Abstract
Introduction
Primary progressive aphasia (PPA) is a neurodegenerative disorder characterized by progressive deterioration of speech and language. A growing body of research supports the utility of speech and language intervention in individuals with PPA, although access to these services remains limited. One potential means of increasing treatment accessibility is the delivery of treatment via telemedicine. Evidence supports the use of teletherapy in stroke-induced aphasia, but research examining the application of teletherapy in PPA is limited. In the current study, a non-randomized group comparison design was used to evaluate the feasibility and utility of treatment delivered via teletherapy relative to treatment administered in person for individuals with PPA.
Methods
Two treatment protocols were administered as part of a larger study investigating treatment for speech and language deficits in PPA. Participants with semantic (n=10) and logopenic (n=11) PPA received lexical retrieval treatment and individuals with nonfluent/agrammatic PPA (n=10) received video-implemented script training for aphasia designed to promote speech production and fluency. Treatment was administered via teletherapy for approximately half of the participants receiving each intervention. Treatment outcomes and performance on standardized tests were assessed at pre-treatment and post-treatment, as well as 3, 6, and 12 months post-treatment.
Results
Overall, both treatment approaches resulted in significant gains for primary outcome measures. Critically, comparison of in-person and teletherapy groups revealed comparable outcomes. Generalization to untrained targets and tasks and maintenance of treatment-induced gains were also comparable for traditional vs teletherapy participants.
Conclusion
Overall, treatment outcomes were largely equivalent for individuals receiving treatment via teletherapy vs traditional, in-person delivery. Results support the application of teletherapy for administering restitutive interventions to individuals with mild-to-moderate PPA. Potential implications for using teletherapy in the treatment of cognitive-linguistic and motoric impairments in other disorders and suggestions for administering treatment via telemedicine are discussed.
Keywords: PPA, lexical retrieval treatment, script training, telemedicine, telerehabilitation, telepractice
Introduction
Primary progressive aphasia (PPA) is a neurodegenerative disorder in which speech and language abilities progressively deteriorate. Although other cognitive and/or motoric functions are affected in later stages of PPA, speech and language are the most prominent deficits in the initial stages of disease and remain the most impaired domains throughout disease progression.1 Relative to individuals with stroke-induced aphasia, those with a diagnosis of PPA are less likely to be referred for or offered behavioral treatment for speech-language deficits. This is due, in part, to negative perceptions concerning the efficacy of behavioral treatment in neurodegenerative disease, as well as referring and treating clinicians’ lack of training and experience with the disorder.2,3
Access to clinical care may also be affected by considerations that are unique to neurodegenerative disorders. Specifically, as PPA progresses, the severity of symptoms worsens and cognitive and motoric functions beyond speech and language are implicated, with many individuals progressing to a more global dementia.4–7 Moreover, patients may become increasingly context dependent, making new, unfamiliar environments challenging to navigate for both patient and caregiver.8 These factors may affect the ease of travel and navigation from appointment to appointment, further limiting the availability and feasibility of speech–language treatment for individuals with PPA. As such, it is incumbent on the clinical and research communities to explore modes of service delivery that can circumvent these issues and promote access to care for individuals with neurodegenerative disorders such as PPA.
Teletherapy, as a replacement for or complement to in-person treatment, may help to mitigate constraints on service delivery that currently limit access to care for patients with neurodegenerative disease. However, there is very little research investigating the utility of teletherapy in PPA. In the current study, we sought to directly examine treatment outcomes following teletherapy as compared to traditional, in-person treatment for individuals with PPA. Before moving into the specific details of the current study, we briefly review relevant treatment literature in PPA, followed by a discussion of teletherapy in primary progressive and stroke-induced aphasia.
Treatment research in PPA
There is now a modest but growing evidence base supporting speech–language treatment in the three PPA variants (ie, semantic PPA [svPPA], logopenic PPA [lvPPA], and nonfluent/agrammatic PPA [nfvPPA]), each of which is characterized by a distinct profile of speech and language characteristics. Individuals with svPPA present with impaired lexical retrieval, single-word comprehension, and object knowledge, attributed to bilateral (typically left greater than right) atrophy in the anterior temporal lobes,1,9,10 which are critically important for semantic processing.11 Individuals with lvPPA present with phonological deficits, which manifest clinically as impairments in lexical retrieval (often with phonological paraphasias) and repetition, caused by atrophy in left temporoparietal cortex,1,12,13 a region involved in phonological processing.13,14 Finally, individuals with nfvPPA present with agrammatism and/or deficits in motor speech caused by atrophy in left lateralized frontoinsular cortex,1,2,15 a region critical for grammatical processing and speech production.16,17
The majority of treatment studies in PPA have addressed lexical retrieval in svPPA, with fewer studies dedicated to lvPPA and nfvPPA.18–22 With svPPA patients, researchers have implemented a variety of training protocols designed to promote successful naming via cued retrieval of semantic, phonological, orthographic, and/or episodic representations, typically in response to pictured items.9,23–39 These studies have documented significantly improved naming performance for trained items, with some studies showing generalization to untrained items and contexts9,23–25,36,37 and a few studies reporting maintenance up to 6 months post-treatment.30,37–39
There are fewer studies investigating lexical retrieval treatment (LRT) in lvPPA, but results are promising. As in svPPA treatment studies, intervention studies in lvPPA have used cueing hierarchies or strategic training to promote the activation of residual semantic, phonological, orthographic, and/or episodic representations in response to pictured items.9,27,37,40–42 In these studies, significant treatment effects were observed on trained items, with some studies showing improved naming of untrained items and maintenance of gains up to 6 months post-treatment.9,23,28,37
Individuals with nfvPPA have also received limited attention in the treatment literature, with the majority of research focusing on LRT. In several studies, LRT was implemented such that a picture was paired and rehearsed with the word form,27,40,42,43 with one study also using semantic feature analysis.44 In these studies, significant treatment effects were observed for treated items, but generalization was not observed for untreated items; maintenance at 1 month was observed in some participants.27,42,43
Other treatment studies in nfvPPA have focused on a range of linguistic deficits associated with the disorder, such as verb and sentence production,45,46 apraxia of speech,2 and phonological processing,47 with results indicating significant improvement on trained items and generalization to untrained items and tasks. Finally, in a recent study, Henry et al48 utilized video-implemented script training to target grammatical and motor speech impairments in nfvPPA. Treatment resulted in significant gains for trained content and generalization to untrained content for some outcome measures; gains for trained material were maintained up to 1-year post-treatment.
Across clinical variants and treatment protocols, behavioral intervention for speech–language deficits has resulted in immediate gains for trained items and tasks. In some instances, generalization to untrained items, tasks, and contexts, as well as maintenance of gains, has also been observed. Despite this solid and growing evidence base showing restitutive treatment to be feasible and worthwhile in individuals with PPA, issues of access to services remain. Teletherapy may be an important and viable tool for overcoming this barrier.
Aphasia treatment utilizing teletherapy
In the last decade, teletherapy has increasingly been considered in the management of adult neurogenic communication disorders as a potential means to overcome the barriers of access to treatment caused by distance, lack of specialist availability, and impaired mobility in patients.49,50 Teletherapy allows the individual to access services from the comfort of their home and shows promising outcomes in the management of chronic aphasia, dysarthria, voice disorders and Alzheimer’s dementia.49,50,51,52
In stroke-induced aphasia, several studies have demonstrated the utility of teletherapy for the treatment of anomia.53–57 Following LRT delivered via teleconference, gains were observed on trained items relative to untrained items,53–55,57 with one study reporting generalization to untrained items and tasks.55 In addition, one study observed a reduction in aphasia severity following teletherapy, as captured by improvements on the Western Aphasia Battery (WAB)58 Aphasia Quotient (AQ).56 Of the aforementioned studies, those who have utilized either a crossover design or a quasi-randomized controlled design to allow the comparison of teletherapy with traditional, in-person treatment reported roughly equivalent gains for the two treatment conditions.53,56,57
Although these studies confirm that teletherapy is a viable and effective means of delivering naming interventions to individuals with stroke-induced anomia, fewer studies have examined interventions addressing linguistic deficits such as syntax, discourse, and apraxia of speech, the core deficits for individuals with nfvPPA. A study from Goldberg et al59 addressed sentence production deficits by implementing a script training protocol (repetition, choral production, and independent production of individualized scripts) in two participants with stroke-induced aphasia using a combination of in-person and teletherapy sessions. Positive effects were noted for speech rate, percentage of correct scripted words, and number of disfluencies, leading the authors to conclude that teletherapy is an efficacious method of delivering script training intervention, at least when augmented by occasional in-person sessions. Lasker et al60 also combined in-person and teletherapy sessions in a feasibility study to address severe speech apraxia in an individual with chronic apraxia and aphasia following stroke. The intervention utilized a motor learning-guided approach with imitation, immediate and delayed repetition, reading aloud, and home practice with a speech-generating device to increase production accuracy for target words and phrases. Following treatment, there was no difference between items treated via teletherapy vs in-person in terms of intelligibility or naturalness ratings. Similar to Goldberg et al,59 Lasker et al60 concluded that teletherapy may be as effective as in-person treatment. Taken together, the current literature on teletherapy implementation for stroke-induced apraxia and aphasia suggests that gains following teletherapy are comparable to those observed after traditional, in-person treatment.
To our knowledge, there are only two studies documenting teletherapy outcomes in individuals with PPA.42,61 Rogalski et al61 utilized a teletherapy platform for 34 individuals with PPA (n=31) or dementia (n=3), addressing speech and language deficits based on individual participant needs using a variety of targets and tasks (eg, script training, picture naming, oral reading). They observed objective gains on standardized measures of speech–language function as well as self-reported benefits, including increased confidence, following treatment. Moreover, self-reported use of trained strategies was maintained up to 6 months. This feasibility study documented the utility of personalized treatment delivered via teleconference in PPA; however, there was no comparison of the results of teletherapy to traditional, in-person treatment and details regarding the intervention procedures were not provided.
Meyer et al42 utilized a LRT protocol in which a picture was paired and rehearsed with the written (orthographic) or spoken (phonological) word form in individuals with nfvPPA (n=1 teletherapy, n=4 in-person), lvPPA (n=1 teletherapy, n=7 in-person), and svPPA (n=1 teletherapy, n=3 in-person). Comparisons of in-person vs teletherapy outcomes in each variant at 1 month post-treatment revealed equivalent or somewhat larger treatment effects for the individuals who received teletherapy. There was, however, no attempt to control for demographic factors between the teletherapy and in-person groups, and only one individual from each clinical variant received teletherapy. As such, larger, matched samples are needed to provide conclusive evidence regarding the benefits of teletherapy in this population.
Current study
As telecommunications technology continues to become more advanced, telepractice will undoubtedly play a greater role in the management of chronic health conditions, including aphasia and dementia. Therefore, it is important to establish the feasibility and utility of telepractice in specific clinical populations by directly comparing treatment outcomes following traditional, in-person interventions to those resulting from teletherapy in larger, matched samples. In order to do this, we analyzed archival data, collected as part of a larger research study investigating restitutive interventions for mild-to-moderate PPA.37,48 Data comprised matched samples of individuals with PPA who underwent treatment either via teletherapy or traditional, in-person treatment, allowing us to compare treatment outcomes between the two treatment delivery modalities.
SvPPA and lvPPA participants received a lexical retrieval training hierarchy to promote word retrieval (adapted from the Arizona Naming Cascade),9,37 whereas nfvPPA participants received video-implemented script training to treat grammatical and motor speech deficits.48 Treatment outcomes following teletherapy were compared to outcomes following traditional, in-person treatment in groups of demographically matched participants with PPA to determine whether comparable gains can be achieved via teletherapy across clinical variants and training protocols. Furthermore, we sought to examine whether differences in the generalization or maintenance of gains existed between teletherapy and in-person treatment. We hypothesized that teletherapy outcomes would be comparable to traditional forms of treatment in terms of immediate and longer-term outcomes, a finding which, if upheld, would have significant implications for improved access to rehabilitation services for individuals in this traditionally underserved clinical population.
General methods
Participants
All participants underwent a comprehensive evaluation at either the Aphasia Research and Treatment Laboratory at the University of Texas at Austin, or the University of California, San Francisco, Memory and Aging Center and were subsequently enrolled in a speech–language treatment study. To qualify for entry into the treatment study, participants with language impairment were required to meet current diagnostic criteria for PPA.1 A PPA diagnosis stipulates insidious onset and gradual deterioration of speech and language functions, with speech and language deficits being the most prominent impairments throughout the initial stages of disease. Diagnosis according to variant was made by consensus following a review of the patient’s medical history, a multidisciplinary evaluation encompassing speech–language and neuropsychological testing (refer to Henry et al13 for an overview of assessment procedures), and neurological examination. Additionally, to be eligible for enrollment in the treatment study, participants were required to have Mini–Mental State Examination (MMSE)62 scores of 15 or higher. This research was approved by the Institutional Review Boards of the University of Texas at Austin and the University of California, San Francisco, and written informed consent was obtained for all participants prior to enrollment in the study.
A total of 31 individuals with PPA were included (n=10 nfvPPA, n=10 svPPA, and n=11 lvPPA). At the time of data analysis, all participants had completed pre-treatment and post-treatment assessments. All participants with nfvPPA had also completed 3- and 6-month follow-up assessments, and the majority (n=9) had completed a 12-month follow-up assessment (n=1 teletherapy participant unable to complete 12-month follow-up due to illness). A majority of the svPPA and lvPPA participants had also completed 3-month (n=9 svPPA and n=9 lvPPA), 6-month (n=8 svPPA and n=10 lvPPA), and 12-month (n=9 svPPA and n=10 lvPPA) followup assessments. One individual with svPPA (in-person treatment condition) passed away after post-treatment testing, and another individual with svPPA (in-person treatment condition) was unavailable at 6-month follow-up but completed the 12-month follow-up. One individual with lvPPA (in-person treatment) was unavailable at 3-month follow-up but completed 6- and 12-month follow-up, and one individual with lvPPA (teletherapy) passed away after post-treatment testing. Participants were nonrandomly assigned to teletherapy vs traditional, in-person treatment according to patient needs and logistical considerations (eg, transportation/mobility issues or remote geographical location relative to the research sites).
In order to be eligible for teletherapy, participants were required to have high-speed Internet access and basic experience using a computer, or access to reliable assistance from someone who could provide support, as needed, during testing and treatment sessions. No participants were excluded for failing to meet these criteria, although, in some instances, there was a delay between screening and enrollment while participants acquired adequate Internet service and computer access. Additionally, participants were required to have adequate hearing to allow the accurate perception of speech presented in person or via computer. This was evaluated on a case-by-case basis, as follows: when possible, a hearing screening was conducted. For participants seen remotely, audiological examination reports and an in-house minimal pairs discrimination task (with a performance criterion of 90% correct; slightly more conservative than the 85% performance criterion used in Henry et al13) were used. If individuals demonstrated a degree of hearing loss that could not be accommodated using a personal amplification device or by increasing the intensity and using headphones (if seen via teleconference), they were not enrolled in the treatment study. Prominent, uncorrected visual acuity impairment was also an exclusionary criterion.
Of the 31 participants, 17 participants received traditional, in-person treatment (n=5 nfvPPA, n=6 svPPA, and n=6 lvPPA) and 14 participants received teletherapy (n=5 nfvPPA, n=4 svPPA, and n=5 lvPPA). (Note that data from 18 of the current study’s 21 svPPA and lvPPA participants [n=9 svPPA and n=9 lvPPA] were included in a study examining the utility of lexical retrieval treatment for facilitating word retrieval in svPPA and lvPPA37 and data from all 10 of the current study’s nfvPPA participants were included in a study examining the utility of speech entrainment for facilitating grammaticality, intelligibility, and fluency of connected speech in nfvPPA.48) Tables 1 and 2 present demographic characteristics and relevant speech and language measures from pre-treatment, post-treatment, and follow-up assessments. Testing sessions were conducted in the same delivery modality in which treatment was administered (ie, in-person and via teleconference) either by the individual who administered treatment or another member of the research team.
Table 1.
Demographic characteristics for participants according to treatment protocol and treatment delivery condition
| Demographic | LRT: in-person | LRT: teletherapy | VISTA: in-person | VISTA: teletherapy |
|---|---|---|---|---|
|
| ||||
| Age | 68.9 (7.1) | 61.0 (6.3) | 67.6 (3.9) | 67.8 (7.3) |
| Sex | 4M, 8F | 5M, 4F | 2M, 3F | 2M, 3F |
| Education (years) | 17.9 (2.7) | 17.2 (3.3) | 15.6 (2.6) | 15.6 (1.8) |
| Handedness | 8R, 2L, 2A | 7R, 2L | 5R | 5R |
| MMSE (30a) | 23.8 (4.3) | 24.8 (3.6) | 26.0 (2.4) | 27.6 (2.1) |
Notes: Age and MMSE reflect baseline. Data shown as mean (SD).
The total points available on the MMSE.
Abbreviations: LRT, lexical retrieval treatment; VISTA, video-implemented script training for aphasia; M, male; F, female; R, right; L, left; A, ambidextrous; MMSE, Mini Mental State Examination.
Table 2.
Group performance on speech and language measures according to treatment protocol and treatment delivery condition
| LRT: in-person | LRT: teletherapy | VISTA: in-person | VISTA: teletherapy | |
|---|---|---|---|---|
|
| ||||
| Pre-Tx BNT (%)b | 40.8 (25.1) | 43.1 (26.8) | N/A | N/A |
| Post-Tx BNT (%)b | 43.5 (24.1) | 45.5 (27.4) | N/A | N/A |
| 3-month BNT (%)b | 40.3 (23.6) (n=10)a | 45.4 (31.6) (n=8)a | N/A | N/A |
| 6-month BNT (%)b | 40.0 (18.5) (n=9)a | 38.3 (27.3) (n=8)a | N/A | N/A |
| 12-month BNT (%)b | 31.5 (22.9) (n=9)a | 35.4 (23.6) (n=8)a | N/A | N/A |
| Pre-Tx NAT (%)b | N/A | N/A | 58.3 (25.0) (n=4)a | 70.0 (15.1) |
| Post-Tx NAT (%)b | N/A | N/A | 71.3 (20.9) | 77.3 (22.8) |
| 3-month NAT (%)b | N/A | N/A | 64.7 (23.4) | 68.3 (33.0) |
| 6-month NAT (%)b | N/A | N/A | 38.7 (33.8) | 70.0 (29.8) |
| 12-month NAT (%)b | N/A | N/A | 29.2 (39.4) (n=4)a | 56.1 (35.7) (n=4)a |
| Pre-Tx WAB AQ (100) | 80.9 (10.6) | 83.7 (7.3) | 82.9 (5.7) | 85.7 (7.4) |
| Post-Tx WAB AQ (100) | 80.3 (10.2) | 83.8 (6.7) | 83.8 (5.7) | 87.6 (6.5) |
| 3-month WAB AQ (100) | 80.1 (10.0) (n=10)a | 81.6 (8.5) (n=8)a | 78.5 (8.2) | 84.8 (8.1) |
| 6-month WAB AQ (100) | 77.7 (12.4) (n=10)a | 79.0 (10.9) (n=8)a | 76.7 (7.1) | 83.2 (10.1) |
| 12-month WAB AQ (100) | 73.2 (14.0) (n=9)a | 71.5 (13.6) (n=8)a | 70.5 (10.5) | 81.8 (11.7) (n=4)a |
Notes: Data shown as mean (SD).
Differences in n as not all participants had completed all assessments or due to missing data.
Generalization measures were selected according to deficits targeted by treatment protocol. The BNT was used to examine generalization of naming ability in LRT while the NAT examined generalization of grammatical skills in VISTA.
Abbreviations: Tx, treatment; LRT, lexical retrieval treatment; VISTA, video-implemented script training for aphasia; BNT, Boston Naming Test; N/A, not applicable; NAT, Northwestern Anagram Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
The treatment approach varied by PPA subtype, in order to address core speech and language deficits associated with each progressive aphasia phenotype. A total of three treatment protocols were used to treat participants. Two of these protocols addressed naming impairment in svPPA and lvPPA and were nearly identical: 1) the LRT (LRT-1) as implemented by Henry et al9 and 2) a modified version of LRT with dosage modifications (LRT-2). The third approach, video-implemented script training in aphasia (VISTA), addressed speech production and fluency in individuals with nfvPPA. Treatment protocols and materials were nearly identical for in-person treatment and teletherapy, with minor modifications, as needed, to accommodate the teleconference set-up (eg, asking patients to present written responses to the webcam in order to be viewed by the clinician). Each treatment approach will be briefly presented below (for a more detailed discussion of the treatment approaches, refer to Henry et al for LRT9,37 and Henry et al for VISTA48).
Apparatus/software
In-person treatment
For participants receiving in-person treatment, testing and treatment materials were presented using paper/pencil tests or via computer, depending on the task.
Teletherapy
For participants receiving teletherapy, testing and treatment sessions were adapted to be conducted on-line via HIPAA-compliant videoconferencing software: either Adobe Connect© (www.adobe.com/products/adobeconnect.html) or Fuze© (www.fuze.com). To assist with software installation, participants were provided with a document containing written instructions and screenshots of each step of the installation process. Additional assistance was provided, as needed, either via phone or by using TeamViewer© (www.teamviewer.com), which allowed short-term remote access to the participant’s computer. For participants who did not own a computer or who did not have a webcam, an iPad Air (2013, 16 GB) was provided with relevant software installed.
LRT: materials and methods
Participants were trained to use word-finding strategies capitalizing on the retrieval of residual semantic, phonological, orthographic, and autobiographical or episodic knowledge. The treatment utilized a cueing hierarchy that guides the participant through a series of tasks to strengthen and activate central components of language processing, while also training self-cueing techniques (Table 3). As previously noted, there were two variations of the same LRT utilized in the current study. Differences between the two treatment protocols involved the number of items trained (LRT-1: 20 items, LRT-2: 40 items), the number of sessions conducted per week (LRT-1: one session and LRT-2: two sessions), and whether or not a booster dose was administered at 3 months post-treatment (booster dose administered for LRT-2). Ten of the 21 participants with svPPA (n=3 in-person and n=2 teletherapy) or lvPPA (n=4 in-person and n=1 teletherapy) received LRT-1,9 and 11 participants with svPPA (n=3 in-person and n=2 teletherapy) or lvPPA (n=2 in-person and n=4 teletherapy) underwent LRT-2.37 In both LRT-1 and LRT-2, participants completed modified Copy and Recall Treatment (CART) as daily homework.63 This involved repeated copy and spoken production of target words (10 times), followed by recall of spoken and written word forms from memory.
Table 3.
Lexical retrieval treatment cueing hierarchy
| Treatment steps | Procedurea |
|---|---|
|
| |
| 1. Present picture for naming | 1. Participant attempts to name the picture |
| 2. Semantic self-cue | 2. Guided semantic feature analysis/generation of episodic/autobiographical information |
| 3. Orthographic/phonemic self-cue (if unnamed step 1) | 3. 1st letter and initial sound elicited/provided |
| 4. Spoken and written repetition | 4. Clinician provides written model; participant says/writes three times |
| 5. Semantic plausibility judgments (×5) | 5. Example: “Is this something that you find in the kitchen?” |
| 6. Recall | 6. Recall two semantic features + spoken and written name |
Notes:
Procedure adapted from the Arizona Naming Cascade in Henry ML, Rising K, Demarco AT, Miller BL, Gorno-Tempini ML, Beeson PM. Examining the value of lexical retrieval treatment in primary progressive aphasia: two positive cases. Brain Lang. 2013;127(2):145–156. Copyright © 2013 Elsevier Inc. All rights reserved.9
In order to determine whether the LRT-1 and LRT-2 groups could be combined into one LRT group for the purpose of comparing traditional vs teletherapy outcomes in the current study, we compared treatment outcomes in LRT-1 to LRT-2 cohorts. As reported in Henry et al37 (where there were three fewer participants than in the current study), no differences were observed in naming performance for trained items between LRT-1 and LRT-2 groups (refer to Table S1 for statistical details). Given the comparable outcomes for LRT-1 and LRT-2 groups, data from both LRT groups were combined into one dataset for subsequent analyses in the current study. This resulted in a group of 21 participants, with 12 receiving in-person treatment and nine receiving teletherapy.
Outcome measures and statistical analysis
The primary outcome measure for naming treatment was a change score calculated as the difference between average percent correct from the last two pre-treatment probes relative to average percent correct from the first two post-treatment probes for trained items. (Note that Henry et al utilized three pre-treatment probes to compute change scores for LRT.37 However, in the current study, we utilized two pre-treatment probes in order to be consistent with how change scores were computed for VISTA.48) Secondary outcome measures, intended to capture generalization, included pre- to post-treatment change scores on untrained items (the difference between average percent correct from the last two pre-treatment probes relative to average percent correct from the first two post-treatment probes) and pre- to post-treatment change scores on the Boston Naming Test (BNT).64 The WAB AQ was used to assess the stability of language profile over time.65 Change scores (the difference between performance at 3-, 6-, and 12-month follow-up relative to post-treatment) for trained sets, untrained sets, and the BNT were used to examine maintenance of treatment effects, and change scores on the WAB AQ were calculated in order to examine changes in overall language profile over time. For the BNT, modified short versions were administered to prevent practice effects and fatigue and to fit testing within allotted time constraints. Equivalent scores were calculated by comparing common items across versions to ensure valid comparison across time points, with a minimum of 15 of the 60 items included at any given time point.
The distribution of the data failed to meet the assumption of normality, so we conducted nonparametric permutation tests in R (Version 3.3.1; 2016) using a complete enumeration of all possible permutations. Permutation tests for paired data were used to examine performance across time points (for more specific details on paired samples analyses, refer to Henry et al37,48 and Table S2). In the current study, the critical comparison was between the group of participants who received in-person treatment and those who received treatment via teletherapy. For these between-groups comparisons, two-tailed permutation tests for independent samples were utilized, with P-values calculated as the proportion of mean differences in the permutation distribution more extreme than the observed mean difference. The 95% CIs were computed by calculating the difference between each permuted mean difference and the observed mean difference and obtaining the 2.5 and 97.5 percentiles of the resulting distribution. The large number of statistical comparisons might typically warrant a multiple comparisons correction. However, our hypothesis predicted no differences in treatment outcomes for individuals receiving in-person treatment relative to those receiving teletherapy (ie, a “null effect”). Because correcting for multiple comparisons results in a smaller alpha for determining statistical significance, rejection of the null hypothesis is rendered less likely. As such, we opted for the more conservative approach and did not correct for multiple comparisons.
LRT: results
Treatment effects following LRT
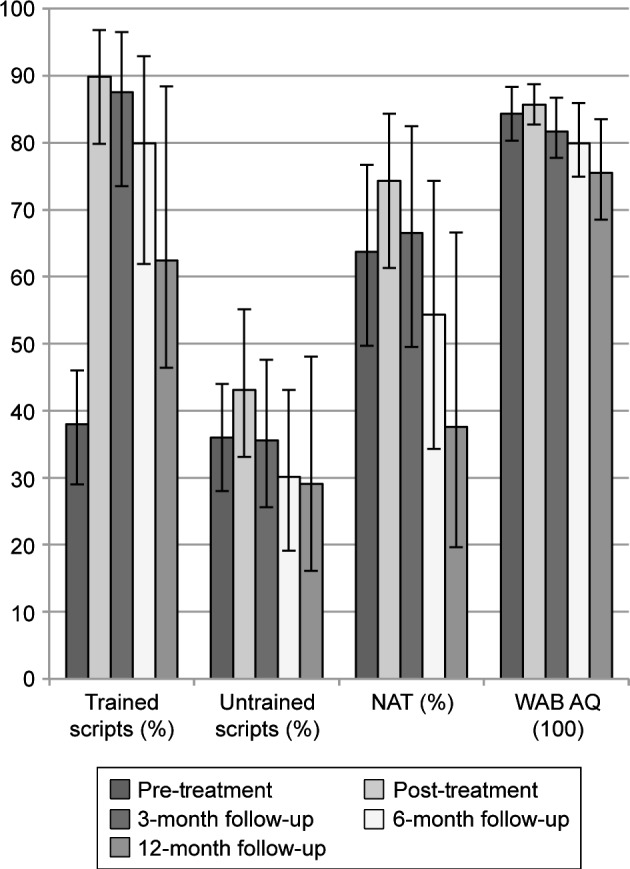
Group mean performance on all pre-treatment, post-treatment, and follow-up measures is presented in Figure 1. Consistent with Henry et al,37 naming of trained items was significantly better than pre-treatment at all time points, with generalization observed for untrained items up to 6 months post-treatment and for the BNT at post-treatment (for statistical details of within-group analyses, refer to Table S2).
Figure 1.
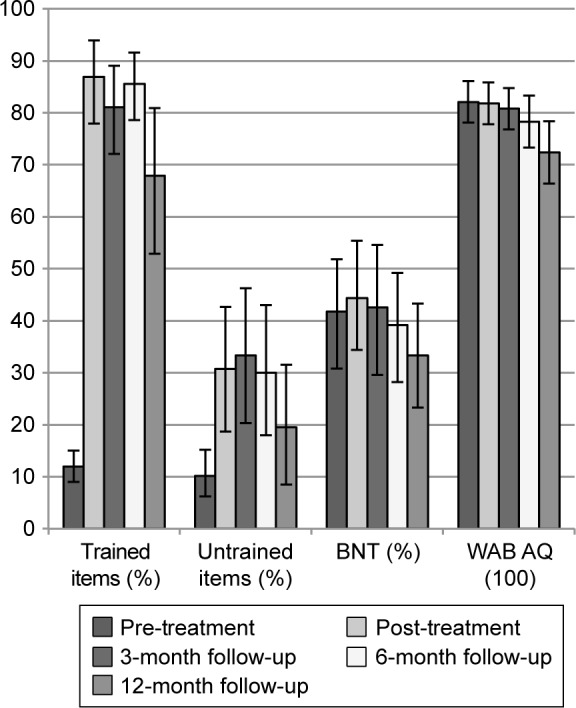
Mean performance on pre-treatment, post-treatment, and follow-up measures for all LRT participants.
Note: 95% confidence intervals around the mean were derived using n=1000 bootstrapped samples.
Abbreviations: LRT, lexical retrieval treatment; BNT, Boston Naming Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
LRT treatment effects for in-person treatment vs teletherapy
Mean performance on all pre-treatment and post-treatment measures by treatment type is presented in Tables 2 and 4, and the corresponding change scores are presented in Figure 2.
Table 4.
Mean performance on trained and untrained items (SDs) for LRT participants by treatment type
| Pre-treatment, in-person (n=12) | Pre-treatment, teletherapy (n=9) | Post-treatment, in-person (n=12) | Post-treatment, teletherapy (n=9) | 3-month follow-up, in-person (n=10) | 3-month follow-up, teletherapy (n=8) | 6-month follow-up, in-person (n=10) | 6-month follow-up, teletherapy (n=8) | 12-month follow-up, in-person (n=11) | 12-month follow-up, teletherapy (n=8) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Trained items (%) | 12.8 (7.9) | 11.0 (7.6) | 85.1 (20.8) | 89.3 (17.5) | 73.8 (24.3) | 90.3 (8.9) | 87.8 (9.9) | 82.8 (19.5) | 66.1 (33.2) | 70.3 (26.7) |
| Untrained items (%) | 11.7 (9.4) | 8.3 (12.8) | 21.7 (24.5) | 42.8 (32.3) | 21.0 (24.7) | 48.8 (26.4) | 19.0 (21.8) | 43.8 (32.5) | 7.3 (12.7) | 36.3 (29.3) |
Abbreviation: LRT, lexical retrieval treatment.
Figure 2.
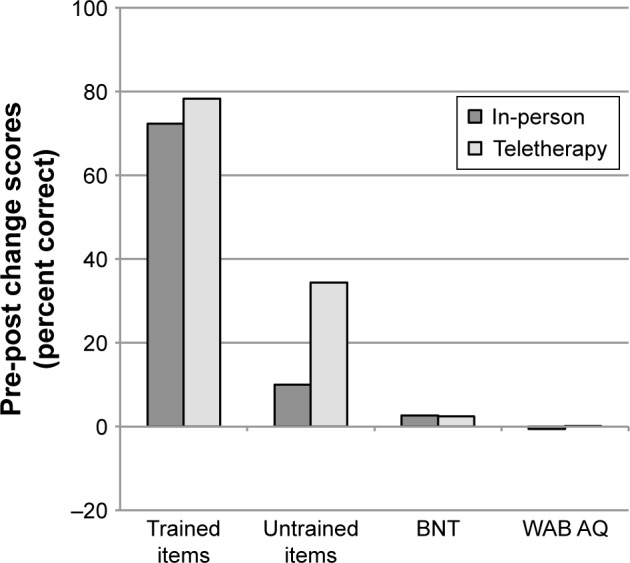
Mean pre- to post-treatment change scores (post minus pre) for participants completing LRT in person or via teletherapy.
Notes: Positive values indicate higher scores at post-treatment than pre-treatment. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: LRT, lexical retrieval treatment; BNT, Boston Naming Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
In order to determine whether demographic factors and severity of deficits at pre-treatment were matched between the two groups, we compared age, education, and pre-treatment BNT, MMSE, and WAB AQ between the in-person and teletherapy groups. Whereas the two groups were not significantly different in education (t=0.51, P=0.610, 95% CI [−3.11, 1.94]), pre-treatment MMSE score (t=−0.60, P=0.591, 95% CI [−2.33, 4.47]), pre-treatment BNT (t=-0.37, P=0.714, 95% CI [−19.09, 28.43]), or pre-treatment WAB AQ (t=−0.71, P=0.504, 95% CI [−5.04, 10.77]), the two groups did differ significantly in age (t=2.69, P=0.018, 95% CI [−14.58, −1.17]), with teletherapy participants being slightly younger (M=61.0 years) than participants treated in person (M=68.9 years).
No significant difference was observed for pre–post change scores for trained items for treatment administered in-person (M=72.3%) compared to teletherapy (M=78.3%; t=−0.64, P=0.560, 95% CI [−10.94, 24.55]); however, a significant difference was observed for pre–post change scores for untrained items for in-person treatment (M=10.0%) compared to teletherapy (M=34.4%; t=−2.23, P=0.031, 95% CI [1.94, 46.67]), with teletherapy participants showing greater generalization. Generalization to the BNT and stability of overall language performance, as documented by the WAB AQ, were comparable between in-person treatment (M=2.7% and −0.59, respectively) and teletherapy (M=2.4% and 0.11, respectively), as changes from pre-treatment to post-treatment on neither the BNT (t=0.11, P=0.927, 95% CI [-5.85, 5.20]) nor the WAB AQ (t=−0.42, P=0.672, 95% CI [−2.45, 3.83]) were significantly different between the two groups.
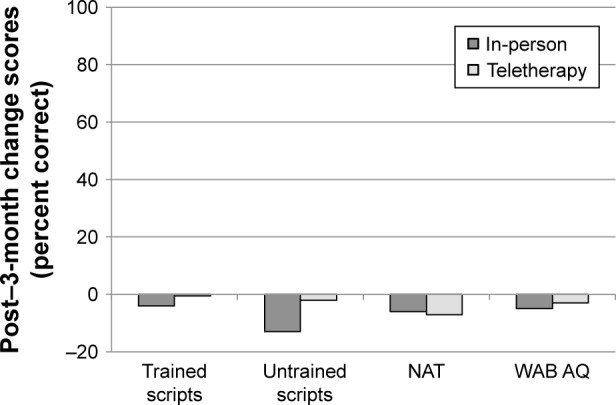
Maintenance of treatment gains following LRT for in-person treatment vs teletherapy
Group mean performance on all post-treatment and follow-up measures is presented in Tables 2 and 4, respectively. Maintenance of gains was examined by comparing change scores from post-treatment to follow-up between the in-person and teletherapy groups. Figure 3 presents mean change scores from post-treatment to 3-month follow-up by treatment type. Post-treatment to 3-month follow-up change scores for trained items for in-person (M=−10.6%) and teletherapy (M=1.1%) were not significantly different (t=−1.54, P=0.141, 95% CI [−3.09, 26.44]). Similarly, post-treatment to 3-month follow-up change scores for untrained items for in-person (M=-5.0%) and teletherapy (M=6.3%) were not significantly different (t=−0.85, P=0.447, 95% CI [-12.38, 33.75]). Finally, maintenance on both the BNT and change in overall language status (WAB AQ) were also comparable between in-person (M=−7.2% and −2.4, respectively) and teletherapy (M=−2.1% and −2.3, respectively), as the post-treatment to 3-month follow-up change scores neither for the BNT (t=-0.92, P=0.366, 95% CI [−5.63, 15.76]) nor for the WAB AQ (t=−0.05, P=0.963, 95% CI [−2.56, 2.74]) were significantly different between the two groups.
Figure 3.
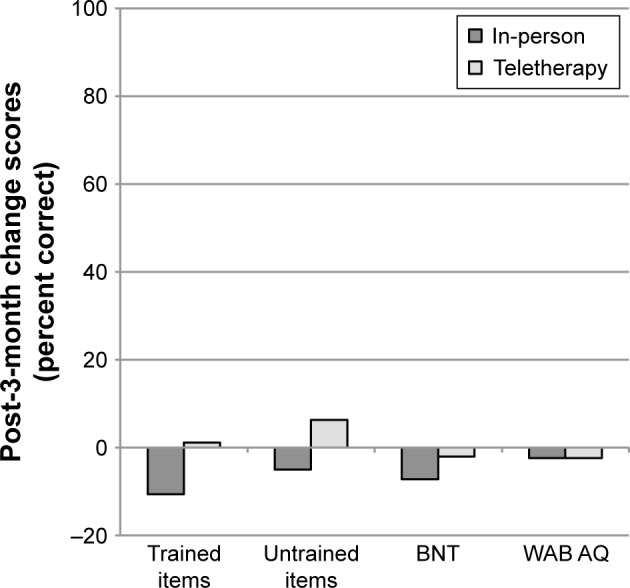
Mean change scores from post-treatment to 3-month follow-up (3 months minus post) for participants completing LRT in-person or via teletherapy.
Notes: Negative values indicate higher scores at post-treatment than 3-month follow-up. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: LRT, lexical retrieval treatment; BNT, Boston Naming Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
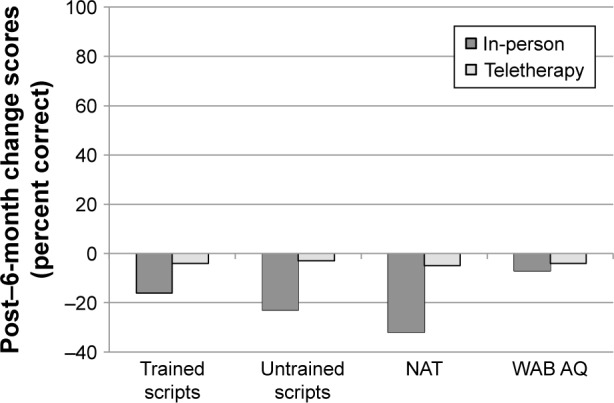
Mean change scores from post-treatment to 6-month follow-up were also compared between the groups (Figure 4). Post-treatment to 6-month follow-up change scores for trained items for in-person (M=−4.0%) and teletherapy (M=-6.4%) were not significantly different (t=0.48, P=0.636, 95% CI [−11.81, 7.03]). Similarly, post-treatment to 6-month follow-up change scores for untrained items for in-person (M=−5.0%) and teletherapy (M=1.3%) were not significantly different (t=−0.44, P=0.682, 95% CI [-18.08, 30.38]). Maintenance on the BNT and change in overall language status (WAB AQ) were also comparable between in-person (M=−9.6% and −4.6, respectively) and teletherapy (M=−5.0% and −4.9, respectively), as the post-treatment to 6-month follow-up change scores neither for the BNT (t=−1.22, P=0.255, 95% CI [−3.13, 12.57]) nor for the WAB AQ (t=0.11, P=0.922, 95% CI [−5.26, 4.88]) were significantly different between the two groups.
Figure 4.

Mean change scores from post-treatment to 6-month follow-up (6 months minus post) for participants completing LRT in-person or via teletherapy.
Notes: Negative values indicate higher scores at post-treatment than 6-month follow-up. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: LRT, lexical retrieval treatment; BNT, Boston Naming Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
Finally, mean change scores from post-treatment to 12-month follow-up were compared between the groups (Figure 5). Post-treatment to 12-month follow-up change scores for trained items for in-person (M=−19.4%) and teletherapy (M=-18.9%) were not significantly different (t=−0.05, P=0.971, 95% CI [−18.62, 20.78]). Similarly, post-treatment to 12-month follow-up change scores for untrained items for in-person (M=−16.4%) and teletherapy (M=-6.3%) were not significantly different (t=−0.68, P=0.535, 95% CI [−15.11, 34.55]). Maintenance on the BNT and change in overall language status (WAB AQ) were also comparable between in-person (M=−18.9% and −10.0, respectively) and teletherapy (M=−12.1% and −12.4, respectively), as the post-treatment to 12-month follow-up change scores neither for the BNT (t=−0.85, P=0.415, 95% CI [−8.66, 22.04]) nor for the WAB AQ (t=0.58, P=0.572, 95% CI [−10.29, 5.57]) were significantly different between the two groups.
Figure 5.
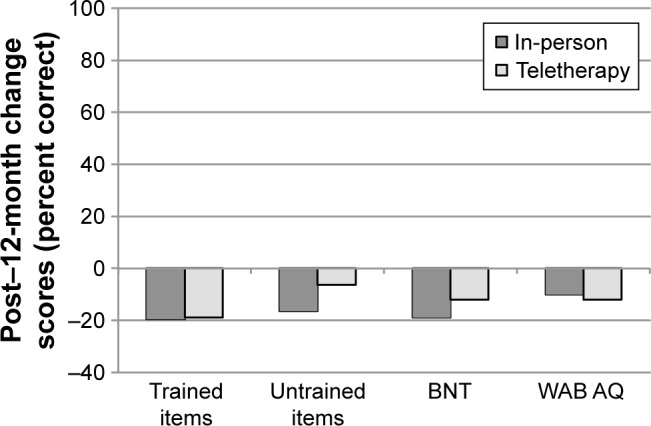
Mean change scores from post-treatment to 12-month follow-up (12 months minus post) for participants completing LRT in-person or via teletherapy.
Notes: Negative values indicate higher scores at post-treatment than 12-month follow-up. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: LRT, lexical retrieval treatment; BNT, Boston Naming Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
VISTA: materials and methods
Participants with nfvPPA underwent VISTA, a treatment approach designed to facilitate grammaticality, intelligibility, and fluency of connected speech.48 This treatment used script training, an approach that involves repeated practice of phrases or sentences in either a monolog or dialog.66,67 VISTA is a largely homework-based protocol that is implemented via “speech entrainment,” a technique that utilizes repeated practice with an audiovisual model of a healthy speaker, which participants attempt to mimic in real time.68 This daily practice is complemented by sessions with a clinician that target articulatory and grammatical aspects of script production. In addition to targeting speech production skills, sessions target memorization and conversational usage of scripted materials via a treatment hierarchy moving from structured to more functional tasks (Table 5). Speech entrainment homework consisted of unison speech production practice with the video model for a minimum of 30 min/day (refer to Henry et al48 for details regarding stimuli and treatment procedures).
Table 5.
VISTA clinician-guided treatment hierarchy
| Treatment steps | Procedure | |
|---|---|---|
|
| ||
| Structured treatment | 1. Recall/recognize | Participant chooses correct trained script sentence from four foil sentences |
|
2. Organize/construct | After selecting correct sentences, participant must put the sentences in order of the script |
| 3. Read | Participant reads script aloud | |
| 4. Respond to questions in scripted order | Clinician prompts each sentence with a question (eg, “Where were you born?”), with cues provided as needed to complete sentence | |
| 5. Produce script from memory | Clinician prompts for script with “Tell me about…” | |
| Functional application | 6. Respond to questions with scripted sentences | Clinician asks questions on the scripted topic in a conversation and elicits scripted sentences out of order |
Notes: Feedback regarding articulation and grammar occur during steps 3–6, with targeted practice as needed. During the second treatment session for a given script, a novel communication partner has an unscripted conversation with the participant to promote the conversational usage of scripted material.
Abbreviation: VISTA, video-implemented script training for aphasia.
Outcome measures and statistical analysis
The primary outcome measure, percent correct intelligible scripted words, was analyzed using change scores, defined as the difference between the average of the last two pretreatment probes relative to the average from the first two post-treatment probes for four trained scripts. Generalization was captured using change scores from pre- to post-treatment on two matched, untrained scripts and the Northwestern Anagram Test (NAT), an assessment of syntactic production.69 Changes in overall language profile were examined via WAB AQ scores. Change scores (the difference between performance at 3-, 6-, and 12-month follow-up assessments relative to post-treatment) for trained scripts, untrained scripts, the NAT, and the WAB AQ were also calculated in order to examine maintenance of treatment gains and stability of overall language profile over time.
As with the LRT data, the distribution of the data failed to meet normality assumptions, so change scores were analyzed using nonparametric permutation tests. Permutation tests for paired data were used to examine performance across time points (for more specific details on paired samples analyses, refer to Henry et al48 and Table S3). In the current study, the critical comparison was between the group of participants who received in-person treatment and those who received treatment via teletherapy. As with LRT analyses, for these between-groups comparisons, two-tailed permutation tests for independent samples were utilized and correction for multiple comparisons was not implemented.
VISTA: results
Treatment effects following VISTA
Group mean performance on all pre-treatment, post-treatment, and follow-up measures is presented in Figure 6. As reported in Henry et al,48 analyses of group data revealed a significant improvement in the production of correct, intelligible scripted words for trained scripts relative to pre-treatment, with gains maintained up to 1-year follow-up. No significant changes were observed on the NAT or WAB AQ. Despite significant declines in performance relative to post-treatment, no measures showed a significant decline in performance relative to pre-treatment at any time point.
Figure 6.
Mean performance on pre-treatment, post-treatment, and follow-up measures for VISTA participants.
Note: 95% confidence intervals around the mean were derived using n=1,000 bootstrapped samples.
Abbreviations: VISTA, video-implemented script training for aphasia; NAT, Northwestern Anagram Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
VISTA treatment effects for in-person treatment vs teletherapy
Mean performance on all pre-treatment and post-treatment measures by treatment administration type is presented in Tables 2 and 6 and Figure 7. In order to determine whether patient characteristics, including severity of deficits, were matched at pre-treatment between the two groups, we compared age, education, and pre-treatment NAT, MMSE, WAB AQ and a measure of apraxia of speech and dysarthria severity (motor speech evaluation)70 between the in-person and teletherapy groups. The two groups were not significantly different with regard to age (t=−0.05, P=1.000, 95% CI [−6.40, 6.80]), education (t=0.00, P=1.000, 95% CI [-2.40, 2.40]), pre-treatment NAT (t=−0.63, P=0.548, 95% CI [−17.81, 38.43]), pre-treatment MMSE (t=−1.14, P=0.310, 95% CI [−1.20, 4.40]), pre-treatment WAB AQ (t=−0.65, P=0.540, 95% CI [−4.76, 10.20]), or apraxia/dysarthria severity (t=−0.38, P=0.873, 95% CI [−2.40, 3.60]).
Table 6.
Mean performance on trained and untrained scripts (SDs) for VISTA participants by treatment type
| Pre-treatment, in-person (n=5) | Pre-treatment, teletherapy (n=5) | Post-treatment, in-person (n=5) | Post-treatment, teletherapy (n=5) | 3-month follow-up, in-person (n=5) | 3-month follow-up, teletherapy (n=5) | 6-month follow-up, in-person (n=5) | 6-month follow-up, teletherapy (n=5) | 12-month follow-up, in-person (n=5) | 12-month follow-up, teletherapy (n=4)a | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Trained scripts (%) | 40.3 (8.4) | 35.8 (18.4) | 88.3 (18.3) | 91.3 (12.0) | 84.3 (25.3) | 90.8 (13.9) | 72.6 (35.2) | 87.2 (17.1) | 54.3 (39.9) | 72.6 (48.0) |
| Untrained scripts (%) | 37.5 (7.2) | 34.7 (20.7) | 41.4 (16.3) | 44.7 (24.8) | 28.6 (15.2) | 42.7 (22.9) | 18.7 (13.0) | 41.4 (20.6) | 20.7 (15.8) | 39.7 (38.3) |
Note:
Differences in n as not all participants had completed all assessments or due to missing data.
Abbreviation: VISTA, video-implemented script training for aphasia.
Figure 7.

Mean pre- to post-treatment change scores (post minus pre) for participants completing VISTA in person or via teletherapy.
Notes: Positive values indicate higher scores at post-treatment than pre-treatment. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: VISTA, video-implemented script training for aphasia; NAT, Northwestern Anagram Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
We compared change scores from pre-treatment to post-treatment between the in-person and teletherapy groups. No significant differences were observed for pre– post change scores for trained scripts (in-person M=48.0%, teletherapy M=55.5%; t=−0.61, P=0.587, 95% CI [−14.36, 29.30]), untrained scripts (in-person M=4.0%, teletherapy M=10.1%; t=-0.71, P=0.500, 95% CI [−9.44, 21.63]), the NAT (in-person M=15.8%, teletherapy M=7.0%; t=0.79, P=0.444, 95% CI [−29.72, 10.41]), or the WAB AQ (in-person M=0.9, teletherapy M=1.9; t=−0.57, P=0.556, 95% CI [−1.98, 4.10]).
Maintenance of treatment gains following VISTA for in-person treatment vs teletherapy
Group mean performance on all post-treatment and follow-up measures is presented in Tables 2 and 6. Maintenance of gains was examined by comparing changes from post-treatment to follow-up between the in-person and teletherapy groups. Figure 8 presents mean change scores from post-treatment to 3-month follow-up by treatment type. No significant differences were observed between the two groups for trained scripts (in-person M=-4.0%, teletherapy M=-0.48%; t=−0.89, P=0.452, 95% CI [−3.37, 10.49]), untrained scripts (in-person M=−12.9%, teletherapy M=−2.1%; t=−1.86, P=0.095, 95% CI [−1.20, 22.80]), the NAT (in-person M=−6.0%, teletherapy M=−6.7%; t=0.04, P=0.992, 95% CI [−29.33, 28.00]), or the WAB AQ (in-person M=-4.9, teletherapy M=−2.8; t=−0.83, P=0.532, 95% CI [−2.04, 6.32]).
Figure 8.
Mean change scores from post-treatment to 3-month follow-up (3 months minus post) for participants completing VISTA in-person or via teletherapy.
Notes: Negative values indicate higher scores at post-treatment than 3-month follow-up. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: VISTA, video-implemented script training for aphasia; NAT, Northwestern Anagram Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
Mean change scores from post-treatment to 6-month follow-up were also compared between the groups (Figure 9). There were no significant differences between the in-person and teletherapy groups on trained scripts (in-person M=−15.7%, teletherapy M=−4.1%; t=−1.28, P=0.262, 95% CI [−4.39, 27.68]), the NAT (in-person M=-32.0%, teletherapy M=−5.0%; t=−1.79, P=0.151, 95% CI [−6.67, 60.65]), or the WAB AQ (in-person M=−7.1, teletherapy M=−4.4; t=−0.70, P=0.516, 95% CI [−4.41, 9.85]). However, there was a significant difference for untrained scripts, where the in-person group (M=−22.7%) declined significantly more than the teletherapy group (M=−3.3%; t=−4.30, P=0.008, 95% CI [5.45, 33.32]).
Figure 9.
Mean change scores from post-treatment to 6-month follow-up (6 months minus post) for participants completing VISTA in-person or via teletherapy.
Notes: Negative values indicate higher scores at post-treatment than 6-month follow-up. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: VISTA, video-implemented script training for aphasia; NAT, Northwestern Anagram Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
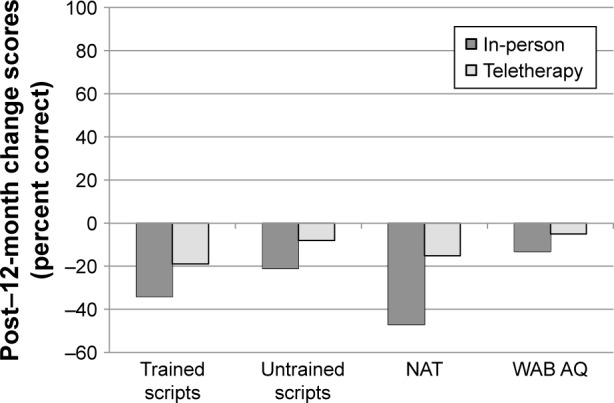
Finally, mean change scores from post-treatment to 12-month follow-up were compared between the groups (Figure 10). As at 3-month follow-up, no significant differences were observed between the two groups for trained scripts (in-person M=-34.0%, teletherapy M=-18.7%; t=−0.66, P=0.524, 95% CI [−23.85, 57.02]), untrained scripts (in-person M=−20.7%, teletherapy M=−8.4%; t=−1.03, P=0.349, 95% CI [−8.81, 32.94]), the NAT (in-person M=−46.7%, teletherapy M=−14.8%; t=−1.47, P=0.257, 95% CI [−4.26, 68.09]), or the WAB AQ (in-person M=−13.3, teletherapy M=−5.4; t=−1.59, P=0.175, 95% CI [−1.56, 17.88]).
Figure 10.
Mean change scores from post-treatment to 12-month follow-up (12 months minus post) for participants completing VISTA in-person or via teletherapy.
Notes: Negative values indicate higher scores at post-treatment than 12-month follow-up. Error bars are not presented as significance was derived via permutation tests. The 95% CIs obtained via permutation are presented in the text.
Abbreviations: VISTA, video-implemented script training for aphasia; NAT, Northwestern Anagram Test; WAB AQ, Western Aphasia Battery Aphasia Quotient.
Discussion
There is a paucity of research comparing treatment outcomes in traditional, in-person treatment vs teletherapy for individuals with PPA. In the current study, we investigated the utility of teletherapy for the delivery of intervention to individuals with PPA using a lexical retrieval training protocol (LRT) and a script training protocol (VISTA). A total of 31 participants underwent treatment, with protocol assignment based on the core speech and language deficits associated with their PPA subtype. LRT was implemented for individuals with svPPA or lvPPA, for whom naming impairment is a core feature. VISTA was implemented for individuals with nfvPPA, who show deficits in speech production and fluency. Within each treatment protocol, one group of participants received treatment via teletherapy, while the other group received treatment via traditional, in-person treatment sessions.
Overall, both treatment approaches (ie, LRT and VISTA) resulted in significant gains from pre-treatment to post-treatment for trained word sets and scripts, respectively. Moreover, performance on trained sets and scripts was significantly better than pre-treatment at all follow-up visits. In general, both interventions had a selective treatment effect on the linguistic skills targeted by the protocol (ie, word retrieval in LRT and fluency/grammar in VISTA).
Our primary question for this study was whether the magnitude of change following treatment was comparable for treatment administered in-person vs that administered via teletherapy. Research in stroke-induced aphasia has suggested that treatment outcomes between teletherapy and traditional in-person treatment are equivalent.50,51 In PPA, teletherapy has also been shown to be efficacious,42,61 with gains comparable to those observed for treatment administered in person.42 The current study sought to extend these findings to a larger, matched sample of individuals with PPA using LRT and VISTA treatment protocols and to examine the generalization and maintenance of gains up to 12 months post-treatment. We predicted that outcomes of teletherapy and treatment delivered via traditional means would be comparable.
As predicted, analyses revealed that, regardless of treatment approach (LRT or VISTA), treatment effects and generalization to untrained items and standardized tests in participants receiving teletherapy were comparable to those observed for participants receiving traditional, in-person treatment. In the few instances where there were significant differences or a trend toward a significant difference, the teletherapy group performed better. Thus, there is no indication that teletherapy is less effective than in-person treatment.
Observed maintenance of gains and/or decline in abilities over time was also comparable across treatment delivery modalities for both treatment protocols. Again, there were a few instances where there were significant differences or a trend toward a significant difference, but in each of these cases, the teletherapy group demonstrated numerically better outcomes. Overall, these findings indicate that teletherapy is a feasible and effective method for delivering intervention for individuals with PPA, as it offers treatment benefits comparable to those observed following traditional treatment.
This study provides the strongest evidence thus far for the utility of teletherapy as a treatment delivery medium in PPA, a patient population that is widely considered to be underserved by speech-language pathologists.3 To date, teletherapy has not been thoroughly investigated in patients with this clinical diagnosis, despite related work in stroke-induced aphasia demonstrating the feasibility of teletherapy for addressing neurogenic speech and language disorders. Furthermore, this study explored teletherapy outcomes for a treatment protocol targeting linguistic skills other than lexical retrieval (ie, speech production and fluency). Interventions for naming have been the focus of most in-person PPA treatment studies as well as the one previous study that compared traditional vs teletherapy outcomes in PPA.42 As such, our findings broaden the evidence base regarding both treatment for PPA more generally, as well as the utility of teletherapy across treatment paradigms.
Special considerations/suggestions
In this study, we document the benefits of treatment delivered via teletherapy for individuals with each of the clinical variants of PPA. Whereas teletherapy resulted in comparable gains relative to traditional treatment, we acknowledge that this mode of treatment delivery is not appropriate for all individuals or all treatment techniques. First, it is worth noting that our study was restricted to participants with mild-to-moderate cognitive and linguistic deficits and, thus, we are unable to address the utility of teletherapy for more cognitively, motorically, or linguistically impaired individuals. The benefits of teletherapy for more severely impaired patients may be limited given the increased technical and cognitive-linguistic demands imposed by this medium. Given the degraded auditory and visual signal afforded by teleconference relative to face-to-face interaction, patient factors such as visual or hearing acuity deficits and linguistic comprehension deficits may be limiting or even exclusionary characteristics. An additional consideration concerns individuals with motoric impairments that may impact the ability to interact with or navigate a computer. Limb apraxia, for example, may hinder an individual’s ability to appropriately use the computer mouse and keyboard. Furthermore, concomitant cognitive impairments may affect an individual’s ability to participate in and benefit from teletherapy. For example, executive function deficits, which have been documented as emergent features with disease progression in PPA,71–73 may reduce an individual’s ability to maintain focused attention during treatment sessions. It is important that clinicians assess, on an individual basis, the degree to which concomitant deficits (eg, hearing acuity, auditory comprehension, visual processing, motor functions, and executive functioning) may mitigate the benefits of teletherapy.
We note, however, that the positive outcomes observed in the current study were not constrained by PPA subtype. While clinical variant was not an explicit variable manipulated or analyzed in our study, teletherapy and in-person treatment groups included participants from all three clinical phenotypes, suggesting that speech–language profile is not a limiting factor, per se. Furthermore, our findings indicate that both linguistic (lexical retrieval and grammar) and motoric (speech production) deficits are amenable to treatment via teletherapy. This finding has potential implications for the use of telemedicine in the treatment of cognitive-linguistic and motoric impairments in other neurodegenerative disorders, including Alzheimer’s and other dementias, as well as degenerative movement disorders that affect speech production, such as Parkinson’s disease.74,75 Of note, telemedicine has been shown to be feasible for use in diagnostic evaluations,76–79 as well as behavioral interventions,52,80 in dementia. More research is needed to explore the possible extension of teletherapy to individuals with severe deficits and to individuals with neurodegenerative disorders beyond PPA.
The LRT and VISTA approaches that we utilized were both easily adapted for and implemented via videoconferencing software. Other treatment approaches may not be as easily adaptable to implementation via teletherapy due to the nature of the training tasks or the specific skills being targeted. For example, interventions training augmentative and alternative communication (AAC) may be difficult to translate to a teletherapy context due to the need for hands-on demonstration of communication strategies or device use. If new treatment approaches are to be implemented via teletherapy, it will be important to determine the feasibility of telepractice implementation on a case-by-case basis. Future studies should address the potential adaptation of treatment protocols for administration to participants with more severe deficits as well as expansion of the range of targeted interventions.
It is important that practicing clinicians consider regulations governing administration and billing for teletherapy services within and across states and internationally. At the time of writing, many states (eg, Arkansas, California, Iowa, New York, and Texas) require that speech–language pathologists have licensure to administer treatment in the state where the patient resides; by contrast, other states (eg, Arizona, New Jersey, Maine, and Vermont) have not implemented any special regulations for teletherapy. Additionally, Medicare does not currently cover costs of speech and language treatment administered via teletherapy.
Limitations
A limitation of the current study concerns the small number of participants. Although comparable treatment effects, generalization of gains, and maintenance effects were observed for teletherapy and in-person treatment platforms, it is possible that there was not enough power to detect significant differences between the two treatment groups, although the use of permutation tests to analyze group mean differences somewhat mitigates this concern. Even so, the results are promising and warrant further consideration. Replication of this study in a larger sample of individuals with PPA would offer confirmatory evidence regarding the relative benefits of treatment administered in each delivery medium. In addition, although comparable outcomes were observed between the in-person and teletherapy groups, no information was collected regarding acceptability of mode of treatment delivery. In future research, qualitative information should be collected to assess participants’ views regarding receiving treatment via teletherapy.
Another limitation stems from the fact that participants were not randomly selected to receive treatment via video-conference or in person; instead, participant needs dictated group membership, as would be the case in actual clinical practice. Furthermore, as assessments were delivered in the same mode as treatment, test delivery was not blind to group assignment. Finally, a potential confound in interpreting the outcomes of this study is that the LRT participants who received treatment via teletherapy were significantly younger than those who received treatment in person. However, research suggests that age does not significantly affect response to treatment delivered in-person81 or performance on cognitive-linguistic assessments delivered via telepractice.82 Moreover, teletherapy participants were not significantly different from in-person participants in terms of baseline cognitive-linguistic abilities (MMSE, BNT, or WAB-AQ), indicating that the two groups were matched for pre-treatment severity of deficit. For these reasons, it is unlikely that participant’s age significantly influenced treatment response in the teletherapy group.
Conclusion
Our research supports the use of teletherapy as a feasible and efficacious method of speech–language treatment delivery for individuals with PPA. Teletherapy has already begun to emerge as a viable treatment option for various speech and language disorders, including stroke-induced aphasia. The advantages of teletherapy for promoting the accessibility of treatment will prove especially beneficial in populations who have been historically underserved due to patient factors (cognitive or motoric limitations) or lack of access to specialists due to geographical constraints. As research continues to bolster the evidence base in favor of teletherapy as a viable alternative to traditional treatment delivery models, there will be real potential to expand the reach of clinical services for patients with PPA and other neurodegenerative disorders.
Supplementary materials
Table S1.
Demographic characteristics and change scores for trained items for LRT-1 and LRT-2 participants
| Variable | LRT-1 (n=10) | LRT-2 (n=11) | T | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 63.2 (8.2) | 67.6 (7.0) | 1.33 | 0.200 |
| Education | 17.3 (2.6) | 17.9 (3.3) | 0.47 | 0.666 |
| MMSE | 24.9 (4.2) | 23.6 (3.8) | −0.77 | 0.455 |
| Pre–post change score (%) trained items | 80.5 (13.6) | 69.8 (26.3) | −1.19 | 0.861 |
| 3-month follow-up vs post-treatment change score (%) trained items | −7.8 (12.7) | −3.5 (19.4) | 0.57 | 0.346 |
| 6-month follow-up vs post-treatment change score (%) trained items | −6.7 (11.5) | −3.5 (9.3) | 0.65 | 0.258 |
| 12-month follow-up vs post-treatment change score (%) trained items | −18.3 (27.5) | −20.0 (17.9) | −0.16 | 0.581 |
Notes: Two-tailed independent samples permutation tests were used for demographic comparisons. Because there were specific predictions for LRT-1 vs LRT-2 for trained items (ie, that increased dosage would lead to larger gains), one-tailed tests were used for these comparisons. Data shown as mean (SD). LRT-1: lexical retreival treatment as implemented by Henry et al2 (2013); LRT-2 a modified version of lexical retrieval treatment with dosage modifications. Details provided in full text.
Abbreviations: LRT, lexical retrieval treatment; MMSE, Mini–Mental State Examination.
Table S2.
Results of paired-samples permutation tests comparing treatment outcome measures and standardized test scores at each time point relative to pre-treatment and post-treatment for participants who underwent LRT
| Pre-treatment performance vs | Post-treatment performance vs | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | |
|
| |||||||
| Trained | |||||||
| n | 21 | 18 | 18 | 19 | 18 | 18 | 19 |
| t | −16.00 | −11.92 | −18.22 | −7.98 | 1.40 | 2.10 | 3.76 |
| P-value | <0.001* | <0.001* | <0.001* | <0.001* | 0.071 | 0.264 | 0.037 |
|
| |||||||
| Untrained | |||||||
| n | 21 | 18 | 18 | 19 | 18 | 18 | 19 |
| t | −3.55 | −3.43 | −2.92 | −1.45 | 0.00 | 0.34 | 1.81 |
| P-value | <0.001* | 0.002* | 0.007* | 0.189 | 1.00 | 0.784 | 0.095 |
|
| |||||||
| BNT | |||||||
| n | 21 | 17 | 16 | 16 | 18 | 16 | 17 |
| t | −1.78 | 1.00 | 2.60 | 3.27 | 1.79 | 4.17 | 3.93 |
| P-value | 0.010* | 0.448 | 0.061 | 0.061 | 0.264 | 0.013* | 0.031 |
|
| |||||||
| WAB | |||||||
| n | 21 | 18 | 18 | 17 | 18 | 18 | 17 |
| t | 0.36 | 2.68 | 3.40 | 5.23 | 3.48 | 3.63 | 5.45 |
| P-value | 0.164 | 0.071 | 0.016 | <0.001* | 0.016* | 0.003* | <0.001* |
Notes: One-tailed tests were used for trained items for all pre-treatment comparisons as well as for the pre–post comparison for untrained items. All other comparisons utilized two-tailed tests. Bonferroni-corrected thresholds for significance: P<0.013 for comparisons to pre-treatment performance and P<0.017 for comparisons relative to post-treatment performance. Analyses were conducted using the exactRankTests package1 in R (Version 3.3.1; 2016).
Significance at Bonferroni-corrected P-value <0.05.
Abbreviations: BNT, Boston Naming Test; LRT, lexical retrieval treatment; WAB, Western Aphasia Battery.
Table S3.
Results of two-tailed paired-samples permutation tests comparing treatment outcome measures and standardized test scores at each time point relative to post-treatment for participants who underwent VISTA
| Post-treatment performance vs
|
|||
|---|---|---|---|
| 3-month follow-up | 6-month follow-up | 12-month follow-up | |
|
| |||
| Trained | |||
| n | 10 | 10 | 9 |
| t | 1.14 | 2.10 | 2.13 |
| P-value | 0.680 | 0.012* | 0.086 |
|
| |||
| Untrained | |||
| n | 10 | 10 | 9 |
| t | 2.27 | 3.36 | 2.33 |
| P-value | 0.184 | 0.012* | 0.023 |
|
| |||
| NAT | |||
| n | 10 | 10 | 8 |
| t | 0.79 | 2.20 | 2.62 |
| P-value | 0.184 | 0.680 | 0.672 |
|
| |||
| WAB | |||
| n | 10 | 10 | 9 |
| t | 3.16 | 3.03 | 3.45 |
| P-value | 0.004* | 0.043 | 0.008* |
Notes: Bonferroni-corrected threshold for significance was P<0.017. Analyses were conducted using the exactRankTests package1 in R (Version 3.3.1; 2016).
Significance at Bonferroni-corrected P-value <0.05.
Abbreviations: NAT, Northwestern Anagram Test; VISTA, video-implemented script training for aphasia; WAB, Western Aphasia Battery.
References
- 1.Hothorn T, Hornik K. exactRankTests: exact distributions for rank and permutation tests. R package Version 0.8-17. 2006. [Accessed November 16, 2018]. Available from: http://CRAN.R-project.org/package=exactRankTests.
- 2.Henry ML, Rising K, Demarco AT, Miller BL, Gorno-Tempini ML, Beeson PM. Examining the value of lexical retrieval treatment in primary progressive aphasia: two positive cases. Brain Lang. 2013;127(2):145–156. doi: 10.1016/j.bandl.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
We wish to the thank members of the University of Texas at Austin Aphasia Research and Treatment Lab and the University of California, San Francisco, Memory and Aging Center who contributed to patient assessment and care. We also thank Gregory Hixon for statistical consultation. Finally, we wish to thank all of our participants with PPA and their families for the time and effort that they have devoted to our research. This work was funded by grants from the National Institutes of Health (NIDCD R01 DC016291 and NIDCD R03 DC013403 to MLH and NINDS R01 NS050915 and NIDCD K24 DC015544 to MLGT) and the Darrell K Royal Research Fund for Alzheimer’s Disease (to MLH).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry ML, Meese MV, Truong S, Babiak MC, Miller BL, Gorno-Tempini ML. Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behav Neurol. 2013;26(1–2):77–88. doi: 10.3233/BEN-2012-120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor C, Kingma RM, Croot K, Nickels L. Speech pathology services for primary progressive aphasia: exploring an emerging area of practice. Aphasiology. 2009;23(2):161–174. [Google Scholar]
- 4.Kertesz A, Blair M, Mcmonagle P, Munoz DG. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2007;21(2):155–163. doi: 10.1097/WAD.0b013e31806547eb. [DOI] [PubMed] [Google Scholar]
- 5.Le Rhun E, Richard F, Pasquier F. Natural history of primary progressive aphasia. Neurology. 2005;65(6):887–891. doi: 10.1212/01.wnl.0000175982.57472.84. [DOI] [PubMed] [Google Scholar]
- 6.Rogalski EJ, Mesulam MM. Clinical trajectories and biological features of primary progressive aphasia (PPA) Curr Alzheimer Res. 2009;6(4):331–336. doi: 10.2174/156720509788929264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapolsky D, Domoto-Reilly K, Negreira A, Brickhouse M, Mcginnis S, Dickerson BC. Monitoring progression of primary progressive aphasia: current approaches and future directions. Neurodegener Dis Manag. 2011;1(1):43–55. [Google Scholar]
- 8.Rutherford S. Our journey with primary progressive aphasia. Aphasiology. 2014;28(8–9):900–908. [Google Scholar]
- 9.Henry ML, Rising K, Demarco AT, Miller BL, Gorno-Tempini ML, Beeson PM. Examining the value of lexical retrieval treatment in primary progressive aphasia: two positive cases. Brain Lang. 2013;127(2):145–156. doi: 10.1016/j.bandl.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- 11.Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsychol Soc. 2009;15(5):645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol. 2010;23(6):633–637. doi: 10.1097/WCO.0b013e32833fb93e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry ML, Wilson SM, Babiak MC, et al. Phonological processing in primary progressive aphasia. J Cogn Neurosci. 2016;28(2):210–222. doi: 10.1162/jocn_a_00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci. 2001;25:663–678. [Google Scholar]
- 15.Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(Pt 7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6(2):78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- 17.Friederici AD, Rüschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 18.Croot K, Nickels L, Laurence F, Manning M. Impairment- and activity/participation-directed interventions in progressive language impairment: clinical and theoretical issues. Aphasiology. 2009;23(2):125–160. [Google Scholar]
- 19.Jokel R, Graham NL, Rochon E, Leonard C. Word retrieval therapies in primary progressive aphasia. Aphasiology. 2014;28(8–9):1038–1068. [Google Scholar]
- 20.Kortte KB, Rogalski EJ. Behavioural interventions for enhancing life participation in behavioural variant frontotemporal dementia and primary progressive aphasia. Int Rev Psychiatry. 2013;25(2):237–245. doi: 10.3109/09540261.2012.751017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rising K. Treatment for lexical retrieval in primary progressive aphasia. Perspect Neurophysiol Neurogenic Speech Lang Disord. 2014;24(4):137–144. [Google Scholar]
- 22.Tippett DC, Hillis AE, Tsapkini K. Treatment of primary progressive aphasia. Curr Treat Options Neurol. 2015;17(8):362. doi: 10.1007/s11940-015-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beales A, Cartwright J, Whitworth A, Panegyres PK. Exploring generalisation processes following lexical retrieval intervention in primary progressive aphasia. Int J Speech Lang Pathol. 2016;18(3):299–314. doi: 10.3109/17549507.2016.1151936. [DOI] [PubMed] [Google Scholar]
- 24.Jokel R, Anderson ND. Quest for the best: effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychol Rehabil. 2012;22(2):187–214. doi: 10.1080/09602011.2011.639626. [DOI] [PubMed] [Google Scholar]
- 25.Jokel R, Rochon E, Anderson ND. Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychol Rehabil. 2010;20(1):16–41. doi: 10.1080/09602010902879859. [DOI] [PubMed] [Google Scholar]
- 26.Mayberry EJ, Sage K, Ehsan S, Ralph MA. Relearning in semantic dementia reflects contributions from both medial temporal lobe episodic and degraded neocortical semantic systems: evidence in support of the complementary learning systems theory. Neuropsychologia. 2011;49(13):3591–3598. doi: 10.1016/j.neuropsychologia.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Meyer AM, Faria AV, Tippett DC, Hillis AE, Friedman RB. The relationship between baseline volume in temporal areas and post-treatment naming accuracy in primary progressive aphasia. Aphasiology. 2017;31(9):1059–1077. doi: 10.1080/02687038.2017.1296557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newhart M, Davis C, Kannan V, Heidlergary J, Cloutman L, Hillis AE. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7–8):823–834. [Google Scholar]
- 29.Savage SA, Ballard KJ, Piguet O, Hodges JR. Bringing words back to mind – Improving word production in semantic dementia. Cortex. 2013;49(7):1823–1832. doi: 10.1016/j.cortex.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Savage SA, Piguet O, Hodges JR. Cognitive intervention in semantic dementia: maintaining words over time. Alzheimer Dis Assoc Disord. 2015;29(1):55–62. doi: 10.1097/WAD.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 31.Snowden JS, Neary D. Relearning of verbal labels in semantic dementia. Neuropsychologia. 2002;40(10):1715–1728. doi: 10.1016/s0028-3932(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 32.Bier N, Macoir J, Gagnon L, van der Linden M, Louveaux S, Desrosiers J. Known, lost, and recovered: efficacy of formal-semantic therapy and spaced retrieval method in a case of semantic dementia. Aphasiology. 2009;23(2):210–235. [Google Scholar]
- 33.Dressel K, Huber W, Frings L, et al. Model-oriented naming therapy in semantic dementia: a single-case fMRI study. Aphasiology. 2010;24(12):1537–1558. [Google Scholar]
- 34.Graham KS, Patterson K, Pratt KH, Hodges JR. Relearning and subsequent forgetting of semantic category exemplars in a case of semantic dementia. Neuropsychology. 1999;13(3):359–380. doi: 10.1037//0894-4105.13.3.359. [DOI] [PubMed] [Google Scholar]
- 35.Graham KS, Patterson K, Pratt KH, Hodges JR. Can repeated exposure to “forgotten” vocabulary help alleviate word-finding difficulties in semantic dementia? An illustrative case study. Neuropsychol Rehabil. 2001;11(3–4):429–454. [Google Scholar]
- 36.Henry ML, Beeson PM, Rapcsak SZ. Treatment for lexical retrieval in progressive aphasia. Aphasiology. 2008;22(7–8):826–838. doi: 10.1080/02687030701820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry MH, Hubbard HI, Grasso SM, et al. J Speech Lang Hear Res. 2019. Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: immediate and long-term outcomes. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heredia CG, Sage K, Ralph MAL, Berthier ML. Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology. 2009;23(2):192–209. [Google Scholar]
- 39.Jokel R, Rochon E, Leonard C. Treating anomia in semantic dementia: improvement, maintenance, or both? Neuropsychol Rehabil. 2006;16(3):241–256. doi: 10.1080/09602010500176757. [DOI] [PubMed] [Google Scholar]
- 40.Croot K, Taylor C, Abel S, et al. Measuring gains in connected speech following treatment for word retrieval: a study with two participants with primary progressive aphasia. Aphasiology. 2015;29(11):1265–1288. [Google Scholar]
- 41.Meyer AM, Snider SF, Eckmann CB, Friedman RB. Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: cross-language transfer. Aphasiology. 2015;29(9):1062–1081. doi: 10.1080/02687038.2015.1028327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer AM, Getz HR, Brennan DM, Hu TM, Friedman RB. Telerehabilitation of anomia in primary progressive aphasia. Aphasiology. 2016;30(4):483–507. doi: 10.1080/02687038.2015.1081142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jokel R, Cupit J, Rochon E, Leonard C. Relearning lost vocabulary in nonfluent progressive aphasia with MossTalk Words®. Aphasiology. 2009;23(2):175–191. [Google Scholar]
- 44.Marcotte K, Ansaldo AI. The neural correlates of semantic feature analysis in chronic aphasia: discordant patterns according to the etiology. Semin Speech Lang. 2010;31(1):52–63. doi: 10.1055/s-0029-1244953. [DOI] [PubMed] [Google Scholar]
- 45.Hameister I, Nickels L, Abel S, Croot K. “Do you have mowing the lawn?” – improvements in word retrieval and grammar following constraint-induced language therapy in primary progressive aphasia. Aphasiology. 2017;31(3):308–331. [Google Scholar]
- 46.Schneider SL, Thompson CK, Luring B. Effects of verbal plus gestural matrix training on sentence production in a patient with primary progressive aphasia. Aphasiology. 1996;10(3):297–317. [Google Scholar]
- 47.Louis M, Espesser R, Rey V, Daffaure V, di Cristo A, Habib M. Intensive training of phonological skills in progressive aphasia: a model of brain plasticity in neurodegenerative disease. Brain Cogn. 2001;46(1–2):197–201. doi: 10.1016/s0278-2626(01)80065-8. [DOI] [PubMed] [Google Scholar]
- 48.Henry ML, Hubbard HI, Grasso SM, et al. Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain. 2018;141(6):1799–1814. doi: 10.1093/brain/awy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards M, Stredler-Brown A, Houston KT. Expanding use of tele-practice in speech-language pathology and audiology. Volta Rev. 2012;112(3):227–242. [Google Scholar]
- 50.Hall N, Boisvert M, Steele R. Telepractice in the assessment and treatment of individuals with aphasia: a systematic review. Int J Telerehabil. 2013;5(1):27–38. doi: 10.5195/ijt.2013.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherney LR, van Vuuren S. Telerehabilitation, virtual therapists, and acquired neurologic speech and language disorders. Semin Speech Lang. 2012;33(3):243–258. doi: 10.1055/s-0032-1320044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jelcic N, Agostini M, Meneghello F, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: a pilot study. Clin Interv Aging. 2014;9(9):1605–1611. doi: 10.2147/CIA.S68145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agostini M, Garzon M, Benavides-Varela S, et al. Telerehabilitation in poststroke anomia. Biomed Res Int. 2014;2014:1–6. doi: 10.1155/2014/706909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dechêne L, Tousignant M, Boissy P, et al. Simulated in-home teletreatment for anomia. Int J Telerehabil. 2011;3(2):3–10. doi: 10.5195/IJT.2011.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furnas DW, Edmonds LA. The effect of computerised Verb Network Strengthening Treatment on lexical retrieval in aphasia. Aphasiology. 2014;28(4):401–420. [Google Scholar]
- 56.Fridler N, Rosen K, Menahemi-Falkov M, et al. Tele-rehabilitation therapy vs face-to-face therapy for aphasic patients. ETELEMED 2012: The Fourth International Conference on EHealth, Telemedicine, and Social Medicine; Valencia: IARIA; 2012. pp. 18–23. [Google Scholar]
- 57.Woolf C, Caute A, Haigh Z, et al. A comparison of remote therapy, face to face therapy and an attention control intervention for people with aphasia: a quasi-randomised controlled feasibility study. Clin Rehabil. 2016;30(4):359–373. doi: 10.1177/0269215515582074. [DOI] [PubMed] [Google Scholar]
- 58.Kertesz A. Western Aphasia Battery. Orlando, FL: Grune and Stratton; 1982. [Google Scholar]
- 59.Goldberg S, Haley KL, Jacks A. Script Training and Generalization for People With Aphasia. Am J Speech Lang Pathol. 2012;21(3):222. doi: 10.1044/1058-0360(2012/11-0056). [DOI] [PubMed] [Google Scholar]
- 60.Lasker JP, Stierwalt JAG, Spence M, Cavin-Root C. Using webcam interactive technology to implement treatment for severe apraxia: a case example. J Med Speech-Lang Pathol. 2010;18(4):71–76. [Google Scholar]
- 61.Rogalski EJ, Saxon M, Mckenna H, et al. Communication Bridge: a pilot feasibility study of Internet-based speech-language therapy for individuals with progressive aphasia. Alzheimers Dement. 2016;2(4):213–221. doi: 10.1016/j.trci.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 63.Beeson PM, Egnor H. Combining treatment for written and spoken naming. J Int Neuropsychol Soc. 2006;12(6):816–827. doi: 10.1017/S1355617706061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan E, Goodglass H, Weintraub S. In: Boston Naming Test. Febiger L, editor. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 65.Kertesz A, Raven JC. In: WAB-R: Western Aphasia Battery-Revised. Firm P, editor. San Antonio, TX: PsychCorp; 2007. [Google Scholar]
- 66.Cherney LR, Halper AS, Holland AL, Cole R. Computerized script training for aphasia: preliminary results. Am J Speech Lang Pathol. 2008;17(1):19–34. doi: 10.1044/1058-0360(2008/003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Youmans G, Holland A, Muñoz M, Bourgeois M. Script training and automaticity in two individuals with aphasia. Aphasiology. 2005;19(3–5):435–450. [Google Scholar]
- 68.Fridriksson J, Hubbard HI, Hudspeth SG, et al. Speech entrainment enables patients with Broca’s aphasia to produce fluent speech. Brain. 2012;135(Pt 12):3815–3829. doi: 10.1093/brain/aws301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24(5):408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of Speech in Adults: The Disorder and Its Management. Orlando, FL: Grune and Stratton; 1984. [Google Scholar]
- 71.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 72.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhee J, Antiquena P, Grossman M. Verb comprehension in frontotemporal degeneration: the role of grammatical, semantic and executive components. Neurocase. 2001;7(2):173–184. doi: 10.1093/neucas/7.2.173. [DOI] [PubMed] [Google Scholar]
- 74.Constantinescu G, Theodoros D, Russell T, Ward E, Wilson S, Wootton R. Treating disordered speech and voice in Parkinson’s disease online: a randomized controlled non-inferiority trial. Int J Lang Commun Disord. 2011;46(1):1–16. doi: 10.3109/13682822.2010.484848. [DOI] [PubMed] [Google Scholar]
- 75.Constantinescu G, Russell T, Ward E, Wilson S, Wootton R. Treating the speech disorder in Parkinson’s disease online. J Telemed Telecare. 2006;12(Suppl 3):S3:88–S3:91. [Google Scholar]
- 76.Barton C, Morris R, Rothlind J, Yaffe K. Video-telemedicine in a memory disorders clinic: evaluation and management of rural elders with cognitive impairment. Telemed J E Health. 2011;17(10):789–793. doi: 10.1089/tmj.2011.0083. [DOI] [PubMed] [Google Scholar]
- 77.Cullum CM, Weiner MF, Gehrmann HR, Hynan LS. Feasibility of telecognitive assessment in dementia. Assessment. 2006;13(4):385–390. doi: 10.1177/1073191106289065. [DOI] [PubMed] [Google Scholar]
- 78.Martin-Khan M, Flicker L, Wootton R, et al. The diagnostic accuracy of telegeriatrics for the diagnosis of dementia via video conferencing. J Am Med Dir Assoc. 2012;13(5):487.e19–e24. doi: 10.1016/j.jamda.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 79.Vestal L, Smith-Olinde L, Hicks G, Hutton T, Hart J. Efficacy of language assessment in Alzheimer’s disease: comparing in-person examination and telemedicine. Clin Interv Aging. 2006;1(4):467–471. doi: 10.2147/ciia.2006.1.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cotelli M, Manenti R, Brambilla M, et al. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer’s disease and frontotemporal dementia: a systematic review. J Telemed Telecare. 2017 Jan 1; doi: 10.1177/1357633X17740390. Epub. [DOI] [PubMed] [Google Scholar]
- 81.de Riesthal M, Wertz R. Prognosis for aphasia: relationship between selected biographical and behavioural variables and outcome and improvement. Aphasiology. 2004;18(10):899–915. [Google Scholar]
- 82.Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147–154. doi: 10.1089/tmj.2004.10.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Demographic characteristics and change scores for trained items for LRT-1 and LRT-2 participants
| Variable | LRT-1 (n=10) | LRT-2 (n=11) | T | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 63.2 (8.2) | 67.6 (7.0) | 1.33 | 0.200 |
| Education | 17.3 (2.6) | 17.9 (3.3) | 0.47 | 0.666 |
| MMSE | 24.9 (4.2) | 23.6 (3.8) | −0.77 | 0.455 |
| Pre–post change score (%) trained items | 80.5 (13.6) | 69.8 (26.3) | −1.19 | 0.861 |
| 3-month follow-up vs post-treatment change score (%) trained items | −7.8 (12.7) | −3.5 (19.4) | 0.57 | 0.346 |
| 6-month follow-up vs post-treatment change score (%) trained items | −6.7 (11.5) | −3.5 (9.3) | 0.65 | 0.258 |
| 12-month follow-up vs post-treatment change score (%) trained items | −18.3 (27.5) | −20.0 (17.9) | −0.16 | 0.581 |
Notes: Two-tailed independent samples permutation tests were used for demographic comparisons. Because there were specific predictions for LRT-1 vs LRT-2 for trained items (ie, that increased dosage would lead to larger gains), one-tailed tests were used for these comparisons. Data shown as mean (SD). LRT-1: lexical retreival treatment as implemented by Henry et al2 (2013); LRT-2 a modified version of lexical retrieval treatment with dosage modifications. Details provided in full text.
Abbreviations: LRT, lexical retrieval treatment; MMSE, Mini–Mental State Examination.
Table S2.
Results of paired-samples permutation tests comparing treatment outcome measures and standardized test scores at each time point relative to pre-treatment and post-treatment for participants who underwent LRT
| Pre-treatment performance vs | Post-treatment performance vs | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | |
|
| |||||||
| Trained | |||||||
| n | 21 | 18 | 18 | 19 | 18 | 18 | 19 |
| t | −16.00 | −11.92 | −18.22 | −7.98 | 1.40 | 2.10 | 3.76 |
| P-value | <0.001* | <0.001* | <0.001* | <0.001* | 0.071 | 0.264 | 0.037 |
|
| |||||||
| Untrained | |||||||
| n | 21 | 18 | 18 | 19 | 18 | 18 | 19 |
| t | −3.55 | −3.43 | −2.92 | −1.45 | 0.00 | 0.34 | 1.81 |
| P-value | <0.001* | 0.002* | 0.007* | 0.189 | 1.00 | 0.784 | 0.095 |
|
| |||||||
| BNT | |||||||
| n | 21 | 17 | 16 | 16 | 18 | 16 | 17 |
| t | −1.78 | 1.00 | 2.60 | 3.27 | 1.79 | 4.17 | 3.93 |
| P-value | 0.010* | 0.448 | 0.061 | 0.061 | 0.264 | 0.013* | 0.031 |
|
| |||||||
| WAB | |||||||
| n | 21 | 18 | 18 | 17 | 18 | 18 | 17 |
| t | 0.36 | 2.68 | 3.40 | 5.23 | 3.48 | 3.63 | 5.45 |
| P-value | 0.164 | 0.071 | 0.016 | <0.001* | 0.016* | 0.003* | <0.001* |
Notes: One-tailed tests were used for trained items for all pre-treatment comparisons as well as for the pre–post comparison for untrained items. All other comparisons utilized two-tailed tests. Bonferroni-corrected thresholds for significance: P<0.013 for comparisons to pre-treatment performance and P<0.017 for comparisons relative to post-treatment performance. Analyses were conducted using the exactRankTests package1 in R (Version 3.3.1; 2016).
Significance at Bonferroni-corrected P-value <0.05.
Abbreviations: BNT, Boston Naming Test; LRT, lexical retrieval treatment; WAB, Western Aphasia Battery.
Table S3.
Results of two-tailed paired-samples permutation tests comparing treatment outcome measures and standardized test scores at each time point relative to post-treatment for participants who underwent VISTA
| Post-treatment performance vs
|
|||
|---|---|---|---|
| 3-month follow-up | 6-month follow-up | 12-month follow-up | |
|
| |||
| Trained | |||
| n | 10 | 10 | 9 |
| t | 1.14 | 2.10 | 2.13 |
| P-value | 0.680 | 0.012* | 0.086 |
|
| |||
| Untrained | |||
| n | 10 | 10 | 9 |
| t | 2.27 | 3.36 | 2.33 |
| P-value | 0.184 | 0.012* | 0.023 |
|
| |||
| NAT | |||
| n | 10 | 10 | 8 |
| t | 0.79 | 2.20 | 2.62 |
| P-value | 0.184 | 0.680 | 0.672 |
|
| |||
| WAB | |||
| n | 10 | 10 | 9 |
| t | 3.16 | 3.03 | 3.45 |
| P-value | 0.004* | 0.043 | 0.008* |
Notes: Bonferroni-corrected threshold for significance was P<0.017. Analyses were conducted using the exactRankTests package1 in R (Version 3.3.1; 2016).
Significance at Bonferroni-corrected P-value <0.05.
Abbreviations: NAT, Northwestern Anagram Test; VISTA, video-implemented script training for aphasia; WAB, Western Aphasia Battery.


