Abstract
Background:
The healed status (end-point of treatment) in tuberculosis (TB) spine is not defined; hence optimum antitubercular therapy (ATT) duration is unresolved. We, for the first time, prospectively evaluated the healed status in TB spine by fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) and contrast magnetic resonance imaging (MRI) with the objective to define end-point of treatment in TB spine.
Materials and Methods:
Thirty seven patients of TB spine diagnosed on clinicoradio imaging/cytology/histologically/molecular methods were enrolled, treated and were evaluated radiologically, by contrast MRI and FDG-PET/CT at 9 months. ATT was stopped on contrast MRI-based healing or absence of FDG uptake on PET-CT. ATT was continued in active/resolving lesion. Repeat evaluation was done at 12, 18, 24, and 30 months till healing is demonstrated. In this research work, we got contrast MRI and FDG-PET/CT done for the patients from government institution free of cost, so patients did not have to bear the burden of cost of these investigations.
Results:
Twenty-eight patients achieved healed status out of which 11 demonstrated healed status on contrast MRI and FDG-PET/CT both, 6 were MRI active (contrast enhancement) but FDG-PET/CT healed, 2 were MRI healed but FDG-PET/CT active (soft-tissue standardized uptake value <2.0), 9 patients were MRI incompatible due to stainless steel implants (n = 6), and in 3 patients MRI could not be done due to financial constraints and were declared healed on FDG-PET/CT. FDG-PET/CT showed healed bone lesion in 28/28 (100%) and on MRI 13/19 (68.42%), respectively. We had 6 patients whose spine was stabilized with stainless steel implants where MRI could not be performed, MRI was useful in 13/25 cases (52%) to demonstrate healed lesion. 7, 6, 6, 5, 1, 2, and 1 cases achieved healed status at 9, 12, 18, 24, 30, 36, and 48 months of ATT intake, respectively.
Conclusions:
FDG-PET/CT is more useful to demonstrate the healed status than MRI and is the only imaging to demonstrate healed status when MRI could not be performed due to metallic implants. All patients achieved healed status at variable length of ATT intake; hence TB spine should be treated by ATT till healed status (end-point of treatment) is demonstrated by FDG-PET/CT (absence of FDG uptake) or contrast MRI.
Keywords: Spine, tuberculosis, antitubercular therapy duration, contrast magnetic resonance imaging, fluorodeoxyglucose-positron emission tomography/computed tomography, Healed tuberculosis spine
Introduction
Spinal tuberculosis (TB) is a medical disease treated by antitubercular chemotherapy (ATT), while surgery is indicated to prevent/treat the complications, that is, neurological deficit, deformity, and presumptive drug resistance.1 The optimum duration of ATT in spinal TB lacks consensus as radiological signs of healing lags the clinical improvement. No serological and/or immunological markers exist to guide the end-point of treatment to stop ATT. Clinicoradiological healing at completion of ATT with no relapse at 2-year followup is defined as healed status.1,2
The remineralization of the vertebral body (VB), sharpening of disc margins, reduction of pre-/paravertebral shadows, and osseous/fibro-osseous fusion are signs of healing on X-rays but are unreliable.1,3,4 The discernible remineralization in tuberculous lesion can be appreciated after 2–3 months of ATT.5
Complete resolution of pre-/paravertebral collections, marrow edema of vertebra, and its replacement by fat/calcification (isointense T1WI and T2WI) on contrast magnetic resonance imaging (MRI) are observed with healing of spine TB.6,7,8 On MRI, the inflammatory response is observed in active disease/healing of lesion, hence contrast MRI can continue to show enhancement even in healed lesion.9
Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) is a radionuclide scan showing increased activity at lesion due to increased glucose uptake (infections, inflammations, and malignancies). The measurement of uptake is standardized uptake value (SUV) characterizing the relative concentration of radiotracer in the lesion. Thus, FDG-PET/CT allows noninvasive quantitative assessment of biochemical and functional processes.10 Zinn compared FDG-PET/CT and CT in diagnosis of 16 cases of spinal TB and found superiority of FDG PET/CT in diagnosis as well as monitoring response to treatment.11 However, use of FDG-PET/CT to evaluate the healed status in TB spine has never been reported hence this study.
Materials and Methods
After approval from the institutional ethical committee, 37 patients of spinal TB having paradisical affection, with/without paraplegia aged 8 years or more with mean age 32.36 (range 8–65 years) of either sex (11 males and 26 females) were enrolled. These cases were diagnosed clinicoradiologically and by MRI and/or histopathology/fine-needle aspiration cytology/bacteriology/molecular tests. The freshly diagnosed cases (n = 25) (on ATT for less than 9 months) who were prospectively treated and patients who were in various stages of treatment and were diagnosed as per criteria described above (n = 12) were also included. The known cases of chronic illnesses, claustrophobic patients, cases with single VB lesion, isolated intraspinal granuloma or posterior complex involvement, isolated craniovertebral or sacral TB were excluded from the study. Complete blood count, erythrocyte sedimentation rate (ESR), liver function test, and kidney function test were performed.
The diagnostic findings on X-rays were demineralization of the vertebra, fuzzy paradiscal margins, reduction/obliteration of disc spaces, anterior scalloping/height loss of VB, kyphotic deformity, and paravertebral shadows.1,3,4 The MRI was performed with 3 Tesla magnetic field strengths. Sagittal, axial, and coronal T1 and T2W fast spin-echo images with additional coronal and sagittal STIR fat suppression sequence were obtained. The contiguous VB involvement, preserved disc height, marrow edema of VB with end plate erosions and discitis, with pre-/paravertebral septate loculated collection (including intraosseous abscess), and epidural extension were considered diagnostic of spinal TB.12,13,14,15,16
All cases were treated with ATT intermittent dose regimen (thrice weekly) as advocated by Revised National TB Control Programme17 consisting of rifampicin (R) 450 mg (10 mg/kg, maximum 600 mg), isoniazid (H) 300 mg (10 mg/kg) (maximum 600 mg), pyrazinamide (Z) 1500 mg (35 mg/kg with maximum dose of 2000 mg), and ethambutol (E) 1200 mg (30 mg/kg, maximum 1600 mg) for 2 months followed by isoniazid (H) and rifampicin (R) for the 7 months. The blood investigations and X-rays were performed every 2 months. Surgery was undertaken for diagnostic dilemma, persistence/worsening of neurological deficit, unstable spine, and progressive kyphotic deformity during course of treatment. Healing was evaluated clinicoradiologically, by contrast MRI and FDG-PET/CT at 9 months. Pain reduction, improved constitutional symptoms, weight gain, absence of cold abscesses, healed sinuses, ESR reduction, and rise in hemoglobin were considered clinical improvement. On X-rays, remineralization of VB, reappearance of bone trabeculae, sharpening of cortical margins, and vertebral fusion were observed on healing.1,3,4 Complete resolution of pre-/paravertebral abscess, replacement of marrow edema of VB by fat/calcification, and absence of contrast enhancement was considered healed lesion on MRI.6,7,8 The decreased abscess size and marrow edema, partial fatty replacement of marrow and persistent contrast enhancement were labeled resolving but active lesion.
FDG-PET/CT was performed after 60 min following intravenous (IV) injection of 370 MBq of 18FFDG (fluorinated deoxyglucose). Images from the skull to mid-thigh were acquired in a whole-body full ring PET CT scanner (discovery STE16 camera). A low dose CT was obtained on the same area without IV contrast for attenuation, correction and co-registration. Images were reconstructed using a three-dimensional virtual unenhanced algorithm and slices were reformatted into axial, coronal and sagittal views.10 Absence of FDG uptake measured in terms of SUV (SUV value – 0 in both bone and soft tissues) was considered healed lesion in FDG-PET/CT. ATT was stopped in patients showing healed lesion on Contrast MRI and/or FDG-PET/CT. These patients were evaluated every 6 months clinically and by X-rays till 2 years after completion of treatment to see any relapse of disease.
ATT was continued in patients showing active uptake of FDG-PET/CT. The lesion was again evaluated at 12, 18, and 24 months till healed status is attained by contrast MRI and/or on FDG-PET/CT. In this research work, we got contrast MRI and FDG-PET/CT done for the patients from government institution free of cost so patients did not have to bear the burden of cost of these investigations.
Results
Thirty-seven patients of TB spine were enrolled. All patients (n = 37) had constitutional symptoms. 28 cases were diagnosed by clinicoimaging including MRI finding. Nine patients were diagnosed on histopathology/acid–fast bacilli (AFB) smear/culture after CT-guided biopsy (n = 4), lymph node biopsy (n = 1), open biopsy (n = 1) and surgical debridement (n = 3). The histopathological diagnosis was available in 9/9 while culture in 3/9 patients and AFB smear in 1/9 patient.
A total of 101 vertebral bodies (mean: 2.75; range: 2–8) were affected. Lumbar spine was involved in 17 patients, dorsal spine was involved in 16 patients followed by cervical spine and multiple levels of vertebrae involvement in two each.
A total of 18 patients were managed nonoperatively with ATT, bed rest, and spinal braces. 19 patients required surgical intervention for diagnostic dilemma (n = 1), persistence/worsening of neurological deficit (n = 11), unstable spine (n = 4), and progressive kyphotic deformity (n = 3). They were operated for decompression only (n = 2), decompression + instrumentation (n = 13), deformity correction (n = 3), and open biopsy (n = 1).
In total [Table 1], 28/37 patients (75.6%) achieved healed status with average duration of 19.85 months (range 9–47 months) ATT. Average MRI performed per patient were 2.48 (range 1–4) and average FDG-PET/CT performed were 1.40 (range: 1–3), 9/37 patients are still on treatment.
Table 1.
The final observation on contrast magnetic resonance imaging and 18-fluorodeoxyglucose-positron emission tomography/computed tomography
| Observation of last contrast MRI | Observation of last FDG-PET/CT | Number of patients (n=37) |
|---|---|---|
| Healed lesion | No FDG activity (SUV bone and soft tissues- Zero) | 11 |
| Resolving lesion | No FDG activity (SUV bone and soft tissues- Zero) | 6 |
| Healed lesion | FDG activity in soft tissue (SUV <2.0), SUV bone- Zero | 2 |
| Not performed (incompatible implants [n=6]/financial constraints [n=3]) | No FDG activity (SUV bone and soft tissues- Zero) | 9 |
| Resolving lesion (on followup) | FDG activity in bone and soft tissues seen | 9 |
FDG=Fluorodeoxyglucose, PET=Positron emission tomography, CT=Computed tomography, SUV=Standardized uptake value, MRI=Magnetic resonance imaging
Eleven patients declared healed on contrast MRI and FDG-PET/CT both. The duration of ATT was 9 months (n = 4), 12 months (n = 1), 18 (n = 3), 24 months (n = 2), and 27 months (n = 1).
Six patients showed resolving lesion on MRI, but FDG-PET/CT showed no FDG uptake suggestive of healed lesion [Figure 1]. The duration of ATT was 9 months (n = 2) while 14, 18, 36, and 47 months in one patient each. This patient who received 47 months of ATT was treated elsewhere and came to us with ATT intake of 47 months with contrast MRI done outside showing resolving but active lesion, we kept clinical suspicion of drug resistance in our mind and CT-guided biopsy was done to ascertain diagnosis and for drug sensitivity testing. Biopsy suggested necrotizing granuloma with rifampicin and isoniazid sensitivity on cartridge-based nucleic acid amplification test and line probe assay. However, FDG-PET/CT showed no activity hence ATT was stopped.
Figure 1A.
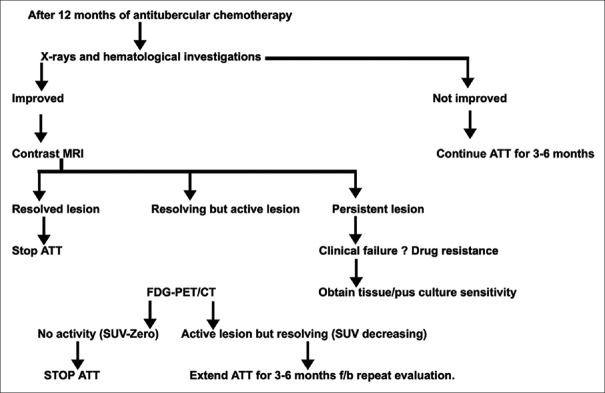
(At 0 months): A 54-year-old female with complaint of neck pain and weakness in the upper and lower limbs with X-ray cervical spine (a) showing reduction of disc space with C5–C7 vertebral body destruction with regional osteoporosis; magnetic resonance imaging cervical spine (b-e) showing altered signal intensity of C5–C7 vertebral bodies with evidence of subligamentous spread of prevertebral abscess beneath anterior longitudinal ligament, axial image (d and e) showing septate pre- and paravertebral collections was diagnosed as a case of Potts spine C5–C7 in January 2015 on clinicoimaging basis and was subsequently started on antitubercular therapy
Figure 1B.
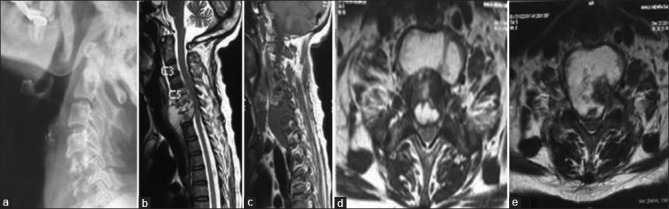
(At 0 + 9 months): X-ray cervical spine (a) shows sharpening of vertebral margins with sclerosis of endplates; contrast magnetic resonance imaging cervical spine (b-e), (b and c), sagittal T2WI and T1WI shows complete resolution of prevertebral and paravertebral collection with hyperintense signal in T1WI suggestive of fatty replacement of bone marrow. (d) Sagittal postcontrast image shows minimal contrast enhancement. (e) Axial T2WI shows complete resolution of all collections with marrow edema suggestive of resolving lesion on MRI; whole-body fluorodeoxyglucose-positron emission tomography/computed tomography (f) shows no fluorodeoxyglucose uptake in the body. Antitubercular therapy was stopped on basis of healing on fluorodeoxyglucose-positron emission tomography/computed tomography at 12 months. At 2-year followup, the patient is asymptomatic and no signs of reactivation
Two patients showed healed lesion on MRI while PET/CT showed no activity in VB (healed bone lesion) and SUV <2.0 in soft tissues, hence were considered healed lesion [Figure 2]. They attained healed lesion at 12 and 22 months of ATT.
Figure 2A.
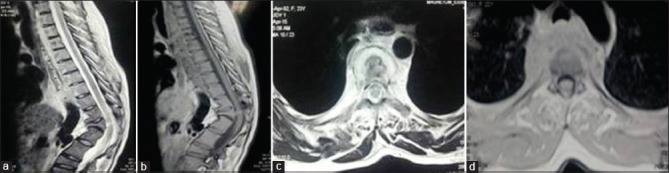
(0 months) – 24-year-old female with complaint of backache and kyphotic deformity with magnetic resonance imaging dorsal spine (a-d) showing altered signal intensity involving D1–D8 vertebrae with subligamentous spread of prevertebral collection and kyphotic deformity at D11–D12 (a and b), axial image (c and d) showing large prevertebral collection and patient was diagnosed as a case of Potts spine D1–D8 on clinicoimaging basis, which was confirmed by histopathology and molecular tests (BACTEC) when patient underwent anterolateral decompression in June 2015 due to suspicion of drug resistance as patient had taken 12 months of antitubercular therapy 10 years back for same disease. The mycobacterium was isolated and was sensitive to the first line antitubercular therapy drugs. The patient was started on Category 2 antitubercular therapy
Figure 2B.
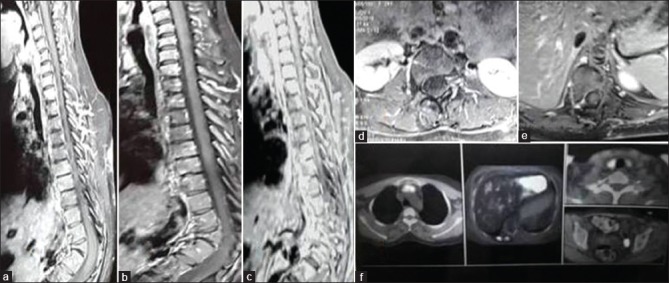
(0 + 12 months) Contrast magnetic resonance imaging Dorsal spine (a-e), (a and b) T2WI and T1WI sagittal shows D11 and D12 block vertebrae with gibbus formation, no evidence of pre-/paravertebral/epidural collection with fatty replacement seen as hyperintensity in T1W1 (b-d) postcontrast sagittal and axial image shows no contrast enhancement; whole-body fluorodeoxyglucose-positron emission tomography/computed tomography (f) shows low-grade fluorodeoxyglucose uptake along paraspinal muscles corresponding to D10 vertebrae (standardized uptake value max-2.0). Antitubercular therapy stopped on the basis of healing of lesion seen on contrast magnetic resonance imaging
Figure 2C.
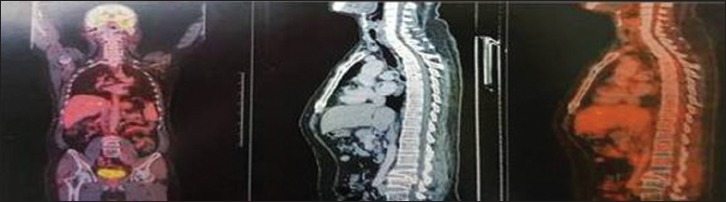
(3 months followup) – whole-body fluorodeoxyglucose-positron emission tomography/computed tomography – no metabolic activity noted
MRI evaluation on followup could not be performed in 9 patients, six due to use of stainless steel for instrumented stabilization [Figure 3] and in three due to financial constraints. PET/CT showed healed lesion in all. The duration of ATT was 9 months (n = 1), 12 months (n = 3), 18 months (n = 2), and 27, 30, and 36 months in one each.
Figure 3A.
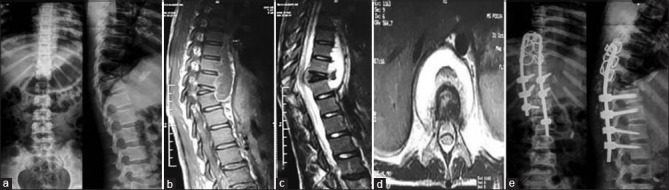
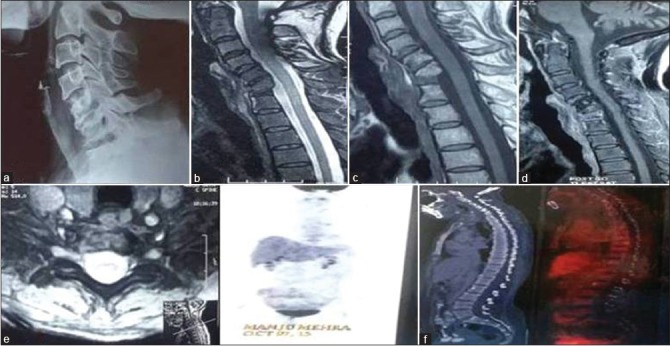
A 11-year-old female with complaints of back pain and weakness of bilateral lower limbs with X-ray dorsal spine (a) showing wedge collapse of D10 vertebrae with reduction in disc space D10–D11 with regional osteoporosis; magnetic resonance imaging dorsal spine (b-d) showing altered signal intensity involving D10–D11 vertebrae with subligamentous spread of prevertebral collections was diagnosed as a case of Potts spine D10–D11 in January 2015 on clinicoimaging basis. The patient underwent decompression with instrumented stabilization with pedicle screw (D11–L1) and sublaminar wires with Hartshill implant (stainless steel), pedicle holes in proximal vertebrae showed pus on probing and was subsequently started on Category 1 antitubercular therapy
Figure 3B.
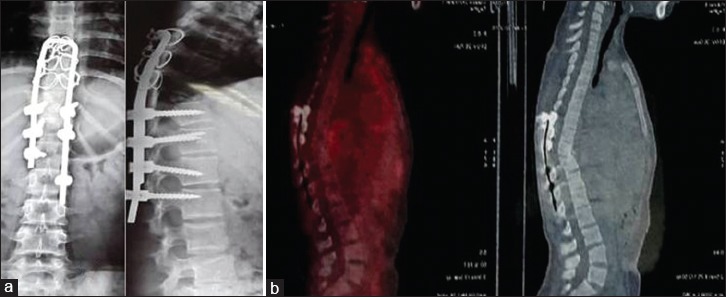
Xray dorsal spine at 12 months (a) shows stainless steel implant in situ . Whole body 18-FDG PET/CT (b) shows no uptake in as magnetic resonance imaging could not be done as stainless steel implants are incompatible with magnetic resonance imaging. Antitubercular therapy was stopped on the basis healing demonstrated on positron emission tomography scan at 12 months
Nine patients are still on treatment as they showed resolving lesion on contrast MRI and FDG activity on FDG-PET/CT. On an average, 3.331,2,3,4,5,6 MRI and 2.2 (range 1–5) FDG-PET/CT were performed. The average duration of ATT was 26.11 months of treatment (range 15–39 months). Average SUV on last FDG-PET/CT was 3.01 (range 1.6–6.1). The size of abscess reduced in 3 patients at 13, 30, and 25 months, respectively, but had persistent marrow edema and contrast enhancement without fat replacement on MRI. The SUV value on their respective last FDG-PET/CT were 1.7, 3.85, and 6.1 in vertebral bodies, hence ATT was continued. One patient at 17-month ATT on MRI showed small abscess, fat replacement of marrow, marrow edema, and contrast enhancement with 2.8 SUV on FDG-PET/CT. Two patients at 32 and 16 months treatment, respectively, with complete resolution of abscess, persistent marrow edema, fat replacement of marrow, and contrast enhancement had active lesion in their last FDG-PET/CT with SUV 1.6 and 3.8 in VB's, respectively. In two patients at 18 months and 9 months duration of treatment, respectively, MRI could not be performed due to stainless steel implant and SUV in their last FDG-PET/CT was 3.8 and 1.5, respectively, hence ATT was continued.
Ninth patient with L3–L4 TB showed resolving lesion on contrast MRI at 32 months. The SUV was decreasing in successive FDG-PET/CT (last L3–L4 SUV-2.5), ATT was stopped and the patient was evaluated every 6 months with no clinicoradiological relapse at 14 months. Out of 27 healed cases, 7/28 (25.0%) healed at mean 9 months, 13/28 (46.4%) at 12 months, 19/28 (67.8%) at 18 months, 24/28 (85.7%) at 24 months, 25/28 (89.2%) at 30 months, 27/28 (96.4%) at 36 months, and all cases achieved healing by 48 months. The ATT intake more than 18 months in 9/28 (32.1%) cases.
FDG-PET/CT demonstrated healed bone lesion in 28/28 (100%) cases (SUV <2.0 observed in soft tissues in 2 cases), MRI could not be done in 9 of these cases. MRI demonstrated healed response in 13/19 (68.42%) patients. If we include 6 patients who had spine stabilized with stainless steel implants incompatible with MRI, MRI was useful in 13/25 cases (52%) to show healed lesion.
FDG-PET/CT demonstrated healed lesion (n = 6) where contrast MRI showed resolving lesion. Thus, FDG-PET/CT was more useful to objectively define healed status. Nine patients where the lesion was active on FDG-PET/CT also showed resolving active lesion on contrast MRI.
In six patients, TB lesions with stainless steel implants incompatible for performing MRI, FDG-PET/CT was only imaging modality to demonstrate healed status.
Discussion
The optimum duration of ATT intake to achieve healed status is unresolved due to lack of definition of end-point of treatment. The 18 months ATT was associated with drug-related toxicity, poor compliance, while short course with persistence of disease/relapse/emergence of drug resistance.18 Ramachandran (2005) reported relapse in five/eight patients with 6-month regime, while none/30 with ATT 9 months or longer.19 In pulmonary TB, the presence of AFB in sputum and its absence on ATT is the key to decide optimum duration of ATT, which is difficult or almost impossible in paucibacillary deep-seated spinal lesions.18 Thus, imaging modalities are used to monitor the treatment.
Early response to ATT can be appreciated on followup X-rays,1,3,4 hence healed status was defined by clinical improvement and radiological signs of healing at completion of predefined ATT regimen without relapse at 2-year followup.1
The healing of lesion can be appreciated on MRI.6,7,8 The inflammatory response of active disease and of repair cannot be differentiated on MRI. The perivertebral abscesses, area of osseous destruction, and bone edema may increase at 6 months ATT.9 The contrast enhancement may continue for many months despite healed lesion. Kramer6 reported progressive healing as increasing signal intensity in T1WI due to focal fat marrow along with decreasing signal in T2WI. Enhancement following GdDTPA administration also decreased with healing. Gillams7 reported MR findings in retrospective analysis (n = 20) of infectious spondylitis (TB = 2), median followup 8 weeks (range 2–104 weeks). Fourteen patients improved on treatment. The reduction in the inflammatory soft tissue (8/14, 57%) was the first sign of treatment response. Bone/disc changes progressed in six/eight patients (75%), new disc level involved in 4 (50%). High signal peripheral rim in T1WI of bone as definite sign of healing was noted in 5 (36%). Gadolinium enhancement persisted for median 17.5 weeks (range 8–80 weeks) after resolution of soft-tissue changes. Jain8 evaluated (n = 51) the efficacy of extended directly observed treatment, short-course (DOTS) (8 months) regimen by contrast MRI. The complete resolution of pre-/paravertebral collections, resolution of vertebral marrow edema, and replacement by fat/calcification (isointense T1WI and T2WI) on contrast MRI were considered healed lesion.
In the present study, we considered the absence of contrast enhancement as criteria of healed lesion. Persistence of contrast enhancement on MRI despite resolution of abscess, resolution of marrow edema (isointense on T1- and T2-weighted images), and fatty replacement of marrow was considered resolving lesion. FDG-PET/CT measures biological activity as SUV due to increased glucose uptake in infections, inflammations, and malignancies. FDG-PET/CT can accurately detect lesion activity and may be useful to decide end-point of treatment.10,11 FDG-PET has not been evaluated for healing of TB spondylitis although reduced SUV values have been reported after ATT.20,21,22 Serial FDG-PET/CT to define end-point of treatment of spinal TB is reported for the first time. Park23 reported decreased maximal SUV from 2.6 to undetectable value in 187 days and from 4.8 to 1.1 in 192 days in one patient each of tuberculoma. Harisankar24 reported 60% reduction in FDG uptake in the right lower paratracheal, subcarinal lymph nodes, L2 vertebra, and right sacroiliac joint after 8 weeks ATT indicating good metabolic response in a case of idiopathic aplastic anemia who developed extrapulmonary TB following bone marrow transplantation. Tian25 reported retrospective review of 3 patients with Extrapulmonary multifocal TB including bones. Pretreatment SUV were 13.6, 17.7, and 13.9 which decreased to undetected value on repeated FDG-PET/CT scans with intervals of 318 days, 258 days, and 182 days, respectively. None of the above studies focused on vertebral TB.
Kim20 prospectively evaluated 30 patients with spinal infections (SIs) (TB-11, pyogenic-19). The residual SI was determined by the presence of preoperative symptoms, hematological infection marker, and radiologic findings. The SUV decreased with treatment in residual (pretreatment 2.85 ± 1.17 vs. posttreatment 2.06 ± 1.03; P < 0.0001) and nonresidual SIs (pretreatment4.31 ± 2.07 vs. posttreatment 1.44 ± 0.46; P < 0.0001). The author concluded that percentage change in SUVmax is a potent predictor of residual SI. Dureja22 evaluated spinal TB (n = 33) on FDG-PET/CT before initiation and 6, 12, and 18 months of ATT. The mean peak SUV max was 14.8 (range 5.9–30.3) which declined 6.3 (range 2.1–17.1), 3.0 (range: 1.6–5.3), and 1.8 (range: 1.1–2.4) at 6, 12, and 18 months, respectively. This study does not entail complete healing of lesion to conclude end-point of treatment, although have shown decline in SUV with treatment in spinal TB.
The optimum duration of ATT intake is controversial due to the absence of definition of end-point of treatment. The retrospective studies have evaluated healing of tubercular lesion.6,7,23 Ours is the only prospective study to evaluate healing in spinal TB with well-defined inclusion criteria and protocol for investigation and treatment. MRI-based healing criteria and observations with FDG-PET/CT are correlated and reported for the first time.
No uptake was observed on FDG-PET/CT when MRI showed healed lesion (n = 11), hence FDG-PET/CT findings with no uptake can be considered as a marker of healed lesion. FDG-PET/CT showed no uptake in vertebral lesions (n = 6), in spite of persistent contrast enhancement on MRI suggesting that enhancement was due to inflammation of healing and not of disease activity. Thus, FDG-PET/CT can be useful to determine the end-point of therapy for individual patient. FDG-PET/CT was the only modality to determine healed lesion in 6 patients with MRI incompatible stainless steel implants.
However, FDG-PET/CT continued to show FDG uptake in soft tissues (SUV value less <2.0) with no uptake in VB in 2 patients, while MRI showed no disease activity could be attributed most likely to physiological uptake in brown fat.26 Furthermore, these two patients on further followup showed no signs of remission of disease showing right decision to stop ATT even with insignificant soft-tissue activity noted in PET/CT.
Overall FDG-PET/CT was useful to decide end-point of treatment in 28/28 (100%) cases, while contrast MRI in 13/25 cases (52%), In six cases where stainless steel implant was used for instrumented stabilization, FDG-PET/CT was the only method to decipher end-point of treatment. 28/37 patients showed healed lesion at varying ATT intake, 7/28 (25%) achieved healing at mean 9 months. 13/28 (46.4%), 19/28 (67.8%), 24/28 (85.7%), 25/28 (89,2%), 27/28 (96,4%) achieved healing at 12, 18, 24, 30, and 36 months, respectively, while all healed by 48 months. Hence, we reiterate that it is unscientific to stop ATT by fixed time frame10 and treatment should be extended till the healing of lesion is not demonstrated on MRI/FDG-PET/CT.
Conclusion
Small number of patients is the limitation of the study. In conclusion, we found FDG-PET/CT to be a better modality than contrast MRI to evaluate healing in spinal TB and only imaging modality to evaluate healed status where stainless steel implants are used for instrumented stabilization in spine TB. It is unscientific to stop the ATT duration of ATT and treatment should be extended till healed status of lesion is demonstrated on MRI/FDG-PET/CT.
We do not intend to suggest that in every patient, this number of MRI and FDG-PET/CT should be performed. We are conducting a research work to define the end-point of treatment and suggest the guidelines of treatment with minimum use of MRI and FDG-PET/CT [Figure 4].
Figure 4.
Flow chart to reach end point
Research involving human participants
All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jain AK. Tuberculosis of the spine: A fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–13. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 2.Tuli SM. Results of treatment of spinal tuberculosis by “middle-path” regime. J Bone Joint Surg Br. 1975;57:13–23. [PubMed] [Google Scholar]
- 3.Shanley DJ. Tuberculosis of the spine: Imaging features. AJR Am J Roentgenol. 1995;164:659–64. doi: 10.2214/ajr.164.3.7863889. [DOI] [PubMed] [Google Scholar]
- 4.Boxer DI, Pratt C, Hine AL, McNicol M. Radiological features during and following treatment of spinal tuberculosis. Br J Radiol. 1992;65:476–9. doi: 10.1259/0007-1285-65-774-476. [DOI] [PubMed] [Google Scholar]
- 5.Jain AK, Dhammi IK, Modi P, Kumar J, Sreenivasan R, Saini NS, et al. Tuberculosis spine: Therapeutically refractory disease. Indian J Orthop. 2012;46:171–8. doi: 10.4103/0019-5413.93685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer J, Stiglbauer R, Wimberger D, Imhof H. MRI of spondylitis. Bildgebung. 1992;59:147–51. [PubMed] [Google Scholar]
- 7.Gillams AR, Chaddha B, Carter AP. MR appearances of the temporal evolution and resolution of infectious spondylitis. AJR Am J Roentgenol. 1996;166:903–7. doi: 10.2214/ajr.166.4.8610571. [DOI] [PubMed] [Google Scholar]
- 8.Jain AK, Srivastava A, Saini NS, Dhammi IK, Sreenivasan R, Kumar S, et al. Efficacy of extended DOTS category I chemotherapy in spinal tuberculosis based on MRI-based healed status. Indian J Orthop. 2012;46:633–9. doi: 10.4103/0019-5413.104191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuli SM. Historical aspects of Pott's disease (spinal tuberculosis) management. Eur Spine J. 2013;22(Suppl 4):529–38. doi: 10.1007/s00586-012-2388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinn C, Vorster M, Sathekge MM. Spinal tuberculosis evaluated by means of 18F-FDGPET/CT: Pilot study. Open Nucl Med. 2014;6:6–11. [Google Scholar]
- 12.Jain AK, Sreenivasan R, Saini NS, Kumar S, Jain S, Dhammi IK, et al. Magnetic resonance evaluation of tubercular lesion in spine. Int Orthop. 2012;36:261–9. doi: 10.1007/s00264-011-1380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AS, Weinstein MA, Mizushima A, Coughlin B, Hayden SP, Lakin MM, et al. MR imaging characteristics of tuberculous spondylitis vs. vertebral osteomyelitis. AJR Am J Roentgenol. 1989;153:399–405. doi: 10.2214/ajr.153.2.399. [DOI] [PubMed] [Google Scholar]
- 14.Sharif HS, Clark DC, Aabed MY, Haddad MC, al Deeb SM, Yaqub B, et al. Granulomatous spinal infections: MR imaging. Radiology. 1990;177:101–7. doi: 10.1148/radiology.177.1.2399306. [DOI] [PubMed] [Google Scholar]
- 15.Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br. 1994;76:863–9. [PubMed] [Google Scholar]
- 16.Andronikou S, Jadwat S, Douis H. Patterns of disease on MRI in 53 children with tuberculous spondylitis and the role of gadolinium. Pediatr Radiol. 2002;32:798–805. doi: 10.1007/s00247-002-0766-8. [DOI] [PubMed] [Google Scholar]
- 17.Treatment of Extrapulmonary TB issue by Department of Health, Republic of South Africa. NTCP management Guidelines. Clause 7.5. 2014. [Last accessed on 2018 Apr 16]. p. 41. Available from: http://www.tbonline.info/media/uploads/documents/ntcp_adult_tb-guidelines-27.5.2014.pdf .
- 18.Park K. 18th ed. Jabalpur, India: Banarasidas Bhanot Publishers; 2005. Park's Textbook of Preventive and Social Medicine; pp. 146–161. [Google Scholar]
- 19.Ramachandran S, Clifton IJ, Collyns TA, Watson JP, Pearson SB. The treatment of spinal tuberculosis: A retrospective study. Int J Tuberc Lung Dis. 2005;9:541–4. [PubMed] [Google Scholar]
- 20.Kim SJ, Kim IJ, Suh KT, Kim YK, Lee JS. Prediction of residual disease of spine infection using F-18 FDG PET/CT. Spine (Phila Pa 1976) 2009;34:2424–30. doi: 10.1097/BRS.0b013e3181b1fd33. [DOI] [PubMed] [Google Scholar]
- 21.Martinez V, Castilla-Lievre MA, Guillet-Caruba C, Grenier G, Fior R, Desarnaud S, et al. (18)F-FDG PET/CT in tuberculosis: An early non-invasive marker of therapeutic response. Int J Tuberc Lung Dis. 2012;16:1180–5. doi: 10.5588/ijtld.12.0010. [DOI] [PubMed] [Google Scholar]
- 22.Dureja S, Sen IB, Acharya S. Potential role of F18 FDG PET-CT as an imaging biomarker for the noninvasive evaluation in uncomplicated skeletal tuberculosis: A prospective clinical observational study. Eur Spine J. 2014;23:2449–54. doi: 10.1007/s00586-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 23.Park IN, Ryu JS, Shim TS. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med. 2008;33:1–3. doi: 10.1097/RLU.0b013e31815c5128. [DOI] [PubMed] [Google Scholar]
- 24.Harisankar C, Mittal BR, Bhattacharya A, Singh B. FDG-PET/CT in diagnosis and early response evaluation of extra-pulmonary tuberculosis in a patient with aplastic anemia. J Postgrad Med. 2010;56:219–21. doi: 10.4103/0022-3859.68639. [DOI] [PubMed] [Google Scholar]
- 25.Tian G, Xiao Y, Chen B, Xia J, Guan H, Deng Q, et al. FDG PET/CT for therapeutic response monitoring in multi-site non-respiratory tuberculosis. Acta Radiol. 2010;51:1002–6. doi: 10.3109/02841851.2010.504744. [DOI] [PubMed] [Google Scholar]
- 26.Long NM, Smith CS. Causes and imaging features of false positives and false negatives on F-PET/CT in oncologic imaging. Insights Imaging. 2011;2:679–98. doi: 10.1007/s13244-010-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]