Abstract
Background:
Cervical carcinoma is the leading cause of cancer mortality in women. C-reactive protein (CRP), albumin (ALB), globulin (GLB), lactate dehydrogenase (LDH), and albumin-to-globulin ratio (AGR) are indicators of systemic inflammation response correlated with tumor outcomes.
Methods:
This study recruited 110 patients with cervical cancer. The patients were divided into 2 groups according to pretreatment median values of CRP, ALB, GLB, LDH, and AGR. The post/preradiotherapy or post/pretreatment ratios were defined as rates of pretreatment CRP, ALB, GLB, LDH, and AGR values and the corresponding ones obtained after radiotherapy or whole treatment.
Results:
Higher pretreatment CRP or LDH levels were correlated with worse progression-free survival (PFS) and overall survival (OS). Increased post/preradiotherapy CRP ratio was correlated with worse PFS and OS, increased post/preradiotherapy LDH ratio was correlated with worse PFS. Increased post/pretreatment CRP ratio was correlated with worse PFS and OS, not-increased post/pretreatment AGR ratio was correlated with worse OS. Cox regression analysis model indicated that, moderately or poorly of differentiation, higher pretreatment CRP or LDH levels were independently associated with worse PFS, higher pretreatment CRP or LDH levels and increased post/pretreatment CRP ratio were independently associated with worse OS.
Conclusion:
CRP, LDH, or AGR are correlated with outcomes of resectable cervical cancer.
Keywords: cervical cancer, systemic inflammation response, prognosis, overall survival
Introduction
With an estimated 528 000 new cases and 266 000 deaths occur annually, cervical cancer accounts for 12% of female cancers and ranks the fourth most common female cancer globally, especially in developing countries.1 Notably, on the account of large population with disequilibrium in financial resources and health care, China is bearing a heavy global burden of cervical cancer.2 As the most common gynecologic cancer in female, most of the invasive cervical cancers are the evolution of dysplasia originating in cervical columnar epithelium, and high-risk human papillomavirus (HPV) infection is widely recognized as the major etiologic factor of cervical cancer.3-5 Due to the popularization of early screening such as cervical scraping smear and liquid-based cytology, as well as HPV vaccination at young age in developed countries, it is not difficult to explain the high morbidity and mortality of cervical cancer in developing countries.5-8 Aside from the improvement in reproductive health education, early detection and prevention measures, surgery including trachelectomy or hysterectomy, radiotherapy with or without chemotherapy, as well as prognostic evaluation are emerging to improve the outcome of cervical cancer.
Systemic inflammation response (SIR) has been proved to contribute to the progressive decline in nutritional and functional status, as well as bad prognosis in many solid tumors.9,10 Thus, in order to forecast and improve the outcomes of resectable cervical cancer, investigation of available predictive markers demands prompt discussion. Regulated by pro-inflammatory cytokines, C-reactive protein (CRP) arises and plays a crucial role in SIR.11 Elevated serum CRP level has been identified to be a poor prognostic factor of cervical cancer. Albumin (ALB) and globulin (GLB) are major components of serum proteins. Previous studies have proved that low serum ALB level was an independent risk factor of poor survival in patients with several cancer types such as non-small-cell lung cancer, pancreatic cancer, and gastric cancer.12-14 The GLB reflects inflammatory status and plays important parts in SIR.15 As a combination of ALB and GLB, albumin-to-globulin ratio (AGR) has been identified as a convenient and useful predictive biomarker for prognosis in several cancers including breast cancer, colorectal cancer, and esophageal squamous cell carcinoma.16-18 To date, the prognostic value of AGR in cervical cancer has not been discussed. Serum lactate dehydrogenase (LDH), a key enzyme that catalyzes the conversion from glucose to lactate,19 serves as a nonspecific indicator of cellular death in many diseases and reflects the aggressiveness of various neoplasms including cervical cancer.20 A recent study has revealed that a high baseline LDH level was an independent prognostic factor for patients with locally advanced cervical cancer.21
In this study, we have investigated several SIR-related parameters and evaluated whether these parameters could be available prognostic indicators in patients with resectable cervical cancer.
Materials and Methods
Patients and Inclusion Criteria
This study was conducted as a retrospective investigation of resectable cervical cancer that had been referred to the Affiliated Suzhou Hospital of Nanjing Medical University (Jiangsu, China) between November 2012 and Jul 2014. Approval for the study was granted by the Medical Ethics Committees of the Affiliated Suzhou Hospital of Nanjing Medical University. All patients have signed informed consent. The inclusion criteria were as follows: (1) those with histologically or cytologically confirmed resectable cervical cancer; (2) age 18 to 70 years; (3) Karnofsky performance status score of ≥70; (4) those who met the following laboratory criteria: white blood cells (WBC) ≥4.0 × 109/L; absolute neutrophil count ≥2.0 × 109/L; PLT ≥100 × 109/L; and (5) histopathology confirmed as squamous cell carcinoma. The exclusion criteria were as follows: (1) patient failed to complete radiotherapy after surgery; (2) histopathology confirmed as adenocarcinoma; and (3) patients with new infections within 2 weeks or chronic infection diseases, autoimmune diseases, organ dysfunction, hematological diseases, and accompanied with another type of tumor. All the patients received modified radical hysterectomy plus pelvic lymph node dissection and external irradiation (45-50 Gy administered in 25 fractions over 5 weeks; 4-field box technique). Clinical and pathological records of all the patients participating in the study were reviewed periodically, the first follow-up was 3 months after radiotherapy, and the last time was July 2014.
In total, 110 patients with resectable cervical cancer were recruited in this study. All cases were confirmed by surgery and pathology. Patient characteristics are detailed in Table 1. The median age of the 110 patients was 51.5 years (range, 25-70 years). The staging of cancer was made according to International Federation of Gynecology and Obstetrics (FIGO) recommendations. The prognostic analyses were performed regarding progression-free survival (PFS) and overall survival (OS).
Table 1.
Clinicopathologic Features.
| Clinicopathologic Features | n | CRP | ALB | GLB | LDH | AGR | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low, n | High, n | χ2 | P Value | Low, n | High, n | χ2 | P Value | Low, n | High, n | χ2 | P Value | Low, n | High, n | χ2 | P Value | Low, n | High, n | χ2 | P Value | ||
| 101 | |||||||||||||||||||||
| Age | |||||||||||||||||||||
| ≤51.5 | 56 | 29 | 26 | 0.327 | .567 | 29 | 26 | 0.327 | .567 | 29 | 26 | 0.146 | .703 | 31 | 24 | 1.309 | .253 | 28 | 27 | 0.036 | .849 |
| >51.5 | 45 | 26 | 29 | 26 | 29 | 27 | 28 | 25 | 30 | 27 | 28 | ||||||||||
| Tumor size (cm) | |||||||||||||||||||||
| ≤4 | 72 | 26 | 36 | 3.670 | .055 | 31 | 31 | 0 | 1 | 28 | 34 | 3.677 | .055 | 29 | 33 | 0.972 | .324 | 34 | 28 | 1.331 | .249 |
| >4 | 48 | 29 | 19 | 24 | 24 | 34 | 20 | 27 | 21 | 21 | 27 | ||||||||||
| FIGO | 101 | ||||||||||||||||||||
| I | 77 | 36 | 24 | 5.280 | .022a | 31 | 29 | 0.147 | .702 | 43 | 34 | 3.709 | .054 | 26 | 34 | 3.031 | .082 | 31 | 29 | 0.147 | .702 |
| II | 24 | 19 | 31 | 24 | 26 | 8 | 16 | 30 | 20 | 24 | 26 | ||||||||||
| N stage | |||||||||||||||||||||
| None | 68 | 33 | 27 | 1.320 | .251 | 25 | 35 | 3.667 | .056 | 32 | 28 | 0.310 | .577 | 29 | 31 | 0.350 | .554 | 28 | 32 | 0.587 | .444 |
| Have | 33 | 22 | 28 | 30 | 20 | 24 | 26 | 27 | 23 | 27 | 23 | ||||||||||
| Differentiation | |||||||||||||||||||||
| Highly | 48 | 24 | 24 | 0 | 1 | 25 | 23 | 0.148 | .701 | 27 | 33 | 1.844 | .174 | 26 | 22 | 0.362 | .548 | 21 | 27 | 1.331 | .247 |
| Moderately or poorly | 37 | 31 | 31 | 30 | 32 | 29 | 21 | 30 | 32 | 34 | 28 | ||||||||||
Abbreviations: AGR, albumin-to-globulin ratio; ALB, albumin; CRP, C-reactive protein; FIGO, Federation of Gynecology and Obstetrics; GLB, globulin; LDH, lactate dehydrogenase.
aP < 0.05.
Blood Samples
Peripheral venous blood (5-7 mL) was collected into a sterile ethylenediaminetetraacetic acid tube; patients were fasted 8 hours and samples were obtained from elbow venous between 6:30 and 7:30 am in order to standardize the known impact of circulating hormones (circadian rhythm) on the number and subtype distribution of the various WBC indices. Blood samples were analyzed using a hematology analyzer (Sysmex XE-2100; Sysmex, Kobe, Japan). The patients were divided into 2 groups according to the median values. The post/preradiotherapy ratios were defined as the rate of preradiotherapy CRP, ALB, GLB, LDH, and AGR values and the corresponding ones obtained after radiotherapy. The post/pretreatment ratios were defined as the rate of pretreatment CRP, ALB, GLB, LDH, and AGR values and the corresponding ones obtained after whole treatment. Blood samples from all patients were obtained 1 month after surgery and 3 months after adjuvant radiotherapy. In our article, all patients underwent surgery and adjuvant radiotherapy.
Evaluation
Computed tomography scan was performed for the assessment of response every 3 months and evaluated according to the criteria of Response Evaluation Criteria in Solid Tumors 1.1.22
Follow-Up
Survival time was measured from the diagnosed date until death or last clinical evaluation. The prognostic analyses were performed regarding PFS or OS. Patients were followed up regularly for 36 months.
Statistical Analysis
All statistical analyses were performed using SPSS 19.0 software (Chicago, Illinois). For analysis of survival data, Kaplan-Meier curves were constructed, and statistical analysis was carried out using the log-rank test. The associations between blood parameters status and clinicopathologic features were explored by the χ2 tests. The relationships between changes in the blood parameters status and surgery or radiotherapy were assessed by the paired samples test. Univariate and multivariate Cox regression analysis model was employed to identify the independent risk factors associated with cervical cancer. All values of P <.05 were considered statistically significant.
Results
High Pretreatment CRP or LDH Levels had Worse Prognosis on OS of Patients With Resectable Cervical Cancer
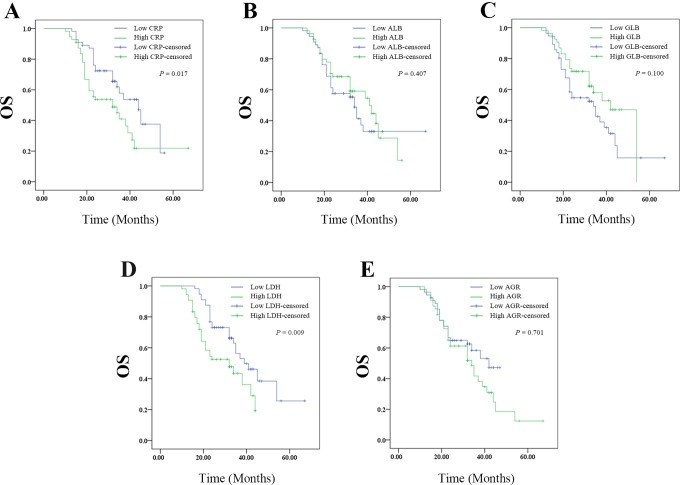
The Kaplan-Meier plots were used to determine the effect of CRP, ALB, GLB, LDH, or ARB levels on OS (Figure 1A-E). The patients were divided into 2 groups according to the median levels of CRP, ALB, GLB, LDH, or ARB. The median OS of the high CRP group was 32 (95% confidence interval [CI]: 20.774-43.226) months, while that of the low CRP group was 44 (95% CI: 34.714-53.286) months (P = .017). The median OS was 41 (95% CI: 29.456-52.544) months in the high ALB group and 34 (95% CI: 30.888-37.112 months in the low ALB group (P = .407). The median OS was 42 (95% CI: 35.643-48.357) months in the high GLB group and 34 (95% CI: 23.430-44.570) months in the low GLB group (P = .100). The median OS of the high LDH group was 32 (95% CI: 19.972-44.028) months, while that of the low LDH group was 39 (95% CI: 30.127-47.873) months (P = .009). The median OS of the high AGR group was 34 (95% CI: 25.917-42.083) months, while that of the low AGR group was 42 (95% CI: 33.244-51.296) months (P = .178). Thus, pretreatment high levels of CRP or LDH had worse prognosis, whereas ALB, GLB, or AGR had no effect on OS.
Figure 1.
The relationship between pretreatment SIR-related indicators values and OS of patients with resectable cervical cancer: (A) the OS according to CPR, (B) the OS according to ALB, (C) the OS according to GLB, (D) the OS according to LDH, and (E) the OS according to AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CPR, cardiopulmonary resuscitation; GLB, globulin; LDH, lactate dehydrogenase; OS, overall survival; SIR, systemic inflammation response.
High Pretreatment CRP or LDH Levels had Worse Prognosis on PFS of Patients With Resectable Cervical Cancer
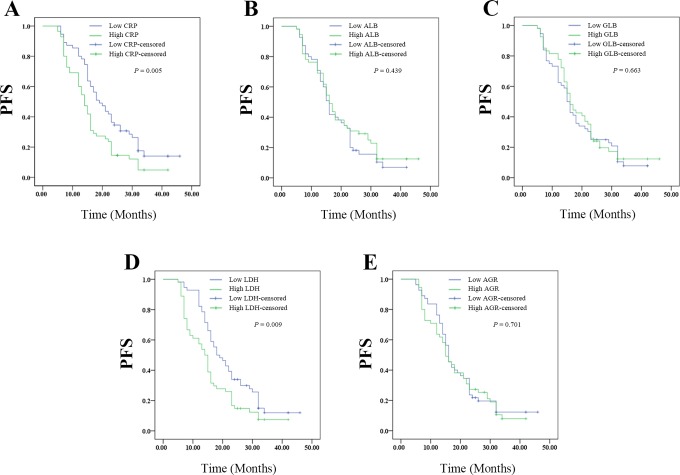
The Kaplan-Meier plots were used to determine the effect of CRP, ALB, GLB, LDH, or ARB levels on PFS (Figure 2A-E). The patients were divided into 2 groups according to the median levels of CRP, ALB, GLB, LDH, or ARB. The median PFS of the high CRP group was 14 (95% CI: 11.823-16.177) months, while that of the low CRP group was 19 (95% CI: 14.848-23.152) months (P = .005). The median PFS was 16 (95% CI: 13.82-18.180) months in the high ALB group and 16 (95% CI: 14.566-17.434) months in the low ALB group (P = .439). The median PFS was 16 (95% CI: 13.601-18.399) months in the high GLB group and 15 (95% CI: 13.370-16.630) months in the low PDW group (P = .663). The median PFS of the high LDH group was 14 (95% CI: 11.840-16.160) months, while that of the low LDH group was 18 (95% CI: 13.809-22.191) months (P = .009). The median PFS of the high AGR group was 15 (95% CI: 12.275-17.725) months, while that of the low AGR group was 16 (95% CI: 14.191-17.809) months (P = .701). Thus, pretreatment high levels of CRP or LDH had shorter PFS, whereas ALB, GLB, or AGR had no effect on PFS.
Figure 2.
The relationship between pretreatment SIR-related indicators values and PFS of patients with resectable cervical cancer: (A) the PFS according to CPR, (B) the PFS according to ALB, (C) the PFS according to GLB, (D) the PFS according to LDH, and (E) the PFS according to AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CPR, cardiopulmonary resuscitation; GLB, globulin; LDH, lactate dehydrogenase; PFS, progression-free survival; SIR, systemic inflammation response.
Effects of Radiotherapy on the CRP, ALB, GLB, LDH, and AGR
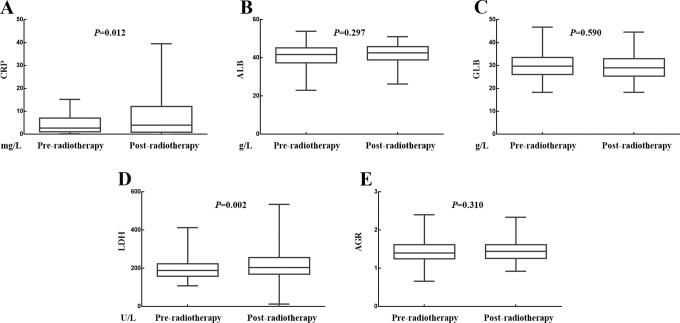
The effects of radiotherapy on the CRP, ALB, GLB, LDH, and AGR levels were presented in Figure 3A to E, respectively. The media value of CRP was 3.135 mg/L before radiotherapy and 3.210 mg/L after radiotherapy (P = .012). The media value of ALB was 41.800 g/L before radiotherapy and 42.650 radiotherapy after radiotherapy (P = .297). The media value of GLB was 29.750 g/L before radiotherapy and 29.000 g/L after radiotherapy (P = .590). The media value of LDH was 189.500 U/L before radiotherapy and 191.500 U/L after radiotherapy (P = .002). The media value of AGR was 1.402 before radiotherapy and 1.451 after radiotherapy (P = .310).Therefore, radiotherapy had significant increased the value of CPR or LDH, whereas had no significant impact on the values of ALB, GLB, or AGR.
Figure 3.
Relationship between changes in SIR-related indicators values and radiotherapy. A, Radiotherapy increased the value of CRP. B, Radiotherapy had no influence on the value of ALB. C, Radiotherapy had no influence on the value of GLB. D, Radiotherapy increased the value of LDH. E, Radiotherapy had no influence on the value of AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CRP, C-reactive protein; GLB, globulin; LDH, lactate dehydrogenase; SIR, systemic inflammation response.
Effects of Whole Treatment (Surgery Plus Radiotherapy) on the Values of CRP, ALB, GLB, LDH, and AGR
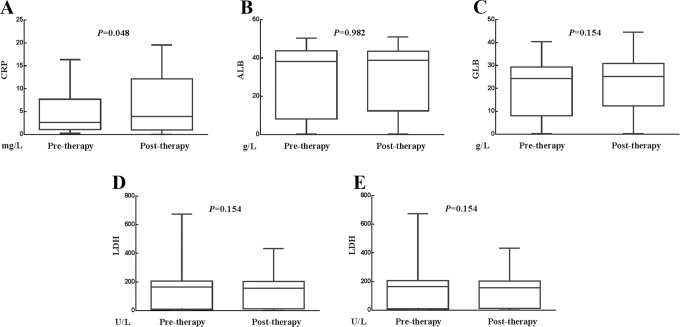
The effects of whole treatment on the CRP, ALB, GLB, LDH, and AGR levels were shown in Figure 4A to E. The media value of CRP was 2.840 mg/L before whole treatment and 3.210 mg/L after whole treatment (P = .048). The media value of ALB was 42.100 g/L before whole treatment and 42.650 g/L after whole treatment (P = .982). The media value of GLB was 27.700 g/L before whole treatment and 29.000 g/L after whole treatment (P = .154). The media value of LDH was 192.000 U/L before whole treatment and 191.500 U/L after whole treatment (P = .154). The media value of AGR was 1.473 before whole treatment and 1.451 after whole treatment (P = .167). Therefore, whole treatment had significant increase on the value of CRP, whereas it had no significant impact on the values of ALB, GLB, LDH, or AGR.
Figure 4.
Relationship between changes in SIR-related indicators values and whole treatment (surgery plus radiotherapy). A, Whole treatment increased the value of CRP. B, Whole treatment had no influence on the value of ALB. C, Whole treatment had no influence on the value of GLB. D, Whole treatment had no influence on the value of LDH. E, Whole treatment had no influence on the value of AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CRP, C-reactive protein; GLB, globulin; LDH, lactate dehydrogenase; SIR, systemic inflammation response.
Changes in CRP Level After Radiotherapy Predicted OS of Patients With Resectable Cervical Cancer
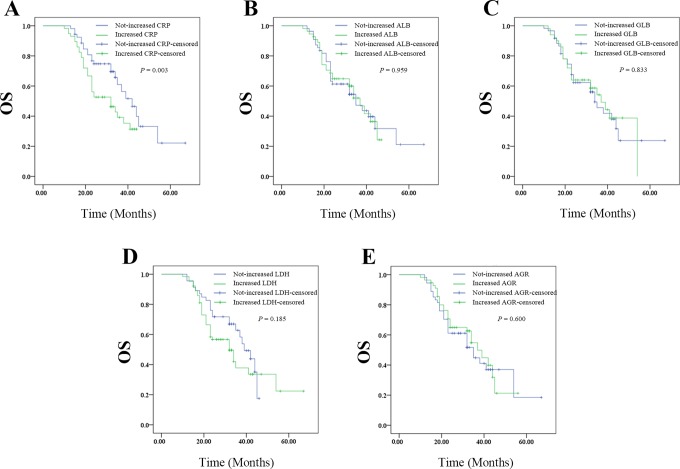
The Kaplan-Meier plots were used to determine the effect of changes of CRP, ALB, GLB, LDH, and AGR status on OS (Figure 5A-E). The median OS of patients whose CRP level decreased following radiotherapy was 42 (33.985-50.015) months, while that of the increased group was 32 (21.128-42.872) months (P = .032). The median OS of patients whose ALB level decreased following radiotherapy was 35 (23.083-46.917) months, while that of the not-decreased group was 37 (30.526-43.474) months (P = .959). The median OS of patients whose GLB level decreased following radiotherapy was 34 (27.927-40.073) months, while that with not-decreased GLB was 37 (28.780-45.220) months (P = .838). The median OS of patients whose LDH level decreased following radiotherapy was 39 (32.778-45.222) months, while that with not-decreased LDH was 32 (23.073-40.927) months (P = .185). The median OS of patients whose AGR level decreased following radiotherapy was 35 (23.979-46.021) months, while that with not-decreased AGR was 37 (30.417-43.583) months (P = .600).Thus, the patients whose CRP level decreased after therapy had increased survival ratio. However, changes in ALB, GLB, LDH, or AGR levels had no effects on OS.
Figure 5.
The relationship between change in pre- and pro-radiotherapy SIR-related indicators values with OS of patients with resectable cervical cancer: (A) the OS according to CPR, (B) the OS according to ALB, (C) the OS according to GLB, (D) the OS according to LDH, and (E) the OS according to AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CPR, cardiopulmonary resuscitation; GLB, globulin; LDH, lactate dehydrogenase; OS, overall survival; SIR, systemic inflammation response.
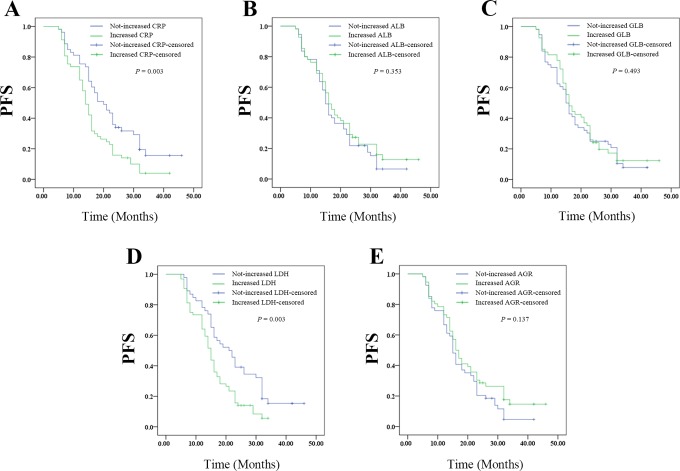
Changes in CRP or LDH Level After Radiotherapy Predicted PFS of Patients With Resectable Cervical Cancer
The Kaplan-Meier plots were used to determine the effect of changes of CRP, ALB, GLB, LDH, and AGR status on PFS (Figure 6A-E). The median PFS of patients whose CRP level decreased following radiotherapy was 20 (14.905-25.095) months, while that of the not-decreased group was 14 (12.356-15.644) months (P = .003). The median PFS of patients whose ALB level decreased following radiotherapy was 15 (13.385-16.615) months, while that of the not-decreased group was 16 (13.275-18.725) months (P = .353). The median PFS of patients whose GLB level decreased following radiotherapy was 15 (13.103-16.897) months, while that with not-decreased GLB was 16 (12.043-19.957) months (P = .493). The median OS of patients whose LDH level decreased following radiotherapy was 21 (16.015-25.985) months, while that with not-decreased LDH was 15 (13.444-16.556) months (P = .003). The median PFS of patients whose AGR level decreased following radiotherapy was 15 (13.561-16.439) months, while that with not-decreased AGR was 16 (13.905-18.095) months (P = .137). Thus, the patients whose CRP or LDH level decreased after therapy had better PFS. However, changes in ALB, GLB, or AGR levels had no effects on PFS.
Figure 6.
The relationship between change in pre- and pro-radiotherapy SIR-related indicators values with PFS of patients with resectable cervical cancer: (A) the PFS according to CPR, (B) the PFS according to ALB, (C) the PFS according to GLB, (D) the PFS according to LDH, and (E) the PFS according to AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CPR, cardiopulmonary resuscitation; GLB, globulin; LDH, lactate dehydrogenase; PFS, progression-free survival; SIR, systemic inflammation response.
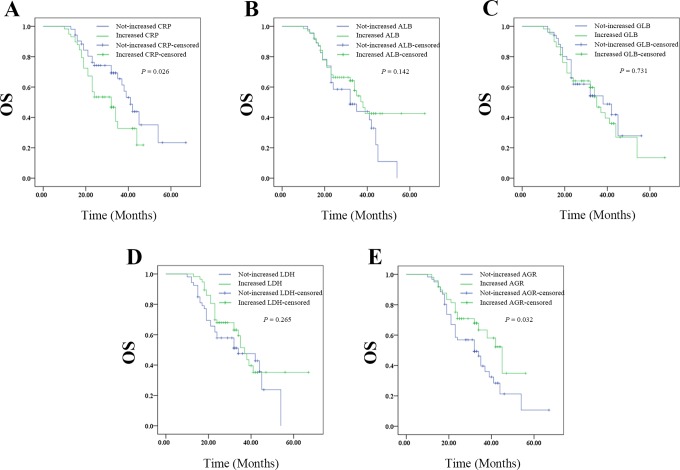
Changes in CRP Level After Whole Treatment Predicted Outcomes on OS of Patients With Resectable Cervical Cancer
The Kaplan-Meier plots were used to determine the effect of changes of CRP, ALB, GLB, LDH, and AGR status on OS (Figure 7A-E). The median OS of patients whose CRP level decreased following whole treatment was 41 (35.697-46.303) months, while that of the not-decreased group was 32 (24.506-39.494) months (P = .026). The median OS of patients whose ALB level decreased following whole treatment was 32 (20.907-43.093) months, while that of the not-decreased group was 38 (32.745-43.255) months (P = .142). The median OS of patients whose GLB level decreased following whole treatment was 38 (23.342-52.658) months, while that with not-decreased GLB was 35 (30.443-39.557) months (P = .731). The median OS of patients whose LDH level decreased following whole treatment was 34 (16.611-51.389 months, while that with not-decreased LDH was 37 (32.825-41.175) months (P = .265). The median OS of patients whose AGR level decreased following whole treatment was 32 (23.806-40.194) months, while that with not-decreased AGR was 45 (35.784-54.216) months (P = .032).Thus, the patients whose decreased CRP level or not-decreased AGR level after therapy had increased survival ratio. However, changes in ALB, GLB, or LDH levels had no effects on OS.
Figure 7.
The relationship between change in pretreatment (surgery plus radiotherapy) and posttreatment SIR-related indicators values with OS of patients with resectable cervical cancer: (A) the OS according to CPR, (B) the OS according to ALB, (C) the OS according to GLB, (D) the OS according to LDH, and (E) the OS according to AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CPR, cardiopulmonary resuscitation; GLB, globulin; LDH, lactate dehydrogenase; OS, overall survival; SIR, systemic inflammation response.
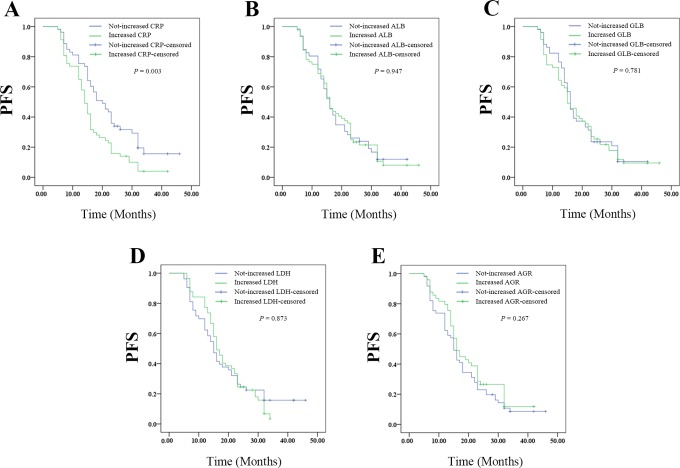
Changes in CRP Level After Whole Treatment Predicted Outcomes on PFS of Patients With Resectable Cervical Cancer
The Kaplan-Meier plots were used to determine the effect of changes of CRP, ALB, GLB, LDH, and AGR status after whole treatment on PFS (Figure 8A-E). The median PFS of patients whose CRP level decreased following whole treatment was 18 (12.111-23.889) months, while that of the not-decreased group was 15 (13.522-16.478) months (P = .020). The median PFS of patients whose ALB level decreased following whole treatment was 16 (13.517-18.483) months, while that of the not-decreased group was 16 (14.049-17.951) months (P = .947). The median PFS of patients whose GLB level decreased following whole treatment was 16 (14.452-17.548) months, while that with not-decreased GLB was 15 (12.263-17.737) months (P = .781). The median OS of patients whose LDH level decreased following whole treatment was 15 (12.626-17.374) months, while that with not-decreased LDH was 16 (13.781-18.219) months (P = .873). The median PFS of patients whose AGR level decreased following whole treatment was 15 (13.299-16.701) months, while that with not-decreased AGR was 16 (13.714-18.286) months (P = .267). Thus, the patients whose CRP level decreased after therapy had better PFS. However, changes in ALB, GLB, LDH, or AGR levels had no effects on PFS.
Figure 8.
The relationship between change in pretreatment (surgery plus radiotherapy) and posttreatment SIR-related indicators values with PFS of patients with resectable cervical cancer: (A) the PFS according to CPR, (B) the PFS according to ALB, (C) the PFS according to GLB, (D) the PFS according to LDH, and (E) the PFS according to AGR. AGR indicates albumin-to-globulin ratio; ALB, albumin; CPR, cardiopulmonary resuscitation; GLB, globulin; LDH, lactate dehydrogenase; PFS, progression-free survival; SIR, systemic inflammation response.
Prognostic Factors of PFS for Patients With Resectable Cervical Cancer
Univariate analyses (Table 2) demonstrated that moderately or poorly of differentiation (hazard ratio [HR]: 1.720; 95% CI: 1.142-2.592; P = .009), larger tumor size (>5 cm; HR 1.561; 95% CI: 1.039-2.346; P = .032), higher FIGO stage (II; HR: 1.615; 95% CI: 1.076-2.424; P = .021), higher pretreatment CPR level (HR: 1.721; 95% CI: 1.148-2.581; P = .009), higher pretreatment LDH level (HR: 1.656; 95% CI: 1.107-2.478; P = .014), increased post/preradiotherapy CRP ratio (≥1; HR: 1.773; 95% CI: 1.179-2.665; P = .006), increased post/preradiotherapy LDH ratio (≥1; HR: 1.839; 95% CI: 1.204-2.810; P = .005), and increased post/pretreatment CRP ratio (≥1; HR: 1.576; 95% CI: 1.050-2.367; P = .028) were significant risk factors for a poor prognosis (Table 2). In multivariate analysis (Table 2), moderately or poorly of differentiation (HR: 1.709; 95% CI: 1.114-2.621; P = .014), higher pretreatment CPR level (HR: 1682; 95% CI: 1.048-2.700; P = .031), and higher pretreatment LDH level (HR: 1.759; 95% CI: 1.155-2.678; P = .009) were found to be independently associated with poor survival.
Table 2.
Univariate and Multivariate Logistic Regression Analyses of Resectable Cervical Cancer Risk Factors.
| Risk Factors | Overall Survival (OS) | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (>51.5 years or ≤51.5 years) | 1.040 (0.618-1.752) | .882 | – | – |
| Tumor size (> 4 or ≤ 4), cm | 1.433 (0.850-2.415) | .176 | – | – |
| Lymphonodus metastasis (have or none) | 1.553 (0.923-2.615) | .098 | – | – |
| FIGO stage (II or I) | 1.980 (1.170-3.353) | .011a | 1.748 (0.971-3.149) | .063 |
| Differentiation (highly or > moderately and poorly) | 1.453 (0.854-2.473) | .168 | – | – |
| Pretreatment CRP level (>2.62 or ≤2.62), mg/L | 1.872 (1.099-3.188) | .021a | 1.959 (1.066-3.597) | 0.030a |
| Pretreatment ALB level (≤42.1 or >42.1), g/L | 0.805 (0.477-1. 35) | .417 | – | – |
| Pretreatment GLB level (> 27.7 or ≤ 27.7), g/L | 0.649 (0.382-1.102) | .110 | – | – |
| Pretreatment LDH level (>193 or ≤193), U/L | 1.994 (1.165-3.413) | .012a | 2.074 (1.202-3.581) | .009b |
| Pretreatment AGR level (>1.47 or ≤1.47) | 1.429 (0.838-2.434) | .190 | – | – |
| Post/preradiotherapy CRP ratio (>1or ≤1) | 1.781 (1.031-3.075) | .038a | 1.023 (0.544-1.921) | .945 |
| Post/preradiotherapy ALB ratio (>1or ≤1) | 0.987 (0.584-1.668) | .960 | – | – |
| Post/preradiotherapy GLB ratio (>1or ≤1) | 0.948 (0.561-1.601) | .841 | ||
| Post/preradiotherapy LDH ratio (>1or ≤1) | 1.428 (0.832-2.452) | .196 | – | – |
| Post/preradiotherapy AGR ratio (>1or ≤1) | 0.872 (0.518-1.469) | .607 | – | – |
| Post/pretreatment CRP ratio (>1 or ≤1) | 1.815 (1.053-3.128) | .032a | 2.081 (1.096-3.953) | .025a |
| Post/pretreatment ALB ratio (> 1or ≤ 1) | 0.684 (0.407-1.151) | .152 | – | – |
| Post/pretreatment GLB ratio (>1 or ≤1) | 1.094 (0.648-1.849) | .736 | ||
| Post/pretreatment LDH ratio (>1 or ≤1) | 0.748 (0.444-1.261) | .276 | – | – |
| Post/pretreatment AGR ratio (>1 or ≤1) | 0.558 (0. 321-0. 969) | .038a | 0.646 (0.365-1.145) | .134 |
Abbreviations: AGR, albumin-to-globulin ratio; ALB, albumin; CI, confidence interval; CRP, C-reactive protein; FIGO, Federation of Gynecology and Obstetrics; GLB, globulin; LDH, lactate dehydrogenase; OR, odds ratio; OS, overall survival.
aP < .05.
bP < .01.
Prognostic Factors of OS for Patients With Resectable Cervical Cancer
Univariate analyses (Table 3) demonstrated that higher FIGO stage (II; HR: 1.980; 95% CI: 1.170-3.352; P = .011), higher pretreatment CPR level (HR: 1872; 95% CI: 1.099-3.188; P = .021), higher pretreatment LDH level (HR: 1.994; 95% CI: 1.165-3.413; P = .012), not-decreased post/preradiotherapy CRP ratio (≥1; HR: 1.839; 95% CI: 1.031-3.075; P = .038), not-decreased post/pretreatment CRP ratio (≥1; HR: 1.815; 95% CI: 1.053-3.128; P = .032), and not-decreased post/pretreatment CRP ratio (≥ 1; HR: 0.558; 95% CI: 0.321-3.128; P = . 969) were significant risk factors for a poor prognosis (Table 2). In multivariate analysis (Table 3), moderately or poorly of differentiation (HR: 1.709; 95% CI: 1.114-2.621; P = .014), higher pretreatment CPR level (HR: 1.959; 95% CI: 1.066-3.597; P = .030), higher pretreatment LDH level (HR: 2.074; 95% CI: 1.202-3.581; P = .009), and post/pretreatment CRP ratio (≥1; HR: 2.081; 95% CI: 1.096-3.953; P = .025) were found to be independently associated with poor survival.
Table 3.
Univariate and Multivariate Logistic Regression Analyses of Resectable Cervical Cancer Risk Factors.
| Risk Factors | Overall Survival (PFS) | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (>51.5 or ≤51.5 years) | 1.046 (0.700-1.562) | 0.630 | – | – |
| Tumor size (> 4 or ≤ 4), cm | 1.561 (1.039-2.346) | 0.032a | 1.424 (0.913-2.222) | .119 |
| Lymphonodus metastasis (have or none) | 1.104 (0.738-1.652) | 0.630 | – | – |
| FIGO stage (II or I) | 1.615 (1.076-2.424) | 0.021a | 1.709 (1.114-2.621) | .121 |
| Differentiation (moderately, poorly or highly) | 1.720 (1.142-2.592) | 0.009b | 1.682 (1.048-2.700) | .014a |
| Pretreatment CRP level (>2.62 or ≤2.62), mg/L | 1.721 (1.148-2.581) | 0.009b | 1.682 (1.048-2.700) | .031a |
| Pretreatment ALB level (≤42.1 or > 42.1), g/L | 0.860 (0.575-1.287) | 0.463 | – | – |
| Pretreatment GLB level (>27.7 or ≤27.7), g/L | 0.919 (0.615-1.373) | 0.680 | – | – |
| Pretreatment LDH level (>193 or ≤193), U/L | 1.656 (1.107-2.478) | 0.014a | 1.759 (1.155-2.678) | .009b |
| Pretreatment AGR level (>1.47 or ≤1.47) | 1.077 (0.721-1.609) | 0.716 | – | – |
| Post/preradiotherapy CRP ratio (>1 or ≤1) | 1.773 (1.179-2.665) | 0.006b | 1.410 (0.875-2.272) | .158 |
| Post/preradiotherapy ALB ratio (>1 or ≤1) | 0.835 (0.559-1.247) | 0.379 | – | – |
| Post/preradiotherapy GLB ratio (>1 or ≤1) | 0.516 (0.585-1.309) | 0.516 | ||
| Post/preradiotherapy LDH ratio (>1 or ≤1) | 1.839 (1.204-2.810) | 0.005b | 1.514 (0.948-2.418) | .080 |
| Post/preradiotherapy AGR ratio (>1 or ≤1) | 0.748 (0.500-1.121) | 0.159 | – | – |
| Post/pretreatment CRP ratio (>1 or ≤1) | 1.576 (1.050-2.367) | 0.028a | 1.423 (0.866-2.338) | .164 |
| Post/pretreatment ALB ratio (>1 or ≤1) | 0.978 (0.657-1.482) | 0.950 | – | – |
| Post/pretreatment GLB ratio (>1 or ≤1) | 1.055 (0.706-1.578) | 0.793 | ||
| Post/pretreatment LDH ratio (>1 or ≤1) | 1.032 (0.689-1.261) | 1.544 | – | – |
| Post/pretreatment AGR ratio (>1 or ≤1) | 0.805 (0.536-1.207) | 0.293 | – | – |
Abbreviation: AGR, albumin-to-globulin ratio; ALB, albumin; CRP, C-reactive protein; CI, confidence interval; FIGO, Federation of Gynecology and Obstetrics; GLB, globulin; LDH, lactate dehydrogenase; OR, odds ratio; PFS, progression-free survival.
aP < .05.
bP < .01.
Discussion
Emerging evidence reveals that oncologic outcome is not only mainly determined by malignant behaviors but also influenced by host SIRs.23 The SIR is widely participated in the initiation and progression of solid tumors including cervical cancer, such as malignant proliferation, survival, invasion, angiogenesis, and metastasis.24-27 Previous studies have reignited the interest of cancer researchers in the concept of a correlation between SIR and tumor outcomes.9,10,28,29 Accordingly, previous researches have undertaken in-depth studies involving SIR-based predictive indicators such as CRP,30 platelet-to-lymphocyte ratio,31 neutrophil-to-lymphocyte ratio,31,32 and modified Glasgow prognostic score (mGPS)33,34 in the outcomes of cervical cancer. By the researches currently, the mechanisms for SIR development in cervical malignant progression are not exactly unveiled.25 Nevertheless, tumor necrosis–related hypoxia, DNA damage and genetic mutation resulted from oxidative stress, secretion of pro-inflammatory cytokines, and acute-phase protein production are incriminated.35-37 The present study aims to evaluate a series of SIR parameters and seek for accurate and comprehensive predictive parameters for resectable cervical cancer.
The CRP, a kind of acute-phase protein, is mainly synthesized in hepatocytes in response to multiple pro-inflammatory cytokines and widely accepted as a sensitive marker of SIR.38-40 The CRP is reemphasized as a significant predictive indicator in various types of cancer such as colorectal cancer, pancreatic cancer, and gastric cancer, as well as cervical cancer.30,41-44 For instance, Chmura et al have demonstrated that high baseline CRP was correlated with adverse prognosis in patients with resectable cervical cancer.45 Moreover, in a recent retrospective research of Polterauer et al, patients with higher CRP level (>5mg/L) had worse outcomes. Multivariable analysis suggested higher CRP level as an independent risk factor affecting PFS and OS.30 The possible mechanisms by which CRP is correlated with the outcome of cervical cancer are listed as follows. Firstly, SIR is supposed to be secondary to tumor necrosis and local tissue damage caused by malignant cells.44 Thus, an increased CRP level partly reflects malignant aggressiveness and progression. Second, an increased secretion of vascular endothelial growth factors (VEGFs) in response to elevated serum CRP level may promote tumor growth, angiogenesis, and metastases.46 Moreover, one study indicated that tumor subclinical metastases that could not be detected either by traditional imaging studies or pathologic biopsies already arose in patients with high CRP level.44 In the present study, both whole treatment and radiotherapy increased the level of CRP. Higher pretreatment CRP level was independently correlated with worse OS and PFS, while increased post/pretreatment CRP ratio was independently associated with worse OS.
Recent researches have defined ALB as a SIR-related parameter reflecting both nutritional status and chronic inflammatory status.47 Additionally, chronic inflammation and malnutrition are accepted as adverse factors for tumor prognosis.48,49 Thus, decreased ALB level is supposed to be an unfavorable prognostic factor in patients with several kinds of malignant tumors.14,49,50 Moreover, ALB is integrated with other markers such as CRP to create new prognostic markers, for example, the mGPS.34 For instance, in a recent study of He et al, pretreatment CRP/ALB ratio was confirmed as an independent predictor of OS in patients with cervical cancer.51 However, to our knowledge, previous studies have not attached importance on the correlation between ALB and patient survival in cervical cancer and it was the first time to dig into the predictive value of ALB independently in resectable cervical cancer. There are several pieces of evidence to support our speculation. Firstly, ALB has been proved to play a crucial anticancer role by stabilitating cell growth and DNA replication, as well as buffering sex hormone homeostasis.52 Secondly, chronic inflammatory response is proved to suppress ALB synthesis and eventually lead to a low serum ALB level and malnutrition, which may weaken host immune defense mechanisms in patients suffering malignant tumors.53,54 Thirdly, in addition to attenuation of ALB synthesis, a long-term SIR may lead to vascular endothelial damage and increases the vascular permeability, which in turn attributes to decreased serum ALB level and forms a vicious circle.55,56
In response to various pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor α, the level of serum GLB arises and reflects the occurrence of SIR.38,57 Previous studies have shown that high GLB level not only indicates inflammatory response but also predict correlated with tumor initiation and recurrence, as well as poor outcomes.38,58-60 Unfortunately, barely a few studies have unveiled the predictive value of GLB in patients with resectable cervical cancer. In the present study, both whole treatment and radiotherapy had no effect on ALB or GLB. Neither ALB nor GLB had any effect on OS and PFS in patients with resectable cervical cancer.
As a combination of ALB and GLB, AGR reflects both nutritional status and inflammatory response.47 Strong evidence suggests that low AGR level is significantly correlated with poor outcomes in several types of malignant tumors.61-63 To the best of our knowledge, this is the first study to specifically focus on the predictive value of AGR in resectable cervical cancer. We propose that AGR is a quite plausible predictive parameter on account of a couple of reasons. Firstly, decreased AGR level may attribute to decreased ALB level and/or increased GLB level, which combines these 2 important unfavorable predictors and may provide more accurate prediction than 2 single parameters. Secondly, difference is, AGR is less influenced by conditions such as dehydration and fluid retention, which may leads to a fluctuation of ALB and GLB levels. In the present study, pretreatment AGR had no impact on PFS and OS. Both whole treatment and radiotherapy had no significant effect on the level of AGR. Univariate analysis demonstrated that increased post/pretreatment ratio of AGR was correlated with better OS.
The LDH, a ubiquitous cellular enzyme, is proved to ensure an efficient anaerobic glycolysis and supply most of energy for malignant cells even under aerobic condition, such phenomenon is widely accepted as the Warburg effect.64,65 Thus, serum LDH level directly mirrors severe tumor hypoxia. Hypoxia and an upregulation of serum LDH level is observed in the majority of patients with cervical cancer and is tightly associated with poor outcomes.21,66 In the present study, lower baseline LDH level was correlated with better PFS and OS. Radiotherapy significantly increased the level of LDH, while surgery had no impact on LDH. Increased post/preradiotherapy LDH ratio was correlated with worse PFS. Multivariate analysis revealed that high baseline LDH level was independently correlated with poor OS and PFS. Several mechanisms underlying the correlation between serum LDH level and outcomes of patient with cancer have been proposed. Firstly, according to the Warburg effect, a high serum LDH level reflects high rate of glucose uptake and hypermetabolism of tumor cells, as well as malignant aggressiveness.67 Secondly, upregulation of LDH may lead to hypoxia-inducible factor 1α (HIF-α) cascade, which promotes the secretion of VEGFs and facilitates angiogenesis. Moreover, activated HIF-α in turn enhances LDH activity, building a microenvironment in favor of tumor angiogenesis and hematogenous metastasis.68 In view of these enumerated evidences, we reckoned that high serum LDH level indicated unfavorable outcomes in cervical cancer. Actually, in a retrospective study of Li et al, high baseline serum LDH level was independently correlated with outcomes in patients with locally advanced cervical cancer.21
However, the present study had several limitations. Firstly, it was a retrospective study with relatively small sample size, and all patients came from a single center. Secondly, HPV screening was not routinely proceeded in the study and HPV infection status of patients might be a source of heterogeneity. Giving the mentioned limitations, further investigation and multicenter study were required to confirm our results.
In summary, it is the first study to obtain an in-depth look at the predictive value of a series of SIR-related present study, high baseline CRP and LDH levels had an adverse impact on PFS and OS. Moreover, not-increased post/preradiotherapy CRP ratio, as well as post/pretreatment CRP ratio, was significantly correlated with better PFS and OS. Patients with not-increased post/preradiotherapy LDH level had better PFS. Considering the mentioned parameters are routinely detected in blood tests, we suggest that these convenient and inexpensive clinical parameters can be incorporated in the routine practice in cervical cancer and have a broad application prospect.
Acknowledgments
The authors thank Chunhua Zhao for his assistance in completing the statistics section.
Authors’ Note: Wen-Jie Wang, Ying Li, and Jie Zhu contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Education for Health Foundation of Suzhou for Youth (Grant Numbers kjxw2018030 and kjxw2018032), the Science and Technology Project Foundation of Suzhou (Grant Numbers SS201651), and the Education Research Project Foundation of Nanjing Medical University (Grant Number FZS-ZD-201701).
References
- 1. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. [DOI] [PubMed] [Google Scholar]
- 2. Di J, Rutherford S, Chu C. Review of the cervical cancer burden and population-based cervical cancer screening in China. Asian Pac J Cancer Prev. 2015;16(17):7401–7407. [DOI] [PubMed] [Google Scholar]
- 3. Zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989;49(17):4677–4681. [PubMed] [Google Scholar]
- 4. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 5. Sherman ME, Wang SS, Carreon J, Devesa SS. Mortality trends for cervical squamous and adenocarcinoma in the United States. Relation to incidence and survival. Cancer. 2005;103(6):1258–1264. [DOI] [PubMed] [Google Scholar]
- 6. Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes Control CCC. 2009;20(7):1129–1138. [DOI] [PubMed] [Google Scholar]
- 7. Gomez SL, Noone AM, Lichtensztajn DY, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J Natl Cancer Inst. 2013;105(15):1096–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dinkelspiel H, Kinney W. State of the science: cervical cancer screening in transition. Gynecol Oncol. 2014;133(3):389–393. [DOI] [PubMed] [Google Scholar]
- 9. Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87(3):264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yildirim M, Yildiz M, Duman E, Goktas S, Kaya V. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J BUON. 2013;18(3):728–732. [PubMed] [Google Scholar]
- 11. McKeown DJ, Brown DJ, Kelly A, Wallace AM, McMillan DC. The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer. Br J Cancer. 2004;91(12):1993–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun H, Hu P, Shen H, et al. Albumin and neutrophil combined prognostic grade as a new prognostic factor in non-small cell lung cancer: results from a large consecutive cohort. PLoS One. 2015;10(12):e0144663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang HH, Li AJ, Tang EJ, et al. Prognostic value of the combination of preoperative hemoglobin, lymphocyte, albumin, and neutrophil in patients with locally advanced colorectal cancer. Med Sci Monit. 2016;22:4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruiz-Tovar J, Martin-Perez E, Fernandez-Contreras ME, Reguero-Callejas ME, Gamallo-Amat C. Impact of preoperative levels of hemoglobin and albumin on the survival of pancreatic carcinoma. Rev Esp Enferm Dig. 2010;102(11):631–636. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Zhou Y, Xu Y, Zhu HY, Shi YQ. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumour Biol. 2016;37(3):3905–3911. [DOI] [PubMed] [Google Scholar]
- 16. Azab BN, Bhatt VR, Vonfrolio S, et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. Am J Surg. 2013;206(5):764–770. [DOI] [PubMed] [Google Scholar]
- 17. Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis. 2013;28(12):1629–1636. [DOI] [PubMed] [Google Scholar]
- 18. Zhang F, Sun P, Wang ZQ, et al. Low preoperative albumin-globulin score predicts favorable survival in esophageal squamous cell carcinoma. Oncotarget.2016;7(21):30550–30560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. [DOI] [PubMed] [Google Scholar]
- 20. Gerolymatos A, Fotiou S, Tserkezoglou A, et al. Evaluation of lactate dehydrogenase activity as an index of cervical malignancy. Eur J Gynaecol Oncol. 1991;12(6):471–476. [PubMed] [Google Scholar]
- 21. Li J, Wu MF, Lu HW, Chen Q, Lin ZQ, Wang LJ. Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer. Cancer Med. 2016;5(8):1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxf, Engl: 1990). 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 23. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Kempen LC, de Visser KE, Coussens LM. Inflammation, proteases and cancer. Eur J Cancer (Oxf, Engl: 1990). 2006;42(6):728–734. [DOI] [PubMed] [Google Scholar]
- 25. Lages EL, Belo AV, Andrade SP, et al. Analysis of systemic inflammatory response in the carcinogenic process of uterine cervical neoplasia. Biomed Pharmacother. 2011;65(7):496–499. [DOI] [PubMed] [Google Scholar]
- 26. Zheng RR, Huang M, Jin C, et al. Cervical cancer systemic inflammation score: a novel predictor of prognosis. Oncotarget. 2016;7(12):15230–15242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(suppl 1):S79–S84. [DOI] [PubMed] [Google Scholar]
- 28. Falconer JS, Fearon KC, Ross JA, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75(8):2077–2082. [DOI] [PubMed] [Google Scholar]
- 29. Lamb GW, McMillan DC, Ramsey S, Aitchison M. The relationship between the preoperative systemic inflammatory response and cancer-specific survival in patients undergoing potentially curative resection for renal clear cell cancer. Br J Cancer. 2006;94(6):781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polterauer S, Grimm C, Zeillinger R, et al. Association of C-reactive protein (CRP) gene polymorphisms, serum CRP levels and cervical cancer prognosis. Anticancer Res. 2011;31(6):2259–2264. [PubMed] [Google Scholar]
- 31. Zhang Y, Wang L, Liu Y, et al. Preoperative neutrophil–lymphocyte ratio before platelet–lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. Int J Gynecol Cancer. 2014;24(7):1319–1325. [DOI] [PubMed] [Google Scholar]
- 32. Wang D, Wu M, Feng FZ, et al. Pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios do not predict survival in patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy. Chin Med J. 2013;126(8):1464–1468. [PubMed] [Google Scholar]
- 33. Ni XC, Yi Y, Fu YP, et al. Prognostic value of the modified Glasgow Prognostic Score in patients undergoing radical surgery for hepatocellular carcinoma. Med. 2015;94(36):e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104(4):726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370. [DOI] [PubMed] [Google Scholar]
- 36. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 37. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. [DOI] [PubMed] [Google Scholar]
- 38. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. [DOI] [PubMed] [Google Scholar]
- 39. Weinhold B, Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997;327(pt 2):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38(2-3):189–197. [DOI] [PubMed] [Google Scholar]
- 41. Koike Y, Miki C, Okugawa Y, et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol. 2008;98(7):540–544. [DOI] [PubMed] [Google Scholar]
- 42. Shimura T, Kitagawa M, Yamada T, et al. C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer Res. 2012;32(2):491–496. [PubMed] [Google Scholar]
- 43. Chapple JP, Bros-Facer V, Butler R, Gallo JM. Focal distortion of the nuclear envelope by huntingtin aggregates revealed by lamin immunostaining. Neurosci Lett. 2008;447(2-3):172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hara M, Matsuzaki Y, Shimuzu T, et al. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res. 2007;27(4c):3001–3004. [PubMed] [Google Scholar]
- 45. Chmura A, Wojcieszek A, Mrochem J, et al. [Usefulness of the SCC, CEA, CYFRA 21.1, and CRP markers for the diagnosis and monitoring of cervical squamous cell carcinoma]. Ginekol Pol. 2009;80(5):361–366. [PubMed] [Google Scholar]
- 46. Pine JK, Fusai KG, Young R, et al. Serum C-reactive protein concentration and the prognosis of ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2009;35(6):605–610. [DOI] [PubMed] [Google Scholar]
- 47. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6 suppl 4):S118–S125. [DOI] [PubMed] [Google Scholar]
- 48. McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. [DOI] [PubMed] [Google Scholar]
- 49. Kanda M, Mizuno A, Tanaka C, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine. 2016;95(24):e3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin Y, Zhao L, Peng F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics. 2013;68(5):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He X, Li JP, Liu XH, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer. 2018;9(10):1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seaton K. Albumin concentration controls cancer. J Nat Med Assoc. 2001;93(12):490–493. [PMC free article] [PubMed] [Google Scholar]
- 53. Chandra RK. Nutrition and immunology: from the clinic to cellular biology and back again. Proc Nutr Soc. 1999;58(3):681–683. [DOI] [PubMed] [Google Scholar]
- 54. Al-Shaiba R, McMillan DC, Angerson WJ, Leen E, McArdle CS, Horgan P. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer. 2004;91(2):205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pfensig C, Dominik A, Borufka L, Hinz M, Stange J, Eggert M. A new application for albumin dialysis in extracorporeal organ support: characterization of a putative interaction between human albumin and proinflammatory cytokines IL-6 and TNFalpha. Artif Organs. 2016;40(4):397–402. [DOI] [PubMed] [Google Scholar]
- 56. Mao MJ, Wei XL, Sheng H, et al. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed Res Int. 2017;2017:3083267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Du XJ, Tang LL, Mao YP, et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One. 2014;9(4):e94473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol. 1997;100(2):151–157. [DOI] [PubMed] [Google Scholar]
- 59. Alacacioglu A, Tarhan O, Alacacioglu I, Dirican A, Yilmaz U. Depression and anxiety in cancer patients and their relatives. J BUON. 2013;18(3):767–774. [PubMed] [Google Scholar]
- 60. Qu X, Pang Z, Yi W, et al. High percentage of alpha1-globulin in serum protein is associated with unfavorable prognosis in non-small cell lung cancer. Med Oncol (Northwood, Lond, Engl). 2014;31(10):238. [DOI] [PubMed] [Google Scholar]
- 61. Arrieta O, Michel Ortega RM, Villanueva-Rodriguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer. 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fujii T, Sutoh T, Morita H, et al. Serum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancer. Nutr Cancer. 2012;64(8):1169–1173. [DOI] [PubMed] [Google Scholar]
- 63. Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41(10):1324–1332. [DOI] [PubMed] [Google Scholar]
- 64. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed] [Google Scholar]
- 65. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 66. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276. [DOI] [PubMed] [Google Scholar]
- 67. Sun X, Sun Z, Zhu Z, et al. Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS One. 2014;9(3):e91068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–23115. [DOI] [PubMed] [Google Scholar]