Abstract
Background:
Filling bone defect after debridement of infected nonunion is an orthopedic challenge. Since the volume of autologous bone graft available is limited, allograft, demineralized bone matrix, and calcium phosphate ceramic-based bone graft substitutes have come up as potential autograft expanders. This study was conducted to analyze the use of beta tri-calcium phosphate (B-TCP)-based composite ceramic as autologous bone-graft expander in the management of postinfective segmental gap nonunion of long bones managed with two-stage Masquelet's technique.
Materials and Methods:
42 consecutive patients with postinfective segmental long bone defects of 4–12 cm managed with Masquelet's-induced membrane technique, operated between February 2012 and June 2015, were included in this prospective case series. During the second stage bone-grafting procedure, iliac crest autograft alone or mixed with B-TCP granules (ratio not exceeding >1:1) was used along with appropriate internal-fixation. Bony union (defined clinicoradiologically as ability to painlessly bear weight on affected limb without support along with bridging of 3 cortices on X-rays) was evaluated.
Results:
Union was achieved in 80.9% patients (34/42) with index bone grafting. 100% union rate was achieved in patients where only autograft was used (15/15) and in nonsmoker femoral nonunion patients with the use of B-TCP (13/13). The use of B-TCP was associated with higher rate of nonunion in smokers (6/8, 75%) and in tibial nonunions (4/9, 55.5%). All, but one, of 8 patients with nonunion, united after the second-bone grafting procedure.
Conclusion:
B-TCP is an efficacious and safe autologous bone graft expander in Masquelet's two-stage management of post infective segmental gap nonunion of long bones. Patients should be counseled regarding increased risk of nonunion and need for repeat grafting with its use, especially if they are smokers or site of involvement is tibia.
Keywords: Beta tri-calcium phosphate, bone grafting, Masquelet's technique, segmental gap nonunion
Introduction
Infected gap nonunion of long bones is a significant reconstructive challenge. Ilizarov's bone-transport, vascularized fibula and Masquelet's-induced membrane technique are the primary modalities of limb-salvage in these cases.1 Masquelet's technique has gained ground in the last decade because of its relatively simple technique which has given excellent results in multiple hands other than the originator.2 The biggest challenge currently with this technique is the requirement of large volume of bone graft to fill the segmental defect in 2nd stage. Cancellous autograft from iliaccrest is the best available material, but the supply is limited.3 Reamer-irrigation-aspiration (RIA) system has been used to harvest autograft of high quality from intramedullary canal.4 This has solved the problem of volume but has the potential of causing significant morbidity.3
Induced membrane, by virtue of its highly vascular nature and presence of growth factors,1 presents an excellent opportunity for the use of osteoconductive bone-graft substitutes (allograft, demineralized bone matrix [DBM], and synthetic calcium-phosphate ceramics) as autograft volume expander. Allograft and DBM have been reported to be successfully used in this scenario.3,5 Synthetic calcium-phosphates, especially beta tri-calcium phosphate (B-TCP), have been successfully used as graft expander in spinal fusions, impaction bone grafting in revision total hip replacement and to fill bone cavity after curettage of bone tumors.6,7,8,9,10,11,12 They have also been shown to incorporate predictably in animal models of segmental long bone defect.13 Despite sound experimental and clinical evidence, there is no report in literature which has analyzed their use specifically in the reconstruction of infected segmental gap nonunion treated with Masquelet's technique.
We present our experience of treatment of infected gap nonunion of long bones with Masquelet's technique. We used B-TCP based composite ceramic (40% TCP and 60% HA) (Triosite, Zimmer Biomet, USA.) as bone autograft expander wherever necessary. The aim of the study was to critically analyze the use of B-TCP as autologous graft expander with respect to correct indication and method of its use in such clinical situations and to present overall results of Masquelet's technique in our hands.
Materials and Methods
42 consecutive patients with postinfective segmental long bone defects of 4–12 cm managed with Masquelet's-induced membrane technique, operated between February 2012 and June 2015, were included in this prospective case series. All patients were treated at a single tertiary level teaching hospital by a single surgeon. Inclusion criteria were adult patients (>18 years) with post infective segmental gap nonunion of long bones (femur, tibia, humerus, and both-bones forearm) who consented for treatment with Masquelet's technique. A total of 49 patients were identified from operation theater records. 3 patients (1 tibia and 2 femur) were excluded as they were amputated after first stage on account of uncontrolled infection and patient's reluctance to continue with limb salvage. 4 patients (2 femur, 1 tibia, and 1 humerus) were excluded as they had a defect of <3 cm (with no shortening) and were treated with primary shortening without cement spacer. That left us with 42 patients with a male-to-female ratio of 20:1 (40 males and 2 females). The distribution of patient according to site of involvement was: 24 femur, 16 tibia, and 2 humerus [Figure 1]. Nonunion score (NUS) was calculated on presentation.14,15
Figure 1.
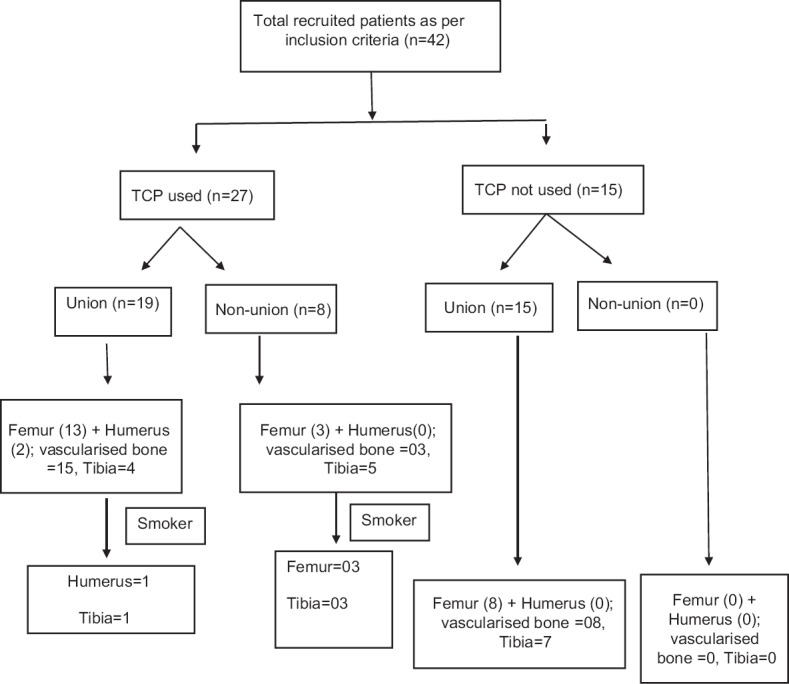
Flow chart showing distribution of patients as per bone involved, use of beta tri-calcium phosphate, union, and smoking
Operative procedure
Patients were asked to stop all antibiotics at least 10 days before first surgery. Detailed informed consent was taken. The first stage involved radical debridement of bone and soft-tissues. Samples for deep-culture were taken and sent for culture-sensitivity. The fracture was adequately stabilized. Femur fracture was stabilized using either antibiotic-loaded cement coated interlock nail or locked plate. Tibia was stabilized with bilateral-uniplanar external fixator along with intra-medullary 2–3 stacked steinmann pins (wherever possible). Humerus was stabilized with intramedullary 2 stacked Steinmann pins. Cement spacer was then made using PMMA cement (Palacos, Zimmer Biomet, USA). A total of 3 g of powder vancomycin and 3 g of powder imipenem were utilized per 40 g batch of cement. Careful position of spacer with overlapping of both bone ends was ensured. Normal saline irrigation was continued to dissipate heat during cement setting. Primary closure was achieved wherever possible. Primary closure was not possible in 5 patients with tibial fractures. All 5 patients underwent same stage rotational flaps (4 gastrocnemius and 1 fasciocutaneous). Postoperative intervenous (i/v) cefuroxime and amikacin were started in weight-adjusted therapeutic dosages and changed according to culture report. Same antibiotics were continued in case of negative culture. I/V antibiotics were continued till stitch removal at 14 days. Oral antibiotics were given for another 7–10 days. Patients were followed every week and control of infection evaluated using C-reactive protein (CRP), erythrocyte sedimentation rate, and local clinical examination. A total of 3 patients (2 femur and 1 tibia) underwent one stage-1 repeat debridement. 1 patient (femur) required two stage-1 repeat debridement procedures. Patients were planned for the second-stage reconstruction. At least 2 weeks of antibiotic holiday was ensured before proceeding with the second stage. External fixator (wherever used) was removed at least 10 days before second-stage surgery to ensure healing of pin-tracks. All patients had a repeat CRP done a day before second stage, and normalization ensured (<10 mg/l). The second stage involved using old incision. Membrane was carefully cut and preserved. Cement spacer was removed. Deep cultures were sent. Medullary canal was opened both proximally and distally. Fracture ends were freshened. The fracture was stabilized with adequate internal fixation. Bone-graft was harvested from iliac-crest. In 15 of these 42 patients, volume of autograft harvested was sufficient to fill the defect in itself as visually confirmed. 27 patients had harvested autograft volume which was not sufficient to fill the defect as visually analyzed. An estimate of defect volume was made using the method to calculate the volume of cylinder, i.e., πr2h (“r” being the average of bone radius at either end and “h” being the bony defect calculated on table). Autograft volume was measured using 50 cc syringe by technique described in literature.16 The autograft volume was built up to desired volume by adding appropriate amount of B-TCP crystals. Care was taken not to exceed ratio of bone graft substitute to bone autograft to >1:1. The mixed graft was loosely packed in the gap and layered closure without drain was done. Postoperative antibiotics were given for two days. Patients were allowed nonweight bearing mobilization on day 3 and stitch removal was done on 14th day. All patients were advised followup visits every 6 weeks and radiographs done to see the progress of union. Toe-touch weight-bearing (with walker) was started typically around 6 weeks. Patients were progressively allowed weight bearing as per pain tolerance after around 12 weeks. All patients were followed clinically and radiologically for a minimum of 12 months. Fracture was considered united if the patient was able to fully bear weight on the extremity without pain and radiographs showed consolidation at 3/4 cortices in orthogonal views after first bone-grafting [Figures 2 and 3] A case showing failure (breakage of implant) in tibia is demonstrated in Figure 4.
Figure 2.
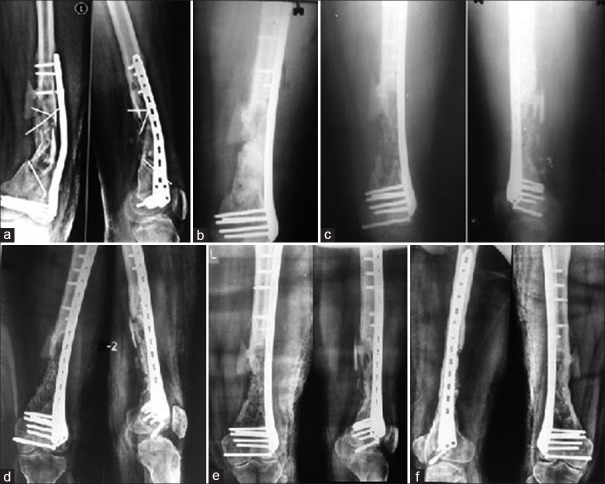
(a) A case of infected nonunion of femur (b) treated with antibiotic-loaded cement coated interlock nail and antibiotic cement spacer (c) filling of gap with bone graft mixed with beta-tricalcium phosphate based composite ceramic as expander (d) follow up at 6 weeks (e) follow up at 6 months (f) follow up at 1 year
Figure 3.
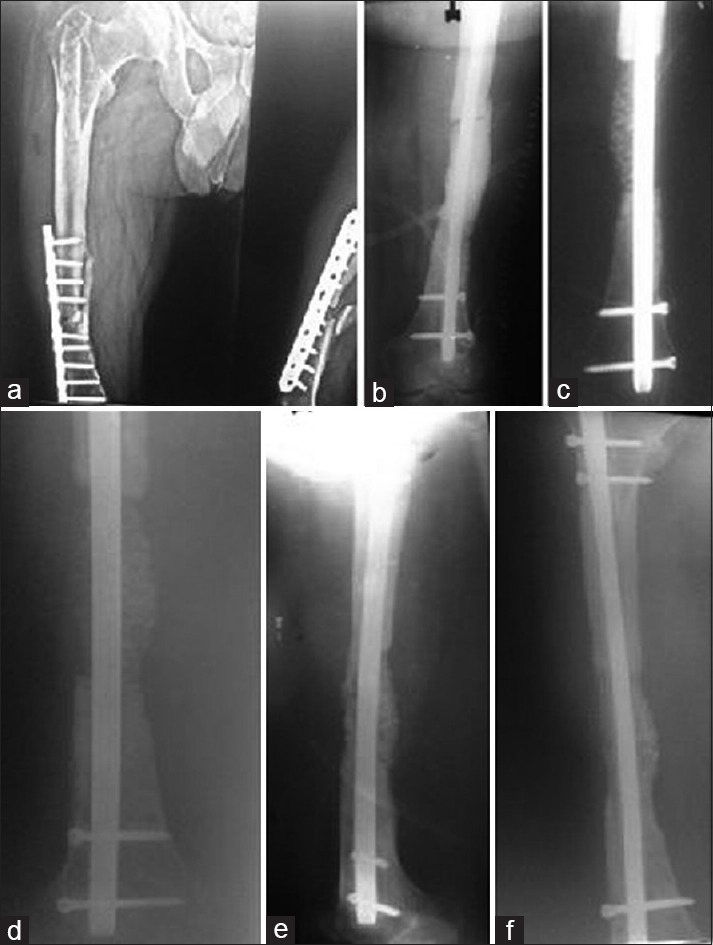
(a) A case of infected nonunion of femur (b) treated with distal femoral locking plate and antibiotic cement spacer (c) filling of gap with bone graft mixed with beta-tricalcium phosphate based composite ceramic as expander (d) follow up at 6 weeks (e) follow up at 6 months (f) follow up at 1 year
Figure 4.
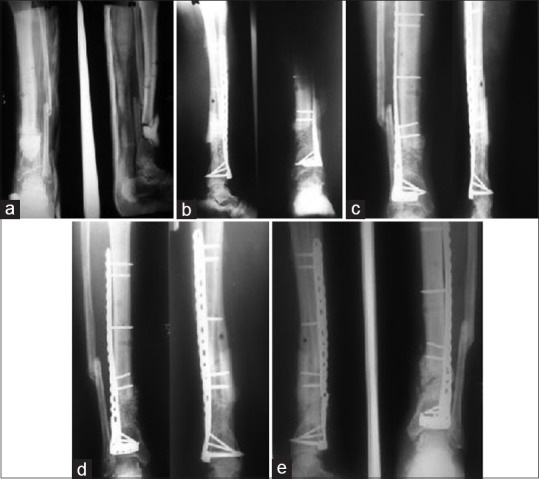
(a) A case of infected nonunion of tibia treated with debridement and antibiotic loaded cement spacer and above knee slab (b) filling of gap with bone graft mixed with beta-tricalcium phosphate based composite ceramic as expander and using distal tibia plate for stability (c) follow up at 6 weeks (d) follow up at 6 months (e) follow up at 9 months showing failure due to breakage of plate
Statistics
Quantitative data were presented as mean ± standard deviation. Normality of data was checked by Kolmogorov–Smirnov tests of normality. For skewed data or scores, Mann–Whitney test was applied. For normally distributed data, independent t-test or Chi-square test/Fisher's exact test was applied, as appropriate. All calculations were performed using SPSS version 22 (Statistical Packages for the Social Sciences, Chicago, IL). P < 0.05 was considered to indicate statistical significance.
Results
A total of 42 patients were recruited in the study. Out of these, average age of the patients enrolled in the study was 36.08 years (range 18–67 years). The average NUS was 64.24 (range 54–74 years). Average number of surgeries per patient till final union was 2.38 (2–4). Average followup duration was 27.7 months (range 12–48 months). Average time to union overall was 9 months (range 6–15 months). B-TCP was used for autograft augmentation in 27 patients (16/24 in femur, 2/2 humerus, and 9/16 tibia), (ratio range 1:4.2–1:1.35) [Table 1].
Table 1.
Summary of relevant study data
| Anatomic area | Number of patients | Average age (Years) | Average nonunion score | Time between 2 stages (days) | Average time to union (months) | Number of nonunion | Percent united | Percent united after 2nd bone grafting |
|---|---|---|---|---|---|---|---|---|
| well vascularized (femur + humerus) | 8 | 33 (22-49) | 64 (52-76) | 59 (36-76) | 7.125 | 0 | 100 | |
| well vascularized (femur + humerus) with TCP | 18 | 31.77 (18-67) | 62.44 (56-74) | 54.33 (37-87) | 9.1 | 3 | 83.34 | 100 |
| Tibia | 7 | 40.57 (20-62) | 63.43 (54-72) | 52.85 (38-66) | 7.5 | 0 | 100 | |
| Tibia with TCP | 9 | 39 (18-56) | 67.11 (56-72) | 50.66 (37-74) | 7.875 | 5 | 44.55 | 88.88 |
TCP=Tri-calcium phosphate
Union was achieved in 80.9% patients (34/42) with index bone grafting. 100% union rate was achieved in patients where only autograft was used (15/15) and in nonsmoker femoral nonunion patients with use of B-TCP (13/13). Use of B-TCP was associated with higher rate of nonunion in smokers (6/8, 75%).
Subgroup analysis
Union rates of patients after first bone-grafting, with or without augmentation of bone-graft with B-TCP, were analyzed. The comparison showed statistically significant difference (P = 0.035 with a likelihood ratio of 8.085).
Preoperative NUS, as a potential confounder in Beta-TCP group, was studied by comparing the patients who united with those who did not and there was insignificant difference (P = 0.423) [Table 2]. Time to second-stage bone-grafting, as a potential confounding factor was analyzed and there was statistically insignificant difference between the groups (P = 0.498) [Table 2].
Table 2.
Potential confounding variables
| Variable | TCP used (union) | TCP used (nonunion) | P |
|---|---|---|---|
| Age | VB 30.6±13.7 | VB 37.67±8.08 | 0.293 |
| Tibia 29.5±12.3 | Tibia 40±9.18 | 0.071 | |
| NUS | 63.26±6.903 | 65.75±4.590 | 0.423 |
| Time to second surgery (days) | 53.84±13.409 | 50.13±11.128 | 0.498 |
NUS=Nonunion score, TCP=Tri-calcium phosphate, VB=Vascularized bone
Union rates of bones with good muscle cover, i.e., femur and humerus, were independently analyzed, and there was no statistically significant difference between union rates with or without use of B-TCP (P = 0.529) [Table 3]. Union rates of tibia (after first bone-grafting) were independently analyzed with regard to whether B-TCP was used or not. Patients with B-TCP use, had a statistically higher rate of nonunion (P = 0.034, likelihood ratio of 7.509) [Table 4].
Table 3.
Union/nonunion in vascularized bone with/without use of beta tri-calcium phosphate
| VB (with/without TCP) | Union | Nonunion | P | Likelihood ratio |
|---|---|---|---|---|
| TCP used-VB | 15 | 3 | 0.529 | 2.376 |
| TCP not used-VB | 8 | 0 |
TCP=Tri-calcium phosphate, VB=Vascularized bone
Table 4.
Union/nonunion in tibia with/without use of beta tri-calcium
| Tibia (with/without TCP) | Union | Nonunion | P | Likelihood ratio |
|---|---|---|---|---|
| TCP used-tibia | 4 | 5 | 0.034 | 7.509 |
| TCP not used-tibia | 7 | 0 |
TCP=Tri-calcium phosphate
The effect of smoking as a risk factor for nonunion with B-TCP use was analyzed in bones with good muscle cover (femur and humerus) and statistically “highly” significant difference was found between the two groups (P = 0.005) [Table 5].
Table 5.
Union/nonunion in vascularized bone with use of beta tri-calcium in smokers/nonsmokers
| VB + TCP ± smoker | Number of total patients | Union | Nonunion | P |
|---|---|---|---|---|
| TCP used-VB-smoker | 4 | 1 | 3 | 0.005 |
| TCP used-VB-nonsmoker | 14 | 14 | 0 |
TCP=Tri-calcium phosphate, VB=vascularized bone
A detailed comparison was done to compare the age of patients who underwent union with those who could not achieve union with B-TCP use. There was no statistically significant difference among the various subgroups in terms of age [Table 2].
Complications
Nonunion was observed in eight cases after index procedure. Seven cases underwent repeat bone grafting at nonunion site and went on to achieve union. One patient refused to undergo bone grafting and was given patellar tendon-bearing (PTB) cast. At final followup (2 years) patient was walking with PTB-brace with 7° of varus deformity. Four cases (all femur) had recurrence of infection which manifested in the form of pus discharging sinuses. All of them resolved with appropriate implant removal and debridement.
Discussion
This is the first study to critically evaluate the use of monophasic ca-p crystals (B-TCP) as bone-autograft expander in Masquelet's technique. We studied 42 cases and used B-TCP in 27 cases. Union was achieved in 80.9% patients (34/42) with index bone grafting. 100% union rate was achieved in patients where only autograft was used (15/15) and in nonsmoker femoral nonunion patients with use of B-TCP (13/13). Use of B-TCP was associated with higher rate of nonunion in smokers (6/8, 75%). Sub-group analysis also showed significantly higher nonunion rate (55.5%, 5/9) with use of B-TCP in tibial nonunions. Age, time to bone-grafting after 1st stage and severity of nonunion (NUS) were not found to be significant factors affecting union with/without bone-graft substitute. All 8 un-united cases showed reasonable consolidation of intercalary graft with nonunion at one end. 7 out of these 8 cases united after further bone-grafting of ununited end. 3 patients (all femur) had recurrence of infection after union. We achieved an overall union rate of 97.6% (41/42) and overall infection control rate of 92.8% (39/42). Average number of surgeries required from presentation to final followup was 2.35 (range 2–4).
The findings of our study have significant implications. Masquelet's technique is an excellent procedure. The only major limitation is the requirement of large amount of autologous bone-graft in second stage. Iliac–crest and RIA are the sites for autograft harvest. Although Masquelet and Beguehave reported successful harvest of autograft from all 4 iliac-crest sites for reconstruction of segmental defect up to 20 cm,2 the feasibility of such a procedure is doubtful in most surgeons’ hands because of the time and extent of surgery involved. Moreover, many of these patients in our setting have already had 1 or 2 of their sites already harvested. To circumvent the problem of volume, use of allograft, and DBM as autograft expander has been reported in literature.3,5 The problem with them is availability and potential for antigenic reaction and disease-transmission. Reamer-irrigation-aspirator system has been successfully used to harvest high volume of autograft but is associated with morbidity and potentially fatal complications.3,4 Moreover, it has a high recurring cost of equipment which is a barrier to its use in most developing countries.
The induced membrane is highly vascular with demonstrated the presence of osteogenic factors.1,2 This presents a unique opportunity for use of ceramics such sa B-TCP as bone-graft expander. Calcium-Phosphate ceramics, especially B-TCP, have microstructure similar to cancellous bone.5 Being synthetic in nature, it is available in unlimited quantity and has no potential for disease transmission and antigenic reaction. B-TCP has an established role as autograft-expander in posterior-spinal fusions.6,7 They have also been successfully used as graft-expanders in impaction bone-grafting and to fill bone-cavities after tumor-curretage.8,9,10,11,12 Experimental studies have also shown predictable uptake of B-TCP in animal model of segmental long bone-defects.13 Despite the potential advantages and evidence for use; there is no report in literature mentioning the use of B-TCP as bone autograft expander in reconstruction of postinfective segmental gap nonunion of long bones with Masquelet's technique. With this study, we have been able to show successful use of B-TCP as bone autograft expander, especially in femur and humerus. This adds significant dimension to this procedure as it can greatly ease the second-stage surgery without the need for aggressive autograft harvesting.
We tried to analyze the reasons of failure with use of B-TCP. Smoking was found to be one of the risk factors for failure. Nonsmoker with involvement of bones with good muscle cover (humerus and femur) achieved 100% union rate (14/14) even with use of B-TCP. Moreover, the average time to union was also not too different (9.1 months) when compared with patients where pure autograft was used (7.1 months). These cases also showed progressive remodeling after union and complete uptake of granules at 2 years from grafting procedure. All the failures in bones with good muscle cover (humerus and femur) occured in those who were active smokers. This is not surprising as the studies on posterior spinal fusion have also shown decreased rate of graft-consolidation in smokers when B-TCP was used as graft-expander.6,7 Smoking has also been shown to result in delayed and unpredictable consolidation of regenerate even with distraction osteogenesis.17 The other risk factor was tibia as site of nonunion (5/9). This was surprising as despite having inherently poor muscle cover, graft uptake should have been predictable given the osteoinductive and vascular nature of induced membrane. Due to small number of cases, we could not definitely comment as to whether any other confounding factor apart from B-TCP use was responsible for this finding because all these cases showed reasonable consolidation of intercalary graft with nonunion at one end. Concomitant smoking could be one of the reasons as 3 cases were smokers. Time to grafting or severity of injury (as determined by NUS) was no different as compared to cases who showed union. We preoperatively assigned NUS to all patients. This scoring system was introduced by Calori et al. and has been further validated to reflect the severity of nonunion.14,15 The average NUS in our series was 63.9 (range 52–76) which reflects the complex nature of the patients treated. The average number of surgeries required from presentation to union was 2.35 (range 2–4). This is less as compared to literature.1,2 We are not sure whether it is due to difference in severity of cases as other series have not utilized objective scoring system as utilized here. In our opinion, the low number of surgeries required to achieve union in our series can be attributed to aggressive first-stage debridement with careful spacer insertion, early soft-tissue cover (wherever required), and emphasis on highly stable internal fixation in second stage. These 3 factors should be considered cornerstone of management.
Overall union rate (97.6%) in our study is comparable to that of literature and further emphasize the utility of this procedure.1,2 The ratio of bone-substitute to bone-graft used in our series is 1:2.34 (1:1.35–1:4.4) and is similar to what has been used in spinal fusions.6,7 The ratio up to 1:1 does not seem to affect the rate and time of graft consolidation. These substitutes require rigid fixation for their uptake. All our cases were internally fixed with stable fixation which is one of the factors for success. Recurrence of infection after union is known with this technique and our rates ([4/42], 10%) are similar to that in literature, despite using Beta-TCP in large majority of our cases.1,2
Our study does have a few shortcomings. It is a prospective study with consecutive cases, but of a single-surgeon. A large multicenter study prospectively evaluating different ceramics, allograft, and autograft are warranted so as to realize the full spectrum of indications as well as unmask the bias inherent in small single-surgeon study. Despite this, our study does provide an emphatic baseline evidence to potential success of B-TCP as autograft expander in Masquelet's technique, especially when used in nonsmoking patients with involvement. Its use in tibia and smokers should be done with caution and patient should be counseled regarding high likelihood of requiring additional bone grafting.
Conclusion
Masquelet's technique is a highly effective option of managing infected gap nonunion of long bones in indicated patients. B-TCP is an efficacious and safe autologous bone graft expander in Masquelet's two-stage management of post infective segmental gap nonunion of long bones. Patients should be counseled regarding increased risk of nonunion and need for repeat grafting with its use, especially if they are smokers or site of involvement is tibia.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Karger C, Kishi T, Schneider L, Fitoussi F, Masquelet AC French Society of Orthopaedic Surgery and Traumatology (SoFCOT) Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Betz RR. Limitations of autograft and allograft: New synthetic solutions. Orthopedics. 2002;25:s561–70. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 4.Stafford PR, Norris BL. Reamer-irrigator-aspirator bone graft and bi masquelet technique for segmental bone defect nonunions: A review of 25 cases. Injury. 2010;41(Suppl 2):S72–7. doi: 10.1016/S0020-1383(10)70014-0. [DOI] [PubMed] [Google Scholar]
- 5.Van Heest A, Swiontkowski M. Bone-graft substitutes. Lancet. 1999;353(Suppl 1):SI28–9. doi: 10.1016/s0140-6736(99)90228-3. [DOI] [PubMed] [Google Scholar]
- 6.Muschik M, Ludwig R, Halbhübner S, Bursche K, Stoll T. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: Preliminary results of a prospective clinical study. Eur Spine J. 2001;10(Suppl 2):S178–84. doi: 10.1007/s005860100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424–9. doi: 10.1097/00024720-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 8.van Haaren EH, Smit TH, Phipps K, Wuisman PI, Blunn G, Heyligers IC, et al. Tricalcium-phosphate and hydroxyapatite bone-graft extender for use in impaction grafting revision surgery. An in vitro study on human femora. J Bone Joint Surg Br. 2005;87:267–71. doi: 10.1302/0301-620x.87b2.14749. [DOI] [PubMed] [Google Scholar]
- 9.Saikia KC, Bhattacharya TD, Bhuyan SK, Talukdar DJ, Saikia SP, Jitesh P, et al. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J Orthop. 2008;42:169–72. doi: 10.4103/0019-5413.39588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogose A, Hotta T, Kawashima H, Kondo N, Gu W, Kamura T, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res B Appl Biomater. 2005;72:94–101. doi: 10.1002/jbm.b.30136. [DOI] [PubMed] [Google Scholar]
- 11.Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K, et al. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990;72:298–302. doi: 10.1302/0301-620X.72B2.2155908. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Onga T, Marui T, Mizuno K. Use of hydroxyapatite to fill cavities after excision of benign bone tumours. Clinical results. J Bone Joint Surg Br. 2000;82:1117–20. doi: 10.1302/0301-620x.82b8.11194. [DOI] [PubMed] [Google Scholar]
- 13.Grundel RE, Chapman MW, Yee T, Moore DC. Autogeneic bone marrow and porous biphasic calcium phosphate ceramic for segmental bone defects in the canine ulna. Clin Orthop Relat Res. 1991;266:244–58. [PubMed] [Google Scholar]
- 14.Calori GM, Phillips M, Jeetle S, Tagliabue L, Giannoudis PV. Classification of nonunion: Need for a new scoring system? Injury. 2008;39(Suppl 2):S59–63. doi: 10.1016/S0020-1383(08)70016-0. [DOI] [PubMed] [Google Scholar]
- 15.Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Marelli N, et al. Validation of the nonunion scoring system in 300 long bone nonunions. Injury. 2014;45(Suppl 6):S93–7. doi: 10.1016/j.injury.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Carragee EJ, Comer GC, Smith MW. Local bone graft harvesting and volumes in posterolateral lumbar fusion: A technical report. Spine J. 2011;11:540–4. doi: 10.1016/j.spinee.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Zheng LW, Sham MH, Cheung LK. Uncoupled angiogenesis and osteogenesis in nicotine-compromised bone healing. J Bone Miner Res. 2010;25:1305–13. doi: 10.1002/jbmr.19. [DOI] [PubMed] [Google Scholar]