Abstract
Low back pain (LBP) is now regarded as the first cause of disability worldwide and should be a priority for future research on prevention and therapy. Intervertebral disc (IVD) degeneration is an important pathogenesis of LBP. Platelet-rich plasma (PRP) is an autologous blood concentrate that contains a natural concentration of autologous growth factors and cytokines and is currently widely used in the clinical setting for tissue regeneration and repair. PRP has great potential to stimulate cell proliferation and metabolic activity of IVD cells in vitro. Several animal studies have shown that the injection of PRP into degenerated IVDs is effective in restoring structural changes (IVD height) and improving the matrix integrity of degenerated IVDs as evaluated by magnetic resonance imaging (MRI) and histology. The results of this basic research have shown the great possibility that PRP has significant biological effects for tissue repair to counteract IVD degeneration. Clinical studies for evaluating the effects of the injection of PRP into degenerated IVDs for patients with discogenic LBP have been reviewed. Although there was only one double-blind randomized controlled trial, all the studies reported that PRP was safe and effective in reducing back pain. While the clinical evidence of tissue repair of IVDs by PRP treatment is currently lacking, there is a great possibility that the application of PRP has the potential to lead to a feasible intradiscal therapy for the treatment of degenerative disc diseases. Further large-scale studies may be required to confirm the clinical evidence of PRP for the treatment of discogenic LBP.
Keywords: intervertebral disc, intervertebral disc degeneration, platelet-rich plasma, PRP, low back pain
Epidemiology of low back pain (LBP) and its association with intervertebral disc (IVD) degeneration
LBP, an extremely common symptom in populations of all ages from children to the elderly, is significantly associated with personal, social, and economic burdens worldwide. In 2012, a systematic review of the global prevalence of LBP reported that the point prevalence of activity-limiting LBP was estimated to bê12%, whereas the 1-month prevalence was 23%.1 The prevalence of LBP was higher among females than among males across all age groups and was relatively high during adolescence. An international survey of pain from the data of the Health Behavior in School-aged Children: WHO collaborative cross-national survey (HBSC) showed that 37.0% of the adolescents reported LBP monthly or more frequently.2 Several epidemiological studies have shown that the prevalence of LBP was highest during middle age. Therefore, LBP has a major societal economic impact.
More recently, the Global Burden of Disease (GBD) in 2015 reported that the global point prevalence of activity-limiting LBP was 7.3% (540 million people in the world), and LBP is now regarded as the first cause of disability worldwide.3 The authors of GDB 2015 suggest that LBP should be a priority for future research on prevention and therapy.
Traditionally, the notion that the cause of LBP is unclear in about 85% of the patients, referred to as having “nonspecific LBP”, has been perpetrated over recent decades. However, recent epidemiological, radiological, and clinical studies have shown accumulating evidence that the specific nociceptive origin of LBP can be identified by a comprehensive diagnosis including radiological, interventional, and physical examinations by spine and/or orthopedic specialists.4–10
Epidemiological studies on large population samples have recently provided evidence that LBP has a significant association with lumbar disc degeneration.8,9,11 A cross-sectional study of young individuals from 13 to 20 years of age showed a stronger correlation between disc degeneration and LBP than that of adult populations.8,12 DePalma et al10 also reported that younger patients tend to have a higher probability of having a discogenic origin of LBP.
The progression of IVD degeneration is known to lead to ruptures (including tears and/or cleft formation) within IVD tissues. Because of the absence of blood supply, IVD tissues have little potential for self-repair. A previous report showed that 39% of the chronic LBP patients had the presence of internal disc disruption evaluated by computed tomography (CT) images.13 On the other hand, annulus fibrosus (AF) tears in the posterior AF area are known as high-intensity zones (HIZs) and are observed as high-intensity signals on T2-weighted magnetic resonance (MR). Previous reports showed that HIZs were identified in 28%–59% of the cases among symptomatic LBP patients (see review by Jha et al14). Peng et al15,16 reported that the formation of vascularized granulation tissue from the NP to the outer AF along the fissures, in which immunoreactive nerve fibers were identified, was found in the HIZ area collected from lumbar surgery. In addition, Dongfeng et al17 reported that the presence of TNF-α and CD68-positive cells was found in the HIZ area, suggesting that an HIZ may be a specific signal for the inflammatory reaction of painful IVDs. Aoki et al18 have shown that nerve fibers (protein gene product 9.5 – immunoreactive) were observed in scar tissues (extruded disc tissues) in the rabbit annular-puncture disc degeneration model. These previous reports suggest that disc rupture would not only induce inflammatory tissue reaction but also nociceptive nerve growth around tissue scars that would be associated with the chronic pain of discogenic origin.
Pathological mechanism of IVD degeneration and its association with pain
Biochemical features of IVD degeneration
The IVD consists of an outer AF, which is rich in collagens accounting for its tensile strength, and an inner nucleus pulposus (NP), which contains large proteoglycans that retain water to maintain the osmotic pressure required for resistance against loading by compression. A normal (healthy) IVD is basically an avascular tissue and only the most outer layer of the AF is innervated by sinuvertebral nerves consisting of spinal sensory and postganglionic sympathetic fibers.19–21
Although the exact mechanism of IVD degeneration remains unknown, the biochemical changes typical of the degenerative IVD are known to include progressive decreases in proteoglycan and collagen type II contents with subsequent dehydration and increased content of collagen type I leading to tissue fibrosis.22,23 Importantly, pro-inflammatory cytokines, such as IL-1 and tumor necrosis factor-alpha (TNF-α), are thought to significantly affect matrix homeostasis during IVD degeneration by stimulating the production of nitric oxide, matrix metalloproteinases (MMPs), and aggrecanases, finally resulting in the destruction of the extracellular matrix (ECM) of both AF and NP tissues.22,24–26
In the process of disc degeneration, these pro-inflammatory cytokines and inflammation-related molecules, including neurotrophic factors, are considered to be responsible for the pathogenesis of discogenic LBP.19
Neurotrophic factors have been shown to play a significant role in the transmission of physiological and pathological pain (see review by García-Cosamalón et al21). Among these, the neurotrophin family is known to play a role in inflammatory responses and pain transmission by increasing the expression of pain-related peptides in response to inflammation in local tissues.21
The neurotrophin (NT) family, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and NT-3 and its receptor, has also been shown to be expressed in the IVD; these expressions are upregulated in degenerated and painful discs.27,28 These expressions of the NT family have been shown to be regulated by pro-inflammatory stimuli, such as IL-1β and TNF-α. The NGF released in inflammatory IVDs may act directly on nociceptive nerve fibers and sensory neurons to trigger hyperalgesic effects. The mutual interaction between pro-inflammatory cytokines and the NT family is considered to play a central role in the pathomechanism of discogenic LBP.
Platelet-rich plasma (PRP)
Definition of PRP and clinical application in orthopedic fields
PRP is an autologous blood concentrate that contains a natural concentration of autologous growth factors and cytokines. PRP has been widely used in the clinical setting for tissue regeneration and repair.29,30 Recently, especially in the field of sports medicine and orthopedics, PRP has demonstrated regenerative ability to repair injured tissues, including tendons, ligaments, and cartilage, all of which have a low intrinsic healing potential.30–34
PRP has recently been applied in the treatment of Achilles and patella tendinopathies. These common sport-related injuries often resist conservative medical management because the tendon is an avascular tissue with low intrinsic healing potential.31 In their systemic review of the clinical effects of PRP injection on Achilles tendinopathy (12 papers) and patella tendinopathy (three papers), di Matteo et al31 reported that, although there are few randomized controlled trials, most studies showed that the intratendinous injection of PRP significantly reduced pain and improved functional scores.
PRP has also been applied for the treatment of osteoarthritis (OA). A recent meta-analysis of Level I randomized controlled trials (10 trials with a total of 1,069 patients) found that PRP injections were more effective for pain relief and functional improvement than injections of placebo (saline) and hyaluronic acid (HA) in the treatment of knee OA. Specifically, one study showed significant improvement in pain and functional scores than the placebo (saline) at 6 and 12 months post-injection and HA at 12 months post-injection.35 From the results of these clinical studies, the authors of the meta-analysis suggest that PRP is a promising treatment for cartilage injuries and relieving pain symptoms.
Biology of PRP and its classification
The main function of platelets is to contribute to hemostasis through adhesion, activation, and the aggregation process. In response to vessel injury, platelets are activated and their granules release coagulation factors that generate a fibrin clot. In addition to the factors that coagulate blood, activated platelets release growth factors. These growth factors increase inflammation and revascularization and accelerate epithelial regeneration in the inflammatory and proliferative stages of wound healing.29
Platelets contain the following three types of secretory granules: α-granules, dense granules, and lysosomes. Recent proteomic analyses revealed that >3,000 bioactive proteins are released from activated platelets. Among the three types of granules, α-granules are the major storage organelles for secreted proteins, containing adhesion proteins, clotting factors and inhibitors, fibrinolytic factors and their inhibitors, proteases and anti-proteases, growth factors and mitogenic factors, chemokines and cytokines, anti-microbial proteins, and others.36 Lysosomes are known to contain proteases, such as cathepsins and elastases, and other enzymes, including phosphatases and glycosidases.36
Once platelets are activated, these bioactive proteins are generated and released to the damaged tissues, synergistically regulating multiple pathways, including cell proliferation, cellular chemotaxis, angiogenesis, cell differentiation, and ECM synthesis.29,30,36
PRP has been classified by Dohan Ehrenfest et al37 and DeLong et al.38 These classifications are based on the number of leukocytes and fibrin content and include four types of PRP preparation. Leukocyte-poor platelet-rich plasma (P-PRP) contains low numbers of leukocytes and low fibrin density.37 Leukocyte and platelet-rich plasma (L-PRP) contains high numbers of leukocytes and low fibrin density.37 Pure platelet-rich fibrin (P-PRF) contains low number of leukocytes and high fibrin density. Leukocyte- and platelet-rich fibrin (L-PRF) contains high numbers of leukocytes and high fibrin density37 (Table 1). DeLong et al developed the PAW classification based on the number of platelets (P), the activation system (A), and the presence of white blood cells (leukocytes), as described in Table 1. It is extremely important to include this information to evaluate the effect of PRP in vitro, in vivo, and in clinical trials. Unfortunately, only 10% of the published articles include this comprehensive information.39 The type of PRP prepared by commercially available equipment is well summarized in the original classification papers37,38 and review book chapter.40
Table 1.
Classification of PRP
| Dohan Ehrenfest Classification60 | |||
|---|---|---|---|
| PRP type | Leukocyte | Fibrin density | |
| Pure platelet-rich plasma | Low | Low | |
| Leukocyte- and platelet-rich plasma | High | Low | |
| Pure platelet-rich fibrin | Low | High | |
| Leukocyte- and platelet-rich fibrin | High | High | |
| PAW Classification61 | |||
| Platelet | Exogenous activation | WBC | |
| Total WBC | Neutrophil | ||
| P1≤ baseline (1´) | x | A: above baseline (buffy-coat system) | α: above baseline |
| P2 baseline – 0.75 M/μL | (empty) Endogenous activation | B: below baseline (plasma based-system) | β: below baseline |
| P3 0.75 M – 1.25 M/μL | |||
| P4≥1.25 M/μL | |||
Note: Example: P2-x-Bβ.
Abbreviations: M, million; P, platelet; PRP, platelet-rich plasma; WBC, white blood cell; x, exogenous.
PRP activation
PRP activation is one of the major factors that influence the outcome of studies. For in vitro studies, it is important to activate and use soluble components for culture, unless the cultures are in contact with a collagen matrix, for example, which induces endogenous activation. For in vivo and clinical studies, exogenous activation or endogenous activation techniques have been used. Activation will result in degranulation of platelets and release of α granules that contain growth factors. Activation also induces fibrinogen cleavage that promotes matrix formation.40 PRP activation is usually induced by the addition of calcium chloride and/or thrombin, freezing and thawing, or exposure to collagen (especially likely to occur in vivo). For in vitro studies, a freezing and thawing procedure (>6 times is recommended) is suitable because no additional component needs to be added. In vivo collagen activation also does not require an additional component, such as thrombin, but the extent of release of growth factors may differ.41 These differences in the activation method for clinical trials make it difficult to accurately assess the result of the studies.
PRP for IVD repair
The use of PRP containing multiple growth factors concentrated at high levels has grown in orthopedic practice even though its biological mechanisms need further exploration. Currently, biological molecules prepared in an autologous fashion are not regulated for clinical use by the US Food and Drug Administration (FDA).42,43 However, the preparation of PRP produced by various apparatuses for “point of care” separation of a patient’s own blood in the operating room is regulated as an FDA 510(k) cleared device. Alternatively, PRP can be produced by local blood banks.
However, the preparation of PRP varies greatly and may be the cause for the differing biological activities reported among the studies.44 It has even been reported that different activation methods of PRP affect its physical form and can also change the release of bioactive molecules.41 A systematic review performed on PRP literature from 2006 to 2016 by Chahla et al39 suggested that there was a need to standardize PRP preparation and composition so that studies are reproducible and comparison among different studies could be achieved. Overall, most studies focusing on the effects of PRP in IVD tissues demonstrate significant biological effects.
In vitro effects of PRP on IVD cells
Studies focusing on the in vitro effects of PRP on IVD cells seem promising, many suggesting that PRP has the potential to be a great therapeutic agent for IVD degeneration (Table 2). The effects of PRP (growth factors) are also well summarized in several review articles.45–47
Table 2.
In vitro effects of PRP on IVD cells
| Study | Agent | Target | Dose and ACT | Effects |
|---|---|---|---|---|
|
| ||||
| Akeda (2006)48 | PRP (leukocyte poor) | Porcine IVD cells; alginate beads | 10% porcine PRP ACT; thrombin + CaC 2 l |
Mild increase in cell proliferation; marked increase in PG and collagen synthesis, and PG accumulation. |
| Chen (2006)49 | PRP | Human NP cells | PRP (defined as TGF-β1 equivalent) ACT; thrombin |
NP cell proliferation and aggregation; optimum at 1 ng TGF-β1 concentration in PRP. Formation of three-dimensional tissue construct ↑ COL2, AGN, SOX-9 mRNA ↑, GAG accumulation ↑ Phosphorylation of Smad 2/3 ↑, apoptosis ↓ |
| Chen (2009)50 | PRP | Porcine IVD organ culture, degeneration induced with chymopapain | Porcine PRP (10%, cell culture) or IVD (not known) ACT; thrombin |
Promoted NP cell viability, chondrogenic redifferentiation in cell cultures (details not presented). Significant recovery of COL2 AGN mRNA expression after chymopapain treatment in organ culture |
| Mietsch (2013)56 | TGF-β1 and PRP | Human MSCs and NP cells | 10% human PRP 10 ng/mL TGF-β1 ACT; freeze and thaw only | MSC chondrocytic differentiation PRP< TGF-β1, mainly proliferation |
| Liu (2014)58 | PRP | Immortalized human NP cells with LPS | PRP equivalent to 1 ng/mL TGF-β1 | Restored diminished chondrogenic markers (SOX9, COL2, and AGN) and deleterious inflammatory responses (IL-1, TNF, and MMP3) induced by LPS. |
| Kim (2014)57 | PRP releasate | Human NP cells on collagen matrix | 5% and 10% PRP ACT; CaC 2 l |
Counteracted inflammatory effects (AGN, COL2 ↓, MMP3, COX2 ↑) by IL-1 and TNF-α in gene expressions |
| Pirvu (2014)51 | PRP and platelet lysate | Bovine AF cells, bovine IVD organ culture | 25%–50% human PRP 25%–50% human platelet lysate ACT: sonication and freezing |
50% platelet lysate – DNA and GAG ↑ Organ culture – spherical cell morphology |
| Khalaf (2015) and Nikkhoo (2017)52,53 | PRP | Porcine IVD organ culture, degeneration induced with trypsin | 1 mL porcine PRP | PRP recovered mechanical properties (elastic modulus and hydraulic permeability) and GAG content of denatured discs |
| Yang (2016)54 | PRP | Rabbit NP cells | 2.5% rabbit PRP releasate ACT: thrombin+CaC 2 l |
COL2, AGN, SOX9 mRNA ↑, COLX ↓ TGF-β1 inhibitor (blocks TGF-β1/Smad2/3 signaling pathway) counteracted PRP effects |
| Cho (2016)59 | PRP | Porcine AF cells with TNF-α | Porcine PRP | COL2 and AGN mRNA ↑, MMP1 mRNA, protein ↓ |
| Yamada (2017)60 | PRP releasate | Human disc cells from spine surgery patients | Human PRP releasate ACT: CaC 2 l |
IL-1β, MMP13, NGF mRNA ↓, TGF-β1 mRNA ↑ |
| Wang (2018)62 | P-PRP and R-PRP | Rabbit NP-derived stem cells | 5%–20% rabbit P-PRP or R-PRP ACT: not described |
P-PRP: AGN, COL2 mRNA and protein ↑, IL- 1β, TNF-α, IL-6, -8, MMP1, MMP13 mRNA ↓, IL-1β, TNF-α production ↓ R-PRP: similar to P-PRP, but to a lesser extent |
| Hondke (2018)55 | PRP | Human early and advanced AF cells in PGA-HA scaffold | 5% PRP ACT: freezing and thawing |
Stimulated migration and cell viability in early and advanced AF cells. COL2 mRNA ↑, COL1 and 3 mRNA ↓ |
Abbreviations: ACT, activation; AF, annulus fibrosus; AGN, aggrecan; BMP-2, bone morphogenetic protein-2; COL2, type II collagen; COX-2, cyclooxygenase-2; GAG, glycosaminoglycan; IVD, intervertebral disc; LPS, lipopolysaccharide; MMP; metalloproteinase; MSC, mesenchymal stem cell; NGF, nerve growth factor; NP, nucleus pulposus; PG, proteoglycan; PGA-HA, polyglycolic acid–hyaluronan; PRP, platelet-rich plasma; P-PRP, Leukocyte-poor PRP; R-PRP, leukocyte-rich PRP; TNF, tumor necrosis factor.
Akeda et al reported that a PRP soluble releasate stimulated cell proliferation and increased proteoglycan and collagen synthesis in alginate-cultured porcine IVD cells without affecting their phenotypic stability.48 PRP had stronger stimulative effects in the AF region compared with the NP. Chen et al49 demonstrated the PRP-stimulated proliferation of NP cells and reported that the optimum concentration of PRP designated by TGF-β1 was 1 ng/mL. They also showed the enhancement of three-dimensional (3D)-engineered tissue formation and matrix gene expression (COL2, SOX9, and AGN), as well as accumulation of glycosaminoglycans (GAGs) and that the signaling pathway, SMAD2/3 phosphorylation, was activated by PRP. The same group reported that PRP promotes recovery of COL2, AGN mRNA expression in degenerated discs after chymopapain treatment using a porcine whole-disc culture system.50 Pirvu et al51 reported that a platelet lysate increased cell proliferation and GAG accumulation in monolayer cultures of bovine AF cells and the maintenance of the spherical shape of cells in whole-disc organ culture. Khalaf et al52 determined the effects of PRP on the biomechanical properties of whole-disc organ cultures treated with trypsin.53 PRP reversed the loss of mechanical properties (elastic modulus and hydraulic permeability) and GAG content of degenerated discs induced by trypsin. Yang et al54 tested the effects of rabbit PRP and found the stimulative effects of PRP on COL2, AGN, SOX9 gene expression and suppression of COLX gene expression. These effects were inhibited by a TGF-β1/Smad2/3 signaling pathway inhibitor. Hondke et al55 examined the effects of PRP on AF cells from early stages of degeneration (Pfirrmann grades 2–3) and advanced degeneration (Pfirrmann grades 4–5). PRP stimulated cell migration and cell viability in both early and advanced degeneration AF cells, but the response was stronger in the early grade AF cells than in the advanced stage cells.
On the other hand, Mietsch et al56 reported that PRP has stimulative effects on cell proliferation in NP and mesenchymal stem cell (MSC) cultures but did not stimulate chondrocytic differentiation when compared to TGF-β1. They concluded that the application of PRP in human NP tissue engineering is not suitable. In their experiment, the activation was a single freezing and thawing after irradiation, and the concentration of TGF-β1 was not analyzed in this treatment but after acetic acid/urea treatment. For in vitro experiments, the spontaneous activation with a collagen matrix is not expected; the differences in activation methods may result in varied conclusions.41 Further studies may be needed to confirm the concentration of TGF-β1 in specific PRP preparations.
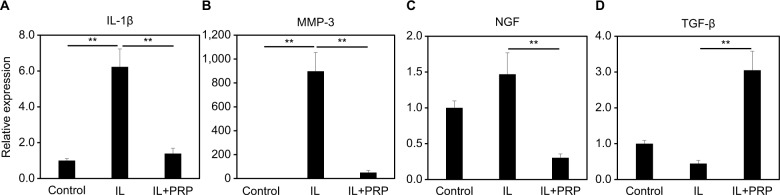
The anti-inflammatory effects of PRP have also been reported as one mechanism for therapeutic effects by several groups. PRP was demonstrated to have anti-inflammatory effects in inflammatory conditions, typically increasing chondrogenic markers and suppressing catabolic pathways. Kim et al57 reported that PRP releasate counteracted inflammatory effects, such as the suppression of AGN and COL2 expressions and stimulation of MMP3 and COX2 expressions. Using immortalized human NP cells, Liu et al58 also demonstrated that PRP restored diminished chondrogenic markers (SOX9, COL2, and AGN) and diminished inflammatory responses (IL-1β, TNF-α, and MMP3) induced by lipopolysaccharide. Cho et al59 reported that PRP stimulated COL2 and AGN gene expressions and inhibited MMP-1 expression under TNF-α-stimulated conditions; the production of MMP-1 induced by TNF-α was also inhibited. Yamada et al60 also reported that PRP releasate significantly suppressed gene expressions of IL-1β, MMP3, and NGF and stimulated that of TGF-β1 using human disc cells obtained from spine surgery samples (seven donors, 25–78-year old). In this in vitro study, stored PRP releasates that were prepared for a clinical trial61 and extra PRP releasates were used (Figure 1).
Figure 1.
Effect of PRP releasate on the mRNA expression of IL-1β (A), MMP-3 (B), NGF (C), and TGF-β (D) by human IVD cells.
Notes: IL: IL-1β (1.0 ng/mL), IL + PRP: IL-1β (1.0 ng/mL) + 10% PRP. **P<0.05.
Abbreviations: IVD, intervertebral disc; MMP, matrix metalloproteinase; NGF, nerve growth factor; PRP, platelet-rich plasma.
Wang et al62 performed a study using rabbit NP-derived stem cells obtained from degenerated discs of rabbit and compared the effects of P-PRP and leukocyte-rich PRP (R-PRP). P-PRP stimulated AGN and COL2 at both mRNA and protein levels and inhibited TNF-α, IL-6, -8, MMP1, and MMP13 mRNA expressions, as well as IL-1β and TNF-α protein production. These results illustrate the importance of the characteristics of the PRP used. Further studies using human PRP and IVD cells may provide further clinically relevant information.
Overall, PRP was effective in stimulating cell proliferation and ECM metabolism. The anti-apoptotic and anti-inflammatory effects of PRP may contribute to disc repair and symptom relief in patients. Given the risks of using animal serum for tissue engineering and limited clinical development in growth factor therapy, autologous blood may gain favor as a source of growth factors and serum supplements needed for stimulating cells to regenerate IVD tissues. However, as discussed earlier, the standardization of study reports and quality assessment of PRP preparation are essential to bridge the gap between in vitro studies and in vivo and clinical studies.
In vivo effects of PRP in IVD degeneration animal models
Many in vivo studies reported similar results with those found in vitro, showing the restorative effects of PRP in joint and musculoskeletal degeneration (Table 3).63 However, because there is minimal standardization in terms of the activation and preparation of PRP, variance in results is expected.39 Additionally, some animal studies use combinatorial treatments of PRP with other inhibitors/carriers to examine the pathways in which PRP helps retard IVD degeneration.
Table 3.
In vivo effects of PRP in IVD degeneration animal models
| Study | Agent (volume) | Species | Samplesize (N) | Injection site | Model Timing of Tx Follow-up | Effects | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Nagae (2007)64 | Rabbit PRP in gelatin hydrogel microsphere (20 μL) ACT: none |
Rabbit | 36 | Lumbar | Nucleotomy Tx 2 W later 2, 4, and 8 W follow-up |
PRP + GHM group had less degeneration and increased PG; PRP + PBS group showed no differences | |
| Sawamura (2009)65 | Rabbit PRP in gelatin hydrogel microsphere (20 μL) ACT: none |
Rabbit | 128 | Lumbar | Nucleotomy Tx 2 W later 2, 4, 8 W follow-up |
PRP + GHM had greater disc height, water content, AGN and COL2 mRNA ↑ n; fewer apoptotic cells in NP | |
| Chen (2009)50 | PRP (volume NA) ACT: not reported |
Porcine | 14 | Thoracic lumbar | Chymopapain Tx 1 W later 4, 8 W follow-up |
Increased AGN and COL2 gene expression, histological improvement, and disc height increase | |
| Gullung (2011)66 | PRP (100 μL) ACT: not reported |
Rat | 18 | Lumbar | Needle puncture Tx 0, 2 W later 2, 4 W follow-up |
Disc height increased in the immediate injection group. Histological improvement. Earlier intervention – better. | |
| Obata (2012)67 | PRP releasate (20 μL) ACT: auto-serum + CaCl2 |
Rabbit | 12 | Lumbar | Needle puncture (18G) Tx 4 W later 2, 4, 6, 8 W follow-up |
Disc height increased, no significant MRI T2 signal | |
| Gui (2015)68 | PRP (100 μL) ACT: thrombin |
Rabbit | 36 | Lumbar | Needle puncture Tx 2, 4 W later 2 W follow-up |
Disc height maintained, NP signal intensity maintained. Significantly low MRI grading | |
| Wang (2016)69 | PRP (200 μL) ACT: no activation |
Rabbit | 40 | Lumbar | Needle puncture (21G) Tx 2 W later 1, 2, 8 W follow-up |
PRP – moderate effect on MRI intensity, disc height PRP + BMSC better than PRP: well-preserved ECM. Cell density increased T2 signal intensity, MRI grading, and COL2 mRNA expression I | |
| Yang (2016)54 | PRP (15 μL) ACT: thrombin + CaCl2 |
Rabbit | 24 | Lumbar | Needle puncture (18G) Tx 4 W later 0, 4, 8, 12 W follow-up |
T2 signal intensity: PRP> control or PRP + TGF-β inhibitor Histology: PRP-less degeneration, strong COL2 staining, and SMAD2/3-positive cells | |
| Hou (2016)70 | PRP (40 μL) PRP + BMSC PRP + BMP2 – BMSC BMP2 – BMSC ACT: thrombin + CaCl2 injection after agents |
Rabbit | 60 | Lumbar | Needle puncture (21G Tx 2 W later 4, 8 W follow-up |
Col2 and PG staining and MRI grade PRP + BMP2 – BMSC> PRP + BMSC > PRP |
|
Abbreviations: ACT, activation, AGN, aggrecan, BMSC, bone marrow-derived mesenchymal stem cell; BMP2, bone morphogenetic protein 2; COL2, type II collagen; ECM, extracellular matrix; GHM, gelatin hydrogel microsphere; IVD, intervertebral disc; MRI, magnetic resonance imaging; PG, proteoglycan; PRP, platelet-rich plasma; TX, treatment; W, weeks.
Nagae et al64 reported, for the first time, that PRP-impregnated gelatin hydrogel microspheres (PRP-GHM) were effective in retarding IVD degeneration, as assessed by histological grading and increasing proteoglycan content in the rabbit nucleotomy disc degeneration model. In this experiment, an injection of PRP without microspheres did not show positive therapeutic effects. They, therefore, concluded that the use of gelatin hydrogel microspheres that immobilized growth factors provided a better therapeutic effect compared to PRP alone. No activation was performed for the PRP alone group. They also suggested that the time of impregnation into gelatin microspheres allowed released growth factors from platelets to be immobilized. A subsequent study by Sawamura et al65 revealed that disc height and MR imaging (MRI) grading scores after an injection of PRP-GHM were significantly higher than the nucleotomy only group or the PRP group. These results were associated with increased AGN and COL2 mRNA expressions and decreased numbers of apoptotic cells 2 weeks after the injection.
Two months after PRP injections, Chen et al50 found increased expressions of collagen II mRNA and aggrecan, improvement in disc height index, and differentiation of osteogenic MSCs in the porcine disc degeneration model induced by chymopapain. Gullung et al66 showed that morphology and high fluid content and disc height remained normal immediately after an injection of PRP in the rat lumbar disc needle puncture model. Additionally, Obata et al67 found that using only PRP releasate also showed similar restorative results in a rabbit needle puncture model, suggesting that there are ways to optimize PRP injection treatments. Gui et al68 reported similar results using thrombin-activated PRP, but also a positive effect on MRI signal intensity, in the rabbit annular puncture model at both 2 and 4 weeks post injury.
Yang et al54 confirmed their in vitro experiment results presented earlier in the rabbit model for the involvement of the TGF-β signaling pathway in the effects of PRP. The positive effects of PRP on MRI and collagen staining and Smad2/3 cell numbers were significantly suppressed by the co-injection of a TGF-β1 inhibitor.
Wang et al69 injected PRP with bone marrow-derived mesenchymal stem cells (BMSCs) in the rabbit annular puncture model and found that the ECM and cell density were well preserved, as well as an increase in T2 signal intensity, MRI grading, and strong immunopositive staining for Col II. MRI scores of the PRP group were similar to those of the PRP+ BMSC group for 2 weeks, but the efficacy was diminished at 8 weeks. They predicted that the injected PRP was activated by the surrounding tissues and interacted with BMSCs.
Hou et al70 reported that BMSCs transduced with bone morphogenetic protein 2 (BMP2) in PRP gel inhibited disc degeneration and enhanced production of the ECM in the NP.
PRP treatment is potentially effective in restoring disc height of mice, rabbit, and rat models, reducing histological degeneration grade, and increasing MRI T2 image signals. The recent meta-analysis of the animal experiment data supports the restoration of disc height and histology, as well as an increase in MRI signal intensity.71 As such, PRP injection may be a promising therapy for retarding disc degeneration. A consensus shows that PRP, as well as its releasate, is effective in retarding and possibly reversing the effects of IVD degeneration. More studies are now focusing on either combining PRP injection with other agents or PRP activation pathways to determine how PRP is effective and to discover limitations as well as ways to optimize this potential treatment.
Clinical studies of PRP for LBP patients
Intradiscal injection
The first prospective preliminary clinical trial for the safety and efficacy of intradiscal injection therapy using PRP for discogenic LBP was reported in 2011 (Table 4).72 This study used PRP releasate prepared by a local blood center that was activated with autologous serum and CaCl2 and included six patients who had chronic LBP for >3 months. The results of this study showed that mean pain scores (VAS and Roland-Morris Disability Questionnaire [RDQ]) before treatment were significantly decreased at 1 month, and these were sustained for 6 months after treatment, although mean T2 values did not significantly change. A subsequent report including 14 patients with an average period of 10 months follow-up also showed the analgesic effect of PRP releasate.61 In the later study, the mean pain scores of over 70% of the patients before treatment were significantly decreased at 1 month, and this was generally sustained throughout the observation period, although VAS scores of two patients and the RDQ score of one patient returned to higher levels following treatment. No patient experienced adverse events or significant narrowing of disc height. In this study, platelets were isolated by the buffy coat (BC) method, which contributes to fewer white blood cells and lower concentrations of pro-inflammatory cytokines. According to the PRP classification system reported by Dohan Ehrenfest et al,37 the PRP preparation process used in these studies by Akeda et al is classified as “pure PRP”, which contains fewer white blood cells. The PRP was activated with CaCl2 and autologous serum, and the soluble releasate following centrifugation was used for the intradiscal injection of patients. Importantly, the soluble releasate used in these studies could be sterilized using a membrane filter while being injected into the target discs, conferring the advantage of reducing the risk of infection associated with the injection procedure. Second, the soluble releasate can be stored at –80°C and prepared for the future treatment of recurrent pain.
Table 4.
Clinical studies of PRP for LBP patients
| Study | Study design | Number of patients | Diagnostic discography | Injection method | PRP preparation method | Characteristics of PRP | Volume of whole blood | Injection volume of PRP | Follow-up period | Outcome measure | Maximum improvement time point | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Intradiscal injection | ||||||||||||
|
| ||||||||||||
| Akeda et al (2011)72 | Prospective preliminary trial | 6 | Yes | Intradiscal | Buffy coat method | P-PRP releasate ACT: CaCl + 2 autoserum | 200 mL | 2 mL | 6 M | VAS (7.1→1.8**) and RDQ (11→3.2**) | 6 M | |
| Bodor et al (2014)73 | Case series | 35 | Yes | Intradiscal | Cascade Autologous Platelet System (ConMed Linvatec) | P-PRP ACT: no | 9 mL | 2 mL | 2–10 M | NRS and ODI (positive response of 2/3 patients) | 1 W–2 M | |
| Navani and Hames (2015)74 | Case series | 6 | Yes | Intradiscal | P-PRP system (EmCyte) | L-PRP ACT: no | 60 mL | 1.5–3 mL | 24 W | VPS (50% decrease in 6 patients) | 24 W | |
| Levi et al (2016)75 | Prospective trial | 22 | Partial | Intradiscal | Smartprep (Harvest) | L-PRP ACT: no | 30 or 60 mL | 1.5 mL | 6 M | 47% patients (both VAS 50% and ODI 30% improvement) | 6 M | PRP injection just after 0.6 mL of contrast, 0.4 mL of gentamicin, and 0.5 mL of lidocaine |
| Tuakli-Wosornu et al (2016)77 Monfett et al (2016)78 | Double-blind randomized controlled trial | 36 treatments and 22 controls | Yes | Intradiscal | Smartprep (Harvest) | L-PRP ACT: no | 30 mL | 1–2 mL | 8 W (RCT) | FRI (control 44–PRP 38*), NRS, SF-36, and modified NASS Outcome Questionnaire | 1–2 years | PRP injection just after 1–2 mL injection of contrast |
| Akeda et al (2017)61 | Prospective trial | 14 | Yes | Intradiscal | Buffy coat method | P-PRP releasate ACT: CaCl + auto 2 serum | 200 mL | 2 mL | 10 M | VAS (7.5→3.1**) and RDQ (12.6→5.1**) T2-value: no change | 1 M | Discography on different days from PRP injection |
| Lutz (2017)90 | Single case report | 1 | Yes | Intradiscal | Arteriocyte (Magellan) | L-PRP ACT: N/D | N/D | 1.5 mL | 12 M | Improvement T2 nuclear signal intensity↑, type I Modic changes↓ | N/A | PRP injection just after injection of contrast |
|
| ||||||||||||
| Non-intradiscal injections (facet, epidural, and intramuscular) | ||||||||||||
|
| ||||||||||||
| Aufiero et al (2015)79 | Case report | 5 | N/A | Intraarticular (facet) | N/D | P-PRP ACT: no | N/D | Multiple injection | 6–12 M | VAS | N/A | |
| Kirchner and Anitua (2016)80,81 | Observational retrospective pilot study | 86 | Yes | Intradiscal and intraarticular | PRGF system IV (BTI-Biotechnology Institute, Vitoria, Álava, Spain) | PRGF ACT: CaC 2 l | 9 mL | 4 mL (disc) and 2 mL (facet) | 6 M | VAS (8.4→0.8**) 91% excellent 8.1% moderate |
6 M | |
| Wu et al (2016)82 | Prospective study | 19 | N/A | Intraarticular (facet) | Centrifuge 2 times soft spin | N/D | 5–10 mL | 0.5 mL | 3 M | VAS (7.05→2.63, P<0.05) RDQ (P<0.05) ODI (54.3→26.3, P<0.05) |
3 M | |
| Bhatia and Chopra (2016)84 | Pilot study | 10 | N/A | Epidural | Blood bank No detail | N/D | 100 mL | 5 mL | 3 M | VAS, MODQ index, and SLRT (most of the patients improved in all evaluations) | 3 M | |
| Hussein and Hussein (2016)85 | Prospective trial | 104 | N/A | Intramuscular (weekly injection for 6 weeks) | PRP method | L-PRP ACT: CaC 2 l | 50 mL | 2.5 mL | 2 years | NRS (8.81→3.5**) and ODI (36.7→14.7**) | 12–18 M | |
| Wu et al (2017)83 | Randomized controlled study | 21 (PRP) 23 (betamethasone) | N/A | Intraarticular (facet) | Centrifuge 2 times soft spin | N/D | 5–10 mL | 0.5 mL | 6 M | PRP> betamethasone (VAS, RDQ, and ODI) | 6 M | |
| Cameron and Thielen (2017)86 | Prospective trial | 50 | N/A | Circumferential manner into posterior spine area | N/D | N/D | N/D | N/D | 5 years | VAS (–77% improvement) and ODI | No description | LBP caused by spinal disc herniation |
| Comella et al (2017)87 | Prospective trial | 15 | No | Intradiscal | N/D | PRP mixed with stromal vascular fraction | N/D | 1 mL | 6 M (safety 12 M) | VAS (5.6→3.6**), PPI (2.6→1.8*), BDI, ODI, SM-MPQ, SF-12, and DPQ | 6 M | |
Note:
P<0.05 and
P<0.01.
Abbreviations: ACT, activation; BDI, Beck Depression Inventory; DPQ, Dallas Pain Questionnaire; FRI, Functional Rating Index; LBP, low back pain; L-PRP, leukocyte- and platelet-rich plasma; M, months; MODQ, Modified Oswestry Disability Questionnaire; N/A, not applicable; N/D, no description; NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; PPI, present pain intensity; P-PRP, Leukocyte-poor PRP; PRP, platelet-rich plasma; RDQ, Roland-Morris Disability Questionnaire; SF, Short Form; SF-MPQ, Short-form McGill Pain Questionnaire; SLRT, Straight Leg Raising Test; VPS, verbal pain scale; W, weeks.
Bodor et al73 included case examples in their book chapters. These authors performed intradiscal PRP injections for 47 thoracic or lumbar discs of 35 patients.73 PRP without leukocytes or erythrocytes was prepared using a commercial kit (Cascade Autologous Platelet System; ConMed Linvatec; MTF biologics, Edison, NJ, USA), and no activators (CaCl2 or thrombin) were used. Two-thirds of the patients reported positive responses in numerical rating scales (NRS) and the Oswestry Disability Index (ODI) scores. In their study, most patients showed improvement of LBP at 1 week to 2 months that was sustained. However, the report lacks details of their clinical outcomes.
Navani et al74 reported a case series study to examine the effects of PRP intradiscal injection on six patients. Fifty percentage of the verbal pain scale (VPS) scores of all patients decreased by 3 months, and low pain levels were maintained until the 6-month follow-up. The short forum (SF)-36 also improved in both physical and mental scores.
Levi et al75 reported a prospective clinical trial of 22 patients investigating the effects of intradiscal PRP injections on discogenic back pain. The PRP was prepared using a Smartprep (Harvest, MA, USA) procedure pack, which makes L-PRP.76 At the 6-month follow-up, 47% had a successful outcome defined as at least 50% improvement in VAS and at least 30% improvement in ODI. Before the 1.5 mL injection of PRP, 0.6 mL of contrast, 0.4 mL of gentamicin (40 mg/mL), and 0.5 mL of 4% lidocaine were injected to confirm the needle position and for discitis prophylaxis and pain control, respectively. The authors speculated that the addition of these substances might have had an adverse effect on their results.
For the first time, Tuakli-Wosornu et al77 reported a double-blind randomized controlled trial of intradiscal PRP therapy for discogenic LBP.78 The participants were randomized into treatment or control groups in a 2:1 ratio, respectively. PRP was prepared using a commercial kit (Harvest). The activation procedure was not described. It is worth noting that they used a contrast to test the presence of concordant pain before the injection of PRP. At the 8-week follow-up, statistically significant improvements in NRS best pain, functional rating index (FRI), and patient satisfaction (NASS outcome Questionnaire) were observed in the treatment group, compared to the control group. Fifty-six percentage (15/27) of the participants were satisfied (NASS Outcome Questionnaire) with the treatment, whereas only 18% (3/17) of control participants were satisfied. The majority of patients (68.2%) in the control group requested that they receive PRP treatment. The significant effects of PRP in NRS worst pain, FRI function, and SF-36 pain and SF-36 function scores were sustained for 2 years.78 However, the outcomes were not compared after 8 weeks because of the lack of control group follow-up.
In summary, six clinical studies that evaluated the effect of intradiscal injection of PRP on low back patients have been reported. However, these previous reports included one single case report, two case series, two prospective trails, and only one double blind randomized controlled trial. Therefore, at present, the clinical evidence of intradiscal therapy of PRP for treatment of discogenic LBP remains insufficient. More clinical studies, especially randomized controlled trials, are needed to evaluate whether PRP is effective for treating degenerative disc disease.
Other injection procedures for LBP
PRP has been used in several different injection methods to treat LBP. Aufiero et al79 reported a case series study showing that P-PRP facet joint injections improved >50% of the symptoms of all five patients. Kirchner et al80,81 performed intradiscal and intraarticular facet infiltrations for 86 patients with plasma rich in growth factor (PRGF), which includes a twofold increase in the platelet count of peripheral blood with scarce numbers of leukocytes. The PRGF was prepared using the PRGF system IV (BTI-Biotechnology Institute, Vitoria, Álava, Spain) and activated with 10% weight/volume calcium chloride immediately before injection. VAS scores significantly decreased from 8.4±1.1 before treatment to 4.0, 1.7, and 0.8 at 1, 3, and 6 months after treatment, respectively. Ninety percentage of patients showed excellent (VAS 0–3) results.
Wu et al82 conducted a prospective study to investigate autologous PRP intraarticular injections for lumbar facet joint syndrome patients. During the 3-month follow up, VAS, RDQ, and ODI scores gradually and significantly improved (–4.42, P<0.05; no data shown, P<0.05; and –28.0, P<0.05, respectively). They also reported a randomized controlled study to compare the efficacy of PRP injection to that of corticosteroid (5 mg/mL betamethasone).83 Both PRP and corticosteroid injection showed significant analgesic effects at all time points of the 6-month follow-up in VAS, RDQ, and ODI scores (P<0.01). Interestingly, those scores of the corticosteroid group reversed and worsened at the 2-month time point after injection, whereas those of the PRP group showed a sustained trend of improvement. At the 1-week and 1-month time points, although those scores of the corticosteroid group were significantly better than those of the PRP group (P<0.05), opposite results were seen at 6 months (P<0.05).
Bhatia and Chopra84 reported an efficacy study of the epidural injection of autologous PRP for patients with lumbar disc herniation/prolapse on MRI, who also had >4 weeks LBP and a positive straight leg raising test (SLRT). After an injection of PRP, patients showed a gradual improvement of symptoms, in terms of VAS scores, Modified Oswestry Disability Questionnaire (MODQ) index, and SLRT that were sustained for 3 months. VAS scores did not change during the first hour but decreased significantly at the 3-week and 3-month time points. Most patients also showed MODQ scores <30% and improved SLRTs >70 at 3 months.
Hussein and Hussein85 showed the effects of autologous platelet, leukocyte-rich plasma injections for LBP accompanied with atrophied lumbar multifidus muscle and monosegmental degenerated IVDs. Patients were treated with weekly injections for 6 weeks and were followed-up for 24 months. Patients demonstrated a significant gradual improvement of NRS scores from 8.8 to 3.45 and ODI scores from 36.7 to 14.6 by 12 months. These improvements were sustained until the 24-month follow-up.
Cameron and Thielen86 reported that PRP injected in a circumferential manner subfascially into the lateral masses, facet joints, and the other areas around the posterior spine contributed to a significant improvement of VAS by 77%. Sixty-eight percentage of the patients reported more than a 75% improvement of symptoms.
Comella et al87 determined the efficacy of an intradiscal injection in 15 patients with stromal vascular fraction (SVF) resuspended in PRP. SVF was prepared with an adipose SVF preparation kit (US Stem Cell, Inc. Sunrise, FL, USA), but there was no description about the preparation of PRP. Patients demonstrated statistically significant improvements in several parameters, including flexion, pain ratings, VAS, present pain intensity (PPI), and Short-form McGill Pain (SF-MPQ) Questionnaire, and Short-form 12-physical component summary.
Discussion
Because activated platelets can release more than several thousand bioactive proteins, those concentrates known as PRP must have multiple important molecular functions, including inflammation, angiogenesis, cell migration, and metabolism for tissue repair and regeneration.29 Interestingly, in vitro studies by cells from the musculoskeletal system showed that PRP has potential to stimulate cell proliferation and metabolic activity.63 It should be emphasized that these cell-activating effects of PRP were also identified by cells from IVDs that are an avascular tissue with a low intrinsic healing potential.48,49,51,55
Our review of the in vitro effects of PRP on IVD cells showed that PRP has not only anabolic effects but also anti-inflammatory effects. It has been reported that the anti-inflammatory properties of PRP are associated with its inhibitory effects on the nuclear factor-κB (NF-κB) signaling pathway in multiple cell types.88,89 In addition, bioactive molecules released from PRP would also induce cells to produce and secrete additional biologically active molecules. We speculate that these complex and harmonized biological functions of PRP would change the pathologic conditions of degenerated IVDs to anabolic and anti-inflammatory conditions by the local injection of PRP. The results of the updated review of animal studies showed that the intradiscal injection of PRP would be effective in restoring structural changes and improving the matrix integrity of degenerated IVDs; this would further support the anabolic effects of PRP within degenerated IVDs in these animal models.
Clinical studies of PRP for LBP patients commonly showed that the intradiscal injection of PRP was effective in reducing back pain; however, a double-blind randomized controlled trial has only been conducted by Tuakli-Wosornu et al.77,78 Furthermore, only a few clinical trials that evaluate the radiological changes of disc degeneration have been reported.61,90 Clinical image findings that PRP particularly stimulated tissue repair within degenerated IVDs have not been confirmed. Further large-scale studies, such as multicenter and/or society-led clinical trials using the same isolation and/or activating protocol of PRP, may be required to confirm the clinical evidence of PRP for the treatment of LBP resulting from degenerative disc disease.
Conclusion
In this review, we describe the effects of PRP with a focus on intradiscal therapy from basic to clinical research for the treatment of discogenic LBP caused by degenerative disc diseases. In vitro and in vivo (animal) studies revealed that PRP has significant biological effects to stimulate IVD cells to repair tissues.
In general, previous clinical reports suggested that an intradiscal injection of various preparations of PRP (leukocyte rich- or poor-PRP, with/without activation, and platelets themselves or platelet releasate) into degenerated discs of patients with LBP provided positive effects on pain relief. It should be noted that only one double-blinded study with contrast agents in a limited number of patients and without characterization of PRP preparation showed some positive effects in limited outcome measures.77 Therefore, it remains to be answered whether PRP has specific biological effects on pain generation in LBP patients.
From a safety standpoint, the injection of a releasate (only the fluid element, not the cellular element) isolated from activated PRP may be advantageous because of the possible use of the pre-filtration technique. The pharmacokinetics of factors released from PRP or PRP releasate have not been reported. The distribution of leukocytes and platelets in discs may be limited, and the activation mechanism of platelets in situ is not well known. These points should be clarified in preclinical studies.
Because the preparation of autologous PRP using a commercial PRP concentration system as a point of care product is a simple and short-time procedure, intradiscal therapy with PRP itself is highly feasible in clinical use if the mechanism of action is completely revealed. To support this contention, well-designed clinical trials should be conducted. Comparisons between leukocyte-rich and poor PRP (including various preparation equipment/devices) and pre-activation or in situ activation should be performed. Other clinical factors, such as the simultaneous injection of contrast agents, especially in consideration of recently reported deleterious effects of contrast agents, must be taken into consideration. The practical limitations of these studies to compare various PRP preparations include the requirement of devices from multiple manufacturers and lack of funding for clinical trials supported by these manufacturers. Therefore, contributions from academic societies or governmental bodies (such as the NIH or European commission) are essential.
In conclusion, large-scale double-blind randomized studies with well-controlled preparation conditions and sufficient power, as well as proper analyses of PRP components, will be required to establish an evidence-based and standardized treatment of LBP.
Acknowledgments
The authors thank Mary Ellen Lenz for her assistance in the preparation of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 2.Swain MS, Henschke N, Kamper SJ, et al. An international survey of pain in adolescents. BMC Public Health. 2014;14(1):447. doi: 10.1186/1471-2458-14-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DALYs GBD, Collaborators H Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE)1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, Wang HL, Ma XS, et al. The value of radiographic indexes in the diagnosis of discogenic low back pain: a retrospective analysis of imaging results. Oncotarget. 2017;8(36):60558–60567. doi: 10.18632/oncotarget.18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi D, Carnahan D, Horn S, Levin J. Is a history of severe episodic low back pain an indicator of a discogenic etiology? Pain Med. 2018;19(7):1334–1339. doi: 10.1093/pm/pnx147. [DOI] [PubMed] [Google Scholar]
- 6.Tonosu J, Inanami H, Oka H, et al. Diagnosing discogenic low back pain associated with degenerative disc disease using a medical interview. PLoS One. 2016;11(11):e0166031. doi: 10.1371/journal.pone.0166031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki H, Kanchiku T, Imajo Y, et al. Diagnosis and characters of nonspecific low back pain in Japan: the Yamaguchi low back pain study. PLoS One. 2016;11(8):e0160454. doi: 10.1371/journal.pone.0160454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teraguchi M, Yoshimura N, Hashizume H, et al. The association of combination of disc degeneration, end plate signal change, and Schmorl node with low back pain in a large population study: the Wakayama spine study. Spine J. 2015;15(4):622–628. doi: 10.1016/j.spinee.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Teraguchi M, Yoshimura N, Hashizume H, et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama spine study. Osteoarthritis Cartilage. 2014;22(1):104–110. doi: 10.1016/j.joca.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Depalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12(2):224–233. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34(9):934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 12.Samartzis D, Karppinen J, Mok F, et al. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93(7):662–670. doi: 10.2106/JBJS.I.01568. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzer AC, Aprill CN, Derby R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20(17):1878–1883. doi: 10.1097/00007632-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Jha SC, Higashino K, Sakai T, et al. Clinical significance of high-intensity zone for discogenic low back pain: a review. J Med Invest. 2016;63(1–2):1–7. doi: 10.2152/jmi.63.1. [DOI] [PubMed] [Google Scholar]
- 15.Peng B, Hou S, Wu W, Zhang C, Yang Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J. 2006;15(5):583–587. doi: 10.1007/s00586-005-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng B, Wu W, Hou S, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87-B(1):62–67. [PubMed] [Google Scholar]
- 17.Dongfeng R, Hou S, Wu W, et al. The expression of tumor necrosis factor-α and CD68 in high-intensity zone of lumbar intervertebral disc on magnetic resonance image in the patients with low back pain. Spine. 2011;36(6):E429–E433. doi: 10.1097/BRS.0b013e3181dfce9e. [DOI] [PubMed] [Google Scholar]
- 18.Aoki Y, Akeda K, An H, et al. Nerve fiber ingrowth into scar tissue formed following nucleus pulposus extrusion in the rabbit anular-puncture disc degeneration model: effects of depth of puncture. Spine. 2006;31(21):E774–E780. doi: 10.1097/01.brs.0000238681.71537.41. [DOI] [PubMed] [Google Scholar]
- 19.Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347–1355. doi: 10.1016/j.spinee.2013.07.490. [DOI] [PubMed] [Google Scholar]
- 20.Simon J, Mcauliffe M, Shamim F, Vuong N, Tahaei A. Discogenic low back pain. Phys Med Rehabil Clin N Am. 2014;25(2):305–317. doi: 10.1016/j.pmr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 21.García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217(1):1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34(8):1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millward-Sadler SJ, Costello PW, Freemont AJ, Hoyland JA. Regulation of catabolic gene expression in normal and degenerate human inter-vertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11(3):R65. doi: 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takegami N, Akeda K, Yamada J, et al. RANK/RANKL/OPG system in the intervertebral disc. Arthritis Res Ther. 2017;19(1):121. doi: 10.1186/s13075-017-1332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology. 2008;47(6):809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 26.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732–745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32(6):635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 28.Gruber HE, Rhyne AL, Hansen KJ, et al. Deleterious effects of discography radiocontrast solution on human annulus cell in vitro: changes in cell viability, proliferation, and apoptosis in exposed cells. Spine J. 2012;12(4):329–335. doi: 10.1016/j.spinee.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 30.Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24(3):173–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 31.di Matteo B, Filardo G, Kon E, Marcacci M. Platelet-rich plasma: evidence for the treatment of Patellar and Achilles tendinopathy—a systematic review. Musculoskelet Surg. 2015;99(1):1–9. doi: 10.1007/s12306-014-0340-1. [DOI] [PubMed] [Google Scholar]
- 32.Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204. doi: 10.1186/ar4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Y, Zhou X, Mao S, Zhang J, Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279–287. doi: 10.1016/j.ijsu.2018.03.078. [DOI] [PubMed] [Google Scholar]
- 34.Zhang HF, Wang CG, Li H, Huang YT, Li ZJ. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Drug Des Devel Ther. 2018;12:445–453. doi: 10.2147/DDDT.S156724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):e651:659–670. doi: 10.1016/j.arthro.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 37.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 38.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the paw classification system. Arthroscopy. 2012;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 39.Chahla J, Cinque ME, Piuzzi NS, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99(20):1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 40.Wasterlain AS, Braun HJ, Dragoo JL. Contents and formulations of platelet rich plasma. In: Maffulli N, editor. Platelet Rich Plasma in Musculoskeletal Practice. London: Springer London; 2016. pp. 1–29. [Google Scholar]
- 41.Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016(1):1–7. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beitzel K, Allen D, Apostolakos J, et al. Us definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28(01):029–034. doi: 10.1055/s-0034-1390030. [DOI] [PubMed] [Google Scholar]
- 43.Center for Biologics Evaluation and Research F Regulatory considerations for human cells, tissues, and cellular and TissueBased products: minimal manipulation and homologous useAvailable from: https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecompliance-regulatoryinformation/guidances/cellularandgenetherapy/ucm585403.pdf
- 44.Masuda K, Kato K. Treatment of degenerative disc Disease/Disc regeneration: growth factors and platelet rich plasma. In: ärtl R, Bonassar LJ, editors. Biological Approaches to Spinal Disc Repair and Regeneration for Clinicians. New York: Thieme; 2017. pp. 101–109. [Google Scholar]
- 45.Wang SZ, Chang Q, Lu J, Wang C. Growth factors and platelet-rich plasma: promising biological strategies for early intervertebral disc degeneration. Int Orthop. 2015;39(5):927–934. doi: 10.1007/s00264-014-2664-8. [DOI] [PubMed] [Google Scholar]
- 46.Wang SZ, Rui YF, Tan Q, Wang C. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15(5):220. doi: 10.1186/ar4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anitua E, Padilla S. Biologic therapies to enhance intervertebral disc repair. Regen Med. 2018;13(1):55–72. doi: 10.2217/rme-2017-0111. [DOI] [PubMed] [Google Scholar]
- 48.Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Chen WH, Lo WC, Lee JJ, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209(3):744–754. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 50.Chen WH, Liu HY, Lo WC, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30(29):5523–5533. doi: 10.1016/j.biomaterials.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Pirvu TN, Schroeder JE, Peroglio M, et al. Platelet-rich plasma induces annulus fibrosus cell proliferation and matrix production. Eur Spine J. 2014;23(4):745–753. doi: 10.1007/s00586-014-3198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khalaf K, Nikkhoo M, Kuo Ya-Wen, et al. Recovering the mechanical properties of denatured intervertebral discs through platelet-rich plasma therapy. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:933–936. doi: 10.1109/EMBC.2015.7318516. [DOI] [PubMed] [Google Scholar]
- 53.Nikkhoo M, Wang JL, Abdollahi M, et al. A regenerative approach towards recovering the mechanical properties of degenerated intervertebral discs: Genipin and platelet-rich plasma therapies. Proc Inst Mech Eng H. 2017;231(2):127–137. doi: 10.1177/0954411916681597. [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Yuan C, Wu C, et al. The role of TGF-β1/Smad2/3 pathway in platelet-rich plasma in retarding intervertebral disc degeneration. J Cell Mol Med. 2016;20(8):1542–1549. doi: 10.1111/jcmm.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hondke S, Cabraja M, Krüger JP, et al. Proliferation, migration, and ECM formation potential of human annulus fibrosus cells is independent of degeneration status. CARTILAGE. 2018;30(1):194760351876426. doi: 10.1177/1947603518764265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mietsch A, Neidlinger-Wilke C, Schrezenmeier H, et al. Evaluation of platelet-rich plasma and hydrostatic pressure regarding cell differentiation in nucleus pulposus tissue engineering. J Tissue Eng Regen Med. 2013;7(3):244–252. doi: 10.1002/term.524. [DOI] [PubMed] [Google Scholar]
- 57.Kim HJ, Yeom JS, Koh YG, et al. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J Orthop Res. 2014;32(4):551–556. doi: 10.1002/jor.22532. [DOI] [PubMed] [Google Scholar]
- 58.Liu MC, Chen WH, Wu LC, et al. Establishment of a promising human nucleus pulposus cell line for intervertebral disc tissue engineering. Tissue Eng Part C Methods. 2014;20(1):1–10. doi: 10.1089/ten.TEC.2013.0048. [DOI] [PubMed] [Google Scholar]
- 59.Cho H, Holt DC, Smith R, et al. The effects of platelet-rich plasma on halting the progression in porcine intervertebral disc degeneration. Artif Organs. 2016;40(2):190–195. doi: 10.1111/aor.12530. [DOI] [PubMed] [Google Scholar]
- 60.Yamada J, Akeda K, Takegami N, et al. Anti-inflammatory properties of platelet rich plasma-releasate on human intervertebral disc cells. Paper presented at: Orthopaedic Research Society Annual Meeting 2017; San Diego, CA. [Google Scholar]
- 61.Akeda K, Ohishi K, Masuda K, et al. Intradiscal injection of autologous platelet-rich plasma Releasate to treat discogenic low back pain: a preliminary clinical trial. Asian Spine J. 2017;11(3):380–389. doi: 10.4184/asj.2017.11.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang SZ, Fan WM, Jia J, et al. Is exclusion of leukocytes from platelet-rich plasma (PrP) a better choice for early intervertebral disc regeneration? Stem Cell Res Ther. 2018;9(1):199. doi: 10.1186/s13287-018-0937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hussain N, Johal H, Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics - a review of the literature. Sicot J. 2017;3(1):57. doi: 10.1051/sicotj/2017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13(1):147–158. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 65.Sawamura K, Ikeda T, Nagae M, et al. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15(12):3719–3727. doi: 10.1089/ten.TEA.2008.0697. [DOI] [PubMed] [Google Scholar]
- 66.Gullung GB, Woodall JW, Tucci MA, et al. Platelet-rich plasma effects on degenerative disc disease: analysis of histology and imaging in an animal model. Evid Based Spine Care J. 2011;2(4):13–18. doi: 10.1055/s-0031-1274752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Obata S, Akeda K, Imanishi T, et al. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012;14(6):R241. doi: 10.1186/ar4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gui K, Ren W, Yu Y, et al. Inhibitory effects of platelet-rich plasma on intervertebral disc degeneration: a preclinical study in a rabbit model. Med Sci Monit. 2015;21:1368–1375. doi: 10.12659/MSM.892510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang SZ, Jin JY, Guo YD, et al. Intervertebral disc regeneration using platelet-rich plasma-containing bone marrow-derived mesenchymal stem cells: a preliminary investigation. Mol Med Rep. 2016;13(4):3475–3481. doi: 10.3892/mmr.2016.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou Y, Shi G, Shi J, et al. Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration. Int Orthop. 2016;40(6):1143–1155. doi: 10.1007/s00264-015-2840-5. [DOI] [PubMed] [Google Scholar]
- 71.Li P, Zhang R, Zhou Q. Efficacy of platelet-rich plasma in retarding intervertebral disc degeneration: a meta-analysis of animal studies. Biomed Res Int. 2017;2017(2):1–10. doi: 10.1155/2017/7919201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akeda K, Imanishi T, Ohishi K, et al. Intradiscal injection of autologous serum isolated from platelet-rich-plasma for the treatment of discogenic low back pain: preliminary prospective clinical trial. poster presentation of the ISSLS meeting; June 14–18, 2011; Gothenburg, Sweden. [Google Scholar]
- 73.Bodor M, Toy A, Aufiero D. Disc regeneration with platelets and growth factors. In: Lana JFSD, Andrade Santana MH, Dias Belangero W, Malheiros Luzo AC, editors. Platelet-Rich Plasma: Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. pp. 265–279. [Google Scholar]
- 74.Navani A, Hames A. Platelet-rich plasma injections for lumbar discogenic pain: a preliminary assessment of structural and functional changes. Tech Reg Anesth Pain Manag. 2015;19(1-2):38–44. [Google Scholar]
- 75.Levi D, Horn S, Tyszko S, et al. Intradiscal platelet-rich plasma injection for chronic discogenic low back pain: preliminary results from a prospective trial. Pain Med. 2016;17(6):1010–1022. doi: 10.1093/pm/pnv053. [DOI] [PubMed] [Google Scholar]
- 76.Fitzpatrick J, Bulsara MK, Mccrory PR, Richardson MD, Zheng MH. Analysis of platelet-rich plasma extraction: variations in platelet and blood components between 4 common commercial kits. Orthop J Sports Med. 2017;5(1):2325967116675272. doi: 10.1177/2325967116675272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal platelet-rich plasma (PrP) injections: a prospective, double-blind, randomized controlled study. PMR. 2016;8(1):1–10. doi: 10.1016/j.pmrj.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Monfett M, Harrison J, Boachie-Adjei K, Lutz G. Intradiscal platelet-rich plasma (PrP) injections for discogenic low back pain: an update. Int Orthop. 2016;40(6):1321–1328. doi: 10.1007/s00264-016-3178-3. [DOI] [PubMed] [Google Scholar]
- 79.Aufiero D, Vincent H, Sampson S, et al. Regenerative injection treatment in the spine: review and case series with platelet rich plasma. J Stem Cells Res Rev Rep. 2015;2(1):1019. [Google Scholar]
- 80.Kirchner F, Anitua E. Intradiscal and intra-articular facet infiltrations with plasma rich in growth factors reduce pain in patients with chronic low back pain. J Craniovertebr Junction Spine. 2016;7(4):250–256. doi: 10.4103/0974-8237.193260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirchner F, Anitua E. Minimally invasive PRGF treatment for low back pain and degenerative disc disease. In: Anitua E, Cugat R, Sánchez M, editors. Platelet Rich Plasma in Orthopaedics and Sports Medicine. Cham: Springer International Publishing; 2018. pp. 259–275. [Google Scholar]
- 82.Wu J, du Z, Lv Y, et al. A new technique for the treatment of lumbar facet joint syndrome using intra-articular injection with autologous platelet rich plasma. Pain Physician. 2016;19(8):617–625. [PubMed] [Google Scholar]
- 83.Wu J, Zhou J, Liu C, et al. A Prospective study comparing platelet-rich plasma and local anesthetic (LA)/corticosteroid in intra-articular injection for the treatment of lumbar facet joint syndrome. Pain Pract. 2017;17(7):914–924. doi: 10.1111/papr.12544. [DOI] [PubMed] [Google Scholar]
- 84.Bhatia R, Chopra G. Efficacy of platelet rich plasma via lumbar epidural route in chronic prolapsed intervertebral disc Patients-A pilot study. J Clin Diagn Res. 2016;10(9):UC05–UC07. doi: 10.7860/JCDR/2016/21863.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussein M, Hussein T. Effect of autologous platelet leukocyte rich plasma injections on atrophied lumbar multifidus muscle in low back pain patients with monosegmental degenerative disc disease. Sicot J. 2016;2(1):12. doi: 10.1051/sicotj/2016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cameron JA, Thielen KM. Autologous platelet rich plasma for neck and lower back pain secondary to spinal disc herniation: midterm results. Spine Res. 2017;03(02):10. [Google Scholar]
- 87.Comella K, Silbert R, Parlo M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med. 2017;15(1):12. doi: 10.1186/s12967-016-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 89.Andia I, Abate M. Platelet-rich plasma: combinational treatment modalities for musculoskeletal conditions. Front. Med. 2018;12(2):139–152. doi: 10.1007/s11684-017-0551-6. [DOI] [PubMed] [Google Scholar]
- 90.Lutz GE. Increased nuclear T2 signal intensity and improved function and pain in a patient one year after an intradiscal platelet-rich plasma injection. Pain Med. 2017;18(6):1197–1199. doi: 10.1093/pm/pnw299. [DOI] [PubMed] [Google Scholar]