Abstract
At present, allogeneic hematopoietic stem cell transplantation (allo-SCT) is the only curative treatment for patients with myelofibrosis (MF). Unfortunately a significant proportion of candidate patients are considered transplant ineligible due to their poor general condition and advanced age at time of diagnosis. The approval of the first JAK inhibitor, Ruxolitinib, for patients with advanced MF in 2011 has had a qualified impact on the treatment algorithm. The drug affords substantial improvement in MF-associated symptoms and splenomegaly but no major effect on the natural history. There has, therefore, been considerable support to assess the drug’s candidacy in the peri-transplant period. The drug’s precise impact on clinical outcome following allo-SCT is currently not known; nor is the drug’s long term efficacy and safety known. Considering the rarity of MF and the small proportion of patients who are undergoing allo-SCT, well designed collaborative efforts are required. In order to address some of the principal challenges, an expert panel of laboratory and clinical experts in this field was established, and an independent workshop held during the 54th American Society of Hematology’s meeting in New Orleans, USA, on 6th December 2013 and the European Hematology Association meeting in Milan, Italy on 13th June 2014. This document summarizes the results of these efforts.
Keywords: Myelofibrosis, Allogeneic Stem Cell Transplantation, JAK inhibitors, Reduced-intensity conditioning
Introduction
Although allogeneic hematopoietic stem cell transplant (allo-SCT) is the only treatment which accords long term remission and potential cure for patients diagnosed with myelofibrosis (MF), it is not available for a significant majority of patients due to the advanced age at diagnosis (1). The term MF comprises a rather motley collection of several clonal but clinically distinct, BCR-ABL1-negative myeloproliferative neoplasms (MPNs) subtypes (2). The World Health Organization (WHO) classifies MF as primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF) or post-essential thrombocythemia myelofibrosis (PET-MF) (3). Following the unravelling of somatic mutations in JAK2, MPL and CALR genes over the past decade, we have an improved understanding of the complex genomic landscape of MPNs, though precise details on driver mutations remain incomplete (4–7). These seminal observations confirmed the principal role of an hyperactive JAK-signal transducers and activators of transcription (STAT) intracellular signaling in the pathogenesis of these disorders (8). Thereafter, many efforts led to the introduction of JAK inhibitors into the clinics in 2007 (9).
There was considerable enthusiasm surrounding this, largely based on the very impressive results achieved in patients with the BCR-ABL1-positive MPN, chronic myeloid leukemia (CML), following treatment with tyrosine kinase inhibitors (TKIs) (10). Current clinical experience, however, has not yielded as impressive results with JAK2 inhibitors, but rather a qualified progress leading to the licensing of ruxolitinib, a selective JAK1 and JAK2 inhibitor, for patients with advanced MF (11–13). The drug affords substantial symptomatic benefits, reduction in splenomegaly and improved survival, but no significant impact on the malignant clone. Furthermore approximately 50% of patients discontinue ruxolitinib therapy at 3 years due to loss of clinical benefit or adverse side effects (11).
Allo-SCT remains the only treatment which accords long term remission and potential cure to MF patients. The historical results of conventional allo-SCT have, however, for the most part, been confounded by substantial non-relapse transplant-related mortality (NRM) (14–16). This is probably due, at least in part, to the older age of patients with MF and the increasing presence of significant co-morbid conditions. Efforts to improve these results have led to general improvements in allo-SCT technology and an increased use of reduced-intensity conditioning (RIC) preparative regimens. RIC is associated with a lower NRM, but a higher risk of relapse, compared with standard myeloablative conditioning (MAC), and the current results suggest a qualified success (Table 1) (17–21). The notion of using ruxolitinib in order to reduce constitutional symptoms and splenomegaly prior to allo-SCT appears attractive. What is not known is the drug’s impact on short and long-term outcomes following allo-SCT; nor is the precise timing of allo-SCT or the role of allo-SCT specific prognostic and predictive scores in the JAK inhibitor era known. In order to address some of these challenges, an expert panel comprising of laboratory and clinical experts in MPNs was established, and an independent workshop held during the 54th American Society of Hematology’s annual meeting in New Orleans, USA, on 6th December 2013. Several discussion groups were held thereafter with the penultimate at the European Haematology Association’s annual meeting in Milan, Italy, on 13th June 2014; additionally a search of published literature (popular search engines, including PubMed, Google scholar and the NCI database) since 1990 was performed. Herein, we summarize our findings and assess the suitability of JAK inhibitors for patients with MF who are candidates for allo-SCT.
Table 1:
Comparative analysis of current retrospective studies of reduced intensity conditioning (RIC) and myeloablative conditioning (MAC) allo-SCT for myelofibrosis (MF) patients
| Reference | N | Years | Preparative regimen | Median age (range) | OS | GVHD | NRM | Relapse |
|---|---|---|---|---|---|---|---|---|
| Guardiola et al.14 | 55 | 1979–1997 | MAC (TBI, 63%) | 42 (4–53) | 5 years, 47% | aGVHD, 60% cGVHD, 60% | 27% | 18% |
| Kerbauy et al.15 | 104 | 1990–2005 | MAC, 95; RIC, 9 | 49 (18–70) | 2 years, 70%; 5 years, 61% | aGVHD, 64% cGVHD, 84% | 32% | 10.5% |
| Abelsson et al.16 | 92 | 1982–2009 | MAC, 40; RIC, 52 | 46 MAC 55 RIC | 2 years, 55%/75%; 5 years, 49%/59% | aGVHD, 75% MAC 28% RIC | 32% 24% | 10% |
| Nivison-Smith et al.17 | 57 | 1993–2005 | MAC, 40; RIC, 17 | 47 (16–71) | 2 years, 59%/66%; 5 years, 58% | aGVHD, 37% | 25% | 12% |
| Kroger et al.19 | 103 | 2002–2007 | RIC, all (BU/Flu) | 55 (32–68) | 5 years, 67% | aGVHD, 27% cGVHD, 49% | 19% | 29% |
| Gupta et al.20 | 233 | 1997–2010 | RIC, all | 55 (19–79) | 5 years, 47% | aGVHD, 37% (day 100) cGVHD-42% | 18% | 43% |
Clinical impact of bone marrow histology
While bone marrow (BM) histology clearly plays a critical role in the diagnosis and classification of MF (22,23), there is some debate as to the methodology used in reporting BM fibrosis and also the integration of serial BM samples in the optimal treatment algorithm of MF patients. Importantly, although MF is an acquired clonal hematopoietic stem cell disorder, BM fibrosis represents a reactive phenomenon characterized by the presence of increased collagen fibers in a disorderly manner (24). Histologically this is best assessed by reticulin and collagen (trichrome) stains of the bone marrow trephine biopsy, and graded by the four-grade semi-quantitative European consensus system (25,26). A recent International and European LeukemiaNet (ELN) effort has proposed a set of BM features that characterize therapy response by BM morphology (27). In analogy to the WHO grading concept for fibrosis, this proposal defines reproducible scoring systems for the grading of collagen deposition and osteosclerosis. Other abnormalities include increased microvessel density and new bone formation, with the eventual development of thickened bone trabeculae (osteosclerosis) Following a successful allo-SCT for MF, the clonal hematopoeisis is promptly replaced by normal donor stem cells that colonize the marrow and produce normal hematopoiesis (Figure 1A and 1B); in contrast, changes in the bone marrow stroma, which remains of the recipient origin, take considerably longer (28). Several studies, both prospective and retrospective, have examined these changes and their time course after allo-SCT, and these data are summarized in Table 2 (29,30).
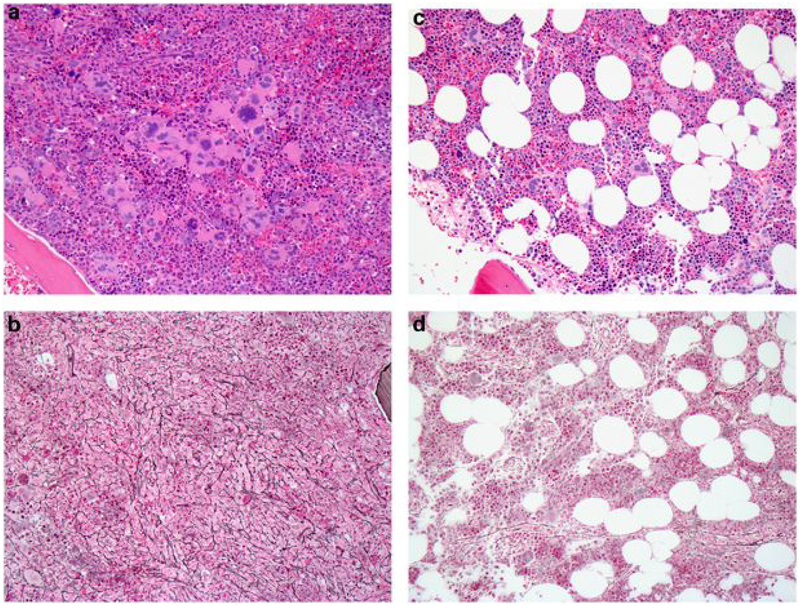
Figure 1:
A,B: Bone marrow biopsy from 50-year old female with post-PV MF prior to allo-SCT. The marrow is markedly hypercellular for age (100% cellularity) and contains large clusters of abnormal megakaryocytes with hyperchromatic and “cloud-like” nuclei, as well as smaller forms with hyperlobulated nuclei. Reticulin stain shows grade 2 of 3 fibrosis (A, hematoxylin & eosin; B, reticulin stain). C,D: Bone marrow biopsy taken 12 months after allo-SCT shows cellularity with normal range for the patient’s age (60% celluarity), with a normal myeloid:erythroid ratio and morphologically normal megakaryocytes that do not cluster. Reticulin stain shows resolution of reticulin fibrosis (grade 0). Chimerism studies on bone marrow showed >98% donor origin (C, hematoxylin & eosin; D, reticulin stain).
Table 2:
Pathology of primary myelofibrosis (PMF) and changes in pathologic features observed after allo-SCT
| Feature | Abnormality in PMF | Resolution after allo-SCT | Time when resolution occurs |
|---|---|---|---|
| Cellularity | Increased | Yes (up to 100%) | Upon engraftment |
| Megakaryocyte morphology | Hyperchromatic, ‘cloud-like’ nuclei, tight clustering | Variable; proportion with resolution unknown | Upon engraftment |
| Myeloid:erythroid ratio | Increased | Variable; proportion with resolution unknown | Upon engraftment |
| Reticulin fibrosis | Increased | Yes (40–93%) | 6–23 months |
| Microvasculature | Increased density | Yes (50–100%) | 3–12 months |
| Bone trabeculae | Thickened with new bone formation | Variable (0–50% at 12 months) | 12 months or longer |
Following a successful allo-SCT, the initial change in BM stroma is a reduction in the reticulin fibrosis grade, which may exhibit heterogeneity and has posed challenges in reporting (Figure 2A and 2B) (31). This heterogeneity may impede determination of any reduction in fibrosis grade in sequential post-SCT biopsies in comparison to the pre-SCT biopsy. Regardless, complete resolution of reticulin fibrosis has been confirmed in about 90% of MF patients by 12 months following both MAC and RIC transplants (14,17,32–34). Resolution of osteosclerosis is more controversial, and has been reported to occur in some studies, but not others (33,35).
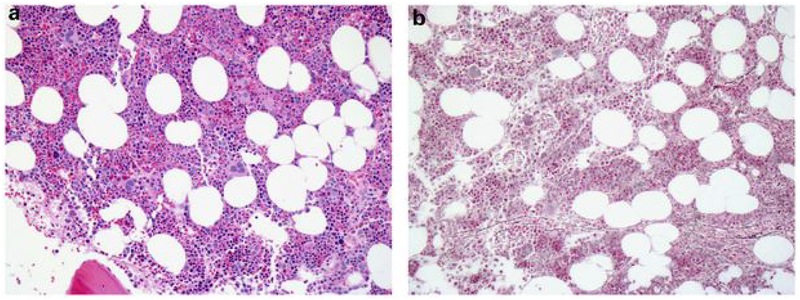
Figure 2:
A,B: Bone marrow biopsy taken 12 months after SCT shows cellularity with normal range for the patient’s age (60% cellularity), with a normal myeloid:erythroid ratio and normal megakaryocytes that do not cluster. Reticulin stain shows resolution of reticulin fibrosis (grade 0). Chimerism studies on bone marrow showed >98% donor origin (A, hematoxylin & eosin; B, reticulin stain).
While successful allo-SCT in patients with MF thus leads to an eventual reversal of the abnormal BM stromal changes and fibrosis in most patients, conversely, relapse of the MPN clone following allo-SCT is associated with return of bone marrow stromal abnormalities stimulated by the abnormal clone. Although some studies use complete resolution of reticulin fibrosis to define a complete ‘histopathologic’ response to allo-SCT, in the presence of full donor chimerism, the significance of persistent fibrosis >12 months after allo-SCT is uncertain and may not necessarily be indicative of persistent or relapsed disease (14,36). More recently, the importance of the rate of decline of the BM fibrosis has been demonstrated, with patients having a rapid regression have the best outcomes (Table 3) (15,37). In the study conducted by Krӧger and colleagues, patients who achieved a complete or near-complete resolution of fibrosis on day +100 post allo-SCT had the best overall survival and this was independent of the IPSS score at the time of transplant.
Table 3:
Dynamics of bone marrow (BM) fibrosis regression (adapted, with permission, from Krӧger et al, Biol Blood Marrow Transplant, 2014;20:812–815)
| Time (days from allo-SCT) | Day 0 | Day +30 | Day +100 |
|---|---|---|---|
| BM fibrosis—grade 0 | 0% | 6% | 25% |
| BM fibrosis—grade 1 | 0% | 15% | 29% |
| BM fibrosis—grade 2 | 28% | 35% | 27% |
| BM fibrosis—grade 3 | 72% | 44% | 18% |
Impact of the timing of transplant
Prior to the introduction of JAK inhibitors into the clinics, it was generally accepted that allo-SCT should be offered to MF patients, perhaps as early as possible following the diagnosis, in order to abrogate the dire consequences of transformation to acute myeloid leukemia (AML). Following the qualified responses accorded by ruxolitinib, there has been considerable uncertainty about the precise timing of allo-SCT. Additionally there is much uncertainty about discontinuing ruxolitinib prior to allo-SCT. Interruption or discontinuation of ruxolitinib, for any reason, often leads to the return of constitutional symptoms to baseline in less than a week and regrowth of spleen shortly thereafter. There are also reports of serious withdrawal symptoms and thus it would appear desirable to continue the drug for as long as possible prior to transplantation (38). These challenges are now being addressed in a prospective multi-center phase II study, MPD-RC114 [NCT01790295], in which ruxolitinb is administered to eligible MF patients for 60 days prior to definitive allo-SCT employing RIC with fludarabine (Flu) and busulfan (Bu) RIC for matched donors and Flu-Bu and antithymocyte globulin (ATG) for mismatched related and unrelated donors. The study mandates ruxolitinib taper to commence 9 days prior to stem cell infusion and continues slowly over a period of 4 days, with the last dose of ruxolitinib to be taken 1 day prior to start of conditioning therapy.
Results following an allo-SCT in general appear to be the best in younger patients who are transplanted at an earlier disease stage and better risk-scores, with the notable exception of the recent European Group for Blood and Marrow Transplantation (EBMT) study (discussed below) (15,39,40). In patients in whom transplant is delayed, there is an increasing risk of progressive splenomegaly, portal hypertension, and iron overload as a consequence of frequent transfusions and the risk of transformation to poor-risk AML.
Impact of pre-transplant conventional therapy
The standard initial treatment for most, if not all, MF patients who present with symptomatic disease is oral hydroxyurea (hydroxycarbamide) at a dose of 500mg to 2000mg per day or subcutaneous alpha interferon (IFN), particularly pegylated-IFN (41–43). Hydroxyurea has not been considered to impact on the clinical outcome of allo-SCT, nor does it appear to have any substantial effect on the natural history of MF, although there remains some debate as to its contribution towards leukemic transformation. In contrast, pegylated-IFN has shown to improve BM fibrosis, reduce splenomegaly and lead to a molecular response in several small studies (44,45). Neither drug appears to affect the time to engraftment, nor affect the incidence or severity of acute or chronic graft-versus-host disease (GvHD).
Impact of targeting the JAK/STAT pathway
Currently there is a paucity of clinical data for patients with MF who have received initial therapy with JAK inhibitors and are subsequently treated with allo-SCT. Several on-going efforts, such as the MPD-RC 114 study (discussed above) are now assessing the potential impact of the use of ruxolitinib during the peritransplantation period for MF patients. The notion of using ruxolitinib as initial therapy leading to a definitive allo-SCT is supported by the substantial and durable improvement in constitutional symptoms and reduction of splenomegaly in two randomized phase III trials (11,12); these results were confirmed on longer follow-up and some intriguing data on the drug’s effects on BM fibrosis noted (Figure 2A and 2B) (46). The adverse events attributed to ruxolitinib appear to be relatively mild and generally manageable. Anemia is the most common side effect, sometimes with thrombocytopenia, and may require dose adjustments. It is therefore possible, though not confirmed, that initial treatment with this drug for a pre-determined period, might improve the general condition, splenomegaly and performance status of candidate patients, factors which may also improve allo-SCT results.
An interesting compromise would be to offer a transplant only to patients who have ruxolitinib resistance or intolerance or patients who start developing significant cytopenias or transfusion dependency on JAK inhibitor therapy. This may allow patients responding to ruxolitinib to continue until resistance/intolerance, or indeed, progression of disease, are noted. The disadvantages with such an approach are the potential risks for leukemic transformation and the prolonged use of a therapy which has yet to demonstrate an effect on the malignant clone itself. There are additional financial implications and the longer term effects of the drug are not known. A recent report documented four patients who developed AML and one who developed chronic myelomonocytic leukemia on ruxolitinib for 3 to 18 months. The time form diagnosis to leukemic transformation to AML was 2, 3, 4, 10 and 20 years. Four of these 5 patients had been treated with hydroxyurea for varying duration, ranging from few months (2 patients) to several years (2 patients). Four of the 5 patients manifested extra-medullary disease without bone marrow involvement (3 patients with leukemia cutis and one with granulocytic sarcoma) (47). With these few patients, and variable duration of prior hydroxyurea exposure, it is not possible to establish a definitive causal relationship between prior exposure to cytotoxic therapy and the emeregence of the myeloid sarcoma. This raises the possibility of evolution of a more aggressive clone in some patients whilst on ruxolitinib therapy and also raises the concern that in patients who become resistant to ruxolitinib, the phenotypic features of disease may be more aggressive and conceivably, make a previously transplant-eligible patient ineligible. Clearly additional work is required to establish the long-term efficacy and safety of the drug in the clinics.
There are additional dilemmas with regards to the potential use of pre-transplant JAK inhibitors. Ruxolitinib has remarkable anti-inflammatory and immunomodulating activity, but the drug also affects dendritic cell differentiation and impairs T-cell and NK cell activation (48,49). The dendritic cell impairments might result in increased infection rates, while the NK cell dysfunction may impair graft-versus-leukemia (GvL) effect (50). Conversely, JAK inhibitors appear to reduce acute GvHD in murine models by increasing T-regulatory cells (Treg), which has led to the successful treatment of six patients with ruxolitinib for steroid-refractory acute GvHD (51). Another JAK inhibitor, tofactinib, a selective JAK3 inhibitor, has been found to reverse CD8+ T-cell mediated mucocutaneous GvHD-like disease in a murine model (52).
Impact of targeting the epigenome
Studies on the remarkable complexity of genetic architecture in MPNs have discovered an unexpected degree of differences in individual cases at the epigenetic level. However, mutations in genes encoding epigenetic regulators are uncommon in MPN and no clear patterns have emerged so far (53). Aberrations in ASXL1, EZH2, SRSF2 and IDH1/2 appear to have a predictive impact on the overall and leukemia-free survival, suggesting that the MPN epigenome is clinically relevant; the greatest impact appears to be with the ASXL1 mutation (13,53). In addition, promoter specific hypermethylation of candidate genes such as the chemokine receptor CXCR4 has been linked to the constitutive migration of CD34+ cells in PMF (54). Global methylation profiling in MF revealed a distinct methylation signature, and in patients who transform to AML, it has been noted that the number of differentially methylated regions increase significantly and the aberrant genes are involved in the ‘interferon pathway’ (55, 56). Collectively, these and related observations suggest the importance of the epigenome in MF patients and there are now several research efforts assessing the potential role of DNA methyl transferase (DNMT) inhibitor and histone deacetylase (HDAC) inhibitors in MF.
Preclinical studies utilizing a DNMT inhibitor and an HDAC inhibitor sequentially in primary MF cells in vitro and in NOD/SCID mouse models in vivo reveal that these agents can inhibit the malignant clone, upregulate the transcription of CXCR4 in primary MF CD34+ cells and reverse the abnormal stem cell trafficking in MF, resulting in homing of stem cells to the marrow, rather than the spleen (57,58). These findings suggest a possible role of hypomethylating therapy prior to allo-SCT in an effort to improve outcomes. Several pilot clinical studies suggest clinical activity in both early and late phases of the disease (59–63). These agents also have the potential to harness an immunomodulatory effect that could synergize with the GvL effect, making them of additional interest (64,65). The notion of administering these agents at a lower dose/intensity to harness their immune modulatory effects which include the up-regulation of cancer testes antigens and augmentation of Tregs, following allo-SCT is additionally attractive (66).
At present there are several on-going clinical trials, assessing a combination of JAK inhibitors and epigenetic modulators in both transplant and non-transplant settings, based on pre-clinical evidence of synergy (67). Recent small series of MF patients suggest the efficacy and safety of a combination of ruxolitinib and DNMT inhibitors (68). In addition, work is underway to identify specific inhibitors of mutated components of the epigenome, and such inhibitors are now in early phase clinical trials (69,70). The recent observation of the induction of cellular differentiation by an IDH1 mutant inhibitor in a refractory AML xenograft model and the candidacy of TET2 mutations as a predictive biomarker for response to hypomethylating agents in MDS patients are also of some interest (71,72).
Impact of patient-related factors
At present the very considerable advances in the understanding of the genomic landscape in MF appear not to have been validated sufficiently for adaptation in treatment algorithms to assess candidacy for allo-SCT compared to conventional therapy (73). The Lille score, first introduced in 1996 for patients with PMF, remains the best studied risk-score tool for transplant candidates with BCR-ABL1 negative MPNs (74). More recently, the International Prognostic Scoring System (IPSS) was introduced to assess PMF patients at time of diagnosis (75). The Dynamic-IPSS (DIPSS) and DIPSS-plus (DIPSS plus cytogenetic information) were subsequently introduced to help refine prognosis for these patients at any time during disease evolution (Table 4) (76,77). These risk-scores are applied at diagnosis and are based on hematological parameters, such as anemia, leukocytosis and peripheral blood blasts, as well as clinical features, such as age and presence of constitutional symptoms. Comparative studies of the usefulness of the various scoring systems in allo-SCT suggest a qualified application at best and most experts advocate the use of transplant-specific risk-models instead. The Seattle group and the Essen group have demonstrated the usefulness of the DIPSS score in terms of predicting NRM, relapse and survival (78,79). Krӧger and colleagues proposed a relatively simple model which added age, JAK2 status and constitutional symptoms to the Lille model to predict for survival following a RIC-allo-SCT (80). The Genoa group developed a RIC-allo-SCT risk-model based on donor type, transfusion requirement and splenomegaly, but this has not attracted wider use (81). Finally, the Gratwohl risk-score is a useful tool for risk-assessment for patients with most hematological malignancies being considered for an allo-SCT, but unfortunately no patients with MF were included in its development (82)
Table 4:
Risk Stratification for Myelofibrosis (MF); (courtesy of Prof A Rambaldi)
| Variable | Lille | IPSS | DIPSS | DIPSS plus |
|---|---|---|---|---|
| Age >65 years | √ | √ | √ | |
| Constitutional symptoms | √ | √ | √ | |
| Hb <10 g/dL | √ | √ | √ | √ |
| Leukocyte counta | √ | √ | √ | √ |
| Circulating blasts ⩾1% | √ | √ | √ | |
| Platelet count <100 × 109/L | √ | |||
| RBC transfusion need | √ | |||
| Unfavorable karyotype +8, −7/7q-, i(17q), inv(3), −5/5q-, 12p-, 11q23 rearr. | √ |
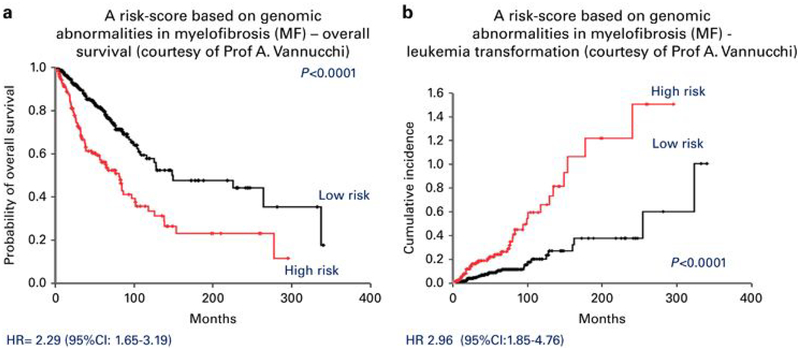
It is actually quite remarkable that risk stratification methods for patients with MF per se have not been studied in a validated prospective manner; rather the risk scores tested in patients with PMF, have been extrapolated into the treatment-decision making process for all MF patients, a cohort which also includes patients with PPV-MF and PET-MF. There is therefore an urgent need to develop MF-specific risk scores not only for potential allo-SCT candidates, but also for non-transplant therapies. Efforts to develop risk scores, including transplant-specific scores, based on integrated molecular-risk profiles, for all MPNs are now being developed (Figure 3).
Figure 3:
A Risk-Score based on Genomic Abnormalities in Myelofibrosis (MF); although mutations in ASXL1, SRSF2, EZH2, predicted poorer outomes (high-risk), only ASXL1 mutations were considered significant in the context of IPSS, DIPSS and DIPSS-plus; (Courtesy of Prof A Vannucchi)
The impact of disease-associated splenomegaly on allo-SCT outcomes in hematological malignancies has been debated often. In general, patients with splenomegaly appear to have delayed engraftment, which nevertheless does not appear to affect the overall survival (36,83). Interestingly, a French study noted a favorable survival for male patients who underwent pre-transplant splenectomy, while a German study demonstrated a higher relapse rate in the splenectomized patients (17,84). Additionally, there appears to be no agreement as to the actual size of the spleen which might, or might not, serve as a trigger for pre-transplant therapy. There is now an emerging consensus to avoid splenic irradiation or splenectomy immediately prior to transplant due to the intrinsic complications of radiation and splenectomy and thus the notion of reducing splenomegaly medically is indeed attractive.
Results of treatments for MF patients who are either in frank AML transformation, or at high risk of transformation, including conventional cytotoxic induction regimes or novel induction regimens, of both higher and lower intensity prior to an allo-SCT, remain suboptimal, with a median survival of less than 5 months (85–87). Current efforts are investigating diverse agents, including hypomethylating agents, JAK and MEK inhibitors, BCL-XL and BCL-2 inhibitors and clofarabine (88–93).
Impact of the transplant-procedure and donor related factors
Several prospective multicenter studies have now confirmed the usefulness of RIC-SCT using related or unrelated donors for younger and older patients with MF. In a large retrospective EBMT study, outcomes from 250 consecutive patients (median age 56 years) with PPV-MF (n=120) or PET-MF (n=130) who received allo-SCT, either in MF ‘chronic’ phase (n=193) or MF transformed to AML (n=57), were analyzed (39). The study accords an opportunity to assess the impact of the various transplant-procedure related factors in a ‘real-world’ cohort of MF patients, including those who had transformed to AML. It is of interest that the non-transplant risk scores of 61% of the study cohort were unknown at the time of transplant. 117 patients received allografts (115 matched; 2 mismatched) and 133 unrelated allografts (124 matched; 9 mismatched); 80 patients underwent conventional conditioning and 170 received RIC. Over half of the transplanted patients had an interval from diagnosis to allograft of 10 years or more. The 3-year overall survival was 55%, with a relapse incidence of 32%, following a median post-transplant follow-up period of 13 months. In uni-variante analysis, older age (>55-years), transplant in AML, and an unrelated donor, were the factors which affected survival (Figure 5). In this study, there appear to be no statistically significant difference between the conventional and RIC regimens, nor was there a significant impact on outcomes in T-depleted versus the non-T-depleted cohorts.
The Center for International Blood and Marrow Transplant Research (CIBMTR) published a report on the outcomes of 233 patients with PMF who received RIC-allo-SCT (20). Since the study excluded patients with PPV-MF, PET-MF and those who had transformed to AML, it offers an assessment of the role of RIC allo-SCT in PMF, using a validated risk-assessment tool (DIPSS) which addresses the course of disease. The study cohort DIPSS-risk stratification was: low (12%), intermediate-1 (49%), intermediate-2 (37%), high (1%) and unknown (1%). 79 patients received fully matched related allografts and 154 received unrelated allografts: 104 fully matched, 40 partially matched and 10 mismatched. The median age was 55years (range 19–79) and the median follow-up of survivors was 50 months (range 3–134). A variety of RIC regimens were used: Flu-Total Body Irradiation (22%); Flu-Melphalan (Flu-Mel) (28%); Flu-Bu (38%); ‘others’ (12%). 123 of 233 patients (53%) received in vivo T-cell depletion: ATG (110 patients) and alemtuzumab (13 patients). The 5-year overall survival was 56% for matched related, 48% for matched unrelated and 34% for partially matched or mismatched unrelated cohorts. Multivariable analysis confirmed donor type as the sole independent factor influencing survival; importantly, age at transplant did not appear to affect survival. There was a trend for patients with intermediate-2 disease to have higher NRM, mainly from GvHD, infections and organ failure, resulting in an inferior survival compared to intermediate-1 and low risk patients. The study lends support to the use of RIC allo-SCT for PMF with the best results accorded to the low and intermediate-1 risk categories and those who have a suitably matched related donor available. The recruitment of just 1% DIPPS-high risk patient in this CIBMTR effort is of note.
Another international effort, the MPD-RC 101 study, has analyzed outcomes in a smaller cohort of 66 patients who received RIC-allografts between 2007 and 2011 (94). The study cohort comprised all MF subtypes and included about 35% intermediate-2/high risk DIPSS. Following a median follow-up of 25 months, 24 of 32 patients who had received a related allograft (30 matched; 2 mismatched) following Flu-Mel conditioning, were alive compared to 11 of 34 who had received an unrelated allograft (25 matched; 9 mismatched) following Flu-Mel-ATG conditioning. The NRM was 22% and 59%, and the secondary graft failure 3% and 12%, for related versus unrelated cohorts, respectively. Survival did not appear to correlate with the degree of donor matching, age at transplant, or JAK2 status at diagnosis. The investigators speculated that the increased risk of graft failure and death in the unrelated cohort might be due to the in vivo T-cell depletion, which was not offered to the related patients.
Efforts have also assessed the suitability of umbilical cord blood (UCB) allo-SCT for adults with MF (95). The results of 35 patients with MF, including 7 who had transformed to AML, who received UCB-allo-SCT were reported to Eurocord; 24 of 35patients received RIC with Flu-cyclophosphamide and total body irradiation, with or without ATG. The median age of this cohort was 54 years. Following a median follow-up of 24 months, the overall survival was 44%; 15 of 24 patients relapsed. It is of interest that in this small series of patients, outcomes for patients with AML was similar to those in chronic phase. The results of this Eurocord series are sufficiently encouraging to suggest further formal exploration of this donor source.
The subtype of MF, patient CMV status, use of T-cell depletion and specific RIC regimen do not appear to influence post-allo-SCT outcomes (38). Currently there appears to be no firm consensus with regards to the prognostic significance of the JAK2, CALR and MPL mutations, one of which is present in most MF patients, on the outcomes following an allo-SCT. There is one preliminary report suggestive of best outcomes in CALR positive and worst outcomes in JAK2/MPL/CALR negative (‘triple-negative’) patients following allo-SCT (96). In this effort comprising of 119 patients with MF, a multivariable analysis revealed shorter survival and higher NRM for the ‘triple-negative’ cohort compared to patients with any mutations. These observations have now been validated by several recent efforts confirming the notion of patients with JAK2 wild-type (WT), MPL WT and CALR WT, to have poor outcomes (97,98).
Impact of patient reported outcomes
Despite the integration and subsequent validation of patient reported outcomes (PROs) in the non-transplant treatment algorithms for MF, their use in the clinical decision-making process for transplant remains a challenge. There are few studies assessing quality of life, financial burden and symptoms in MF patients undergoing allo-SCT. The development of the Myelofibrosis Symptom Assessment Form (MF-SAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. facilitated a platform for the objective quantification of these parameters and was incorporated in ruxolitinib MF-registration studies (11,12). In an effort to assess the role of PROs in assessing MF patients” candidacy and post-transplant progress, SYMPTOMS, a prospective questionnaire-based study was recently commenced (99–102). The study evaluates the quality of life of patients undergoing allo-SCT, utilizing MPN-SAF TSS, the FACT-BMT, Global Impression of Change, and a financial questionnaire at baseline, day +30, day +100 and day +365.
Conclusion
Following the qualified results accorded by ruxolitinib for patients with MF, many efforts are appraising the current treatment algorithm for newly diagnosed patients and assess the impact of JAK inhibition and other candidate therapies on transplant outcomes (103–107). Currently allo-SCT remains the only treatment modality which appears to be associated with long term remissions and potential cure. Transplant-related mortality and morbidity, both GvHD- and non-GvHD-associated, remains a problem with both RIC and MAC regimens. The notion of having access to a drug which results in substantial symptomatic benefit, significant reduction in splenomegaly, and an improvement in survival appears attractive. However, the fact that ruxolitinib does not appear to affect the risk of AML and does not reduce the malignant clone, suggest that allo-SCT performed as early as possible affords the best chance of a long term remission and potential cure for most patients. Indeed, recent assessment of transplant activity suggests an increase in the utilization of allo-SCT, particularly with RIC regimens.
There is now an urgent need to develop suitable risk stratification methodology to optimize the decision-making process for treating patients with MF. This should encompass both transplant and non-transplant treatments, since none of the current methods have been validated for patients with PPV-MF and PET-MF, who comprise about 60% of all MF patients. Efforts are underway to develop a molecular-based risk score which should help navigate the treatment algorithms commensurate to AML transformation-risks. MF patients have distinct subclones which persist for years and emerging evidence suggest that the order in which the various mutations are acquired influences the timing of disease presentation and may also affect response to therapy. Furthermore, novel molecular insights into the evolution of the various MPN genotypes and pathologic subtypes into MF and AML pose interesting clinical questions. A principle challenge now is to decide how exactly allo-SCT efforts can be improved; topical challenges include refinement in risk stratification tools prior to transplant, role of peri-transplant JAK inhibition and indeed other candidate therapies, evaluation of alternative donor sources, and reduction in NRM and primary and secondary graft failure. In addition, several significant challenges have been identified in the methodology and the selection of the surrogate end-points of some of the recent clinical trials. A recent proposal led by the European LeukemiaNet (ELN) and the International Working Group for MPN Research and Treatment (IWG-MRT) appears to be a reasonable starting point to develop prospective randomized trials which may help to develop definitive guidance for the selection of suitable peri-transplant therapy, if any, in due course (108).
Acknowledgements:
The authors wish to thank Alpine Oncology Foundation, in particular Dr. Alpa Parmar, who helped organize the workshop and Incyte Corporation (USA) for their unrestricted educational support.
Footnotes
Conflict of interest Disclosures
V Fauble: No conflicts of interest
G Finazzi: No conflicts of interest
S Giralt: No conflicts of interest
V Gupta: Scientific advisory board: Novartis and Incyte; Research support: Novartis and Incyte
R Hasserjian: Scientific advisory board: Incyte
R Hoffman: No conflicts of interest
J Mascarenhas: Research support: Novartis and Incyte
TI Mughal: Consultancy: Novartis & Incyte
O Odenike: Scientific advisory board: Sanofi-Aventis, Incyte, Spectrum Pharmaceuticals, Suneisis Pharmaceuticals, Algeta Pharmaceuticals; Research support: Eisai Pharmaceuticals, Topotarget Pharmaceuticals
F Pane: No conflicts of interest
J Prchal: No conflicts of interest
D Rondelli: No conflicts of interest
R Tamari: No conflicts of interest
References:
- 1.Tefferi A Myelofibrosis with myeloid metaplasia. N Engl J Med 2000;342(17):1255–1265 [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Vainchenker W. Myeloproliferative Neoplasms:Molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol 2011;29(5):573–582 [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:937–951 [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Thiele J, Vannucchi AM, Barbui T. An overview of CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia 10.1038/leu.2014.35 [DOI] [PubMed] [Google Scholar]
- 5.Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood 2014; 10.1182/blood-2014-03-530865 [DOI] [PubMed] [Google Scholar]
- 6.Klampf T, Gisslinger H, Hanutyunyan A, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N Engl J Med 2013; 369:2379–2390 [DOI] [PubMed] [Google Scholar]
- 7.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N Engl J Med 2013;369:2391–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mughal TI, Girnius S, Rosen ST, Kumar S, Wiestner A, Abdel-Wahab O, et al. Emerging therapeutic paradigms to target the dysregulated Janus kinase/signal transducer and activator of transcription pathway in hematological malignancies.Leuk & Lymphoma 2014; 10.3109/10428194.2013.863307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA,et al. safety and Efficacy of INCB018424, a JAK1 and JAK2 Inhibitor, in Myelofibrosis. N Engl J Med 2010;363:1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Druker B, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM,et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031–1042 [DOI] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366(9):799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366(9):787–798 [DOI] [PubMed] [Google Scholar]
- 13.Mughal TI, Vannucchi AM, Soverini S, Bazeos A, Tibes R, Saglio G, et al. Current pre-clinical and clinical advances in the BCR-ABL1-positive and −negative chronic myeloproliferative neoplasms. Haematologica 2014;99:797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guardiola P, Anderson JE, Bandini G, Cervantes F, Runde V, Arcese W, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Société Française de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood 1999;93:2831–2838. [PubMed] [Google Scholar]
- 15.Kerbauy DM, Gooley TA, Sale GE, Flowers ME, Doney KC, Georges GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant 2007;13:355–65. [DOI] [PubMed] [Google Scholar]
- 16.Abelsson J, Merup M, Birgegård G, WeisBjerrum O, Brinch L, Brune M et al. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant 2012;47:380–6. [DOI] [PubMed] [Google Scholar]
- 17.Nivison-Smith I, Dodds AJ, Butler J, Bradstock KF, Ma DD, Simpson JM et al. Allogeienic hematopoietic cell transplantation for chronic myelofibrosis in Austrelia and New Zeland: older recipients receiving myeloablative conditioning at increased mortality risk. Biol Blood Marrow Transplant 2012;18:302–8 [DOI] [PubMed] [Google Scholar]
- 18.Rondelli D, Barosi G, Bacigalupo A, Prchal JT, Popat U, Alessandrino EP, et al. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate- or high-risk patients with myelofibrosis with myeloid metaplasia. Blood 2005;105(10):115–119. [DOI] [PubMed] [Google Scholar]
- 19.Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H,et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009;114:5264–70 [DOI] [PubMed] [Google Scholar]
- 20.Gupta V, Malone AK, Hari PN, Ahn KW, Hu ZH, Gale RP, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2014;20:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merup M, Lazarevic V, Nahi H, Andreasson B, Malm C, Nilsson L, et al. Different outcome of allogeneic transplantation in myelofibrosis using conventional or reduced-intensity conditioning regimens. Br J Haematol 2006;135(3):367–373 [DOI] [PubMed] [Google Scholar]
- 22.Thiele J, Kvasnicka HM, Vardiman J: Bone marrow histopathology in the diagnosis of chronic myeloproliferative disorders: a forgotten pearl. Best Pract Res Clin Haematol 2006;19:413–437 [DOI] [PubMed] [Google Scholar]
- 23.Boiocchi L, Mathew S, Gianelli U, Iurlo A, Radice T, Barouk-Fox S, et al. Morphologic and cytogenetic differences between post-polycythemic myelofibrosis and primary myelofibrosis in fibrotic stage. Mod Pathol 2013;26:1577–1585 [DOI] [PubMed] [Google Scholar]
- 24.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP, Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol 2007;139:351–362. [DOI] [PubMed] [Google Scholar]
- 25.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A.. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005;90:1128–1132. [PubMed] [Google Scholar]
- 26.Thiele J, Kvasnicka HM, Diehl V: Standardization of bone marrow features - does it work in hematopathology for histological discrimination of different disease patterns? Histol Histopathol 2005;20:633–644 [DOI] [PubMed] [Google Scholar]
- 27.Kvasnicka HM, Thiele J, Orazi A, et al. : European LeukemiaNet (ELN) consensus criteria for therapy response based on bone marrow features in patients with myelofibrosis. Leukemia, 2014, in press. [Google Scholar]
- 28.Bacher U, Asenova S, Badbaran A, Zander AR, Alchalby H, Fehse B, et al. Bone marrow mesenchymal stromal cells remain of recipient origin after allogeneic SCT and do not harbor the JAK2V671F mutation in patients with myelofibrosis. Clin Exp Med 2010;10:205–208. [DOI] [PubMed] [Google Scholar]
- 29.Thiele J, Varus E, Siebolts U, Kvasnicka HM, Wickenhauser C, Metz KA, et al. Dualism of mixed chimerism between hematopoiesis and stroma in chronic idiopathic myelofibrosis after allogeneic stem cell transplantation. Histol Histopathol 2007;22:365–372. [DOI] [PubMed] [Google Scholar]
- 30.Ni H, Barosi G, Hoffman R. Quantitative evaluation of bone marrow angiogenesis in idiopathic myelofibrosis. Am J Clin Pathol 2006;126:241–247. [DOI] [PubMed] [Google Scholar]
- 31.Sale GE, Deeg HJ, Porter BA. Regression of myelofibrosis and osteosclerosis following hematopoietic cell transplantation assessed by magnetic resonance imaging and histologic grading. Biol Blood Bone Marrow Transpl 2006;12:1285–1294. [DOI] [PubMed] [Google Scholar]
- 32.Devine SM, Hoffman R, Verma A, Shah R, Bradlow BA, Stock W, et al. Allogeneic blood cell transplantation following reduced-intensity conditioning is effective therapy for older patients with myelofibrosis with myeloid metaplasia. Blood 2002;99:2255–2258. [DOI] [PubMed] [Google Scholar]
- 33.Thiele J, Kvasnicka HM, Dietrich H, Stein G, Hann M, Kaminski A, et al. Dynamics of bone marrow changes in patients with chronic idiopathic myelofibrosis following allogeneic stem cell transplantation. Histol Histopathol 2005;20:879–889. [DOI] [PubMed] [Google Scholar]
- 34.Kröger N, Thiele J, Zander A, Schwerdtfeger R, Kobbe G, Bornhäuser M, et al. Rapid regression of bone marrow fibrosis after dose-reduced allogeneic stem cell transplantation in patients with primary myelofibrosis. Exp Hematol 2007;35:1719–1722. [DOI] [PubMed] [Google Scholar]
- 35.Tanner ML, Hoh CK, Bashey A, Holman P, Sun C, Broome HE, et al. FLAG chemotherapy followed by allogenetic stem cell transplant using nonmyeloablative conditioning induces regression of myelofibrosis with myeloid metaplasia. Bone Marrow Transpl 2003;32:581–585. [DOI] [PubMed] [Google Scholar]
- 36.Shanavas M, Messner HA, Atenafu EG, Kim DH, Kuruvilla J, Lipton JH, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis using fludarabine, intravenous busulfan and low dose TBI-based conditioning. Bone Marrow Transplant 2014; 10.1038/bmt.2014131 [DOI] [PubMed] [Google Scholar]
- 37.Kröger N, Zabelina T, Alchalby H, Stübig T, Wolschke C, Ayuk F, et al. Dynamic of bone marrow fibrosis regression predicts survival after allogeneic stem cell transplantation for myelofibrosis. Biol Blood Marrow Transplant 2014;20:812–815 [DOI] [PubMed] [Google Scholar]
- 38.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med 2011;365:1455–1457 [DOI] [PubMed] [Google Scholar]
- 39.Lussana F, Rambaldi A, Finazzi MC, van Biezen A, Scholten M, Oldani E, et al. Allogeneic hematopoietic stem cell transplantation in patients with polycythemia or essential thrombocythemia transformed to myelofibrosis or acute myeloid leukemia: report from MPN subcommittee of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Haematologica 2014;99(5):916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 2010;16(3):358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative Classical Myeloproliferative Neoplasms: Critical Concepts and Management Recommendations from the European LeukemiaNet. J Clin Oncol 2011;29(6):761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reilly JT, McMullin MF, Beer PA, Butt N, Conneally E, Duncombe AS, et al. Use of JAK inhibitors in the management of myelofibrosis: a revision of the British Committee for Standards in Haematology Guidelines for Investigation and Mangaement of Myelofibrosis 2012. Br J Haematol 2014; 10.1111/bjh.12985 [DOI] [PubMed] [Google Scholar]
- 43.Hasselbalch HC, Kiladjian JJ, Silver RT. Interferon alfa in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. J Clin Oncol 2011;29(18):564–565 [DOI] [PubMed] [Google Scholar]
- 44.Ianotto JC, Boyer-Perrard F, Gyan E, Laribi K, Cony-Makhoul P, Demory JL, et al. Efficacy and safety of pegylated interferonα−2a in myelofibrosis: A study by the FIM and GEM French Cooperative Groups. Br J Haematol 2013;162(6):783–791 [DOI] [PubMed] [Google Scholar]
- 45.Silver RT, Lascu E, Feldman EJ, Ritchie E, Roboz GJ, De Sancho MT, et al. Recombinant interferon alfa (rIFN) may retard progression of early myelofibrosis by reducing splenomegaly and by decreasing marrow fibrosis. Blood 2013;122:4053- [Google Scholar]
- 46.Kvasnicka HM, Thiele J, Bueso-Ramos C, Sun W, Cortes JE, Kantarjian HM, et al. Effects of 5-years of Ruxolitinib therapy on bone marrow morphology in patients with Myelofibrosis and comparison with Best Available Therapy. Blood 2013;122(21);4055- [Google Scholar]
- 47.Kremyanskaya M, Mascarenhas J, Rampal R, Hoffman R. Development of extramedullary sites of leukemia during ruxolitinib therapy for myelofibrsosis. Brit J Haem 2014; 10.1111/bjh.12948 [DOI] [PubMed] [Google Scholar]
- 48.Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and invivo. Blood 2013;122(7):1192–1202 [DOI] [PubMed] [Google Scholar]
- 49.Schӧnberg K, Rudolph J, Cornez I, Brossart P, Wolf D. The JAK1/JAK2-inihibitor ruxolitinib substantially affects NK cell biology. Blood 2013;122:16- [Google Scholar]
- 50.Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest 2013;143(5):1478–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK1/2 blockade in graft-versus-host-disease. Blood 2014; 10.1182/blood-2013-12-543736 [DOI] [PubMed] [Google Scholar]
- 52.Okiyama N, Furumoto Y, Villarroel VA, Linton JT, Tsai WL, Gutermuth J, et al. Reversal of CD8 T-cell mediated mucocutaneous graftversus-host-like disease by the JAK inhibitor Tofacitibib. J Invest Dermatol 2014;134(4):992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013;27:1861–9. [DOI] [PubMed] [Google Scholar]
- 54.Bogani C, Ponziani V, Guglielmelli P, Desterke C, Rosti V, Bosi A, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells 2008; 26:1920–1930. [DOI] [PubMed] [Google Scholar]
- 55.Nischal S, Bhattacharyya S, Christopeit M, Yu Y, Zhou L, Bhagat TD, et al. Methylome profiling reveals distinct alterations in phenotypic and mutational subgroups of myeloproliferative neoplasms. Cancer Research February 1 2013;73(3):1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pérez C, Pascual M, Martín-Subero JI, Bellosillo B, Segura V, Delabesse E, et al. Aberrant DNA methylation profile of chronic and transformed classic Philadelphia-negative myeloproliferative neoplasms. Haematologica 2013;98:1414–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Zhang W, Ishii T, Sozer S, Wang J, Xu M, et al. Correction of the abnormal trafficking of primary myelofibrosis CD34+ cells by treatment with chromatin-modifying agents. Cancer Research October 1 2009;69(19):7612–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Zhang W, Tripodi J, Lu M, Xu M, Najfeld V, et al. Sequential treatment of CD34+ cells from patients with primary myelofibrosis with chromatin-modifying agents eliminate JAK2V617F-positive NOD/SCID marrow repopulating cells. Blood 2010,116:5972–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quintás-Cardama A, Tong W, Kantarjian H, Thomas D, Ravandi F, Kornblau S, et al. A phase II study of 5-azacitidine for patients with primary and post-essential thrombocythemia/polycythemia vera myelofibrosis. Leukemia May 2008;22(5):965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odenike OM, Godwin JE, Van Besien K, et al. Phase II Trial of Low Dose, Subcutaneous Decitabine in Myelofibrosis. Blood 2008;112(11):2809-. [DOI] [PubMed] [Google Scholar]
- 61.Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Brit J Haem 2010;150(4):446–455. [DOI] [PubMed] [Google Scholar]
- 62.Quintas-Cardama A, Kantarjian H, Estrov Z, Borthakur G, Cortes J, Verstovsek S.Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leukemia research. Sep 2012;36(9):1124–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mascarenhas J, Lu M, Li T, Petersen B, Hochman T, Najfeld V, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Brit J Haem 2013;161(1):68–75. [DOI] [PubMed] [Google Scholar]
- 64.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 2010;116:1908–1918 [DOI] [PubMed] [Google Scholar]
- 65.Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012;119:3361–3369 [DOI] [PubMed] [Google Scholar]
- 66.Atanackovic D, Luetkens T, Kloth B, Fuchs G, Cao Y, Hildebrandt Y, et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am. J. Hematol 2011;86:918–22 [DOI] [PubMed] [Google Scholar]
- 67.Baffert F, Evrot E, Ebel N, et al. Improved Efficacy Upon Combined JAK1/2 and Pan-Deacetylase Inhibition Using Ruxolitinib (INC424) and Panobinostat (LBH589) in Preclinical Mouse Models of JAK2V617F-Driven Disease. Blood 2011;118(21):798-. [Google Scholar]
- 68.Tabarroki A, Saunthararajah Y, Visconte V, Cinalli T, Colaluca K, Rogers HJ, et al. Ruxolitinib in combination with DNA methyltransferase inhibitors: clinic responses in patients with symptomatic myelofibrosis with cytopenias and elevated blast(s) counts. Leuk Lymphoma 2014; pre-pub online May 21:1–3 [DOI] [PubMed] [Google Scholar]
- 69.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 2013;339(6127):1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science May 3 2013;340(6132):622–626. [DOI] [PubMed] [Google Scholar]
- 71.Ellwood-Yen K, Chen Y, Wang F, Lemieux R, Popovici-Muller J, Yang H, et al. IDH1 mutant inhibitor induces cellular differentiation and offers a combination benefit with Ara-C in a primary human Idh1 mutant AML xenograft model. Cancer Res 2014;74:1194- [Google Scholar]
- 72.Bejar R, Lord A, Stevenson K, Bar-Natan M, Perez-Ladaga A, Zaneveld J, et al. TET2 mutations predict response to hypomethylating agents in MDS patients. Blood 2014; 10.1182/blood-2014-06-582809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mughal TI, Barbui T, Abdel-Wahab O, Kralovics R, Jamieson C, Kvasnicka HM, et al. Novel Insights into the Biology and Treatment of Chronic Myeloproliferative Neoplasms. Leuk Lymphoma 2014, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupriez B, Morel P, Demory JL, Lai JL, Simon M, Plantier I, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood 1996;88(3):1013–8. [PubMed] [Google Scholar]
- 75.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working group for Myelofibrosis Research and Treatment. Blood 2009;113:2985–2901 [DOI] [PubMed] [Google Scholar]
- 76.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010;115(9):1703–1708. [DOI] [PubMed] [Google Scholar]
- 77.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011;29(4):392–397. [DOI] [PubMed] [Google Scholar]
- 78.Scott BL, Gooley TA, Sorror ML, Rezvani AR, Linenberger ML, Grim J, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood 2012;119(11):2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ditschkowski M, Elmaagacli AH, Trenschel R, Gromke T, Steckel NK, Koldehoff M, et al. Dynamic International Prognostic Scoring System scores, pretransplant therapy and chronic graft-versus-host disease determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica 2012;97(10):1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alchalby H, Yunus D-R, Zabelina T, Kobbe G, Holler E, Bornhäuser M, et al. Risk models predicting survival after reduced-intensity transplantation for myelofibrosis. Brit J Haematol 2012;157:75–85. [DOI] [PubMed] [Google Scholar]
- 81.Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hematopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant 2009:45(3):458–463 [DOI] [PubMed] [Google Scholar]
- 82.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer 2009;115:4715–4726 [DOI] [PubMed] [Google Scholar]
- 83.Li Z, Gooley T, Applebaum FR, Deeg HJ. Splenectomy and hemopoietic stem cell transplantation for myelofibrosis. Blood 2001; 97(7):2180–2181 [DOI] [PubMed] [Google Scholar]
- 84.Robin M, Tabrizi R, Mohty M, Furst S, Michallet M, Bay JO, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis: a report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Brit J Haematol 2011;152:331–339 [DOI] [PubMed] [Google Scholar]
- 85.Alchalby H, Zabelina T, Stübig T, et al. Allogeneic stem cell transpalntation for myelofibrosis with leukemic transformation: A study from the Myeloproliferative Neoplasm Subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2014;20:279–287. [DOI] [PubMed] [Google Scholar]
- 86.Mesa RA, Tibes R. MPN blast phase: clinical challenge and assessing response. Leuk Res 2012;36:1496–1497. [DOI] [PubMed] [Google Scholar]
- 87.Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86. [DOI] [PubMed] [Google Scholar]
- 88.Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–3742. [DOI] [PubMed] [Google Scholar]
- 89.Mascarenhas J, Navada S, Malone A, Rodriguez A, Najfeld V, Hoffman R. Therapeutic options for patients with myelofibrosis in blast phase. Leuk Res 2014;34:1246–1249. [DOI] [PubMed] [Google Scholar]
- 90.Cherington C, Slack JL, Leis J, et al. Allogeneic stem cell transplantation for myeloproliferative neoplasm in blast phase. Leuk Res 2012;36:1147–1151. [DOI] [PubMed] [Google Scholar]
- 91.Eghtedar A, Verstovsek S, Estrov Z, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood 2012;119:4614–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borthakur GPL, Kirschbaum MH et al. ,. Phase I/II trial of the MEK1/2 inhibitor GSK1120212 (GSK212) in patients (pts) with relapsed/refractory myeloid malignancies: Evidence of activity in pts with RAS mutation. J Clin Oncol 2011;29:6506-. [Google Scholar]
- 93.Tibes R, Bogenberger JM, Geyer HL, Mesa RA. JAK2 inhibitors in the treatment of myeloproliferative neoplasms. Expert Opin Investig Drugs 2012;21:1755–1774. [DOI] [PubMed] [Google Scholar]
- 94.Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC101 prospective study of Reduced Intensity Allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood 2014; 10.1182/blood-2014-04-572545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robin M, Giannotti F, Deconinck E, Mohty M, Michallet M, Sanz G, et al. Unrelated Cord Blood Transplantation for patients with primary or secondary myelofibrosis. Biol Blood Transplant 2014; 10.1016/j.bbmt.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 96.Panagiota V, Thol F, Markus B, Fehse B, Alchalby H, Badbaran A, et al. Prognostic effect of calreticulin mutations in patoents with myelofibrosis after allogeneic hematopoietic stem cell transplantation. Leukemia 2014; 10.1038/leu.2014.66 [DOI] [PubMed] [Google Scholar]
- 97.Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 2014; 10.1038/leu.2014.3 [DOI] [PubMed] [Google Scholar]
- 98.Rumi E, Harutyunyan AS, Pietra D, Milosevic JD, Casetti IC, Bellini M, et al. CALR exon 9 mutations are somaticallyacquired events in familial cases of essential thrombocythemia or primary myelofibrosis. Blood 2014;123:2416–2419. [DOI] [PubMed] [Google Scholar]
- 99.Fauble V, Emanuel RM, Geyer HL, Dueck AC, Kroeger N, Scott BL MD et al. Myeloproliferative Neoplasm Quality Of Life (MPN-QOL) Study Group: Observational Study Of Quality Of Life and Symptomatic Response In Myelofibrosis Patients Receiving Undergoing Treatment With Conventional Therapy, The Measures Trial and Allogeneic Stem Cell Transplant, The Symptoms Trial. Blood 2013;122:4090-24100448 [Google Scholar]
- 100.Mesa RA, Schwager S, Radia D, Cheville A, Hussein K, Niblack J, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res 2009;33:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–408. [DOI] [PubMed] [Google Scholar]
- 102.Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood 2012;120(7):1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta V, Gotlib J, Radich JP, Kröger NM, Rondelli D, Verstovsek S, et al. Janus kinase inhibitors and Allogeneic Stem Cell Transplantation for Myelofibrosis. Biol Blood Marrow Transplant 2014; 10.1016/j.bbmt.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaekel N, Behre G, Behning A, Wickenhauser C, Lange T, Niederwieser D, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis in patients pretreated with JAK1 and JAK2 inhibitor ruxolitinib. Bone Marrow Transplant 2014;49(2):179–184 [DOI] [PubMed] [Google Scholar]
- 106.Stübig T, Alchalby H, Ditschkowski M, Wolf D, Wulf G, Zabelina T, et al. JAK inhibition with ruxolitinib as pretreatment for allogeneic stem cell transplantation in primary or post ET/PV myelofibrosis. Leukemia 2014; 10.1038/leu.2014.86 [DOI] [PubMed] [Google Scholar]
- 107.Kröger N, Alchalby H, Ditschkowski M, Wolf D, Wulf G, Zabelina T, et al. Ruxolitinib as pretreatment before allogeneic stem cell transplantation for myelofibrosis. Blood 2013;122(21):392- [DOI] [PubMed] [Google Scholar]
- 108.Barosi G, Tefferi A, Besses C, Birgegard G, Cervantes F, Finazzi G, et al. Clinical endpoints for drug treatment trials in BCR-ABL1-negative classic myeloproliferative neoplasms: consensus statements from European Leukemia-NET (ELN) and International Working Group-Myeloprolifetive Neoplasms Researh and Treatment (IWG-MRT). Leukemia 2014; 10.1038/leu.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]