To the Editor:
Idiopathic pulmonary fibrosis (IPF) is a chronic interstitial lung disease (ILD) of unknown cause, with median survival of 3 to 5 years (1). In 2014, two treatments that slow IPF progression gained U.S. Food and Drug Administration approval, highlighting the need for early, accurate diagnosis (2–4). Establishing a diagnosis of IPF can be challenging, with misdiagnoses and delays commonly reported (5–7). Despite evidence of diagnostic delays, developing a real-world estimate of this delay is difficult because of inclusion of data in IPF registries only after diagnosis, poor recall of symptom onset in surveys, and the disease’s relative rarity (annual prevalence, 4.6–11.3/100,000) (8), preventing identification of adequate sample populations. Using Medicare claims data, which capture health service utilization for Americans 65 years and older, we examined patterns of diagnostic respiratory testing and pulmonologist visits that precede IPF diagnosis to investigate potential diagnostic delays. This research was previously presented at the American Thoracic Society 2017 conference, the 2017Aspen Lung Conference, and the CHEST 2017 meeting.
Methods
We analyzed claims data for all Medicare beneficiaries who were diagnosed with IPF in 2012. On the basis of a published algorithm for identifying beneficiaries with IPF in claims data (9, 10), beneficiaries were included if they: 1) had one or more inpatient or two outpatient claims with IPF as a listed diagnosis in 2012 (3), 2) had no claim with another ILD code (3) after the last observed IPF claim, 3) had no claims with IPF within 5 years before the first qualifying IPF claim in 2012 (index date), and 4) had one or more chest computerized tomography (CT) scans before index diagnosis.
Among included beneficiaries, we counted the following tests in the 5 years before IPF diagnosis: pulmonary function tests (PFTs), chest radiographs, chest CT scans, fiberoptic bronchoscopy, autoimmune serologies, 6-minute-walk test, cardiopulmonary exercise test, precipitin panels, arterial blood gas, oxygen saturation, and surgical lung biopsies. Time from first recorded test to diagnosis of IPF was measured and illustrated as cumulative probability curves. We reviewed claims for evaluation and management services to identify provider specialty; pulmonologist visits were reported similar to testing above. The study was exempted from institutional review board review.
Results
Among 33,780 Medicare beneficiaries with a qualified claims-based diagnosis of IPF in 2012, 7,306 met all criteria and formed the final sample. Beneficiary characteristics are summarized in Table 1. All beneficiaries had at least one diagnostic test of interest during the 5-year prediagnosis period, with the most common tests being chest radiographs (99.2%) and PFTs (75.0%) (the full list is provided in Table 2). Tests were associated with many provider specialties, often other than pulmonology. The time between tests and initial IPF diagnosis varied, although testing and pulmonologist visit frequency increased immediately before diagnosis (Figure 1).
Table 1.
Patient demographics of patients newly diagnosed with idiopathic pulmonary fibrosis (N = 7,306)
| Characteristic | Patients Newly Diagnosed with IPF |
|---|---|
| Age, yr | |
| Mean (standard deviation) [median] | 80.8 (6.2) [81] |
| Minimum–maximum | 70–105 |
| Female | 3,559 (48.7) |
| Race | |
| White | 6,894 (94.4) |
| Black | 212 (2.9) |
| Hispanic | 56 (0.8) |
| Asian | 40 (0.5) |
| Other/unknown | 104 (1.4) |
| Region | |
| Midwest | 1,880 (25.7) |
| Northeast | 1,412 (19.3) |
| South | 2,962 (40.5) |
| West/other/unknown | 1,052 (14.4) |
Definition of abbreviation: IPF = idiopathic pulmonary fibrosis.
Data presented as No. (%) unless otherwise noted.
Table 2.
Frequency distributions of first diagnostic test and first pulmonologist visit in 5 years before diagnosis of idiopathic pulmonary fibrosis among newly diagnosed Medicare beneficiaries (N = 7,306)
| Year before Diagnosis when First Test Occurred |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fifth Year |
Fourth Year |
Third Year |
Second Year |
First Year* |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | Cumulative % | |
| Any diagnostic test | 4,217 | 57.7 | 1,365 | 18.7 | 695 | 9.5 | 460 | 6.3 | 569 | 7.8 | 100.0 |
| Pulmonary function testing | 1,219 | 16.7 | 867 | 11.9 | 696 | 9.5 | 752 | 10.3 | 1,942 | 26.6 | 75.0 |
| Chest radiograph | 3,542 | 48.5 | 1,531 | 21.0 | 823 | 11.3 | 571 | 7.8 | 782 | 10.7 | 99.2 |
| CT scan of the chest | 1,399 | 19.1 | 978 | 13.4 | 864 | 11.8 | 962 | 13.2 | 3,103 | 42.5 | 100.0 |
| Fiberoptic bronchoscopy | 115 | 1.6 | 100 | 1.4 | 116 | 1.6 | 168 | 2.3 | 770 | 10.5 | 17.4 |
| Autoimmune serologies | 484 | 6.6 | 420 | 5.7 | 414 | 5.7 | 479 | 6.6 | 1,157 | 15.8 | 40.4 |
| 6-minute-walk test | 142 | 1.9 | 127 | 1.7 | 156 | 2.1 | 229 | 3.1 | 742 | 10.2 | 19.1 |
| Cardiopulmonary exercise testing† | N/A | N/A | N/A | N/A | 33 | 0.5 | 1.1 | ||||
| Precipitin panel | 22 | 0.3 | 26 | 0.4 | 26 | 0.4 | 47 | 0.6 | 244 | 3.3 | 5.0 |
| Arterial blood gas | 186 | 2.5 | 156 | 2.1 | 181 | 2.5 | 214 | 2.9 | 525 | 7.2 | 17.3 |
| Oxygen saturation | 753 | 10.3 | 600 | 8.2 | 566 | 7.7 | 618 | 8.5 | 1,176 | 16.1 | 50.8 |
| Surgical lung biopsy | 60 | 0.8 | 73 | 1.0 | 65 | 0.9 | 104 | 1.4 | 474 | 6.5 | 10.6 |
| Pulmonologist visit | 1,245 | 17.0 | 543 | 7.4 | 520 | 7.1 | 625 | 8.6 | 2,221 | 30.4 | 70.5 |
Definition of abbreviations: CT = computed tomography; N/A = not applicable.
Including index date (the date of diagnosis of idiopathic pulmonary fibrosis).
Four cell counts suppressed because of three cell sizes with counts less than 11.
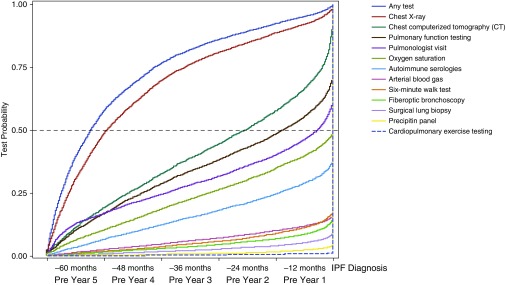
Figure 1.
Cumulative probability curves illustrate the time from first recorded diagnostic test and first pulmonologist visit to diagnosis of idiopathic pulmonary fibrosis (IPF) among Medicare beneficiaries (N = 7,306).
The majority of beneficiaries (n = 5,154, 70.5%) had a pulmonologist visit within 5 years before IPF diagnosis (Table 2). Of these 5,154 beneficiaries, 34.7% had their initial pulmonologist visit more than 3 years before diagnosis.
The first chest CT scan was observed throughout the 5-year prediagnosis period, with 19.1% of beneficiaries having their first scan more than 4 years before IPF diagnosis, 32.5% more than 3 years before diagnosis, and 57.5% more than 1 year before diagnosis (Table 2). Repeated scans were common before index diagnosis.
Discussion
In a large, nationally representative sample of Medicare beneficiaries with IPF, chest imaging, PFTs, and pulmonologist visits were commonly performed in the 5 years before a diagnostic code for IPF appeared. Nearly one-third of beneficiaries had their first CT scan more than 3 years before diagnosis, with slightly more seeing a pulmonologist in this period. If these are proxies for development of IPF-related respiratory symptoms, our findings may mean that diagnosis of IPF is frequently delayed, even after evaluation by a pulmonologist.
Our results are consistent with previous studies (7). Lamas and colleagues (7) found a median delay of 2.2 years before an accurate diagnosis. Furthermore, diagnostic delay was associated with comorbidities such as coronary artery disease and gastroesophageal disease, to which symptoms of IPF may have been initially attributed. A survey study of patients with IPF reported more than half of patients were initially misdiagnosed, and 43% recalled a delay of 1 year or more from symptoms to diagnosis (6). Our study supports these findings by suggesting that diagnostic imaging and PFT are ordered often years before initial diagnosis, and that delayed diagnosis may potentially occur even after diagnostic tests and pulmonologist evaluation. This may point to a need to improve IPF diagnostic tests, testing algorithms, and/or test interpretation, even among pulmonologists. In addition, 30% of patients did not see a pulmonologist before diagnosis, possibly indicating the need for better access to subspecialty care. Improvement in chest CT scan capabilities and interpretation and early referral to an ILD center may reduce potential delays and expedite appropriate treatment.
Our study is limited, in that even with use of chest CT scans (not specific to high-resolution scans) and other respiratory testing, earlier diagnosis of IPF may not have been possible. The test results may have been unavailable, initially normal, or insufficient to diagnose IPF; beneficiaries may have initially lacked a radiographic usual interstitial pneumonia (UIP) pattern recommended for definite diagnosis or had testing that preceded the development of IPF diagnostic criteria on the basis of confidence of radiographic UIP pattern (11). In addition, surgical lung biopsy, itself a risk among elderly patients, who predominated our study, may have been deferred (11, 12). Second, the long observation period pre-index could have introduced early testing that was unrelated to IPF diagnosis; we minimized this (immortal time) bias by including specialized testing that was likely related to a later diagnosis. Third, beneficiaries identified as having IPF may not have IPF, because of misclassification and/or miscoding (10, 13); however, our method for identifying IPF beneficiaries was derived from a modified code-based algorithm (10), with improved positive predictive value over prior algorithms. Finally, as an artifact of our study’s 5-year look-back period, the final sample was older than typical beneficiaries with IPF.
Our study examined Medicare beneficiaries with IPF to show that diagnostic delays may occur, even after chest imaging, pulmonary function studies, and a pulmonologist’s evaluation. Further study among ILD specialty centers and within the era of antifibrotic therapy may help confirm whether patients could be diagnosed earlier and if earlier diagnosis leads to improved clinical outcomes.
Supplementary Material
Footnotes
Genentech, Inc. provided financial support for this research.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 2.Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med. 2014;108:819–829. doi: 10.1016/j.rmed.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan SD, Shlobin OA, Weir N, Ahmad S, Kaldjob JM, Battle E, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140:221–229. doi: 10.1378/chest.10-2572. [DOI] [PubMed] [Google Scholar]
- 4.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol. 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells AU, Costabel U, Poletti V, Crestani B, Egan J, Margaritopoulos G, et al. Challenges in IPF diagnosis, current management and future perspectives. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:28–35. [PubMed] [Google Scholar]
- 6.Cosgrove GP, Bianchi P, Danese S, Lederer DJ. Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey. BMC Pulm Med. 2018;18:9. doi: 10.1186/s12890-017-0560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184:842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghu G, Chen S-Y, Hou Q, Yeh W-S, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Chen S-Y, Yeh W-S, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 10.Ley B, Urbania T, Husson G, Vittinghoff E, Brush DR, Eisner MD, et al. Code-based diagnostic algorithms for idiopathic pulmonary fibrosis. case validation and improvement. Ann Am Thorac Soc. 2017;14:880–887. doi: 10.1513/AnnalsATS.201610-764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161–1167. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 13.Esposito DB, Lanes S, Donneyong M, Holick CN, Lasky JA, Lederer D, et al. Idiopathic pulmonary fibrosis in United States automated claims. incidence, prevalence, and algorithm validation. Am J Respir Crit Care Med. 2015;192:1200–1207. doi: 10.1164/rccm.201504-0818OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.