Abstract
Background:
Vitamin D deficiency (VDD) is seen in all races, age groups, and ethnic backgrounds. VDD estimated to affect >1 billion people worldwide. The purpose of the present study is to characterize the extent of Vitamin D inadequacy and parathyroid hormone (PTH) levels among patients presenting to us for fracture management.
Materials and Methods:
A prospective case-control study was performed whereby serum Vitamin D levels and corresponding PTH levels were measured. The study subjects included patients >45 years of age irrespective of sex who presented with fracture as a result of trivial trauma. A total of 102 cases (34 intertrochanteric fracture, 66 fracture neck of femur, and 2 Colle's fracture) and 100 controls were included in the study.
Result:
Average serum hydroxy-vitamin D and serum PTH of cases 15.8 ± 5.25 ng/mL and 91.15 ± 6.03 pg/mL, respectively. Average S.25-OH Vitamin D and serum PTH of controls was 25.49 ± 3.79 ng/mL and 23.46 ± 3.79 pg/mL, respectively. Eighty (78.4%) cases were in insufficient range (Vitamin D between 10–30 ng/mL), 16 (15.6%) were deficient (Vitamin D <10 ng/mL), and only 6 (5.8%) were sufficient (Vitamin D >30 ng/mL). While in controls, 28% had sufficient and 72% had insufficient Vitamin D levels. There was no deficient control. Serum PTH levels were significantly raised in cases when compared to controls consistent with secondary hyperparathyroidism.
Conclusion:
This study gives us very important data regarding the prevalence of VDD and rise of PTH secondary to the former and this hormonal milieu in our body is an independent risk factor for increased incidence of fracture.
Keywords: Fracture, hyperparathyroidism, prevalence rate, Vitamin D deficiency
Introduction
Orthopedic surgeons tend to think of the skeleton primarily in its mechanical role as the body's framework, often overlooking its metabolic aspects. Bone is dynamic tissue, and disturbances in its metabolic activity may have significant consequences for the skeleton and the body as a whole. Vitamin D deficiency (VDD) is estimated to affect >1 billion population worldwide.1 It is well known that severe deficiency causes rickets (in children) and osteomalacia (“softening of the bones”) in adults. VDD causes paresthesia in hands and feet as well as aching muscles and bones.2,3,4 Muscle weakness particularly proximal myopathy causing difficulty getting up from a chair without using arms and by walking on stairs has been found to be caused by VDD muscle.5,6
VDD is believed to cause secondary hyperparathyroidism leading to an increase in the bone turnover and bone loss.7 While the majority of studies of this nature have involved postmenopausal women with fragility or hip fractures, to the best of our knowledge, no studies to date have described the prevalence of status of Vitamin D in the adult orthopedic population, irrespective of sex.
Given the critical role of Vitamin D in musculoskeletal health and function as well as the potential negative consequences of Vitamin-D deficiency in the operative and postoperative settings, data revealing the prevalence of Vitamin-D deficiency in this patient population may be of great value. The purpose of the present study is to characterize the extent of Vitamin-D inadequacy and parathyroid hormone (PTH) levels among patients presenting to our outpatient department (OPD) or casualty department for fracture management. The hypothesis kept for the study was elderly patients with fracture due to trivial trauma had lower serum Vitamin D levels and higher PTH levels as compared to controls.
Materials and Methods
A prospective case-control study was conducted from January 2014 to December 2015 which included 102 (42 females and 60 males) patients as cases. All cases were above the age of 45 years who presented to our OPD and casualty following fragility fracture to hip and wrist and admitted for operative intervention. A fragility fracture may be defined as one that occurred as a result of minimal trauma, such as fall from a standing height or less, or that occurred without any identifiable trauma. A total of 100 controls were included in the study (49 females and 51 males) [Figure 1]. The controls were chosen from healthy persons in the same age group mainly nurses, hospital staff, and otherwise healthy patients visiting OPD for minor orthopedic problems such as a low backache, weakness, and generalized body ache without any fracture or comorbidity.
Figure 1.
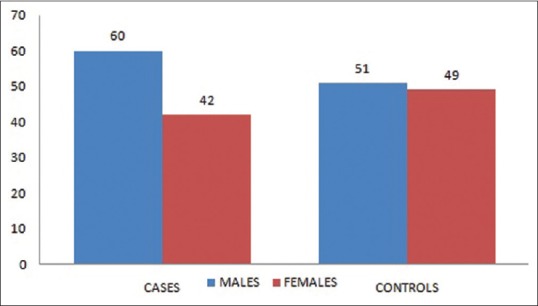
Number of case and controls included in the study (male and female separately)
Serum 25 hydroxy-vitamin D (25 OH Vitamin D) and PTH levels before surgery were measured as part of routine preoperative care. The study was approved by the board of studies and the protocol was approved by the local institutional review committee. Informed consent was obtained from all subjects involved in the study. A detailed history and physical examination were carried out for every subject who entered into the study as per a predesigned pro forma covering all known risk factors and conditions leading to decrease in bone stock and secondary osteoporosis.
Patients with deranged renal function (serum creatinine >1.5 mg %) or renal calculi., abnormal thyroid function (serum thyroid-stimulating hormone between 0.5–5.0 mIU/L) significant liver disease, history of cancer, peptic ulcer, or oesophageal disease requiring prescription medication within the previous 5 years, on regular therapy with a phosphate binding antacid, on estrogen replacement therapy within the previous 9–12 months, or on therapy with any drugs that affect skeleton such as steroids anticonvulsants and anticoagulants, or on prolonged glucocorticoid intake defined as use of prednisolone in a dosage of more than 5 mg/day for at least 3 months were excluded from the study.
Out of 102 cases included in the study, 34 were of intertrochantric fracture, 66 were of fracture neck of femur, and 2 were of Colle's fracture [Figure 2]. All the cases included in the study were admitted in orthopedic wards, and their blood sample was collected in ice cooled syringes (0°C–4°C temperature). A volume of 4 mL of blood was collected in plain vials. Moreover, samples were sent to our endocrinology laboratory within 1–2 h of collection maintaining cold chain (samples were kept in ice bags and then transferred to laboratory). After reaching the laboratory, the vials were subjected to centrifugation at 1200 rotations/minute for 10 min. Then, the serum collected at the top of vial was meticulously separated in two separate sterile vials with the help of a pipette. One sample for Vitamin D and the other for PTH estimation was simultaneously subjected for hormonal assay. The controls were asked to get their Vitamin D and serum PTH done by visiting the collection center of same laboratory and data were collected in subsequent followup to our orthopedic OPD.
Figure 2.
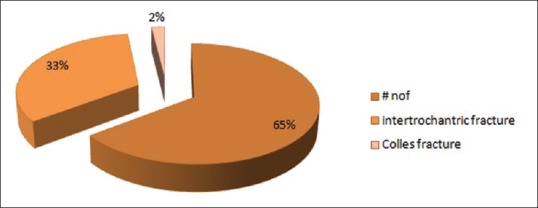
Distribution of fractures among cases included in the study
The methodology for estimating 25 OH Vitamin D used in this study was a direct competitive chemiluminescence immunoassay (CLIA) for quantitative determination of total 25 OH Vitamin D in serum. In this technique, a light signal is measured by a photomultiplier as relative light units and is inversely proportional to the concentration of 25 OH Vitamin D present in calibrators, controls, or samples. The operating instructions for the test were strictly adhered to, for ensuring proper measurement of Vitamin D in samples. The Vitamin D ranges considered in this study as normal/Vitamin D sufficient between 30 and 100 ng/mL, Vitamin D Excess >150 ng/mL, Vitamin D Insufficient between 10 and 30 ng/mL, and Vitamin D deficient <10 ng/mL. Similarly, serum PTH was measured using LIAISON N-tact PTH CLIA. This is a two-site immunoenzymatic assay. The amount of PTH in the sample is determined from a stored, multi-point calibration curve. The Normal value of PTH was taken between 10 and 65 pg/mL, patients were labelled hyper-parathyroid if PTH was >65 pg/mL and hypo parathyroid when PTH was <10 pg/mL.
Statistical analysis was performed using SPSS 20.0 (IBM Corp. Released 2011. Version 20.0. Armonk, NY). statistical package. Continuous variables were expressed as mean ± standard deviation (Gaussian/normal distribution curve or range) and qualitative data were expressed in percentages. Unpaired and paired t-test for independent and dependent samples were used in comparing continuous data between the groups. All tests were two tailed, and the value of P ≤ 0.05 was considered statistically significant. All confidence intervals were calculated at 95% level.
Result
Average S.25-OH Vitamin D and serum PTH of cases were 15.8 ± 5.25 ng/mL and 91.15 ± 6.03 pg/mL, respectively. Moreover, average serum 25-OH Vitamin D and serum PTH of controls were 25.49 ± 3.79 ng/mL and 23.46 ± 3.79 pg/mL. Average serum 25-OH Vitamin D of male cases was 17.05 ± 5.39 ng/mL and that of female cases was 14.03 ± 4.54 ng/mL. Average serum PTH level of male and female cases was 91.88 ± 5.82 and 90.10 ± 6.16 pg/mL, respectively. Average serum 25 OH Vitamin D of male and female controls was 26.16 ± 4.26 and 24.80 ± 3.09 ng/mL, respectively. Average serum PTH of male and female controls was 22.3 ± 3.48 and 24.65 ± 3.73 pg/mL, respectively [Figure 3].
Figure 3.
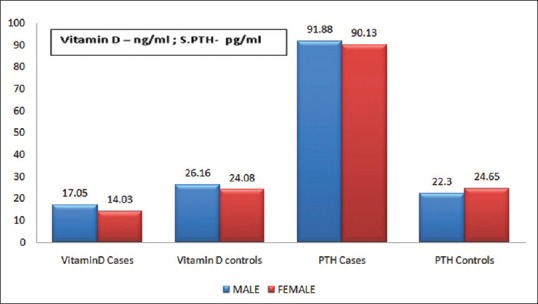
The bar graph shows the mean values of serum Vitamin D and serum parathyroid hormone of the case and controls (males and females separately)
The mean age of female cases was 61.64 ± 13.07 years and that of male cases was 60.13 ± 13.69 years. The mean age of female controls was 56.5 ± 11.2 years, and that of male controls was 56.6 ± 9.13 years [Tables 1–3]. Fifty-five percent of patients included in the study were urban residents while 45% were rural resident. The value of S. 25 OH Vitamin D according to residential status of the cases included in the study was rural patients were having 16.55 ng/mL, whereas urban patients were having 14.1 ng/mL [Table 4].
Table 1.
Differences in base line characteristics between males and females
| Base line characteristics | Male | Females |
|---|---|---|
| Total number of cases | 60 | 42 |
| Total number of controls | 51 | 49 |
| Mean age of cases | 60.1±13.69 | 61.64±13.07 |
| Mean age of controls | 56.6±9.13 | 56.5±11.2 |
| Mean Vitamin D of cases (ng/mL) | 17.05±5.39 | 14.03±4.54 |
| Mean Vitamin D of controls (ng/mL) | 26.16±4.26 | 24.14±3.09 |
| Mean serum PTH of cases (pg/mL) | 91.88±5.82 | 90.13±6.16 |
| Mean serum PTH of controls (pg/mL) | 22.3±3.48 | 24.65±3.73 |
PTH=Parathyroid hormone
Table 3.
Values of baseline variables based on age distribution
| Variables | 45-60 years | 61-70 years | >70 years |
|---|---|---|---|
| Number of females cases | 22 | 9 | 11 |
| Number of male cases | 35 | 10 | 15 |
| Number of female controls | 34 | 8 | 6 |
| Number of male controls | 28 | 12 | 9 |
| Mean serum Vitamin D of male cases (ng/mL) | 19.5 | 13.8 | 12.9 |
| Mean serum Vitamin D of female cases (ng/mL) | 17.25 | 10.2 | 10.7 |
| Mean serum PTH of male cases (pg/mL) | 93.29 | 87.13 | 95.14 |
| Mean serum PTH of female case (pg/mL) | 89.44 | 88.36 | 92.93 |
| Mean serum Vitamin D of male controls (ng/mL) | 28.1 | 22.4 | 20.2 |
| Mean serum Vitamin D of female controls (ng/mL) | 25.5 | 24.7 | 22.9 |
| Mean serum PTH of male controls (pg/mL) | 21.8 | 26.1 | 28.6 |
| Mean serum PTH of female controls (pg/mL) | 23.6 | 26.1 | 28.6 |
PTH=Parathyroid hormone
Table 4.
Residential status of cases and controls and their serum Vitamin D levels
| Residential status | Sex | Vitamin D levels (ng/dL) |
|---|---|---|
| Rural | ||
| Number of cases (56) | Male | 17.2 |
| Female | 15.9 | |
| Number of controls (15) | Male | 25.6 |
| Female | 23.4 | |
| Urban | ||
| Number of cases (46) | Male | 15.8 |
| Female | 12.4 | |
| Number of controls (85) | Male | 24.3 |
| Female | 22.6 | |
Table 2.
Base line characteristics and the distribution of cases and controls
| Baseline characteristics | Number of cases | Number of controls |
|---|---|---|
| Age (years) | ||
| 45-60 | 57 | 69 |
| 61-70 | 18 | 20 |
| >70 | 26 | 11 |
| Sex | ||
| Male | 60 | 49 |
| Female | 42 | 51 |
| Residential status | ||
| Rural | 56 | 15 |
| Urban | 46 | 85 |
| Menopausal status | ||
| Premenopause | 8 | 10 |
| Postmenopause | 34 | 41 |
| Exposure to sunlight | ||
| >15 min daily | 85 | 70 |
| ≤15 min daily | 17 | 30 |
| Serum Vitamin D | ||
| Sufficient (>30 ng/dL) | 6 | 28 |
| Insufficient (10-30 ng/dL) | 80 | 72 |
| Deficient (<10 ng/dL) | 16 | 0 |
| Serum PTH | ||
| Hypoparathyroid (<10 pg/dL) | 0 | 0 |
| Normoparathyroid (10-65 pg/dL) | 34 | 75 |
| Hyperparathyroid (>65 pg/dL) | 68 | 20 |
| BMI (kg/m2) | 25.8±3.6 | 24.3±3.1 |
BMI=Body mass index, PTH=Parathyroid hormone
Out of 102 cases included in the study 6 (5.8%) patients were in the sufficient range (>30 ng/mL), 80 (78.4%) were insufficient (between 10 and 30 ng/mL) and 16 patients (15.6%) patient were deficient in serum Vitamin D (<10 ng/mL) [Figure 4]. Cases were divided into 3 age groups and mean Vitamin D level of males and females were separately calculated, in 45–60 year age group males had a mean of 19.5 ng/mL of serum Vitamin D, whereas females had 17.25 ng/mL of serum Vitamin D. Those between 61 and 70 years of age, the males had a mean value of 13.8 ng/mL of Vitamin D while females had 10.2 ng/mL. Moreover, those above 70 years of age, males had a mean Vitamin D of 12.9 ng/mL and females had 10.7 ng/mL [Figure 5]. It was inferred from the above values that serum Vitamin D followed decreasing age-wise trend and females had lesser values as compared to their male counterparts.
Figure 4.
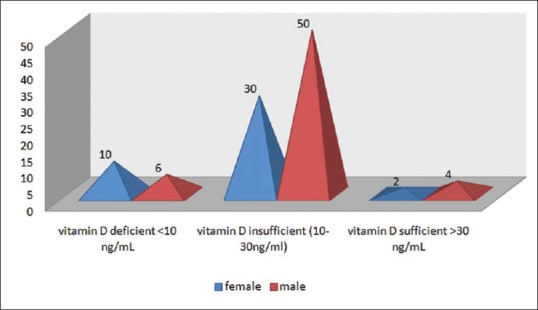
Pyramid graph showing sex-wise number of cases in respective Vitamin D ranges
Figure 5.
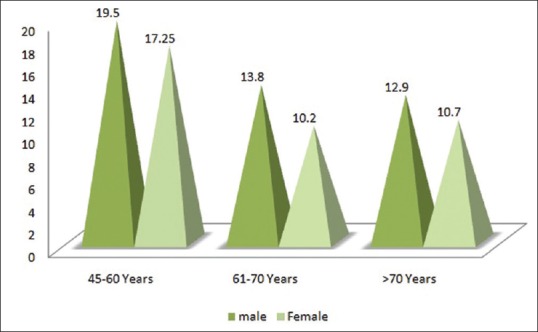
The pyramid graph shows the age-wise mean S Vitamin D level of male and female cases
On comparing the serum Vitamin D of the cases with that of controls, unpaired t-test was applied and the P < 0.0001 (t = −14.9; degree of freedom = 200) which showed that Vitamin D levels were significantly low among cases [Figure 6]. On comparison of serum PTH of cases with controls, unpaired t-test was applied and the P < 0.0001 (t = 94.8; degree of freedom = 200) which shows that the secondary hyperparathyroidism in the cases included in the study was highly significant [Figure 7].
Figure 6.
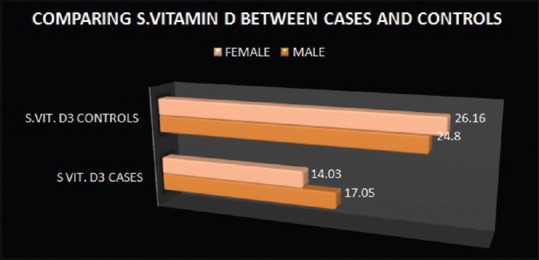
Comparison of serum Vitamin D levels between cases and controls
Figure 7.
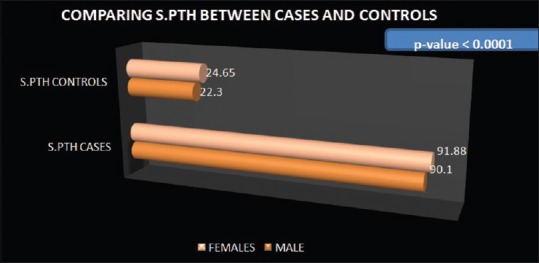
Comparison of serum parathyroid hormone levels between cases and controls
Chi-square test using (SPSS 20.0 (IBM Corp. Released 2011. Version 20.0. Armonk, NY).) was applied to see the effect of age on Vitamin D level, and it was found that age was a significant determinant of hypo-vitaminosis D (P < 0.0001), i.e., decrease in serum Vitamin D with increasing age is significantly related to each other.
Discussion
Fracture around the hip, vertebral fractures and other fractures resulting from trivial trauma mainly in elderly population has become a major problem for the health sector in India today and knowing about risk factors associated with these fractures is really the need of the hour because it would help reduce the already overburdened health sector and would go a long way in improving the quality of life of the elderly population affected by these debilitating fractures.8 There have been many studies on the risk factors associated with trivial and osteoporotic fractures, but all of them were done in either western population or other Asian countries, so there are very few studies about these risk factors in Indian population. The orthopedic surgery patients, especially those who are >45 years of age have very high prevalence of VDD and secondary hyperparathyroidism.9 Of the 102 cases included in this study only 6 (5.8%) had serum Vitamin D level in the sufficient range and all the rest were having their serum Vitamin D below 30 ng/mL that is insufficient. Of all the cases, 15.6% were deficient that is they had their serum Vitamin D <10 ng/mL. When the levels of Vitamin D are below 10 ng/mL chances of associated osteomalacia, impaired muscle function and secondary hyperparathyroidism are very high.10 The study shows similar results as other studies which investigate the prevalence rate of serum Vitamin D and secondary hyperparathyroidism due to VDD. Many studies have proved high prevalence rates of VDD in the fracture patients in this age group. More than 90% of fractures occur as a result of a fall and the rate of fall increases with age as muscle strength and function decrease with advancing age. So this noble hormone Vitamin D prevents both fall and the resultant fracture which is of significant clinical importance. Several studies have proven the role of Vitamin D in maintaining proper muscle health. It is the proximal muscle weakness which is more pronounced as a result of the VDD.11 VDD myopathy also leads to widespread muscle pain and waddling gait. Many studies in the literature have shown the positive and significant improvement of muscle function after Vitamin D supplementation in terms of improvement of muscle strength,12,13 decreased body sway,14,15 an increase in gait speed,16 and an improvement in aggregated physical abilities. Vitamin D receptors have been found in human muscle tissue, and their activation leads to increase in the synthesis of muscle protein. When Vitamin D binds to its receptors, it leads to stimulation of intracellular uptake of the inorganic phosphates which are utilized for generating energy-rich phosphate compounds necessary for sustaining muscle contractility.17,18,19 In a study done on elderly women who were admitted to the hospital for an acute hip fracture and there was no other cause for the bone loss, >50% had Vitamin-D deficiency and elevated PTH levels.20 Fracture around the hip and vertebral fractures due to osteoporosis leads to physical impairment, a decrease in quality of life, increased hospitalization and also increased mortality, especially in the elderly. The Indian population is more prone to osteoporosis and hip fractures. Poor calcium intake and VDD, female sex, early menopause, sedentary lifestyle, and poor sun exposure are the major risk factors leading to these fractures.
In our study, increasing age and sedentary lifestyle have been significantly related with VDD. In Europe, a study by Johnell et al. found that late onset of menarche, poor mental function, low BMI, lesser physical activity, less exposure to sunlight, and low consumption of calcium were significantly associated with the increased risk of hip fracture.21 In the Asian population, Lau et al. showed that low dietary calcium intake, lesser physical activity, alcoholism, and tobacco smoking were significant risk factors for hip fracture.22 Similarly, Fujiwara et al. performed their study in Japan and showed a low BMI, regular alcohol intake, having 5 or more children and a low milk intake to be associated with risk of hip fracture.23 When the calcium available from the diet is not in sufficient quantity, and there is also VDD serum PTH is increased to maintain calcium concentration in the blood so that more of the calcium is withdrawn from bone; it is mainly the cortical bone from which the calcium is eluted leading to decreased bone strength and hence contributes to the pathogenesis of osteoporosis and predisposes the patient to fragility fractures. Although secondary hyperparathyroidism, muscle weakness, and osteomalacia are more pronounced in VDD states, these conditions can be associated with Vitamin D insufficiency also. India is located between 8.4 and 37.61N latitude with the majority of its population is living in regions with ample sunlight throughout the year. However, there are numerous reports of widespread VDD (<20 ng/mL) and/or insufficiency (20–30 ng/mL) in India. This deficiency is seen among both the genders, various socioeconomic groups, young as well as elderly, different ethnicities, rural and urban areas as well as different professions.24
In the present study serum PTH was found to be increasing with age and was inversely related to serum Vitamin D. Recent studies have stated that a serum Vitamin D levels of 30 ng/mL should be used as the cut off value for Vitamin D inadequacy. In the study analyzed in those reviews, serum intact PTH (iPTH) showed a plateau at serum Vitamin D of approximately 30 ng/mL. However, other recent studies showed that the serum Vitamin D level at which iPTH levels attain a plateau cannot be used by itself to estimate the cut off value of Vitamin D inadequacy.25 According to Kim et al. when femur neck BMD values were compared around specific serum Vitamin D values from 10 ng/mL to 30 ng/mL. The BMD values differ most significantly when they compared male and female groups who had more than 15 ng/mL and the other who had <15 ng/mL of S Vitamin D. The iPTH concentration was found to be greatest in both men and women when serum Vitamin D concentration was 15 ng/mL.26
Some studies have shown that intestinal calcium absorption decreases with aging, probably due to intestinal resistance to 1,25 Dihydroxy-Vitamin D, which leads to compensatory increases in iPTH secretion and serum Vitamin D production, to maintain calcium absorption and serum ionic calcium.27,28 Such factors may have contributed to the relatively high iPTH levels in the older individuals in our study. A high serum PTH level in older women may be because of the fact that they had the lowest serum Vitamin D levels as compared to their male counterparts and younger age group females. The low serum Vitamin D levels, in turn, would have probably resulted from the limited outdoor activities of old women compared to the rest of the individuals in the winter season. Since there were relatively small numbers of individuals aged ≥80 years, more study subjects would be required to further evaluate this phenomenon.
Limitations of the study is the standard deviation used to calculate the sample size is based on single pilot case-control study8 and due to the dearth of similar studies adequate sample size calculation can only done by large-scale multicenter studies. Matching age and sex perfectly was also a challenge for us. Sex matching was difficult to obtain as the number of the male case were high and finding an equal amount of male controls was difficult, and hence, we had 60 male cases as compared to 49 male in control group. Similarly, female cases presenting were less in number and finding female control was easier so we had to take 42 female cases and 51 female controls were kept to satisfy the sample size, hence the power of the study. Furthermore, exact one to one matching could not be done for all age groups as finding controls >70 years age group was not easy and we had slightly more cases in >70 years age group as they voluntarily presented to us hence higher in number (26 case versus 11 controls). We chose controls from a healthy population and their comorbidity status was mainly ruled out by detailed clinical history and examination, so we feel that subclinical comorbidities like hypothyroidism which have a bearing on Vitamin D and PTH levels may have been missed, this could affect our result to a limited extent. It was a single center experience with study population belonging to a particular geographical pocket, and most them were hospital based who themselves came for treatment. Unlike this, a large population-based study is the need of the hour so that the prevalence of this burden can be ascertained with higher and accurate confidence. Finally, we feel that our single center experience would pave way for a longitudinal multicenter study involving a larger number of patients to confirm or negate our observations.
Conclusion
The study gives us very important data regarding the prevalence of VDD and rise of serum PTH secondary to the VDD in patient population >45 years in age presenting with fracture due to trivial trauma and this hormonal milieu in our body is an independent significant risk factor for increased incidence of fracture. The study also sheds light on the need of supplementation of Vitamin D stores in elderly population in order to lessen the hazards of fracture resulting from the trivial trauma.
Patient declaration statement
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed W, Khan N, Glueck CJ, Pandey S, Wang P, Goldenberg N, et al. Low serum 25 (OH) Vitamin D levels (< 32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl Res. 2009;153:11–6. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–94. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 4.Glerup H, Eriksen EF. Acroparaesthesia – A typical finding in Vitamin D deficiency. Rheumatology (Oxford) 1999;38:482. doi: 10.1093/rheumatology/38.5.482. [DOI] [PubMed] [Google Scholar]
- 5.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1:626–9. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 6.Skaria J, Katiyar BC, Srivastava TP, Dube B. Myopathy and neuropathy associated with osteomalacia. Acta Neurol Scand. 1975;51:37–58. doi: 10.1111/j.1600-0404.1975.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra N, Mithal A. Osteoporosis in Indians. Indian J Med Res. 2008;127:263–8. [PubMed] [Google Scholar]
- 8.Khadgawat R, Brar KS, Gahlo M, Yadav CS, Malhotra R, Guptat N, et al. High prevalence of Vitamin D deficiency in Asian-Indian patients with fragility hip fracture: A pilot study. J Assoc Physicians India. 2010;58:539–42. [PubMed] [Google Scholar]
- 9.Rao DS, Agarwal G, Talpos GB, Phillips ER, Bandeira F, Mishra SK, et al. Role of Vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: A global perspective. J Bone Miner Res. 2002;17(Suppl 2):N75–80. [PubMed] [Google Scholar]
- 10.LeBoff MS, Hawkes WG, Glowacki J, Yu-Yahiro J, Hurwitz S, Magaziner J, et al. Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos Int. 2008;19:1283–90. doi: 10.1007/s00198-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose Vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: A randomized controlled trial. Cerebrovasc Dis. 2005;20:187–92. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 13.Moreira-Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti-Castro M. Treatment of Vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: A randomized double-blind controlled trial. Ann Nutr Metab. 2009;54:291–300. doi: 10.1159/000235874. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C, et al. Effects of a short-term Vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–8. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 15.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 16.Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, Avendaño M, et al. Effects of Vitamin D supplementation and exercise training on physical performance in Chilean Vitamin D deficient elderly subjects. Exp Gerontol. 2006;41:746–52. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Marcinkowska E. A run for a membrane Vitamin D receptor. Biol Signals Recept. 2001;10:341–9. doi: 10.1159/000046902. [DOI] [PubMed] [Google Scholar]
- 18.Bellido T, Boland R. Effects of 1,25-dihydroxy-Vitamin D3 on phosphate accumulation by myoblasts. Horm Metab Res. 1991;23:113–6. [PubMed] [Google Scholar]
- 19.Rodman JS, Baker T. Changes in the kinetics of muscle contraction in Vitamin D-depleted rats. Kidney Int. 1978;13:189–93. doi: 10.1038/ki.1978.28. [DOI] [PubMed] [Google Scholar]
- 20.LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J, et al. Occult Vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281:1505–11. doi: 10.1001/jama.281.16.1505. [DOI] [PubMed] [Google Scholar]
- 21.Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, et al. Risk factors for hip fracture in European women: The MEDOS study. Mediterranean osteoporosis study. J Bone Miner Res. 1995;10:1802–15. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- 22.Lau EM, Suriwongpaisal P, Lee JK, Das De S, Festin MR, Saw SM, et al. Risk factors for hip fracture in Asian men and women: The Asian osteoporosis study. J Bone Miner Res. 2001;16:572–80. doi: 10.1359/jbmr.2001.16.3.572. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara S. Epidemiology of osteoporosis in Japan. J Bone Miner Metab. 2005;23(Suppl):81–3. doi: 10.1007/BF03026329. [DOI] [PubMed] [Google Scholar]
- 24.Harinarayan CV, Joshi SR. Vitamin D status in India – Its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8. [PubMed] [Google Scholar]
- 25.Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between Vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96:E436–46. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim G, Oh KW, Jang EH, Kim MK, Lim DJ, Kwon HS, et al. Relationship between Vitamin D, parathyroid hormone, and bone mineral density in elderly Koreans. J Korean Med Sci. 2012;27:636–43. doi: 10.3346/jkms.2012.27.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL, et al. Evidence of an age-related decrease in intestinal responsiveness to Vitamin D: Relationship between serum 1,25-dihydroxyvitamin D3 and intestinal Vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75:176–82. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 28.Pattanaungkul S, Riggs BL, Yergey AL, Vieira NE, O’Fallon WM, Khosla S, et al. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH) 2D] levels in young versus elderly women: Evidence for age-related intestinal resistance to 1,25(OH) 2D action. J Clin Endocrinol Metab. 2000;85:4023–7. doi: 10.1210/jcem.85.11.6938. [DOI] [PubMed] [Google Scholar]


