Abstract
Objective
To evaluate the effects of surfactant administration on the neonatal brain using 3-channel neonatal electroencephalography (EEG).
Study design
A prospective cohort of 30 infants had scalp electrodes placed to record brain waves using 3-channel EEG (Fp1-O1, C3-C4, and Fp2-O2). Sixty-second EEG epochs were collected from a 10-minute medication-free baseline, during premedication for endotracheal intubation, at surfactant administration, and at 10, 20, and 30 minutes after surfactant administration for amplitude comparisons. Oxygen saturation and heart rate were monitored continuously. Blood pressure and transcutaneous carbon dioxide were recorded every 5 minutes.
Results
Eighteen of 29 infants (62%) exhibited brain wave suppression on EEG after surfactant administration (P ≤ .008). Four of those 18 infants did not receive premedication. Nine infants exhibited evidence of EEG suppression during endotracheal intubation, all of whom received premedication before intubation. Five infants had EEG suppression during endotracheal suctioning. Oxygen saturation, heart rate, and blood pressure were not independent predictors of brain wave suppression.
Conclusion
Eighteen of 29 intubated infants (62%) had evidence of brain wave suppression on raw EEG after surfactant administration. Nine patients had evidence of brief EEG suppression with endotracheal intubation alone, a finding not previously reported in neonates. Intubation and surfactant administration have the potential to alter cerebral function in neonates.
Endotracheal surfactant administration has been shown to reduce neonatal mortality and improve lung function in infants with respiratory distress syndrome.1 After being approved by the US Food and Drug Administration in 1990, surfactant replacement is now routinely used in most neonatal intensive care units for infants with respiratory distress syndrome, meconium aspiration syndrome, and other etiologies.2 Infants with respiratory distress syndrome and meconium aspiration syndrome typically exhibit a significant improvement in pulmonary mechanics along with improved chest radiography findings shortly after surfactant administration.3 However, delivery of surfactant via an endotracheal tube (ETT) has been associated with cerebroelectrical depression as recorded by single-channel amplitude-integrated electroencephalography (aEEG).4–7 The duration of aEEG changes after surfactant administration have been reported to be as short as 10–20 minutes by Skov et al5 and as long as 24 hours by van den Berg et al6; in both of those studies, surfactant was administered as a single bolus via an ETT. The etiology of the changes in electrical brain wave activity occurring with surfactant administration is unclear, and there are no published long-term studies on neurodevelopmental outcomes.
ETT placement in newborns is known to be a painful procedure and is also associated with adverse physiological effects including bradycardia,8,9 alterations in blood pressure (BP),10,12–15 and increased intracranial pressure.11,16 Furthermore, surfactant administration is known to be associated with changes in cerebral blood flow velocity,5,17,18 BP,5,18 oxygen saturation measured by preductal pulse oximetry (SpO2),5 and carbon dioxide measured transcutaneously (TcCO2).7 The objective of the present study was to use 3-channel electroencephalography (EEG) to further characterize the effects of surfactant administration and related procedures on the neonatal brain.
Methods
A prospective cohort of 30 infants (24 preterm and 6 full term) who were admitted to the University of California San Diego’s Infant Special Care Center and who underwent endotracheal intubation and/or surfactant administration were studied using 3-channel EEG between December 2009 and December 2010. The University’s Institutional Review Board approved the study, and previous informed consent was obtained from a parent of each enrolled infant.
Any newborn in the Infant Special Care Center who required surfactant administration and was not emergent was a candidate for this study, including infants previously intubated and on ventilatory support. Each infant was studied only once during surfactant administration. Infants with a facial or airway anomaly that could interfere with ventilation or intubation, the need for emergent intubation, known chromosomal abnormality, congenital brain abnormality, or intraventricular hemorrhage grade III or greater were excluded. No infant had received antiepileptic drug therapy or was receiving a continuous narcotic infusion while participating in the study.
Heart rate and SpO2 data were collected continuously, and BP and TcCO2 were recorded every 5 minutes. For infants without a BP transducer in place, BP was obtained by oscillometry and recorded every 5 minutes until completion of the study. A data acquisition system (BioPac Systems, Goleta, California) recorded analog physiological data. Analog signals from the oximeter (Radical; Masimo, Irvine, California) were analyzed using AcqKnowledge software (BioPac Systems), which linked them in time. For pulse rate and SpO2, the exact duration of any change from baseline, with medians, means, maxima, and minima, were calculated and stored for subsequent analysis using PROFOX software (PROFOX Associates, Escondido, California). BP, TcCO2, and demographic data were recorded by a staff member (a registered nurse, registered respiratory therapist, pediatric resident, or neonatal fellow). Blood gas analysis was not performed for this study.
EEG Monitoring and Analysis
Once the clinical team decided to intubate and/or provide surfactant administration to an enrolled patient, a modified neonatal 3-channel EEG (Fp1–01, C3-C4, and Fp2-O2) montage was used to record continuously before, during, and after surfactant administration. Raw EEG brain wave activity data were collected with the Bio-Logic digital EEG data collection system (Bio-Logic Systems, Mundelein, Illinois). Scalp electrodes were placed according to the International 10–20 System by a single neonatology fellow previously registered with the American Board of Registration of Electroencephalographic and Evoked Potential Technologists. Electrode impedance levels were kept below 10 kΩ. Low-pass and high-pass filters were set at 1 Hz and 70 Hz, respectively. The 60-Hz notch filter was not engaged. The 3-channel montage used in this study records EEG activity from both hemispheres and has been validated as comparable to routine 9-channel neonatal montage in seizure detection.19
A 10-minute baseline EEG recording was obtained before the administration of any medication or surfactant. EEG recordings were made continuously throughout each study and continued for at least 30 minutes after completion of surfactant administration or until the EEG returned to near baseline values. A single child neurologist (R.H.) qualitatively analyzed EEG data for changes in amplitude, frequency, and seizure activity. For quantitative EEG analysis, the median amplitude in microvolts over a 60-second epoch from the start of the recording (baseline), during premedication, at surfactant administration, and at 10, 20, and 30 minutes after surfactant administration was selected and analyzed using Persyst Insight II EEG software (Persyst Development, Prescott, Arizona). Median EEG amplitudes were collected from all EEG channels for a comparison of values before and after surfactant administration.
Surfactant and Intubation Procedure
In our neonatal unit, surfactant therapy involved either Survanta (beractant; Abbott Nutrition, Columbus, Ohio) or Curosurf (poractant alfa; Cornerstone Therapeutics, Cary, North Carolina) administered as an intratracheal suspension via ETT. Survanta was given at a dose of 4 mL/kg via ETT. If a repeat dose was necessary, it was given 6 hours after the initial dose. The standard dose of Curosurf was 2.5 mL/kg via ETT, with a repeat dose of 1.25 mL/kg/dose every 12 hours if necessary to increase fraction of inspired oxygen. After confirmation of ETT placement, a 5 Fr multi-access catheter (Ballard, Roswell, Georgia) was placed inline to the ventilator and was connected to the ETT for surfactant administration. Infants were given half the aliquot of surfactant in each lung. The attending neonatologist and/or neonatal fellow/neonatal nurse practitioner chose the type of surfactant used at the time of intubation.
The current standard of care for elective endotracheal intubation in our neonatal unit includes the use of a combination of premedication agents, including a short-acting muscle relaxant. Oxygen is administered via a flow-inflating resuscitation bag, with the fraction of inspired oxygen adjusted to maintain saturation at ≥95% and <100% before endotracheal intubation, followed by atropine (20 μg/kg) to prevent vagolytic response and a narcotic for pain management. Fentanyl (2 μg/kg) and then cisatracurium (up to 200 μg/kg) were given 1 minute before endotracheal intubation. Intubation was performed by an attending neonatologist, neonatal fellow, pediatric intern/resident, or neonatal nurse practitioner using the appropriate-sized laryngoscope and ETT. Proper ETT placement was confirmed with a Pedi-Cap colorimetric qualitative CO2 detector, (Nellcor, Pleasanton, California) and/or chest auscultation and radiography. All endotracheal intubation attempts were limited to 30 seconds, including the duration of intubation. Brief endotracheal suctioning via a Ballard Multi-Access Catheter connected to 100-mmHg wall suction was performed before surfactant administration.
Statistical Analyses
Minimum, maximum, mean, median, and SD amplitudes from all EEG channels were collected before and after surfactant administration. Data are expressed as median voltage of amplitude in microvolts. The Wilcoxon matched-pair signed rank test was used for quantitative data analysis. Statistical significance was defined as P≤ .05. Logistic regression was used to compare heart rate, SpO2, BP, and TcCO2 values. PASW Statistics GradPack version 18 (IBM, Chicago, Illinois) was used for data analysis.
Results
The study cohort included 30 patients admitted to the University of California San Diego’s Infant Special Care Center between December 2009 and December 2010 (Table). One term infant whose records were not retrievable from the digital recording storage media was excluded from the analysis; thus, 29 infants were analyzed. Birth weight ranged from 590 to 4425 g (mean, 2085 ± 986 g), and gestational age at birth ranged from 25.5 to 41.6 weeks (mean, 33 ± 4.3 weeks). EEG recordings were collected between 1 and 30 hours after birth (median, 4 hours). No infant had an intraventricular hemorrhage.
Table.
Characteristics of the study population (n = 30)
| Characteristic | Value |
|---|---|
| Gestational age, n | |
| Term | 6 |
| Preterm | 24 |
| Birth weight, g, mean ± SD | 2085 ± 986 |
| Males/females, n | 16/14 |
| Race, n (%) | |
| Black | 1 (3) |
| White | 18(60) |
| Hispanic | 7(23) |
| Asian | 3(10) |
| Other | 1 (3) |
| Apgar score, median (range) | |
| 1-minute | 6 (0–9) |
| 5-minute | 8 (4–9) |
| Primary diagnosis, n (%) | |
| Respiratory distress syndrome | 26 (87) |
| Meconium aspiration syndrome | 4(13) |
| Antenatal steroid use, n (%) | 19(63) |
| Cesarean delivery, n (%) | 24 (80) |
| Multiple birth | 11 |
| Intraventricular hemorrhage, n (%) | 0 |
| Previous intubation for other indications, n (%) | 8 (27) |
| Poractant alfa/Beractant administration, n | 26/4 |
| Delivery of surfactant, mean time from birth | 6 hours, 26 minutes |
| Premedication for endotracheal intubation, n (%) | 23 (77) |
EEG Data
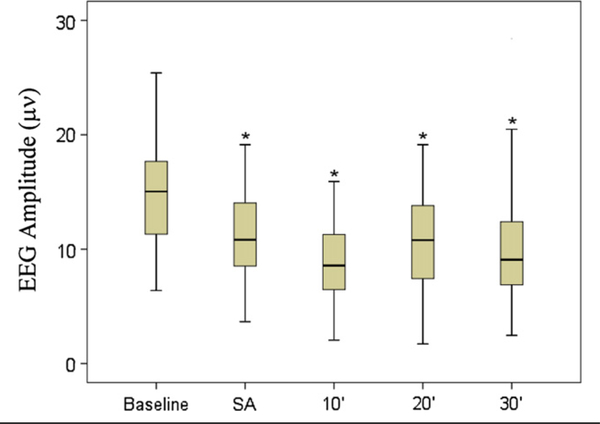
Eighteen of 29 infants (62%) exhibited brain wave suppression on EEG after surfactant administration (P≤ .008). Of those 18 infants, 14 (78%) had received premedication for endotracheal intubation before surfactant administration. In all 29 infants, the median baseline EEG amplitude and median EEG amplitude at the time of premedication for endotracheal intubation were not significantly different (P = .224). However, the median EEG amplitude at baseline was significantly higher than that measured at the time of surfactant administration and at 10, 20, and 30 minutes after surfactant administration (P ≤ .008). Figure 1 shows amplitude changes in the midline C3-C4 EEG channel at baseline, at surfactant administration, and at 10, 20, and 30 minutes after surfactant administration. Twenty-five infants received Curosurf, and 4 infants received Survanta. Two of the 4 infants who received Survanta did not receive premedication for endotracheal intubation within 4 hours and had evidence of brain wave suppression after surfactant administration.
Figure 1.
EEG amplitude for channel C3-C4 in microvolts. Baseline amplitude is medication/surfactant free recording during first 10 minutes of study, followed by surfactant administration 10, 20, and 30 minutes post-surfactant administration amplitudes. * Indicates significant level of P ≤ .008. Outliers not shown on box-plot. SA, surfactant administration.
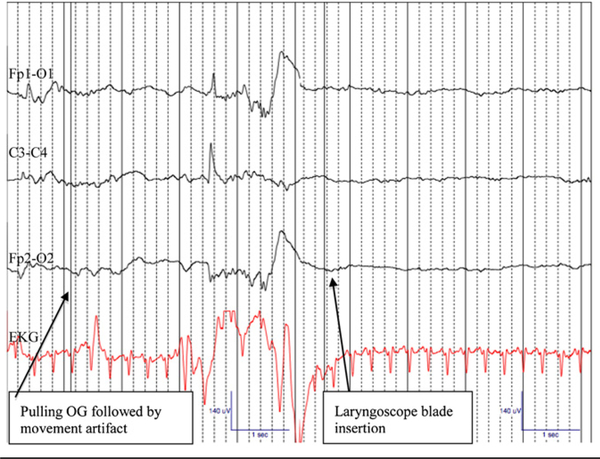
Brain wave suppression on endotracheal intubation was detected in 9 infants who had received standard premedication (Figure 2). Although this brain wave suppression was brief in all infants (range, 5–12 seconds) and not statistically significant, it was readily recognizable on EEG just after laryngoscopic blade insertion. The mean duration for 34 recorded intubation attempts was 24.6 seconds. Five infants exhibited evidence of brain wave suppression after endotracheal suctioning and before surfactant administration. Again, the brain wave suppression was brief and not statistically significant, but was identifiable on raw EEG. No infant exhibited EEG or clinical evidence of seizure, or any signs of hemispheric asymmetry.
Figure 2.
Amplitude suppression and background pattern changes on EEG Channels: Fp1-O1, C3-C4, Fp2-O2, EKG following laryngoscope blade insertion for endotracheal intubation in a 31 week female (1855 g) premedicated with atropine, narcotic, and paralytic prior to endotracheal intubation for respiratory distress syndrome. Artifact noted on EEG following removal of orogastric tube and just before laryngoscope blade insertion. Gain: 7 mm, Low Frequency Filter of 1 Hz, High Frequency Filter of 20 Hz. Recording speed 30 mm/ sec. OG, orogastric tube.
SpO2, BP, and TcCO2 Monitoring
Mean SpO2 at the time of surfactant administration was 91% in 14 infants who exhibited brain wave suppression on EEG and 83% in 6 infants with no evidence of brain wave suppression (P = .065). Overall, 12 of 20 infants (60%) had SpO2 <90% at some time during surfactant administration (range, 34%−89%), and 11 of 13 (85%) had SpO2 ≤90% during endotracheal intubation (range, 55%−90%). Ten of 15 infants (67%) had a mean SpO2 ≤90% (range, 48%−90%) during endotracheal suctioning; 5 of those 10 infants (50%) exhibited concurrent EEG suppression. The mean heart rate was 147 ± 26 bpm at baseline and 158 ± 36 bpm at the time of surfactant administration, a statistically insignificant difference. Mean BP was 44 ± 8 mmHg at baseline, 43 ± 12 mmHg at 5 minutes after surfactant administration, and 40.5 ± 9 mmHg at 10 minutes after surfactant administration; again, these differences were not statistically significant. The mean TcCO2 was 54 ± 14 mmHg at baseline, 57 ± 12 mmHg at 5 minutes after surfactant administration, and 55 ± 14 mmHg at 10 minutes after surfactant administration. The changes in SpO2, heart rate, BP, and TcCO2 were not independent predictors of EEG suppression on logistic regression analysis (P > .05).
Discussion
This prospective study found significant brain wave suppression on multichannel EEG after surfactant administration in the majority of infants studied. Our finding of amplitude suppression using multichannel EEG after surfactant administration provides a more detailed look at brain wave suppression than previous studies, which used only single-channel aEEG.4–7 Brain wave suppression was not correlated with SpO2, BP, heart rate, or TcCO2. Interestingly, 14 of the 18 infants demonstrating brain wave suppression after surfactant administration also had higher mean SpO2 values at the time of surfactant administration.
Furthermore, because we looked at raw EEG rather than aEEG alone, we uncovered brain wave suppression with endotracheal intubation and suctioning, a finding not previously reported in neonates. We found brief EEG suppression with endotracheal intubation in 9 infants, which although not statistically significant, is in contrast to previous studies reporting increased electrical brain wave activity with this procedure.20,21 Those studies were in adult populations undergoing endotracheal intubation after anesthesia. Another plausible explanation for the brief EEG suppression was transient obstruction of the airway or perhaps a vagal response detected by EEG. However, all 9 infants received anticholinergic medication before intubation, so any vagal response should have been blunted.
Our unit’s protocol is to premedicate all infants with an anticholinergic, narcotic, and paralytic agent before intubation to increase patient comfort and facilitate intubation. We previously demonstrated that such premedication decreases the time and number of attempts required for successful intubation while also reducing the incidence of severe oxygen desaturation.22 There were no statistically significant differences in the median amplitude of EEG between baseline and the time of premedication or surfactant administration. We used minimal doses of narcotics and paralytics, so the effect of medication should not have affected the EEG for prolonged periods. It is possible that some infants did not metabolize the medications quickly, thus explaining the longer time for EEG readings to return to near baseline in some infants. Another possible factor in the prolonged EEG suppression in some infants is the additive effects of the premedication used for endotracheal intubation and the surfactant itself, accounting for the continued electrocerebral depression beyond 30 minutes after surfactant administration.
Some medications commonly used in neonates, including morphine, phenobarbital, midazolam, and even sufentanil, have been shown to alter EEG activity.23 Morphine delivered by continuous infusion has been associated with periods of electrical quiescence, interictal epileptiform activity, and generalized burst-suppression activity, which resolves once the medication is stopped.24 We speculate that the narcotics and paralytics administered before endotracheal intubation provided no significant contribution to the EEG suppression after surfactant administration. We base this speculation on the lack of significant changes in median amplitude from baseline to after premedication and before endotracheal intubation, and on the fact that no infant received continuous infusion of either a narcotic or a paralytic, which have the potential to alter EEG readings.
Eighteen infants exhibited immediate brain wave suppression after surfactant administration that persisted beyond 30 minutes after administration. This might be related to a sudden decrease in pulmonary vascular resistance after surfactant administration. Because all of the infants likely had a patent ductus arteriosus at time of surfactant administration, they might have experienced an increase in left-to-right ductal shunting during surfactant administration (systemic to pulmonary), resulting in changes in cerebral blood flow and thus altered EEG amplitudes. Another possible explanation for the brain wave suppression seen on EEG is other changes in cardiac hemodynamics associated with prematurity. West et al25 reported that lower EEG amplitude was associated with lower right ventricular output (<282 mL/ kg/min) in a small cohort of preterm infants. Surfactant administration might further reduce right ventricular output, possibly contributing to a series of events leading to decreased EEG amplitude.
Kaiser et al17 reported an increase in mean cerebral blood flow velocity after surfactant administration in a small group of very low birth weight infants; however, they also demonstrated a strong association with a concurrent rise in PaCO2. In that study, surfactant was administered via a multi-access catheter tube, and infants were briefly disconnected from the ventilator during catheter removal. This might have resulted in loss of functional residual capacity and increased PaCO2, with subsequent changes in EEG activity. Suppression of EEG waveforms has been associated with increased PaCO2 values.26 We might have avoided fluctuations in functional residual capacity, and thus PaCO2, by using an in-line Ballard catheter and not discontinuing ventilation unless clinically indicated. We found no significant difference in TcCO2 values before or after surfactant administration; however, some infants did exhibit a transient TcCO2 elevation immediately after surfactant administration, which could be explained by transient ETT obstruction. A previous study found increased TcCO2 in all patients after surfactant administration, but that study used a higher dose of surfactant compared with the present study.7
In contrast to our findings, Chalak et al27 reported increased electrical excitability on aEEG after surfactant administration and suctioning in a cohort of 30 extremely low birth weight infants. However, these authors did not study raw EEG data, and they administered surfactant via 4 bolus aliquots, which might have caused fluctuations in cerebral blood flow that produced the changes seen on aEEG. They noted no change in mean arterial BP during surfactant administration, and speculated that the lack of BP fluctuation alone might have accounted for the lack of electrocerebral depression seen on aEEG. Although our infants also demonstrated stable mean BP, most of them exhibited significant brain wave suppression after surfactant administration. Perhaps a better explanation is related to the higher SpO2 resulting in decreased cerebral blood flow or extraction, possibly leading to suppression.
Our finding of EEG suppression after surfactant administration might represent compensatory physiological and/or biochemical changes secondary to changes in global or local cerebral blood flow, cerebral oxygenation, cardiac/pulmonary hemodynamics, airway obstruction, or a combination of one or more of these changes. The mechanism of the brain wave changes seen with this procedure merit further investigation, given the possible effects of such suppression on short-term and long-term neurologic outcomes. Acute-stage EEG abnormalities (lower amplitude, increased discontinuity, and decreased faster frequencies) have been related to brain injury.28 Such brain injury may be transient, and serial EEG recordings might be useful. The same group studied the relationship between serial EEG findings and outcomes in preterm infants born at <33 weeks gestational age and reported that mildly low voltages, prolonged inter-burst intervals, and attenuated alpha, beta, and theta frequencies on EEG were associated with normal or border-line outcomes in 40% of the infants and with mild cerebral palsy in 30%.28
As we have demonstrated, surfactant administration has the potential to alter EEG signals. This may or may not have bearing on neurodevelopmental outcomes. Aerosol administration of surfactant is a potential approach to alleviate EEG abnormalities. Rabbits with severe respiratory failure given a nebulized form of surfactant exhibited minimal changes in cerebral blood flow with no changes in mean arterial BP.29 Thus, aerosolized surfactant might have less significant effects on neonatal hemodynamics, such as mean arterial BP and cerebral blood flow.
The strengths of the present study include its prospective design, use of raw EEG data, and observations during endotracheal intubation. Study limitations include the small sample size and the large percent of patients who received premedication for endotracheal intubation, which might be a confounding factor but represents current recommended practice.30 In addition, neurodevelopmental follow-up was not part of our study.
In this study, more than one-half of the infants exhibited evidence of brain wave suppression on raw EEG after surfactant administration. Nine patients had evidence of EEG suppression with endotracheal intubation alone, a finding not previously reported in neonates. Surfactant administration has the potential to alter cerebral function, and further studies are needed to examine whether such EEG alterations are associated with significant short-term and long-term effects. ■
Acknowledgments
We thank all of the infants’ families for their willingness to participate in this study. This project would not have been possible without the dedication of the registered nurses, respiratory therapists, and Infant Special Care Center staff at the University of California San Diego Medical Center.
Glossary
- aEEG
Amplitude-integrated electroencephalography
- BP
Blood pressure
- EEG
Electroencephalography
- ETT
Endotracheal tube
- SpO2
Oxygen saturation measured by pulse oximetry
- TcCO2
Transcutaneous carbon dioxide
Footnotes
The authors declare no conflicts of interest
References
- 1.Collaborative European Multicenter Study Group. Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Pediatrics 1988; 82:683–91. [PubMed] [Google Scholar]
- 2.Warren JB, Anderson JD. Core concepts: respiratory distress syndrome. NeoReviews 2009;10:e351–61. [Google Scholar]
- 3.Stevens TP, Sinkin RA. Surfactant replacement therapy. Chest 2007;131: 1577–82. [DOI] [PubMed] [Google Scholar]
- 4.Hellstrom-Westas L, Bell AH, Skov L, Greisen G, Svenningsen NW. Cerebroelectrical depression following surfactant treatment in preterm neonates. Pediatrics 1992;89:643–7. [PubMed] [Google Scholar]
- 5.Skov L, Bell A, Greisen G. Surfactant administration and the cerebral circulation. Biol Neonate 1992;61:31–6. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg E, Lemmers PM, Toet MC, Klaessens JH, van Bel F. Effect of the “InSurE” procedure on cerebral oxygenation and electrical brain activity of the preterm infant. Arch Dis Child 2010;95:53–8. [DOI] [PubMed] [Google Scholar]
- 7.Lundstrom KE, Greisen G. Changes in EEG, systemic circulation and blood gas parameters following two or six aliquots of porcine surfactant. Acta Paediatr 1996;85:708–12. [DOI] [PubMed] [Google Scholar]
- 8.Marshall TA, Deeder R, Pai S, Berkowitz GP, Austin TL. Physiologic changes associated with endotracheal intubation in preterm infants. Crit Care Med 1984;12:501–3. [DOI] [PubMed] [Google Scholar]
- 9.Kelly MA, Finer NN. Nasotracheal intubation in the neonate: physiologic responses and effects of atropine and pancuronium. J Pediatr 1984;105:303–9. [DOI] [PubMed] [Google Scholar]
- 10.Friesen RH, Honda AT, Thieme RE. Changes in anterior fontanel pressure in preterm neonates during tracheal intubation. Anesth Analg 1987;66:874–8. [PubMed] [Google Scholar]
- 11.Stow PJ MM, Burrows FA, Creighton RE. Anterior fontanelle pressure responses to tracheal intubation and anesthesia in infants. Br J Anaesth 1988;60:167–70. [DOI] [PubMed] [Google Scholar]
- 12.Khammash HM, O’Brien K, Dunn MS, Jefferies AL, Perlman M. Blunting of hypertensive response to endotracheal intubation in neonates by premedication [abstract]. Pediatr Res 1993;33:218A. [Google Scholar]
- 13.Millar C, Bissonnette B. Awake intubation increases intracranial pressure without affecting cerebral blood flow velocity in infants. Can J Anaesth 1994;41:281–7. [DOI] [PubMed] [Google Scholar]
- 14.Pokela ML, Koivisto M. Physiological changes, plasma beta-endorphin and cortisol responses to tracheal intubation in neonates. Acta Paediatr 1994;83:151–6. [DOI] [PubMed] [Google Scholar]
- 15.Barrington KJ, Finner NN, Etches PC. Succinylcholine and atropine for premedication of the newborn before nasotracheal intubation; a randomized, control trial. Crit Care Med 1989;17:1293–6. [DOI] [PubMed] [Google Scholar]
- 16.Raju TN, Vidyasagar D, Torres C, Grundy D, Bennett EJ. Intracranial pressure during intubation and anesthesia in infants. J Pediatr 1980; 96:860–2. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very low birth weight infants. J Pediatr 2004;144:809–14. [DOI] [PubMed] [Google Scholar]
- 18.Terry MH, Merritt TA, Harding B, Schroeder H, Merrill-Henry J, Mazela J, et al. Pulmonary distribution of lucinactant and poractant alfa and their peridosing hemodynamic effects in a preterm lamb model of respiratory distress syndrome. Pediatr Res 2010;68:193–8. [DOI] [PubMed] [Google Scholar]
- 19.Zimbric MR, Sharpe CM, Albright KC, Nespeca MP. Three-channel electroencephalogram montage in neonatal seizure detection and quantification. Pediatr Neurol 2011;44:31–4. [DOI] [PubMed] [Google Scholar]
- 20.Kox WJ, von Heymann C, Heinze J, Prichep LS, John ER, Rundshagen I. Electroencephalographic mapping during routine clinical practice: cortical arousal during tracheal intubation? Anesth Analg 2006;102:825–31. [DOI] [PubMed] [Google Scholar]
- 21.Rundshagen I, Schroder T, Prichep LS, John ER, Kox WJ. Changes in cortical electrical activity during induction of anaesthesia with thiopental/fentanyl and tracheal intubation: a quantitative electroencephalographic analysis. Br J Anaesth 2004;92:33–8. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KD, Leone TA, Edwards WH, Rich WD, Finer NN. Premedication for nonemergent neonatal intubations: a randomized, controlled trial comparing atropine and fentanyl to atropine, fentanyl, and mivacurium. Pediatrics 2006;118:1583–91. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen The Tich S, Vecchierini MF, Debillon T, Pereon Y. Effects of sufentanil on electroencephalogram in very and extremely preterm neonates. Pediatrics 2003;111:123–8. [DOI] [PubMed] [Google Scholar]
- 24.Young GB, da Silva OP. Effects of morphine on the electroencephalograms of neonates: a prospective, observational study. Clin Neurophysiol 2000;111:1955–60. [DOI] [PubMed] [Google Scholar]
- 25.West CR, Groves AM, Williams CE, Hardings JE, Skinner JR, Kuschel CA, et al. Early low cardiac output is associated with compromised electroencephalographic activity in very preterm infants. Pediatr Res 2006;59:610–5. [DOI] [PubMed] [Google Scholar]
- 26.Victor S, Appleton RE, Beirne M, Marson AG, Weindling AM. Effect of carbon dioxide on background cerebral electrical activity and fractional oxygen extraction in very low birth weight infants just after birth. Pediatr Res 2005;58:579–85. [DOI] [PubMed] [Google Scholar]
- 27.Chalak LF, Sikes NC, Mason MJ, Kaiser JR. Low-voltage aEEG as predictor of intracranial hemorrhage in preterm infants. Pediatr Neurol 2011; 44:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K, Hayakawa F, Okumur A. Neonatal EEG: a powerful tool in the assessment of brain damage in preterm infants. Brain Dev 1999;21: 361–72. [DOI] [PubMed] [Google Scholar]
- 29.Dijk PH, Heilkamp A, Bambang Oetomo S. Surfactant nebulisation prevents the adverse effects of surfactant therapy on blood pressure and cerebral blood flow in rabbits with severe respiratory failure. Intensive Care Med 1997;23:1077–81. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Denson SE, Mancuso TJ. Clinical report: premedication for nonemergency endotracheal intubation in the neonate. Pediatrics 2010;125:608–15. [DOI] [PubMed] [Google Scholar]