ABSTRACT
The present study investigated the anti-microbial and anti-mycotoxigenic activities of the ethyl acetate extract (EA) and a bioactive compound obtained from an endophytic fungus Alternaria alternata isolated from Catharanthus roseus leaves. A. alternata was identified using PCR-based 5.8S rDNA sequencing. The EA and bioactive compound, p-Coumaric acid (PC), showed concentration-dependent broad-spectrum anti-microbial activity against the tested bacteria, yeast, and fungi with MICs ranging from 7.8 to 250 µg/mL. The in vitro production of aflatoxin B1 (AFB1) from Aspergillus flavus and fumonisin B1 (FB1) from Fusarium verticillioides was completely inhibited by EA and PC at 400 µg/mL. The synthesis of the membrane-bound ergosterol from A. flavus and F. verticillioides was strongly inhibited by PC at 200 µg/mL. The EA and PC were found to show significant anti-microbial and anti-mycotoxigenic activities, hence, they could be explored as protective agents for preventing microbial deterioration and mycotoxins accumulation in food and feedstuffs during pre- and post-harvest and storage.
KEYWORDS: Endophytic Alternaria alternata, ethyl acetate extract, p-Coumaric acid, anti-microbial activity, aflatoxin B1, fumonisin B1
Introduction
The xerophilic filamentous fungi belong to the genera Aspergillus, Fusarium and Penicillium. They are predominantly associated with food- and feed stuffs during pre- and post-harvest and storage, and make them unfit for human consumption by destroying the nutritive values due to the synthesis of mycotoxins (Lutfullah et al. 2012; Abhishek et al. 2015). The most important groups of mycotoxins that are a major threat to human and animal health, and occur quite often in foodstuffs are aflatoxin B1 (AFB1) and fumonisin B1 (FB1) produced by toxigenic strains of A. flavus and F. verticillioides, respectively (Garcia et al. 2012; Mohana et al. 2016). In addition, the increasing multidrug-resistant (MDR) food-borne human pathogens, particularly the strains of Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae and Candida albicans are the greatest and most urgent global risk (Xu et al. 2014). Nowadays, many antibiotics and pesticides of synthetic origin are being widely used to manage problems associated with MDR microbes, moulds and mycotoxins, but their continuous and indiscriminate use has intensified the development of MDR micro-organisms and resulted in many ecological problems (Calbo et al. 2011). In this scenario, there is an urgent need to search alternative strategies that are eco-friendly and effective.
Endophytic fungi are omnipresent plant symbionts, reside inter- or intra-cellularly inside a host plant without causing any conspicuous infection (Guo et al. 2000; Ayob et al. 2016). The endophytic fungi indirectly benefit plant growth by producing many vital compounds including bioactive secondary metabolites (Kaul et al. 2012; Lou et al. 2013). Even though, many valuable bioactive compounds have been successfully identified from endophytic fungi (Yu et al. 2010; Bhagat et al. 2016), only a few investigations have been focused on the exploration of endophytic fungi and their bioactive molecules in food, agriculture and pharmaceuticals (Ezra et al. 2004; Yu et al. 2010; Kaul et al. 2012; Palem et al. 2015). In this backdrop, the present investigation was undertaken to evaluate the anti-microbial and anti-mycotoxigenic properties of A. alternata, an endophytic fungus isolated from the leaves of Catharanthus roseus (L.) G. Don., which is a well-known medicinal plant belonging to the family Apocynaceae.
Material and methods
Isolation of endophytic fungi
Endophytic fungi were isolated from the leaves of C. roseus following the procedure of Ezra et al. (2004). Briefly, fresh plant samples of C. roseus were collected from Charaka-Sushruta Vana, Bengaluru (India), and authenticated by Dr. Seetharam, Professor, Department of Biological Sciences, Bangalore University, Bengaluru (India). The authenticated voucher specimen (BUB/MB-BT/DCM/2016/08) was deposited in the Department of Microbiology and Biotechnology, Bangalore University, Bengaluru (India).
The collected leaf samples were cut into small pieces (0.5 × 0.5 cm), surface sterilised successively with 1% sodium hypochlorite and 90% ethanol for 3 min, and placed in potato-dextrose-agar (PDA) plates. The plates were incubated up to 7 days at 28 ± 2°C. The hyphal tips emerging out of the leaf samples were picked up using stereomicroscope (SZ-PT, Olympus Corporation, Tokyo, Japan) and sub-cultured on fresh PDA. The pure culture of the isolate DCM-EFS-11 showing the highest anti-microbial activity was selected for identification using the 5.8S rDNA sequence analysis followed by a binocular compound microscopic (CX41RF, Olympus Corporation, Tokyo, Japan) images analysis (Nagamani et al. 2006; Watanabe et al. 2010).
5.8S rDNA sequence analysis of the isolate DCM-EFS-11
The 5.8S rDNA sequence of the isolate DCM-EFS-11 was analysed following the procedure of Mohana et al. (2016). Briefly, the genomic DNA from fresh culture DCM-EFS-11 was isolated using genomic DNA isolation kit procured from Aristogene Bioscience Pvt. Ltd. (Bengaluru, India). The isolated DNA was amplified using a PCR machine (Q cycler, CM 6050, Quanta Biotech, England). The fungal-specific internal transcribed spacer (ITS) regions (5´TGATCCTTCYGCAGGTTCAC3´ and 5´ACCTGGTTGATCCTGCCAG3´) were used as forward and backward primers to amplify a fragment within the gene coding for 5.8S rRNA. The PCR products were analysed by electrophoresis and the separated bands were subjected to genome sequencing at Eurofins Genomics Pvt. Ltd., Bengaluru (India). The collected 5.8S rDNA sequences were searched against GenBank using NCBI-BLAST search tool and a phylogenetic tree was constructed based on the similarities between the related taxa. The isolate fungal strain DCM-EFS-11 was identified using a phylogenetic tree followed by microscopic image analysis and the base sequence of the identified fungal strain was deposited in NCBI-GenBank (accession no. MG551266).
Ethyl acetate extraction and bioactive compound isolation from the culture filtrates of endophytic A. alternata
Ethyl acetate extract was prepared from the culture filtrate of A. alternata following the procedure of Srivastava and Anandrao (2015). Briefly, A. alternata was grown in 500 mL PDB up to 15 days at 28°C and filtered through a Whatman grade 1 filter paper. The collected filtrate (100 mL) was extracted with the same amount of ethyl acetate using a separatory funnel, and the collected ethyl acetate fraction was concentrated to dryness using a vacuo evaporator (Lyoquest-85, Telstar Technologies, S.L. Terrassa, Spain). The dried crude ethyl acetate extract (EA) was collected (45 mg) and then subjected to bioactivity evaluation followed by compound isolation using column chromatography as described by Mohana et al. (2010). Briefly, a silica gel chromatographic column (mesh size 60–120, SRL, India) loaded with EA was eluted sequentially with the mixtures of CHCl3 and MeOH (10:0, 7.5:2.5, 1:1, 2.5:7.5, 0:10, v/v). The collected chromatogram fractions were allowed to dry and all the fractions were subjected for anti-microbial activity evaluation. The fraction showing the highest activity was selected and further purified using TLC where CHCl3: MeOH (9:1) served as the mobile phase. The TLC bands were scraped out, dissolved individually in MeOH, filtered using Whatman grade 1 filter paper, and allowed to dry. The pure crystals (08 mg) obtained from the third band were collected and subjected to anti-microbial evaluation followed by ESI-MS and FT-IR spectral analysis. The bioactive compound was identified by comparing the spectral data with the published literature.
Evaluation of anti-microbial activities of EA and PC
Microbes tested
In order to test the anti-microbial activity of EA and PC, following microbes were used: seven bacterial species, viz., Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus and Streptococcus faecalis; two yeast species, viz., Candida albicans and Cryptococcus neoformans; and 15 species of fungi, viz., Alternaria brassicicola, Alternaria geophila, Aspergillus flavus (aflatoxigenic strain), Aspergillus fumigatus, Aspergillus ochraceus, Aspergillus tamarii, Aspergillus terreus, Curvularia tetramera, Fusarium oxysporum, Fusarium lateritium, Fusarium equiseti, Fusarium udum, Fusarium verticillioides (fumonisinogenic strain), Penicillium citrinum and Penicillium expansum. The collection centres and sources of these isolates have been described earlier (Thippeswamy et al. 2014; Abhishek et al. 2015). The cultures, grown for 24 h, 48 h and 7 days for bacteria, yeast and fungi, respectively, were used for the assay.
Evaluation of anti-bacterial and anti-yeast activities
The disc diffusion method was employed for the evaluation of anti-bacterial and anti-yeast activities of EA and PC against seven bacterial and two yeast species (Hajji et al. 2010). Briefly, the discs (6 mm in diameter) impregnated with EA (150 µg/disc) and PC (100 µg/disc) were placed on the pre-inoculated MHA (108 CFU/mL of bacteria) and MGYPA (106 CFU/mL of yeast) plates and incubated at 37°C for 24 h for bacteria and 48 h for yeast. The disc impregnated with the same amount of DMSO served as a negative control and the antibiotics erythromycin (15 mcg) and itraconazole (10 mcg) served as positive control for bacterial and yeast species, respectively. The anti-microbial activities of EA and PC were determined by measuring the zone of inhibition (ZOI) around the discs.
Evaluation of anti-fungal activity
The poisoned food technique was employed for the evaluation of anti-fungal activities of EA and PC against 15 seed-borne fungal species following the procedure of Mohana et al. (2010). Briefly, mycelial discs (5 mm in diameter) of the test fungi were placed on SDA impregnated with EA (150 µg/mL) and PC (100 µg/mL), and incubated at 28 ± 2°C for 7 days. The SDA impregnated with the same amount of DMSO served as a negative control and zinc ethylene bisthiocarbamate (Indofil Z-78) (2 mg/mL) served as a positive control. The anti-fungal activities of EA and PC were measured by calculating the percentage of mycelial growth inhibition (%MI) using the formula given below.
where C is the diameter of mycelial growth in control plate and T is the diameter of mycelial growth in a treated plate.
Determination of MICs of EA and PC against microbes
The broth dilution technique was employed for the determination of MICs of EA and PC (Hajji et al. 2010). Briefly, 15 µL of bacteria (108 CFU/mL), yeast (106 CFU/mL), and fungi (104 CFU/mL) were independently inoculated into a microtiter plate containing 200 µL of MHB, MGYPB, and SDB impregnated with twofold dilution of EA and PC (3.9 µg/mL–2 mg/mL), respectively, and incubated for a specific period and at temperature as explained above. After incubation, 50 µL of iodo-nitro-tetrazolium chloride (INT 2 mg/mL) was added to each well and incubated further for 30 min. The pale yellow-coloured INT was reduced to pink indicating the presence of viable cells and no change in colour indicated the inhibition of microbial growth. The lowest concentration at which the colour remained unchanged was considered as MIC. DMSO served as a negative control.
Evaluation of anti-mycotoxigenic activities of EA and PC
The in vitro anti-aflatoxin B1 and anti-fumonisin B1 activities of EA and PC were determined following the procedure of Abhishek et al. (2015). Briefly, 100 µL of spore suspension (104 CFU/mL) of a toxigenic strain (A. flavus) was inoculated into the SMKYB, and F. verticillioides was inoculated into the SDA containing the requisite amount of EA and PC (50, 100, 200 and 400 µg/mL), and incubated at 28°C for 10 days. The medium devoid of EA and PC served as control. After incubation, AFB1 was extracted from the culture filtrate of A. flavus by adding an equal volume of CHCl3 (Shukla et al. 2008), and FB1 was extracted from a culture of F. verticillioides by adding an equal amount of acetonitrile-water mixture (1:1, v/v) (Baily et al. 2005).
The extracted AFB1 was spotted on the TLC plate adjacent to AFB1 standard (Sigma, Germany), then eluted using CHCl3-acetone (96:4, v/v) as the mobile phase and observed under ultraviolet light at 365 nm (UV-cabinet, Labline, India). AFB1 was estimated qualitatively by visual comparison of the fluorescence intensity of the samples with standard spots, and quantitatively by measuring the light absorbance of the samples using a spectrophotometer at 600 nm wavelengths (UV-1800, Shimadzu, Japan). The amount of AFB1 content was calculated by the formula given below.
where D is absorbance, M is the molecular weight of AFB1 (312), E is the molar extinction coefficient of AFB1 (21,800) and L is the path length (1 cm).
The extracted FB1 was spotted on the TLC plate adjacent to standard FB1 (Sigma, Germany) and eluted using a mixture of butanol-acetic acid-water (20:10:10, v/v/v) as the mobile phase. The solution of p-anisaldehyde in methanol-acetic acid-H2SO4 (85:10:0.5, v/v/v) (0.5%) was sprayed on the chromatogram and incubated at 110°C for 10 min. The amount of FB1 on chromatogram was estimated by comparing the band’s intensity with standard spots using a spectrophoto-densitometer at 600 nm wavelengths (BioRad, Universal Hood II 720BR/02170, USA).
The in vivo efficacy of EA and PC on inhibition of AFB1 and FB1 production was estimated using viable maize seed samples following the procedure of Bailly et al. (2005). Briefly, 100 µL spore suspension of toxigenic strains of A. flavus and F. verticillioides (104 CFU/mL) was inoculated separately into the maize samples treated with different amount of EA and PC (50, 100, 200 and 400 µg/g). The maize seeds impregnated with EA and PC without spore inoculum of A. flavus and F. verticillioides were used for natural mycoflora analysis and seedling-vigour estimation following the procedure of ISTA (2006). The maize seeds without EA and PC served as control. Both treated and control maize samples were stored in plastic containers (200 g pack–1) separately up to 15 days at 25 ± 2°C. The water activity (aw) was adjusted to 0.95 by adding sterile distilled water (Mohana et al. 2014). After 15 days, all samples were powdered separately, A. flavus treated samples were used for AFB1 extraction followed by quantification, whereas F. verticillioides-treated maize samples were used for FB1 extraction followed by quantification using the same procedure as explained above in in vitro studies.
Mode of anti-fungal action of PC against ergosterol synthesis in A. flavus and F. verticillioides
The mode of action of PC on the inhibition of ergosterol synthesis from the toxigenic strains of A. flavus and F. verticillioides was determined following the procedure of Tian et al. (2012). Briefly, 50 µL of spore suspension of the toxigenic strains of A. flavus (104 spores/mL) was separately inoculated with SDB containing desired concentrations of PC (50, 100, 150 and 200 µg/mL) and incubated at 28 ± 2°C for 4 days. After incubation, the mycelial mat was collected and subjected to ergosterol extraction by adding a solution of alcoholic potassium hydroxide (7.5:2.5 v/v) and n-heptane. The extracted ergosterol was analysed by scanning between 230 and 300 nm in a spectrophotometer. The SDB without PC served as a positive control and SDB with only PC (without A. flavus and F. verticillioides) served as a blank. A characteristic curve at 230 and 282 nm indicates the presence of ergosterol. The percent ergosterol content was calculated using the formula given below.
where 290 and 518 are the E values (in percentages per centimetre) of crystalline ergosterol and 24(28)-dehydroergosterol, respectively, and pellet weight is the net wet weight (g).
Results
Among the 14 different endophytic fungi isolated from the leaf of C. roseus, an isolate DCM-EFS-11 showing the highest anti-microbial activity, was selected for 5.8S rDNA sequence analysis for identification. The sequence analysis of the isolate showed 99.0% similarity with A. alternata and 97–98% similarity with other Alternaria spp. The sequence analysis along with the microscopic image analysis identified the isolate to be A. alternata. The complete 5.8S rDNA nucleotide sequence of A. alternata was submitted to the NCBI GenBank (India), and an accession number (MG551266) was given.
In the column chromatography separation, a total of 21 fractions (10 mL per fraction) were collected from EA, of which, eleventh fraction showed anti-microbial activity. Thus it was selected for further purification of the bioactive compound using the TLC system. In the TLC separation, a total of six bands were eluted with the Rf values of 0.22, 0.30, 0.45, 0.63, 0.75 and 0.90. Among them, the third band with an Rf value of 0.45 and showing anti-microbial activity was collected from the TLC, and subjected to ESI-MS and FT-IR analysis for identification. In the positive mode ESI-MS analysis, the isolated bioactive compound showed a molecular weight of 165.05 [M + H]+ (Figure 1). In the FT-IR spectra, the broad signals at 3376.86 (OH stretching) and 2927.93 (C-H aromatic + (CH)C=C), sharp peak at 1679.88 (-CC aromatic), 1519.61 (COO-) and 1348.51 (β (CH)C=C + βOH) medium signals 1072.23 [β (CH)], 671.50 (COO–), 665.02 (CC), 455.31 (CC) and 442.72(CH) were observed. Based on a comparison of ESI-MS and FT-IR with the reported values in the literature (Ruelas et al. 2006; Swislocka et al. 2012; Umashankar et al. 2014, 2015; Akdemir et al. 2017), the bioactive compound was predicted to be p-Coumaric acid (C9H8O3) (PC) with a molecular weight of 164.04 Da (Figure 1).
Figure 1.
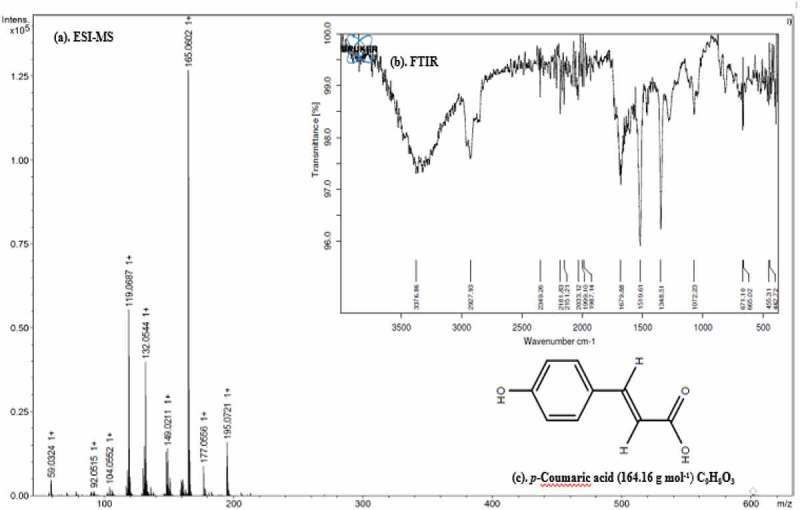
ESI-MS spectra (a), FT-IR spectra (b) and chemical structure of the p-Coumaric acid.
The anti-bacterial and anti-yeast activities of EA and PC were determined qualitatively and quantitatively by measuring ZOIs and MICs against three Gram-positive bacteria, five Gram-negative bacteria and two yeast species (Table 1). For both EA and PC, the ZOIs and MICs ranged from 12.5 to 24.5 mm and from 7.8 to 125 µg/mL, respectively. Among the bacteria tested, the most susceptible bacterium against EA and PC was S. faecalis (MICs 15.6 and 7.8 µg/mL, respectively) and the most resistant bacterium was P. aeruginosa (MICs 125 and 62.5 µg/mL), respectively. Further, EA and PC also showed strong anti-yeast activity against C. albicans and C. neoformans with the ZOIs and MICs ranging from 16.6 to 23.3 mm and from 7.8 to 125 µg/L, respectively. The anti-microbial activities of PC were comparable with the standard drugs, erythromycin and itraconazole.
Table 1.
Anti-bacterial and anti-yeast activities of EA, PC, erythromycin and itraconazole against some food-borne human pathogenic bacteria and yeast.
| EA |
PC |
Erythromycin/Itraconazole |
|||
|---|---|---|---|---|---|
| Microbial isolates | ZOI | MIC | ZOI | MIC | ZOI |
| E. coli | 13.5 ± 0.6 | 125 | 16.8 ± 1.0 | 62.5 | 10.1 ± 0.3 |
| K. pneumoniae | 17.2 ± 1.2 | 31.2 | 21.6 ± 1.3 | 15.6 | 15.5 ± 0.7 |
| P. vulgaris | 16.8 ± 0.8 | 62.5 | 19.6 ± 0.9 | 15.6 | 8.5 ± 0.4 |
| P. aeruginosa | 12.9 ± 0.6 | 125 | 16.5 ± 0.7 | 62.5 | 11.8 ± 0.6 |
| S. typhi | 17.9 ± 0.9 | 31.2 | 20.5 ± 0.8 | 31.2 | 19.5 ± 0.6 |
| S. aureus | 18.5 ± 1.2 | 31.2 | 21.4 ± 0.9 | 15.6 | 17.2 ± 0.5 |
| S. faecalis | 21.5 ± 1.4 | 15.6 | 24.5 ± 1.3 | 07.8 | 19.6 ± 0.7 |
| C. albicans | 16.6 ± 0.8 | 125 | 21.6 ± 1.1 | 31.2 | 13.5 ± 0.6 |
| C. neoformans | 20.7 ± 1.0 | 62.5 | 23.3 ± 1.0 | 07.8 | 14.8 ± 0.4 |
The data given are the means of four replicates ± standard error (p ≤ 0.05). The ZOIs values of EA (150 µg/disc) and PC (100 µg/disc) expressed in mm, and MICs values in µg/mL. Erythromycin (15 mcg/disc) served as positive control for bacteria and Itraconazole (10 mcg/disc) for yeast.
The EA and PC showed significant anti-fungal activities against 15 different seed-borne plant pathogenic fungi with %MI and MICs values ranging from 23.6 to 82.6 mm and from 7.8 to 250 µg/mL, respectively (Table 2). Among the fungi tested, the most susceptible fungus was F. udum (%MI 68.5% and 82.6%) followed by C. tetramera (%MI 63.2% and 80.4%), whereas P. expansum (%MI 23.6% and 54.6%) and A. flavus (%MI 28.9% and 58.4%) were recorded as the most resistant organisms. On a comparative evaluation of EA and PC with indofil Z-78, the %MI values of EA and PC were comparable with indofil Z-78.
Table 2.
Anti-fungal activity of EA, PC, and indofil Z-78 against different field and storage fungi.
| EA |
PC |
Indofil Z-78 |
|||
|---|---|---|---|---|---|
| Fungal species | %MI | MIC | %MI | MIC | %MI |
| A. brassicicola | 57.4 ± 2.8 | 31.2 | 73.4 ± 1.4 | 15.6 | 70.6 ± 0.8 |
| A. geophila | 51.5 ± 2.2 | 62.5 | 70.6 ± 1.9 | 31.2 | 67.4 ± 0.6 |
| A. flavus† | 28.9 ± 1.4 | 250 | 58.4 ± 1.0 | 125 | 51.5 ± 1.1 |
| A. fumigatus | 48.5 ± 2.1 | 125 | 65.6 ± 0.9 | 62.5 | 61.2 ± 0.9 |
| A. ochraceous | 50.6 ± 0.6 | 62.5 | 69.8 ± 0.8 | 31.2 | 58.6 ± 0.5 |
| A. tamarii | 28.5 ± 0.3 | 250 | 56.6 ± 0.6 | 125 | 52.6 ± 0.8 |
| A. terreus | 49.5 ± 1.0 | 125 | 66.8 ± 0.8 | 62.5 | 64.6 ± 1.2 |
| C. tetramera | 63.2 ± 2.3 | 31.2 | 80.4 ± 1.7 | 15.6 | 79.8 ± 1.0 |
| F. equiseti | 58.1 ± 1.5 | 31.2 | 82.2 ± 1.2 | 15.6 | 80.2 ± 1. |
| F. lateritium | 51.8 ± 1.2 | 62.5 | 73.5 ± 0.7 | 31.2 | 68.4 ± 0.7 |
| F. oxysporum | 41.8 ± 1.0 | 125 | 75.8 ± 1.5 | 62.5 | 70.3 ± 0.7 |
| F. udum | 68.5 ± 1.3 | 15.6 | 82.6 ± 1.2 | 07.8 | 82.4 ± 1.5 |
| F. verticillioides‡ | 59.8 ± 0.9 | 62.5 | 71.6 ± 1.0 | 31.2 | 65.6 ± 0.9 |
| P. citrinum | 28.5 ± 0.6 | 125 | 59.8 ± 0.8 | 125 | 56.8 ± 0.5 |
| P. expansum | 23.6 ± 0.8 | 250 | 54.6 ± 1.3 | 125 | 51.4 ± 0.7 |
The data given are the means of three replicates ± standard error (p ≤ 0.05). The %MIs values of EA (150 µg/mL) and PC (100 µg/mL) are expressed in percentage, and MICs values are expressed in µg/mL. Indofil Z-78 (2 mg/mL) served as a positive control. †Aflatoxin B1 producing strain and ‡Fumonisin B1 producing strain.
The AFB1 production from A. flavus and FB1 production from F. verticillioides were significantly inhibited by EA and PC (Table 3). The control sets showed the presence of the highest amount of AFB1 and FB1 but the in vitro production of these toxins was completely inhibited by EA and PC at 400 µg/mL. In the viable maize model assay, both EA and PC significantly protected maize seeds from a wide range of fungal infestation and also inhibited AFB1 and FB1 synthesis from A. flavus and F. verticillioides at 400 µg g–1. Based on the values of percent incidences (PI), Alternaria (PI 38.7%), Aspergillus (PI 82.6%), Cladosporium (PI 21.5%), Curvularia (PI 26.7%), Fusarium (PI 71.6%), Penicillium (PI 59.6%), Rhizopus (PI 18.6%) and Trichoderma (PI 15.0%) were recorded as the dominant fungi in the control seed samples, but the emergence of these fungal species viz., Alternaria, Aspergillus, Cladosporium, Curvularia, Fusarium, Penicillium, Rhizopus and Trichoderma were significantly inhibited by EA and PC with PI values 10%, 35%, 0%, 9%, 15%, 32%, 0% and 2% in case of EA, and 0%, 10%, 0%, 0%, 0%, 14%, 0% and 0% in case of PC, respectively. Compared to the seedling-vigour index of the control samples (2245), the seedling-vigour index in EA (2472) and PC (2580) treated maize samples was significantly increased at 400 µg kg–1 treatment. The ergosterol synthesis from A. flavus and F. verticillioides was significantly inhibited by PC (Figure 2) and the percent reduction in the ergosterol in comparison with the control was 24.56, 37.56, 46.84 and 58.45%, and 46.89, 58.34, 68.95 and 76.93% at 50, 100, 150 and 200 µg/mL, respectively.
Table 3.
Efficacy of EA and PC on AFB1 production from A. flavus and FB1 production F. verticillioides.
| AFB1 production from A. flavus |
FB1 production from F. verticillioides |
|||||||
|---|---|---|---|---|---|---|---|---|
|
In vitro |
In vivo |
In vitro |
In vivo |
|||||
| Extract concentrations (EA/PC) | EA | PC | EA | PC | EA | PC | EA | PC |
| Control | 1455.6 ± 8.9 | 1455.6 ± 8.9 | 1586.8 ± 9.7 | 1586.8 ± 9.7 | 82.9 ± 3.8 | 82.9 ± 3.8 | 50.5 ± 3.7 | 50.5 ± 3.7 |
| 50 | 1005.2 ± 7.6 | 768.4 ± 7.6 | 1285.6 ± 8.8 | 1145 ± 4.7 | 41.6 ± 2.5 | 22.7 ± 1.8 | 41.6 ± 2.5 | 33.7 ± 2.1 |
| 100 | 614.6 ± 3.9 | 143 ± 1.8 | 895.5 ± 4.5 | 685 ± 4.5 | 10.8 ± 0.7 | 0.0 ± 0.0 | 25.4 ± 2.2 | 14.4 ± 0.8 |
| 200 | 135.8 ± 2.6 | 0.0 ± 0.0 | 564.4 ± 2.7 | 145 ± 2.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 12.9 ± 1.7 | 0.0 ± 0.0 |
| 400 | 0.0 ± 0.0 | 0.0 ± 0.0 | 195.7 ± 1.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
The data given are the means of three replicates ± standard error (p ≤ 0.05). The values of AFB1 production are expressed in µg/mL under in vitro and µg/g under in vivo. The values of FB1 production are expressed in mg/mL under in vitro and mg/g under in vivo.
Figure 2.
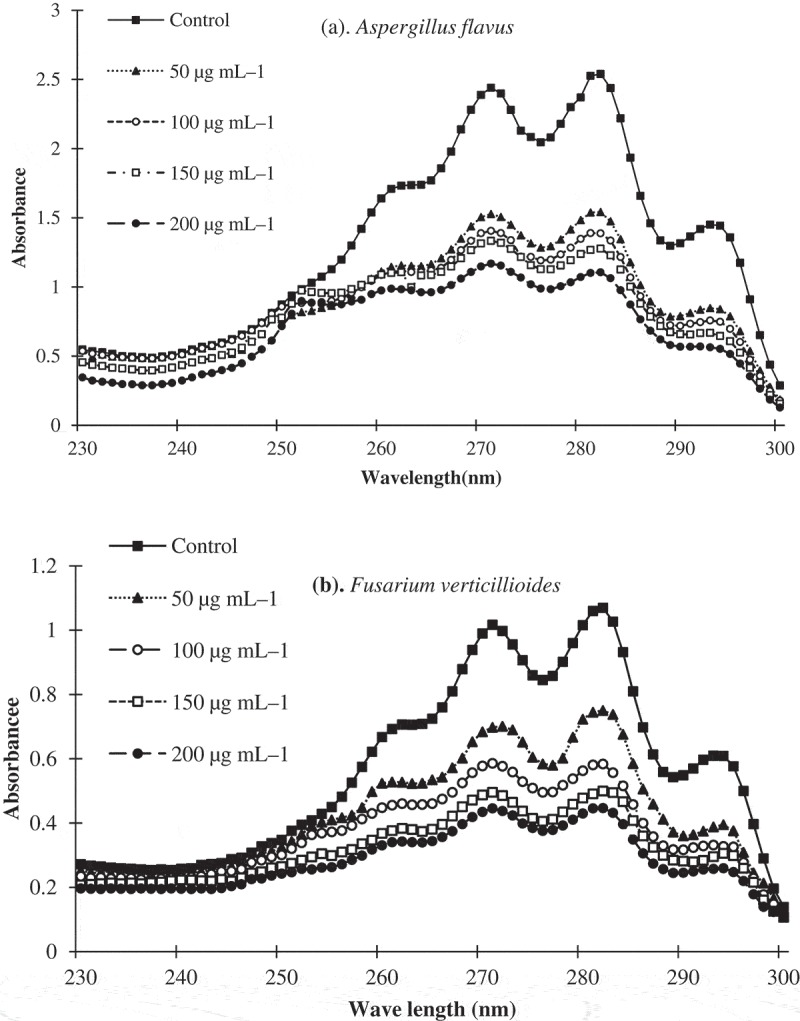
Inhibitory effect of p-Coumaric acid on the synthesis of ergosterol from A. flavus (a) and F. verticillioides (b).
Discussion
The present study confirms that both EA and PC show broad-spectrum anti-microbial activity against bacteria, yeast and fungi. Among the bacteria tested, the Gram-positive bacteria are more susceptible than the Gram-negative ones. Similarly, the field fungi were more susceptible than the storage fungi. The obtained results confirm the potential of EA and PC in the management of diseases caused by Gram-positive bacteria and field fungi. The broth dilution method was employed for the determination of MICs of EA and PC because this method facilitates better interaction between extracts and micro-organisms than the diffusion techniques (Prakash et al. 2014). The values of MIC of EA and PC were comparable or greater than the synthetic agents erythromycin, itraconazole, and Indofil Z-78, indicating possible exploitation of endophytic A. alternata for the production of large-scale chemotherapeutic and anti-mycotoxigenic agents. Maize is a nutritionally important staple food for human but it is susceptible to fungal infestation either in the field before harvest or at a post-harvest stage such as storage and processing. The common fungal flora reported in maize samples worldwide includes the species of Alternaria, Aspergillus, Chaetomium, Cladosporium, Curvularia, Drechslera, Fusarium Penicillium, Phoma and Trichoderma (Soares et al. 2013; Mohana et al. 2016). In the in vivo studies, the conditions used in the viable maize model were similar to the conditions prevalent during the storage. The obtained results confirm that both EA and PC were effective in suppressing a wide number of seed-borne fungi. The inhibition of mycotoxin production suggests their possible use as protective agents during storage of food grains and feed-stuffs. Ergosterol is a fungal-specific sterol component in the cell membrane and also one of the potential target sites for the action of many anti-fungal drugs (Tian et al. 2012). The depletion of ergosterol alters the activity of several membrane-bound enzymes related to nutrient transport and chitin synthesis. The present study confirms that the ergosterol synthesis from A. flavus was strongly inhibited by PC, which indicates ergosterol bound to the plasma membrane is a possible target for the anti-fungal action of PC.
A high number of bioactive secondary metabolites have been isolated from Alternaria (Gu et al. 2009; Lou et al. 2013; Bhagat et al. 2016). The extracts of endophytic A. alternata and A. brassicicola have shown anti-microbial activity against B. subtilis, E. coli, Mycobacterium tuberculosis, P. fluorescens and C. albicans (Qiao et al. 2007; Fernades et al. 2009; Sonaimuthu et al. 2011; Srivastava et al. 2015). Bhagat et al. (2016) reported that the bioactive compound, altenuene, isolated from A. alternata an endophytic fungus of Catharanthus roseus shows significant antioxidant and insecticidal activity. Umashankar et al. (2014; 2015) reported that the bioactive compound p-Coumaric acid isolated from endophytic Alternaria species shows anti-cancer activity against HeLa cancer cell line. As per best of our knowledge, there is no report on the anti-microbial activities of EA and PC from A. alternata isolated from C. roseus against a wide-range of food-borne bacteria, yeast and fungi, and further this is also the first report on the anti-aflatoxigenic and anti-fumonisinic properties of EA and PC. Hence, EA and PC could be explored as anti-microbial agents for the protection of food and feedstuff against microbial deterioration and mycotoxins contamination.
Acknowledgements
This work was financially supported by the Bangalore University, Bengaluru. The authors wish to thank the Indian Institute of Science, Bengaluru (India) for ESI-MS analysis and interpretation. The authors also wish to thank the Institute of Excellence, University of Mysore, Mysore (India) for providing FT-IR analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abhishek RU, Thippeswamy S, Manjunath K, Mohana DC.. 2015. Antifungal and antimycotoxigenic potency of Solanum torvum Swartz. leaf extract: isolation and identification of compound active against mycotoxigenic strains of Aspergillus flavus and Fusarium verticillioides. J Appl Microbiol. 119:1624–1636. [DOI] [PubMed] [Google Scholar]
- Akdemir FNE, Albayrak M, Calik M, Bayir Y, Gulcin I. 2017. The protective effects of p-Coumaric acid on acute liver and kidney damages induced by Cisplatin. Biomedicines. 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayob FW, Simarani K. 2016. Endophytic filamentous fungi from a Catharanthus roseus: identification and its hydrolytic enzymes. Saudi Pharm J. 24:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly JD, Querin A, Tardieu D, Guerre P. 2005. Production and purification of fumonisins from a highly toxigenic Fusarium verticilloides strain. Revue Med Vet. 156:547–554. [Google Scholar]
- Bhagat J, Kaur A, Kaur R, Yadav AK. 2016. Cholinesterase inhibitor (Altenuene) from an endophytic fungus Alternaria alternata: optimization, purification and characterization. J Appl Microbiol. 121:1015–1025. [DOI] [PubMed] [Google Scholar]
- Calbo E, Freixas N, Xercavins M, Riera M, Nicolas C, Monistrol O, Sole MDM, Sala MR, Vila J, Garau J. 2011. Foodborne nosocomial outbreak of SHV1 and CTX-M–15-producing Klebsiella pneumoniae: epidemiology and control. Clin Infect Dis. 52:743–749. [DOI] [PubMed] [Google Scholar]
- Ezra D, Hess WM, Strobel GA. 2004. New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology. 150:4023–4031. [DOI] [PubMed] [Google Scholar]
- Fernandes MDRV, Silva TACE, Pfenning LH, Costa-Neto CMD, Heinrich TA, Alencar SMD, Lima MAD, Ikegaki M. 2009. Biological activities of fermentation extract of the endophytic fungus Alternaria alternata isolated from Coffea arabica L. Braz J Pharm Sci. 45:677–685. [Google Scholar]
- Garcia D, Ramos AJ, Sanchis V, Marin S. 2012. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. Int J Food Microbiol. 153:21–27. [DOI] [PubMed] [Google Scholar]
- Gu W. 2009. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Mallus halliana. World J Microbiol Biotechnol. 25:1677–1683. [Google Scholar]
- Guo LD, Hyde KD, Liew ECY. 2000. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 147:617–630. [DOI] [PubMed] [Google Scholar]
- Hajji M, Masmoudi O, Souissi N, Triki Y. 2010. Chemical composition, angiotensin i-converting enzyme (ace) inhibitory, antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chem. 121:724–731. [Google Scholar]
- ISTA 2006. International rules for seed testing. Seed Sci Technol. 21:25–70. [Google Scholar]
- Kaul S, Gupta S, Ahmed M, Dhar MK. 2012. Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem Rev. 11:487–505. [Google Scholar]
- Lou J, Fu L, Peng Y, Zhou L. 2013. Metabolites from Alternaria fungi and their bioactivities. Molecules. 18:5891–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfullah G, Hussain A. 2012. Studies on contamination level of aflatoxins in some cereals and beans of Pakistan. Food Control. 23:32–36. [Google Scholar]
- Mohana DC, Raveesha KA. 2010. Antimycotic, antibiodeteriorative and antiaflatoxigenic potency of 2-hydroxy–4-methoxybenzaldehyde isolated from Decalepis hamiltonii on fungi causing biodeterioration of maize and sorghum grains. J Mycol Plant Pathol. 40:197–206. [Google Scholar]
- Mohana DC, Thippeswamy S, Abhishek RU, Manjunath K. 2014. Natural occurrence of Aspergillus flavus and Fusarium verticillioides, and AFB1 and FB1 contamination in maize grown in Southern Karnataka (India). Can J Plant Protect. 2:17–20. [Google Scholar]
- Mohana DC, Thippeswamy S, Abhishek RU, Shobha B. 2016. Studies on seed-borne mycoflora and aflatoxin B1 contaminations in food based seed samples: molecular detection of mycotoxigenic Aspergillus flavus and their management. Int Food Res J. 23:2689–2694. [Google Scholar]
- Nagamani A, Kunwar IK, Manoharachary C. 2006. Handbook of soil fungi. 1st ed. New Delhi: I. K International Pvt Ltd; p. 6–436. [Google Scholar]
- Palem PP, Kuriakose GC, Jayabaskaran C. 2015. An endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. Plos One. 10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash B, Mishra PK, Kedia A, Dubey NK. 2014. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT-Food Sci Technol. 56:240–247. [Google Scholar]
- Qiao L, Yuan L, Gao J, Shen Y. 2007. Tricyloalternarene derivatives produced by an endophyte Alternaria alternata isolated from Maytenus hookeri. J Basic Microbiol. 47:340–343. [DOI] [PubMed] [Google Scholar]
- Ruelas C, Tiznado‐Hernández ME, Sánchez‐Estrada A, Robles‐Burgueno MR, Troncoso‐Rojas R. 2006. Changes in phenolic acid content during Alternaria alternata infection in tomato fruit. J Phytopathol. 154:236–244. [Google Scholar]
- Shukla R, Kumar A, Prasad CS, Srivastava B. 2008. Antimycotic and antiaflatoxigenic potency of Adenocalymma alliaceum Miers. on fungi causing biodeterioration of food commodities and raw herbal drugs. Int Biodeterior Biodegradation. 62:348–351. [Google Scholar]
- Soares C, Calado T, Venancio A. 2013. Mycotoxin production by Aspergillus niger aggregate strains isolated from harvested maize in three Portuguese regions. Rev Iberoam Micol. 30:9–13. [DOI] [PubMed] [Google Scholar]
- Sonaimuthu V, Parihar S, Thakur JP, Luqman S. 2011. Tenuazonic acid: a promising antitubercular principle from Alternaria alternata. Microbiol Res. 2:63–65. [Google Scholar]
- Srivastava A, Anandrao RK. 2015. Antimicrobial potential of fungal endophytes isolated from leaves of Prosopis juliflora (SW.) DC. an important weed. Int J Pharm Pharmacol Sci. 7:128–136. [Google Scholar]
- Swislocka R, Kowczyk-Sadowy M, Kalinowska M, Lewandowski W. 2012. Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. J Spectrosc. 27(1):35–48. [Google Scholar]
- Thippeswamy S, Mohana DC, Abhishek RU, Manjunath K. 2014. Inhibitory effect of alkaloids of Albizia amara and Albizia saman on growth and fumonisin B1 production by Fusarium verticillioides. Int Food Res J. 21:947–952. [Google Scholar]
- Tian J, Ban XQ, Zeng H, He JS. 2012. The mechanism of antifungal action of essential oil from Dill (Anethum graveolens L.) on Aspergillus flavus. Plos One. 7:e30147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umashankar T, Govindappa M, Ramachandra YL. 2014. In vitro antioxidant and antimicrobial activity of partially purified coumarins from fungal endophytes of Crotalariapallida. Int J Curr Microbiol App Sci. 3:58–72. [Google Scholar]
- Umashankar T, Govindappa M, Ramachandra YL, Padmalatha RS. 2015. Isolation and characterization of coumarin isolated from endophyte, Alternaria Species–1 of Crotalariapallida and its apoptotic action on HeLa cancer cell line. Metabolomics. 5:1–8. [Google Scholar]
- Watanabe T. 2010. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. New York (NY): CRC press, Taylor and Francis group; p. 29–135. [Google Scholar]
- Xu CM, Yagiz Y, Hsu WY, Simonne A. 2014. Antioxidant, antibacterial and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J Agric Food Chem. 62:6640–6649. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhang L, Li L, Zheng C. 2010. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res. 165:437–449. [DOI] [PubMed] [Google Scholar]


