Abstract
Background:
Catheter-directed interventions for the treatment of patients with submassive pulmonary embolism (sPE) have shown promise in rapidly improving right-sided heart strain and preventing decompensation to massive pulmonary embolism. Among various catheter interventions, ultrasound-assisted thrombolysis (USAT) has attracted interest as potentially having more efficient lytic effect that could achieve thrombolysis faster and with a reduced lytic dose. However, based on clinical evidence, it is unclear whether USAT is superior to standard catheter-directed thrombolysis (SCDT). We herein describe the study design of the Standard vs UltrasouNd-assiSted CathEter Thrombolysis for Sub-massive Pulmonary Embolism (SUNSET sPE) trial, an ongoing randomized clinical trial designed to address this question.
Methods:
Adults with sPE presenting or referred to our institution are considered for enrollment in the trial. At the discretion of the treatment team, all patients undergo a catheter-directed intervention plus concomitant therapeutic anticoagulation. Participants are randomized 1:1 to a USAT catheter or an SCDT catheter. Study assessors are blinded to treatment group. The primary outcome is clearance of pulmonary thrombus burden, assessed by postprocedure computed tomography angiography. Secondary outcomes include resolution of right ventricular strain by echocardiography; improvement in pulmonary artery pressures; and 3- and 12-month improvement in echocardiographic, functional capacity, and quality of life measures. The study is powered to detect a 50% improvement in pulmonary artery thrombus clearance. Our enrollment target is 40 patients per treatment arm.
Conclusions:
SUNSET sPE is an ongoing randomized, head-to-head, single-blinded clinical trial with the goal of assessing whether USAT results in superior thrombus clearance compared with SCDT in patients with sPE. We expect the results of our study to inform future guidelines on choice of thrombolysis modality in this population of challenging patients.
Acute pulmonary embolism (PE) carries a high morbidity and is the third leading cause of cardiovascular mortality in the Western world. It accounts for 5% to 10% of in-hospital deaths, which for the United States translates to 200,000 deaths per year.1Recent registries and cohort studies suggest that approximately 10% of all patients with acute PE die during the first 1 to 3 months after diagnosis.1–5 Studies that have observed survivors for >3 months have reported an incidence of chronic thromboembolic pulmonary hypertension (CTEPH) up to 5% as a result of residual thrombi causing increased pulmonary vascular resistance. CTEPH is an incapacitating long-term complication with a significant impact on the patient’s quality of life and prognosis.6–10
Once PE is diagnosed, risk stratification is necessary to define appropriate management. It is typically stratified into three risk categories: high risk or massive, intermediate risk or submassive, and low risk. The distinction between these three groups is primarily based on hemo-dynamics and the presence of right-sided heart strain, which reflects the acute increase in pulmonary vascular resistance. Massive PE is characterized by circulatory shock and hypotension. Submassive PE (sPE) is characterized by clinical, radiographic, or biochemical evidence of right-sided heart strain in the absence of hypotension. Patients without any evidence of right-sided heart strain are classified as having low-risk PE.3
TREATMENT OF PE
The goals of treatment in patients with acute PE include prevention of decompensation to hemodynamic instability (if stable) and short- and long-term mortality9,11,12 as well as potential prevention of CTEPH.13–15 These outcomes have been linked to successful clearance of arterial thrombus burden.3,9,11,12,14,15 Initial systemic anticoagulation (AC) is the standard of care and is used in nearly all patients. In patients with a low-risk PE, AC alone is sufficient to enable endogenous reduction of thrombus. However, in patients with evidence of right-sided heart strain or hemodynamic changes, treatment may be escalated with thrombolysis targeting faster thrombus reduction.
The introduction of catheter-directed therapies has provided an alternative to the use of systemic thrombolysis, which is effective in clearing thrombus but is plagued with high bleeding rates.11,12 Proponents of catheter-directed therapies for PE suggest that they may provide therapeutic benefits similar to systemic thrombolysis but with lower doses of thrombolytic agent, thus potentially reducing the rate of bleeding events. The American Heart Association and European Society of Cardiology have both acknowledged catheter-directed therapies as a viable alternative to systemic thrombolysis, particularly in patients at high risk for a bleeding complication.3,5 Standard catheter-directed thrombolysis (SCDT) requires placement of a multi-side hole infusion catheter within the pulmonary artery (PA) thrombus under angiographic guidance. Thrombolytic agents are slowly infused through the catheter, which is left in place for the duration of the treatment. Ultrasound-assisted thrombolysis (USAT) is a modification of this therapy using a proprietary system of local high-frequency, low-power ultrasound waves to dissociate the fibrin matrix of the thrombus, allowing deeper penetration of lytic medication.
Several observational noncontrolled series have demonstrated the efficacy of catheter-directed therapies in improving clinical and hemodynamic parameters and reducing clot burden in patients with sPE.16–22 The Ultra-sound Accelerated Thrombolysis of Pulmonary Embolism (ULTIMA) trial was the first randomized controlled trial comparing USAT plus AC with AC alone in the treatment of sPE in 59 patients.23The investigators found that the right ventricular to left ventricular (RV/LV) diameter ratio, the most commonly used echocardiographic measure of right-sided heart strain, was significantly reduced at 24 hours in the USAT group but not in the control group, although this difference was not evident at 90 days. In both study groups, there were no major bleeding events, and minor bleeding complications were rare. A Prospective, Single-arm, Multicenter Trial of EkoSonic Endovascular System and Activase for Treatment of Acute Pulmonary Embolism (SEATTLE II) trial evaluated the effectiveness of USAT in patients with sPE, and it also showed an improvement in RV/LV ratio at 48 hours.24In our outcomes evaluation of the National Inpatient Sample, catheter-directed interventions for PE were associated with similar rates of in-hospital mortality but a significant reduction in the rate of hemorrhagic stroke compared with systemic thrombolysis.25
USAT VS SCDT
In vitro studies have demonstrated the improved penetration of thrombolytic agents with USAT.26,27 The purported clinical benefit of this technology is that similar thrombus clearance may be achieved using lower doses of lytic agents or shorter duration of therapy. This, in turn, would be expected to decrease the rate of bleeding complications.
However, USAT compared with SCDT is costly and requires special equipment, adding some complexity. Little is known about whether USAT is superior to SCDT in the treatment of sPE in the clinical setting. Both the ULTIMA and SEATTLE II trials along with multiple other series used USAT only and did not enable any assessment of the contribution of ultrasound to clinical outcomes. Although favorable compared with systemic thrombolysis, these studies were associated with an estimated 3.5% major bleeding rate.23,24,28,29 One series of 33 patients is the largest study to show equal thrombus clearance with reduced thrombolytic infusion time and treatment-related complications with USAT compared with SCDT.28A large prospective multicenter registry, on the other hand, noted no difference in outcomes by modality used.21Our retrospective analysis of 102 patients showed similar rates of survival, hemodynamic stabilization, and echocardiographic parameters between the two treatments.22A recent systematic review and meta-analysis of available data concluded that current evidence did not support the superiority of USAT over SCDT.29
To date, there are no randomized controlled trials comparing USAT with SCDT in patients with sPE. However, the BERN Ultrasound-enhanced Thrombolysis for Ilio-Femoral Deep Vein Thrombosis versUs Standard Catheter Directed thromboLysis (BERNUTIFUL) trial did compare USAT with SCDT in the treatment of 48 patients with acute iliofemoral deep venous thrombosis. The investigators found no difference in thrombus load reduction, venous patency, or symptoms of post-thrombotic syndrome to support an incremental benefit of USAT over SCDT.16Whether similar results would be found for sPE remains unknown. In an era of increasing focus on quality and cost-consciousness, the use of USAT over SCDT should be justified by prospectively demonstrated improvements in efficacy and safety. The Standard vs Ultrasound-assisted Catheter Thrombolysis for Submassive Pulmonary Embolism (SUNSET sPE) trial is an ongoing randomized, head-to-head, single-blinded clinical trial designed to address these objectives.
STUDY OBJECTIVES
Our primary objective is to determine whether USAT is associated with superior thrombus load reduction compared with SCDT in patients presenting with sPE. Our secondary objectives are to determine the change in RV function within 48 hours, in-hospital clinical outcomes, functional status and quality of life at 3 and 12 months, and cost-effectiveness of a USAT-based treatment strategy compared with SCDT.
PARTICIPANTS
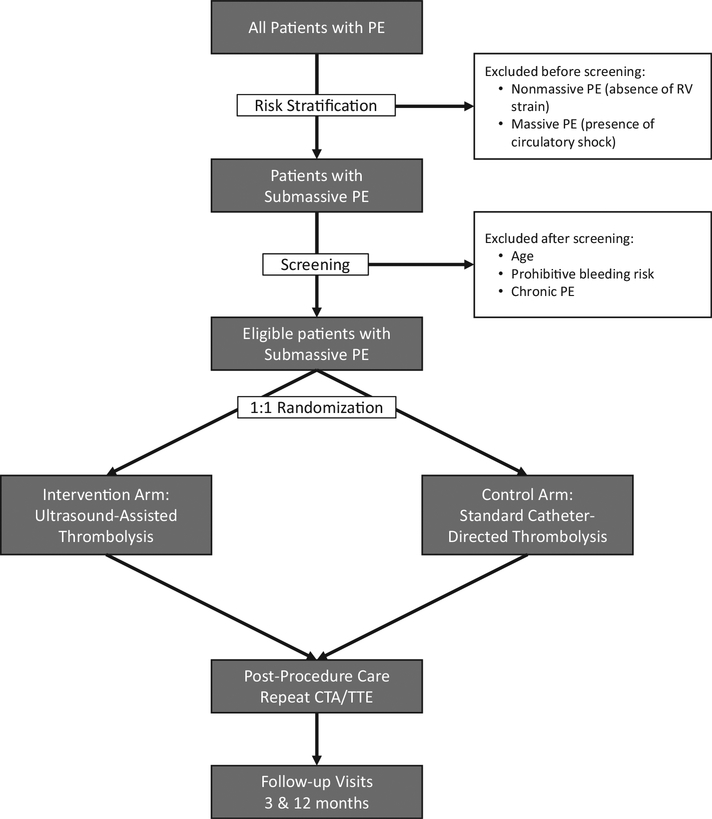
Our institution is a tertiary care referral center with a PE response team (PERT) consisting of pulmonology, cardiac surgery, and either vascular surgery or interventional cardiology teams on call. Patients who either present with PE or are transferred with this working diagnosis are evaluated by the PERT. After initial diagnostic evaluation, patients deemed eligible for SCDT are approached for participation in the trial (Fig).
Fig.
Flow chart of study events. CTA, Computed tomography angiography; PE, pulmonary embolism; RV, right ventricle; TTE, transthoracic echocardiography.
Patients meet inclusion criteria if they are diagnosed with sPE, defined as a combination of the following: PE diagnosed by computed tomography (CT) angiography; RV strain as diagnosed by RV/LV ratio >1 by either CT angiography or echocardiography and elevated cardiac biomarkers; and absence of circulatory shock as defined by cardiac arrest, persistent hypotension (systolic blood pressure <90 mm Hg), requirement of vasoactive medications, or evidence of end-organ hypoperfusion. Patients are excluded for age <18 years, symptoms for >14 days, elevated bleeding risk (any prior intracranial hemorrhage, known structural intracranial cerebrovascular disease or neoplasm, ischemic stroke within 3 months, suspected aortic dissection, active bleeding or bleeding diathesis, recent spinal or cranial/brain surgery, recent closed head or facial trauma with bone fracture or brain injury), participation in any other investigational drug or device study, life expectancy <90 days, or inability to comply with study assessments (Table I).
Table I.
Inclusion and exclusion criteria for participation in the Standard vs Ultrasound-assisted Catheter Thrombolysis for Submassive Pulmonary Embolism (SUNSET sPE) study
| Inclusion criteria | Exclusion criteria |
|---|---|
PE demonstrated by pulmonary CT angiography RV strain, defined by
|
Age <18 years Symptom duration >14 days High bleeding risk, defined by
|
Absence of circulatory shock, defined by
|
Active participation in another study Life expectancy <90 days Inability to comply with assessments |
BNP, Brain natriuretic peptide; CT, computed tomography; LV, left ventricle; PE, pulmonary embolism; RV, right ventricle; SBP, systolic blood pressure; TTE, transthoracic echocardiography.
STUDY DESIGN AND INTERVENTIONS
Patients are recruited through the emergency departments, unless they are inpatients, of three hospitals within our institution that are capable of providing interventional therapies for sPE. Many of these patients are initially evaluated at other hospitals before transfer to one of these referral centers. The PERT evaluates each patient and applies established institutional algorithms to determine whether the patient should receive AC alone, a catheter-directed therapy, or an open surgical procedure. As part of this decision-making process, patients receive baseline CT angiography of the chest, transthoracic echocardiography (TTE), and cardiac biomarker determinations including troponin I and brain natriuretic peptide levels. In addition, baseline vital signs, including heart rate, pulse oximetry, and oxygen requirements at rest, are documented. If the patient is an appropriate candidate for SCDT and is agreeable to such a procedure, the trial is discussed with the patient at this time, and informed consent to participate is obtained.
Patients are randomized 1:1 to either the USAT or the SCDT arm. Randomization occurs after the patient is screened, has consented, and has enrolled in the trial. The randomization schedule is generated by a permuted block algorithm with random block sizes of four or six participants. Treatment assignments are stored in sequentially numbered, sealed, opaque envelopes accessible only to study investigators and designated research coordinators.
The study treatment is performed by the same physician group regardless of which of the three hospitals is providing periprocedural care. All participants are taken to the interventional suite to undergo the study procedure, which involves positioning of one (for unilateral PE) or two PA infusion catheters, one into each main PA, under fluoroscopic guidance through percutaneous transvenous access. The specific catheters used differ by intervention arm; the experimental arm receives the EKOS USAT catheter (EKOS Corporation, Bothell, Wash), and the control arm receives a standard Cragg-McNamara multi-side hole catheter (Medtronic, Minneapolis, Minn). Invasive PA systolic and diastolic pressures are transduced and documented intraprocedurally. Alteplase, a recombinant tissue plasminogen activator (tPA), is the drug infused in all patients.
Technical details of the procedure, including choice of access site, concomitant inferior vena cava filter placement, and intraoperative and postoperative tPA dosing, are left to the discretion of the treating physician. However, study protocol dictates that the maximum tPA dosing should not exceed 24 mg total. In May 2017, preliminary results from the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Pulmonary Embolism (OPTALYSE) study, which assessed four different tPA dosing and duration protocols in USAT, showed that shorter periods of thrombolysis and smaller tPA doses may be sufficient to achieve thrombolysis.30Based on these findings, we amended the study protocol to include a recommendation to treating physicians that 4 to 6 mg of tPA should be given through each catheter during a 4- to 6-hour period with no loading dose. Termination should be considered at this point, provided the patient’s hemo-dynamic or respiratory parameters have improved. This recommendation applies to both the SCDT and USAT arms of the study.
All patients remain in the intensive care unit while tPA is being administered. Heparin is administered concomitantly, with dosages determined by a hospital-defined nomogram that protocolizes dosing to target a therapeutic anti-Xa level or partial thromboplastin time. Before catheter removal, TTE is repeated and PA pressures are transduced. Infusion catheters are removed at the bedside. Care after lysis follows standard institutional algorithms for patients with PE. AC is continued after discharge in all patients. Bleeding risk, insurance coverage, prior AC history, preference of the patient, and other factors are considered, and the choice of specific agent (which may include warfarin, enoxaparin, or a novel oral anticoagulant agent) is individualized to the specific needs of the patient.
Two follow-up visits are planned at 3 months and 12 months after the study procedure. All patients are asked to return to the PERT follow-up clinic. Follow-up visits include repeated TTE, 6-minute walk test, and quality of life questionnaires as detailed later. To enhance patient retention after discharge, a small honorarium is provided to patients who return for follow-up. Those who do not return for follow-up are asked to see their primary care provider, with attempts made to coordinate completion of the required testing at this visit.
Because of inherent differences in the two catheter types, the participants and treating physicians are not blinded to treatment allocation. However, all outcome assessors will be blinded, including the radiologists interpreting the CT angiograms, the cardiologists interpreting the TTEs, and the statistician performing the analysis.
OUTCOMES
The primary outcome, thrombus load reduction, is measured by change in the CT obstruction index. This index is a numeric score measured by dividing the PA tree into 10 segmental arteries per lung (3 for the upper lobes, 2 for the middle lobe or lingula, and 5 for the lower lobes). The CT obstruction index has been previously described and validated as a quantifiable measure of pulmonary clot burden.31,32 The degree of thrombus load reduction is recorded as the percentage change in the CT obstruction index, based on repeated CT angiography performed within 48 hours after SCDT (Table II).
Table II.
Outcome measures
| Immediate postprocedure outcomes | Follow-up outcomes |
|---|---|
| Imaging | |
| Change in CT obstruction index (CT | RV/LV ratio (TTE) |
| angiography) | |
| RV/LV ratio (CT and TTE) | |
| Hemodynamic | |
| Invasive PA pressures | |
| Clinical | |
| Mortality | |
| Decompensation to massive PE | Mortality |
| Major bleeding | Recurrent VTE |
| Minor bleeding | Quality of life |
| Length of ICU and hospital stay |
CT, Computed tomography; ICU, intensive care unit; LV, left ventricle; PA, pulmonary artery; PE, pulmonary embolism; RV, right ventricle; TTE, transthoracic echocardiography; VTE, venous thromboembolism.
The primary outcome is change in CT obstruction index.
Our secondary effectiveness outcome, clinical success, is defined as survival plus freedom from PE decompensation at 90 days. Our secondary safety outcomes include the following: in-hospital and 90-day mortality; decompensation to massive PE (with circulatory shock as defined before); International Society on Thrombosis and Haemostasis major bleeding, defined as a decrease in hemoglobin of >2.0 g/dL, transfusion requirement of at least 2 units of packed red blood cells, or involvement of a critical site (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome); minor bleeding (any bleeding not classified as major bleeding); recurrent venous thromboembolic event within 90 days; any other major adverse events identified or reported to study staff; and intensive care unit length of stay (Table II).
Secondary hemodynamic outcomes are measured by echocardiography and invasive PA pressure measurements. PA pressures are measured through the thrombolytic catheters immediately before and after infusion of thrombolytic. Echocardiographic RV/LV ratio and tricuspid annular plane systolic excursion are also measured before and after the procedure.
At each follow-up visit, patients undergo assessment of right-sided heart function, functional status, and quality of life. These secondary end points are measured by TTE, a 6-minute walk test performed according to published guidelines,33,34 and three questionnaires: 36-Item Short Form Health Survey, PE Quality of Life, and San Diego Shortness of Breath35–37 (Table II).
DATA MANAGEMENT AND ANALYTICAL METHODS
Data are collected by a combination of the study investigators, nurse research coordinators, and trained residents and fellows. Baseline characteristics and clinical data, procedural data, and postprocedure outcomes are prospectively entered into a secure database. Longer term outcomes are identified at follow-up visits, but periodic review of the electronic medical record is used to identify uncaptured outcome events. Data safety monitoring is performed by independent nurse coordinators who assess the accuracy and completeness of collected data and ensure timely capture and reporting of any adverse events in study participants.
Although we expect little crossover, given the nature of the treatment being studied and the proximity between randomization and treatment allocation, all statistical analysis will be done in an intention-to-treat fashion. No interim analyses are planned. For all statistical tests, a P value of .05 will be considered significant. The primary outcome, change in the CT obstruction index, is an intervally scaled variable. A Student t-test will provide an unadjusted estimate of the difference between treatment arms. Analysis of covariance, a more robust method that allows control for key differences in baseline characteristics, will be used to provide adjusted comparisons. Binary secondary outcomes (such as mortality or decompensation) will be modeled by logistic regression. Like the primary outcome, continuous secondary outcomes (such as RV/LV ratio) will be modeled by t-tests and analysis of covariance. Survival models for long-term complication rates will identify time-to-event differences. As an exploratory analysis, we additionally plan to determine whether the use of USAT is cost-effective from a societal perspective, using our results to estimate the incremental benefit of USAT over SCDT with regard to clinical outcomes and quality of life, and to estimate incremental cost based on both device cost and any differences in in-hospital and out-of-hospital resource utilization (outpatient nursing care, loss of work, outpatient testing and follow-up).
SAMPLE SIZE ESTIMATION
Sample size estimation was based on the primary end point of change in CT obstruction index. Population parameters for this measure have not been established in the literature but were estimated from similar studies reporting Miller scores.38,39 Thus, our sample size estimation was modeled after the methodologically similar BERNUTIFUL trial.16In vitro studies have shown ultra-sound assistance to increase the degree of thrombus clearance by approximately 50%.26,27 As in the BERNUTIFUL trial, we assumed 43% thrombus clearance in the SCDT group and 64.5% thrombus clearance in the USAT group. To detect an improvement of at least 50% in thrombus clearance with USAT, at a significance level of .05 and 80% power, we will require 40 patients per group.
ETHICAL CONSIDERATIONS
This study was approved by our Institutional Review Board before enrollment of the first patient. All potential risks and benefits (those related specifically to the study and those related to interventional PE therapy) are discussed with potential study subjects, and participants provide appropriate informed consent before enrollment in the study. All patient data collected as part of this study will remain secure and confidential. In accordance with our study protocol, no raw data, even deidentified, will be disseminated to groups outside of our institution. No blood or tissue samples are being retained as a result of this study.
This study is being funded by a grant provided by the University of Pittsburgh Medical Center Vascular Medicine Institute, a group with no financial interests in the results of our trial. No industry funding has been obtained for the conduct of our research, and the study investigators have no relevant financial interests in the outcome of our study.
CONCLUSIONS
Current guidelines support the use of SCDT in patients with sPE, but to date there are few data favoring the use of USAT over an SCDT catheter. The SUNSET sPE trial has been actively enrolling since May 2016, and the anticipated completion date is December 2018. The trial will determine whether USAT provides improved thrombus clearance compared with SCDT and guide future recommendations on choice of treatment modality in patients who undergo catheter-directed therapy for sPE.
Acknowledgments
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Footnotes
Clinical Trial registration: NCT02758574.
Author conflict of interest: none.
REFERENCES
- 1.Kahn SR, Houweling AH, Granton J, Rudski L, Dennie C, Hirsch A. Long-term outcomes after pulmonary embolism: current knowledge and future research. Blood Coagul Fibrinolysis 2014;25:407–15. [DOI] [PubMed] [Google Scholar]
- 2.Avgerinos ED, Chaer RA. Catheter-directed interventions for acute pulmonary embolism. J Vasc Surg 2015;61:559–65. [DOI] [PubMed] [Google Scholar]
- 3.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788–830. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–73.25173341 [Google Scholar]
- 6.Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Taliani MR, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006;130:172–5. [DOI] [PubMed] [Google Scholar]
- 7.Meyer G, Planquette B, Sanchez O. Long-term outcome of pulmonary embolism. Curr Opin Hematol 2008;15:499–503. [DOI] [PubMed] [Google Scholar]
- 8.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257–64. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008;29:1569–77. [DOI] [PubMed] [Google Scholar]
- 10.Guérin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Haemost 2014;112:598–605. [DOI] [PubMed] [Google Scholar]
- 11.Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage. JAMA 2014;311:2414. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides SV, Vicaut E, Danays T, Becattini C, Bertoletti L, Beyer-Westendorf J, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol 2017;69:1536–44. [DOI] [PubMed] [Google Scholar]
- 14.Kline JA, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, Rondina MT, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014;12: 459–68. [DOI] [PubMed] [Google Scholar]
- 15.Fasullo S, Scalzo S, Maringhini G, Ganci F, Cannizzaro S, Terrazzino G, et al. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci 2011;341:33–9. [DOI] [PubMed] [Google Scholar]
- 16.Engelberger RP, Spirk D, Willenberg T, Alatri A, Do DD, Baumgartner I, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv 2015;8:e002027. [DOI] [PubMed] [Google Scholar]
- 17.Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009;20: 1431–40. [DOI] [PubMed] [Google Scholar]
- 18.Bagla S, Smirniotopoulos JB, van Breda A, Sheridan MJ, Sterling KM. Ultrasound-accelerated catheter-directed thrombolysis for acute submassive pulmonary embolism. J Vasc Interv Radiol 2015;26:1–6. [DOI] [PubMed] [Google Scholar]
- 19.George B, Wallace EL, Charnigo R, Wingerter KE, Kapadia P, Gurley JC, et al. A retrospective analysis of catheter-based thrombolytic therapy for acute submassive and massive pulmonary embolism. Vasc Med 2015;20:122–30. [DOI] [PubMed] [Google Scholar]
- 20.McCabe JM, Huang PH, Riedl L, Eisenhauer AC, Sobieszczyk P. Usefulness and safety of ultrasound-assisted catheter-directed thrombolysis for submassive pulmonary emboli. Am J Cardiol 2015;115:821–4. [DOI] [PubMed] [Google Scholar]
- 21.Kuo WT, Banerjee A, Kim PS, DeMarco FJ, Levy JR, Facchini FR, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015;148:667–73. [DOI] [PubMed] [Google Scholar]
- 22.Avgerinos ED, Abou Ali AN, Liang NL, Genovese E, Singh MJ, Makaroun MS, et al. Predictors of failure and complications of catheter-directed interventions for pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2017;5:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479–86. [DOI] [PubMed] [Google Scholar]
- 24.Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015;8:1382–92. [DOI] [PubMed] [Google Scholar]
- 25.Liang NL, Avgerinos ED, Marone LK, Singh MJ, Makaroun MS, Chaer RA. Equivalent outcomes between ultrasound-assisted thrombolysis and standard catheter-directed thrombolysis for the treatment of acute pulmonary embolism. Vasc Endovascular Surg 2016;50:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood 1993;81:2636–43. [PubMed] [Google Scholar]
- 27.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol 1995;21:419–24. [DOI] [PubMed] [Google Scholar]
- 28.Lin PH, Annambhotla S, Bechara CF, Athamneh H, Weakley SM, Kobayashi K, et al. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular 2009;17(Suppl 3):S137–47. [DOI] [PubMed] [Google Scholar]
- 29.Tafur AJ, Shamoun FE, Patel SI, Tafur D, Donna F, Murad MH. Catheter-directed treatment of pulmonary embolism. Clin Appl Thromb Hemost 2017;23:821–9. [DOI] [PubMed] [Google Scholar]
- 30.BTG. OPTALYSE PE study demonstrates safety and efficacy of shorter, lower dose EKOS therapy for pulmonary embolism. Available at: https://www.btg-im.com/en-US/EKOS/News-Events/Press-Releases/View?pressOPTALYSE-PE-Study-Demonstrates-Safety-and-Efficacy. Accessed September 7, 2017. [Google Scholar]
- 31.Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism. AJR Am J Roentgenol 2001;176:1415–20. [DOI] [PubMed] [Google Scholar]
- 32.Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, et al. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 2003;13: 29–35. [DOI] [PubMed] [Google Scholar]
- 33.Gorkin L, Norvell NK, Rosen RC, Charles E, Shumaker SA, McIntyre KM, et al. Assessment of quality of life as observed from the baseline data of the Studies of Left Ventricular Dysfunction (SOLVD) trial quality-of-life substudy. Am J Cardiol 1993;71:1069–73. [DOI] [PubMed] [Google Scholar]
- 34.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 36.Klok FA, Cohn DM, Middeldorp S, Scharloo M, Büller HR, van Kralingen KW, et al. Quality of life after pulmonary embolism: validation of the PEmb-QoL Questionnaire. J Thromb Haemost 2010;8:523–32. [DOI] [PubMed] [Google Scholar]
- 37.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113:619–24. [DOI] [PubMed] [Google Scholar]
- 38.Lin PH, Annambhotla S, Bechara CF, Athamneh H, Weakley SM, Kobayashi K, et al. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular 2009;17:S137–47. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy RJ, Kenney HH, Dunfee BL. Thrombus resolution and hemodynamic recovery using ultrasound-accelerated thrombolysis in acute pulmonary embolism. J Vasc Interv Radiol 2013;24:841–8. [DOI] [PubMed] [Google Scholar]