have important roles in regulation of apoptotic germ cell death.79,80 However, Liu et al found that no statistical correlation between apoptotic cell death and Bcl-2 and Bax protein expression in germ cells exposed to 25 to 200 mGy LDR.76 This finding demonstrates induction of testicular apoptosis by LDR via a Bax-independent pathway. In addition, Liu et al examined alterations in Fas expression in the testes of Kunming mice exposed to 25 to 200 mGy. Significant increases in Fas expression were detected, and a positive correlation between Fas expression and apoptosis was observed.12 It is well known the mitochondrial pathway is another apoptosis pathway found in both somatic and germ cells. Fang et al found that when male Kunming mice were exposed to whole-body LDR, the total NO synthase, ROS levels, and expression of apoptotic factors, such as cytochrome C, caspase-9, and caspase-3, were increased and ATPase activity and mitochondrial membrane potential in testicular cells were decreased, suggesting LDR can induce testicular cell apoptosis through a mitochondrial signaling pathway.81 All of the above studies suggest that LDR-induced apoptosis of testicular cells may be directly correlated with the p53, Fas, and mitochondrial signaling pathways. Many reports have suggested apoptosis plays an important role in eliminating overproduced, genetically abnormal, or accidentally damaged germ cells.80,82,83 Along these lines, apoptosis induced by LDR may serve as a checkpoint to control and eliminate abnormal cells caused by LDR.
Low-Dose Radiation–Induced Genomic and Inheritable Effects in Germ Cells
Apoptosis of germ cells can be induced by LDR. However, when preexposed to LDR, apoptosis of germ cells is reduced in response to subsequent HDR. Genetic material can be passed on to offspring by germ cells. Therefore, the reduction in apoptosis by LDR-induced AR in male germ cells should be investigated in terms of its genomic and inheritable effects.
In 1990, Cai and Liu demonstrated LDR can attenuate chromosomal damage caused by HDR. They found the rates of chromatid aberrations were reduced in spermatocytes from male Kunming mice exposed to whole-body LDR of 10 to 150 mGy that were subsequently exposed to HDR of 0.75 to 1.5 Gy compared to the spermatocytes of males exposed to HDR alone.84 The researchers also observed translocation of chromosomes was significantly reduced in spermatogonia from male Kunming mice exposed to whole-body LDR of 10 to 150 mGy that were subsequently exposed to HDR of 1.5 Gy compared to the spermatogonia from males exposed to HDR alone. In addition, the researchers, who found HDR induction of chromosomal aberrations was markedly reduced when Kunming mice were exposed to prechronic LDR compared to HDR only, suggested that ARs from LDR damage to chromosomes in male germ cells were induced not only by acute LDR but also by chronic LDR.85 Subsequently, Cai et al found the incidence of dominant lethal mutations was markedly decreased in adapted males compared to nonadapted males when they were mated to nonirradiated females; here, the fertilizing sperm was irradiated during the premeiotic stages of development, suggesting preexposure of male germ cells to LDR can protect against inheritable mutations in germ cell DNA. In addition to attenuation of chromosomal damage, Zhang et al observed testicular morphological changes were reduced in B6C3F1 hybrid strain male mice preexposed to LDR and then exposed to HDR compared to mice exposed to HDR only.86
Furthermore, the mechanisms by which LDR causes an AR that can reduce chromosomal and DNA damage were investigated. Many studies have suggested activation of DNA repair and antioxidant activity may be the major mechanisms responsible for LDR-induced ARs in germ cells. Zhang et al found a significant increase in superoxide dismutase activity in testes pre-irradiated with LDR and then exposed to HDR and a significant decrease in peroxidized lipid substrates compared to testes exposed to HDR alone.87 In addition to triggering antioxidant protective mechanisms, Cai and Wang suggested that LDR may induce selective apoptosis of cells that harbor genetic abnormalities.85,88
Biological Effects of LDR on Tumor Cells
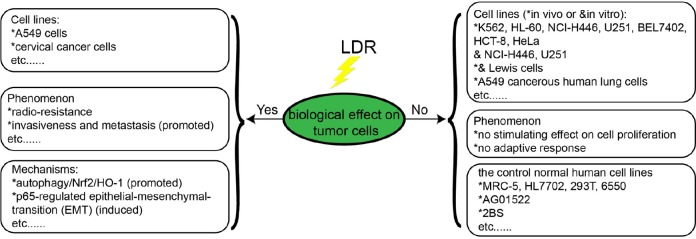
Low-dose radiation–induced biological effects, such as hormesis, adaptive effects, and bystander effects, have been extensively documented by many investigators in different experimental models, including cultured cells and experimental animals. However, it is unclear whether such LDR-induced biological effects can also occur in tumor cells. Over the past 2 decades, Chinese researchers have done a lot of work investigating the biological effects of LDR on tumor cells using cultured tumor cells in vitro and tumor-bearing animal models. Here, we review the progress of Chinese researchers in this field (Figure 3).
Figure 3.
Research on the different biological effects of low-dose radiation (LDR) on tumor cells by Chinese scholars.
Jiang et al demonstrated that LDR does not induce tumor cells proliferation in vitro or in vivo. In their study, they found a stimulating effect on 4 normal human cell lines (MRC-5, HL7702, 293T, and 6550), but not on all of the human tumor cells lines (K56, HL-60, NCI-H446, U251, BEL7402, HCT-8, and HeLa) and the tumor-bearing mice (NCI-H446 and U251) when they were exposed to LDR (25-200 mGy X-rays for cells and 75 mGy for tumor-bearing mice).89 In addition, Jiang et al demonstrated LDR does not induce an AR in tumor cells either in vitro or in vivo.90 Yu et al also found that there was a stimulating effect on the normal cell line AG01522, but not the cancer cell line Lewis cells when they were exposed to LDR in vitro and in vivo. And, lack of an LDR-induced AR in tumor cells was also observed in tumor-bearing mice. Furthermore, they found a higher apoptotic effect and lower expression of the antiapoptotic gene Bcl-2 in tumor cells of tumor-bearing mice exposed to D1 + D2 than those exposed to D2 alone.91
Liang et al proved LDR can induce cell proliferation in the human embryonic lung fibroblast cells 2BS, but not in the lung cancer cells NCI-H446 in response to 20 to 75 mGy X-rays. Using specific inhibitor, they also suggest LDR stimulates cell proliferation via the activation of both the MAPK/ERK and P13K/Akt signaling pathways in 2BS cells, but not in NCI-H446 cells.92 Yang et al demonstrated LDR can induce distinct biological effects on HBE135-E6E7 normal lung epithelial cells and A549 cancerous human lung cells through ataxia-telangiectasia mutated (ATM) signaling. They found the activation of ATM/Akt/GSK-3β signaling pathway, nuclear accumulation of nuclear factor erythroid 2-related factor 2, and the expression of antioxidant were induced by LDR in normal lung epithelial cells (HBE135-E6E7), which can mitigate cellular damage from excessive HDR-induced ROS productions. However, all of these effects were not observed in A549 cells and the failure to activate these pathways may explain the distinction between normal and cancer cells in response to LDR.93
However, some studies have reported an opposite phenotype, where LDR can induce radioresistance in cancer cells. For example, Chen et al showed exposure to 50 mGy α particles can induce radioresistance following exposure to 750 mGy α-particles radiation in human lung adenocarcinoma A549 cells. They also suggested that ROS elevation in response to LDR may promote autophagy/Nrf2/HO-1 and confer radioresistance in A549 cells.94 Yan et al found LDR can induce p65-regulated epithelial–mesenchymal transition in cervical cancer cell lines Siha and C33A, thus promoting invasiveness and metastasis of cervical cancer cells.95 Some researchers speculate these discrepancies may be due to differences in cancer cell lines, LET, and experimental time points.93
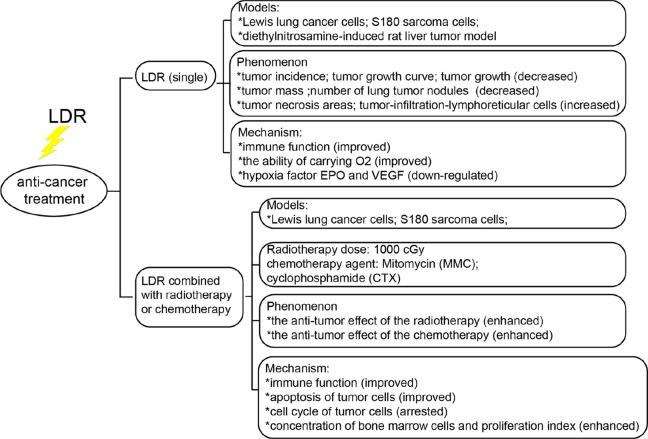
In recent years, laboratory and clinical studies have shown the occurrence and development of malignant tumors is closely related with immune dysfunction or suppression. Therefore, effectively improving the immune functioning of patients with cancer is considered an important means of anticancer treatment. It has been demonstrated that LDR can induce hormesis and ARs in the immune system. In addition, many researchers have suggested LDR can induce distinct biological effects on normal and cancerous cells. Therefore, the hypothesis that LDR may have antitumor effects in vivo has been proposed. Over the past 30 years, Chinese scientists have conducted extensive research on this hypothesis (Figure 4).
Figure 4.
Research on the models, phenomena, and mechanisms of low-dose radiation (LDR)-induced anticancer treatment by Chinese scholars.
In 1995, Yin et al reported the tumor incidence in Kunming mice and C57BL/6J mice irradiated with 50 mGy before tumor inoculation (78.31%) was significantly lower than in nonirradiated direct inoculation tumor mice (91.7%) on day 12 after tumor inoculation. In the LDR-irradiated group, tumor growth was slower and tumor mass was smaller than in the group not exposed to LDR.96 Fu et al found when C57BL/6J mice were irradiated with 50 to 150 mGy X-rays and then inoculated with Lewis lung cancer cells, the mean number of lung tumor nodules was significantly lower than in the LDR nonirradiated tumor-inoculated mice 14 days after LDR irradiation. Furthermore, IL-2 secretion and NK cell activity in the LDR group were significantly higher than in the nonirradiated tumor inoculation group.97 Yu et al demonstrated that LDR (75 mGy) markedly increases antitumor abilities in tumor-bearing Kunming strain mice and improves erythrocyte immune function and the ability to carry O2.98 Wang et al demonstrated low-dose splenic radiation can inhibit liver tumor development in Sprague-Dawley (SD) male rats through functional changes in CD4+CD25+ T regulatory cells.99
Li et al observed that, in C57BL/6J mice subcutaneously transplanted with S180 tumor cells were preexposed to 75 mGy whole-body LDR and then irradiated with 10 Gy, the tumor growth rate was significantly lower than in tumor-bearing mice only exposed to 10 Gy irradiation. Natural killer and lymphokine-activated killer cell activity in the spleen in the group preexposed to LDR and then irradiated with 10 Gy were significantly higher than in the group only exposed to 10 Gy.100 Fu et al found that preexposure to 75 mGy before mitomycin C systemic chemotherapy significantly improved the effect of the chemotherapy in an experimental model utilizing C57BL/6J mice inoculated with Lewis lung cancer cells. At the same time, evaluation of immune indicators revealed a decrease in the number of spleen cells, NK cell and cytotoxic T-lymphocyte activity, phagocytosis by macrophages, and the responses of splenocytes to ConA due to chemotherapy in the tumor-bearing mice. However, all of the abovementioned immune indicators surpassed the chemotherapy-alone group when tumor-bearing mice were preexposed to LDR before chemotherapy.101 Yu et al showed LDR can enhance the antitumor effect of the chemotherapy agent cyclophosphamide (CTX) in S180 sarcoma-bearing mice. In their study, Kunming mice implanted with S180 sarcoma cells were exposed to 75 mGy whole-body γ rays and then 300 mg/kg CTX was administered by intraperitoneal injection after the LDR. Tumor growth was discovered to be significantly reduced and tumor cell apoptosis significantly increased in the group exposed to CTX in addition to LDR. Increased cell-cycle arrest was observed in mice exposed to LDR followed by CTX than in mice exposed only to LDR or CTX. In addition, BMCs concentrations and proliferation in CTX + LDR mice were higher than in the untreated mice. Therefore, LDR was suggested to significantly protect the hematopoietic functions of the bone marrow, which may be of practical significance for adjuvant chemotherapy.102
All the above studies showed LDR may have antitumor effects in vivo that are perhaps related to enhancements in immune function or others LDR-induced functions. These findings imply LDR has potential for protecting normal tissues from radiotherapy while enhancing or not diminishing the efficacy of tumor therapy.
Biological Effects of LDR on Diabetes
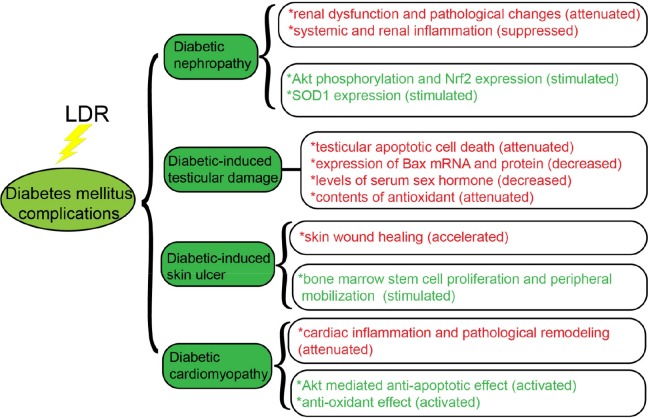
Diabetes mellitus, including types 1 and 2, which is characterized by destruction of insulin-producing cells of the pancreas (called β cells), has dramatically increased worldwide.103 Oxidative stress is now known to be involved in almost of all pathological states of pancreatic β cells in diabetes.104 In addition, secondary oxidative stress caused by diabetic hyperglycemia, hyperlipidemia, and inflammation plays a critical role in almost all diabetic complications.105 This raises an important issue: whether LDR can prevent the development of diabetes and its various complications. Researchers in China have conducted extensive studies on this issue, especially on the prevention of diabetic complications, including on diabetic nephropathy (DN), and diabetes-induced testicular damage, cardiomyopathy, and skin ulcers. Therefore, we collected the available from these studies and described it below (Figure 5).
Figure 5.
Research on the biological effects of low-dose radiation (LDR) on diabetes mellitus complications by Chinese scholars.
Diabetic nephropathy is a major microvascular complication in patients with diabetes. Renal oxidative damage induced by systemic inflammation caused by hyperglycemia and hyperlipidemia plays an important role in the initiation of DN.106–108 Zhang et al showed multiple exposures to LDR can attenuate diabetes-induced renal dysfunction and this effect is associated with the suppression of systemic and renal inflammation. In their study, when diabetic male C57BL/6J mice were exposed to whole-body 25 mGy X-rays, diabetes-induced renal dysfunction and pathological changes were markedly attenuated. In addition, multiple exposures to LDR increased TNF-α, intercellular adhesion molecule 1 (ICAM-1), IL-18, monocyte chemoattractant protein 1 (MCP-1), and plasminogen activator inhibitor 1 (PAI-I) levels in the serum and kidneys.109 Xing et al suggested LDR can prevent DN by stimulating Akt phosphorylation and upregulating Nrf2 expression and function.110 Zhang et al also proved a single 75 mGy and accumulated 75 mGy can stimulate SOD1 expression and activity; this may be one of the mechanisms preventing DN.111 Shao et al demonstrated exposure to LDR (50 or 75 mGy) can significantly prevent type 2 diabetes-induced kidney injury characterized by renal dysfunction and pathological changes. They also suggested the protective mechanisms of LDR can be mainly attributed to the attenuation of dyslipidemia the subsequent lipotoxicity-induced insulin resistance, inflammation, and oxidative stress.112
In recent years, many studies have demonstrated diabetes has a significant impact on the fertility of men, including through erectile dysfunction and reduced sperm motility and semen volume. In 2000, Cai et al reported a significant increase in apoptotic cell death in the testes of diabetic male SD rats.113 Low-dose radiation (less than 100 mGy) was found to induce genomic damage and cell death in the testes.12,76,84,114 Therefore, it was hypothesized exposure to LDR can attenuate diabetes-induced testicular damage. Zhao et al found repeated exposure to LDR significantly attenuates testicular apoptotic cell death, decreases expressions of Bax mRNA and protein, decreases levels of serum sex hormones (testosterone, luteinizing hormone, and follicle-stimulating hormone), and attenuates antioxidant levels (lipid peroxides) and oxidative damage both in the serum and testes in a type 1 diabetic experimental male Wistar rat model, where diabetes is induced with a single injection of streptozotocin (STZ). Their results suggest diabetes-induced testicular cell death may be mediated by increased oxidative stress and LDR protection from the cell death is most likely mediated through preservation of antioxidants.115
In addition, some studies have studied impaired wound healing as a complication associated with diabetes.116,117 The lack of cellular and molecular signals required for normal wound repair processes, including angiogenesis, epithelialization, and remodeling, is likely the main factor contributing to impaired wound healing in patients with diabetes.118,119 In 2010, Guo et al investigated the biological effects of repeated LDR exposure (75 mGy X-rays) on skin wound healing in a male Wistar rat model of diabetes. Their results suggest repeated exposure of diabetic rats to LDR can significantly accelerate skin wound healing compared to nonirradiated diabetic rats. They also demonstrated the LDR-induced improvement in wound healing was associated with increases in bone marrow and circulating CD31+/CD34+ stem cells, vessel regeneration, and cell proliferation in the wound tissue and the expression of matrix metalloproteinases 2 and 9. They concluded LDR-induced acceleration of wound healing in diabetic rats is associated with stimulation of bone marrow stem cell proliferation and peripheral mobilization.120
Diabetic cardiomyopathy, characterized by cardiac remodeling, including profibrotic changes and cardiac hypertrophy associated with cardiac dysfunction, is another severe complication of diabetes.121,122 Diabetes-induced inflammation, oxidative stress, and apoptosis are thought to be the main features of diabetic cardiomyopathy.123,124 In 2009, Zhang et al investigated the preventive effects of repeated LDR exposure on diabetes-induced cardiac inflammation and damage in a C57BL/6J mice model of diabetes. In their study, they observed diabetes caused significant increases in cardiac inflammation, as indicated by increases in IL-18, TNF-α, ICAM-1, PAI-1, and MCP-1 mRNA and protein levels. Compared to nonirradiated diabetic mice, repeated exposure to LDR significantly reduced diabetes-enhanced cardiac expression of IL-18, TNF-α, MCP-1, and PAI-1. There was also a lesser extent of cardiac histopathological abnormalities, oxidative damage, and fibrosis in diabetic mice exposed repeatedly to LDR than in those that were not. Their results suggest LDR can attenuate diabetes-induced cardiac inflammation and pathological remodeling.125 However, some studies found significant inflammation was normally observed in short-term rather than long-term diabetes.126,127 Therefore, the same group investigated whether LDR can prevent late-stage diabetic cardiomyopathy and whether this protection is due to induction of antiapoptotic and antioxidant pathways. In the study, they found LDR can prevent cardiomyopathy in C57BL/6J mice with STZ-induced diabetes treated with whole-body LDR. In addition, they observed this protection induced by LDR associated with p53 inactivation, Nrf2 function, and Akt activation enhancement.128 The above studies by Chinese scholars indicate LDR may be an effective treatment for diabetes-induced complications.
Stochastic Effects of LDR
At present, a consensus has been reached that HDR could have injurious effects; however, whether the effects of LDR are beneficial or injurious still remains controversial.129 The beneficial effects include hormesis and adaptive effects, which were reviewed above. The possible injurious effects include bystander effect, stochastic effect, and so on. The stochastic effect means the potential possibility of carcinogenesis resulting from radiation-induced DNA mutations and damage. Nowadays, it remains unclear whether multi-exposure to LDR has any risk of increasing tumorigenesis. Scientists in China have done many works on the stochastic effects of LDR. Here, we summarized the works in this field.
In 2000, Tao et al estimated the cancer risk associated with the LDR exposure of average annual effective dose of 6.4 mSv in the high-background radiation areas in Yangjiang, China.130 They observed 1 698 316 person-years by following up 125 079 patients, and accumulated 10 415 deaths, among which 1003 were caused by cancer during period 1979 to 1995. In their study, they did not find any increased cancer risk associated with the high levels of natural radiation in high-background radiation areas. And, on the contrary, they found that the mortality of all cancers in high-background radiation areas was generally lower than that in the control area, but not statistically significant. In 2009, Yu et al investigated the effects of multiexposure to LDR on tumorigenesis using a C57BL/6J mouse model. Their results suggested that 0.1 Gy, even after multiple exposures (0.1 Gy × 10), does not increase tumorigenesis.131
However, there were also many researches indicating that LDR could induce stochastic effect. Hwang et al assessed the cancer risk in a population who received prolong LDR for about 10 years as a result of occupying building containing 60Co-contaminated steel in Taiwan. Their results indicated that protracted LDR could higher cancer risks in the general public, especially for leukemia.132 In the study of Wang et al, the relative risk of developing different types of cancers among medical X-ray workers in China was determined.133 They found the significant relationship between the risk of malignant and occupational radiation factor. Their results also suggested that the risk of lung cancer in medical diagnostic X-ray workers was significantly higher than that in control group. In 2010, Feng et al measured the radiation dose from computed tomography (CT) scans in anthropomorphic phantom using a 64-slice multiple detector CT and estimated the associated cancer risk.134 They concluded that the effective doses from these common pediatric CT examinations ranged from 0.7 to 3.5 mSv, and the cancer risks were found to be up to 0.16% with some organs of higher radiosensitivity, including the breast, thyroid gland, colon, and lungs. And these above researches indicated that it is still controversial whether LDR induces stochastic effect. This may be due to the insufficient sample size. Compared with HDR, the risk of LDR is likely to be lower, and progressively larger epidemiological studies are required to quantify the risk.
Conclusions
In this review, we summarized research progress by Chinese scholars on LDR-induced biological effects, including hormesis, ARs, bystander effects, and stochastic effect, as well as on germ cells, tumor cells, and diabetes. Low-dose radiation–induced hormesis has been extensively observed in different biological systems, especially immune and hematopoietic systems. The research progress made by Chinese scholars on enhancement of immunity and hematopoiesis by LDR was reviewed with an emphasis on associated cellular and molecular mechanisms. The LDR-induced AR is described as the induction of cellular resistance to genotoxic effects caused by subsequent exposure to HDR. Here, the research progress by Chinese scientists on LDR-induced AR is reviewed.
In addition, the data available from Chinese scientists on LDR-induced bystander effects, which refer to the induction of damage in cells not directly hit by radiation, were collected and evaluated. Although fundamental scientific evidence on the biological effects of LDR exposure is already available, further studies are required to illustrate both the phenomenological features and mechanisms underlying the biological effects of LDR, which can be studied through genomics and proteomics research. Animal models are also necessary methods of research.
Furthermore, the biological effects of LDR on germ cells, tumor cells, and diabetes have also been studied by Chinese researchers. Although there are fewer studies on the biological effects of LDR on germ cells than somatic cells, it is clear that the biological effects of LDR on germ cells cannot be simply extrapolated from those observed in somatic cells. Apoptotic cell death and genomic and inheritable effects induced by LDR in germ cells have been investigated by Chinese scientists. Further investigations on associated mechanisms are urgently needed to provide further insight into the biological effects of LDR on germ cells. Considerable evidence gathered over nearly half a century suggests that LDR may be used as a treatment for cancer and diabetic complications. And, the controversy of the stochastic effects and cancer risk induced by LDR were also discussed. The resolution of the controversy may depend on the future epidemiological investigation with large sample size.
We hope these beneficial applications of LDR will be achieved soon and become commonplace in treating cancer and diabetic complications. Although the beneficial biological effects of LDR on different biological systems were reviewed in this article, the potential risks of LDR need to be considered in future applications.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (31670859), CAMS Innovation Fund for Medical Science (2017-I2M-1-016), China Postdoctoral Science Foundation (2018M630106), Natural Science Foundation of Tianjin (18JCYBJC26800, 18JCQNJC12300), and the Fundamental Research Funds for the Central Universities (10023201601602).
ORCID iD: Kaihua Ji  https://orcid.org/0000-0002-0155-9506
https://orcid.org/0000-0002-0155-9506
References
- 1. Ji KH, Fang LY, Zhao H, et al. Ginger oleoresin alleviated gamma-ray irradiation-induced reactive oxygen species via the Nrf2 protective response in human mesenchymal stem cells. Oxid Med Cell Longev. 2017. doi.org/10.1155/2017/1480294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules – mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21(2):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu SZ. On radiation hormesis expressed in the immune system. Crit Rev Toxicol. 2003;33(3-4):431–441. [DOI] [PubMed] [Google Scholar]
- 4. Munteanu AC, Uivarosi V, Andries A. Recent progress in understanding the molecular mechanisms of radioresistance in Deinococcus bacteria. Extremophiles. 2015;19(4):707–719. [DOI] [PubMed] [Google Scholar]
- 5. Ostrau C, Hülsenbeck J, Herzog M, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92(3):492. [DOI] [PubMed] [Google Scholar]
- 6. Horie K, Kubo K, Yonezawa M. . p53 dependency of radio-adaptive responses in endogenous spleen colonies and peripheral blood-cell counts in C57BL mice. J Radiat Res. 2002;43(4):353. [DOI] [PubMed] [Google Scholar]
- 7. Yang G, Kong Q, Wang G, et al. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother Radiopharm. 2014;29(10):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai L. Research of the adaptive response induced by low-dose radiation: where have we been and where should we go. Hum Exp Toxicol. 1999;18(7):419–425. [DOI] [PubMed] [Google Scholar]
- 9. Liu SZ. Nonlinear dose–response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinearity Biol Toxicol Med. 2003;1(1):71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43(6):771. [DOI] [PubMed] [Google Scholar]
- 11. Yang G, Li W, Jiang H, et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int J Cancer. 2016;139(10):2157–2168. [DOI] [PubMed] [Google Scholar]
- 12. Liu G, Gong P, Bernstein LR, et al. Apoptotic cell death induced by low-dose radiation in male germ cells: hormesis and adaptation. Crit Rev Toxicol. 2007;37(7):587–605. [DOI] [PubMed] [Google Scholar]
- 13. United Nations Scientific Committee on the Effects of Atomic Radiation. Adaptive responses to radiation in cells and organisms. Unscear report sources & effects of ionizing radiation. 1994. New York, NY: United Nations. [Google Scholar]
- 14. Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223(4636):594–597. [DOI] [PubMed] [Google Scholar]
- 15. Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 1992;52(22):6394–6396. [PubMed] [Google Scholar]
- 16. Liu SZ. Discussion on some problems of the mechanisms of hormesis effect of low dose radiation. Chin J Radiol Med Prot. 2003;23(6):393–398. [Google Scholar]
- 17. Zhang L, Tian Y, Wu Y, et al. Low-dose radiation-induced hormetic effect on hematopoietic reconstitution. Int J Radiat Biol. 2010;86(4):329–333. [DOI] [PubMed] [Google Scholar]
- 18. Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol. 2008;84(1):1. [DOI] [PubMed] [Google Scholar]
- 19. Liu S. Current status of research on radiation hormesis in the immune system after low level radiation. J Radiat Res Radiat Proc. 1995;13(3):129–139. [Google Scholar]
- 20. Fan X, Liu S. Radiation effect on splenic natural killer cells in mice. 1989;15(6):551–553. [Google Scholar]
- 21. Liu SZ, Zhang YC, Mu Y, Su X, Jian-Xiang L. Thymocyte apoptosis in response to low-dose radiation. Mutat Res. 1996;358(2):185–191. [DOI] [PubMed] [Google Scholar]
- 22. Liu SZ. Biological defense and adaptation induced by low dose radiation. Hum Ecol Risk Assess. 1998;4(5):1217–1254. [Google Scholar]
- 23. Su X, Zhang YC, Liu SZ. Effects on subsets of thymic lymphocytes after X-irradiation with different doses. Chin J Radiol Med Prot. 1995;15(2):103–105. [Google Scholar]
- 24. Liu S, Zhang Y, Qi J. Thymocyte renewal and differentiation in mice following low dose ionizing radiation. J Norman Bethune Univ Med Sci. 1992;18(5):405–408. [Google Scholar]
- 25. Liu XD, Ma SM, Liu SZ. Effects of 0.075 Gy x-ray irradiation on the expression of IL-10 and IL-12 in mice. Phys Med Biol. 2003;48(13):2041–2049. [DOI] [PubMed] [Google Scholar]
- 26. Liu SZ, Jin SZ, Liu XD, Sun YM. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol. 2001;2(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu S, Feng X. Involvement of the Ca2+-protein kinase C and adenylate cyclace signal pathways in the activation of thymocytes in response to whole-body irradiation with low dose X-rays. Acta Acad Med Sin. 2000;15(1):1–7. [PubMed] [Google Scholar]
- 28. Buckner CD. Autologous bone marrow transplants to hematopoietic stem cell support with peripheral blood stem cells: a historical perspective. J Hematother. 1999;8(3):233–236. [DOI] [PubMed] [Google Scholar]
- 29. Zaucha JM, Knopińskaposłuszny W, Bieniaszewska M, Myśliwski A, Hellmann A. The effect of short G-CSF administration on the numbers and clonogenic efficiency of hematopoietic progenitor cells in bone marrow and peripheral blood of normal donors. Ann Transplant. 2000;5(4):20. [PubMed] [Google Scholar]
- 30. Mouthon MA, Meeren AVD, Vandamme M, Squiban C, Gaugler MH. Thrombopoietin protects mice from mortality and myelosuppression following high-dose irradiation: importance of time scheduling. Can J Physiol Pharmacol. 2002;80(7):717–721. [DOI] [PubMed] [Google Scholar]
- 31. Li W, Wang G, Cui J, Xue L, Cai L. Low-dose radiation (LDR) induces hematopoietic hormesis: LDR-induced mobilization of hematopoietic progenitor cells into peripheral blood circulation. Exp Hematol. 2004;32(11):1088–1096. [DOI] [PubMed] [Google Scholar]
- 32. Zhang HL. Stimulation of low dose radiation on hematopoietic system. Zhonghua Yi Xue Za Zhi. 1993;73(2):99. [PubMed] [Google Scholar]
- 33. Zhang L. The hormesis of blood samples irradiated in vitro by X-rays to low doses. J Radiat Res Radiat Proc. 2004;22(5):315–317. [Google Scholar]
- 34. Wang GJ, Cai L. Induction of cell-proliferation hormesis and cell-survival adaptive response in mouse hematopoietic cells by whole-body low-dose radiation. Toxicol Sci. 2000;53(2):369–376. [DOI] [PubMed] [Google Scholar]
- 35. Sensebé L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87(9 suppl):49–53. [DOI] [PubMed] [Google Scholar]
- 36. Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106(6):984. [DOI] [PubMed] [Google Scholar]
- 37. Liang XY, So YH, Cui JW, et al. The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK_ERK pathway in rat cultured mesenchymal stem cells. J Radiat Res. 2011;52(3):380–386. [DOI] [PubMed] [Google Scholar]
- 38. Cheng GH, Wu N, Jiang DF, et al. Increased Levels of p53 and PARP-1 in EL-4 cells probably related with the immune adaptive response induced by low dose ionizing radiation in vitro. Biomed Environ Sci. 2010;23(6):487–495. [DOI] [PubMed] [Google Scholar]
- 39. Cortés F, Dominguez I, Pi’Ero J, Mateos JC. Adaptive response in human lymphocytes conditioned with hydrogen peroxide before irradiation with X-rays. Mutagenesis. 1990;5(6):555–557. [DOI] [PubMed] [Google Scholar]
- 40. Liu SZ, Cai L, Sun JB. Effect of low-dose radiation on repair of DNA and chromosome damage. Acta Biol Hung. 1990;41(1-3):149. [PubMed] [Google Scholar]
- 41. Cai L, Meng QY. Studies on the induction of cross-resistance by low dose radiation or by low concentrations of chemicals. Biomed Environ Sci. 1994;7(3):241. [PubMed] [Google Scholar]
- 42. Gong SL, Lu Z, Liu SC, et al. Time effect of adaptive response of mouse thymocyte apoptosis and cell cycle progression induced by low dose radiation. J Jilin Univ (Med Ed). 2001;27(1):1–5. [Google Scholar]
- 43. Cai L, Jiang J. Mild hyperthermia can induce adaptation to cytogenetic damage caused by subsequent X irradiation. Radiat Res. 1995;143(1):26–33. [PubMed] [Google Scholar]
- 44. Yamaoka K, Kojima S, Takahashi M, Nomura T, Iriyama K. Change of glutathione peroxidase synthesis along with that of superoxide dismutase synthesis in mice spleens after low-dose X-ray irradiation. Biochim Biophys Acta. 1998;1381(2):265. [DOI] [PubMed] [Google Scholar]
- 45. Cai L, Satoh M, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132(2-3):85. [DOI] [PubMed] [Google Scholar]
- 46. Chankova GS, Matos JA, Simoes F, Bryant PE. Adaptive response of a new radioresistant strain of Chlamydomonas reinhardtii and correlation with increased DNA double-strandbreak rejoining. Int J Radiat Biol. 2005;81(7):509–514. [DOI] [PubMed] [Google Scholar]
- 47. Raaphorst GP, Li LF, Yang DP. Evaluation of adaptive responses to cisplatin in normal and mutant cell lines with mutations in recombination repair pathways. Anticancer Res. 2006;26(26):1183–1187. [PubMed] [Google Scholar]
- 48. Ikushima T, Aritomi H, Morisita J. Radioadaptive response: efficient repair of radiation-induced DNA damage in adapted cells. Mutat Res. 1996;358(2):193–198. [DOI] [PubMed] [Google Scholar]
- 49. Yu X, Wang H, Wang P, Chen BPC, Wang Y. The Ku-dependent non-homologous end-joining pathway contributes to low-dose radiation-stimulated cell survival. J Cell Physiol. 2011;226(2):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ning HS. Low dose radiation induced adaptive response of apoptosis in mouse spleen cells. Chin-German J Clin Oncol. 2010;9(4):235–238. [Google Scholar]
- 51. Goldberg Z, Lehnert BE. Radiation-induced effects in unirradiated cells: a review and implications in cancer. Int J Oncol. 2002;21(2):337. [PubMed] [Google Scholar]
- 52. Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res. 2011;176(2):139. [DOI] [PubMed] [Google Scholar]
- 53. Hall EJ. Radiation, the two-edged sword: cancer risks at high and low doses. Cancer J. 2000;6(6):343. [PubMed] [Google Scholar]
- 54. Bonner WM. Low-dose radiation: thresholds, bystander effects, and adaptive responses. Proc Natl Acad Sci U S A. 2003;100(9):4973–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ballarini F, Biaggi M, Ottolenghi A, Sapora O. Cellular communication and bystander effects: a critical review for modelling low-dose radiation action-mutation research. Mutat Res. 2002;501(1-2):1–12. [DOI] [PubMed] [Google Scholar]
- 56. Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100(24):13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing cancer risks of low-dose radiation. Nat Rev Cancer. 2009;9(8):596–604. [DOI] [PubMed] [Google Scholar]
- 58. Wang H, Yu KN, Hou J, Liu Q, Han W. Radiation-induced bystander effect: early process and rapid assessment. Cancer Lett. 2015;356(1):137–144. [DOI] [PubMed] [Google Scholar]
- 59. Hu B, Han W, Wu L, et al. In situ visualization of DSBs to assess the extranuclear/extracellular effects induced by low-dose alpha-particle irradiation. Radiat Res. 2005;164(3):286–291. [DOI] [PubMed] [Google Scholar]
- 60. Han W, Chen S, Yu KN, Wu L. Nitric oxide mediated DNA double strand breaks induced in proliferating bystander cells after alpha-particle irradiation. Mutat Res. 2010;684(1-2):81–89. [DOI] [PubMed] [Google Scholar]
- 61. Hu B, Wu L, Han W, et al. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27(2):245–251. [DOI] [PubMed] [Google Scholar]
- 62. Chen S, Zhao Y, Zhao G, et al. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutat Res. 2009;666(1-2):68–73. [DOI] [PubMed] [Google Scholar]
- 63. Chen S, Zhao Y, Han W, et al. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br J Cancer. 2008;98(11):1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shao C, Folkard M, Prise KM. Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008;27(4):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57(18):3963. [PubMed] [Google Scholar]
- 66. Xu S, Ding N, Pei H, et al. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014;11(9):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hu W, Xu S, Yao B, et al. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol. 2014;11(9):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suzuki K, Kodama S, Watanabe M. Extremely low-dose ionizing radiation causes activation of mitogen-activated protein kinase pathway and enhances proliferation of normal human diploid cells. Cancer Res. 2001;61(14):5396–5401. [PubMed] [Google Scholar]
- 69. Planel H, Soleilhavoup JP, Tixador R, et al. Influence on cell proliferation of background radiation or exposure to very low, chronic gamma radiation. Health Phys. 1987;52(5):571–578. [DOI] [PubMed] [Google Scholar]
- 70. Chen SL, Cai L, Meng QY, Xu S, Wan H, Liu SZ. Low-dose whole-body irradiation (LD-WBI) changes protein expression of mouse thymocytes: effect of a LD-WBI-enhanced protein RIP10 on cell proliferation and spontaneous or radiation-induced thymocyte apoptosis. Toxicol Sci. 1999;55(1):97–106. [DOI] [PubMed] [Google Scholar]
- 71. Chen Z, Sakai K. Enhancement of radiation-induced apoptosis by preirradiation with low-dose X-rays in human leukemia MOLT-4 cells. J Radiat Res. 2004;45(2):239–243. [DOI] [PubMed] [Google Scholar]
- 72. Mohammadi S, Taghavi-Dehaghani M, Gharaati MR, Masoomi R, Ghiassi-Nejad M. Adaptive response of blood lymphocytes of inhabitants residing in high background radiation areas of ramsar-micronuclei, apoptosis and comet assays. J Radiat Res. 2006;47(3-4):279. [DOI] [PubMed] [Google Scholar]
- 73. Gong SL, Liu SC, Liu JX, Zhang YC. Adaptive response of thymocyte apoptosis and cell cycle progression induced by low dose X-ray irradiation in mice. Biomed Environ Sci. 2000;13(3):180–188. [PubMed] [Google Scholar]
- 74. Hamer G, Roepersgajadien HL, Gademan IS, Kal HB, De Rooij DG. Intercellular bridges and apoptosis in clones of male germ cells. Int J Androl. 2003;26(6):348–353. [DOI] [PubMed] [Google Scholar]
- 75. Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56(6):1490. [DOI] [PubMed] [Google Scholar]
- 76. Liu G, Gong P, Zhao H, Wang Z, Gong S, Cai L. Effect of low-level radiation on the death of male germ cells. Radiat Res. 2006;165(4):379. [DOI] [PubMed] [Google Scholar]
- 77. Kim YM, Yang I, Lee J, Koo HS. Deficiency of Bloom’s syndrome protein causes hypersensitivity of C. elegans to ionizing radiation but not to UV radiation, and induces p53-dependent physiological apoptosis. Mol Cells. 2005;20(2):228–234. [PubMed] [Google Scholar]
- 78. Lambrot R, Coffigny H, Pairault C, et al. High radiosensitivity of germ cells in human male fetus. J Clin Endocrinol Metab. 2007;92(7):2632–2639. [DOI] [PubMed] [Google Scholar]
- 79. Beumer TL, Roepersgajadien HL, Gademan IS, Lock TM, Kal HB, De Rooij DG. Apoptosis regulation in the testis: involvement of Bcl-2 family members. Mol Reprod Dev. 2000;56(3):353. [DOI] [PubMed] [Google Scholar]
- 80. Koji T, Hishikawa Y. Germ cell apoptosis and its molecular trigger in mouse testes. Arch Histol Cytol. 2003;66(1):1–16. [DOI] [PubMed] [Google Scholar]
- 81. Fang F, Gong PS, Zhao HG, et al. Mitochondrial modulation of apoptosis induced by low-dose radiation in mouse testicular cells. Biomed Environ Sci. 2013;26(10):820–830. [DOI] [PubMed] [Google Scholar]
- 82. Castanares M, Vera Y, Erkkilä K, et al. Minocycline up-regulates BCL-2 levels in mitochondria and attenuates male germ cell apoptosis. Biochem Biophys Res Commun. 2005;337(2):663–669. [DOI] [PubMed] [Google Scholar]
- 83. Sasagawa I, Yazawa H, Suzuki Y, Nakada T. Stress and testicular germ cell apoptosis. Arch Androl. 2001;47(3):211–216. [DOI] [PubMed] [Google Scholar]
- 84. Cai L, Liu SZ. Induction of cytogenetic adaptive response of somatic and germ cells in vivo and in vitro by low-dose X-irradiation. Int J Radiat Biol. 1990;58(1):187–194. [DOI] [PubMed] [Google Scholar]
- 85. Cai L, Wang P. Induction of a cytogenetic adaptive response in germ cells of irradiated mice with very low-dose rate of chronic gamma-irradiation and its biological influence on radiation-induced DNA or chromosomal damage and cell killing in their male offspring. Mutagenesis. 1995;10(2):95–100. [DOI] [PubMed] [Google Scholar]
- 86. Hong Z, Zhou Q, Li W, et al. Alleviation of pre-exposure of mouse brain with low-dose ∼(12)C∼(6+) ion or ∼(60)Co γ-ray on male reproductive endocrine damages induced by subsequent high-dose irradiation. Int J Androl. 2006;29(6):592–596. [DOI] [PubMed] [Google Scholar]
- 87. Zhang H, Zheng RL, Wei ZQ, et al. Effects of pre-exposure of mouse testis with low-dose (16)O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. Int J Radiat Biol. 1998;73(2):163–167. [DOI] [PubMed] [Google Scholar]
- 88. Cai L, Wang P, Piao XG. Cytogenetic adaptive response with multiple small X-ray doses in mouse germ cells and its biological influence on the offspring of adapted males. Mutat Res Lett. 1994;324(1-2):13–17. [DOI] [PubMed] [Google Scholar]
- 89. Jiang HY, Xu Y, Li W, Ma K, Cai L, Wang G. Low-dose radiation does not induce proliferation in tumor cells in vitro and in vivo. Radiat Res. 2008;170(4):477–487. [DOI] [PubMed] [Google Scholar]
- 90. Jiang HY, Li W, Li XY, Cai L, Wang G. Low-dose radiation induces adaptive response in normal cells, but not in tumor cells: in vitro and in vivo studies. J Radiat Res. 2008;49(3):219–230. [DOI] [PubMed] [Google Scholar]
- 91. Yu H, Liu N, Wang H, Shang Q, Jiang P, Zhang Y. Different responses of tumor and normal cells to low-dose radiation. Contemp Oncol (Pozn). 2013;17(4):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liang X, Gu J, Yu D, et al. Low-dose radiation induces cell proliferation in human embryonic lung fibroblasts but not in lung cancer cells: importance of ERK1/2 and AKT signaling pathways. Dose Response. 2016;14(1). doi:10.1177/1559325815622174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yang G, Yu D, Li W, et al. Distinct biological effects of low-dose radiation on normal and cancerous human lung cells are mediated by ATM signaling. Oncotarget. 2016;7(44):71856–71872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen N, Wu L, Yuan H, Wang J. ROS/autophagy/Nrf2 pathway mediated low-dose radiation induced radio-resistance in human lung adenocarcinoma A549 cell. Int J Biol Sci. 2015;11(7):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yan S, Wang Y, Yang Q, et al. Low-dose radiation-induced epithelial–mesenchymal transition through NF-κB in cervical cancer cells. Int J Oncol. 2013;42(5):1801–1806. [DOI] [PubMed] [Google Scholar]
- 96. Yin H, Yu K, Wang XL. Inhibitory effect of low dose radiation on the implanted tumor and its influence on genetic substances. J Norman Bethune Univ Med Sci. 1995;21(1):33–35. [Google Scholar]
- 97. Fu HQ, Li XY, Li YJ, Liu SZ. Whole-body low dose irradiation suppresses cancer cell dissemination in mice. Chin J Radiol Med Prot. 1996;16(5):307–309. [Google Scholar]
- 98. Yu HS, Liu ZM, Yu XY, Song AQ, Liu N, Wang H. Low dose radiation induces antitumor effects and erythrocyte system hormesis. Asian Pac J Cancer Prev 2013;14(7):4121. [DOI] [PubMed] [Google Scholar]
- 99. Wang B, Li B, Dai Z, et al. Low-dose splenic radiation inhibits liver tumor development of rats through functional changes in CD4+CD25+Treg cells. Int J Biochem Cell Biol. 2014;55:98–108. [DOI] [PubMed] [Google Scholar]
- 100. Li XY, Fu HQ, Liu SZ. Whole-body lose dose irradiation enhances the tumor-suppressive effect of high dose local irradiation given to the tumor site. J Jilin Univ (Med Ed). 1995;(6):559–562. [Google Scholar]
- 101. Fu Q, Wang LJ, Chen J, Song LS. Effects of low-dose radiation immunostimulatory effects on chemotherapy side effects in tumor bearing mice. Chin J Radiol Med Prot. 1999;19(2):127–128. [Google Scholar]
- 102. Yu HS, Xue HW, Guo CB, et al. Low dose radiation increased the therapeutic efficacy of cyclophosphamide on S180 sarcoma bearing mice. J Radiat Res. 2007;48(4):281–288. [DOI] [PubMed] [Google Scholar]
- 103. Bardsley JK, Want LL. Overview of diabetes. Crit Care Nurs Q. 2004;27(2):106–112. [DOI] [PubMed] [Google Scholar]
- 104. Haskins K, Bradley B, Powers K, et al. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005(1):43. [DOI] [PubMed] [Google Scholar]
- 105. Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy. Cardiovasc Toxicol. 2001;1(3):181. [DOI] [PubMed] [Google Scholar]
- 106. Ichinose K, Kawasaki E, Eguchi K. Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol. 2007;27(6):554–564. [DOI] [PubMed] [Google Scholar]
- 107. Lin J, Glynn RJ, Rifai N, et al. Inflammation and progressive nephropathy in type 1 diabetes in the diabetes control and complications trial. Diabetes Care. 2008;31(12):2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433. [DOI] [PubMed] [Google Scholar]
- 109. Zhang C, Tan Y, Guo W, et al. Attenuation of diabetes-induced renal dysfunction by multiple exposures to low-dose radiation is associated with the suppression of systemic and renal inflammation. Am J Physiol Endocrinol Metab. 2009;297(6): E1366–E1377. [DOI] [PubMed] [Google Scholar]
- 110. Xing X, Zhang C, Shao M, et al. Low-dose radiation activates Akt and Nrf2 in the kidney of diabetic mice: a potential mechanism to prevent diabetic nephropathy. Oxid Med Cell Longev. 2012. doi:10.1155/2012/291087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang C, Xing X, Zhang F, et al. Low-dose radiation induces renal SOD1 expression and activity in type 1 diabetic mice. Int J Radiat Biol. 2014;90(3):224–230. [DOI] [PubMed] [Google Scholar]
- 112. Shao M, Lu X, Cong W, et al. Multiple low-dose radiation prevents type 2 diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PLoS One. 2014;9(3):e92574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Evans T. Apoptotic germ-cell death and testicular damage in experimental diabetes: prevention by endothelin antagonism. Urol Res. 2000;28(5):342. [DOI] [PubMed] [Google Scholar]
- 114. Cai L, Wang J, Li Y, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54(6):1829. [DOI] [PubMed] [Google Scholar]
- 115. Zhao H, Xu S, Wang Z, et al. Repetitive exposures to low-dose X-rays attenuate testicular apoptotic cell death in streptozotocin-induced diabetes rats. Toxicol Lett. 2010;192(3):356–364. [DOI] [PubMed] [Google Scholar]
- 116. Brem H, Tomiccanic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pavlović MD, Milenković T, Dinić M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964–1967. [DOI] [PubMed] [Google Scholar]
- 118. Blakytny R, Jude EB, Martin GJ, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2015;190(5):589–594. [DOI] [PubMed] [Google Scholar]
- 119. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23(6):594. [DOI] [PubMed] [Google Scholar]
- 120. Guo WY, Wang GJ, Wang P, Chen Q, Tan Y, Cai L. Acceleration of diabetic wound healing by low-dose radiation is associated with peripheral mobilization of bone marrow stem cells. Radiat Res. 2010;174(4):467–479. [DOI] [PubMed] [Google Scholar]
- 121. Feng B, Chen S, George B, Feng Q, Chakrabarti S. . miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes. 2010;26(1):40. [DOI] [PubMed] [Google Scholar]
- 122. Chang SH, Liu CJ, Kuo CH, et al. Garlic oil alleviates MAPKs- and IL-6-mediated diabetes-related cardiac hypertrophy in STZ-induced DM rats. Evid Based Complement Alternat Med. 2011;2011:950150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang Y, Sun W, Du B, et al. Therapeutic effect of MG-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: roles of Nrf2 and NF-κB. Am J Physiol Heart Circ Physiol. 2013;304(4):H567. [DOI] [PubMed] [Google Scholar]
- 124. Guan SJ, Ma ZH, Wu YL, et al. Long-term administration of fasudil improves cardiomyopathy in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2012;50(6):1874–1882. [DOI] [PubMed] [Google Scholar]
- 125. Zhang C, Jin S, Guo W, et al. Attenuation of diabetes-induced cardiac inflammation and pathological remodeling by low-dose radiation. Radiat Res. 2011;175(3):307–321. [DOI] [PubMed] [Google Scholar]
- 126. Westermann D, Van LS, Dhayat S, et al. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes. 2007;56(7):1834–1841. [DOI] [PubMed] [Google Scholar]
- 127. Westermann D, Rutschow S, Jager S, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56(3):641. [DOI] [PubMed] [Google Scholar]
- 128. Zhang F, Lin X, Yu L, et al. Low-dose radiation prevents type 1 diabetes-induced cardiomyopathy via activation of AKT mediated anti-apoptotic and anti-oxidant effects. J Cell Mol Med. 2016;20(7):1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ma S, Kong B, Liu B, Liu X. Biological effects of low-dose radiation from computed tomography scanning. Int J Radiat Biol. 2013;89(5):326–333. [DOI] [PubMed] [Google Scholar]
- 130. Tao ZZ, Sun Y, Akiba S, et al. Cancer mortality in the high background radiation areas of Yangjiang, China during the period between 1979 and 1995. J Radiat Res. 2000;41:31–41. [DOI] [PubMed] [Google Scholar]
- 131. Yu XY, Lu L, Wen SY, et al. The effects of Fhit on tumorigenesis after multi-exposure to low-dose radiation. Int J Clin Exp Med. 2009;2:348–353. [PMC free article] [PubMed] [Google Scholar]
- 132. Hwang S, Guo HR, Hsieh W, et al. Cancer risk analysis of low-dose radiation exposure. Int Congress Ser. 2007;1299:87–97. [Google Scholar]
- 133. Wang FR, Sun QF, Wang J, Yu NL. Risk of developing cancers due to low-dose radiation exposure among medical X-ray workers in China – results of a prospective study. Int J Clin Exp Pathol. 2016;9(11):11897–11903. [Google Scholar]
- 134. Feng ST, Law MWM, Huang BS, et al. Radiation dose and cancer risk from pediatric CT examinations on 64-slice CT: a phantom study. Eur J Radiol. 2010;76:e19–e23. [DOI] [PubMed] [Google Scholar]