Abstract
Rationale: In the United States, an algorithm known as the “match-run” creates an ordered ranking of potential recipients for available lung allografts. A potential recipient’s match-run position, or “sequence number,” is available to the transplant center when contacted with a lung offer. Lung offers with higher sequence numbers may be interpreted as a crowd-sourced evaluation of poor organ quality, though the association between the sequence number at which a lung is accepted and its recipient’s post-transplant outcomes is unclear.
Objectives: We sought to evaluate the primary reasons provided when a lung offer was refused by a transplant center, transplant center and donor/organ factors associated with a higher sequence number at acceptance, and the association of the sequence number at acceptance with post-transplant mortality and graft failure.
Methods: Match-run outcomes for lung offers that occurred in the United States from May 2007 through June 2014 were merged with recipient follow-up data through December 2017. Associations between the sequence number at the time of acceptance and selected transplant center and donor characteristics were estimated using multivariable logistic and multinomial regression models. The associations between the final sequence number and recipient survival and graft survival were estimated using multivariable time-to-event models.
Results: Of 10,981 lung offer acceptances, nearly 70% were accepted by one of the top 10 ranked candidates. Higher median annual center volume and potential indicators of organ quality (e.g., abnormal chest radiograph or bronchoscopy) were associated with a higher sequence number at acceptance. There was weak evidence for a small positive relationship between the sequence number at acceptance and both mortality and graft failure. For example, the unadjusted and adjusted hazard ratios for death associated with the log-sequence number at acceptance were 1.019 (95% confidence interval, 1.001–1.038) and 1.011 (95% confidence interval, 0.989–1.033), respectively. On the absolute scale, using the multivariable model, a 10-fold increase in the sequence number translated into a 0.8% absolute decline in the predicted 5-year survival.
Conclusions: Acceptance of a donor lung offer at a later point in the match-run was associated with measurable indicators of organ quality, but not with clinically meaningful differences in post-transplant mortality or graft failure.
Keywords: transplant, survival, organ allocation
Donor lung allocation in the United States is performed through an algorithm that considers medical and biological compatibility, geography, and medical urgency (1, 2). This process, known as the “match-run,” generates a list of prioritized candidates, each with a sequence number that defines where on that list each potential recipient resides. Lung offers are made to transplant centers based on the order in which their patients appear in the match-run, progressing from the candidate with sequence number 1 on to those with higher numbers if the lung offer is not accepted. Criteria for acceptance can be subjective (e.g., a function of a program’s/surgeon’s risk tolerance), and can vary significantly within and between transplant centers. When a lung offer is made to a candidate, information on the candidate’s sequence number in the match-run, along with the reason why prior offers, if they have occurred, have been refused, are made available to the transplant center. Though transplant providers incorporate numerous data when making decisions to accept an organ, some may view a higher sequence number as a crowd-sourced evaluation of organ quality or as a reflection of difficulty in placing an available organ (3). The goals of this study were to examine the lung offer process in the United States and to estimate the association between the sequence number at the time that a lung offer was accepted for transplant and post-transplant outcomes.
Methods
Study Design and Participants
This study used data from the U.S. United Network for Organ Sharing (UNOS). We merged lung transplant recipient follow-up data from the UNOS Standard Transplant Analysis Research (STAR) file, which contained information on waiting list registrations and transplants in the United States through December 31, 2017 with lung offer (match-run) data from the Potential Transplant Recipient (PTR) database from May 1, 2007 through June 30, 2014. Exclusion criteria (age <18 yr at the time of waitlisting, listing for multiorgan transplantation, or a prior transplant) were applied to the STAR file to generate an analytic dataset of lung offers made to adult, first-time lung transplant recipients. The rationale for these restrictions and additional details regarding the study design, methods, and statistical analysis are provided in the Methods in the online supplement.
Analytic Sample and Donor Lung Sequence Number
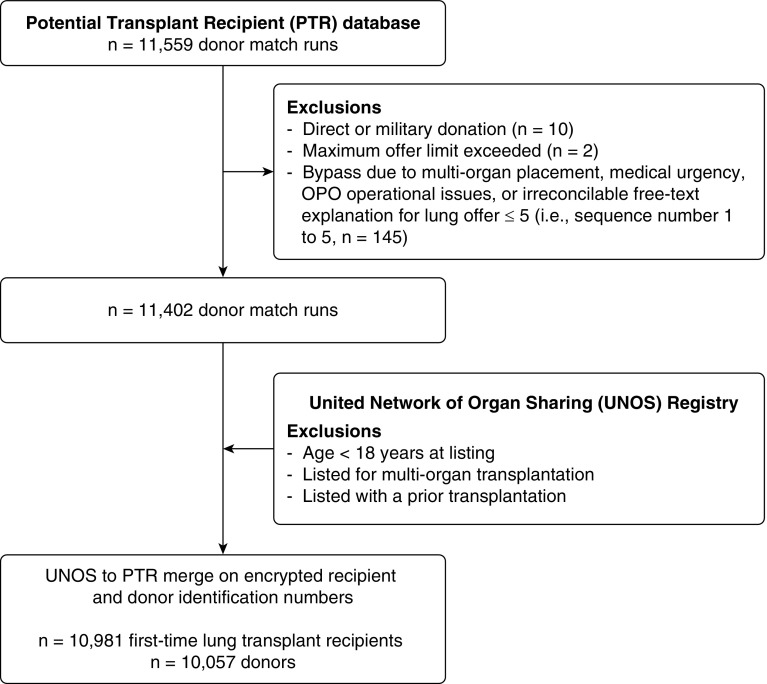
To construct our analytic dataset, we first curated the PTR file (see the Methods in the online supplement). Next, we applied selected restrictions (as summarized previously) to the STAR file. We then merged these two files using both the encrypted donor and recipient identification numbers. A flow diagram summarizing this process is shown in Figure 1.
Figure 1.
Data assembly and participant flow for the primary analysis. OPO = Organ Procurement Organization.
Once generated, a lung offer to each ranked candidate on a match-run can result in a refusal, bypass, or acceptance. A refusal or bypass both result in the progression of the match-run onto the next ranked candidate. Bypasses can occur for numerous reasons, including medical urgency or directed donation. Before applying recipient exclusion criteria, we removed donor match-runs associated with a bypass for direct donation (n = 10) and where the maximum offer limit was exceeded (n = 2). We then evaluated each match-run with a bypass, including the free-text explanations when provided. Based on the observations during the analysis of bypasses, which are summarized in the Methods in the online supplement, we chose to analyze two analytic samples. The primary sample excluded donor match-runs that had their first bypass occur in the first five offers (sequence numbers 1 to 5) for emergent reasons, multiorgan placement, or an irreconcilable free-text field. This methodology defines a cohort in which 50% of organs would normally already have been allocated, so bypasses after this threshold likely still reflect difficulty placing higher-risk organs (see Figure E1 in the online supplement). In addition, no adjustments were made to the sequence number reported in the PTR database to discount bypasses, as the match-run sequence number would remain the same regardless of preceding actions by other centers. Acknowledging that bypasses may create complexities with the interpretation of a sequence number at the time of acceptance, we completed a secondary analysis in which we evaluated a cohort where a bypass did not occur.
Analytic Strategy
We first summarized the primary reason that lung transplant centers provided when a lung offer was refused. Next, among accepted offers, we examined potential preprocurement factors associated with the match-run position (i.e., sequence number) at the time of offer acceptance, as well as the association of this position with mortality and graft failure (a composite outcome indicating allograft failure, retransplantation, or mortality).
Categorization of Lung Offer Refusal Codes
There are three ways that a lung offer refusal reason can be provided in the PTR database: primary reason, secondary reason, or in a free-text field. For this study, we only examined pre-established, numerically submitted, primary reasons for refusal. In certain instances, we collapsed the primary refusal codes provided in the PTR database into alike categories, which are summarized in the Methods in the online supplement.
Factors Associated with Later Acceptance in the Match-Run
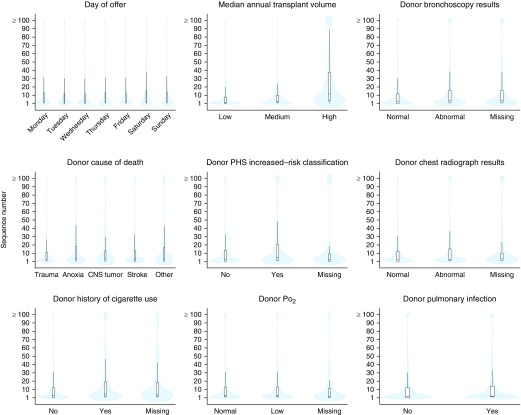
For this analysis, the primary outcome was the sequence number at the time of organ offer acceptance. We selected variables, as available in the STAR file, that we hypothesized could be associated with center or surgeon preprocurement decision-making. These variables comprised donor or organ factors, including bronchoscopy and chest radiograph results, pulmonary infection, cause of death, and partial pressure of oxygen (Po2), as well as yes/no indicators for known donor smoking history and classification of increased risk for blood-borne disease transmission using U.S. Public Health Service criteria. We also hypothesized that center factors, specifically, center volume and the calendar day (Friday, Saturday, or Sunday, compared with other days) of the offer may be associated with organ acceptance. Specifically, we hypothesized that higher volume centers may be more willing to take organs with a higher sequence number, and that smaller-volume centers may be less likely to pursue organ offers that occurred during periods of reduced staffing during the weekend. Center volume was examined as a continuous and categorical variable (median annual lung transplant volume over the study period ≤40 as low, 41–80 as medium, and ≥81 as high), and was calculated before any restriction criteria were applied to best capture true transplant volume.
We first examined unadjusted associations with the aforementioned variables and the sequence number at the time of acceptance using violin plots and boxplots. Next, we estimated several multivariable logistic and multinomial logistic regression models to compare the association of these variables with higher sequence numbers compared with lower sequence numbers at acceptance (see the Methods in the online supplement). We examined several different contrasts, as there was no obvious sequence number cut point for the analysis. The fitted regression models accounted for dependence among observations that were transplanted at the same center or that had the same donor (i.e., two single lungs from one donor; see the Methods in the online supplement).
Association of the Final Sequence Number with Transplantation Outcomes
For this analysis, the primary exposure was the sequence number at the time of lung offer acceptance. The outcomes of clinical interest were all-cause mortality and graft failure. Time to death was defined as the period from a recipient’s date of transplantation through their date of death. In this analysis, recipients that underwent retransplantation, but were still alive at the end of follow-up, were categorized as alive. The time to graft failure was defined as the period from a recipient’s date of transplantation to the date of retransplantation, UNOS recorded graft failure, or death (i.e., a composite outcome capturing the earliest date recorded for any of these events).
Unadjusted survival and graft failure–free survival were first visualized using the Kaplan-Meier method. As some categorization is required for displaying the survival functions in a Kaplan-Meier plot, we present two, albeit arbitrary, categorizations for descriptive visual assessments. Given that there is substantive statistical evidence against the categorization of continuous exposures when there is no natural categorization (4–6), we next used fractional polynomials to model a possible nonlinear association between the accepted organ sequence number and recipient outcomes. The fractional polynomial methodology permits the continuous nature of the sequence number distribution to be preserved while allowing flexible nonlinear relationships to be identified (7–9). The unadjusted fractional polynomial model identified a log-linear–like relationship between the sequence number at acceptance and both outcomes. Thus, we examined multivariable models with both an untransformed and log-transformed sequence number exposure variable.
Next, we constructed mixed-effects multivariable Cox proportional hazards regression models (10) that were informed by published studies, clinical experience, and the Scientific Registry of Transplant Recipients post-lung transplant survival and graft survival prediction models (11). The models included recipient characteristics (age, sex, race, lung allocation score, transplant type [single vs. double lung], primary disease category categorized as obstructive lung disease, pulmonary vascular disease, cystic fibrosis or bronchiectasis lung disease, or restrictive lung disease, type of insurance coverage, highest educational attainment, body mass index, and blood type) and donor characteristics (age, sex, race, body mass index, cause of death, and indicators for abnormal Po2, history of pulmonary infection, tobacco use, and drug abuse). A random effect for a recipient’s transplant center was added to the model, as prior research showed variability in survival across U.S.-based lung transplant centers (12) and because transplant center staff and protocols may be associated with organ acceptance. The Cox regression method has attractive qualities for modeling time-to-event outcomes. However, the resultant estimate, the hazard ratio (HR), can be difficult to interpret and place into a clinical context (13, 14). Therefore, we also used the adjusted mixed-effects Cox regression models to generate 5-year predicted survival and graft survival estimates so that the clinical associations could be assessed on the absolute risk scale.
Analyses were performed using Stata version 15 (StataCorp LLC) and R statistical software package version 3.5.0 (The R Foundation for Statistical Computing).
Results
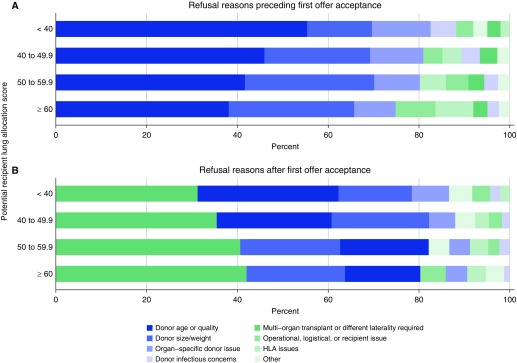
The primary analytic sample included information on lung offers from 10,057 donors that resulted in 10,981 lung transplants (Figure 1). Related lung offer refusal codes were collapsed into discrete categories and were primarily attributed to donor factors (see the Methods in the online supplement and Figure 2). Over 50% of lung offers were accepted by the top 5 ranked candidates, and nearly 70% of lung offers were accepted by the 10th offer (Figure E1). There were 68 lung transplant centers included in the analysis. Higher annual median center volume, abnormal donor bronchoscopy and chest radiograph results, U.S. Public Health Service increased-risk donor status, and known donor smoking history were associated with higher sequence numbers at acceptance (Figure 3, Table E1). These associations, particularly with high annual center volume, generally persisted in multivariable regression analyses (Figures E2–E4). Donor cause of death, presence of a pulmonary infection, and the calendar day of the lung offer (day of the week, or weekday [Monday to Thursday] compared with weekend [Friday to Sunday]) exhibited weaker and less-consistent associations with the sequence number at acceptance.
Figure 2.
Summary of primary lung offer refusal reasons stratified by potential recipient lung allocation score at the time of the lung offer. (A) Summary of refusal reasons preceding the first recorded offer acceptance, and (B) summary of refusal reasons for double lung offers that turned into offers for a single lung thereafter. Categories are further defined in the Methods in the online supplement. HLA = human leukocyte antigen.
Figure 3.
Violin and box and whisker plots depicting the distribution of the sequence number at the time of acceptance by selected variables. Offer acceptances at sequence number 100 (n = 2) or above a sequence number of 100 (n = 371) were combined so that all offers could be visualized. Black bars in the middle of the white boxes indicate the median values. The bottoms and tops of the boxes represent the first and third quartiles (i.e., interquartile range [IQR]), respectively, and the tops and bottoms of the whiskers represent data points that do not extend from the boxes by more than 1.5 times the IQR. The blue shading depicts the density of the distribution of offers accepted. CNS = central nervous system; PHS = Public Health System.
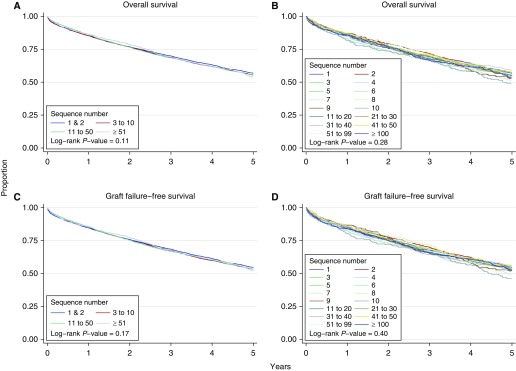
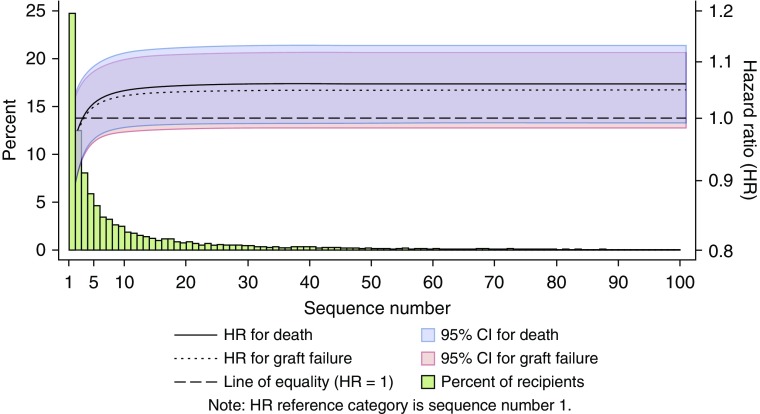
During the follow-up period, 5,534 (50.4%) lung transplant recipients died and 5,873 (53.5%) recipients experienced the composite outcome of graft failure. The Kaplan-Meier plots for overall survival and graft survival for individual sequence numbers and sequence number categories showed considerable overlap, and there was no suggestion of a dose response (i.e., lower survival) with incrementally higher sequence numbers or sequence number categories (Figure 4). The unadjusted fractional polynomial estimate was similar for both outcomes, though weaker for graft failure, and showed a slightly nonlinear relationship and a higher hazard for higher sequence numbers at acceptance compared with a sequence number of 1 (Figure 5). Unadjusted Cox proportional hazards regression analyses of the log-transformed sequence number similarly showed weak evidence of a higher hazard for each outcome with higher sequence numbers at acceptance (HR for the natural logarithm of sequence number of 1.019, 95% confidence interval [CI], 1.001–1.038, P = 0.043 for death and 1.015, 95% CI, 0.997–1.034, P = 0.10 for graft failure). After adjustment for recipient characteristics, transplant center, and donor characteristics, the HR for the sequence number at acceptance was attenuated for both outcomes. For example, the adjusted HR for death associated with one more log (natural logarithm) of the sequence number at acceptance was 1.011 (95% CI, 0.989–1.033) and 1.025 (95% CI, 0.974–1.078) using log10.
Figure 4.
(A–D) Kaplan-Meier estimates of overall survival (A and B) and graft failure-free survival (C and D) for different categorizations of the sequence number at the time of lung offer acceptance.
Figure 5.
Unadjusted fractional polynomial hazard ratio (HR) estimate for death and graft failure for the sequence number at the time of lung offer acceptance relative to sequence number 1. Offer acceptances above sequence number 100 were included in the analysis but not visualized to support readability as the HR estimates do not change. The histogram indicates the percent of offer acceptances at each sequence number out of all 10,981 acceptances included in the analysis. For example, 24.7% of all offers were accepted on the first offer, and 12.5% were accepted on the second offer. CI = confidence interval.
To summarize, we did not find strong statistical evidence of an effect of sequence number in any adjusted model for either patient or graft survival. However, the point estimate of the unadjusted and adjusted HR always remained slightly above 1 in analyses of a log-transformed and non–log-transformed sequence number exposure. Therefore, to contextualize the change in risk that might be associated with a higher sequence number, we calculated predicted 5-year survival probabilities. Let us consider a lung transplant recipient with a predicted 5-year survival equal to the cohort average of 55.7%. The final adjusted model showed that if this recipient had received a lung transplant with a final sequence number 10-fold higher (i.e., offer 1 to 10, by which time nearly 70% of lungs had been accepted or 10 to 100, by which time over 90% of offers have been accepted), this would translate into a 0.8% lower predicted 5-year survival (54.9% instead of 55.7%). In comparison, the predicted 5-year survival for a recipient with identical characteristics and transplanted with the same organ, in the same center, but who was 10 years older, would be 3.7% lower (52.0% instead of 55.7%). Similarly, an equivalent recipient with a lung allocation score score 10 points higher would have a 2.1% lower 5-year survival probability (53.6% instead of 55.7%).
In addition, based on the strong association that we observed between center volume and acceptance, we tested for, but did not find evidence of a center volume–by–sequence number interaction on mortality or graft failure. Finally, we also undertook the above analyses in a sample that only included the sequence number at acceptance from match-runs without any bypasses. The results of these analyses were consistent with the reported effect estimates, and did not suggest different conclusions.
Discussion
In this study of lung allografts accepted for transplantation in the United States from May 2007 to June 2014, almost 70% were accepted by one of the top ten matched candidates (i.e., sequence numbers 1 to 10). The most common reason reported for organ decline was donor quality, followed by donor size (Figure 2). Indicators of poor organ quality were associated with higher sequence numbers at acceptance for transplant. In addition, larger-volume centers were more likely to accept organs with higher sequence numbers than smaller-volume centers. There was weak evidence that a higher sequence number was associated with the day of the week that an offer occurred, suggesting that center-specific human resource limitations were not strong factors in acceptance (Figure 3 and Figures E2–E4). In addition, though there was an apparent increase in the point estimate of the hazard of graft failure and death associated with a higher sequence number at acceptance, this relationship was neither clinically significant nor robust in adjusted statistical models.
There are at least two possible, and not mutually exclusive, interpretations of our study results. First, our findings may suggest that the number of offers preceding the acceptance of a lung offer is not informative beyond traditional donor information. Indeed, we found a correlation between a higher final sequence number and several donor factors (Figure 3 and Figures E2 and E3). However, many of these associations, as well as the association with mortality and graft failure, were attenuated in multivariable regression models when a recipient's transplant center was included (Figure E4). Thus, a second possible mechanism that could explain our results is that centers are selecting the offers they accept appropriately. For example, smaller centers could be appropriately selecting cases to maximize lung transplant outcomes based on potentially limited local center resources. Furthermore, large centers may be using higher-risk organs because of their collective experience and available resource support.
The results of our study complement several recent examinations of the organ offer process for heart, kidney, liver, and lung transplantation (3, 15–20). Studies of the liver (15) and kidney (3, 16) offer processes have not found a meaningful association between the sequence number (match-run position) at acceptance and graft failure or mortality. In regard to the lung offer process, Wey and colleagues (17) documented considerable U.S. program-level variability in lung offer acceptance practices, and found that higher program-level acceptance was associated with a lower incidence of patient removal from the waitlist due to death or becoming too sick. In addition, in a recent analysis of U.S. lung offers, Singh and colleagues (20) found that lungs that were transplanted after having been refused by other centers specifically for reasons of poor donor quality were not associated with worse post-transplant survival. Though the analytic approach of Singh and colleagues differed from that of our study, their findings align with the analyses we present herein. In a response to a letter to the editor regarding their analysis of the kidney match-run, Cohen and colleagues (16) suggested the need for a randomized trial testing whether centers that are blinded to prior lung offer outcomes make different acceptance decisions. Our results, together with other studies of the lung offer process, suggest that a similar study would be informative for the U.S. lung allocation process, potentially revealing mechanisms to improve lung allograft utilization.
There are important considerations and limitations to this study, many of which suggest the need to improve and expand data collection regarding the organ acceptance and refusal process. First, we used an administrative database in which data were sometimes missing or unable to be reconciled. Second, there is the potential for measurement error and misclassification of the reported reasons for an offer refusal due to variation across transplant centers and providers in the rationale for selecting specific refusal reasons (15). It is also possible that some of the codes that were entered do not reflect the actual reason that an offer was declined, as the system is not audited, and a code may be entered in error or for convenience. Third, other donor, candidate, and provider factors that we did not (or could not) examine were likely also associated with offer refusal and acceptance. Mixed-methods research with transplant teams or national surveys will be important to inform future empirical analyses. Fourth, the factors we examined in our acceptance analysis were static, and do not capture changes in available donor information as the allocation process progressed. Fifth, we focused only on mortality and graft failure, which could be considered “hard” endpoints, and thus the lack of a relationship with these outcomes does not necessarily imply a lack of association with other recipient outcomes not included in our study. Sixth, we did not include pediatric, multiorgan, and retransplant recipients in our analysis due to different decision-making and considerations in these populations. Future studies are needed to understand barriers to organ allocation within these patient groups. Finally, different research groups may have approached the primary sample selection process differently. However, our sensitivity analyses, in which we analyzed several different data samples, revealed minimal sampling variability in the effect estimates that we observed.
In conclusion, we examined the reasons for lung offer refusal, the number of refusals (and, in some match-runs, the combined number of refusals and bypasses) before lung offer acceptance, and the association of the sequence number at the time of acceptance with several hypothesized measures of organ quality, as well as post-transplant survival and subsequent graft failure. We conclude that a sequence number in isolation is not a suitable proxy for lung allograft quality, given the small effect size and weak evidence for an association between the sequence number at the time of acceptance and recipient outcomes after transplantation. In light of recent changes to the U.S. lung allocation policy (2, 21, 22), ongoing monitoring of organ acceptance practices is warranted.
Supplementary Material
Acknowledgments
Acknowledgment
This study used data from the U.S. Organ Procurement and Transplantation Network. The authors thank Dr. Meera Harhay and Dr. Rachel Kohn for comments on an earlier draft of this manuscript.
Footnotes
Supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant K99 HL141678 (M.O.H.), research grants from the Agence de Biomédecine grant Appel d’offres Recherche et Greffe 2014 and Vaincre la Mucoviscidose grant RC20140501095 (R.P.), NIH/NHLBI research grants HL115354, HL114626, HL087115, and K24HL115354 (J.D.C.), NIH/NHLBI grant HL121406 (J.M.D.), and by NIH/NHLBI research grants HL116656 and HL135227 (E.C.). This work was also supported in part by Health Resources and Services Administration contract 234-2005-37011C.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Author Contributions: Conception and design—M.O.H., R.P., G.T., M.J.C., T.D., S.R., Z.P., Z.B., J.D.C., J.M.D., and E.C.; acquisition of data—M.O.H. and E.C.; interpretation of data and drafting and revising manuscript—all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Organ Procurement and Transplantation Network. PoliciesGoogle Scholar. January 17, 2018 [accessed 2018 May 26]. Available from: https://optn.transplant.hrsa.gov/governance/policies/
- 2.Egan TM. How should lungs be allocated for transplant? Semin Respir Crit Care Med. 2018;39:126–137. doi: 10.1055/s-0037-1620265. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JB, Shults J, Goldberg DS, Abt PL, Sawinski DL, Reese PP. Kidney allograft offers: Predictors of turndown and the impact of late organ acceptance on allograft survival. Am J Transplant. 2018;18:391–401. doi: 10.1111/ajt.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 5.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dinero TE. Seven reasons why you should not categorize continuous data. J Health Soc Policy. 1996;8:63–72. doi: 10.1300/J045v08n01_06. [DOI] [PubMed] [Google Scholar]
- 7.Royston P, Sauerbrei W.Multivariable model-building: a pragmatic approach to regression anaylsis based on fractional polynomials for modelling continuous variables.Chichester, England: John Wiley & Sons; 2008 [Google Scholar]
- 8.Binder H, Sauerbrei W, Royston P. Comparison between splines and fractional polynomials for multivariable model building with continuous covariates: a simulation study with continuous response. Stat Med. 2013;32:2262–2277. doi: 10.1002/sim.5639. [DOI] [PubMed] [Google Scholar]
- 9.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 10.Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85:185–203. doi: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scientific Registry of Transplant RecipientsSRTR risk adjustment model documentation: posttransplant outcomes. August 2018 [accessed 2018 Aug 24]. Available from: https://www.srtr.org/reports-tools/risk-adjustment-models-posttransplant-outcomes/
- 12.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304:53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 13.Harhay MO, Porcher R, Cantu E, Crowther MJ, Christie JD, Thabut G, et al. An alternative approach for the analysis of time-to-event and survival outcomes in pulmonary medicine. Am J Respir Crit Care Med. 2018;198:684–687. doi: 10.1164/rccm.201801-0189LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutradhar R, Austin PC. Relative rates not relative risks: addressing a widespread misinterpretation of hazard ratios. Ann Epidemiol. 2018;28:54–57. doi: 10.1016/j.annepidem.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DS, French B, Lewis JD, Scott FI, Mamtani R, Gilroy R, et al. Liver transplant center variability in accepting organ offers and its impact on patient survival. J Hepatol. 2016;64:843–851. doi: 10.1016/j.jhep.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen JB, Shults J, Goldberg DS, Abt PL, Sawinski DL, Reese PP. Kidney transplant outcomes: position in the match-run does not seem to matter beyond other donor risk factors. Am J Transplant. 2018;18:1577–1578. doi: 10.1111/ajt.14883. [DOI] [PubMed] [Google Scholar]
- 17.Wey A, Valapour M, Skeans MA, Salkowski N, Colvin M, Kasiske BL, et al. Heart and lung organ offer acceptance practices of transplant programs are associated with waitlist mortality and organ yield. Am J Transplant. 2018;18:2061–2067. doi: 10.1111/ajt.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huml AM, Albert JM, Thornton JD, Sehgal AR. Outcomes of deceased donor kidney offers to patients at the top of the waiting list. Clin J Am Soc Nephrol. 2017;12:1311–1320. doi: 10.2215/CJN.10130916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizwan R, Zafar F, Bryant R, III, Tweddell JS, Lorts A, Chin C, et al. The number of refusals for donor organ quality does not impact heart transplant outcomes in children. Ann Thorac Surg. 2018;105:1223–1230. doi: 10.1016/j.athoracsur.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Singh E, Schecter M, Towe C, Rizwan R, Roosevelt B, Tweddell J, et al. Sequence of refusals for donor quality, organ utilization, and survival after lung transplantation. J Heart Lung Transplant. 2019;38:35–42. doi: 10.1016/j.healun.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan TM. From 6 years to 5 days for organ allocation policy change. J Heart Lung Transplant. 2018;37:675–677. doi: 10.1016/j.healun.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 22.United Network for Organ SharingPolicy modification to lung distribution sequence. November 24, 2017[accessed 2018 Sep 11]. Available from: https://transplantpro.org/news/thoracic/policy-modification-to-lung-distribution-sequence/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.