Abstract
JAK2 V617F mutation and BCR-ABL translocation have been considered to be mutually exclusive. However, many cases where both hits coexisted have been reported. We have personally managed a case too. We believe this hybrid entity is underdiagnosed. Thus, we decided to shed light on this “double hit” disease to improve its diagnosis and optimize its treatment. We reviewed the English literature in PubMed since JAK2 discovery. We found 33 cases reported so far. We summarized patient characteristics and analyzed possible interactions between JAK2 and BCR-ABL clones.
Keywords: myeloproliferative disorder, chronic myelogenous leukemia, JAK2 mutation, BCR-ABL translocation
Introduction
Chronic myelogenous leukemia (CML) and Philadelphia-negative myeloproliferative disorders (MPD) are common hematologic diseases. The prevalence of CML is 1 in 17 000. Polycythemia vera (PV) with a prevalence of 44 to 57 per 100 000 and essential thrombocytosis (ET) with a prevalence of 38 to 57 per 100 000 are much more common than primary myelofibrosis (PMF), which has a prevalence of 4 to 6 per 100 000.1 In CML, hybrid BCR-ABL gene as a result of translocation (9,22) encodes a fusion protein that leads to activation of ABL tyrosine kinase and subsequent uncontrolled production of mature and maturing granulocytes. In Philadelphia-negative MPD, mutated Janus kinase 2 (JAK2) has increased kinase activity. It leads to proliferation of progenitors independently of cytokine stimulation. JAK2 V617F mutation is present in around 95% of PV and 50% of ET and PMF cases. BCR-ABL translocation and JAK2 V617F mutation have been considered to be mutually exclusive. However, several cases of JAK2 mutation coexisting with CML have been reported. In the pre-JAK2 era, it was difficult to confirm the coexistence of CML and Philadelphia-negative MPD due to lack of a marker for the latter. The discovery of JAK2 V617F mutation in 2005 provided the opportunity to document concomitant Philadelphia-positive and Philadelphia-negative MPD with more certainty. We used PubMed to conduct a review of cases published in English where JAK2-positive MPD and CML coexisted. Obviously, the cases were published post JAK2 discovery in 2005. We are also reporting a case that we have personally diagnosed and treated.
Results
We found 33 cases of concomitant JAK2-positive MPD and CML. Patient characteristics are shown in Table 1. We have also personally managed a case of PMF and CML.
Table 1.
Patient Characteristics.
| Age/Sex | Diagnosis (+CML) | WBC (×103) | Hb | PLT | Treatment | Course | Clone Interaction |
|---|---|---|---|---|---|---|---|
| Unknown2 | ET | ? | ? | ? | I | ET, CML 12 years later | ? |
| 50/Male3 | MF | 93 | 15 | 345 | I | CML, MF 4 years later | BCR-ABL disappeared, JAK2 constant |
| 43/Male4 | PV | ? | Hct 49 | ? | INF, HU then I | CML, PV 6 years later | BCR-ABL decreased, JAK2 constant |
| 82/Female | PV | 67 | 9.8 | 605 | Radioactive P, HU, A then I | PV, CML 16 years later | ? |
| 73/Female5 | PV | 214 | 10.2 | 65 | HU then I | PV, CML 15 years later | |
| 66/Male6 | MF | ? | 11.3 | ? | I then HU | Concomitant | ? |
| 55/Male7 | MF | 163 | 11.5 | ? | I | CML, MF 2 years later | BCR-ABL decreased, JAK2 increased |
| 43/Male8 | PV | 100 | Hct 36 | ? | I | PV, CML 16 years later | BCR-ABL decreased, JAK2 increased |
| 49/Male | MF | ? | ? | ? | I then D | CML, MF 2 years later | BCR-ABL and JAK2 decreased |
| 64/Male9 | ? | ? | ? | ? | I then N | Concomitant | BCR-ABL decreased, JAK2 constant |
| 64/Male10 | PV | 15.3 | 24 | 380 | I | Concomitant | BCR-ABL decrease, JAK2 increased |
| 52/Female11 | MF | 193 | 10 | 689 | HU then I | Concomitant | BCR-ABL and JAK2 decreased |
| 58/Male12 | MF | 7.5 | 13.5 | 669 | I then HU | MF, CML 4 years later | BCR-ABL decreased, JAK2 increased |
| 67/Male13 | MF | 35 | 8.9 | 143 | I then HU | Concomitant | ? |
| 32/Male | ET | 35 | ? | 907 | A, HU then I | ET, CML 40 months later | BCR-ABL and JAK2 decreased |
| 58/Female | MF | 83 | 14.5 | 496 | HU, INF | Concomitant | ? |
| 63/Male | PV | 41 | 14.4 | 371 | HU then I | PV, CML 15 years later | BCR-ABL decreased, JAK2 increased |
| 45/Male14 | ? | 57 | 14 | 266 | I | Concomitant | ? |
| 39/Male15 | PV | 66 | 20 | 342 | I then D then N | PV, CML 15 years later | Opposite growth |
| 69/Male16 | ? | 32.5 | 10.9 | 511 | I | Concomitant | BCR-ABL and JAK2 decreased |
| 60/Male17 | PV | 121 | 18.5 | 152 | I then HU | Concomitant | BCR-ABL increased, JAK2 decreased |
| 67/Male18 | PV | 11.9 | 17.2 | 507 | INF, HU then I | CML, PV 10 years later | BCR-ABL decreased, JAK2 increased |
| 70/Male19 | ? | 11.5 | 15.3 | 950 | INF then I | JAK2, 7 years after CML | ? |
| 42/Female20 | ? | 350 | ? | 498 | HU then I then D | JAK2, 5 years after CML | JAK2 increased with CML treatment |
| 53/Female | ET | 36.7 | 12.5 | 983 | HU then INF then I, A | CML, ET 11 years later | ? |
| 60/Female21 | ET | 23 | 8.3 | 220 | I, A then D | CML, ET 2 years later | ? |
| 21/Female22 | ? | 73 | 13.3 | 440 | I | Concomitant | ? |
| 67/Male23 | MF | 22.6 | Hct 42 | 652 | N then D | MF, CML 3 years later | BCR-ABL decreased, JAK2 constant |
| 77/Male24 | ? | 6.2 | 8.7 | 242 | ? | Concomitant | ? |
| 60/Male25 | ? | 30 | Hct 21 | 324 | I | Concomitant | ? |
| 61/Male26 | PV | 108 | 9 | 95 | HU, I | PV, CML 7 years later | JAK2 emerged with decrease in BCR-ABL |
| 36/Male | ET | 9.4 | 13.8 | 830 | HU | Concomitant | BCR-ABL increased |
| 58/Male27 | MF | 19.7 | 13 | 285 | HU, D | Concomitant | ? |
| Our case: 75/Male | MF | 23 | 14.3 | 741 | HU, I | Concomitant | ? |
Abbreviations: A, anagrelide; CML, chronic myelogenous leukemia; D, dasatinib; ET, essential thrombocytosis; Hb, hemoglobin in g/dL; Hct, hematocrit; HU, hydroxyurea; I, imatinib; INF, interferon; JAK2, Janus kinase 2; MF, myelofibrosis; N, nilotinib; P, phosphorus; PLT, platelet count/mm3; PV, polycythemia vera; WBC, white blood cell count/mm3; ?, unknown or unclear.
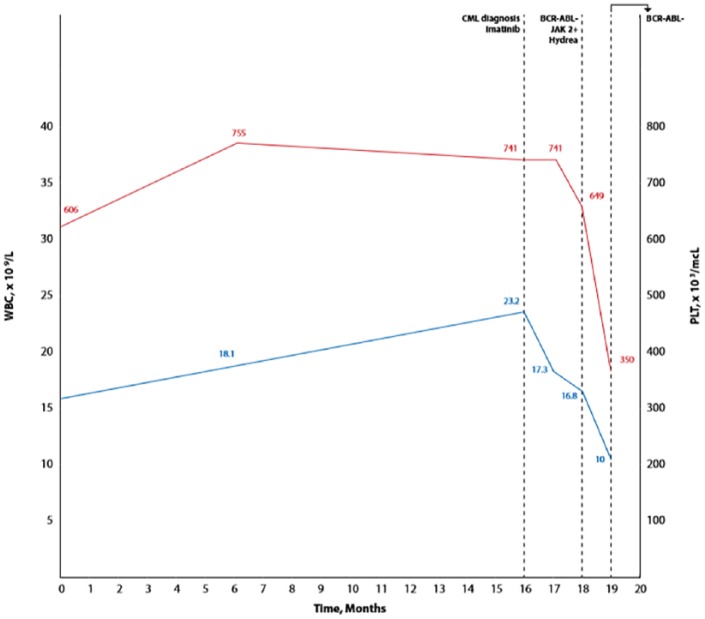
The patient is a 75-year-old male who presented to our clinic in 2017 with progressive weight loss over prior 2 years, night sweats, pruritus, early satiety, and left upper quadrant pain. Physical examination was pertinent for hepatosplenomegaly. His white blood cell (WBC) count was 23 200 cells/mm3 with 92% neutrophils, hemoglobin 14.3 g/dL, and platelet count 741/mm3. He had no blasts in peripheral blood. Per chart review, we noted that he had started to develop neutrophilia and thrombocytosis in 2013 and 2014, respectively. Quantitative reverse transcription polymerase chain reaction was positive for both b2a2 and b3a2 transcripts at 2.1% and 1.2%, respectively. Imatinib was started. Eight weeks later, WBC count was 16.8 cells/mm3, hemoglobin 14.5 g/dL, platelet count 649/mm3, and reverse transcription polymerase chain reaction negative for BCR-ABL transcripts. The fact that the patient had complete molecular response without hematologic response triggered further workup. JAK2 V617F mutation was checked in peripheral blood and was positive. Erythropoietin was low at 1.3 mIU/mL. Examination of the bone marrow showed hypercellularity (90%) with myeloid/erythroid ratio of 7.7, proliferation of atypical megakaryocytes, <1% blasts, and widespread grade 2 reticulin fibrosis. No BCR-ABL translocation was detected by fluorescence in situ hybridization. DIPSS (Dynamic International Prognostic Scoring System) Plus score was 1. Myelofibrosis mutational profile analysis was not available. Hydroxyurea was added on top of imatinib (Figure 1).
Figure 1.
Clinical course.
Including our case, 11 patients had PMF, 10 had PV, and 5 had ET. In 8 cases, the exact nature of the myeloproliferative disorder was not clear. MPD preceded CML in 10 cases, CML preceded MPD in 9 cases, and both were diagnosed concomitantly in 15 cases. Interestingly, when MPD preceded CML, the mean time interval between the diagnoses of the 2 entities was 10.6 years compared with 5.4 years when CML preceded MPD. Interaction between JAK2 mutation and BCR-ABL transcripts levels varies. In most cases, JAK2 and BCR-ABL levels moved in opposite directions. In the remaining cases, JAK2 level decreased or remained constant while BCR-ABL level decreased.
Discussion
BCR-ABL transcripts have been detected at very low levels in healthy persons without clinical features of CML. The amount of BCR-ABL mRNA present in healthy individuals ranged from 5 to 20 copies/5 × 107 to 108 WBCs.28 Our patient had BCR-ABL transcripts level well above this threshold. We believe he had concomitant PMF and CML. As mentioned above, JAK2 mutation and BCR-ABL translocation have been considered to be mutually exclusive. However, several cases of coexistence of both hits have been reported. The prevalence of this “dual disease” is unknown. However, we think this entity is underdiagnosed since it is rarely suspected and checked. Few studies showed surprisingly elevated proportion of this “double hit” in CML patients. In one study, 314 patients with CML were screened for the presence of JAK2 V617F mutation. The latter was present in 8 patients accounting for 2.5%.29 Two studies done in Karachi, Pakistan, showed proportions of CML patients harboring JAK2 V617F mutation as high as 26.7% and 44%.30,31
Clinically, emergence of MPD in CML patients may be mistaken for relapse or drug resistance. MPD must be suspected in CML with atypical course such as erythrocytosis or progressive or persistent thrombocytosis or neutrophilia while CML is in remission. Development of CML must be considered in cases of JAK2-positive MPD with CML-like features such as leukocytosis or progressive splenomegaly years after clinical stability.
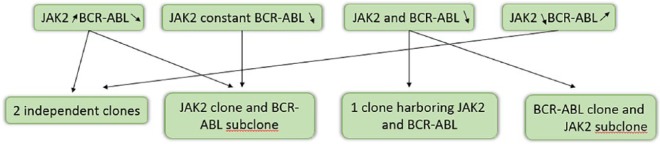
In Figure 2, we show possible interactions between JAK2 mutation and BCR-ABL transcripts levels and corresponding clonal origin. The model where BCR-ABL clone is a subclone of JAK2 one was demonstrated in a patient with CML and PV. BCR-ABL translocation and JAK2 mutation were present in most erythroid and myeloid colonies. Few colonies harbored JAK2 mutation only, and none had BCR-ABL transcripts alone.8 In another case, JAK2 mutation was present in CFU-GM and CFU-E compartments. However, BCR-ABL translocation was present in few CFU-GM colonies and absent in CFU-E.6 In 2 patients with PV and CML32 and a patient with PMF and CML,23 all hematopoietic colonies had JAK2, some had both JAK2 and BCR-ABL, and none had BCR-ABL alone. The model where JAK2 clone is a subclone of BCR-ABL one was also demonstrated. A patient with CML with positive JAK2 was treated with imatinib. Both JAK2 and BCR-ABL disappeared with treatment.16 The model where JAK2 and CML clones are distinct was also shown. BCR-ABL was absent in endogenous erythroid colonies formed without erythropoietin. Progressive decrease in BCR-ABL was associated with JAK2 clone expression.10
Figure 2.
Possible JAK2 and BCR-ABL interactions and clones.
As mentioned above, it took around a decade in average for CML to occur after MPD. However, MPD followed CML in about 5.4 years in average. The difference between time intervals is not clear.
Conclusion
Several cases of concomitant JAK2 MPD and CML have been reported. This hybrid disease is probably more frequent than expected because it is underdiagnosed. Providers must suspect this hybrid disease in atypical clinical course to optimize treatment and avoid transformation to acute leukemia. The World Health Organization’s MPD diagnostic criteria must probably be revised to account for the possibility of this “double hit” disease, which might be a novel clinical entity.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases.
Informed Consent: Verbal informed consent was obtained from the patient for his anonymized information to be published in this article.
ORCID iD: Gilbert Bader  https://orcid.org/0000-0003-3576-7693
https://orcid.org/0000-0003-3576-7693
References
- 1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595-600. [DOI] [PubMed] [Google Scholar]
- 2. Scott LM, Campbell PJ, Baxter EJ, et al. The V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disorders. Blood. 2005;106:2920-2921. [DOI] [PubMed] [Google Scholar]
- 3. Krämer A, Reiter A, Kruth J, et al. JAK2-V617F mutation in a patient with Philadelphia-chromosome-positive chronic myeloid leukaemia. Lancet Oncol. 2007;8:658-660. [DOI] [PubMed] [Google Scholar]
- 4. Inami M, Inokuchi M, Okabe M, et al. Polycythemia associated with the JAK2V617F mutation emerged during treatment of chronic myelogenous leukemia. Leukemia. 2007;21:1103-1104. [DOI] [PubMed] [Google Scholar]
- 5. Mirza I, Frantz C, Clarke G, Voth AJ, Turner R. Transformation of polycythemia vera to chronic myelogenous leukemia. Arch Pathol Lab Med. 2007;131:1719-1724. [DOI] [PubMed] [Google Scholar]
- 6. Bornhäuser M, Mohr B, Oelschlaegel U, et al. Concurrent JAK2(V617F) mutation and BCR-ABL translocation within committed myeloid progenitors in myelofibrosis. Leukemia. 2007;21:1824-1826. [DOI] [PubMed] [Google Scholar]
- 7. Hussein K, Bock O, Seegers A, et al. Myelofibrosis evolving during imatinib treatment of a chronic myeloproliferative disease with coexisting BCR-ABL translocation and JAK2 V617F mutation. Blood. 2007;109:4106-4107. [DOI] [PubMed] [Google Scholar]
- 8. Bocchia M, Vannucchi AM, Gozzetti A, et al. Insights into JAK2-V617F mutation in CML. Lancet Oncol. 2007;8:864-866. [DOI] [PubMed] [Google Scholar]
- 9. Kim YK, Shin MG, Kim HR, et al. Simultaneous occurrence of the JAK2V617F mutation and BCR-ABL gene rearrangement in patients with chronic myeloproliferative disorders. Leuk Res. 2008;32:993-995. [DOI] [PubMed] [Google Scholar]
- 10. Cambier N, Renneville A, Cazaentre T, et al. JAK2V617F-positive polycythemia vera and Philadelphia chromosome-positive chronic myeloid leukemia: one patient with two distinct myeloproliferative disorders. Leukemia. 2008;22:1454-1455. [DOI] [PubMed] [Google Scholar]
- 11. de Conchon MRM, Costa JL, Novaes MMY, et al. Simultaneous detection of JAK2 V617F mutation and BCR-ABL translocation in a patient with chronic myelogenous leukemia. Int J Hematol. 2008;88:243-345. [DOI] [PubMed] [Google Scholar]
- 12. Jallades L, Hayette S, Tigaud I, et al. Emergence of therapy-unrelated CML on a background of BCR-ABL-negative JAK2V617F-positive chronic idiopathic myelofibrosis. Leuk Res. 2008;32:1608-1610. [DOI] [PubMed] [Google Scholar]
- 13. Pardini S, Fozza C, Contini S, et al. A case of coexistence between JAK2V617F and BCR/ABL. Eur J Haematol. 2008;81:75-76. [DOI] [PubMed] [Google Scholar]
- 14. Hussein K, Bock O, Theophile K, et al. Chronic myeloproliferative diseases with concurrent BCR-ABL junction and JAK2V617F mutation. Leukemia. 2008;22:1059-1062. [DOI] [PubMed] [Google Scholar]
- 15. Pingali SR, Mathiason MA, Lovrich SD, Go RS. Emergence of chronic myelogenous leukemia from a background of myeloproliferative disorder: JAK2V617F as a potential risk factor for BCR-ABL translocation. Clin Lymphoma Myeloma. 2009;9:E25-E29. [DOI] [PubMed] [Google Scholar]
- 16. Campiotti L, Appio L, Solbiati F, Ageno W, Venco A. JAK2-V617F mutation and Philadelphia positive chronic myeloid leukemia. Leuk Res. 2009;33:e212-e213. [DOI] [PubMed] [Google Scholar]
- 17. Bee PC, Gan GG, Nadarajan VS, Latiff NA, Menaka N. A man with concomitant polycythemia vera and chronic myeloid leukemia: the dynamics of the two disorders. Int J Hematol. 2010;91:136-139. [DOI] [PubMed] [Google Scholar]
- 18. Tefferi A, Levitt R, Lasho TL, Knudson RA, Ketterling RP. Postimatinib therapy emergence of a new JAK2V617F clone and subsequent development of overt polycythemia vera in a patient with chronic myelogenous leukemia. Eur J Haematol. 2010;85:86-87. [DOI] [PubMed] [Google Scholar]
- 19. Caocci G, Atzeni S, Orrù N, et al. Response to imatinib in a patient with chronic myeloid leukemia simultaneously expressing p190 BCR-ABL oncoprotein and JAK2 V617F mutation. Leuk Res. 2010;34:e27-e29. [DOI] [PubMed] [Google Scholar]
- 20. Pastore F, Schneider S, Christ O, Hiddemann W, Spiekermann K. Impressive thrombocytosis evolving in a patient with a BCR-ABL positive CML in major molecular response during dasatinib treatment unmasks as additional JAK2V617F. Exp Hematol Oncol. 2013;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee YJ, Moon JH, Shin HC, et al. Two CML patients who subsequently developed features of essential thrombocytopenia with JAK2-V617F mutation while in complete cytogenetic remission after treatment with imatinib mesylate. Int J Hematol. 2013;97:804-807. [DOI] [PubMed] [Google Scholar]
- 22. Xu W, Chen B, Tong X. Chronic myeloid leukemia patient with co-occurrence of BCR-ABL junction and JAK2 V617F mutation. Int J Hematol. 2014;99:87-90. [DOI] [PubMed] [Google Scholar]
- 23. Yamada O, Mahfoudhi E, Plo I, et al. Emergence of a BCR-ABL translocation in a patient with the JAK2V617F mutation: evidence for secondary acquisition of BCR-ABL in the JAK2V617F clone. J Clin Oncol. 2014;32:e76-e79. [DOI] [PubMed] [Google Scholar]
- 24. Maerki J, Katava G, Siegel D, Silberberg J, Bhattacharyya PK. Unusual case of simultaneous presentation of plasma cell myeloma, chronic myelogenous leukemia, and a Jak2 positive myeloproliferative disorder. Case Rep Hematol. 2014;2014:738428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hassan A, Dogara LG, Babadoko AA, Awwalu S, Mamman AI. Coexistence of JAK2 and BCR-ABL mutation in patient with myeloproliferative neoplasm. Niger Med J. 2015;56:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ursuleac I, Colita A, Adam T, Jardan C, Ilea A, Coriu D. The concomitant occurrence of JAK2V617F mutation and BCR/ABL transcript with phenotypic expression—an overlapping myeloproliferative disorder or two distinct diseases? Case report. J Med Life. 2013;6:34-37. [PMC free article] [PubMed] [Google Scholar]
- 27. Park SH, Chi HS, Cho YU, et al. Two cases of myeloproliferative neoplasm with a concurrent JAK2 V617F mutation and BCR/ABL translocation without chronic myelogenous leukemia phenotype acquisition during Hydroxyurea treatment. Ann Lab Med. 2013;33:229-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biernaux C, Loos M, Sels A, Huez G, Stryckmans P. Detection of major BCR-ABL gene expression at a very low level in blood cells of some healthy individuals. Blood. 1995;86:3118-3122. [PubMed] [Google Scholar]
- 29. Pieri L, Spolverini A, Scappini B, et al. Concomitant occurrence of BCR-ABL and JAK2V617F mutation. Blood. 2011;118:3445-3446. [DOI] [PubMed] [Google Scholar]
- 30. Pahore ZA, Shamsi TS, Taj M, et al. JAK2V617F mutation in chronic myeloid leukemia predicts early disease progression. J Coll Physicians Surg Pak. 2011;21:472-475. [PubMed] [Google Scholar]
- 31. Tabassum N, Saboor M, Ghani R, Moinuddin M. Frequency of JAK2 V617F mutation in patients with Philadelphia positive chronic myeloid leukemia in Pakistan. Pak J Med Sci. 2014;30:185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Tripodi J, Kremyanskaya M, et al. BCR-ABL1 is a secondary event after JAK2V617F in patients with polycythemia vera who develop chronic myeloid leukemia. Blood. 2013;121:1238-1239. [DOI] [PubMed] [Google Scholar]