Abstract
Rationale: Air pollution may influence sleep through airway inflammation or autonomic nervous system pathway alterations. Epidemiological studies may provide evidence of relationships between chronic air pollution exposure and sleep apnea.
Objectives: To determine whether ambient-derived pollution exposure is associated with obstructive sleep apnea and objective sleep disruption.
Methods: We analyzed data from a sample of participants in MESA (Multi-Ethnic Study of Atherosclerosis) who participated in both the Sleep and Air studies. Mean annual and 5-year exposure levels to nitrogen dioxide (NO2) and particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5) were estimated at participants’ homes using spatiotemporal models based on cohort-specific monitoring. Participants completed in-home full polysomnography and 7 days of wrist actigraphy. We used multivariate models, adjusted for demographics, comorbidities, socioeconomic factors, and site, to assess whether air pollution was associated with sleep apnea (apnea–hypopnea index ≥ 15) and actigraphy-measured sleep efficiency.
Results: The participants (n = 1,974) were an average age of 68 (±9) years, 46% male, 36% white, 24% Hispanic, 28% black, and 12% Asian; 48% had sleep apnea and 25% had a sleep efficiency of ≤88%. A 10 ppb annual increase in NO2 exposure was associated with 39% greater adjusted odds of sleep apnea (95% confidence interval [CI], 1.03–1.87). A 5 μg/m3 greater annual PM2.5 exposure was also associated with 60% greater odds of sleep apnea (95% CI, 0.98–2.62). Sleep efficiency was not associated with air pollution levels in fully adjusted models.
Conclusions: Individuals with higher annual NO2 and PM2.5 exposure levels had a greater odds of sleep apnea. These data suggest that in addition to individual risk factors, environmental factors also contribute to the variation of sleep disorders across groups, possibly contributing to health disparities.
Keywords: environmental pollutants, health status disparities, obstructive sleep apnea
Sleep disruption and obstructive sleep apnea (OSA) are associated with hypertension, diabetes, stroke, ischemic heart disease, cancer, and cardiac death (1–3). Proposed mechanisms include altered autonomic tone, increased inflammation, and metabolic dysregulation. Similarly, air pollution has been linked with cerebrovascular disease, cancer and cardiovascular morbidity, and overall mortality, attributed in part to an increased systemic inflammatory response to fine particles (4–7). Ambient air pollution consists of fine particulate matter (PM) as well as gaseous products of combustion (oxides of nitrogen [NOx]) produced by the burning of fossil fuels (e.g., in automobiles and power plants). Although there is increasing interest in the influence of the environment on sleep, there is limited research evaluating the relationship between sleep and air pollution.
Few studies have examined the epidemiological effects of air pollution on disorders of the upper airway. Epidemiological studies have typically highlighted the consequences of pollution on the distal airways associated with respiratory disease—for example, chronic obstructive lung disease (COPD) and asthma are both worsened by air pollution (8–11). High levels of pollution increase the risk of respiratory infections requiring hospitalization in children and mortality in the elderly (10). Air pollution exposure can impair the pulmonary immune defense and alter normal airway clearance by injury and inflammation of airway mucosa (12). The proximal upper airways, such as the nasopharynx, may respond similarly with inflammation and edema (13). Thus, air pollution levels may contribute to OSA risk and severity through upper airway irritation, edema, and subsequent narrowing. Prior studies showed some association of short-term PM (particulate matter ≤ 10 μm in aerodynamic diameter [PM10]) levels with the apnea–hypopnea index (AHI) and nocturnal hypoxemia (14, 15), but were limited by the available air pollution data and lack of racial diversity among the subjects.
Air pollution exposures differ geographically and may partially explain differences in lung and sleep health by demographics such as socioeconomic status (SES), race, and ethnicity. Disadvantaged neighborhoods with low-SES residents are often exposed to higher ambient air pollution. In studies in the United States and developed countries, a consistent pattern of greater burden of air pollutants was shown to aggregate in lower-SES communities (16). In addition to the upper airway irritation and congestion induced by air pollution, neuroinflammation and neurotoxicity may also contribute to sleep disruption (17). Poor air quality may partially explain observations of reduced sleep quality among residents living in low-SES neighborhoods, in addition to the effects of poor social cohesion, fear of crime, and greater psychosocial stressors (18–20). Air pollution levels therefore may further contribute to sleep health disparities.
Using a cohort from MESA (Multi-Ethnic Study of Atherosclerosis), consisting of subjects who participated in the MESA Air and Sleep studies, we investigated whether ambient air pollution levels were associated with sleep apnea and sleep disruption. We hypothesized that greater levels of long-term nitrous oxide and PM pollution would be associated with a higher risk of OSA and lower sleep efficiency. We also hypothesized that short-term PM levels would have a similar association with sleep metrics.
This work was previously presented at the Association of Professional Sleep Societies Meeting, June 2014, and the American Thoracic Society International Meeting, May 2017 (21, 22).
Methods
Data
MESA is a longitudinal study of cardiovascular disease in adults 45–84 years of age. Subjects without clinical cardiovascular disease at baseline were recruited from the community in six U.S. cities (Los Angeles, California; New York City, New York; Chicago, Illinois; Winston-Salem, North Carolina; St. Paul, Minnesota; and Baltimore, Maryland). The current sample was restricted to individuals with air pollution data who agreed to participate in the MESA Sleep ancillary study that occurred after the 10-year MESA follow-up (Exam 5 in 2010–2013). The institutional review board at each site approved the study and all participants gave written informed consent.
MESA Air
Individual air pollution exposure estimates were calculated from data collected from community and Air Quality System monitoring sites in each metropolitan area (see the online supplement). These data were then integrated with detailed geographical data (including residential location, roadway proximity, population density, vegetative index, industrial pollution sources, and land use) using hierarchical spatiotemporal modeling. Details regarding MESA Air recruitment, exposure assessment, and the methodology for estimating individual-level long-term air pollution exposure have been previously described (23). Exposures include 1- and 5-year averages before the sleep study for particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), nitrogen dioxide (NO2), and NOx. Because NOx distributions were nearly identical to NO2 distributions, only NO2 is reported here. Short-term PM2.5 levels were collected for the day of the study using citywide air quality monitoring station data. These data were preadjusted for city-specific trends by day of week, calendar day, temperature, and dew points using splines to account for seasonality and local meteorology (24).
MESA Sleep
Through the MESA Sleep ancillary study, sleep was assessed in a proportion of MESA subjects via 1-week actigraphy and in-home full polysomnography as described previously (see online data supplement) (19, 25). Objective sleep disruption was measured by actigraphy, and sleep efficiency (the proportion of time asleep over total time in bed) was calculated from the 7-day average of scored actigraphy. The AHI was calculated by including all apneas and hypopneas with 4% or greater desaturation or an arousal on polysomnogram divided by total sleep time.
Covariates
Sociodemographic and comorbidity data were obtained from the MESA health and medical history surveys at the fifth examination (see online supplement). Depressive symptoms were defined as a Center for Epidemiological Studies–Depression score > 16 (26). To account for neighborhood SES at the current residence, we used census-tract–level data from the American Community Survey 5-year estimates for 2007–2011. We created a measure of census-tract SES including >25th percentile of resident families below poverty, >12% of resident males 25 years or older unemployed, and >30% of residents with less than high school education.
Analysis
We evaluated for differences in the cohort sociodemographics from the parent MESA sample and by air pollution exposure by quartile, using the chi-squared test for categorical variables and t tests for continuous variables. We defined OSA as an AHI ≥ 15, a threshold that is considered moderate to severe (27). We compared subjects with OSA with those without OSA by sample characteristics. We defined reduced sleep efficiency as ≤88%, corresponding to the lowest 25th percentile of the 7-day actigraphy average. Sleep efficiency was made dicholomous as its distribution was not normal but highly skewed and to improve interpretability of analysis.
We examined associations of OSA and reduced sleep efficiency with long-term exposure to ambient air pollution (NO2 and PM2.5 exposures averaged over 1 and 5 years before sleep evaluation) and short-term PM2.5 in multivariate logistic regression. For interpretability, we modeled NO2 in units of 10 ppb and PM2.5 by 5 μg/m3; we excluded observations with any missing covariates. The simplest model (model 1) adjusted for age, sex, and body mass index (BMI) (plus OSA in the sleep efficiency analysis). Model 2 added the covariates of individual household income, race/ethnicity, diabetes, hypertension, short sleep duration (in actigraphy outcome only), smoking status, and neighborhood SES features. These covariates were included because they differed by air pollution and/or sleep apnea. Model 3 added adjustment for site.
We performed several additional sensitivity analyses to assess the strength of our findings. We evaluated the association of AHI ≥ 15 using the 4% oxygen desaturation definition of hypopnea (U.S. Centers for Medicare and Medicaid [CMS] criteria) with air pollution. We evaluated whether including the use of medications for reflux and evidence of airflow obstruction on spirometry changed the observed associations. We also performed analyses excluding subjects with mild OSA (AHI 5–15).
In an exploratory analysis, we assessed AHI, modeled continuously, in generalized linear models. These models included the same units of air pollution predictors and covariates as the logistic regression models. Site, residential SES, and race/ethnicity had high collinearity with air pollution levels (and with each other), precluding assessment of their potential effect modification with one another. We also explored the association of air pollution with sleep apnea when stratified by site.
Results
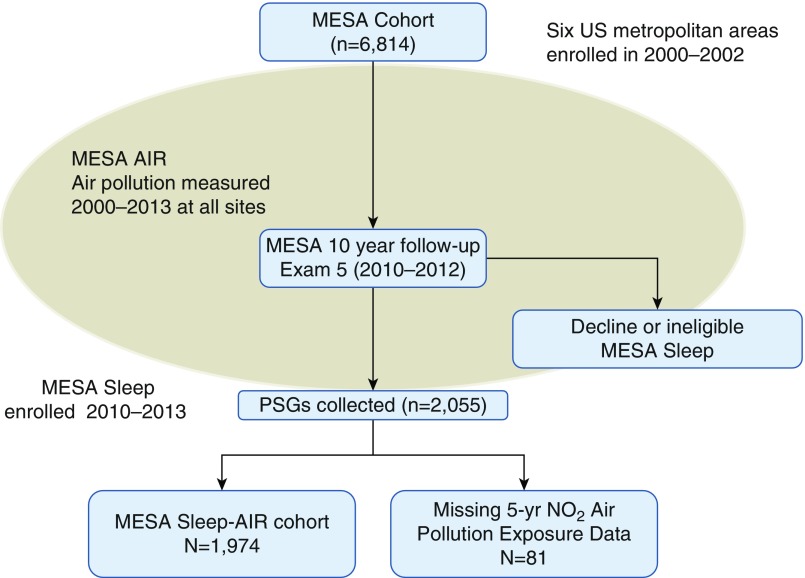
Among the 1,974 MESA subjects with both sleep polysomnography data and air pollution data (Figure 1), the mean age was 68.4 years (± 9.2) and BMI was 28.7 kg/m2 (± 5.6), with racial/ethnic, socioeconomic, and geographic diversity (Table 1). The cohort had greater racial/ethnic diversity but otherwise did not differ significantly from the parent MESA study population by demographics. Air pollution differed substantially by site, with the highest levels and greatest distribution in New York and Los Angeles, and the lowest levels in St. Paul and Winston-Salem areas. Pollution levels also differed significantly by race/ethnicity, education level, income level, and neighborhood SES, with higher pollution exposure levels observed in minorities, subjects with high school or less education, subjects with lower household income, and subjects living in lower SES neighborhoods.
Figure 1.
Flow chart detailing the sample included in this analysis from the parent MESA (Multi-Ethnic Study of Atherosclerosis) cohort. PSG = polysomnography.
Table 1.
MESA Sleep and Air pollution sample characteristics
| Total % (n) N = 1,974 | |
|---|---|
| Age, yr*, mean (SD) | 68.4 (9.2) |
| Percentage men (n)* | 45.9 (905) |
| BMI, kg/m2*, mean (SD) | 28.7 (5.6) |
| Percentage married (n)† | 60.5 (1,176) |
| Percentage smokers (former/current) (n)*† | 45.2 (886) |
| Percentage depressed, CES-D > 16 (n)† | 14.4 (279) |
| Percentage hypertensive*† | 57.5 (1,134) |
| Percentage diabetic*† | 39.9 (780) |
| Percentage race/ethnicity, n*† | |
| White | 35.9 (708) |
| Chinese-American | 12.2 (240) |
| African-American | 28.0 (553) |
| Hispanic | 24.0 (473) |
| Total family income, $† | n |
| <20,000 | 20.6 (394) |
| 20–49,999 | 33.8 (647) |
| 50,000–99,999 | 27.5 (528) |
| ≥100,000 | 18.2 (348) |
| Education level† | n |
| High school or less | 31.6 (622) |
| Some college/technical/associate | 29.1 (574) |
| Bachelor degree or more | 39.3 (774) |
| Site*† | n |
| Winston-Salem, NC (Wake Forest) | 14.7 (291) |
| New York City, NY (Columbia) | 17.9 (354) |
| Baltimore, MD (Johns Hopkins University) | 14.6 (289) |
| St. Paul, MN (University of Minnesota) | 17.1 (338) |
| Chicago, IL (Northwestern University) | 18.9 (373) |
| Los Angeles, CA (University of California, Los Angeles) | 16.7 (329) |
| Census-tract SES† | Median (IQR) |
| Percentage unemployed males > 25 yr old | 6 (3–10) |
| Percentage households below poverty | 11 (6–20) |
| Percentage professional occupation | 34 (23–50) |
| Percentage less than high school education | 19 (10–32) |
| Census-tract low-SES composite† | % n |
| Percentage low-SES neighborhood (n) | 34.7 (685) |
Definition of abbreviations: BMI = body mass index; CES-D = Center for Epidemiological Studies–Depression; IQR = interquartile range; NO2 = nitrogen dioxide; SD = standard deviation; SES = socioeconomic status.
Significant difference by obstructive sleep apnea (apnea–hypopnea index ≥ 15 vs. < 15), P < 0.05.
Significant difference by NO2 quartile, P < 0.05.
The median AHI was 14.4 (interquartile range [IQR], 7.1–27.4) (Table 2). Subjects with OSA were, as expected, more often men, obese, former or current smokers, hypertensive, and diabetic, but did not differ by income level, education level, or residential SES features. OSA was more often identified in subjects tested in the spring and summer months (April–September). The median NO2 exposure estimate averaged over 5 years was 14.8 ppb (IQR, 10–23.7) and was well below the U.S. Environmental Protection Agency (EPA) National Ambient Air Quality Standards standard of <53 ppb (https://www.epa.gov/criteria-air-pollutants/naaqs-table). In contrast, the median PM2.5 exposure estimate over 5 years was 12.3 μg/m3 (IQR, 11.5–13.5), above the EPA standard of 12.0 μg/m3.
Table 2.
MESA Sleep and Air pollution sample characteristics (N = 1,974) presented as median and interquartile range
| Median | IQR | |
|---|---|---|
| Sleep Metrics: Polysomnography Data | ||
| AHI (AASM criterion) events per hour | 14.4 | 7.1–27.2 |
| AHI (4% desaturation) events per hour | 9.1 | 3.2–19.7 |
| 4% ODI events per hour* | 8.2 | 3.2–19.2 |
| Nadir saturation* | 85 | 80–89 |
| Percentage sleep time with <90% saturation | 0.63 | 0.04–3.32 |
| Percentage OSA, AHI > 15 by AASM (n)* | 48.0% | n = 884 |
| Sleep Metrics: Actigraphy Data | ||
| Percentage WASO > 60 min (n)* | 10.8% | n = 201 |
| Percentage short sleeper, <6 h (n)* | 31% | n = 578 |
| Sleep efficiency over 7 days* | 90.5 | 88.0–92.5 |
| Sleep time over 7 days, h* | 6.64 | 5.74–7.38 |
| Air Pollution Levels | Median | IQR |
| Five-year NO2, ppb | 14.8 | 10.0–23.7 |
| One-year NO2, ppb | 13.0 | 9.0–21.4 |
| One-year PM2.5, μg/m3* | 11.0 | 10.3–12.0 |
| Five-year PM2.5, μg/m3 | 12.3 | 11.5–13.5 |
Definition of abbreviations: AASM = American Academy of Sleep Medicine; AHI = apnea–hypopnea index; IQR = interquartile range; NO2 = nitrogen dioxide; ODI = oxygen desaturation index; OSA = obstructive sleep apnea; PM2.5 = particulate matter ≤ 2.5 μm in aerodynamic diameter; WASO = wake after sleep onset.
Significant difference by OSA (AHI > 15 vs. ≤ 15), P < 0.05.
Sleep and NO2
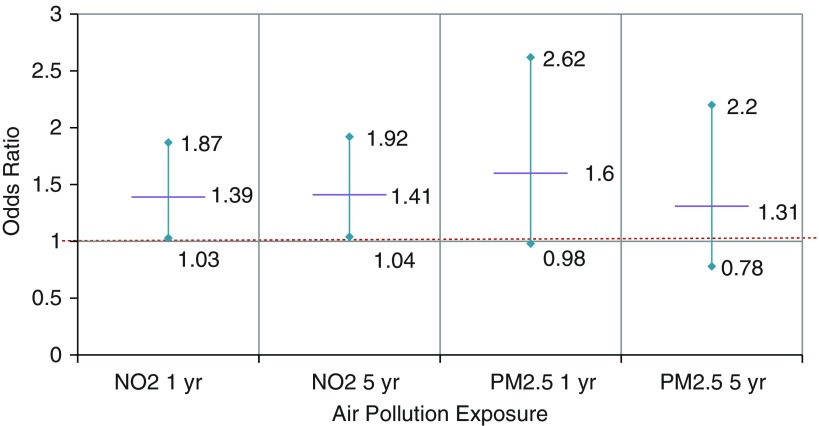
There was an association of NO2 exposure with OSA in fully adjusted models including site (Table 3), but not in the simpler models. A 10 ppb greater NO2 (averaged over either 1 year or 5 years) was associated with a nearly 40% greater odds of OSA (95% confidence interval [CI], 1.03–1.87) (Figure 2). The association was seen but was not significant when the 4% desaturation criterion for hypopneas (CMS definition) for AHI (Table E1 in the online supplement) was used. No association was seen when AHI was modeled continuously (Table E2). When stratified by site, associations persisted only in sites with greater pollution exposure and variation (Los Angeles and New York City) (Table E3).
Table 3.
Outcome of sleep apnea (apnea–hypopnea index > 15 on polysomnography)*
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| One-year | n = 1,961 | n = 1,884 | n = 1,884 |
| NO2, per 10 ppb | 1.05 (0.95–1.16) | 1.00 (0.88–1.14) | 1.39 (1.03–1.87)† |
| Five-year | n = 1,948 | n = 1,871 | n = 1,871 |
| NO2, per 10 ppb | 1.06 (0.96–1.17) | 1.01 (0.89–1.15) | 1.41 (1.04–1.92)† |
Definition of abbreviation: NO2 = nitrogen dioxide.
NO2 average exposure estimates (in 10 ppb units), over 1–5 years before sleep assessment, presented as odds ratios (95% confidence interval).
Adjusting for age, sex, body mass index (model 1); plus diabetes, hypertension, race/ethnicity, household income, smoking status, and residential socioeconomic status (model 2); plus site (model 3).
P < 0.05.
Figure 2.
Adjusted odds of obstructive sleep apnea (apnea–hypopnea index ≥ 15) with ambient air pollution, NO2 (in 10 ppb units), and particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5; in 5 μg/m3 units) exposure estimates averaged over 1 year and 5 years before sleep assessment; odds ratios and 95% confidence intervals are shown. Logistic regression models were adjusted for age, sex, body mass index, diabetes, hypertension, race/ethnicity, household income, smoking status, residential socioeconomic status, and site. NO2 = nitrogen dioxide.
A 10 ppb greater NO2 exposure was associated with 19% greater odds of a low sleep efficiency (Table 4) in minimally adjusted models. With social and residential factors included in the model, the association was no longer significant.
Table 4.
Outcome of reduced sleep efficiency (≤88% on actigraphy)*
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| 1 yr prior | n = 1,854 | n = 1,781 | n = 1,781 |
| NO2, per 10 ppb | 1.19 (1.07–1.33)† | 1.11 (0.96–1.28)‡ | 1.04 (0.74–1.44) |
| 5 yr prior | n = 1,842 | n = 1,769 | n =1,769 |
| NO2, per 10 ppb | 1.19 (1.07–1.33)† | 1.12 (0.97–1.29)‡ | 1.09 (0.77–1.53) |
Definition of abbreviation: NO2 = nitrogen dioxide.
NO2 average exposure estimates (in 10 ppb units), over 1–5 years before sleep assessment, presented as odds ratios (95% confidence interval).
Adjusted for age, sex, body mass index, obstructive sleep apnea (apnea–hypopnea index ≥ 15) (model 1); plus race/ethnicity, income, smoking status, diabetes, hypertension, short sleep duration (< 6 h), and residential socioeconomic status (model 2); plus site (model 3).
P < 0.05.
P < 0.10.
Sleep and PM2.5
An association was observed between 1-year average PM2.5 exposure estimates with OSA, but not with 5-year average PM2.5 exposure estimates. In fully adjusted models, each 5 μg/m3 increase in annual mean PM2.5 exposure was associated with a 60% greater odds of OSA (95% CI, 0.98–2.62) (Figure 2). This was significant when site was not in the model with a narrower CI (Table 5). Short-term PM2.5 levels were not associated with OSA in logistic regression. Using the CMS hypopnea definition (4% oxygen desaturation), each 5 μg/m3 greater annual PM2.5 exposure was associated with 59% greater odds of CMS AHI ≥ 15 OSA (Table E1). When AHI was modeled continuously, there was evidence of association only in the minimally adjusted model (Table E2). When stratified by site, only the Los Angeles site demonstrated a clear association of PM2.5 exposure to sleep apnea (Table E3).
Table 5.
Outcome sleep apnea (apnea–hypopnea index > 15)*
| Long-term PM2.5 | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| PM2.5 1 yr | n = 1,928 | n = 1,853 | n = 1,853 |
| Per 5 μg/m3 PM2.5 | 1.79 (1.25–2.55)† | 1.63 (1.09–2.44)† | 1.60 (0.98–2.62)‡ |
| PM2.5 5 yr | n = 1,950 | n = 1,873 | n = 1,873 |
| Per 5 μg/m3 PM2.5 | 1.24 (0.93–1.65) | 1.14 (0.82–1.58) | 1.31 (0.78–2.20) |
| Short-term PM2.5 | n = 1,916 | n = 1,812 | n = 1,812 |
| PM2.5 day prior* | 1.00 (0.98–1.02) | 1.01 (0.98–1.03) | 1.01 (0.99–1.03) |
| PM2.5 day of polysomnography* | 1.00 (0.98–1.02) | 0.99 (0.97–1.02) | 0.99 (0.97–1.02) |
Definition of abbreviation: PM2.5 = particulate matter ≤ 2.5 μm in aerodynamic diameter.
PM2.5 exposure estimates (in 5 μg/m3 units), averaged over 1–5 years before sleep assessment, presented as odds ratio (95% confidence interval).
Adjusted for age, sex, body mass index (model 1); plus diabetes, hypertension, race/ethnicity, household income, smoking status, residential socioeconomic status (model 2); plus site (model 3).
P < 0.05.
P < 0.10.
For 5-year average PM2.5 exposure, each 5 μg/m3 increase was associated with 51% greater odds of reduced sleep efficiency (95% CI, 1.09–2.09) in the age, sex, BMI, and OSA-only adjusted model. However, there was no evidence of association after adjustment for individual sociodemographics and residential SES (Table 6). One-year PM2.5 exposure levels and short-term PM2.5 levels were not associated with sleep efficiency.
Table 6.
Outcome of reduced sleep efficiency (≤88% on actigraphy)*
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| PM2.5 1 yr prior | n = 1,822 | n = 1,750 | n = 1,750 |
| Per 5 μg/m3 PM2.5 | 1.34 (0.91–1.98) | 1.00 (0.63–1.58) | 0.92 (0.53–1.60) |
| PM2.5 5 yr prior | n = 1,843 | n = 1,770 | n = 1,770 |
| Per 5 μg/m3 PM2.5 | 1.51 (1.09–2.09)‡ | 1.28 (0.88–1.87) | 1.07 (0.59–1.93) |
| Short-term PM2.5 | n = 1,811 | n = 1,711 | |
| PM2.5 day prior† | 1.00 (0.98–1.02) | 1.01 (0.98–1.04) | 1.01 (0.98–1.04) |
| PM2.5 day of polysomnography† | 1.01 (0.98–1.03) | 0.99 (0.97–1.02) | 1.01 (0.98–1.03) |
Definition of abbreviation: PM2.5 = particulate matter ≤ 2.5 μm in aerodynamic diameter.
PM2.5 exposure estimates (in 5 μg/m3 units), averaged over 1–5 years before sleep assessment, presented as odds ratio (95% confidence interval).
Adjusting for age, sex, body mass index, obstructive sleep apnea (apnea–hypopnea index ≥ 15) (model 1); model 1 plus race/ethnicity, income, smoking status, diabetes, hypertension, short sleep duration (<6 h), and residential socioeconomic status (model 2); model 2 plus site (model 3).
Short-term PM2.5 values are citywide levels from the day of and day before overnight polysomnography. The values have been preadjusted for seasonal and meteorological trends.
P < 0.05.
Sensitivity Analyses
Including the use of proton pump inhibitors in the models did not change the association of air pollution with sleep apnea. We also found no difference when spirometry data were included (available for only a subset of the cohort [n = 1,407]). When subjects with mild OSA were excluded (AHI 5–15; n = 719), the association of OSA with air pollution remained robust; the odds ratios were 1.52 (95% CI, 1.09–2.12) for NO2 and 1.65 (95% CI, 0.97–2.81) for PM2.5 in fully adjusted models.
Discussion
This study reveals an association of chronic exposure to ambient derived air pollution with sleep apnea. Higher average yearly levels of NO2 and PM2.5 were associated with increased odds of moderate to severe sleep apnea independently of race/ethnicity, income, diabetes, hypertension, neighborhood SES, and site. The cumulative effects of air pollution exposure over the year may lead to changes to the upper airway. However, PM2.5 exposure averaged over 5 years was not associated with sleep apnea, possibly due to chronic adaptations. Exposures to greater average NO2 and PM2.5 levels were less robustly associated with lower sleep efficiency. This association was attenuated after adjustment for comorbidities, individual demographics, and residential factors, all of which are known to contribute to sleep quality (19, 25).
There are several potential mechanisms linking air pollution to sleep. Experimental models in mammals have demonstrated that ambient air pollutants cause upper airway edema, inflammation, and irritation (28, 29), which may contribute to upper airway obstruction during sleep. Fine PM and NOx are associated with chronic rhinosinusitis, nonallergic and allergic rhinitis (28, 30), and excess risk of upper respiratory infections (31, 32). However, most of the data were obtained in regions with much higher pollution exposure levels than those examined in this study. PM2.5 has been shown in vivo to induce an inflammatory response in human nasal epithelial cells (33). These ambient pollutants are also known to contribute to incident obstructive airway disease and respiratory symptoms in the elderly (34), and this may be another mechanism that contributes to sleep-disordered breathing. Chronic upper airway irritation and inflammation due to air pollution may induce adenoid and tonsillar hypertrophy and consequently upper airway narrowing. Further experimental studies are needed to verify these hypotheses. Fine PM and traffic-related air pollutants represented by NOx may directly penetrate the central nervous system, causing neurotoxicity and neuroinflammation (17), which may affect brain areas involved in the regulation of sleep and control of ventilation. Long-term air pollution exposure has been associated with cognitive impairment, neuroinflammation, and neurodegeneration (17).
Environmental factors in the form of air pollution may increase the risk of OSA and may therefore contribute to sleep health disparities. Prior environmental studies of sleep have predominantly focused on features of the social environment such as low social cohesion, crowding, and neighborhood disorder (19, 35, 36) as explanatory factors contributing to poor sleep in low-SES neighborhoods and sleep disparities. More recently, built-environment and neighborhood-walkability features have been associated with sleep apnea (37). Neighborhood disadvantage, as quantified by census-track indicators of poverty, low education attainment, and family structure, has been associated with an increased risk of sleep apnea in children (38–40), but not yet in adults. These pediatric studies postulated that geographic differences in sleep disorders may relate to air quality, but did not have data to address this hypothesis.
Our results are consistent with the few prior studies evaluating air pollution and sleep. A Swiss study found an association of sleep disturbance, measured by electrocardiogram features, with proximity to roadways (41). Using Sleep Heart Health Study data, Zanobetti and colleagues reported an association of short-term PM10 with an elevation in AHI and reduced sleep efficiency particularly in the summer (15). Greater air pollution (NO2 and PM2.5) exposure in Taiwan was also associated with a greater AHI and oxygen desaturation index, with a similar seasonal component of spring and winter (42). Elevated short-term ozone and temperature were associated with a higher AHI in a European study (43). Several studies also have reported associations with meteorological conditions such as temperature and humidity (44). These studies reported a strong seasonal variation, perhaps reflecting an interaction of pollution with air temperature, environmental allergens such as pollens, and humidity levels. We found a seasonal variation in AHI but no interaction with the association of long-term air pollution exposure and sleep apnea. In this study, we used year-long averages in multiple geographical locations with state-of-the-art modeling to minimize the effect of these regional and temporal variations on pollution.
We observed differences in associations of air pollution with our sleep outcomes depending on exposure durations (daily, annual, and 5-year). Whether this relates to differences in the biological effects of different exposures or to measurement issues is not clear. The lack of clear associations of sleep apnea with 5-year average and short-term PM2.5 exposure may be a falsely null hypothesis due to the overall high prevalence of OSA, the lack of air pollution variation within cities, and the overall low burden of ambient air pollution exposures compared with other urban environments worldwide (45), or to other measurement issues. For example, short-term exposures were citywide measures because it was not possible to obtain individual-level estimates. The null findings for short-term exposure estimates also may reflect the importance of longer-term exposures causing chronic changes in the airway, brain, or other tissues. The stronger association between 1-year PM2.5 compared with 5-year PM2.5 exposure estimates similarly may reflect more airway adaptations with chronic higher pollution exposure over 5 years.
Our primary outcome was based on OSA as defined using the more sensitive American Academy of Sleep Medicine (AASM) definition of hypopnea (46) rather than the strict ≥4% oxygen desaturation hypopnea criterion specified by the CMS. The CMS AHI has been criticized because it may lead to underdiagnosis in women and nonobese individuals, who are less likely to experience deep desaturations with events (47). When we used the more stringent definition of hypopnea (≥4% desaturation), we observed similar associations for PM2.5 but less significant associations for NO2, perhaps reflecting the distinct impacts of these pollutants on the airway, with one leading to more desaturation and the other contributing to arousals. This is consistent with the finding of association of lower sleep efficiency with higher NO2 exposure levels. These differences may reflect the more subtle pathological effects of air pollution on the upper airway, which are detectable only with the more sensitive AASM definition of hypopnea.
The strengths of this study include the detailed individual air pollution metrics with spatiotemporal modeling, the diversity of the subjects (in terms of both racial/ethnic background and geography), the large sample of subjects gathered from the community, and the use of objective sleep measures. The limitations of this study include its observational nature and the fact that sleep was assessed at only one time point, which limited our ability to evaluate causality and gain a full understanding of how exposure durations influence sleep. Pollution exposure differed substantially by site, with low exposures in some areas, which made it difficult to separate site effects from pollution effects. Site was also highly colinear with other sociodemographic features (race/ethnicity and SES). In addition, the MESA participants in our cohort were older, with a mean age of 68.5 years, and our findings may not be generalizable to younger individuals. Although our sample was diverse, there was inadequate power to test for race differences. We adjusted for many measured potential confounders, but other factors, including environmental features associated with air pollution, such as noise and light pollution, may explain the observed relationship with sleep disruption. Noise from traffic is also a known sleep disruptor and has been associated with cortical arousals, sleep fragmentation, insomnia, and self-reported poor sleep (48). Traffic noise rather than traffic-associated pollution (NO2) may explain some of our observed associations, although relationships remained robust in sensitivity models that adjusted for road proximity. Urban living has additional sleep disruptors, such as light pollution (49), which can impact circadian rhythm and melatonin excretion, and result in sleep onset delay and fragmentation.
Although prior studies have largely focused on individual risk factors for sleep apnea, our data suggest that environmental features also contribute to variation in sleep disorders across groups. This has implications for regulatory standards, public health, environmental justice, and health disparities, as higher levels of air pollution are more prevalent in poor, urban areas, as seen in this MESA cohort (50). Populations residing in cities with ambient air pollution above World Health Organization levels may have a greater risk of sleep apnea and sleep disruption, in addition to the other known effects of air pollution on mortality; cardiovascular, pulmonary, and neurodegenerative diseases; and cancer risk (7, 45, 51–52). Efforts to improve air quality could also improve sleep health, potentially decreasing the prevalence and severity of sleep apnea. Furthermore, air quality improvements may reduce sleep health disparities as the poor may be particularly susceptible to air pollution, with less access to protection indoors, lacking air conditioners, air filtration systems, relying on open windows and having greater occupational exposure to the outdoors.
Conclusions
This study demonstrates an association of ambient air pollution exposure with sleep apnea. Chronic exposure to greater levels of air pollution may adversely influence breathing during sleep, suggesting possible etiologies of sleep health disparities. Future studies are needed to distinguish the effects of specific air pollutants from those of other neighborhood and regional features, to explore possible mechanisms, and to evaluate whether improving air quality improves sleep health.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. Additional funding was provided by NHLBI R01 R098433 for MESA Sleep (Redline). This publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Author Contributions: M.E.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.S., N.J., A.G., J.D.K., and S.R.R. contributed substantially to data collection and analysis. M.E.B., D.G., C.P.A., P.J.L., and S.R.R. contributed substantially to the study design, data interpretation, and writing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23:S122–S126. [PubMed] [Google Scholar]
- 4.Gold DR, Mittleman MA. New insights into pollution and the cardiovascular system: 2010 to 2012. Circulation. 2013;127:1903–1913. doi: 10.1161/CIRCULATIONAHA.111.064337. [DOI] [PubMed] [Google Scholar]
- 5.Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188:593–599. doi: 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Goldberg MS, Burnett RT, Jerrett M, Wheeler AJ, Villeneuve PJ. Long-term exposure to traffic-related air pollution and cardiovascular mortality. Epidemiology. 2013;24:35–43. doi: 10.1097/EDE.0b013e318276c005. [DOI] [PubMed] [Google Scholar]
- 7.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the Medicare population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham Heart Study. Am J Respir Crit Care Med. 2015;191:656–664. doi: 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med. 2014;190:914–921. doi: 10.1164/rccm.201403-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoni M, Baldacci S, Maio S, Cerrai S, Sarno G, Viegi G. Adverse effects of outdoor pollution in the elderly. J Thorac Dis. 2015;7:34–45. doi: 10.3978/j.issn.2072-1439.2014.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laumbach RJ, Kipen HM. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol. 2012;129:3–11, quiz 12–13. doi: 10.1016/j.jaci.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med. 2016;22:138–143. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trevino RJ. Air pollution and its effect on the upper respiratory tract and on allergic rhinosinusitis. Otolaryngol Head Neck Surg. 1996;114:239–241. doi: 10.1016/S0194-59989670174-2. [DOI] [PubMed] [Google Scholar]
- 14.DeMeo DL, Zanobetti A, Litonjua AA, Coull BA, Schwartz J, Gold DR. Ambient air pollution and oxygen saturation. Am J Respir Crit Care Med. 2004;170:383–387. doi: 10.1164/rccm.200402-244OC. [DOI] [PubMed] [Google Scholar]
- 15.Zanobetti A, Redline S, Schwartz J, Rosen D, Patel S, O’Connor GT, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med. 2010;182:819–825. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajat A, Hsia C, O’Neill MS. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep. 2015;2:440–450. doi: 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calderón-Garcidueñas L, Leray E, Heydarpour P, Torres-Jardón R, Reis J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Rev Neurol (Paris) 2016;172:69–80. doi: 10.1016/j.neurol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Desantis AS, Diez Roux AV, Moore K, Baron KG, Mujahid MS, Nieto FJ. Associations of neighborhood characteristics with sleep timing and quality: the Multi-Ethnic Study of Atherosclerosis. Sleep (Basel) 2013;36:1543–1551. doi: 10.5665/sleep.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson DA, Simonelli G, Moore K, Billings M, Mujahid MS, Rueschman M, et al. The neighborhood social environment and objective measures of sleep in the Multi-Ethnic Study of Atherosclerosis. Sleep (Basel) 2017;40:40. doi: 10.1093/sleep/zsw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonelli G, Dudley KA, Weng J, Gallo LC, Perreira K, Shah NA, et al. Neighborhood factors as predictors of poor sleep in the Sueño Ancillary Study of the Hispanic Community Health Study/Study of Latinos. Sleep (Basel) 2017;40 doi: 10.1093/sleep/zsw025. doi: 10.1093/sleep/zsw025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings ME, Leary PJ, Gold D, Aaron CP, Kaufman J, Redline S. Relationship of air pollution to sleep disordered breathing and sleep disruption: the multi-ethnic study of atherosclerosis sleep and air studies [abstract] Sleep. 2014;37:0309. [Google Scholar]
- 22.Billings ME, Gold DR, Leary PJ, Szpiro A, Aaron CP, Kaufman JD, et al. The relationship of air pollution to sleep disruption: the multi-ethnic study of atherosclerosis (MESA) sleep and MESA-air studies [abstract] Am J Respir Crit Care Med. 2017;195:A2930. [Google Scholar]
- 23.Keller JP, Olives C, Kim SY, Sheppard L, Sampson PD, Szpiro AA, et al. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect. 2015;123:301–309. doi: 10.1289/ehp.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szpiro AA, Sheppard L, Adar SD, Kaufman JD. Estimating acute air pollution health effects from cohort study data. Biometrics. 2014;70:164–174. doi: 10.1111/biom.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep (Basel) 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27.Sleep-related breathing disorders in adults. recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 28.Gulisano M, Marceddu S, Barbaro A, Pacini A, Buiatti E, Martini A, et al. Damage to the nasopharyngeal mucosa induced by current levels of urban air pollution: a field study in lambs. Eur Respir J. 1997;10:567–572. [PubMed] [Google Scholar]
- 29.Ramanathan M, Jr, London NR, Jr, Tharakan A, Surya N, Sussan TE, Rao X, et al. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am J Respir Cell Mol Biol. 2017;57:59–65. doi: 10.1165/rcmb.2016-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng B, Zhang X, Yi C, Zhang Y, Ye S, Wang Y, et al. The association between ambient air pollution and allergic rhinitis: further epidemiological evidence from Changchun, Northeastern China. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14030226. pii: E226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YR, Xiao CC, Li J, Tang J, Geng XY, Cui LJ, et al. Association between air pollution and upper respiratory tract infection in hospital outpatients aged 0-14 years in Hefei, China: a time series study. Public Health. 2018;156:92–100. doi: 10.1016/j.puhe.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Tam WW, Wong TW, Ng L, Wong SY, Kung KK, Wong AH. Association between air pollution and general outpatient clinic consultations for upper respiratory tract infections in Hong Kong. PLoS One. 2014;9:e86913. doi: 10.1371/journal.pone.0086913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong Z, Guo Z, Zhang R, Xu J, Dong W, Zhuang G, et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016;239:117–125. doi: 10.1620/tjem.239.117. [DOI] [PubMed] [Google Scholar]
- 34.Simoni M, Baldacci S, Maio S, Cerrai S, Sarno G, Viegi G. Adverse effects of outdoor pollution in the elderly. J Thorac Dis. 2015;7:34–45. doi: 10.3978/j.issn.2072-1439.2014.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health Place. 2009;15:1006–1013. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DA, Drake C, Joseph CL, Krajenta R, Hudgel DW, Cassidy-Bushrow AE. Influence of neighbourhood-level crowding on sleep-disordered breathing severity: mediation by body size. J Sleep Res. 2015;24:559–565. doi: 10.1111/jsr.12305. [DOI] [PubMed] [Google Scholar]
- 37.Billings ME, Johnson DA, Simonelli G, Moore K, Patel SR, Diez Roux AV, et al. Neighborhood walking environment and activity level are associated with OSA: the Multi-Ethnic Study of Atherosclerosis. Chest. 2016;150:1042–1049. doi: 10.1016/j.chest.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spilsbury JC, Storfer-Isser A, Kirchner HL, Nelson L, Rosen CL, Drotar D, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Dong Y, Weng J, Kontos EZ, Chervin RD, Rosen CL, et al. Associations among neighborhood, race, and sleep apnea severity in children. A six-city analysis. Ann Am Thorac Soc. 2017;14:76–84. doi: 10.1513/AnnalsATS.201609-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. 2011;158:789–795.e1. doi: 10.1016/j.jpeds.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Gerbase MW, Dratva J, Germond M, Tschopp JM, Pépin JL, Carballo D, et al. Sleep fragmentation and sleep-disordered breathing in individuals living close to main roads: results from a population-based study. Sleep Med. 2014;15:322–328. doi: 10.1016/j.sleep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Shen YL, Liu WT, Lee KY, Chuang HC, Chen HW, Chuang KJ. Association of PM2.5 with sleep-disordered breathing from a population-based study in Northern Taiwan urban areas. Environ Pollut. 2018;233:109–113. doi: 10.1016/j.envpol.2017.10.052. [DOI] [PubMed] [Google Scholar]
- 43.Weinreich G, Wessendorf TE, Pundt N, Weinmayr G, Hennig F, Moebus S, et al. Heinz Nixdorf Recall study group. Association of short-term ozone and temperature with sleep disordered breathing. Eur Respir J. 2015;46:1361–1369. doi: 10.1183/13993003.02255-2014. [DOI] [PubMed] [Google Scholar]
- 44.Cassol CM, Martinez D, da Silva FABS, Fischer MK, Lenz MDCS, Bós ÂJG. Is sleep apnea a winter disease?: meteorologic and sleep laboratory evidence collected over 1 decade. Chest. 2012;142:1499–1507. doi: 10.1378/chest.11-0493. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. Ambient air pollution: a global assessment of exposure and burden of disease. 2016 [accessed 2019 Jan 23]. Available from: http://www.who.int/phe/health_topics/outdoorair/en/
- 46.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campos-Rodriguez F, Martínez-García MA, Reyes-Nuñez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med. 2016;27-28:54–58. doi: 10.1016/j.sleep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Kim M, Chang SI, Seong JC, Holt JB, Park TH, Ko JH, et al. Road traffic noise: annoyance, sleep disturbance, and public health implications. Am J Prev Med. 2012;43:353–360. doi: 10.1016/j.amepre.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015;32:1294–1310. doi: 10.3109/07420528.2015.1073158. [DOI] [PubMed] [Google Scholar]
- 50.Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2013;121:1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Health effects of outdoor air pollution. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 52.Farmer SA, Nelin TD, Falvo MJ, Wold LE. Ambient and household air pollution: complex triggers of disease. Am J Physiol Heart Circ Physiol. 2014;307:H467–H476. doi: 10.1152/ajpheart.00235.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.