Abstract
Rationale: A large portion of asthma morbidity occurs in low- and middle-income countries, and Peru suffers particularly high asthma prevalence. Ambient air exposures are also high, and likely play a role. Most studies of environmental exposures focus on understanding contributors to health care utilization or mortality risk; however, less severe outcomes may still impact quality of life (QOL).
Objectives: To study the association between multiple pollutants and several asthma domains in Peruvian children.
Methods: A total of 484 children aged 9–19 years with asthma were followed for 6–9 months, and evaluated for asthma control, asthma-related QOL, missed school days, and health care utilization. We used geographically distributed monitors to estimate air pollutant concentrations and multivariable generalized linear mixed models to model asthma outcomes as a function of pollutants.
Results: A total of 67% of children had moderate to severe persistent asthma. In multipollutant models, higher particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5), black carbon, and nitrogen dioxide concentrations were independently associated with worse asthma control. For each interquartile range increase in PM2.5 or nitrogen dioxide concentration, there was a 59% or 34% higher odds of uncontrolled asthma, respectively. If the proportion of PM2.5 that was black carbon increased, there were increased odds of uncontrolled asthma. Similarly, pollutants were independently associated with worse asthma-related QOL, and PM exposure was associated with increased risk of health care utilization.
Conclusions: Our study highlights the importance of pollutant exposures on multiple domains of asthma morbidity among Peruvian children, including not only acute exacerbations, but also on general asthma burden, such as worse asthma symptom control and QOL.
Keywords: asthma, air pollution, children, particulate matter, nitrogen dioxide
Asthma is the most common chronic disease in children and over 80% of asthma-related deaths occur in low- and middle-income countries (LMICs) (1). Peru is reported to have a high prevalence of asthma in children, with estimates of asthma prevalence ranging from 14% to 33% based on previous studies (2–4), yet there remains limited information regarding the specific factors driving the asthma epidemic in this population. We have previously shown that Peruvian children living in households closer to a main roadway with a larger burden of diesel traffic had higher odds of current asthma symptoms (5), highlighting the potential of ambient and vehicular traffic air pollution to influence asthma control.
Epidemiological research supports the link between urban air pollutant exposures and exacerbations of asthma symptoms in children, but many of these studies focus on health care utilization, such as emergency department (ED) visits or hospitalizations, or exacerbation risk (6–10). Studies addressing the association between ambient air pollution and less severe, but impactful, asthma outcomes, such as asthma control and health-related quality of life (QOL) have been limited, and have provided less consistent results (11–15). Furthermore, air pollution exposure is likely a strong contributor to high and increasing asthma severity in LMICs due to recent urbanization (16).
We sought to evaluate the impact of several ambient air pollutants, including particulate matter (PM), black carbon (BC), and NO2, on several domains of asthma morbidity (i.e., asthma control, asthma-related QOL, missed school days, and health care utilization) in a cohort of Peruvian children. To achieve this objective, we conducted longitudinal environmental exposure assessments within a small geographic area using multiple study-specific monitoring sites and spatial interpolation as well as validated asthma assessment instruments to understand the contribution of gaseous and particulate ambient air pollutants and their potential joint effects on pediatric asthma morbidity.
Methods
Study Setting and Design
We conducted a longitudinal study of children with asthma as part of the GASP (Genetic Asthma Susceptibility to Indoor Pollution in Peru) study. Children aged 9–19 years were recruited from two adjacent periurban communities in Lima, Pampas de San Juan de Miraflores (Pampas), and Villa El Salvador (Villa), between 2011 and 2014. Participant homes were located on both paved and unpaved streets at varying distances from highly trafficked roadways used primarily by diesel-fueled vehicles (e.g., commuter buses and motorbike taxis) and secondarily by gas-fueled personal motor vehicles. Other sources of ambient pollution include local industrial facilities, residential construction, and trash burning. Although the annual average precipitation in Lima is less than 50 mm per year, the city has high cloud cover for 9 months of the year.
Children with a physician diagnosis of asthma and determined to have current or active asthma as determined by either reporting taking medications for asthma or having active symptoms over the past year were recruited for longitudinal follow-up similar to previous asthma epidemiological studies (17, 18). Children were excluded if they had a history of chronic respiratory conditions other than asthma. Children with active asthma were followed for a total of 9 months in Pampas and 6 months in Villa. Informed consent was obtained from parents or guardians for participants younger than 18 years of age. The study was approved by the Institutional Review Boards of the Johns Hopkins School of Medicine in Baltimore, Maryland, and Asociacion Benefica PRISMA, in Lima, Peru.
Sociodemographics and Characterization of Clinical Outcomes
Baseline questionnaires collecting demographic information, personal and family histories, and asthma status were administered to all children in the presence of caregivers. We created a socioeconomic status score (SES) score based upon a principal component analysis of 12 households assets, 1 household size variable, and 2 variables related to years of parental education, with lower SES score representing higher levels of poverty. Asthma severity was classified according to the National Asthma Education and Prevention Program (NAEPP)-3 guidelines (19). Baseline anthropometry, including height and weight, was based on the average of two repeated measurements, and lung function was assessed using a flow-based portable spirometer (SpiroPro, Jaeger/Cardinal Health, Hoechberg, Germany) according to American Thoracic Society/European Respiratory Society guidelines (20). We determined predicted values and z-scores using multiethnic reference values derived by the Global Lung Health Initiative (21). Using fluorescent enzyme immunoassay (ImmunoCAP250; Phadia), a positive IgE antibody response (IgE level > 0.1 kU/L) to any of the following three mixes indicated atopy: 1) mold allergens (Penicillium notatum, Cladosporium herbarum, Aspergillus fumigatus, Candida albicans, Alternaria alternate/tenius, Helminthospoirum halodes); 2) common house allergens (house dust Hollister-Stier, Dermatophagoides pteronyssinus, Dermatophagoides farina; Blatella germanica, cockroach); and 3) animal epidermal allergens (cat dander and epithelium, dog dander, guinea pig epithelium, rat, and mouse).
Asthma Control
Children were assessed for asthma control monthly (with assessment of asthma control over the previous 4 wk) using the validated Spanish language versions of the pediatric Asthma Control Test (ACT) for children aged 9–11 years and the ACT for adolescents aged 12–19 years (22–24). Therefore, each child had the ACT administered up to nine times (monthly for a 9-mo follow-up period). Asthma control was classified as follows: a score of 20 or greater represents well-controlled asthma and a score less than 20 represents uncontrolled asthma.
Asthma QOL
The Spanish-Peruvian version of the validated Pediatric Asthma Quality of Life Questionnaire (PAQLQ) for children aged 7–17 years by Juniper (25) was also administered monthly, and had a 1-week recall. It was designed to measure functional problems (physical, emotional, and social) and their impact on children with asthma (26, 27). This questionnaire uses a seven-point scale for each of 23 items, where 1 represents severe impairment and 7 represents no impairment during the previous week. The overall PAQLQ score is the mean of 23 responses; total scores were dichotomized into no impairment (score = 7) or some degree of impairment (score <7).
Health Care Utilization and Missed School Days
Information on health care utilization and missed school days was collected by self-report every 2 weeks. Health care utilization was defined as an ED visit or hospitalization due to asthma, and was treated as a binary outcome.
Environmental Assessment
To account for the range of participant exposures in Pampas and Villa, weekly time-weighted average ambient concentrations of PM with aerodynamic diameter less than 2.5 μm (PM2.5), BC, NO2, temperature, and humidity were measured continuously at 15 residential locations spatially distributed throughout each community (n = 30 total) and at varying distances from highly trafficked main roads to cover the entire time period that participants were followed at each location. Monitoring equipment was located on the roofs of houses in a secure and weather-protected environment. PM2.5 was collected gravimetrically with a personal DataRam (pDR model 1200; Thermo Scientific) and a BGI 400 Personal Air Sampling Pump (BGI Inc.) set to a flow rate of 4 L/min. The flow rate was verified before and after sampling using a BIOS Defender 530 calibrator (BIOS International). Each pDR was equipped with a PM2.5 cyclone inlet and had a cassette with a 37-mm Teflon filter (Pall Corp.) downstream of the nephelometer. All filters were pre- and post-weighed at the Johns Hopkins Bloomberg School of Public Health using a Mettler-Toledo UMX2 microbalance (Mettler-Toledo, Inc.). Filters were collected over 1-week periods continuously throughout the study period. Total BC was assessed from the same 37-mm Teflon PM2.5 filters collected using the pDR 1200 and analyzed using the Magee OT-21 SootScan Model Transmissometer (Magee Scientific Corp.). The proportion of PM2.5 that was BC (hereafter, “proportion BC”) was also calculated. NO2 was measured with passive detectors according to standard methods (28–30). We measured ambient temperature and relative humidity in 5-minute intervals for the entire period of sampling using HOBO Data Loggers (Onset Corp.).
Longitude and latitude were recorded for all participating households and pollution monitors. Average measurements for each monitor were determined for the 28-day period before outcomes with a 4-week recall (asthma control and QOL), and a 14-day period for the outcomes with a 2-week recall (health care utilization and missed school days). Using an inverse distance weighting approach, the final individual-level exposure was estimated as a distance-weighted average of measured concentrations at the 15 nearby monitors within each community for each outcome-specific time period.
Biostatistical Analysis
Our primary aim was to determine the relationship between air pollutants (PM2.5, NO2, and BC) and several asthma outcomes. We used a random-effects logistic regression model with a random intercept to evaluate subject-specific associations of air pollutant concentrations with the odds of self-reported uncontrolled asthma as determined by the ACT (as the primary outcome). Initially, unadjusted single-pollutant models estimated the overall association of PM2.5, total BC, and NO2 with the odds of uncontrolled asthma. Next, multivariable multipollutant models were used to evaluate the independent effects of each pollutant after adjustment for other pollutants. These models included PM2.5, proportion BC, and NO2. A priori–specified multivariable models were fitted to adjust for the following baseline variables: age; sex; SES; Cole body mass index cutoff for international, adolescent population (normal, overweight, or obese) (31); forced expiratory volume in 1 second (FEV1) z-score; study site (Pampas vs. Villa); and 2-week or 4-week average ambient temperature (as a proxy for season). Results of regression models were presented as the subject-specific odds ratios (ORs) and corresponding 95% confidence intervals (CIs) per interquartile range (IQR) increase in each pollutant. The association between ambient air pollution concentrations and asthma QOL, health care utilization, and missed school days was also investigated, both in single-pollutant and in multipollutant adjusted models.
Sensitivity analyses were conducted: 1) considering ACT and PAQLQ as continuous measures; and 2) additionally adjusting primary models for atopy, use of inhaled corticosteroids (ICS), and household smoke exposure. All analyses were conducted using STATA, Version 14.1 (STATACorp.). P values of less than 0.05 were considered statistically significant.
Results
Participant and Environmental Characteristics
Children with active asthma (n = 484) were followed longitudinally across both sites, and all but one (missing either exposure or outcome data at all time points) were included in the analyses (259 children were residents of Pampas, and 224 resided in Villa). Overall, children had a mean age of 13.2 years and 55% were female. One-third of the children had mild persistent asthma, 45% had moderate persistent asthma, and 22% had severe persistent asthma. Although over two-thirds of participants had moderate or severe persistent asthma, only 4% of them were using controller inhalers. The majority of children (77%) had allergic sensitization to at least one of the three allergen mixtures. Participant characteristics in Pampas and Villa were not statistically different, except for SES and body mass index, which were lower in Pampas (Table 1).
Table 1.
Baseline participant characteristics
| Characteristics | Overall (n = 483) | Pampas (n = 259) | Villa (n = 224) |
|---|---|---|---|
| Demographic characteristics | |||
| Age, yr | 13.2 ± 2.55 | 13.5 ± 2.60 | 12.9 ± 2.47 |
| Male | 44.3 | 39.4 | 50.0 |
| SES score | 0.27 ± 1.69 | −0.34 ± 1.64 | 0.97 ± 1.44 |
| Number of follow-up visits | 11.1 ± 3.08 | 13.3 ± 2.43 | 8.5 ± 1.14 |
| Household smoking | 11.8 | 11.3 | 12.6 |
| Clinical characteristics | |||
| Cole BMI | |||
| Normal | 47.0 | 52.1 | 41.1 |
| Overweight | 32.9 | 31.7 | 34.4 |
| Obese | 20.1 | 16.2 | 24.6 |
| Baseline FEV1 % predicted | 105.5 ± 14.2 | 105.1 ± 14.4 | 105.9 ± 14.0 |
| Baseline FEV1, raw | 2.8 ± 0.77 | 2.8 ± 0.81 | 2.8 ± 0.73 |
| Baseline FEV1, z-score | 1.1 ± 1.31 | 1.1 ± 1.33 | 1.2 ± 1.29 |
| Asthma severity | |||
| Mild intermittent/mild persistent | 33.8 | 36.7 | 30.1 |
| Moderate persistent | 44.7 | 43.9 | 45.7 |
| Severe persistent | 21.5 | 19.4 | 24.2 |
| Inhaled corticosteroid use | 4.0 | 3.7 | 4.5 |
| Long-acting β agonist use | 0.4 | 0.2 | 0.2 |
| Atopy | 77.3 | 79.6 | 74.9 |
Definition of abbreviations: BMI = body mass index; FEV1 = forced expiratory volume in 1 second; SD = standard deviation; SES = socioeconomic status score.
Data are presented as % or mean ± SD.
Mean (SD) PM2.5, BC, and NO2 concentrations during the study period (June 2012 to October 2014) were 21.2 μg/m3 (3.19), 4.4 μg/m3 (1.53), and 10.9 ppb (0.81), respectively. Median (IQR) PM2.5, BC, and NO2 concentrations during the study period were 21.6 μg/m3 (18.2–23.4), 3.8 μg/m3 (3.0–5.8), and 11.0 ppb (10.5–11.5), respectively. Mean (SD) temperature and humidity (SD) during the study period were 21.2°C (2.44) and 74.5% (4.45), respectively. The correlation (r) between PM2.5 and total BC was 0.40 with lower correlation between PM2.5 and proportion BC (r = 0.15). Similarly, the correlations between NO2 and PM2.5 (r = 0.38) or NO2 and proportion BC (r = 0.19) were statistically significant (P < 0.001).
Association of Pollutants with Asthma Control
In longitudinal, single-pollutant, unadjusted models, higher concentrations of PM2.5 and NO2 were associated with higher odds of having uncontrolled asthma (Table 2). In multipollutant models, we found that all pollutants were independently associated with higher subject-specific odds of uncontrolled asthma after adjustment for confounders (Table 2). Specifically, for an IQR increase in PM2.5 concentrations, there was almost a 60% increase in subject-specific odds of uncontrolled asthma (OR = 1.59, 95% CI = 1.26–2.00). In addition, as the proportion of PM attributed to BC increased, there was increased risk of uncontrolled asthma. There was also a 34% higher subject-specific odds of uncontrolled asthma for each IQR increase in NO2 concentration. Conclusions were similar if analyzing asthma control as a continuous measure. Furthermore, sensitivity analyses after adjustment for atopy, ICS, and household smoking, PM and NO2 exposure continued to be associated with worse asthma control and, although there was still a trend for proportion of BC to contribute to worse asthma control, it was no longer statistically significant (see Tables E1 and E2 in the online supplement).
Table 2.
Single and multipollutant random effects logistic regression models: association of particulate matter, black carbon, and nitrogen dioxide with odds of reporting uncontrolled asthma
| Air Pollutants | Single-Pollutant Unadjusted OR (95% CI) | Multipollutant Adjusted OR (95% CI)* |
|---|---|---|
| PM2.5 | 1.27 (1.12–1.45) | 1.59 (1.26–2.00) |
| Total BC | 1.31 (0.97–1.78) | — |
| Proportion BC | — | 4.89 (1.64–14.62) |
| NO2 | 1.86 (1.53–2.25) | 1.34 (1.05–1.71) |
Definition of abbreviations: BC = black carbon; CI = confidence interval; NO2 = nitrogen dioxide; OR = odds ratio; PM2.5 = particulate matter <2.5 μg in aerodynamic diameter.
Adjusted models include the following covariates: age (continuous), sex (male vs. female), socioeconomic status score, body mass index (normal, overweight, obese), baseline forced expiratory volume z-score, site (Pampas, Villa), temperature (°C), and each pollutant. Proportion BC indicates the proportion of PM2.5 that was BC.
Association of Pollutants with Asthma QOL
In single-pollutant, unadjusted models, all measured pollutants (PM2.5, BC, and NO2) were associated with higher odds of having poor asthma-related QOL, as measured by the PAQLQ.
In adjusted multipollutant models, we found that both PM2.5 and NO2 were independently associated with worse asthma-related QOL (Table 3). Specifically, every IQR increase in PM2.5 concentration was associated with higher odds of having poor asthma-related QOL (OR = 1.34; 95% CI = 1.15–1.56). Similarly, higher NO2 concentrations were associated with worse QOL. There was a trend for proportion BC to be linked to worse QOL, although this did not reach statistical significance. Conclusions were similar after adjustment for atopy, smoking status and medication use or if analyzing asthma-related QOL as a continuous measure (see Tables E1 and E2).
Table 3.
Random effects logistic regression equations evaluating the association of multiple pollutants with the odds of reporting adverse asthma-related quality of life: single and multipollutant models
| Air Pollutants | Single-pollutant Unadjusted OR (95% CI) | Multipollutant Adjusted OR (95% CI)* |
|---|---|---|
| PM2.5 | 1.19 (1.09–1.29) | 1.34 (1.15–1.56) |
| BC | 1.20 (1.02–1.42) | — |
| Proportion BC | — | 1.79 (0.96–3.36) |
| NO2 | 2.12 (1.88–2.38) | 1.58 (1.36–1.83) |
Definition of abbreviations: BC = black carbon; CI = confidence interval; NO2 = nitrogen dioxide; OR = odds ratio; PM2.5 = particulate matter <2.5 μg in aerodynamic diameter.
Adjusted models include the following covariates: age (continuous), sex (male vs. female), socioeconomic status score, body mass index (normal, overweight, obese), baseline forced expiratory volume z-score, site (Pampas, Villa), temperature (°C), and each pollutant. Proportion BC indicates the proportion of PM2.5 that was BC.
Health Care Utilization and School Days Missed
In single-pollutant unadjusted models, higher levels of all measured pollutants (PM2.5, BC, and NO2) displayed a trend toward higher health care utilization, and BC and NO2 exposure were significantly associated with missed school days. In multipollutant models, after adjustment for confounders, both an increase in PM2.5 and an increase in the proportion of BC were significantly associated with increased odds of recent health care utilization. Specifically, an IQR increase in PM2.5 concentrations was associated with a 44% increased odds of health care utilization.
These results showed a similar trend and effect sizes for increased odds of missed school days in multipollutant models, but pollutants were not linked to statistically significant increased odds of missing school after adjustment for confounders (Table 4).
Table 4.
Logistic random effects regression evaluating the association of multiple pollutants with the odds of reporting health care utilization and missed school days
| Air Pollutants | Single-Pollutant Unadjusted OR (95% CI) | Multipollutant Adjusted OR (95% CI)* |
|---|---|---|
| Health care utilization | ||
| PM2.5 | 1.00 (0.84–1.21) | 1.44 (1.08–1.92) |
| BC | 1.17 (0.80–1.71) | — |
| Proportion BC | — | 4.06 (1.38–11.95) |
| NO2 | 1.21 (0.98–1.49) | 0.95 (0.74–1.21) |
| Missed school days | ||
| PM2.5 | 1.16 (0.94–1.42) | 1.32 (0.96–1.82) |
| BC | 1.59 (1.01–2.50) | — |
| Proportion BC | — | 3.12 (0.91–10.66) |
| NO2 | 1.38 (1.08–1.75) | 1.21 (0.91–1.60) |
Definition of abbreviations: BC = black carbon; CI = confidence interval; NO2 = nitrogen dioxide; OR = odds ratio; PM2.5 = particulate matter <2.5 μg in aerodynamic diameter.
Adjusted models include the following covariates: age (continuous), sex (male vs. female), socioeconomic status score, body mass index (normal, overweight, obese), baseline forced expiratory volume z-score, site (Pampas, Villa), temperature (°C), and each pollutant. Proportion BC indicates the proportion of PM2.5 that was BC.
Results were similar after adjustment for atopy, ICS, and household smoking status (see Table E2).
Discussion
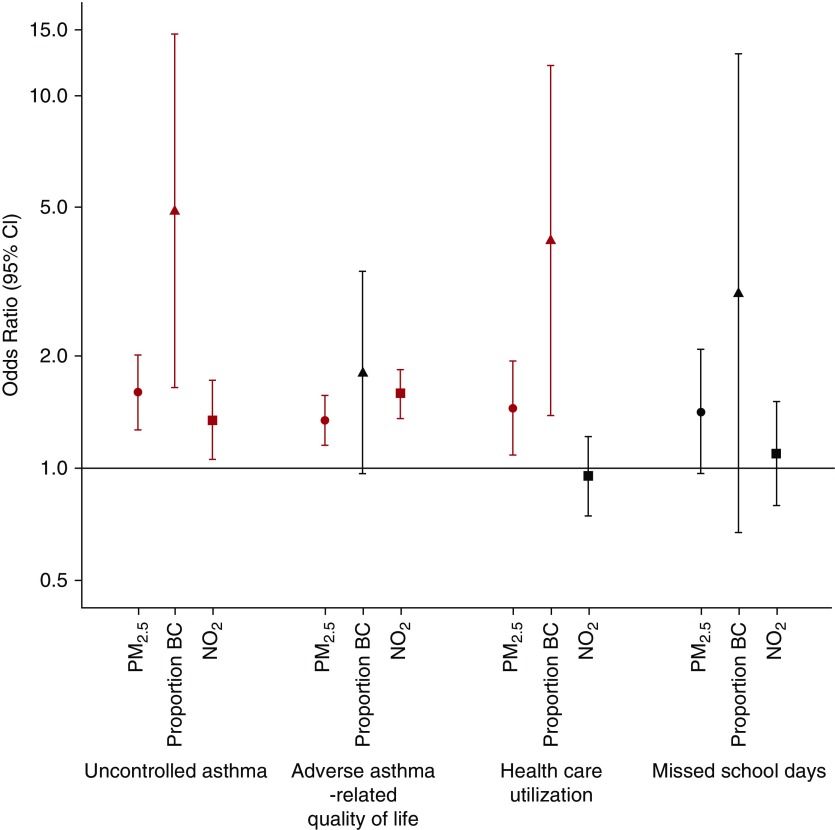
We found high ambient pollutant concentrations in Lima well beyond recommended international guidelines, and we highlight the clinical impact that elevated exposures to ambient pollutants, including PM, BC, and NO2, can have on several domains of asthma morbidity in children living in Lima. By using person-specific spatiotemporal estimates of exposure, our study results underscore the harmful impact of ambient pollution on multiple domains of asthma health in children in addition to health care utilization, including asthma control, asthma-related QOL, and trends toward increased missed school days (Figure 1).
Figure 1.
Association of ambient pollutants (particulate matter <2.5 μg in aerodynamic diameter [PM2.5], proportion black carbon [BC], and NO2) with odds of reporting uncontrolled asthma, adverse asthma-related quality of life, health care utilization, and missed school days. Multiple-pollutant random effects logistic regression models adjusted for age, sex, socioeconomic status score (SES), body mass index (BMI), baseline forced expiratory volume in 1 second (FEV1) z-score, site, and temperature (°C). In the y-axis, we plot odds ratios and corresponding 95% confidence intervals (CI) for uncontrolled asthma, adverse asthma-related quality of life, health care utilization, and missed school days to reflect an interquartile range increase in each pollutant.
The adverse effects of air pollution on asthma health have been shown in several studies. Studies suggest that, even at relatively low concentrations, primary pollutants from traffic sources contribute to the burden of pediatric asthma. However, to date, most studies have focused on the impact of pollutants on ED visits, hospitalizations, or specific asthma symptoms (6–10), rather than general measures of asthma burden, such as asthma control and QOL, using validated questionnaires. Furthermore, most environmental studies were focused on North American or European countries or LMICs with high biomass exposure (32, 33). Fewer have focused on understanding the impact of ambient pollution on urban communities in LMICs in South America, which suffer from high ambient pollutant exposures due to urbanization, and which may have unique cultural contexts (34). Understanding the impact of ambient exposures in Peru is particularly important, as it also suffers from particularly high asthma burden in urban cities like Lima (22.6% of adolescents), even when compared with other LMICs (35).
Ambient pollutant concentrations from our current study in Lima (mean PM2.5 = 21.2 μg/m3) were higher than most U.S. cities and double the World Health Organization annual recommended levels (10 μg/m3) (36). This is not surprising, given that people living in LMICs disproportionately experience higher levels of outdoor air pollution (37). However, the high ambient pollutant concentrations may also partly explain why the impact of ambient pollutants in Lima contribute such a substantial impact on asthma morbidity. Importantly, we show harmful effects of ambient pollution on multiple domains of asthma health in children from Lima. Specifically, we demonstrate that, in addition to contributing to increased health care utilization, ambient air pollution can lead to worse symptom control and lower asthma-related QOL, both of which may have a more substantial impact on the daily life of children with asthma compared with infrequent health care utilization events. We estimate that an IQR increase in PM2.5 exposure, which, in this cohort, corresponded to a 5.2 μg/m3 increase in PM2.5 exposure, was linked with approximately 59% increased odds of having uncontrolled asthma and a 34% increased odds of asthma contributing to worse QOL. Furthermore, even while adjusting for PM2.5 exposure, higher ambient NO2 exposure significantly increased a child’s risk of having uncontrolled asthma and having an asthma burden impact on QOL. These estimates imply that the impact of air pollutants on asthma health may be broader than conclusions based on increased risk of health care utilization alone, and that, to most effectively reduce risk, multipollutant reduction strategies may be most effective.
There is increasing recognition that individuals are exposed to a complex mixture of air pollutants of varying concentrations, depending on sources of exposure, and addressing multipollutant effects is crucial (38). Our study demonstrates that PM and NO2 had independent adverse effects on asthma control and asthma-related QOL, and that estimates of the magnitude of effect of elevated PM2.5 concentrations may vary based on particulate composition. For example, in addition to total PM2.5, the proportion of BC in PM2.5 was independently associated with worse asthma control and increased health care utilization, and trends, although not statistically significant effects, were seen for asthma-related QOL and missed school days. These results are, overall, consistent with those of Peng and colleagues (39) who demonstrated that exposure to elemental and organic carbon components of ambient particles was associated with risk of hospital admissions due to respiratory symptoms. Together, these results support further investigation of the impact of PM2.5 composition on airway diseases to disentangle the contribution of specific particulate constituents.
Among the many strengths of our study, several limitations should be considered when interpreting results. First, our results highlight the importance of accounting for multipollutant exposures when assessing asthmatic health outcomes. Unlike many previous studies using central site monitoring, we employed numerous longitudinal monitors and inverse distance weighting interpolation to capture the intraurban exposure variation among children with asthma residing within a narrow radius (approximately 2 km). Although this approach may improve the precision of the exposure–response relationship between asthma outcomes and measured pollutants, several other pollutants that were not measured in this study, including ozone and sulfur dioxide, have been previously linked to adverse asthma morbidity outcomes (40). Second, our analyses did not account for indoor exposures, which have known adverse effects on asthma morbidity in the United States (41, 42). However, we previously found that indoor and outdoor residential concentrations of PM2.5 and BC in the study area were similar, likely due to the relatively high exchange of outdoor and indoor air in Peruvian homes compared with U.S. homes (43). Finally, although we adjusted for temperature as a proxy for season, this approach does not fully account for seasonal trends related to other meteorological variables (e.g., humidity), behavioral systems (e.g., cooking), or exposure factors (e.g., traffic). Although previous data suggest that use of ICS may not protect children from adverse pollutant effects (44), and we adjusted for ICS in sensitivity analyses, due to the low rate of ICS use in this Peruvian population, we were unable to determine whether there was a differential effect of PM exposure on those with and without ICS. Therefore, we were also unable to ascertain whether improving access to inhalers may attenuate the adverse pollutant effects identified in this study.
Conclusions
In conclusion, we show harmful effects of ambient pollution on multiple domains of asthma morbidity in children from Lima, including asthma control, asthma-related QOL, and health care utilization, with trends toward increased missed days of school. We also show that the adverse effects of PM2.5 exposure may vary depending on PM2.5 composition, particularly the degree to which it is composed of BC, and that PM and NO2 exposure have independent effects on asthma morbidity. These results underscore the broad clinical impact of multipollutant exposures on airway disease, and highlight the importance of pollutant exposures on multiple domains of asthma morbidity, including impacts on not only acute exacerbations of disease, but also on general asthma burden, such as worse asthma symptom control and QOL. These results are particularly relevant for children and adolescents residing in LMICs with rising burdens of pollution and asthma.
Supplementary Material
Acknowledgments
Acknowledgment
The authors gratefully acknowledge the Peru Center for Asthma Research study team at the Asociacion Benefica PRISMA for their support and contributions; they also acknowledge the Laboratorio de Investigacion y Desarrollo at the Universidad Peruana Cayetano Heredia for their support on laboratory analyses, as well all other GASP (Genetic Asthma Susceptibility to Indoor Pollution in Peru) Study Investigators for their contributions.
Other investigators in the GASP study are as follows:
Rocio Galvez, R.D.
Carla Tarazona, R.D.
Jessica Rice, D.O.
Gary Malpartida, B.S.
Jesse Negherbon, Ph.D.
Chen Chen, M.S.P.H.
Footnotes
Supported by National Institute of Environmental Health Sciences/National Institutes of Health (NIH) grants R01ES018845 and R01ES018845-S1, National Institute of Environmental Health Sciences grants P01ES018176 and P50ES018176, and U.S. Environmental Protection Agency grants RD83451001 and RD83615201 contributed with infrastructure support. K.M.R. and S.L.P. were Fogarty Global Health Fellows through the consortium comprised of the University of North Carolina, Johns Hopkins University, Morehouse School of Medicine, and Tulane University during the conduct of this work through grant 5R25TW009340. W.C. was supported by Pathway to Independence Award R00HL096955 from the National Heart, Lung, and Blood Institute/NIH.
Author Contributions: N.N.H. and W.C. take responsibility for the overall content of this work; the manuscript was written primarily by N.N.H. and W.C.; N.N.H., W.C., K.M.R., D’A.W., and P.B. contributed to study design and study coordination; K.J.P. and W.C. conducted statistical analyses; K.M.R. contributed to study design, data analysis, and drafting of the manuscript; C.J. and D’A.W. oversaw laboratory analyses of samples and contributed to data interpretation and critical review of the manuscript; S.L.P., S.B., F.C.C., and K.K. contributed to data interpretation and critical review of manuscript; N.N.H. and W.C. led the study design and conduct, and contributed to data analysis and manuscript writing; all authors were involved in planning various aspects of the study, and all authors reviewed and approved the final manuscript; N.N.H., K.M.R., and W.C. had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: the GASP Study Investigators, Rocio Galvez, Carla Tarazona, Jessica Rice, Gary Malpartida, Jesse Negherbon, and Chen Chen
References
- 1.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 2.Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S International Study of Asthma and Allergies in Childhood Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64:476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 3.Martin M, Sauer T, Alarcon JA, Vinoles J, Walter EC, Ton TG, et al. Prevalence and impact of asthma among school-aged students in Lima, Peru. Int J Tuberc Lung Dis. 2017;21:1201–1205. doi: 10.5588/ijtld.17.0282. [DOI] [PubMed] [Google Scholar]
- 4.Robinson CL, Baumann LM, Romero K, Combe JM, Gomez A, Gilman RH, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax. 2011;66:1051–1057. doi: 10.1136/thx.2011.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann LM, Robinson CL, Combe JM, Gomez A, Romero K, Gilman RH, et al. Effects of distance from a heavily transited avenue on asthma and atopy in a periurban shantytown in Lima, Peru. J Allergy Clin Immunol. 2011;127:875–882. doi: 10.1016/j.jaci.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim H, Kwon HJ, Lim JA, Choi JH, Ha M, Hwang SS, et al. Short-term effect of fine particulate matter on children’s hospital admissions and emergency department visits for asthma: a systematic review and meta-analysis. J Prev Med Public Health. 2016;49:205–219. doi: 10.3961/jpmph.16.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng YY, Rull RP, Wilhelm M, Lombardi C, Balmes J, Ritz B. Outdoor air pollution and uncontrolled asthma in the San Joaquin Valley, California. J Epidemiol Community Health. 2010;64:142–147. doi: 10.1136/jech.2009.083576. [DOI] [PubMed] [Google Scholar]
- 9.Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, et al. The relationship of ambient ozone and PM(2.5) levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch Environ Occup Health. 2012;67:103–108. doi: 10.1080/19338244.2011.598888. [DOI] [PubMed] [Google Scholar]
- 10.Nastos PT, Paliatsos AG, Anthracopoulos MB, Roma ES, Priftis KN. Outdoor particulate matter and childhood asthma admissions in Athens, Greece: a time-series study. Environ Health. 2010;9:45. doi: 10.1186/1476-069X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loftus C, Yost M, Sampson P, Arias G, Torres E, Vasquez VB, et al. Regional PM2.5 and asthma morbidity in an agricultural community: a panel study. Environ Res. 2015;136:505–512. doi: 10.1016/j.envres.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zora JE, Sarnat SE, Raysoni AU, Johnson BA, Li WW, Greenwald R, et al. Associations between urban air pollution and pediatric asthma control in El Paso, Texas. Sci Total Environ. 2013;448:56–65. doi: 10.1016/j.scitotenv.2012.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Sommar JN, Ek A, Middelveld R, Bjerg A, Dahlén SE, Janson C, et al. Quality of life in relation to the traffic pollution indicators NO2 and NOx: results from the Swedish GA(2)LEN survey. BMJ Open Respir Res. 2014;1:e000039. doi: 10.1136/bmjresp-2014-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cano-Garcinuño A, Bercedo-Sanz A, Mora-Gandarillas I, Callén-Blecua MT, Castillo-Laita JA, Forns-Serrallonga D, et al. Association between quality of life in parents and components of asthma control in children. J Asthma. 2014;51:1089–1095. doi: 10.3109/02770903.2014.943372. [DOI] [PubMed] [Google Scholar]
- 15.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 16.Cruz AA, Stelmach R, Ponte EV. Asthma prevalence and severity in low-resource communities. Curr Opin Allergy Clin Immunol. 2017;17:188–193. doi: 10.1097/ACI.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 17.Lødrup Carlsen KC, Håland G, Devulapalli CS, Munthe-Kaas M, Pettersen M, Granum B, et al. Asthma in every fifth child in Oslo, Norway: a 10-year follow up of a birth cohort study. Allergy. 2006;61:454–460. doi: 10.1111/j.1398-9995.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- 18.Håland G, Carlsen KCL, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. ORAACLE. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 19.National Heart, Lung, and Blood InstituteGuidelines for the diagnosis and management of asthma: Expert Panel Report 3. Bethesda: NHLBI Health Information Center; 2007
- 20.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124:719–23.e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend MMAPI Research InstitutePediatric Asthma Quality of Life Questionnaire with standardised activities (PAQLQ(S)) 2002. Spanish version for Peru. QOL Technologies. 2002[accessed 2018 Sep 30]. Available from: www.qoltech.co.uk/paqlq_s.html
- 26.Sanjuàs C, Alonso J, Sanchís J, Casan P, Broquetas JM, Ferrie PJ, et al. The quality-of-life questionnaire with asthma patients: the Spanish version of the Asthma Quality of Life Questionnaire [Article in Spanish] Arch Bronconeumol. 1995;31:219–226. doi: 10.1016/s0300-2896(15)30927-3. [DOI] [PubMed] [Google Scholar]
- 27.Juniper EF, Guyatt GH, Feeny DH, Griffith LE, Ferrie PJ. Minimum skills required by children to complete health-related quality of life instruments for asthma: comparison of measurement properties. Eur Respir J. 1997;10:2285–2294. doi: 10.1183/09031936.97.10102285. [DOI] [PubMed] [Google Scholar]
- 28.Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environ Sci Technol. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- 29.Koutrakis P, Wolfson JM, Bunyaviroch A, Froehlich SE, Kirano K, Mulik JD. Measurement of ambient ozone using a nitrite-coated filter. Anal Chem. 1993;65:209–214. [Google Scholar]
- 30.Palmes ED, Gunnison AF, DiMattio J, Tomczyk C. Personal sampler for nitrogen dioxide. Am Ind Hyg Assoc J. 1976;37:570–577. doi: 10.1080/0002889768507522. [DOI] [PubMed] [Google Scholar]
- 31.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweileh WM, Al-Jabi SW, Zyoud SH, Sawalha AF. Outdoor air pollution and respiratory health: a bibliometric analysis of publications in peer-reviewed journals (1900 - 2017) Multidiscip Respir Med. 2018;13:15. doi: 10.1186/s40248-018-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong GW, Brunekreef B, Ellwood P, Anderson HR, Asher MI, Crane J, et al. ISAAC Phase Three Study Group. Cooking fuels and prevalence of asthma: a global analysis of phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Lancet Respir Med. 2013;1:386–394. doi: 10.1016/S2213-2600(13)70073-0. [DOI] [PubMed] [Google Scholar]
- 34.Gaviola C, Miele CH, Wise RA, Gilman RH, Jaganath D, Miranda JJ, et al. CRONICAS Cohort Study Group. Urbanisation but not biomass fuel smoke exposure is associated with asthma prevalence in four resource-limited settings. Thorax. 2016;71:154–160. doi: 10.1136/thoraxjnl-2015-207584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62:758–766. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health OrganizationWHO Air quality quidelines for particulate matter, ozone, nitrogen dioxide and sulfer dioxide: global update 2005. Geneva: WHO Press; 2006 [Google Scholar]
- 37.World Health OrganizationAir pollution rising in many of the world’s poorest cities. Geneva: WHO Press; 2016 [accessed 2018 Sep 30]. Available from: https://www.who.int/news-room/detail/12-05-2016-air-pollution-levels-rising-in-many-of-the-world-s-poorest-cities [Google Scholar]
- 38.Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21:187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;117:957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng XY, Ding H, Jiang LN, Chen SW, Zheng JP, Qiu M, et al. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One. 2015;10:e0138146. doi: 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116:1428–1432. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. Center for Childhood Asthma in the Urban Environment. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117:294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Underhill LJ, Bose S, Williams DL, Romero KM, Malpartida G, Breysse PN, et al. Association of roadway proximity with indoor air pollution in a peri-urban community in Lima, Peru. Int J Environ Res Public Health. 2015;12:13466–13481. doi: 10.3390/ijerph121013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ierodiakonou D, Zanobetti A, Coull BA, Melly S, Postma DS, Boezen HM, et al. Childhood Asthma Management Program Research Group. Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J Allergy Clin Immunol. 2016;137:390–399. doi: 10.1016/j.jaci.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.