Abstract
Dietary factors that contribute to chronic low-grade metabolic acidosis have been linked to breast cancer risk, but to date no epidemiologic study has examined diet-dependent acid load and breast cancer. We used data from 43,570 Sister Study participants who completed a validated food frequency questionnaire at enrollment (2003–2009) and satisfied eligibility criteria. The Potential Renal Acid Load (PRAL) score was used to estimate diet-dependent acid load. Higher scores reflect greater consumption of protein and phosphorus, and lower consumption of potassium, calcium, and magnesium. The association between PRAL and breast cancer was evaluated using multivariable Cox proportional hazards regression. We identified 1,882 invasive breast cancers diagnosed at least 1 year after enrollment (mean follow-up, 7.6 years). The highest PRAL quartile, reflecting greater acid-forming potential, was associated with increased risk of breast cancer (HRhighest vs. lowest quartile: 1.21 [95% CI, 1.04–1.41], Ptrend=0.04). The association was more pronounced for estrogen receptor (ER)-negative (HRhighest vs. lowest quartile: 1.67 [95% CI, 1.07–2.61], Ptrend=0.03) and triple-negative breast cancer (HRhighest vs. lowest quartile: 2.18 [95% CI, 1.22–3.91], Ptrend=0.02). Negative PRAL scores, representing consumption of alkaline diets, were associated with decreased risk of ER-negative and triple-negative breast cancer, compared to a PRAL score of 0 representing neutral pH. Higher diet-dependent acid load may be a risk factor for breast cancer while alkaline diets may be protective. Since PRAL scores are positively correlated with meat consumption and negatively correlated with fruit and vegetable intake, results also suggest that diets high in fruits and vegetables and low in meat may be protective against hormone receptor negative breast cancer.
Keywords: diet-dependent acid load, potential renal acid load, breast cancer, ER-negative breast cancer, triple-negative breast cancer
INTRODUCTION
Epidemiologic evidence on the association between individual dietary factors and risk of breast cancer is inconclusive,1 with only alcohol consumption being consistenly recognized as a dietary risk factor for breast cancer.2 Since individual nutrients or foods may not reflect the complexity of dietary exposures, the study of dietary patterns or overall diet quality may provide a better measure of the influence of diet on health outcomes.3 Among several dietary patterns or indices, Mediterranean diet and diets composed largely of vegetables have been reported to be associated with a decreased risk of breast cancer.4
It has recently been proposed that diet-dependent acid load, representing consumption of acidogenic diets characterized by higher dietary intake of proteins and minerals5, 6, may play an important role in increasing the risk of metabolic abnormalities such as kidney stone formation7, chronic kidney disease8, loss of lean body mass9 hypertension10, and diabetes mellitus11, as well as mortality.12 Dietary intake can influence the body’s acid-base balance.5, 6 For example, oxidation of the sulfur-containing amino acids in animal proteins and cereal grains can contribute to increasing diet-dependent acid load. On the other hand, consumption of fruits and vegetables that are rich in potassium salts may decrease diet-dependent acid load through metabolism spending hydrogen ions.13
Short-term dietary acid loading may produce temporary acid-base imbalance, but it is quickly corrected and has no significant clinical effects.14 However, prolonged diet-induced low-grade metabolic acidosis over years may predispose to cardio-metabolic abnormalities,15 in particular, insulin resistance, possibly through increased glucocorticoid secretion,16 decreased urinary secretion of citrate,17 and increased urinary secretion of magnesium.18
Insulin resistance, a key mechanism in diabetes mellitus may contribute to risk of breast cancer.19 Acidic pH levels in the extracellular space may enhance the invasive and metastatic potential of cancer cells.20, 21 Evidence also suggests that chronic diet-induced low-grade acidosis, represented by long-term high protein consumption, may increase insulin-like growth factor-1 (IGF-1)14 which is known to be associated with an increased risk of breast cancer.22 However, there is limited epidemiologic evidence on the association between diet-dependent acid load and risk of cancer, especially for breast cancer.23
Therefore, we examined the association between diet-dependent acid load and risk of breast cancer, using data from the nationwide prospective Sister Study cohort. We hypothesized that acidogenic diet would be associated with increased risk of breast cancer.
MATERIALS AND METHODS
Study population
Study participants came from the Sister Study, a nationwide prospective cohort study designed to investigate environmental and genetic risk factors for breast cancer.24,25 During 2003 to 2009, 50,884 US and Puerto Rican women whose sister had been diagnosed with breast cancer were enrolled. At enrollment, participants were ages 35–74 and had never been diagnosed with breast cancer. The Sister Study was approved by the Institutional Review Boards of the National Institute of Environmental Health Sciences/NIH and the Copernicus Group. All participants provided informed consent. Information on demographics, medical and family history, and lifestyle factors was assessed through telephone interview and written questionnaires at enrollment. During the home visit, current height, weight, and hip and waist circumferences were measured by trained study personnel. Study participants were asked to complete detailed follow-up questionnaires every 2–3 years to provide information on risk factors and changes in health status. Response rates were over 90% throughout follow-up26.25 The data presented in the current analyses were obtained from Sister Study data release 5.0 (July 2016), which included incident breast cancer cases diagnosed as of August 14, 2015.
Diet-dependent acid load score assessment
Participants completed a standardized self-administered food frequency questionnaire (FFQ) at baseline, a modified version of the 110-item 1998 Block FFQ (NutritionQuest, Berkeley, CA, USA).27 The questionnaire was structured to collect average food consumption in the past 12 months, calculated by multiplying frequency of consumption (9 possible frequencies, ranging from “never” to “every day”) by the quantity specified (3 or 4 quantity choices per each food item or group of similar food items). Based on the information obtained by FFQ, nutrient consumption was estimated using the United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (FNDDS) for U.S. women.28 All the food items in the FFQ were used to calculate diet-dependent acid load.
We calculated diet-dependent acid load using formulas that have been previously defined and used in other epidemiologic studies: potential renal acid load (PRAL), net endogenous acid production (NEAP)6, net acid excretion (NAE)5, and the ratio of animal protein to potassium (A:P)29. These measures were calculated as follows: PRAL(mEq/day) = (0.4888×total protein[g/day]) + (0.0366×phosphorus[mg/day]) − (0.0205×potassium[mg/day]) − (0.0263×magnesium[mg/day]) − (0.0125×calcium[mg/day]); NEAP(mEq/day) = (54.5×protein[g/day]) / (0.0366×potassium[mEq/day]) – 1.02; and NAE(mEq/day) = PRAL + (body surface area[m2]×41[mEq/day]/1.73 m2) in which body surface area was calculated by the Du Bois formula: 0.007184×height0.725×weight0.425 30; and A:P ratio = animal protein[g/day] / potassium[g/day]. We did not include dietary supplements in the calculation of diet-dependent acid load. Instead, we included the use of multivitamins as a covariate in the models. PRAL and NEAP were validated in independent populations to predict urine pH using nutrient intake data from FFQs.31,32
Negative values of PRAL and lower values of NEAP and NAE reflect base (or alkaline)-forming potential, whereas positive values of PRAL and higher values of NEAP and NAE indicate acid-forming potential. The PRAL score takes into account average intestinal absorption rates for dietary proteins and minerals, ionic dissociation, and sulfur metabolism.5 NEAP and A:P ratio are simpler formulas using only two nutrients included the in PRAL6, and NAE is a variant of PRAL that further includes estimated excretion of organic acids.5 Thus, we used PRAL for our main analysis and carried out sensitivity analyses using NEAP, NAE and A:P ratio. The relative validity of dietary consumption of protein, phosphorus, potassium, magnesium, and calcium used in calculation of diet-dependent acid load has been evaluated using three 4-day records33 and two 24-hour recalls34 (correlation coefficients ranged from 0.48 to 0.59).
Ascertainment of breast cancer
Self-reported incident breast cancers and tumor characteristics have been verified by medical records for more than 80% of cases. Agreement was high between self-reported breast cancer diagnosis and medical records (99.4%). Classification of an invasive cancer (99.3%), and estrogen receptor (ER) positive status (99.3%) were also in high agreement.35,25 Therefore, self-reported cases and tumor characteristics were included in the analyses when medical records were not available. During follow-up (mean, 7.6 years from one year after enrollment), 1,614 invasive breast cancers were diagnosed.
Statistical Analysis
We excluded women who did not provide a FFQ (n=1,145), reported implausibly extreme energy intakes (<600 and >3500 kcals/d) (n=1,469), were pregnant (n=20) at baseline, had extreme body mass index (BMI) values (<15 or >50 kg/m2) (n=284), or had a history of any cancer except non-melanoma skin cancer (n=2,757). To reduce bias from reverse-causality related to undetected tumors present at baseline, we also excluded person-time within 12 months after enrollment (thereby excluding 603 incident breast cancers). In addition, we excluded women who had a chronic disease that could affect chronic acid-base disturbances36, such as chronic kidney disease (n=582), liver cirrhosis (n=90), congestive heart failure (n=335), or chronic obstructive pulmonary disease (n=787). Thus, a total of 43,570 women were included, contributing 240,863 person-years of follow-up. Participants excluded from the analysis had higher PRAL scores and were older, less physically active, had higher BMI and shorter lifetime duration of breastfeeding, and were more likely to be cases (Supplemental Table 1).
We computed hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between diet-dependent acid load and breast cancer risk using Cox proportional hazards regression; Quartiles and a 1 standard deviation (SD) increment of diet-dependent acid load were used to characterize diet measures. Proportional hazards assumptions were evaluated by Schoenfeld residuals with the logarithm of the cumulative hazards function based on Kaplan-Meier estimates for diet-dependent acid load. There was no significant departure from proportionality in hazards over time. Since age was used as the primary time scale, study subjects were entered into the risk set at one year after the age when they finished baseline evaluations (left truncation). Person-time was accrued from then until the age of breast cancer diagnosis or until death, last follow-up or when they dropped out of the study. We focused on invasive breast cancer in the present analysis; women with in situ tumors were censored at the age of diagnosis. HR for cancer defined by ER expression subtype were determined with opposing or undefined ER expression censored at the age of diagnosis.37 Time-varying menopausal status was considered for both incident cases and non-cases.
Potential confounders or effect modifiers were identified a priori based on literature review and presumed causal relationships among the covariates.38 The following covariates at baseline were included in multivariable adjusted models: race/ethnicity (non-Hispanic White, non-Hispanic Black, or other), education (high school or less, some college, or 4-year degree or higher), household income (<$49,999, $50,000-$99,999, $100,000+), measured BMI (<18.5, 18.5 to <25, 25 to <30, 30 to <35, 35 to <40, or ≥40 kg/m2), pack-years of smoking (never smoker, smoker <10 pack-years, smoker ≥10 pack-years), alcohol consumption (never, former, current drinker < 1 drink/day, current drinker 1 drink/day, current drinker 1.1–1.9 drink/day, current drinker ≥2 drink/day), multivitamin use (none, < 1–3 days/week, < 4–6 days/week, every day, missing), total energy intake (kcal/day), self-reported physical activity (metabolic equivalent hours/week, quintile), strong family history of breast cancer (presence versus absence of at least one first degree female relative diagnosed with breast cancer before age 50), recent mammogram screening (<1yr, ≥1yr), breastfeeding history (total number of weeks, quintile), parity (nulliparous or 1, 2–3, ≥4), postmenopausal hormone therapy (none, estrogen only, both estrogen and progesterone), and age at menopause (premenopausal, <40, 40–49, 50–54, ≥55 years based on enrollment information).
Tests for linear trend across quartiles of PRAL scores were performed by modeling an ordinal variable for each quartile. In addition, Cox models using restricted cubic splines with four knots (at the 5th, 35th, 65th, 95th percentiles) with the same covariates were used to evaluate the shape of the exposure response for the relationship between the PRAL score and the risk of breast cancer. The reference value for estimating HRs and 95% CIs was chosen as PRAL= 0 mEq/day, which is considered to be a neutral PRAL score.12 Linearity was evaluated with a likelihood ratio test comparing the results from a linear model with a single term for the continuous measure to the results from a model with cubic spline terms added.
Case-case analysis was carried out to evaluate whether the association between diet-dependent acid load and breast cancer differed by ER and progesterone receptor (PR) expression.39 Potential effect modification by time-varying menopausal status, race/ethnicity, degree of family history of breast cancer, BMI, and physical activity was evaluated through stratified analysis and interaction testing using a likelihood ratio test. In addition, we performed a sensitivity analysis with an additional adjustment for Healthy Eating Index (HEI)-2015 to explore the effect of overall diet quality. The HEI-2015 was developed by the US Department of Agriculture to measure adherence to the Dietary Guidelines for Americans and the Food Guide Pyramid.40 We further analyzed data after excluding women who had type 2 diabetes and/or use of anti-diabetic medications, or hypertension and/or use of anti-hypertensive medications at baseline. Statistical significance was evaluated with two-sided tests, with the level of significance at 0.05. SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
RESULTS
Women with higher PRAL were younger, less physically active, and had higher BMI, shorter lifetime duration of breastfeeding, and younger age at menopause. They were less likely to be non-Hispanic white, and were more likely to have less education and lower income. They also were less likely to consume multivitamins and to have used hormone therapy in the past, but more likely to be former drinkers and to have ever smoked (Table 1). Women with higher PRAL tended to consume more red meat, poultry, added sugar, high fat dairy, protein, and fats, and less fruits, vegetables, and carbohydrates. Higher PRAL scores were correlated with higher consumption of red meat and poultry (r=0.50 and 0.37, respectively) and lower consumption of fruits and vegetables (r= −0.53 and −0.36, respectively), as well as lower HEI-2015 (r= −0.34) (Supplemental Table 2).
Table 1.
Study population characteristics by quartiles of the potential renal acid load score representing diet-dependent acid load at baseline: The Sister Study 2003–2009
| Potential renal acid load score quartiles (mEq/d) | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Characteristic | < −5.33 (n=10,892) | −5.33 to 2.25 (n=10,893) | 2.25 to 9.52 (n=10,893) | > 9.52 (n=10,892) | |
| Mean (SD) | |||||
| Age at baseline, yr | 57.9 (8.6) | 56.2 (8.8) | 54.4 (8.9) | 53.3 (8.7) | |
| BMI, kg/m2 | 26.4 (5.4) | 27.1 (5.6) | 27.6 (5.8) | 28.9 (6.3) | |
| Total MET-hours of physical activity/wk | 57.5 (33.4) | 51.4 (30.6) | 48.3 (29.7) | 47.2 (29.7) | |
| Paritya | 2.43 (1.11) | 2.39 1.10 | 2.39 (1.09) | 2.36 (1.08) | |
| Lifetime duration of breastfeeding, wkb | 71.0 (76.3) | 67.0 (71.9) | 63.1 (70.7) | 62.7 (71.3) | |
| Age at menopausec | 49.9 (6.0) | 49.5 (6.1) | 49.2 (6.4) | 48.7 (6.4) | |
| Proportion (%) | |||||
| Race/ethnicity | |||||
| Non-Hispanic white | 85.8 | 85.2 | 84.8 | 83.5 | |
| Non-Hispanic black | 8.0 | 8.0 | 7.8 | 8.1 | |
| Other | 6.2 | 6.8 | 7.4 | 8.4 | |
| Education | |||||
| High school degree or less | 12.7 | 14.6 | 15.8 | 16.9 | |
| Some college | 29.7 | 32.9 | 34.5 | 35.3 | |
| College degree or higher | 57.6 | 52.5 | 49.7 | 47.8 | |
| Income | |||||
| < $49,999 | 24.3 | 23.3 | 24.8 | 24.9 | |
| $50,000-$99,999 | 39.5 | 41.3 | 41.1 | 42.3 | |
| $100,000+ | 36.2 | 35.4 | 34.1 | 32.7 | |
| Multivitamin use | |||||
| None | 9.1 | 9.3 | 10.2 | 9.9 | |
| < 1–3 days/wk | 9.0 | 10.8 | 11.6 | 12.6 | |
| < 4–6 days/wk | 12.1 | 13.1 | 12.9 | 12.7 | |
| Every day | 51.0 | 43.5 | 38.4 | 35.9 | |
| Missing | 18.8 | 23.4 | 27.0 | 28.9 | |
| Drinking alcohol | |||||
| Never | 4.2 | 3.8 | 3.5 | 3.3 | |
| Former | 13.6 | 14.1 | 14.7 | 15.7 | |
| Current drinker, < 1 drink/day | 65.9 | 68.1 | 68.9 | 69.1 | |
| Current drinker, ≥ 1 and <2 drink/day | 9.8 | 9.2 | 8.7 | 8.0 | |
| Current drinker, ≥ 2 drink/day | 6.6 | 4.8 | 4.3 | 3.8 | |
| Smoking | |||||
| Never smoker | 58.2 | 58.5 | 57.4 | 55.6 | |
| Smoker <10 pack-years | 23.6 | 22.7 | 21.5 | 20.9 | |
| Smoker ≥ 10 pack-years | 18.2 | 18.8 | 21.1 | 23.5 | |
| ≥ 1 First-degree female member diagnosed with breast cancer before age 50 | 52.3 | 55.4 | 60.1 | 62.0 | |
| Mammography within 1 year before baseline | 83.5 | 83.1 | 81.2 | 79.4 | |
| Use of hormone therapy | |||||
| None | 52.6 | 55.5 | 60.7 | 63.7 | |
| Estrogen only | 20.5 | 20.2 | 18.5 | 17.3 | |
| Both estrogen and progesterone | 26.9 | 24.3 | 20.8 | 19.1 | |
Abbreviation: BMI, body mass index; MET, metabolic equivalent.
Data are presented as mean ± standard deviation, or percentage.
Among parous women (n=35,632)
Among women who ever breastfed (n=25,049)
Among women who experienced menopause (n=27,786)
The associations between PRAL quartiles and invasive breast cancer are shown in Table 2. After multivariable adjustment, invasive breast cancer risk was increased in the highest quartile of PRAL compared to the lowest quartile with a trend of increasing risk (HR=1.21, 95% CI,1.04–1.41; P trend=0.04) with increasing PRAL. The association was significant for ER-negative (HRhighest vs. lowest quartile: 1.67 [95% CI, 1.07–2.61], Ptrend=0.03) but not for ER-positive breast cancer (HRhighest vs. lowest quartile: 1.16 [95% CI, 0.97–1.38], Ptrend=0.17). A 1 SD increment (12.6 points) in PRAL was associated with increased risk of ER-negative breast cancer (HR1SD increase: 1.23 [95% CI, 1.05–1.43]), but not ER-positive breast cancer (HR1SD increase: 1.01 [95% CI, 0.95–1.08]); this difference was significant in a case-case analysis (P=0.04).
Table 2.
Hazard ratios (HRs) and 95% CIs for the association between diet-dependent acid load and risk of invasive breast cancer
| PRAL score quartiles (mEq/d) | Continuous PRAL | ||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend | 1 SD increment | ||
| Person-years | 83126 | 82469 | 82077 | 82185 | 329857 | ||
| Total invasive breast cancer | No. of cases | 388 | 409 | 384 | 433 | 1614 | |
| Model 1, HR (95%CI)1 | 1 (ref) | 1.11 (0.97–1.28) | 1.10 (0.95–1.26) | 1.28 (1.11–1.47) | 0.001 | 1.07 (1.02–1.13) | |
| Model 2, HR (95%CI)2 | 1 (ref) | 1.15 (1.00–1.33) | 1.09 (0.94–1.27) | 1.21 (1.04–1.41) | 0.04 | 1.04 (0.99–1.10) | |
| ER+ Invasive breast cancer | No. of cases | 299 | 306 | 290 | 311 | 1206 | |
| Model 1, HR (95%CI)1 | 1 (ref) | 1.08 (0.92–1.27) | 1.08 (0.91–1.27) | 1.19 (1.02–1.40) | 0.04 | 1.03 (0.98–1.10) | |
| Model 2, HR (95%CI)2 | 1 (ref) | 1.13 (0.96–1.34) | 1.09 (0.92–1.30) | 1.16 (0.97–1.38) | 0.17 | 1.01 (0.95–1.08)3 | |
| ER- Invasive breast cancer | No. of cases | 38 | 52 | 51 | 66 | 207 | |
| Model 1, HR (95%CI)1 | 1 (ref) | 1.40 (0.92–2.13) | 1.42 (0.93–2.17) | 1.86 (1.24–2.78) | 0.004 | 1.27 (1.11–1.46) | |
| Model 2, HR (95%CI)2 | 1 (ref) | 1.31 (0.84–2.04) | 1.36 (0.86–2.11) | 1.67 (1.07–2.61) | 0.03 | 1.23 (1.05–1.43)3 | |
Abbreviation: PRAL, potential renal acid load; SD, standard deviation; HR, hazard ratio; 95% CI, 95% confidence interval; ER, estrogen receptor; +, positive; −, negative.
Adjusted for age (age as the primary time scale)
Adjusted for race (non-Hispanic white, non-Hispanic black, Hispanic, others), education (less than high school, completed high school, some college, completed 4-year college or more), household income (<49,999, 50,000–99,999, 100,000+), BMI (<18.5, 18.5 to <25, 25 to <30, 30 to <35, 35 to <40, or ≥40 kg/m2), physical activity (MET-hours/week, quintile), pack-years of smoking (never smoker, smoker <10 pack-years, smoker ≥10 pack-years), alcohol consumption (never, former, current drinker < 1 drink/day, current drinker 1 drink/day, current drinker 1.1–1.9 drink/day, current drinker ≥2 drink/day), total energy intake (kcal/day), recent mammogram screening (<1 yr, ≥ 1 yr), stronger family history of breast cancer (presence or absence of first degree female relatives diagnosis under age 50 with breast cancer), breastfeeding history (total number of weeks, quintile), parity (nulliparous or 1, 2–3, ≥4), postmenopausal hormone therapy (none, estrogen only, both estrogen and progesterone), age at menopause (premenopausal, <40, 40–49, 50–54, ≥55 years based on enrollment information), and multivitamin use (none, < 1–3 days/week, < 4–6 days/week, every day, missing)
HR for ER- BC was significantly different from ER+ breast cancer in case-case analysis (P=0.04).
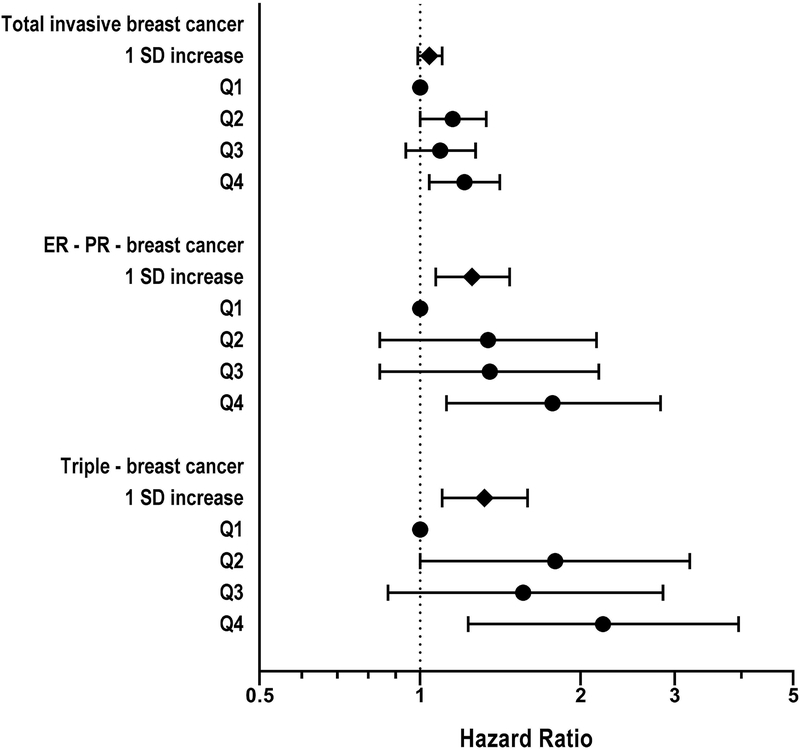
The association with high PRAL was more pronounced for ER-PR- negative and triple negative breast cancer (HRhighest vs. lowest quartile: 1.77 [95% CI, 1.12–2.82], Ptrend=0.02, HRhighest vs. lowest quartile: 2.20 [95% CI, 1.23–3.95], Ptrend=0.02, respectively) (Figure 1). Consistent results were found using the alternative indices for diet-dependent acid load, NAE, NEAP, and A:P ratio (Supplemental Table 3).
Figure 1.
Hazard ratios (HRs) and 95% CIs for the association between potential renal acid load and risk of ER-PR- negative and triple negative breast cancer. The models were adjusted for covariates used in Table 2. SD, standard deviation; HR, hazard ratio; 95% CI, 95% confidence interval; ER, estrogen receptor; PR, progesterone receptor; +, positive; −, negative.
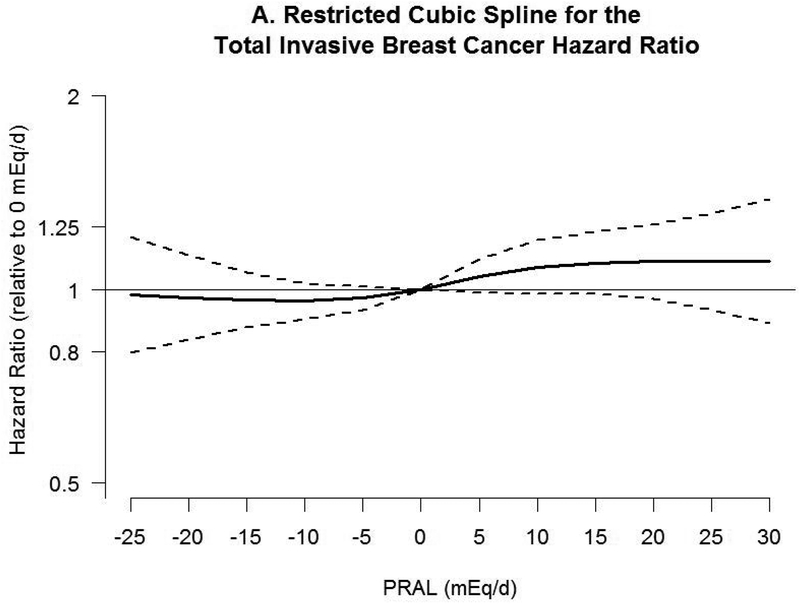
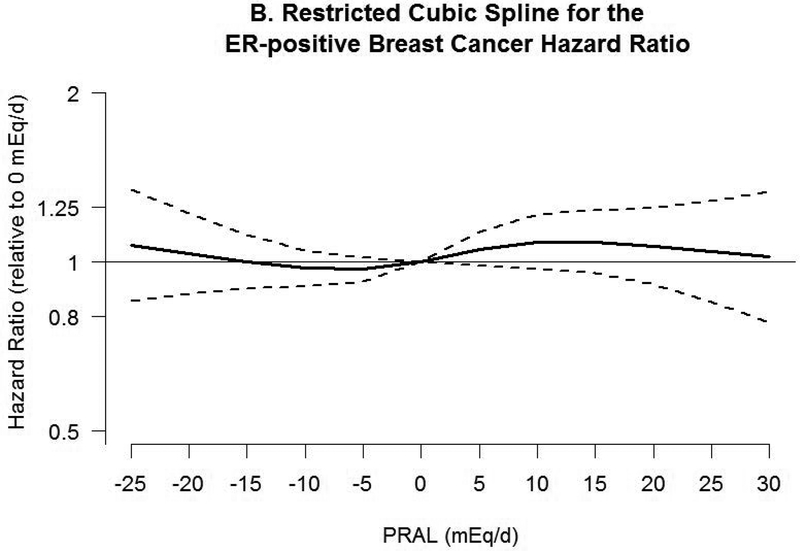
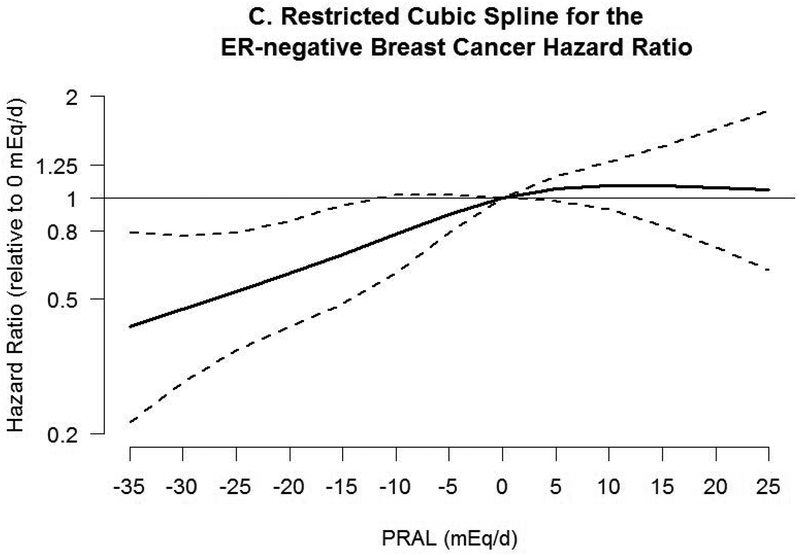
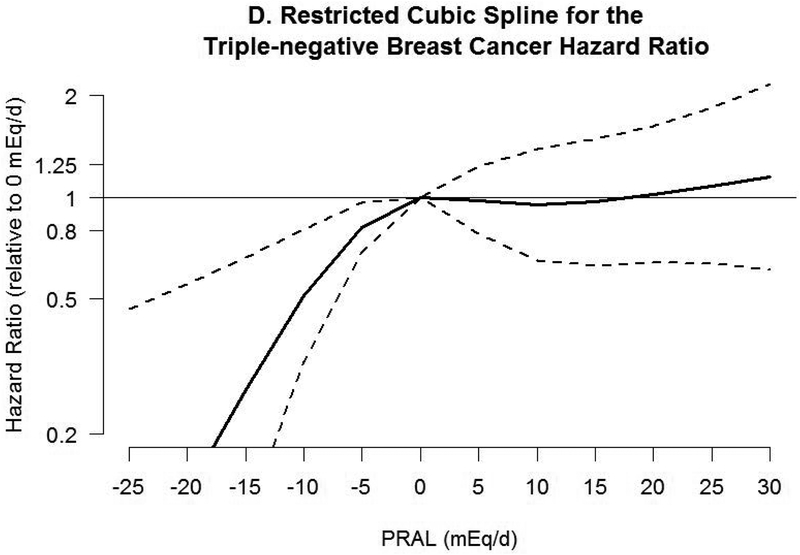
Associations between PRAL and risk of breast cancer with neutral PRAL score (0 mEq/day) as a reference using a restricted cubic spline model are shown in Figure 2. A nonlinear association was observed between PRAL and triple negative breast cancer (P=0.02). No significant association was observed with total invasive and ER-positive breast cancer, whereas inverse associations were observed between negative PRAL values representing alkaline diet consumption and ER-negative and triple negative breast cancer. For example, compared with a PRAL score of “0”, the HRs for the 10th percentile (−13.2 mEq/d) of PRAL were 0.60 (95% CI, 0.44–0.89) for ER-negative breast cancer and 0.35 (95% CI, 0.17–0.71) for triple negative breast cancer.
Figure 2.
Hazard ratios (HRs, solid lines) and 95% CIs (dashed lines) for the association of potential renal acid load (PRAL) with A) total invasive breast cancer, B) ER-positive breast cancer, C) ER-negative breast cancer, and D) triple negative breast cancer. PRAL was modeled by using restricted cubic splines with 4 knots placed at the 5th, 35th, 65th, and 95th percentiles. The reference value was 0 mEq/day. The models were adjusted for covariates used in Table 2. PRAL, potential renal acid load; ER, estrogen receptor; +, positive; −, negative.
In stratified analyses shown in Table 3, although interactions were not significant, associations between the highest quartile of PRAL and invasive breast cancer and trends appeared stronger among postmenopausal women (HRhighest vs. lowest quartile: 1.22 [95% CI, 1.03–1.45], Ptrend=0.03), non-Hispanic Black women (HRhighest vs. lowest quartile: 2.50 [95% CI, 1.25–5.00], Ptrend=0.05), and women with stronger family history (HRhighest vs. lowest quartile: 1.31 [95% CI, 1.07–1.61], Ptrend=0.04). Sensitivity analyses with an additional adjustment for the HEI-2015 did not materially change the overall results (Supplemental Table 4). When we further excluded women who had type 2 diabetes and/or hypertension and use of medications for these conditions at baseline, the associations tended to be strengthened (Supplemental Table 5).
Table 3.
Hazard ratios (HRs) and 95% CIs for the association between diet-dependent acid load and risk of invasive breast cancer stratified by selected factors
| PRAL score quartiles | PRAL score Continuous | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. participants at baseline | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend | 1 SD increment | P interaction | |
| Time varying menopausal status | Premenopausal | 15457 | 1 (ref) | 1.63 (1.12–2.37) | 1.17 (0.80–1.72) | 1.23 (0.84–1.80) | 0.92 | 0.99 (0.87–1.13) | |
| Postmenopausal | 28095 | 1 (ref) | 1.06 (0.90–1.25) | 1.08 (0.91–1.27) | 1.22 (1.03–1.45) | 0.03 | 1.05 (0.99–1.12) | 0.16 | |
| Race/ethnicity | Non-Hispanic White | 36947 | 1 (ref) | 1.08 (0.93–1.27) | 1.08 (0.92–1.27) | 1.13 (0.96–1.33) | 0.17 | 1.03 (0.97–1.09) | |
| Non-Hispanic Black | 3472 | 1 (ref) | 2.04 (1.03–4.03) | 1.05 (0.47–2.34) | 2.50 (1.25–5.00) | 0.05 | 1.29 (1.02–1.63) | ||
| Other | 3141 | 1 (ref) | 1.78 (0.92–3.43) | 1.34 (0.67–2.71) | 1.59 (0.81–3.12) | 0.34 | 1.04 (0.84–1.28) | 0.48 | |
| Stronger family history | No | 18328 | 1 (ref) | 1.01 (0.82–1.26) | 1.07 (0.86–1.34) | 1.09 (0.86–1.38) | 0.41 | 1.02 (0.94–1.11) | |
| Yes1 | 24735 | 1 (ref) | 1.28 (1.05–1.56) | 1.12 (0.91–1.38) | 1.31 (1.07–1.61) | 0.04 | 1.06 (0.99–1.14) | 0.54 | |
| BMI, kg/m2 | Normal weight (<25) | 17183 | 1 (ref) | 1.28 (1.02–1.61) | 1.05 (0.82–1.34) | 1.21 (0.93–1.57) | 0.35 | 1.06 (0.97–1.16) | |
| Overweight/obese (≥25) | 26387 | 1 (ref) | 1.06 (0.87–1.28) | 1.09 (0.90–1.32) | 1.19 (0.98–1.44) | 0.07 | 1.03 (0.96–1.11) | 0.25 | |
| Physical activity | High2 | 10163 | 1 (ref) | 1.26 (0.96–1.65) | 0.97 (0.71–1.33) | 1.22 (0.89–1.67) | 0.44 | 1.05 (0.95–1.17) | |
| Low to moderate | 19353 | 1 (ref) | 1.12 (0.94–1.33) | 1.14 (0.96–1.36) | 1.22 (1.02–1.45) | 0.04 | 1.04 (0.98–1.11) | 0.75 | |
Abbreviation: PRAL, potential renal acid load; HR, hazard ratio; 95% CI, 95% confidence interval; SD, standard deviation; BMI, body mass index;
Adjusted for covariates used in Table 2 except each stratified variable.
Stronger family history of breast cancer defined as presence or absence of at least one first degree female relative diagnosed with breast cancer before age 50.
Total metabolic equivalent hours/week ≥ 21 for leisure-time physical activity, corresponding to the ≥420 min per week of moderate-intensity physical activity.
DISCUSSION
In this nationwide large prospective cohort study, we found that higher diet-dependent acid load was associated with increased risk of invasive breast cancer, especially for ER-negative and triple-negative breast cancer. In addition, negative PRAL scores representing consumption of alkaline diets were associated with decreased risk of ER-negative and triple-negative breast cancer compared to neutral PRAL scores. To our knowledge, this is the first study to evaluate the association between breast cancer risk and acidogenic diet, here represented by scores for diet-dependent acid load.
It has been argued that diet-dependent acid load may be associated with increased risk of cancer, given that acid-base imbalance can affect cellular and molecular activities that stimulate carcinogenesis or tumor progression.14, 23 However, few epidemiological studies have evaluated this association.23 Only one prospective cohort study examined the relationship between diet-dependent acid load and risk of bladder cancer, based on the hypothesis that low urine pH could promote bladder cancer by elevating levels of arylamine-DNA adducts. However, no association between NAE and incident bladder cancer was found except for a suggestive increased risk in smokers.41
Although there is no obvious mechanism to explain the association between diet-dependent acid load and risk of breast cancer, a potential role for hormones or adipokines has been proposed.14 Diet-dependent acid load may contribute to decreased adiponectin,42 which is associated with increased risk of breast cancer.43 Additionally, animal and in vitro studies have shown that increased cortisol bioactivity induced by diet-induced low-grade acidosis may promote insulin resistance through activating cell signaling pathways.44, 45 An experimental study in healthy adults also showed that induction of mild metabolic acidosis decreases insulin sensitivity.46 On the other hand, acidosis induced by long-term high protein consumption over months to years appears to increase IGF-1 concentrations.14 Activated signal transduction pathways through insulin resistance and IGF-1 are the main pathophysiological mechanisms thought to explain the association between type 2 diabetes and breast cancer.47
Multiple epidemiologic studies have explored the association between diet-dependent acid load, insulin resistance48–50 and incident type 2 diabetes.11, 50–52 They have consistently reported positive associations except for a Swedish study limited to participants aged 70 years or older.50 Therefore, underlying mechanisms for the association between diet-dependent acid load and risk of breast cancer might include an indirect effect of alterations in circulating concentrations of insulin and related increasing bioavailability of IGFs.
In the present study, the association between diet-dependent acid load and breast cancer was more pronounced for hormone receptor negative cancer, especially for triple-negative breast cancer, suggesting etiologic heterogeneity. It is unclear whether diet-dependent acid load is exclusively associated with hormone receptor negative breast cancers. ER-negative breast cancers have been shown to have different etiology compared with ER-positive breast cancers. Known reproductive risk factors related to estrogen levels such as nulliparity, delayed childbearing, and early menarche are more associated with ER-positive than ER-negative breast cancers.53 A previous study also showed that non-starchy vegetables and carotenoids are associated with decreased risk of ER-negative breast cancer but not ER-positive breast cancer.2 It has been suggested that cathepsin D is often overexpressed in ER-negative tumors and acts as an autocrine mitogen on breast cancer cells.54 As an extracellular protease, cathepsin D can be activated at acidic pH to degrade extracellular matrix, which in turn stimulate angiogenesis.55
An alternative explanation may be based on the fact that PRAL is inversely correlated with consumption of vegetables and that negative PRAL is associated with decreased risk of ER-negative breast cancer. Epidermal growth factor receptor is known to be a major growth stimulating factor exclusively in ER-negative breast cancer,56 and phytochemicals contained in vegetables may contribute to decrease the level of epidermal growth factor receptor.57, 58 Higher PRAL scores were also correlated with higher consumption of meat and lower consumption of fruits and vegetables in this and other studies.12, 52, 59 It has been suggested that red meat consumption may increase and fruit and vegetable consumption may decrease breast cancer risk.58, 60 Thus, positive association between PRAL and breast cancer risk in our study support that diets high in fruits and vegetables and low in meat may decrease breast cancer risk.
In stratified analyses in the present study, there were suggestively stronger associations between PRAL and breast cancer in non-Hispanic Black women and for women who had a sister(s) diagnosed with breast cancer before age 50 years, especially for ER-negative breast cancer (data not shown). Since ER-negative breast cancer is more common in non-Hispanic Black women and women with family history,61 acidogenic diets might make them more susceptible to developing ER-negative breast cancer. Thus alkaline diets might be useful to reduce the risk of breast cancer in non-Hispanic Black women and women with family history, which could be assessed in further studies.
We observed slightly attenuated, but consistent associations using NEAP and A:P ratio, which was expected because these alternative indices for diet-dependent acid load were highly correlated with PRAL (Supplemental Table 2). Consistent findings among PRAL, NEAP, and A:P ratio were also found in other studies investigating the association between these indices and risk of type 2 diabetes.11, 52 This may indicate that dietary consumption of protein and potassium are strong drivers of diet-dependent acid load.
Strengths of the present study include a prospective design with the large sample size, high rates of follow-up, and standardized data collection. Also, comprehensive information on potential risk factors for breast cancer helps to reduce residual confounding.
This study has several limitations. Since dietary information was not updated during follow-up, we could not account for any changes in dietary consumption over time. Also, self-reported FFQ may be prone to measurement error. However, FFQ data are reproducible and adequately able to rank individuals regarding food and nutrient consumption.62 We did not have information on kidney function, which plays a crucial role in acid-base balance, but tried to reduce the underlying effect of chronic metabolic acidosis or alkalosis by excluding women who had a history of chronic kidney disease, liver cirrhosis, congestive heart failure, or chronic obstructive pulmonary disease. In addition, we observed that the associations were strengthened after further excluding women who had type 2 diabetes and/or hypertension and use of medications for these conditions at baseline. However, there could still be residual confounding due to lack of complete information on use of medications known to affect acid-base disturbance.36
In conclusion, findings from this large prospective study suggest that higher diet-dependent acid load is associated with increased risk of invasive breast cancer and that conversely, alkaline diets or diets that are lower in diet-dependent acid load may be protective, especially for ER-negative breast cancer. Findings are consistent with other evidence suggesting that diets high in fruits and vegetables and low in meat may decrease breast cancer risk. Our findings need to be confirmed in other populations and further research is warranted to understand the underlying mechanisms.
Supplementary Material
Novelty and Impact:
Diet-induced chronic low-grade metabolic acidosis might promote carcinogenesis or tumor progression. Epidemiologic studies have reported associations between diet-dependent acid load and metabolic diseases and pathways that may be linked to breast cancer, but no epidemiologic study of diet-dependent acid load and breast cancer has been conducted. The present study shows that women with higher diet-dependent acid load are at increased risk of breast cancer. Alkaline diets may be protective against breast cancer risk, especially estrogen receptor negative and triple-negative breast cancer.
ACKNOWLEDGMENTS
The authors appreciate the helpful comments of Drs. Katie M. O’Brien and Donna D. Baird.
FUNDING
This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences [Z01-ES044005].
Abbreviations:
- A:P
ratio of animal protein to potassium
- BMI
body mass index
- CI
confidence interval
- ER
estrogen receptor
- FFQ
food frequency questionnaire
- HEI-2015
Healthy Eating Index-2015
- HR
hazard ratio
- IGF
insulin-like growth factor
- NAE
net acid excretion
- NEAP
net endogenous acid production
- PR
progesterone receptor
- PRAL
potential renal acid load
Footnotes
DISCLOSURE
All the authors declare no conflicts of interest.
REFERENCES
- 1.Mourouti N, Kontogianni MD, Papavagelis C, Panagiotakos DB. Diet and breast cancer: a systematic review. International journal of food sciences and nutrition 2015;66: 1–42. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update for project report: diet, nutrition, physical activity and breast cancer. 2017. available at http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/breast-cancer
- 3.Kant AK. Dietary patterns and health outcomes. Journal of the American Dietetic Association 2004;104: 615–35. [DOI] [PubMed] [Google Scholar]
- 4.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. International journal of cancer Journal international du cancer 2014;135: 1884–97. [DOI] [PubMed] [Google Scholar]
- 5.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. American Journal of Clinical Nutrition 1994;59: 1356–61. [DOI] [PubMed] [Google Scholar]
- 6.Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. American Journal of Clinical Nutrition 1998;68: 576–83. [DOI] [PubMed] [Google Scholar]
- 7.Ferraro PM, Mandel EI, Curhan GC, Gambaro G, Taylor EN. Dietary Protein and Potassium, Diet-Dependent Net Acid Load, and Risk of Incident Kidney Stones. Clinical journal of the American Society of Nephrology : CJASN 2016;11: 1834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebholz CM, Coresh J, Grams ME, Steffen LM, Anderson CA, Appel LJ, Crews DC. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. American journal of nephrology 2015;42: 427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welch AA, MacGregor AJ, Skinner J, Spector TD, Moayyeri A, Cassidy A. A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2013;24: 1899–908. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Curhan GC, Forman JP. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension (Dallas, Tex : 1979) 2009;54: 751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiefte-de Jong JC, Li Y, Chen M, Curhan GC, Mattei J, Malik VS, Forman JP, Franco OH, Hu FB. Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia 2017;60: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akter S, Nanri A, Mizoue T, Noda M, Sawada N, Sasazuki S, Tsugane S. Dietary acid load and mortality among Japanese men and women: the Japan Public Health Center-based Prospective Study. The American journal of clinical nutrition 2017. [DOI] [PubMed] [Google Scholar]
- 13.Adeva MM, Souto G. Diet-induced metabolic acidosis. Clinical nutrition (Edinburgh, Scotland) 2011;30: 416–21. [DOI] [PubMed] [Google Scholar]
- 14.Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutrition & metabolism 2012;9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzorno J, Frassetto LA, Katzinger J. Diet-induced acidosis: is it real and clinically relevant? The British journal of nutrition 2010;103: 1185–94. [DOI] [PubMed] [Google Scholar]
- 16.Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. American journal of physiology Renal physiology 2003;284: F32–40. [DOI] [PubMed] [Google Scholar]
- 17.Abate N, Chandalia M, Cabo-Chan AV, Jr., Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney international 2004;65: 386–92. [DOI] [PubMed] [Google Scholar]
- 18.Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension (Dallas, Tex : 1979) 1993;21: 1024–9. [DOI] [PubMed] [Google Scholar]
- 19.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. The American Journal of Clinical Nutrition 2007;86: 823S–35S. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene 2001;20: 3751–6. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer research 2000;60: 4610–6. [PubMed] [Google Scholar]
- 22.Fürstenberger G, Senn H-J. Insulin-like growth factors and cancer. The Lancet Oncology 2002;3: 298–302. [DOI] [PubMed] [Google Scholar]
- 23.Fenton TR, Huang T. Systematic review of the association between dietary acid load, alkaline water and cancer. BMJ open 2016;6: e010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg CR, Shore DL, Umbach DM, Sandler DP. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. American journal of epidemiology 2007;166: 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, Kleeberger CA, Shore DL, DeRoo LA, Taylor JA, Weinberg CR. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environmental health perspectives 2017;125: 127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekenga CC, Parks CG, Sandler DP. A prospective study of occupational physical activity and breast cancer risk. Cancer causes & control : CCC 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124: 453–69. [DOI] [PubMed] [Google Scholar]
- 28.Bowman SA, Clemens JC, Friday JE, Thoerig RC, Moshfegh AJ, Food Patterns Equivalents Database 2011–12: Methodology and User Guide. Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, 2014. [Google Scholar]
- 29.Zwart SR, Hargens AR, Smith SM. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. The American journal of clinical nutrition 2004;80: 1058–65. [DOI] [PubMed] [Google Scholar]
- 30.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition (Burbank, Los Angeles County, Calif) 1989;5: 303–11; discussion 12–3. [PubMed] [Google Scholar]
- 31.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association 1995;95: 791–7. [DOI] [PubMed] [Google Scholar]
- 32.Michaud DS, Troiano RP, Subar AF, Runswick S, Bingham S, Kipnis V, Schatzkin A. Comparison of estimated renal net acid excretion from dietary intake and body size with urine pH. Journal of the American Dietetic Association 2003;103: 1001–7; discussion 7. [DOI] [PubMed] [Google Scholar]
- 33.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. Journal of clinical epidemiology 1990;43: 1327–35. [DOI] [PubMed] [Google Scholar]
- 34.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public health nutrition 2006;9: 84–93. [DOI] [PubMed] [Google Scholar]
- 35.D’Aloisio AA, Nichols HB, Hodgson ME, Deming-Halverson SL, Sandler DP. Validity of self-reported breast cancer characteristics in a nationwide cohort of women with a family history of breast cancer. BMC cancer 2017;17: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayers P, Dixon C, Mays A. Acid-base disorders: learning the basics. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition 2015;30: 14–20. [DOI] [PubMed] [Google Scholar]
- 37.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the sister study. Cancer 2015;121: 3700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999;10: 37–48. [PubMed] [Google Scholar]
- 39.Martinez ME, Cruz GI, Brewster AM, Bondy ML, Thompson PA. What can we learn about disease etiology from case-case analyses? Lessons from breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2010;19: 2710–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. https://epi.grants.cancer.gov/hei/
- 41.Wright ME, Michaud DS, Pietinen P, Taylor PR, Virtamo J, Albanes D. Estimated urine pH and bladder cancer risk in a cohort of male smokers (Finland). Cancer causes & control : CCC 2005;16: 1117–23. [DOI] [PubMed] [Google Scholar]
- 42.Disthabanchong S, Niticharoenpong K, Radinahamed P, Stitchantrakul W, Ongphiphadhanakul B, Hongeng S. Metabolic acidosis lowers circulating adiponectin through inhibition of adiponectin gene transcription. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2011;26: 592–8. [DOI] [PubMed] [Google Scholar]
- 43.Jardé T, Perrier S, Vasson M-P, Caldefie-Chézet F. Molecular mechanisms of leptin and adiponectin in breast cancer. European Journal of Cancer 2011;47: 33–43. [DOI] [PubMed] [Google Scholar]
- 44.Buren J, Liu HX, Jensen J, Eriksson JW. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. European journal of endocrinology 2002;146: 419–29. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds RM, Walker BR. Human insulin resistance: the role of glucocorticoids. Diabetes, obesity & metabolism 2003;5: 5–12. [DOI] [PubMed] [Google Scholar]
- 46.Defronzo RA. Glucose intolerance following chronic metabolic acidosis in man. The American journal of physiology 1978;236: E328–E34. [DOI] [PubMed] [Google Scholar]
- 47.Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. The Lancet Oncology 2005;6: 103–11. [DOI] [PubMed] [Google Scholar]
- 48.Moghadam SK, Bahadoran Z, Mirmiran P, Tohidi M, Azizi F. Association between Dietary Acid Load and Insulin Resistance: Tehran Lipid and Glucose Study. Preventive nutrition and food science 2016;21: 104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akter S, Eguchi M, Kuwahara K, Kochi T, Ito R, Kurotani K, Tsuruoka H, Nanri A, Kabe I, Mizoue T. High dietary acid load is associated with insulin resistance: The Furukawa Nutrition and Health Study. Clinical nutrition (Edinburgh, Scotland) 2016;35: 453–9. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Jia T, Huang X, Riserus U, Cederholm T, Arnlov J, Sjogren P, Lindholm B, Carrero JJ. Dietary acid load, insulin sensitivity and risk of type 2 diabetes in community-dwelling older men. Diabetologia 2014;57: 1561–8. [DOI] [PubMed] [Google Scholar]
- 51.Akter S, Kurotani K, Kashino I, Goto A, Mizoue T, Noda M, Sawada N, Tsugane S. High Dietary Acid Load Score Is Associated with Increased Risk of Type 2 Diabetes in Japanese Men: The Japan Public Health Center-based Prospective Study. The Journal of nutrition 2016;146: 1076–83. [DOI] [PubMed] [Google Scholar]
- 52.Fagherazzi G, Vilier A, Bonnet F, Lajous M, Balkau B, Boutron-Rualt MC, Clavel-Chapelon F. Dietary acid load and risk of type 2 diabetes: the E3N-EPIC cohort study. Diabetologia 2014;57: 313–20. [DOI] [PubMed] [Google Scholar]
- 53.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2004;13: 1558–68. [PubMed] [Google Scholar]
- 54.Tandon AK, Clark GM, Chamness GC, Chirgwin JM, McGuire WL. Cathepsin D and Prognosis in Breast Cancer. New England Journal of Medicine 1990;322: 297–302. [DOI] [PubMed] [Google Scholar]
- 55.Rochefort H, Glondu M, Sahla ME, Platet N, Garcia M. How to target estrogen receptor-negative breast cancer? Endocrine-related cancer 2003;10: 261–6. [DOI] [PubMed] [Google Scholar]
- 56.Biswas DK, Cruz AP, Gansberger E, Pardee AB. Epidermal growth factor-induced nuclear factor kappa B activation: A major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America 2000;97: 8542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moiseeva EP, Heukers R, Manson MM. EGFR and Src are involved in indole-3-carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis 2007;28: 435–45. [DOI] [PubMed] [Google Scholar]
- 58.Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Cerhan JR, Gaudet MM, Giles GG, Goodman G, Hakansson N, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. Journal of the National Cancer Institute 2013;105: 219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engberink MF, Bakker SJ, Brink EJ, van Baak MA, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Dietary acid load and risk of hypertension: the Rotterdam Study. The American journal of clinical nutrition 2012;95: 1438–44. [DOI] [PubMed] [Google Scholar]
- 60.Guo J, Wei W, Zhan L. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast cancer research and treatment 2015;151: 191–8. [DOI] [PubMed] [Google Scholar]
- 61.Gierach GL, Burke A, Anderson WF. Epidemiology of triple negative breast cancers. Breast disease 2010;32: 5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RL, Schatzkin A, Spiegelman D, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 2014;180: 172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.