Abstract
Nicotinic acetylcholine receptors (nAChRs), particularly the α7 nAChR, are implicated in the pathophysiology of both autism spectrum disorder (ASD) and aggressive behavior. We explored the feasibility, tolerability, and preliminary efficacy of targeting nAChRs using transdermal nicotine to reduce aggressive symptoms in adults with ASD. Eight subjects were randomized in a double-blind crossover trial of seven milligrams transdermal nicotine or placebo, each for one week. All participants tolerated nicotine treatment well. Five subjects contributed data to the primary outcome, Aberrant Behavior Checklist-Irritability (ABC-I) subscale change from baseline, which was improved by nicotine compared to placebo. Sleep ratings were also improved by nicotine and correlated with ABC-I improvement. These findings support further investigation of nAChR agonists for aggression and sleep in ASD.
Keywords: Nicotine, nicotinic acetylcholine receptor, autism spectrum disorder, aggression, irritability, adult, sleep
Introduction
Aggression and irritability are prominent sources of morbidity for individuals with Autism Spectrum Disorder (ASD) (Kanne and Mazurek 2011). Poorly controlled aggression may contribute to an increased need for psychiatric services, including hospital admission and the prescription of psychotropic medication (Tsakanikos et al. 2007). Individuals with ASD who display prominent aggressive symptoms may be misdiagnosed as having comorbid psychotic illnesses, potentially misdirecting their subsequent treatment (Van Schalkwyk et al. 2015b). Behavioral treatments are typically considered to be the first-line intervention for this clinical problem (Matson 2009), although a large number of trials support the utility of atypical antipsychotic medications (McCracken et al. 2002; McDougle et al. 2005; Potenza et al. 1999; van Schalkwyk et al. 2017). Atypical antipsychotic usage is limited by significant associated side effects, including weight gain (Ji and Findling 2015), and in clinical practice it remains clear that a subset of individuals will continue to have significant symptoms of irritability and aggression, even with antipsychotic treatment.
The mechanism by which atypical antipsychotics are helpful in ASD is unclear, and may include non-specific factors such as sedation in addition to any primary anti-aggressive, or “serenic” effect. Therefore, there is a need for emerging neurobiological findings of basic mechanisms of neuropsychiatric illness and behavior to inform new approaches in clinical psychopharmacology. Toward this end, several studies have contributed to defining the role of nicotinic acetylcholine receptors (nAChRs) in depression and anxiety (Picciotto et al. 2015), and more recent work has shed light on both the pharmacological and regional mechanism by which nicotine reduces aggressive behavior through its action on the α7 nAChR (Lewis et al. 2015; Lewis et al. 2017).
Accordingly, several clinical studies suggest a potential role for transdermal nicotine (TN) in reducing symptoms of aggression in neuropsychiatric disorders, including in schizophrenia (Allen et al. 2011), ASD (Van Schalkwyk et al. 2015a), and severe dementia (Carmel and Sheitman 2007; Rosin et al. 2001). Targeting the α7 nAChR using the cholinesterase-inhibitor and α7 positive allosteric modulator (PAM) galantamine was also beneficial for reducing aggression in an individual with 15q13.3 microdeletion syndrome, a rare genetic disorder involving deletion of the gene coding for the α7 nAChR frequently resulting in ASD or schizophrenia often with comorbid aggression (Cubells et al. 2011). However, the level of evidence for nAChR-based treatments is largely limited to open-label case reports.
Further study of the potential role of nicotinic agents in treating irritability and aggression in individuals with ASD is thus strongly supported by the significance of the clinical problem and promising findings from preliminary clinical research directed by evidence from basic scientific studies. In this study, we sought to build on existing case reports by conducting a randomized, double-blind, placebo-controlled pilot study in adults with ASD and clinically significant behavioral symptoms. We hypothesized that TN would be a well-tolerated, feasible, and effective intervention to produce a measurable change in caregiver-rated symptoms of irritability and aggression. The aim of our study was to provide further data to inform future, more extensive clinical study of nicotine or related nAChR-targeting compounds for this critical symptom dimension in individuals with ASD.
Methods
Study design
This was an exploratory, three-week, randomized, placebo-controlled crossover study. Recruitment was conducted between May 2015 and November 2017. Study assessments were primarily conducted at the [BLINDED] in [BLINDED], with one subject’s assessments performed at a residential treatment setting in [BLINDED]. Subjects were randomized to receive one week of 7 mg daily TN or placebo, followed by a washout period during which all subjects received one week of transdermal placebo, followed by one week of 7 mg TN or placebo, whichever was not received during the first week. No previous multi-subject trials of TN in adults with ASD have been published to our knowledge. Therefore, because our initial goal was determination of feasibility, tolerability, and safety in individuals with ASD, a dose of 7 mg for the brief time period of one week was chosen to limit potential adverse effects and maximize subject retention. Patches were placed by caregivers upon waking and removed at bedtime. Randomization and preparation of patches was performed by the [BLINDED] Investigational Drug Service. Assessments were conducted at baseline and at completion of study weeks 1 and 3. The study was registered at www.clinicaltrials.gov (NCT02552147) and approved by the [BLINDED] University Human Investigation Committee. All subjects or their legal guardian provided informed consent to participate in the trial. In the case of consent provided by a legal guardian, assent to participate was obtained from all subjects.
Subjects
Inclusion criteria.
Eligible English-speaking male or female participants were age 18 to 60 with prior Diagnostic and Statistical Manual (DSM)-5 diagnosis of ASD or DSM-4 diagnosis of autism, Asperger’s syndrome, or pervasive developmental disorder not otherwise specified. They had symptoms or irritability, agitation, or aggression as reported by a parent or caregiver, with a baseline score on the Aberrant Behavior Checklist (ABC)-Irritability Subscale (ABC-I) of 16 or greater. They were required to live with or be engaged with a caregiver capable of completing written behavioral scales at all three study visits, rating the subject’s behavioral symptoms during a designated time frame. Subjects were required to be on a stable medication regimen with no changes in the past 14 days and to have a body mass index (BMI) of greater than 17.5 and less than 45.
Exclusion criteria.
Exclusionary criteria included current use of tobacco or any nicotine products (transdermal, gum, e-cigarettes), previous allergy to transdermal patches, known serious cardiac rhythm abnormalities, tachy- or bradycardia (heart rate > 100 or < 50), or hyper- or hypotension (systolic blood pressure > 150 or < 95, diastolic blood pressure > 90 or < 50).
Participants (Figure 1).
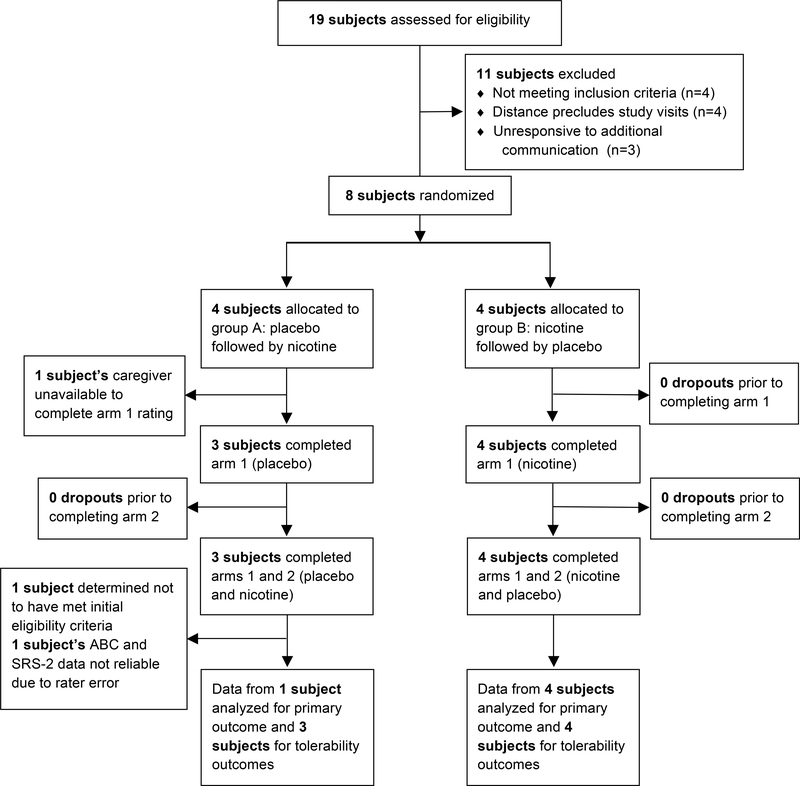
Fig. 1.
Participant flow through the trial.
Nineteen potential subjects or their caregivers corresponded with the study team regarding participation. Four were excluded because of age less than 18, four were excluded because they resided too far from the study location to feasibly present for study assessments, and three did not respond to further communication with the study team to assess eligibility. The remaining eight subjects (7 males, 1 female) were enrolled at the [BLINDED] (n = 7) or at a residential treatment facility in [BLINDED] (n = 1). After completing trial procedures, one subject was determined to not have met inclusion criteria due to age greater than approved range at time of enrollment, and this subject’s data was not included in either tolerability or efficacy analysis. One subject completed all study visits however his caregiver did not provide rating scales for the week 1 assessment or any sleep assessments. Finally, one subject completed all study visits, however upon study completion but prior to unblinding the subject’s caregiver reported rating scales were not confined to an assessment of the time frame specified by the study staff. As such, this subject’s ABC and Social Responsiveness Scale-2 (SRS-2) data were not included in the analysis, though nightly sleep ratings and other adverse effect information were included. Decisions to include or exclude subjects were made prior to unblinding of the data. Overall, five subjects were included for the primary outcome assessment and other efficacy measures, six subjects were included for sleep assessments, and seven subjects included for non-sleep tolerability assessments.
Assessments
At the time of screening, one specific caregiver rater was designated for each subject who affirmed frequent (typically daily) interaction with the subject and agreed to attend all three study visits. At baseline, raters completed the ABC (Aman et al. 1985) and SRS-2 adult form (Constantino and Gruber 2012). We attempted to administer both the State-Trait Anxiety Inventory (STAI) (Spielberger et al. 1983) and the State-Trait Anger Expression Inventory-2 (STAXI-2) (Spielberger 1999), which are both designed for self-report, however most subjects were unable to complete either scale owing to severity of impairment due to ASD. Additionally, we attempted to collect data from a computerized frustration-induction Go-NoGo task (Sukhodolsky et al. 2016), however again most subjects were unable to consistently participate in this task. Raters were also provided with a sleep rating score sheet to assess sleep quality during each of 21 study nights on a scale of 0 (worst) to 10 (best). At weeks 1 and 3, raters were instructed to complete both the ABC and SRS-2 based solely on observed behavior over the prior week. Adverse effects were identified by asking raters and subjects whether they specifically experienced any dermatological, cardiovascular, gastrointestinal, or other side effects. Heart rate and blood pressure were assessed at baseline and at each study visit.
Statistical analysis
A power analysis was performed based on effect sizes observed in a previous trial of risperidone for aggression in ASD (Research Units on Pediatric Psychopharmacology Autism 2005). Based on this analysis, we planned to enroll 16 subjects for appropriate power to detect medium and large effects, however we were unable to achieve this sample size despite a more than two-year recruitment period, therefore the study is underpowered to detect the desired effect sizes. Statistical testing was conducted using the Wilcoxon signed-rank test for paired data and the binomial test to compare observed versus expected percentages. Correlation significance was tested using Pearson correlation. Data are expressed throughout as mean (standard deviation (S.D.)). IBM SPSS Statistics version 24 and Graphpad Prism Version 7 were used for data analysis and plots. G*Power Version 3.1 was used to calculate effect sizes.
Results
Study population
Eight subjects were enrolled and randomized. Prior to unblinding, one subject was determined to have not met eligibility criteria at time of enrollment. The remaining seven subjects (6 males, 1 female) completed both arms of the crossover trial and contributed tolerability data. Five of these subjects contributed reliable rating scales for both arms of the trial and therefore contributed to assessment of the primary outcome, ABC-I change from baseline. Figure 1 shows participant flow through the study. Baseline characteristics are reported in Table 1. Mean ABC-I and other ABC-subscale scores were comparable to previous medication treatment studies to treat aggression in ASD (Erickson et al. 2014; McCracken et al. 2002). Subjects were severely affected by ASD as demonstrated by mean SRS-2 T-score > 90. All subjects were prescribed medications commonly used to treat aggression in ASD, with most subjects prescribed antipsychotics and/or mood stabilizers.
Table 1.
Characteristics of individual participants and overall group randomized and contributing data to the study. Nic, transdermal nicotine; Pbo, transdermal placebo; S.D., standard deviation; SRS, Social Responsiveness Scale.
| Baseline and aggregate (grey bar; mean (S.D.) or n (%)) participant characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Psychotropic medication | Treatment order | Aberrant Behavior Checklist (ABC) Subscales | SRS-2 (total) | ||||
| Irritability | Lethargy/social withdrawal | Stereotypic behavior | Hyperactivity | Inappropriate speech | |||||
| 28 | M | Divalproex, risperidone, haloperidol | Nic→Pbo | 34 | 31 | 10 | 38 | 11 | 202 |
| 201 | M | Divalproex, risperidone, aripiprazole, clonazepam | Pbo→Nic | 23 | 21 | 4 | 30 | 0 | 182 |
| 23 | M | Risperidone | Pbo→Nic | 16 | 9 | 0 | 7 | 3 | 130 |
| 221 | M | Risperidone, clonidine | Nic→Pbo | 21 | 2 | 11 | 29 | 7 | 175 |
| 24 | M | Divalproex, desipramine, methylphenidate | Pbo→Nic | 20 | 31 | 10 | 23 | 4 | 194 |
| 24 | M | Lamotrigine, olanzapine, fluoxetine, clonidine | Nic→Pbo | 39 | 35 | 21 | 38 | 11 | 195 |
| 27 | F | Lithium, trazodone, buspirone, clonazepam, propranolol, gabapentin | Nic→Pbo | 25 | 14 | 6 | 17 | 7 | 159 |
| 24 (3) | 6 M (86%) | Mood stabilizer: 5 (71%) Antipsychotic: 5 (71%) Antidepressant: 3 (43%) Benzodiazepine: 2 (29%) β-blocker: 1 (14%) Stimulant: 1 (14%) α2-agonist: 2 (29%) Other: 1 (14%) |
Nic→Pbo: 4 (57%) Pbo→Nic: 3 (43%) |
25 (8) | 20 (13) | 9 (7) | 26 (11) | 6 (4) | 177 (25)2 |
Subject continued on open-label nicotine patch following conclusion of the study.
T-score > 90.
Feasibility and tolerability
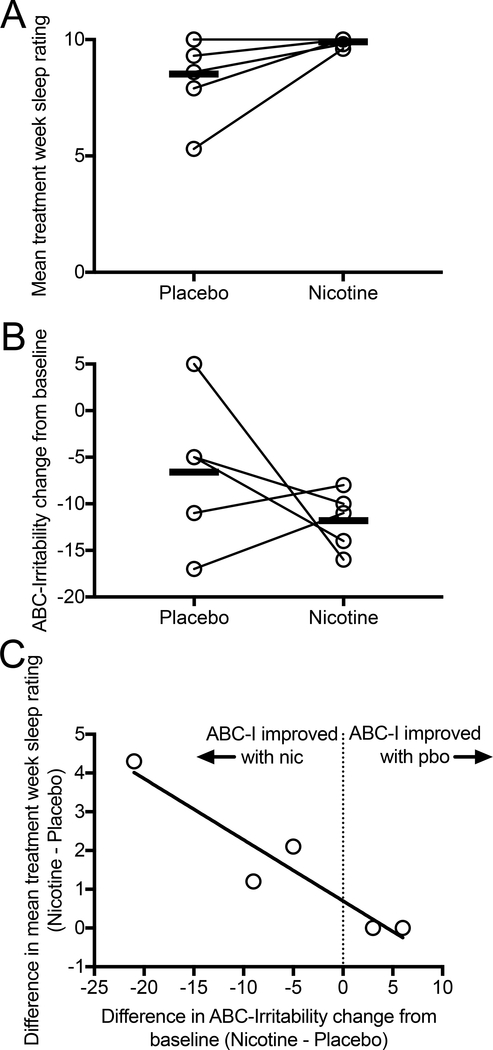
Skin patches containing 7 mg nicotine or placebo were well tolerated by all subjects. No subjects or caregivers reported significant difficulty in maintaining the patch in place throughout the trial. One subject reported mild skin irritation that did not interfere with tolerability. In multiple subjects, caregivers reported placing the skin patches on the subject’s back covered by medical tape. In one subject, caregivers reported nightmares and one day of gastrointestinal symptoms, ultimately determined to occur during the placebo week only. No subjects or caregivers reported cardiovascular adverse effects. There was no significant difference (p ≥ 0.75) in mean change from baseline for heart rate (mean (S.D.) beats per minute: Pbo: −3 (8), Nic: −1 (5)) or blood pressure (mean (S.D.) systolic blood pressure: Pbo: 2 (10), Nic: 2 (6); mean (S.D.) diastolic blood pressure: Pbo: 0.2 (10), Nic: 1 (7)). Sleep, as rated nightly by caregivers, either remained at maximal score in 2 of 6 subjects or improved in 4 of 6 subjects during the nicotine treatment week as compared to placebo week (Table 2, Figure 2A).
Table 2.
Effects of transdermal nicotine versus placebo on efficacy and tolerability measures. S.D., standard deviation; CFB, change from baseline; SRS, Social Responsiveness Scale.
| Measure | Placebo | Nicotine | p-value1 | Effect size (dz)2 |
|---|---|---|---|---|
| ABC-Irritability mean (S.D.) CFB | −7 (8) | −12 (3) | 0.44 | 0.49 |
| ABC-Lethargy/Social withdrawal mean (S.D.) CFB | −7 (4) | −2 (3) | 0.13 | −0.94 |
| ABC-Stereotypic behavior mean (S.D.) CFB | −4 (4) | −2 (3) | 0.50 | −0.44 |
| ABC-Hyperactivity mean (S.D.) CFB | −6 (6) | −7 (6) | 1.0 | 0.12 |
| ABC-Inappropriate speech mean (S.D.) CFB | 0.2 (0.8) | −1 (2) | 0.25 | 0.67 |
| Global aggressive behavior improvement3 | 2 (33%) | 4 (67%) | 0.69 | N/A |
| SRS-2 mean (S.D.) CFB | −15 (23) | −1 (25) | 0.38 | −0.56 |
| Nightly sleep quality | 9 (2) | 10 (0.2) | 0.13 | 0.88 |
Calculated using two-tailed Wilcoxon signed-rank test except for global impression of aggressive behavior, which was calculated using binomial test.
Improvement with drug is positive effect size, improvement with placebo is negative effect size.
One subject was included in this category but not in ABC or SRS-2 categories because of accuracy concerns in rating scale completion (see methods).
Fig. 2.
Comparison of effects of 7 mg transdermal nicotine versus placebo on sleep ratings (A) and ABC-Irritability (ABC-I) subscale (B). Caregivers recorded ratings for nightly sleep quality from 0 (worst) to 10 (best) and these were averaged over the nicotine or placebo week. ABC-I reflects caregiver assessment of behavior integrated over the entire nicotine or placebo week. Change in sleep correlated significantly with change in ABC-I score during the nicotine week versus placebo week (r2 = 0.89, p = 0.016) (C).
Efficacy
Mean ABC-I change from baseline was improved during nicotine treatment as compared to placebo, though this did not achieve statistical significance (Table 2, Figure 2B). We identified a significant correlation between improvement in ABC-I and sleep with nicotine as compared to placebo (r2 = 0.89, p = 0.016, Figure 2C). As compared to placebo, ABC-Inappropriate speech subscale scores improved, while ABC-Lethargy/social withdrawal and ABC-Stereotypic behavior scores worsened during the nicotine treatment week (Table 2). SRS-2 scores were also modestly increased (worsened) during the nicotine week as compared to placebo. None of these differences achieved statistical significance. One subject’s caregiver reported marked reduction in aggressivity during the third week of the trial, however this was not reflected in his ABC-I scores. Prior to unblinding, it was determined that the ratings were not performed based on behaviors over the prior week, deeming them unreliable. This subject’s data was not included in ABC or SRS-2 analysis. However, unblinding revealed that the subject received nicotine in the week of marked improvement, corresponding to the third study week. Therefore, 4 of 6 (67%) study subjects’ caregivers reported overall improvement in aggressive behavior during the nicotine treatment week as compared to placebo week.
Subject and caregiver-initiated open-label continuation
Two subjects with caregiver-reported markedly asymmetric improvement in aggression during the double-blind phase elected to continue treatment with TN after completing the study, purchased over-the-counter. This was performed under the guidance of the subjects’ personal physicians and/or caregiver and was not a formal component of the trial. Treatment allocation remained blinded at the subjects’ decision to continue TN open label, however it was ultimately revealed that both subjects received nicotine during their weeks of marked caregiver-reported improvement. One subject’s dose was escalated from 7 mg to 14 mg and ultimately to 21 mg as each dose was noted to lose effectiveness for aggression over the course of months. This subject has continued to use nicotine for over 16 months without development of adverse effects. His ABC-I score at 16 months was 19 (study baseline = 23), and his caregiver noted being unsure whether TN remains of any benefit. The other subject continues on 7 mg nicotine patch after 14 months of use. His ABC-I score at 14 months was 13 (study baseline = 21) and also developed no known adverse effects. His caregiver reported on multiple occasions forgetting to apply the nicotine patch with resultant worsening in aggression symptoms that resolved upon application of nicotine patch, and believes the subject continues to receive benefit for aggression from TN.
Discussion
The main finding of our study was that use of TN during waking hours in adults with severe symptoms of ASD and moderate levels of aggression and irritability was both highly feasible and tolerable. Our results also suggest that TN may have a beneficial effect on aggression, irritability, and sleep in ASD, though the sample size of this study is too small to make definitive conclusions. As aggression in ASD is common and can severely limit the functioning of affected individuals (Kanne and Mazurek 2011), further investigation into novel treatment methodologies, such as nAChR-targeting pharmacotherapies, is critical. As illustrated in this exploratory study, all subjects were prescribed medications to target aggression and irritability, especially antipsychotics and mood stabilizers. Such agents can confer significant side effects, most notably disorders of metabolism such as obesity and diabetes, as well as movement disorders such as tardive dyskinesia. Most of these effects are dose-dependent, and hence other well-tolerated treatment options that might permit lower doses of these agents or their discontinuation could considerably reduce the burden of adverse effects when considered across the population and lifespan of individuals with ASD.
Surprisingly, few previous studies have assessed the feasibility and tolerability of any form of skin patches in individuals with severe symptoms of ASD. Sensory sensitivities of such individuals might a priori suggest skin patches would be poorly tolerated. However, our study and one other previous small study (Fankhauser et al. 1992) suggest otherwise. In our study, no subjects were unable to tolerate the patch due to sensory sensitivity, and only mild skin irritation was reported in one individual. Transdermal delivery of medication, especially in individuals with severe symptoms of ASD, might be a preferable alternative to traditional oral formulations owing to their simplicity of use, contributing to reduced caregiver burden and improved medication adherence.
Several previous studies have demonstrated the safety and tolerability of TN in neurotypical adult, non-smoking subjects, especially in adults with neurocognitive disorders such as mild cognitive impairment (MCI) or Alzheimer’s disease. In a six-month trial of non-smoking older adults with MCI, subjects randomized to TN (n = 34) demonstrated excellent tolerability, with no withdrawal or dependence symptoms reported (Newhouse et al. 2012). Similarly, other studies of non-smoking individuals to assess TN for cognitive indications demonstrates good tolerability, with dose-related side effects primarily limited to mild gastrointestinal symptoms or lightheadedness (White and Levin 1999; White and Levin 2004). Whether subjects with ASD would be more sensitive to side effects of nicotine was unknown, and was the rationale underlying our use of the low dose 7 mg nicotine patch. Our findings of excellent tolerability support the safety of future studies at higher doses, which has been demonstrated by one subject continuing TN at doses up to 21 mg following this study as well as in a separate patient previously described in a case report (Van Schalkwyk et al. 2015a).
Several lines of evidence from both animal models and humans support the rationale for targeting nAChRs to treat aggression in individuals with ASD, in particular the α7 nAChR. The α7 nAChR is a homomeric, ligand-gated calcium-permeable ion channel present in multiple brain regions, with substantial expression in the cortex and hippocampus in both rodents (Baddick and Marks 2011) and humans (Rubboli et al. 1994). The gene coding for the α7 nAChR, CHRNA7, resides in an unstable region of chromosome 15 prone to copy number variations (CNVs). Numerous deletions in this region have been reported, resulting in a variable neuropsychiatric phenotype, which can include ASD, schizophrenia, and persistent aggression, called 15q13.3 microdeletion syndrome (Gillentine and Schaaf 2015; Sharp et al. 2008; Shinawi et al. 2009). While the size of the deletion in this region is variable, one commonality amongst CNVs resulting in 15q13.3 appears to be deletion of CHRNA7 (Hoppman-Chaney et al. 2013; Masurel-Paulet et al. 2010; Mikhail et al. 2011; Shinawi et al. 2009). A recent study found the prevalence of CHRNA7 deletions to be markedly enriched in a population of children and adolescents treated with the antipsychotic risperidone for aggressive indications, again suggesting an association between CHRNA7 and aggressive behavior (Gillentine et al. 2017). Indeed, the successful use of galantamine, a cholinesterase inhibitor and α7 nAChR PAM, to improve aggression in a man with 15q13.3 microdeletion lends further support to this association (Cubells et al. 2011). Rodent studies also argue a role for the α7 nAChR and Chrna7 dosage in regulation of aggressive behavior. In mice, serenic effects of acute nicotine administration were blocked by the α7 nAChR antagonist methyllycaconitine but not by dihydro-β-erythroidine, an antagonist of heterometric nAChRs. GTS-21, an α7 nAChR partial agonist, was also serenic in mice (Lewis et al. 2015). Short hairpin RNA targeting Chrna7 in bilateral dentate gyrus/hippocampus also shortened aggression latency and blocked the serenic effects of both nicotine and GTS-21 (Lewis et al. 2017). Taken together, animal and human studies support pharmacological targeting of the α7 nAChR as a serenic strategy.
Our finding that sleep improved during TN treatment as compared to placebo was unexpected. Indeed, our intention to monitor sleep quality was to confirm that it was not worsened by TN exposure. There is extensive literature to support the interconnectedness of sleep and aggression and irritability in ASD (Chen et al. 2017; Hill et al. 2014; Mazurek and Sohl 2016). Though the direction of causality is poorly understood, poor sleep appears to clearly correlate with greater degree of aggression and irritability. Cholinergic neurons in the basal forebrain and brainstem play important roles in sleep onset and maintenance, and their degeneration is believed to contribute to sleep disruption in individuals with Parkinson’s disease (Suzuki et al. 2015). Relatedly, several studies report changes in nAChRs in brain regions important for sleep, including reduction in α7 nAChRs in brainstem neurons in a mouse model of Rett Syndrome (Oginsky et al. 2014) and in thalamic neurons of postmortem adults with ASD (Ray et al. 2005). Thus, TN may normalize a pre-existing insufficiency in α7 or other nAChR signaling, or might have a more indirect effect, such as by organizing wakefulness during the day which may enhance delineation of sleep-wake cycles, as has been previously reported using TN in a neurotypical individual with narcolepsy (Bagai and Malow 2010). Drugs capable of normalizing sleep-wake cycles, such as morning use of modafinil (Morgan et al. 2010), may improve nighttime sleep, and lead to an indirect improvement of aggressive behaviors during the day. Future studies of nAChR pharmacotherapy in individuals with ASD should employ rating of sleep features through standardized scales and even the use of laboratory-based sleep studies to help understand this intriguing intersection.
Our exploratory study has several limitations to consider in its interpretation. The small sample size is underpowered to make definitive conclusions regarding the efficacy of TN for aggression in ASD. While recruitment was low, subjects and their caregivers who did participate traveled great distances to the site assessments. The remarkable effort of these subjects and caregivers is a true testament to the detrimental effect that aggression can have on patients with ASD and their families. Furthermore, the use of a one-week treatment period is brief, and behavioral improvements reported during such a short period may be due to a variety of factors independent of the active drug, especially environmental and familial influences. Because this is this first systemic, blinded study of TN in adults with ASD, the feasibility and tolerability of this approach was unknown, arguing for a limited duration trial. Subject allocation at randomization was well balanced, however more subjects ultimately contributed primary outcome data in the group receiving TN first and placebo second (Figure 1). While we cannot rule out an order effect as the primary cause for aggressive symptom improvement, other ABC-subscales either were largely unchanged or worsened during the nicotine period, arguing against this possibility. Our study’s primary outcome, the ABC-I, is a subjective measure that can include expectation bias, and indeed a placebo effect in most measures was noted. More objective outcome measures, such as frequency of as-needed medications or frequency of physical aggressive outbursts, could be performed most accurately in either residential or inpatient settings, and would likely be less susceptible to bias. Conversely, we were unable to reliably ascertain subjective assessment of symptom effects directly from participants using standardized rating scales such as STAI or STAXI-2. Finally, the degree to which the 7 mg daily dose of TN engages the α7 nAChR, the putative mechanistic target, is unknown, both in neurotypical adults and in individuals with ASD. Multiple studies suggest that chronic delivery of nicotine can result in nAChR desensitization rather than activation, leading to “functional antagonism” of the receptor (Picciotto et al. 2008; Reitstetter et al. 1999). Interestingly, a previous study of the non-selective nAChR antagonist mecamylamine in ASD did not demonstrate any effect on aggression symptoms (Arnold et al. 2012), nor did MLA or DHβE when used alone in mice (Lewis et al. 2015). There is limited direct evidence demonstrating activation or desensitization of α7 nAChRs in the human brain by nicotine, and some have argued that doses of nicotine achieved by tobacco smoking would be insufficient to activate α7 nAChRs owing to their low affinity for nicotine (Geerts 2012).
While our study is suggestive of a specific effect of TN on aggression in ASD, future studies may be able to address many of the limitations addressed above through the use of selective α7 nAChR agonists or PAMs. Several α7 nAChR agonists have been developed in both academic and pharmaceutical research efforts primarily to address cognitive impairment in schizophrenia or Alzheimer’s disease (Freedman 2014). Overall, such agents have been well tolerated in both healthy adults and patients, and would likely have a tolerability advantage over higher doses of TN. Along these lines, the use of non-nicotine pharmacological agents may enhance the feasibility of conducting such a trial with a larger sample size owing to reduced concern by subjects and families about nicotine itself, despite several previous studies of TN demonstrating safety in non-smoking subjects. Finally, the use of specific α7 nAChR pharmacotherapy would reduce (though not entirely eliminate) the uncertainty as to mechanism of action in human brain, as opposed to the pan-nAChR agonist nicotine. As an example, the α7 partial agonist GTS-21was recently shown in a placebo-controlled study in two adults with ASD to improve P50 auditory gating as well as improve cognitive measures (Olincy et al. 2016). Encouragingly, GTS-21 was well tolerated in these two subjects, supporting future studies of this agent in larger trials.
Taken together, our study provides evidence for the feasibility and tolerability of TN in a small sample of adults with severe ASD symptoms and pathological chronic aggression and irritability. We also demonstrate a possible effect of TN when applied during waking hours to reduce aggression and improve sleep quality. Given both human and animal evidence for the role of α7 nAChRs in aggressive behavior as well as in ASD, future studies will examine the effects of α7 nAChR agonists or PAMs on this symptom domain. Furthermore, by incorporating clinical genetics, the use of α7 nAChR pharmacotherapy might optimally be used in individuals with CHRNA7 CNVs and aggressive behavior, representing a precision medicine approach to treating a constellation of symptoms that for many individuals with ASD remains poorly addressed.
Acknowledgements
This work was supported by Autism Speaks grant #9699 (ASL), National Institutes of Health grants R01DA14241 and R01MH077681 (MRP), R25MH071584, T32MH019961, and T32MH14276 (ASL), and the Child Study Center Associates and the AACAP Pilot Award for General Psychiatry Residents (GIvS). We thank Lawrence Price, Roger Jou, Michael Bloch, and Philip Smith for constructive feedback on this study and Jeffrey Eilbott for technical assistance.
Funding: This work was supported by Autism Speaks grant #9699, National Institutes of Health grants R01DA14241, R01MH077681, R25MH071584, T32MH019961, and T32MH14276, and the [BLINDED] Associates and the AACAP Pilot Award for General Psychiatry Residents.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Allen MH, Debanne M, Lazignac C, Adam E, Dickinson LM, Damsa C (2011). Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry 168, 395–9 doi: 10.1176/appi.ajp.2010.10040569 [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic 89, 485–91 [PubMed] [Google Scholar]
- Arnold LE, et al. (2012). Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorders. J Child Adolesc Psychopharmacol 22, 198–205 doi: 10.1089/cap.2011.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddick CG, Marks MJ (2011). An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol 82, 828–41 doi: 10.1016/j.bcp.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagai K, Malow BA (2010). A novel approach to treating morning sleep inertia in narcolepsy. J Clin Sleep Med 6, 77–8 [PMC free article] [PubMed] [Google Scholar]
- Carmel H, Sheitman BB (2007). Adjunctive transdermal nicotine reduced behavioral agitation in severe dementia. Am J Geriatr Psychiatry 15, 449 doi: 10.1097/01.JGP.0000235688.05709.e2 [DOI] [PubMed] [Google Scholar]
- Chen C, et al. (2017). Aggressive behaviors and treatable risk factors of preschool children with autism spectrum disorder. Autism Res 10, 1155–1162 doi: 10.1002/aur.1751 [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP (2012). Social responsiveness scale : SRS-2, Second edition edn. Torrane, California, Western Psychological Services. [Google Scholar]
- Cubells JF, et al. (2011). Pharmaco-genetically guided treatment of recurrent rage outbursts in an adult male with 15q13.3 deletion syndrome. Am J Med Genet A 155A, 805–10 doi: 10.1002/ajmg.a.33917 [DOI] [PubMed] [Google Scholar]
- Erickson CA, et al. (2014). STX209 (arbaclofen) for autism spectrum disorders: an 8-week open-label study. J Autism Dev Disord 44, 958–64 doi: 10.1007/s10803-013-1963-z [DOI] [PubMed] [Google Scholar]
- Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD (1992). A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 53, 77–82 [PubMed] [Google Scholar]
- Freedman R (2014). alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med 65, 245–61 doi: 10.1146/annurev-med-092112-142937 [DOI] [PubMed] [Google Scholar]
- Geerts H (2012). alpha7 Nicotinic receptor modulators for cognitive deficits in schizophrenia and Alzheimer’s disease. Expert Opin Investig Drugs 21, 59–65 doi: 10.1517/13543784.2012.633510 [DOI] [PubMed] [Google Scholar]
- Gillentine MA, Schaaf CP (2015). The human clinical phenotypes of altered CHRNA7 copy number. Biochem Pharmacol 97, 352–62 doi: 10.1016/j.bcp.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillentine MA, White JJ, Grochowski CM, Lupski JR, Schaaf CP, Calarge CA (2017). CHRNA7 Deletions are Enriched in Risperidone-Treated Children and Adolescents. J Child Adolesc Psychopharmacol, doi: 10.1089/cap.2017.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AP, et al. (2014). Aggressive Behavior Problems in Children with Autism Spectrum Disorders: Prevalence and Correlates in a Large Clinical Sample. Res Autism Spectr Disord 8, 1121–1133 doi: 10.1016/j.rasd.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppman-Chaney N, Wain K, Seger PR, Superneau DW, Hodge JC (2013). Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. Clin Genet 83, 345–51 doi: 10.1111/j.1399-0004.2012.01925.x [DOI] [PubMed] [Google Scholar]
- Ji N, Findling RL (2015). An update on pharmacotherapy for autism spectrum disorder in children and adolescents. Curr Opin Psychiatry 28, 91–101 doi: 10.1097/YCO.0000000000000132 [DOI] [PubMed] [Google Scholar]
- Kanne SM, Mazurek MO (2011). Aggression in children and adolescents with ASD: prevalence and risk factors. J Autism Dev Disord 41, 926–37 doi: 10.1007/s10803-010-1118-4 [DOI] [PubMed] [Google Scholar]
- Lewis AS, Mineur YS, Smith PH, Cahuzac EL, Picciotto MR (2015). Modulation of aggressive behavior in mice by nicotinic receptor subtypes. Biochem Pharmacol 97, 488–97 doi: 10.1016/j.bcp.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Pittenger ST, Mineur YS, Stout D, Smith PH, Picciotto MR (2017). Bidirectional Regulation of Aggression in Mice by Hippocampal Alpha-7 Nicotinic Acetylcholine Receptors. Neuropsychopharmacology, doi: 10.1038/npp.2017.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurel-Paulet A, et al. (2010). Delineation of 15q13.3 microdeletions. Clin Genet 78, 149–61 doi: 10.1111/j.1399-0004.2010.01374.x [DOI] [PubMed] [Google Scholar]
- Matson J (2009). Aggression and Tantrums in Children with Autism: A Review of Behavioral Treatments and Maintaining Variables. Journal of Mental Health Research in Intellectual Disabilities 2, 169–187 doi: 10.1080/19315860902725875 [DOI] [Google Scholar]
- Mazurek MO, Sohl K (2016). Sleep and Behavioral Problems in Children with Autism Spectrum Disorder. J Autism Dev Disord 46, 1906–15 doi: 10.1007/s10803-016-2723-7 [DOI] [PubMed] [Google Scholar]
- McCracken JT, et al. (2002). Risperidone in children with autism and serious behavioral problems. N Engl J Med 347, 314–21 doi: 10.1056/NEJMoa013171 [DOI] [PubMed] [Google Scholar]
- McDougle CJ, et al. (2005). Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 162, 1142–8 doi: 10.1176/appi.ajp.162.6.1142 [DOI] [PubMed] [Google Scholar]
- Mikhail FM, et al. (2011). Clinically relevant single gene or intragenic deletions encompassing critical neurodevelopmental genes in patients with developmental delay, mental retardation, and/or autism spectrum disorders. Am J Med Genet A 155A, 2386–96 doi: 10.1002/ajmg.a.34177 [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT (2010). Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry 167, 331–40 doi: 10.1176/appi.ajp.2009.09050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, et al. (2012). Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology 78, 91–101 doi: 10.1212/WNL.0b013e31823efcbb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Cui N, Zhong W, Johnson CM, Jiang C (2014). Alterations in the cholinergic system of brain stem neurons in a mouse model of Rett syndrome. Am J Physiol Cell Physiol 307, C508–20 doi: 10.1152/ajpcell.00035.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Blakeley-Smith A, Johnson L, Kem WR, Freedman R (2016). Brief Report: Initial Trial of Alpha7-Nicotinic Receptor Stimulation in Two Adult Patients with Autism Spectrum Disorder. J Autism Dev Disord 46, 3812–3817 doi: 10.1007/s10803-016-2890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008). It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84, 329–42 doi: 10.1016/j.pneurobio.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS (2015). Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology, doi: 10.1016/j.neuropharm.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Holmes JP, Kanes SJ, McDougle CJ (1999). Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 19, 37–44 [DOI] [PubMed] [Google Scholar]
- Ray MA, Graham AJ, Lee M, Perry RH, Court JA, Perry EK (2005). Neuronal nicotinic acetylcholine receptor subunits in autism: an immunohistochemical investigation in the thalamus. Neurobiol Dis 19, 366–77 doi: 10.1016/j.nbd.2005.01.017 [DOI] [PubMed] [Google Scholar]
- Reitstetter R, Lukas RJ, Gruener R (1999). Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 289, 656–60 [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism, N. (2005). Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry 162, 1361–9 doi: 10.1176/appi.ajp.162.7.1361 [DOI] [PubMed] [Google Scholar]
- Rosin RA, Levine MD, Peskind E (2001). Transdermal nicotine for agitation in dementia. Am J Geriatr Psychiatry 9, 443–4 doi: 10.1097/00019442-200111000-00014 [DOI] [PubMed] [Google Scholar]
- Rubboli F, et al. (1994). Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci 6, 1596–604 [DOI] [PubMed] [Google Scholar]
- Sharp AJ, et al. (2008). A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet 40, 322–8 doi: 10.1038/ng.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinawi M, et al. (2009). A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat Genet 41, 1269–71 doi: 10.1038/ng.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1999). The State-Trait Anger Expression Inventory-2 (STAXI-2): Professional manual. Odessa, FL, Psychological Assessment Resources, Inc.. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA, Consulting Psychologists Press. [Google Scholar]
- Sukhodolsky DG, et al. (2016). Neural Mechanisms of Cognitive-Behavioral Therapy for Aggression in Children and Adolescents: Design of a Randomized Controlled Trial Within the National Institute for Mental Health Research Domain Criteria Construct of Frustrative Non-Reward. J Child Adolesc Psychopharmacol 26, 38–48 doi: 10.1089/cap.2015.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Miyamoto M, Miyamoto T, Hirata K (2015). Parkinson’s disease and sleep/wake disturbances. Curr Neurol Neurosci Rep 15, 8 doi: 10.1007/s11910-015-0525-5 [DOI] [PubMed] [Google Scholar]
- Tsakanikos E, Costello H, Holt G, Sturmey P, Bouras N (2007). Behaviour management problems as predictors of psychotropic medication and use of psychiatric services in adults with autism. J Autism Dev Disord 37, 1080–5 doi: 10.1007/s10803-006-0248-1 [DOI] [PubMed] [Google Scholar]
- van Schalkwyk GI, Lewis AS, Beyer C, Johnson J, van Rensburg S, Bloch MH (2017). Efficacy of antipsychotics for irritability and aggression in children: a meta-analysis. Expert Rev Neurother 17, 1045–1053 doi: 10.1080/14737175.2017.1371012 [DOI] [PubMed] [Google Scholar]
- Van Schalkwyk GI, Lewis AS, Qayyum Z, Koslosky K, Picciotto MR, Volkmar FR (2015a). Reduction of Aggressive Episodes After Repeated Transdermal Nicotine Administration in a Hospitalized Adolescent with Autism Spectrum Disorder. J Autism Dev Disord 45, 3061–6 doi: 10.1007/s10803-015-2471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schalkwyk GI, Peluso F, Qayyum Z, McPartland JC, Volkmar FR (2015b). Varieties of misdiagnosis in ASD: an illustrative case series. J Autism Dev Disord 45, 911–8 doi: 10.1007/s10803-014-2239-y [DOI] [PubMed] [Google Scholar]
- White HK, Levin ED (1999). Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 143, 158–65 [DOI] [PubMed] [Google Scholar]
- White HK, Levin ED (2004). Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berl) 171, 465–71 doi: 10.1007/s00213-003-1614-8 [DOI] [PubMed] [Google Scholar]