Abstract
While dehydroepiandrosterone (DHEA) may exert neuroprotective effects in the developing brain, prolonged or excessive elevations in cortisol may exert neurotoxic effects. The ratio between DHEA and cortisol (DC ratio) has been linked to internalizing and externalizing disorders as well as cognitive performance, supporting the clinical relevance of this hormonal ratio during development. However, the brain mechanisms through which these effects may be mediated have not been identified as of yet. Further, while there is evidence that the CNS effects of cortisol may be sexually dimorphic in humans, the opposite is true of DHEA, with human studies showing no sex-specific associations in cortical thickness, cortico-amygdalar or cortico-hippocampal structural covariance. Therefore, it remains unclear whether sex moderates the developmental associations between DC ratio, brain structure, cognition and behavior. Here we examined associations between DC ratio, structural covariance of the hippocampus with whole-brain cortical thickness, and measures of personality, behavior and cognition in a longitudinal sample of typically developing children, adolescents and young adults 6–22 years (N=225 participants (F=128); 355 scans (F=208)), using mixed effects models that accounted for both within- and between-subject variances. We found sex-specific interactions between DC ratio and anterior cingulate cortex-hippocampal structural covariance, with higher DC ratios associated with a more negative covariance between these structures in girls, and a more positive covariance in boys. Further, the negative prefrontal-hippocampal structural covariance found in girls was associated with higher verbal memory and mathematical ability, while the positive covariance found in boys was associated with lower cooperativeness and reward dependence personality traits. These findings support the notion that the ratio between DHEA and cortisol levels may contribute, at least in part, to the development of sex differences in cognitive abilities as well as risk for internalizing/externalizing disorders, through an alteration in prefrontal-hippocampal structure during the transition from childhood to adulthood.
Keywords: Adrenarche, Androgens, Adolescence, Puberty, Cognition
1. INTRODUCTION
Adrenal responses to stress (including an increase in cortisol production in response to hypothalamo-pituitary-adrenal (HPA) axis activation) play an important role in flight-or-fight behaviors across the lifespan and, in an acute situation, represent an adaptive response to external stressors. However, because cortisol increases the body’s catabolic state, drawing upon the brain as well as the body’s energy reserves, a built-in buffering mechanism of this adrenocortical response is necessary to prevent long-term detrimental effects. The adrenal steroid hormone dehydroepiandrosterone (DHEA) may play a part in buffering the adverse effects of a chronic elevation in cortisol levels, in part through its anti-glucocorticoid properties in the liver, the skin and the brain (1). As such, examination of both adrenal hormones may provide a more accurate assessment of the body’s anabolic/catabolic state than either hormone measured alone.
Direct exposure to glucocorticoids in vitro or via stress models which trigger excessive or prolonged cortisol release (such as immobilization or social defeat) have been demonstrated to reduce hippocampal cell volume and arborization of dendritic spines (2). Glucocorticoids may exert this effect by inhibiting proliferation and differentiation of oligodendrocytes, thus inhibiting myelination, and by triggering the transformation of microglial cells toward a phagocytic and neurotoxic phenotype (3). In contrast, DHEA may stimulate neurogenesis and protect against neuronal injury due to excessive or prolonged exposure to glucocorticoids in both hippocampal and cortical structures (4). The buffering and protective effects of DHEA against neurotoxic effects of glucocorticoids may be especially relevant during childhood when the brain experiences an increase in metabolic activity in the context of adrenarche (1)
In line with these in vitro and animal studies, our group found that DHEA may support attentional and working memory processes through its impact on cortico-hippocampal and cortico-amygdalar networks (5). In contrast, prolonged or excessive exposure to cortisol may decrease cognitive performance (in particular, verbal declarative memory and episodic autobiographical memory), modulate risk for depression and conduct disorders, and have significant neurotoxic effects on cortico-limbic systems during development (6). Notably, the extent and magnitude of these developmental effects may only be fully appreciated during middle adulthood or even old age (6), suggesting that some of these hormonal effects may be additive over time. In this context, it is perhaps not surprising that a higher ratio of DHEA relative to cortisol (DC ratio) has been associated in several, though not all, studies with a lower risk of psychiatric disorders (e.g. major depression, conduct disorder, attention-deficit hyperactivity disorder) (7).
The roles of DHEA and cortisol in modulating brain structure and function might vary according to sex. There is prior evidence of sexual dimorphisms in HPA axis regulation in both animals and humans (8). In addition, DHEA was shown to regulate the main source of energy for the brain –glucose uptake- as well as glucose oxidation, in a sex-dependent manner (9), and the process of adrenarche (with its increased production of adrenal steroids such as DHEA and cortisol) begins earlier in girls vs. boys (10).
Similarly, sex differences in human brain structure have been identified. In particular, hippocampal volumes increase linearly in late childhood in both sexes but then follow different trajectories in males and females later during adolescence such that the linear increase continues for males while there may be no change or a slight decrease in hippocampal volumes in females (11–13). Dendritic pruning has also been described in the medial prefrontal cortex (Fig. 1) of female, but not male, rats suggesting sex-specific dendritic pruning in cortical regions as well (11, 14). Surprisingly, it remains unclear to which extent sex differences in the hippocampus are affected by pubertal steroid hormones. On the other hand, prefrontal neurons have been shown to be sensitive to pubertal hormones, and the sex difference in neuronal number favoring males emerges only after puberty (15). Although both sexes lose prefrontal neurons after puberty, females do so to a greater extent (16). Interestingly, structural covariance of the hippocampus also shows distinct patterns in men and women: in men the posterior hippocampus showed reliable structural covariance with the medial and lateral parietal lobes and the prefrontal cortex, whereas in women the anterior hippocampus showed reliable structural covariance with the anterior temporal lobe bilaterally (17). Thus, any effects of pubertal hormones on cortical areas such as the prefrontal cortex could potentially extend to include the hippocampus through their mutual structural connections.
FIGURE 1: Girls: DHEA/Cortisol Ratio and Prefrontal-Hippocampal Structural Covariance.
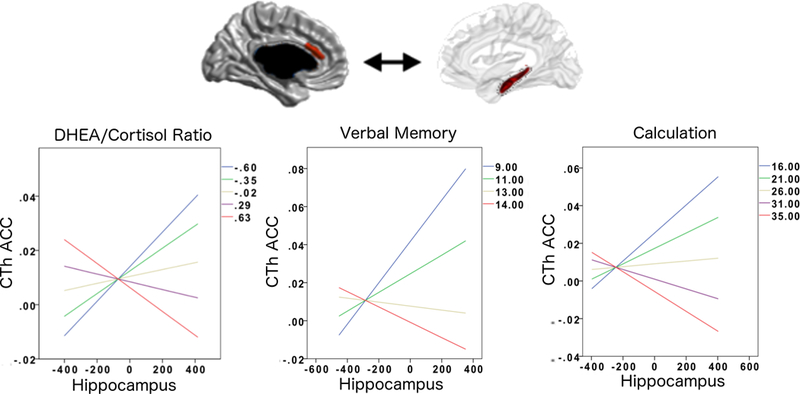
This figure shows the associations between DHEA/Cortisol ratio, ACC-hippocampal structural covariance and verbal as well as calculation skills in girls.
The negative ACC-hippocampal covariance seen at higher DHEA/Cortisol ratios was associated with higher scores on tests of verbal memory (California Verbal Learning Test, immediate free recall, trial 5, number of correct words recalled) and mathematical ability (Woodcock-Johnson Calculation). Overall regression-fit-lines for ACC-hippocampus covariance are displayed for different percentiles (20th, 40th, 60th, 80th, 100th) of DHEA/Cortisol ratios and cognitive/behavioral scores.
Please note that the ACC area shown in this figure is not a pre-defined region-of-interest (ROI). Rather, this is the only region across the whole brain whose sex-specific relationship to the hippocampus significantly varied as a function of DHEA/Cortisol ratio (controlled for multiple comparisons using random field theory). In addition, note that the Y axes of graphs list standardized residuals of cortical thickness (accounting for the effects of age, sex, handedness, scanner, and total brain volume in all analyses, as well as collection time for hormonal analyses).
Several behavioral and cognitive measures show increasing sexual dimorphisms during adolescence. For example, compared with their male counterparts, female adolescents report more symptoms of internalizing disorders (e.g. major depression, generalized anxiety) while male (vs. female) adolescents report more symptoms of externalizing disorders (e.g. conduct disorders); this difference also increases as adolescents mature (18). Girls score higher on most aspects of personality compared to age-matched boys; e.g. higher neuroticism scores starting at age 14, higher openness and conscientiousness scores between 12 and 17 years (19). Overall, female adolescents also tend to outperform their male counterparts in several executive domains, including processing speed and selective attention (18). Sex differences in verbal memory favoring girls have been well documented, but may arise before puberty (20). In contrast, there is a documented robust sex differences in visuo-spatial abilities favoring boys throughout childhood and adolescence (21, 22). It remains controversial whether these sex differences in visuo-spatial abilities extend to mathematical abilities (23). This may be because sex differences in mathematical abilities can vary in direction depending on mathematical domain -e.g. calculation skills may rely more prominently on working memory and retrieval strategy, favoring women, while mathematical fluency may rely more significantly on visuo-spatial skills, favoring men (16). Because of these contrasting differences, there may be in turn no overall sex difference when examining overall mathematical performance (16). Interestingly, even in a context where no sex differences in mathematical abilities are present, structural and functional neural processing of mathematical cognition may be more efficient in women compared to men (24).
Thus, hormonal states, brain structure and cognitive function all display sexual dimorphisms (8–10, 23–26). Although some of these differences may be attributed to sex chromosomes, there is also evidence suggesting causal pathways linking brain-hormone and cognitive development. For example, a lower ratio of DHEA relative to cortisol (DC ratio) or a higher ratio of cortisol to DHEA (CD ratio) have both been associated in several, though not all, studies with a higher risk of internalizing and externalizing symptoms (7). While a decrease in CD ratios throughout the day have been associated with a decrease in personality disorder symptoms in adult women (27), lower CD ratios may be associated with less adaptive coping skills and resilience in children (28). There is also evidence that higher DHEA levels may optimize working memory and executive function, while higher cortisol levels may increase distractibility and as a result, impair executive function (29–31). Similarly, DHEA may have a beneficial effect on verbal abilities, particularly in women (30), while higher cortisol levels have been associated with decreased performance on tests of verbal memory and learning (32). Higher CD ratios have also been associated with lower visuo-spatial memory performance in both men (33); conversely, administration of DHEA improved visuo-spatial abilities in women at a given cortisol level (34). However, both studies examined subjects much older than those include in our study. Finally, DHEA and cortisol were also found to alter performance on a difficult mathematical task, with DHEA playing a more important and beneficial role than cortisol in this regard (35). Despite these demonstrated associations between DC (or CD) ratios and sexually dimorphic cognitive/behavioral measures, prior studies from our group have not demonstrated sex differences in cortico-hippocampal or cortico-amygdalar structural variance related to DHEA in humans (5, 36). Therefore, it remains unclear to which extent DC-related alterations in structural covariance would lead to a sex-specific difference in behavior or cognition, and this has not been formally tested up to now. Still, when taken together, these studies suggest that certain anatomical and functional brain circuits (37) are regulated by DHEA and cortisol, in particular cortico-hippocampal and cortico-amygdalar networks, and that these circuits may, at least in part, contribute to the development of sex differences in cognition and behavior. For example, we found that DHEA may optimize cortical functions related to general attentional and working memory processes, but impair the development of bottom-up, hippocampal-to-cortical connections, resulting in impaired encoding of spatial, and possibly even social, cues (36).
While our group previously documented the relationship between DHEA levels and cortico-hippocampal structural covariance (36), there are no prior studies, to our knowledge, examining the relationship between cortisol and cortico-hippocampal covariance. As such, our hypotheses focus on DC ratio -as opposed to CD ratio-. While the expected direction of associations is difficult to predict given the scarcity of studies on the subject, one could hypothesize, based on the evidence available, that prefrontal-hippocampal structural networks may be particularly sensitive to the effects of both DHEA and cortisol, and that this effect is to likely to be sex-specific. In addition, we hypothesized that DC-related cortico-hippocampal covariance may optimize top-down, cortical functions related to general attention, verbal, mathematical and executive abilities but may impair the development of bottom-up hippocampal function related to spatial and social cues, resulting in an impairment in interoceptive awarenesss, social understanding and emotional processing/empathy. To test these exploratory hypotheses, we examined associations between DC ratio, structural covariance of the hippocampus with whole-brain cortical thickness, and cognitive as well as behavioral measures, in a longitudinal sample of typically developing children, adolescents and young adults 6 to 22 years of age. Relationships between DC ratio, cortico-amygdalar structural covariance and cognitive-behavioral measures are addressed in a separate manuscript (under revision).
2. METHODS AND MATERIALS
2.1. Sampling and Recruitment
The National Institutes of Health (NIH) MRI Study of Normal Brain Development is a multi-site project that aimed to provide a normative database to characterize healthy brain maturation. Participants were recruited across the United States with a population-based sampling method seeking to achieve a representative sample in terms of income level, race and ethnicity. All experiments on human participants were conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the adequate understanding and written parental consent, as well as assent of the participants (or consent, if >=18 years old, see Table 1 for more details). Participants underwent repeated hormonal sampling, magnetic resonance brain imaging (MRI) and cognitive batteries every 2 years, with a maximum of 3 scans over 4 years. The sample was limited to developmentally healthy children with rigorous exclusion criteria. In particular, any individual with a current or past treatment for language disorder (simple articulation disorders not exclusionary); and a lifetime history of Axis I psychiatric disorder (except for simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency) were excluded from the study. After strict quality control of MRI data (see section 2.2) and the exclusion of scans without hormonal measurements or behavioral/cognitive parameters, 225 subjects (128 girls) were used for analyses examining the relationship between DC ratio and cortico-hippocampal covariance (total 355 scans) and 58–203 subjects (31–112 girls) for analyses examining the relationship between cortico-hippocampal covariance and behavioral/cognitive analyses (total 75–293 scans).
Table 1: Sample Characteristics.
Each column shows the characteristics of the sample used for each set of analyses (DC ratio-brain analyses, brain-cognition analyses). The data are divided into 3 visits (as each child was followed longitudinally up to 3 times, every 2 years, for a total of 4 years), and the subdivisions ‘1, 2, and 3’ for each row represent each of these visits, except for the row ‘# Participants per # of Scans completed’, for which each row represents the number of scans completed. F=female; M=male
| Visit | DHEA- Cortisol Ratio |
Woodcock- Johnson Calculation |
California Verbal Learning |
Temperament Character Inventory |
|
|---|---|---|---|---|---|
| # Scans per Visit # | 1 | n = 126 scans (F = 73) | n = 115 scans (F = 64) | n = 18 scans (F = 10) | n = 30 scans (F = 17) |
| 2 | n = 129 scans (F = 80) | n = 93 scans (F = 59) | n = 27 scans (F = 15) | n = 39 scans (F = 23) | |
| 3 | n = 100 scans (F = 55) Total = 355 (F = 208) | n = 85 scans (F = 45) Total = 293 (F = 168) | n = 30 scans (F = 16) Total = 75 (F = 41) | n = 38 scans (F = 21) Total = 107 (F = 61) | |
| # Participants per # Scans Completed | 1 scan | n =122 participants (F = 68) | n = 130 participants (F = 68) | n = 45 participants (F = 23) | n = 55 participants (F = 25) |
| 2 scans | n = 76 participants (F = 40) | n = 56 participants (F = 32) | n = 9 participants (F = 6) | n = 20 participants (F = 15) | |
| 3 scans | n = 27 participants (F = 20) Total = 225 (F = 128) | n = 17 participants (F = 12) Total = 203 (F = 112) | n = 4 participants (F = 2) Total = 58 (F = 31) | n = 4 participants (F = 2) Total = 79 (F = 42) | |
| Testosterone Time 1 (pg/mL) | 1 | n = 126, mean = 66.90, SD = 54.30 | n = 115, mean = 68.52, SD = 55.63 | n = 18, mean = 119.57, SD = 75.71 | n = 30, mean = 108.73, SD = 66.33 |
| 2 | n = 129, mean = 50.32, SD = 31.20 | n = 93, mean = 52.13, SD = 31.97 | n = 27, mean = 72.86, SD = 45.02 | n = 39, mean = 67.28, SD = 40.38 | |
| 3 | n = 100, mean = 69.53, SD = 73.99 | n = 85, mean = 69.37, SD = 77.20 | n = 30, mean = 80.21, SD = 50.61 | n = 38, mean = 75.63, SD = 48.36 | |
| Testosterone Time 2 (pg/mL) | 1 | n = 126, mean = 66.38, SD = 46.50 | n = 115, mean = 62.88, SD = 47.61 | n = 18, mean = 105.95, SD = 64.27 | n = 30, mean = 92.37, SD = 55.75 |
| 2 | n = 129, mean = 45.46, SD = 30.10 | n = 93, mean = 48.55, SD = 30.24 | n = 27, mean = 67.87, SD = 39.05 | n = 39, mean = 64.71, SD = 35.17 | |
| 3 | n = 100, mean = 67.90, SD = 61.42 | n = 85, mean = 67.14, SD = 62.79 | n = 30, mean = 73.97, SD = 43.91 | n = 38, mean = 72.28, SD = 44.78 | |
| Cortisol Time 1 (ug/dL) | 1 | n = 126, mean = 0.221, SD = 0.180 | n = 115, mean = 0.221, SD = 0.184 | n = 18, mean = 0.275, SD = 0.243 | n = 30, mean = 0.256, SD = 0.203 |
| 2 | n = 129, mean = 0.170, SD = 0.113 | n = 93, mean = 0.169, SD = 0.111 | n = 27, mean = 0.168, SD = 0.133 | n = 39, mean = 0.163, SD = 0.121 | |
| 3 | n = 100, mean = 0.208, SD = 0.189 | n = 85, mean = 0.216, SD = 0.198 | n = 30, mean = 0.236, SD = 0.181 | n = 38, mean = 0.245, SD = 0.243 | |
| Cortisol Time 2 (ug/dL) | 1 | n = 126, mean = 0.15, SD = 0.12 | n = 115, mean = 0.16, SD = 0.12 | n = 18, mean = 0.21, SD = 0.13 | n = 30, mean = 0.16, SD = 0.12 |
| 2 | n = 129, mean = 0.11, SD = 0.08 | n = 93, mean = 0.12, SD = 0.09 | n = 27, mean = 0.11, SD = 0.07 | n = 39, mean = 0.12, SD = 0.06 | |
| 3 | n = 100, mean = 0.15, SD = 0.13 | n = 85, mean = 0.16, SD = 0.13 | n = 30, mean = 0.16, SD = 0.14 | n = 38, mean = 0.16, SD = 0.15 | |
| Estradiol Time 1 (pg/mL) | 1 | n = 126, mean = 7.49, SD = 4.17 | n = 115, mean = 7.49, SD = 4.22 | n = 18, mean = 8.52, SD = 5.12 | n = 30, mean = 7.41, SD = 4.53 |
| 2 | n = 129, mean = 8.00, SD = 4.31 | n = 93, mean = 8.05, SD = 4.43 | n = 27, mean = 8.23, SD = 4.31 | n = 39, mean = 7.94, SD = 4.37 | |
| 3 | n = 100, mean = 10.96, SD = 6.88 | n = 85, mean = 11.21, SD = 6.89 | n = 30, mean = 11.17, SD = 6.85 | n = 38, mean = 11.29, SD = 7.47 | |
| Estradiol Time 2 (pg/mL) | 1 | n = 126, mean = 7.17, SD = 4.42 | n = 115, mean = 7.21, SD = 4.38 | n = 18, mean = 8.76, SD = 0.13 | n = 30, mean = 7.77, SD = 4.67 |
| 2 | n = 129, mean = 8.05, SD = 4.71 | n = 93, mean = 8.18, SD = 4.86 | n = 27, mean = 7.15, SD = 3.77 | n = 39, mean = 7.90, SD = 4.51 | |
| 3 | n = 100, mean = 11.53, SD = 6.91 | n = 85, mean = 11.93, SD = 7.02 | n = 30, mean = 12.43, SD = 7.48 | n = 38, mean = 12.25, SD = 7.60 | |
| DHEA Time 1 (pg/mL) | 1 | n = 126, mean = 102.24, SD = 101.81 | n = 115, mean = 106.82, SD = 104.91 | n = 18, mean = 162.13, SD = 75.22 | n = 30, mean = 169.14, SD = 113.93 |
| 2 | n = 129, mean = 194.08, SD = 204.37 | n = 93, mean = 201.81, SD = 209.83 | n = 27, mean = 323.75, SD = 257.45 | n = 39, mean = 316.51, SD = 254.04 | |
| 3 | n = 100, mean = 179.37, SD = 160.26 | n = 85, mean = 181.16, SD = 157.61 | n = 30, mean = 262.52, SD = 187.64 | n = 38, mean = 247.63, SD = 182.89 | |
| DHEA Time 2 (pg/mL) | 1 | n = 126, mean = 102.24, SD = 101.81 | n = 115, mean = 100.94, SD = 114.95 | n = 18, mean = 162.13, SD = 75.22 | n = 30, mean = 169.14, SD = 113.93 |
| 2 | n = 129, mean = 194.08, SD = 204.37 | n = 93, mean =183.19, SD = 184.42 | n = 27, mean = 323.75, SD = 257.45 | n = 39, mean = 316.51, SD = 254.04 | |
| 3 | n = 100, mean = 207.20, SD = 210.27 | n = 85, mean = 212.32, SD = 218.00 | n = 30, mean = 264.96, SD = 233.59 | n = 38, mean = 257.19, SD = 225.54 | |
| Season of sampling | 1 | Spring = 38 Summer = 49 Fall = 16 Winter= 23 Total = 126 | Spring = 33 Summer = 45 Fall = 16 Winter = 21 Total = 115 | Spring = 6 Summer = 7 Fall = 2 Winter = 3 Total = 18 | Spring = 9 Summer = 12 Fall = 3 Winter = 6 Total = 30 |
| 2 | Spring = 33 Summer = 49 Fall = 25 Winter = 22 Total = 129 | Spring = 21 Summer = 43 Fall = 16 Winter = 13 Total = 93 | Spring = 9 Summer = 6 Fall = 5 Winter = 7 Total = 27 | Spring = 11 Summer = 12 Fall = 6 Winter = 10 Total = 39 | |
| 3 | Spring = 33 Summer = 38 Fall = 13 Winter = 16 Total = 100 | Spring = 25 Summer = 34 Fall = 13 Winter = 13 Total = 85 | Spring = 7 Summer = 13 Fall = 4 Winter = 6 Total = 30 | Spring = 8 Summer = 17 Fall = 5 Winter = 8 Total = 38 | |
| Collection Time 1 Before cognitive testing (min after midnight) | 1 | n = 126, mean = 680.09, SD = 137.23 | n = 115, mean = 682.79, SD = 140.84 | n = 18, mean = 709.28, SD = 168.26 | n = 30, mean = 715.03, SD = 155.44 |
| 2 | n = 129, mean = 711.65, SD = 123.23 | n = 93, mean = 710.58, SD = 121.67 | n = 27, mean = 739.22, SD = 109.31 | n = 39, mean = 729.03, SD = 114.44 | |
| 3 | n = 100, mean = 722.32, SD = 105.41 | n = 85, mean = 720.52, SD = 105.51 | n = 30, mean = 727.10, SD = 118.59 | n = 38, mean = 726.34, SD = 109.48 | |
| Collection Time 2 After cognitive testing (min after midnight) | 1 | n = 126, mean = 819.21, SD = 152.99 | n = 115, mean = 824.12, SD = 157.61 | n = 18, mean = 869.61, SD = 61.83 | n = 30, mean = 869.53, SD = 161.42 |
| 2 | n = 129, mean = 869.03, SD = 99.42 | n = 93, mean = 870.38, SD = 96.03 | n = 27, mean = 891.19, SD = 85.93 | n = 39, mean = 885.38, SD = 96.95 | |
| 3 | n = 100, mean = 850.18, SD = 143.52 | n = 85, mean = 846.24, SD = 147.04 | n = 30, mean = 847.83, SD = 166.09 | n = 38, mean = 857.37, SD = 150.92 | |
| Age (years) | 1 | n = 126, mean = 12.47, SD = 3.29 Age range = 4.88 to 18.24 | n = 115, mean = 12.63, SD = 3.19 Age range = 6.09 to 18.24 | n = 18, mean = 17.02, SD = 0.62 Age range = 16.00 to 18.24 | n = 30, mean = 16.51, SD = 0.891 Age range = 15.04 to 18.24 |
| 2 | n = 129, mean = 13.25, SD = 3.60 Age range = 6.79 to 20.17 | n = 93, mean = 13.61, SD = 3.54 Age range = 6.79 to 20.17 | n = 27, mean = 18.30, SD = 1.19 Age range = 16.01 to 20.17 | n = 39, mean = 17.44, SD = 1.57 Age range = 15.09 to 20.17 | |
| 3 | n = 100, mean = 14.40, SD = 3.68 Age range = 9.08 to 22.26 | n = 85, mean = 14.52, SD = 3.84 Age range = 9.08 to 22.26 | n = 30, mean = 18.84, SD = 2.04 Age range = 16.08 to 22.26 | n = 38, mean = 18.14, SD = 2.28 Age range = 15.04 to 22.26 | |
| Gender F = female M = male | 1 | F = 73, M = 53 Total = 126 | F = 64, M = 51 Total = 115 | F = 10, M = 8 Total = 18 | F = 17, M = 13 Total = 30 |
| 2 | F = 80, M = 49 Total = 129 | F = 59, M = 54 Total = 113 | F = 15, M = 12 Total = 27 | F = 23, M = 16 Total = 39 | |
| 3 | F = 55, M = 45 Total = 100 | F = 45, M = 40 Total = 95 | F = 16, M = 14 Total = 30 | F = 21, M = 17 Total = 38 | |
| Pubertal stage | 1 | n = 126, mean = 2.13 SD = 1.16 | n = 115, mean = 2.19 SD = 1.18 | n = 18, mean = 3.56 SD = 0.705 | n = 30, mean = 3.50 SD = 0.777 |
| 2 | n = 128, mean = 2.30, SD = 1.28 | n = 93, mean = 2.42, SD = 1.29 | n = 27, mean = 3.96, SD = 0.706 | n = 39, mean = 3.67, SD = 0.772 | |
| 3 | n = 100, mean = 2.64, SD = 1.38 | n = 85, mean = 2.65, SD = 1.412 | n = 30, mean = 4.07, SD = 0.828 | n = 38, mean = 3.92, SD = 0.818 | |
| Handedness | 1 | L = 11, R = 115 Total = 126 | L = 8, R = 107 Total = 115 | L = 0, R = 18 Total = 18 | L = 1, R = 29 Total = 30 |
| 2 | L = 11, R = 118 Total = 129 | L = 7, R = 86 Total = 93 | L = 1, R = 26 Total = 27 | L = 1, R = 38 Total = 39 | |
| 3 | L = 9, R = 91 Total = 100 | L = 8, R = 77 Total = 85 | L = 3, R = 27 Total = 30 | L = 4, R = 34 Total = 38 | |
| Total brain volume (cm3) | 1 | n = 126, mean = 1266.16 SD = 639.68 | n = 115, mean = 1269.59, SD = 632.74 | n = 18, mean = 1288.05, SD = 572.60 | n = 30, mean = 1294.40, SD = 643.49 |
| 2 | n = 129, mean = 1276.07, SD = 648.14 | n = 93, mean = 1278.05, SD = 642.45 | n = 27, mean = 1310.72, SD = 609.08 | n = 39, mean = 1304.60, SD = 680.92 | |
| 3 | n = 100, mean = 1291.78, SD = 748.61 | n = 85, mean = 1291.79, SD = 772.72 | n = 30, mean = 1310.85, SD = 884.88 | n = 38, mean = 1310.80, SD = 861.10 | |
| Left hippocampus (mm3) | 1 | n = 126, mean = 2940.23 SD = 324.23 | n = 115, mean = 2956.07, SD = 326.92 | n = 18, mean = 2939.55, SD = 351.82 | n = 30, mean = 3010.12, SD = 354.66 |
| 2 | n = 129, mean = 2987.06, SD = 304.70 | n = 93, mean = 2991.27, SD = 298.77 | n = 27, mean = 3038.67, SD = 284.19 | n = 39, mean = 3055.25, SD = 313.63 | |
| 3 | n = 100, mean = 3087.75, SD = 345.36 | n = 85, mean = 3064.34, SD = 357.74 | n = 30, mean = 3071.43, SD = 373.92 | n = 38, mean = 3104.27, SD = 391.26 | |
| Right hippocampus (mm3) | 1 | n = 126, mean = 3028.87 SD = 354.48 | n = 115, mean = 3043.84, SD = 354.86 | n = 18, mean = 3014.47, SD = 370.09 | n = 30, mean = 3102.84, SD = 421.53 |
| 2 | n = 129, mean = 3073.19, SD = 328.94 | n = 93, mean = 3085.12, SD = 330.75 | n = 27, mean = 3137.34, SD = 326.86 | n = 39, mean = 3153.56, SD = 347.05 | |
| 3 | n = 100, mean = 3153.42, SD = 371.67 | n = 85, mean = 3132.58, SD = 379.19 | n = 30, mean = 3162.60, SD = 410.30 | n = 38, mean = 3200.43, SD = 414.76 |
2.2. Neuroimaging Measures
A three-dimensional T1-weighted (T1W) Spoiled Gradient Recalled (SPGR) echo sequence from 1.5 Tesla scanners was obtained on each participant, with 1mm isotropic data acquired sagittally from the entire head for most scanners. In addition, T2-weighted (T2W) and proton density-weighted (PDW) images were acquired using a two-dimensional (2D) multi-slice (2mm) dual echo fast spin echo (FSE) sequence. Fully automated analysis of whole-brain cortical thickness was done through the CIVET pipeline, developed at the Montreal Neurological Institute (MNI). First, a multistage quality control process was implemented, excluding participants with white or gray matter artifacts. All quality-controlled MR images were subsequently processed through the CIVET pipeline. These processing steps have been described at length in other publications.
Volumetric measures of the hippocampus were obtained from MRI data using a fully automated segmentation method validated in human participants (38). This method utilizes a MRI dataset (N=80) of young healthy adults that serves as a template library of manually-labeled hippocampal volumes. Even though the template library of manually labeled hippocampal volumes consisted of data from healthy young adults, using the ANIMAL pipeline combined with this template library results in a method fairly resistant to developmental volumetric deviations, as shown by its high Dice Kappa and Jaccard similarity values (38). In addition, previous comparisons between pediatric and adult structural MRI brain templates detected no systematic bias in comparisons between adults and children over 6 years of age in our NIHPD dataset. The manual segmentation was done by four different raters, and intra-class intra-rater and inter-rater reliability varied between r=0.83 for the right and r=0.95 for the left hippocampus. From this manual segmentation, a fully automated method was derived, characterized by label fusion techniques that combine segmentations from a subset of ‘n’ most similar templates. Specifically, each template is used to produce an independent segmentation of the participant using the ANIMAL pipeline, followed by a thresholding step to eliminate cerebrospinal fluid, which results in ‘n’ different segmentations. To fuse the segmentations at each voxel, a voting strategy is used; the label with the most votes from the ‘n’ templates is assigned to the voxel (38). Combining multiple segmentations minimizes errors and maximizes consistency between segmentations. When using n = 11 templates, the label fusion technique has been shown to yield an optimal median Dice Kappa of 0.886 and Jaccard similarity of 0.796 for the hippocampus (38). Of note, the relationship between DC ratio, cortico-amygdalar structural covariance and cognition will be addressed in a separate manuscript.
Here we use structural covariance between the cortex and hippocampus as a measure of coordinated growth between these structures. If greater cortical thickness correlates with greater hippocampal volume, we term this “positive covariance”. If greater cortical thickness correlates with lower hippocampal volume, we term this “negative covariance”. Structural covariance has been hypothesized to result from the combined influence of mutually trophic (or apoptotic) factors (39). As such, cortico-limbic structural covariance may be uniquely determined in each subject, presumably by a combination of genetic and environmental factors (39). In addition, there is some evidence that structural covariance networks parallel the development of functional networks from childhood to young adulthood (40). As such, cortico-hippocampal structural covariance may also represent a valid measure of structural connectivity and coordinated functional activation between these structures during development.
2.3. Hormonal and Pubertal Measures
Children provided two 1–3 cm3 samples of saliva before cognitive testing, and two samples after cognitive testing (total 4 samples), for each research visit that the child completed, every 2 years. These samples were assayed by enzyme-linked immunosorbent assay (ELISA) methods and time 1 (pre-cognitive testing) and time 2 (post-cognitive testing) samples were averaged separately. Care was taken to sample saliva some time (1–2 hours) after the completion of the MRI scan. Most of these measures were collected in the early morning to the early afternoon, though no waking samples were collected per se. At the next visit, a similar procedure was followed, and the child again provided two separate saliva samples for hormonal measurement before and after cognitive testing (total 4 samples).
Salivary sampling measures the free, non-protein bound, biologically active portions of circulating hormonal levels relevant to studies of brain-hormone associations (41). Salivary cortisol levels correlate highly with serum levels; in turn serum levels correlate highly with ventricular cerebrospinal fluid (CSF) levels. Cortisol shows marked reactivity to the environment, including diurnal rhythms, with morning levels up to 10-fold those collected in the evening. DHEA, as opposed to its sulfated hydrophilic form DHEAS, is easily measured in saliva and crosses the blood-brain barrier due to its lipophilicity (42). Similar to cortisol salivary DHEA levels correlate with serum DHEA levels; in turn, peripheral DHEA levels correlate with CSF DHEA levels (43). DHEA diurnal rhythm is less pronounced than cortisol, e.g. morning levels are about 2-fold those collected in the evening. Androgen levels may also rise in the fall, followed by a slow decrease during the spring and summer (42). Both diurnal and seasonal patterns appear to be more prominent in boys than in girls. To control for these factors, we have included collection time (time 1: when the first set of 2 samples -before cognitive testing- was collected, and time 2: when the second set of 2 samples -after cognitive testing- was collected), sex and season as covariates in hormonal analyses (see section 2.5). Because both hormonal variables exhibited a positive skew to their distribution, we used a log transformation on both of them prior to the calculation of the ratio. This also allows us to standardize the ranking of values within a variable prior to the calculation of the ratio. Notably, cognitive testing in itself may constitute a stressful situation which elicits a cortisol response (44). Elevations in salivary cortisol have been reported in response to cognitive testing specifically (45), suggesting that hormonal measures collected before and after cognitive testing might represent different responses to stress, i.e. anticipatory stress response vs. post-stress hormonal response.
To measure pubertal maturation, the Pubertal Development Scale (PDS) was administered by a physician to all participants included in this study. This scale has been shown to have good reliability (coefficient alpha: 0.77) and validity compared to physical examination (r2=0.61–0.67 for the relationship between PDS scores and Tanner staging based on physical examination). During an interview with the child/adolescent, questions were asked about physical development. We computed a puberty variable consisting of 5 stages, representing increasing levels of physical maturity similar to Tanner staging, previously described. Pubertal stage was also included as a covariate in hormonal analyses (see section 2.5), to account for other pubertal hormones not measured in the study (e.g. androstenedione, progesterone, etc.).
2.4. Measures of Personality, Behavior and Cognition
Measures of temperament, behavior and cognition were collected after the MRI scan and after the first hormonal sampling.
2.4.1. Behavioral Measures
To measure internalizing symptoms, we used the Anxious-Depressed subscale of the Child Behavior Checklist (CBCL) and Young Adult Self-Report (YASR). To measure externalizing symptoms, we used the Aggression and Rule-Breaking subscales of the CBCL and YASR. The CBCL and YASR are instruments extensively used for assessing psychopathology worldwide and are appropriate for the entire age range of 6–22 years old examined here. These questionnaires require parents or young adults themselves to report on specific behaviors exhibited within the previous 6 months (46). The YASR was derived from items on the CBCL and serves as a self-report extension of the CBCL for young adults. Both the CBCL and YASR are reliable measures with high stability over time, validated in multiple cultures, with high internal consistency (Cronbach α values=0.84–0.94 for the subscales used in this study) (46).
2.4.2. Measures of Personality
Given this study includes a typically developing sample and excludes the great majority of clinically significant psychiatric disorders (see section 2.1 for more details), examining personality characteristics that may predispose an individual toward developing internalizing or externalizing disorders may represent a more sensitive method to detect mental health vulnerability than directly measuring these symptoms, particularly in a typically developing population (see below section 2.4.2). To measure temperament/personality traits in our sample, we used the Temperament and Character Inventory (TCI). The TCI is an measure of temperament and character traits that has been validated in adolescents and young adults (47). The TCI measures seven dimensions of personality: four temperaments –Novelty Seeking, Harm Avoidance, Reward Dependence and Persistence; 3 character traits –Self-Directedness, Cooperativeness, and Self-Transcendence (47). Overall, excellent internal consistency were reported with Cronbach α values ranging from 0.76 to 0.89 in adults, and acceptable to good internal consistency in adolescents with Cronbach α values ranging from 0.60 to 0.85 (48). The predictive value of the TCI in terms of mental health vulnerability has been examined in several adult samples, with high levels of harm avoidance and self-transcendence, as well as low levels of reward dependence, cooperativeness, novelty-seeking and self-directness particularly associated with the development of depression (49).
2.4.3. Cognitive Measures
To assess executive function, we used the Behavior-Rated Inventory of Executive Function (BRIEF). The BRIEF uses parent ratings of executive function in the context of everyday problem solving. It measures executive function (including working memory) in an integrated way, outlining the complex, priority-based decision-making that is demanded in real-world situations (50). The BRIEF has demonstrated high test-retest reliability (r ≈0.82 for parent ratings) and high internal consistency (Cronbach’s alphas ≈.80 −.98) (50).
To assess verbal learning and memory, we used the California Verbal Learning Test (CVLT). The CVLT assesses components of immediate, short-term and long-term delayed recall of verbal items (51). This test measures performance with regards to semantic clustering, serial clustering, free vs. cued recall, perseveration and intrusion errors, response bias, response consistency, and learning slope, and yields several sub-scores (51). Here we are focusing on immediate free recall (for up to 5 trials), short-term delay (free and cued) recall, and long-term delay (free and cued) recall. The CVLT is one of the most frequently used children’s measures of verbal learning and memory and has moderate to high test-retest reliability: r ≈ 0.62–0.93. The previously demonstrated construct validity and temporal stability of the CVLT also make it a measure of episodic verbal learning and memory supported by a considerable body of research (51).
To assess spatial abilities, we used the Cambridge Neuropsychological Test Automated Battery [CANTAB] spatial span and spatial working memory subtests (52). The CANTAB is a computerized neuropsychological test battery that includes only nonverbal geometric designs or simple shapes, with minimal required language proficiency. The validity of CANTAB for assessing brain-behavior relations in adults has been established, and results of tests in pediatric populations have shown that children can be tested with the same item sets that are employed in adult studies. Reliability is high in pediatric populations (Cronbach’s alpha coefficients=0.73 for reaction time, and 0.95 for performance on the spatial working memory test) (52). Test-retest stability coefficients are moderate in magnitude and range from 0.6–0.7, and construct validity has been established in pediatric populations (52).
To assess mathematical abilities, we used the Woodcock-Johnson (WJ) Tests of Cognitive Abilities. The WJ is a cognitive battery measuring intelligence level and learning capacities and predicting academic achievement, which uses interpretative scaling to predict how the individual would perform similar tasks in real-life, functional settings (53). It has been validated on large pediatric samples, with a median reliability coefficient around 0.9 (53). Here we used the WJ Calculation and Math Fluency sub-tests.
2.5. Statistical Analyses
Statistical analyses were done using SurfStat (Matlab toolbox designed by Keith J. Worsley) for neuroimaging analyses and SPSS 21.0 (SPSS, Inc., Chicago, Illinois) for behavioral/cognitive analyses. Please see Table 2 for more details on statistical models used in this section. Of note, a correction for multiple comparisons using random field theory (RFT, p<0.05) was applied to all analyses.
TABLE 2: Description of statistical models.
The specific statistical term of interest is underlined in each model; the rest of the terms represent control variables; ‘id’ refers to a specific participant’s identification number: this term is included in order to identify and link all longitudinal data from the same participant; ‘I’ to the identity matrix of the mixed effects model; ‘CTh’ in section 2.5.2 refers to average cortical thickness of the brain regions found to be significant in section 2.5.1
| Methods section | Statistical model |
|---|---|
| 2.5.1 DC Ratio & Cortico-Hippocampal Structural Covariance | (1) Whole-brain CTh = 1 + DHEA/Cortisol*Hipp + DHEA/Cortisol +Hipp + Collection Time + Age + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I |
| (2) Whole-brain CTh = 1 + DHEA/Cortisol*Hipp*Sex + DHEA/Cortisol*Hipp + Hipp*Sex + DHEA/Cortisol*Sex + DHEA/Cortisol +Hipp + Sex + Collection Time + Age + Scanner + Handedness + Total Brain Volume + random (id) + I | |
| (3) Whole-brain CTh = 1 + DHEA/Cortisol*Hipp*Age + DHEA/Cortisol*Hipp + Hipp*Age + DHEA/Cortisol*Age + DHEA/Cortisol +Hipp + Age + Collection Time + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I | |
| (4) Whole-brain CTh = 1 + DHEA/Cortisol*Hipp*Puberty + DHEA/Cortisol*Hipp + Hipp*Puberty + DHEA/Cortisol*Puberty + DHEA/Cortisol +Hipp + Puberty + Age + Collection Time + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I | |
| (5) Whole-brain CTh = 1 + DHEA/Cortisol*Hipp*Sex*Age + DHEA/Cortisol*Hipp*Sex + DHEA/Cortisol*Hipp*Age + Hipp*Sex*Age + Hipp*Sex + Hipp*Age + DHEA/Cortisol*Hipp + DHEA/Cortisol*Sex + DHEA/Cortisol*Age + DHEA/Cortisol +Hipp + Sex + Collection Time + Age + Scanner + Handedness + Total Brain Volume + random (id) + I | |
| (6) Note that in order to limit the number of control variables per model: models (1), (2), (3) were retested while adding testosterone, estradiol, pubertal stage or season of sampling as additional covariates (one at a time) | |
| 2.5.2 Cortico-Hippocampal Structural Covariance & Measures of Personality,Behavior and Cognition | (1) Following significant results with the ′Sex*DHEA/Cortisol Ratio*Hipp′ term in section 2.5.1: Cognitive/Behavioral Scores = 1 +Sex*CTh*Hipp + Sex*CTh + CTh*Hipp + Sex*Hipp + CTh + Hipp + Sex + Age + Scanner + Handedness + Total Brain Volume + random (id) + I |
| 2.5.3 Indirect Effects of DC ratio on Measures of Personality, Behavior and Cognition | (1) Beta coefficients and p-values extracted
from section 2.5.1 (2) Beta coefficients and p-values extracted from section 2.5.2 |
| (3) Beta coefficients and p-values extracted from (1) and (2) were extracted from existing analyses, and entered in the Sobel-Goodman test calculator to formally test indirect effects |
2.5.1. DHEA/Cortisol Ratio and Cortico-Hippocampal Structural Covariance
Mixed effects designs were used to model the relationship between DC ratio and covariance of the hippocampus with whole-brain, native-space CTh, taking into account the within- and between- individual variances in this longitudinal sample, and controlling for the effects of age, sex, total brain volume, scanner, handedness, time of salivary sampling. As such, data from the same children collected across different timepoints are nested within-individuals. In other words, in the context of this statistical procedure, MRI scans from the same child are treated as related measures (with some degree of within-individual variance due to differences in the age at which the scan was completed) and MRI scans from different children as independent measures (accounted by between-individual variance). For every MRI scan, DC ratio collected at time 1 (before cognitive testing) was evaluated in separate models than DC ratio collected at time 2 (after cognitive testing), as they represent different measures of stress response (anticipatory increase vs. post-stress reactivity). All continuous variables were centered using their respective means. To examine associations between DC Ratio and structural covariance of the hippocampus, we examined the significance of the term “DC ratio*Hippocampus”, while controlling for all the aforementioned control variables (see example below, with the terms of interest underlined). To examine any distinct effects of DHEA and cortisol above and beyond those related to estradiol, testosterone or season of collection, these variables were also included as control variables in additional models. Finally, to test for sex, age and pubertal effects on the relationship between DC ratio and cortico-hippocampal networks, we tested for interactions with these variables, e.g. ‘DC ratio*Hippocampus*Sex’; and interactions between sex, age and pubertal stage, e.g. ‘DC ratio*Hippocampus*Age*Sex’, on whole-brain cortical thickness (see Table 2 for more details).
2.5.2. Cortico-Hippocampal Structural Covariance and Cognitive/Behavioral Measures
To examine associations between the brain circuits impacted by DC ratio and cognitive/behavioral measures, we averaged the cortical thickness (CTh) of brain regions found to be significant in section 2.5.1 and examined the significance of the interactions terms found to be significant in section 2.5.1 on cognitive/behavioral measures, while controlling for all the aforementioned control variables (see Table 2 for more details).
2.5.3. Indirect effects of DC ratio through Cortico-Hippocampal Structural Covariance
We formally tested whether DC ratio could have indirect effects on measures of personality, behavior and cognition (through an alteration in cortico-hippocampal covariance:
-
(1)
To examine the relationship between DC ratio and covariance of the hippocampus with the cortical region found to be significant in section 2.5.1, we extracted the coefficients and p-values of the significant interaction term ‘DC Ratio*Hippocampus*Sex’.
-
(2)
To examine the relationship between cortico-hippocampal covariance and cognitive/behavioral measures found to be significant in section 2.5.2, we extracted the coefficients and p-values of the significant interaction term ‘CTh*Hippocampus*Sex’.
-
(3)
Finally, coefficients and p-values were extracted from existing analyses and tested for indirect effects using a formal Sobel’s test (http://quantpsy.org/sobel/sobel.htm).
Because of the complexity of our data (multiple visits completed by each participant, different number of visits available per subject over a different number of timepoints), we did not use bootstrapping, because this procedure cannot be applied simultaneously to all the predictors, mediators, and outcomes of interest in this study while also taking into account their longitudinal nature. The conservative method we selected instead (Sobel test) treats each relationship (between predictor and mediator, and then between mediator and outcome) separately, allowing us to model the longitudinal component of the data for each of these relationships. The Sobel test is very conservative and as a result has low power (http://davidakenny.net/cm/mediate.htm)–but this is counterbalanced by our relatively large sample size. Finally, please note that the same set of control variables (including age), as listed in sections 2.5.1 and 2.5.2 was used to test indirect effects.
3. RESULTS
3.1. Sample Characteristics
Table 1 details sample characteristics, including number of longitudinal scans and covariates of interest. 225 subjects (128 girls) were used for analyses examining the relationship between DC ratio and cortico-hippocampal covariance (total 355 scans) and 58–203 subjects (31–112 girls) for analyses examining the relationship between cortico-hippocampal covariance and behavioral/cognitive analyses (total 75–293 scans). Participants were aged between 6 and 22 years old, with a mean age of 12.36 to 18.21 (SD = 1.50 to 1.65 years), depending on availability of hormonal or cognitive/behavioral data.
3.2. DHEA/Cortisol Ratio and Cortico-Hippocampal Structural Covariance
As shown in Figures 1 and 2, whole-brain analyses showed there was a significant interaction between sex, DC ratio (collected at time 1) and structural covariance between the hippocampus and CTh of the left medial anterior cingulate cortex (ACC, linear models, Brodmann 24, cluster-level p=9.75*10−3, 205 vertices, peak vertex id 7209 [x=−3.32, y=28.6, z=19.1], coefficient=−0.235; standard error=5.25*10−2; p=6.31*10−6). Note that the relationship between this prefrontal region and the hippocampus was present for both the left and right hippocampus. In girls, higher DC ratios were associated with a more negative ACC-hippocampal covariance; in boys, higher DC ratios were associated with a more positive ACC-hippocampal covariance. Of note, there was no sex-specific association between DC collected at time 2 and cortico-hippocampal covariance. In addition, no other brain region met the threshold for significance (RFT, p<0.05) in the analysis involving DC ratio collected at time 2 (post-cognitive testing). Adding pubertal stage, estradiol, testosterone, or season of sampling as control variables did not result in any differences in the above findings. Finally, there were no significant interactions with age or puberty on the sex-specific relationship between DC ratio and cortico-hippocampal structural covariance.
FIGURE 2: Boys: DHEA/Cortisol Ratio and Prefrontal-Hippocampal Structural Covariance.
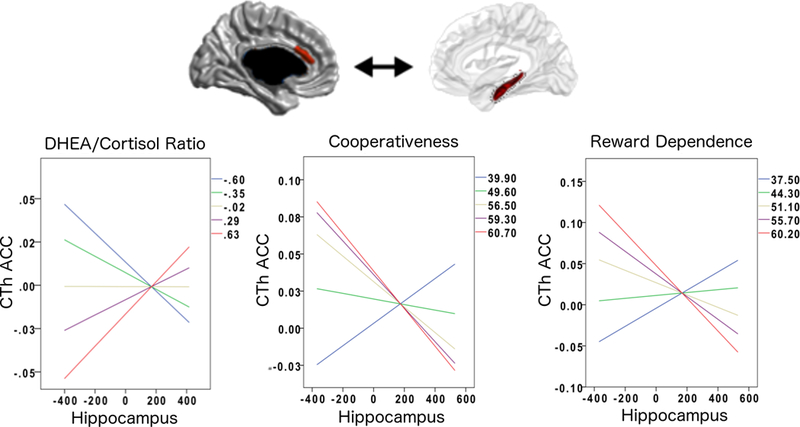
This figure shows the associations between DHEA/Cortisol ratio, ACC-hippocampal structural covariance and personality traits in boys.
The positive ACC-hippocampal covariance seen at higher DHEA/Cortisol ratios was associated with lower scores on the cooperativeness and reward dependence subscales. Overall regression-fit-lines for ACC-hippocampus covariance are displayed for different percentiles (20th, 40th, 60th, 80th, 100th) of DHEA/Cortisol ratios and cognitive/behavioral scores.
Please note that the ACC area shown in this figure is not a pre-defined region-of-interest (ROI). Rather, this is the only region across the whole brain whose sex-specific relationship to the hippocampus significantly varied as a function of DHEA/Cortisol ratio (controlled for multiple comparisons using random field theory). In addition, note that the Y axes of graphs list standardized residuals of cortical thickness (accounting for the effects of age, sex, handedness, scanner, and total brain volume in all analyses, as well as collection time for hormonal analyses).
3.3. Cortico-Hippocampal Structural Covariance and Cognitive/Behavioral Measures
As shown in Figures 1 and 2, there was a significant interaction between sex, ACC-hippocampal structural covariance and specific cognitive and behavioral measures. In girls, the ACC-hippocampal structural covariance previously associated with higher DC ratios (section 3.2) was associated with higher verbal memory (immediate free recall, trial 5, number of words recalled, as measured by CVLT, linear models, cluster-level coefficient=7.59*10−3; standard error=3.57*10−3; p=3.50*10−2) and higher mathematical ability (WJ Calculation, linear models, cluster-level coefficient=−1.30*10−2; standard error=6.44*10−3; p=4.40*10−2), but was not related to measures of personality. In boys, the ACC-hippocampal structural covariance previously associated with higher DC ratios was associated with personality traits (lower cooperativeness; linear models, cluster-level coefficient=−6.50*10−2; standard error=2.52*10−2; p=1.20*10−2; lower reward dependence; linear models, cluster-level coefficient=−6.15*10−2; standard error=2.84*10−2; p=3.30*10−2) but was not related to cognitive measures. No significant relationship emerged between ACC-hippocampal covariance and any cognitive measures related to spatial abilities (CANTAB spatial span or spatial working memory) or any behavioral measures (anxious-depressed symptoms, aggression or rule-breaking behavior as measured by CBCL/YASR).
3.4. Indirect effects of DC Ratio through Cortico-Hippocampal Structural Covariance
As shown in Table 3, DC ratio had significant indirect effects on verbal memory in girls, and on cooperativeness and reward dependence in boys, through its impact on ACC-hippocampal structural covariance (p<=0.05). There was also a trend for the indirect effect of DC ratio on calculation scores in girls (through an alteration in ACC-hippocampal covariance; p≅0.06).
TABLE 3: Indirect Effects.
| Coefficient (test-statistic) |
Standard error | Significance (p-value; *p<0.05) |
|
|---|---|---|---|
| DC Ratio&ACC-hippocampal Covariance | −0.235 | 5.25*10−2 | 6.31*10−6 |
| ACC-hippocampal Covariance&Cognition | WJ-Calculation: -1.30*10−2CVLT-immediate recall: 7.59*10−3TCI-Cooperativeness: -6.50*10−2TCI-Reward Dependence: -6.15*10−2 | WJ-Calculation: 6.44*10−3CVLT-immediate recall: 3.57*10−3 TCI-Cooperativeness: 2.52*10−2 TCI-Reward Dependence: 2.84*10−2 | WJ-Calculation: 4.40*10−2 CVLT-immediate recall: 3.50*10−2 TCI-Cooperativeness: 1.20*10−2 TCI-Reward Dependence: 3.30*10−2 |
| Sobel test for indirect effects *testing whether structural covariance mediates the relationship between DC ratio and cognitive test | WJ-Calculation: 3*10−3 CVLT-immediate recall: -2*10−3TCI-Cooperativeness:1.5*10−2TCI-Reward Dependence:1.4*10−2 | WJ-Calculation:2*10−3CVLT-immediate recall:1*10−3TCI-Cooperativeness:7*10−3TCI-Reward Dependence:8*10−3 | Confidence Interval: WJ-Calculation: [0, 7*10−3] CVLT-immediate recall:[-4*10−3, 0] TCI-Cooperativeness:[3*10−3, 3*10−2] TCI-Reward Dependence:[1*10−3, 3.1*10−2] |
4. DISCUSSION
This study shows sex-specific associations between DC ratio and ACC-hippocampal structural covariance. Higher DC ratios were associated with more negative covariance between hippocampal and medial cingulate areas in girls. In contrast, higher DC ratios were associated with more positive covariance between the same areas in boys. MRI results continue to represent a somewhat crude estimate of the molecular processes occurring in the CNS and cannot be readily interpreted in terms of neurogenesis or glial proliferation. Therefore, we cannot determine from this study whether differences in DC ratio in vivo leads to a difference in neuronal or glial survival. However, based on prior studies of structural covariance and connectivity -see section 2.2 for more details- (40), we could infer from the current results that higher DC ratios have the potential to decrease the functional connectivity, structural covariance, and possibly the density of structural connections, between prefrontal and hippocampal areas in girls, with the opposite effect observed in boys. Whether this process results from strengthening of other, long-range connections (between the hippocampus and cortical regions other than the prefrontal cortex) or the weakening of existing short-range ACC-hippocampal connections remains to be determined by future investigations and is outside the scope of the current study. In addition, it should not be assumed that more positive ACC-hippocampal covariance is necessarily beneficial (and vice versa for negative structural covariance). Indeed, in certain contexts, a pruning of extraneous connections between the ACC-hippocampal connections (potentially linked to negative structural covariance between these structures) may have a beneficial effect on cognitive parameters -as seen here in girls-. Indeed, negative structural covariance may be related to greater brain maturation and cognitive function. Structural connectome mapping has demonstrated that the developing male brain has a high connectivity within brain modules whereas the female brain is characterized by a widely distributed network with weaker connectivity. This distributed network correlates with developmental maturity and may underlie superior cognitive performance in females for higher-level cognitive tasks requiring extensive multi-modal integration (25).
Previous studies have shown that prolonged elevations in stress hormones may lead to loss of neurons and neural connections in both the medial prefrontal cortex (mPFC) and hippocampus, leading to a potential loss in top-down control (54). In turn, these alterations in brain networks have been shown to lead to subsequent problems in cognitive and behavioral development, setting the stage for adult psychiatric disorders later on (55). While our study cannot confirm that DC ratio is a valid marker for prolonged or chronic stress, we found that, across all brain regions, only a very specific region of the prefrontal cortex (the medial anterior cingulate cortex) showed significant covariance with the hippocampus as a function of both DHEA and cortisol levels. The specificity of this finding suggests that the medial ACC-hippocampal anatomical network may be particularly sensitive to the effects of these adrenal hormones and indicate that DC ratio could be a valid marker of anabolic and catabolic state in the CNS.
Further, we found that the ACC-hippocampal structural covariance related to higher DC ratios was also associated with higher verbal memory and mathematical ability in girls; in boys, it was associated with low cooperativeness and reward dependence. These findings underline the critical role that ACC-hippocampal connections may play in the development of sex differences in cognition and personality during adolescence. Both the medial prefrontal cortex and the hippocampus are part of a cortico-limbic networks that regulates goal-directed behavior by integrating hippocampal-dependent contextual information with cognitive information processed in the medial prefrontal cortex (56). Interestingly, specific hippocampal areas (CA1 and subiculum) also send direct projections back to the medial prefrontal cortex, allowing the formation of a direct feedback loop between cortical and subcortical areas during memory encoding and retrieval (56). Alterations in the structural connections between the hippocampus and the medial prefrontal cortex have previously been linked with decreased mnemonic ability as well as disrupted emotional control in the context of various psychiatric disorders (57).
Our results in girls, though preliminary and awaiting replication, underline the importance of prior observations that higher DHEA or DHEA-S levels and lower cortisol levels may alter cognitive performance in a sex-specific manner (30). Specific decreases in verbal memory have already been reported in children exposed to high-dose corticosteroids (58), and there is some evidence that, at least in adults, these toxic effects may extend to other types of memories such as episodic autobiographical memories (59). In contrast, there is evidence from our group that endogenous DHEA levels in children and adolescents may be associated with better visual attention and working memory through cortico-amygdalar and cortico-hippocampal alterations (5, 36) and that higher DHEA or DHEA-S levels may predict better working memory, attention and verbal fluency in women, but not in men (30). In the current study, we find that DC ratio was associated with alterations in ACC-hippocampal covariance that were in turn related to enhanced verbal immediate recall and calculation abilities. Interestingly, this relationship between DC ratio, ACC-hippocampal covariance and WJ Calculation (but not WJ Math Fluency) underscores the fact that some students may have trouble with calculation because of difficulties in retrieving arithmetic facts, rather than because of a lack of understanding of mathematical concepts (60). This supports the notion that certain types of reading and mathematical disabilities may co-occur because of a shared reliance on working memory/retrieval processes (60). Taken together, these findings suggest that the impact of DC ratio on sex-specific reading and mathematical abilities may be a function of its impact on shared working memory processes mediated by ACC-hippocampal connections (59), and support the notion that sex differences in cognition can be tied, at least in part, to hormonal causes.
Our findings in boys are consistent with prior reports that higher DC ratios, or lower CD ratios, may be associated with a higher frequency and severity of both internalizing and externalizing disorders, particularly in male children and adolescents (7). For example, one study reports that lower CD ratios were linked to a higher risk of impaired socio-emotional functioning (lower social competence and higher externalizing problems) (61). Similarly, other reports contend that higher DHEA levels relative to cortisol levels may be associated with a higher risk of conduct-related externalizing problems in children (62–69). However, findings have not been consistent across studies, with several demonstrating a higher risk of psychopathology with lower DC (or higher CD) ratios (7). Several explanations may account for these conflicting findings. First, studies looking at the relationship between DC ratios and major depression (MDD), and finding an inverse relationship between these variables, have primarily examined adolescent girls (more prone to developing MDD) (7). On the other hand, studies looking at the relationship between DC ratios and conduct/externalizing disorders, and finding a positive relationship between these two variables, have primarily examined adolescent boys (more prone to developing conduct disorders) (62–69). Therefore, sex is a confounding/moderating variable that could partly explain some of the conflicting findings in the literature.
Second, as previously postulated by our group (36), the effect of DHEA on the brain is complex and may both be beneficial and detrimental depending on the context as well as the specific genetic/environmental factors that may influence a child’s development. For example, DHEA may improve both attention and overall working memory during development by decreasing the influence of amygdalar and hippocampal afferents on cortical functions, e.g. DHEA may inhibit amygdalar-based functions (e.g. the detection of emotional stimuli), and hippocampal-based functions (e.g. encoding and processing of spatial and social cues). This inhibition could allow for more purely cognitive functions, like attention and overall working memory, to proceed unencumbered. However, this phenomenon could occur at the cost of a DHEA-related impairment in interoceptive awarenesss, social understanding and emotional processing/empathy. This theory would be consistent with the higher rates of disruptive behaviors seen with increased DHEA levels, an effect that may be particularly striking in the context of an abnormal, premature timing of adrenarche (70–74) as well as in the context of low cortisol levels, as demonstrated in the current study.
Regardless of the complex mechanisms underlying the effects of DC ratio on brain structure, brain imaging studies certainly support the notion of sex-specific correlations between structural brain covariance and externalizing symptoms. Female adolescents with conduct disorder demonstrate reduced insula volume relative to controls, whereas male adolescents demonstrate an increased insula volume (75). Aggressive and defiant behavior has been linked to a decreased right ACC volume in boys but not girls (76). In young children (aged 6–9), aggression has been correlated to reduced cortical thickness in regions of the default mode network in boys but increased thickness in girls (77). We tested these associations in both boys and girls and find support for a sex-specific effect. Indeed, ACC-hippocampal covariance (as modulated by DC ratio) was associated with lower cooperativeness and reward dependence only in boys, not girls. Low cooperativeness and reward dependence are, in turn, associated with lower academic achievement and an increased risk of both internalizing disorders (such as mood and anxiety) and externalizing disorders (such as oppositional-defiant and conduct disorders) (78). It is also interesting to note that we previously found effects of DHEA on working memory through alterations in insula-hippocampal structural covariance (36). This effect may compound those of DC ratio on ACC-hippocampal covariance and personality traits, at least in boys.
Possible biological mechanisms for the DC effects seen in this study include the neuroprotective actions of DHEA through its anti-glucocorticoid properties. Part of these DHEA’s anti-glucocorticoid effects may be medicated by 7-oxygenated metabolites, in particular 7-alpha-hydroxy-DHEA (79). This DHEA metabolite was shown to disrupt the conversion of inactive cortisone to active cortisol by 11-beta-hydroxysteroid dehydrogenase (79). However, whatever neuroprotection DHEA may afford most likely varies depending on the specific brain region involved, the developmental stage of the child, and the sex-specific effects of cortisol on the CNS. Indeed, although animal models showed a sexually dimorphic effect of DHEA on glucose uptake and oxidation in the cerebral cortex and hippocampus (9), there is no evidence of such sexually dimorphic effects of DHEA in humans, and brain-hormone studies have shown no sex differences in the impact of DHEA on cortical thickness, cortico-amygdalar or cortico-hippocampal networks. On the other hand, there is well-documented evidence of a sex difference in the density and distribution of glucocorticoid receptors in the human brain (80), as well as sex differences in neural circuits responding to corticotropin signalling (81). In the context of the critical transition from childhood to adulthood, this may lead to more detrimental effects of higher DC ratios in boys than girls, as observed in the current study.
4.1. Strengths and Limitations
Strengths of our study include the large, longitudinal developmental dataset, including the repeated collection of hormonal, neuroimaging and measures of personality, behavior and cognition. On the other hand, lack of association with tests measuring clinically significant behavioral parameters are somewhat perplexing. For example, DC-related alterations in ACC-hippocampal networks were not found to be associated with any of the CBCL/YASR measures related to internalizing or externalizing symptoms (level and frequency of anxious-depressed symptoms, aggression or rule-breaking behaviors). This suggests that, as would be expected, many other influences (environmental, hormonal and genetic) play a role in determining the clinical relevance of varying DC ratios on the development of an individual child. Another unexpected finding was the lack of any significant age or pubertal effects on the impact of DC ratio, in contrast to prior findings that DHEA-related effects on brain structure may be more prominent in pre-pubertal kids ages 4–13 years old (82). This may be because sex differences in verbal memory arise before puberty, with a mean age lower than that of our sample (20). It is also possible that the cognitive and behavioral measures we selected are not sufficiently sensitive to detect sex differences in visuo-spatial abilities, for which robust evidence suggests that the magnitude of the sex differences increases as adolescents mature (22). Yet, other reports suggest that the stress response (and buffering response) as measured by DC ratio may be relatively stable across the pubertal transition, supporting its potential role as a biomarker of mental health vulnerability (83). Only the DHEA and cortisol levels measured in the sample collected before cognitive testing were associated with ACC-hippocampal structural covariance, suggesting that (1) anticipatory levels of these adrenal hormones may have a more significant impact on this circuit than post-stress hormonal levels; and (2) some of the effects of DHEA on this circuit may only become apparent at higher levels of cortisol, as levels of this hormone peak earlier during the day. Nonetheless, we cannot exclude that some other variation in methodological procedures may confound the findings of the present study and explain the lack of associations between ACC-hippocampal covariance and DC ratios collected post-cognitive testing. Another limitation with regards to using DC ratio is that many behavioral studies have used CD, rather than DC, ratio. One ratio cannot simply be regarded as the inverse of the other given the differential sensitivities of these hormones to the process of pubertal maturation (84). Therefore, results from this study may not be directly generalizable to populations previously studied using CD ratio. Regarding potential adjustments for educational or environmental differences, it is difficult to entirely control for these differences. Regardless, it is important to note that the sample is counter-balanced across sites for differences in household income, and thereby findings are quite generalizable to the population of the United States and controlling for household income did not significantly alter the results. Still, even controlling for household income may not fully account for environmental/educational differences, and therefore, confounding due to environmental factors remains a limitation of our study. Finally, the differences in ages among the analyzed sub-samples (i.e. between the sample used for brain-hormone analyses vs. samples used for each cognitive test) limit the generalizability of results from one set of results to the next. However, it must be noted that results of brain-hormone analyses are similar regardless of the age range tested.
5. CONCLUSIONS
We found sex-specific associations between DC ratio and ACC-hippocampal structural covariance. In turn, this structural covariance was associated with higher verbal memory and mathematical ability in girls; in boys, it was associated with low cooperativeness and low reward dependence, personality traits predictive of an increased risk of internalizing and externalizing disorders. These findings support the notion that the ratio between DHEA and cortisol levels may contribute, at least in part, to the development of sex differences in reading and mathematical abilities as well as at-risk personality traits, through an alteration in ACC-hippocampal structure during the transition from childhood to adulthood.
6. ACKNOWLEDGEMENTS:
Dr. Nguyen was supported by the Canadian Institutes of Health Research, the Fonds de Recherche Santé Québec, the Montreal General Foundation and the McGill University Health Center Foundation. Data collection for this study was also supported by Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02–3343, N01-MH9–0002, and N01-NS-9–2314, −2315, −2316, −2317, −2319 and −2320).
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Campbell BC. Adrenarche and middle childhood. Hum Nat. 2011; 22(3): 327–49. [DOI] [PubMed] [Google Scholar]
- 2.Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002; 22(15): 6810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrino. 2014; 40191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin RO, Mason S, Mellon SH, Epel ES, Reus VI, Mahan L, Rosser RL, Hough CM, Burke HM, Mueller SG, Wolkowitz OM. Cortisol/DHEA ratio and hippocampal volume: A pilot study in major depression and healthy controls. Psychoneuroendocrino. 2016; 72139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TV, Gower P, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins L, Ducharme S, McCracken JT. The developmental relationship between DHEA and visual attention is mediated by structural plasticity of cortico-amygdalar networks. Psychoneuroendocrino. 2016; 70122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaysina D, Gardner MP, Richards M, Ben-Shlomo Y. Cortisol and cognitive function in midlife: the role of childhood cognition and educational attainment. Psychoneuroendocrino. 2014; 47189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Br J Psychiatry. 2001; 179243–9. [DOI] [PubMed] [Google Scholar]
- 8.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrino. 2006; 31(2): 151–78. [DOI] [PubMed] [Google Scholar]
- 9.Vieira-Marques C, Arbo BD, Cozer AG, Hoefel AL, Cecconello AL, Zanini P, Niches G, Kucharski LC, Ribeiro MFM. Sex-specific effects of dehydroepiandrosterone (DHEA) on glucose metabolism in the CNS. J Steroid Biochem Mol Biol. 2017; 1711–10. [DOI] [PubMed] [Google Scholar]
- 10.Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev Cogn Neurosci. 2017; 2512–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res. 2017; 95(1–2): 539–62. [DOI] [PubMed] [Google Scholar]
- 12.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011; 21(3): 636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014; 88242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014; 68(2): 61–72. [DOI] [PubMed] [Google Scholar]
- 15.Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol. 2015; 57(3): 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005; 15(1): 97–103. [DOI] [PubMed] [Google Scholar]
- 17.Persson J, Spreng RN, Turner G, Herlitz A, Morell A, Stening E, Wahlund LO, Wikstrom J, Soderlund H. Sex differences in volume and structural covariance of the anterior and posterior hippocampus. Neuroimage. 2014; 99215–25. [DOI] [PubMed] [Google Scholar]
- 18.Paus T, Wong AP, Syme C, Pausova Z. Sex differences in the adolescent brain and body: Findings from the saguenay youth study. J Neurosci Res. 2017; 95(1–2): 362–70. [DOI] [PubMed] [Google Scholar]
- 19.De Bolle M, De Fruyt F, McCrae RR, Lockenhoff CE, Costa PT, Aguilar-Vafaie ME, Ahn CK, Ahn HN, Alcalay L, Allik J, Avdeyeva TV, Bratko D, Brunner-Sciarra M, Cain TR, Chan W, Chittcharat N, Crawford JT, Fehr R, Fickova E, Gelfand MJ, Graf S, Gulgoz S, Hrebickova M, Jussim L, Klinkosz W, Knezevic G, Leibovich de Figueroa N, Lima MP, Martin TA, Marusic I, Mastor KA, Nakazato K, Nansubuga F, Porrata J, Puric D, Realo A, Reategui N, Rolland JP, Schmidt V, Sekowski A, Shakespeare-Finch J, Shimonaka Y, Simonetti F, Siuta J, Szmigielska B, Vanno V, Wang L, Yik M, Terracciano A. The emergence of sex differences in personality traits in early adolescence: A cross-sectional, cross-cultural study. J Pers Soc Psychol. 2015; 108(1): 171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herlitz A, Rehnman J. Sex differences in episodic memory. Curr Dir Psychol Sci. 2008; 17(1): 52–6. [Google Scholar]
- 21.Sneider JT, Hamilton DA, Cohen-Gilbert JE, Crowley DJ, Rosso IM, Silveri MM. Sex differences in spatial navigation and perception in human adolescents and emerging adults. Behav Processes. 2015; 11142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clements-Stephens AM, Rimrodt SL, Cutting LE. Developmental sex differences in basic visuospatial processing: differences in strategy use? Neurosci Lett. 2009; 449(3): 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012; 26(2): 251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999; 19(10): 4065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley JD, Chen EE, Winsell J, Davis EP, Glynn LM, Baram TZ, Sandman CA, Small SL, Solodkin A. Network specialization during adolescence: Hippocampal effective connectivity in boys and girls. Neuroimage. 2018; 175402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC. Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry. 2014; 53(3): 341–50 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jogems-Kosterman BJ, de Knijff DW, Kusters R, van Hoof JJ. Basal cortisol and DHEA levels in women with borderline personality disorder. J Psychiatr Res. 2007; 41(12): 1019–26. [DOI] [PubMed] [Google Scholar]
- 28.Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: a multilevel perspective. Dev Psychopathol. 2007; 19(3): 787–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.do Vale S, Selinger L, Martins JM, Gomes AC, Bicho M, do Carmo I, Escera C. The relationship between dehydroepiandrosterone (DHEA), working memory and distraction--a behavioral and electrophysiological approach. Plos One. 2014; 9(8): e104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Menezes KJ, Peixoto C, Nardi AE, Carta MG, Machado S, Veras AB. Dehydroepiandrosterone, Its Sulfate and Cognitive Functions. Clin Pract Epidemiol Ment Health. 2016; 1224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sontag-Padilla LM, Dorn LD, Tissot A, Susman EJ, Beers SR, Rose SR. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev Psychopathol. 2012; 24(1): 211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007; 64(7): 810–8. [DOI] [PubMed] [Google Scholar]
- 33.van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrino. 2001; 26(6): 591–612. [DOI] [PubMed] [Google Scholar]
- 34.Stangl B, Hirshman E, Verbalis J. Administration of dehydroepiandrosterone (DHEA) enhances visual-spatial performance in postmenopausal women. Behav Neurosci. 2011; 125(5): 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields GS, Lam JC, Trainor BC, Yonelinas AP. Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrino. 2016; 6751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen TV, Wu M, Lew J, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins DL, Campbell BC, Booij L, Herba C, Monnier P, Ducharme S, McCracken JT. Dehydroepiandrosterone impacts working memory by shaping cortico-hippocampal structural covariance during development. Psychoneuroendocrino. 2017; 86110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raznahan A, Lerch Jason P, Lee N, Greenstein D, Wallace Gregory L, Stockman M, Clasen L, Shaw Phillip W, Giedd Jay N. Patterns of Coordinated Anatomical Change in Human Cortical Development: A Longitudinal Neuroimaging Study of Maturational Coupling. Neuron. 2011; 72(5): 873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage. 2010; 52(4): 1355–66. [DOI] [PubMed] [Google Scholar]
- 39.Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005; 25(36): 8303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander-Bloch A, Raznahan A, Bullmore ET, Giedd J. The Convergence of Maturational Change and Structural Covariance in Human Cortical Networks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013; 33(7): 2889-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clinical chemistry. 1990; 36(10): 1769–73. [PubMed] [Google Scholar]
- 42.Stanczyk FZ. Measurement of Androgens in Women. Semin Reprod Med. 2006; 24(02): 078–85. [DOI] [PubMed] [Google Scholar]
- 43.Kancheva R, Hill M, Novák Z, Chrastina J, Kancheva L, Stárka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience. 2011; 191(0): 22–7. [DOI] [PubMed] [Google Scholar]
- 44.Vors O, Marqueste T, Mascret N. The Trier Social Stress Test and the Trier Social Stress Test for groups: Qualitative investigations. Plos One. 2018; 13(4): e0195722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maestripieri D, Baran NM, Sapienza P, Zingales L. Between- and within-sex variation in hormonal responses to psychological stress in a large sample of college students. Stress. 2010; 13(5): 413–24. [DOI] [PubMed] [Google Scholar]
- 46.Achenbach TM. Challenges and benefits of assessment, diagnosis, and taxonomy for clinical practice and research. Aust N Z J Psychiatry. 2001; 35(3): 263–71. [DOI] [PubMed] [Google Scholar]
- 47.Cloninger CR, Svrakic DM, Przybeck TR. A Psychobiological Model of Temperament and Character. Arch Gen Psychiat. 1993; 50(12): 975–90. [DOI] [PubMed] [Google Scholar]
- 48.Garcia D, Lundstrom S, Brandstrom S, Rastam M, Cloninger CR, Kerekes N, Nilsson T, Anckarsater H. Temperament and Character in the Child and Adolescent Twin Study in Sweden (CATSS): Comparison to the General Population, and Genetic Structure Analysis. Plos One. 2013; 8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farmer A, Mahmood A, Redman K, Harris T, Sadler S, McGuffin P. A sib-pair study of the temperament and character inventory scales in major depression. Arch Gen Psychiat. 2003; 60(5): 490–6. [DOI] [PubMed] [Google Scholar]
- 50.Gioia GA, Isquith PK, Guy SC, Kenworthy L, Baron IS. Test review: Behavior rating inventory of executive function. Child Neuropsychology. 2000; 6(3): 235–8. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs ML, Donders J. Performance discrepancies on the California Verbal Learning Test - Second Edition (CVLT-II) after traumatic brain injury. Arch Clin Neuropsych. 2008; 23(1): 113–8. [DOI] [PubMed] [Google Scholar]
- 52.Luciana M Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). Journal of child psychology and psychiatry, and allied disciplines. 2003; 44(5): 649–63. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds MR, Niileksela CR, Schrank FA, McGrew KS, Mather N. Woodcock- Johnson IV Tests of Cognitive Abilities. J Psychoeduc Assess. 2015; 33(4): 381–90. [Google Scholar]
- 54.McEwen BS, Gianaros PJ. Stress- and Allostasis-Induced Brain Plasticity. Annu Rev Med. 2011; 62431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Child NSCotD. Excessive Stress Disrupts the Architecture of the Developing Brain: Working Paper #3. 2011. [Google Scholar]
- 56.Weilbacher RA, Gluth S. The Interplay of Hippocampus and Ventromedial Prefrontal Cortex in Memory-Based Decision Making. Brain Sci. 2016; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin JJ, Maren S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front Syst Neurosci. 2015; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bender BG, Lerner JA, Kollasch E. Mood and memory changes in asthmatic children receiving corticosteroids. J Am Acad Child Adolesc Psychiatry. 1988; 27(6): 720–5. [DOI] [PubMed] [Google Scholar]
- 59.Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl). 2006; 188(4): 541–51. [DOI] [PubMed] [Google Scholar]
- 60.Geary DC, Saults SJ, Liu F, Hoard MK. Sex differences in spatial cognition, computational fluency, and arithmetical reasoning. J Exp Child Psychol. 2000; 77(4): 337–53. [DOI] [PubMed] [Google Scholar]
- 61.Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Development and Psychopathology. 2007; 19(3): 787–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiat. 2007; 64(10): 1172–9. [DOI] [PubMed] [Google Scholar]
- 63.Dorn LD, Kolko DJ, Susman EJ, Huang B, Stein H, Music E, Bukstein OG. Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biological Psychology. 2009; 81(1): 31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiat Res. 2005; 134(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 65.Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005; 17(1): 167–84. [DOI] [PubMed] [Google Scholar]
- 66.McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, Lahey BB. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biol Psychiat. 2005; 57(10): 1109–16. [DOI] [PubMed] [Google Scholar]
- 67.Dmitrieva TN, Oades RD, Hauffa BP, Eggers C. Dehydroepiandrosterone sulphate and corticotropin levels are high in young male patients with conduct disorder: Comparisons for growth factors, thyroid and gonadal hormones. Neuropsychobiology. 2001; 43(3): 134–40. [DOI] [PubMed] [Google Scholar]
- 68.Golubchik P, Mozes T, Maayan R, Weizman A. Neurosteroid blood levels in delinquent adolescent boys with conduct disorder. Eur Neuropsychopharm. 2009; 19(1): 49–52. [DOI] [PubMed] [Google Scholar]
- 69.Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003; 44(3): 242–57. [DOI] [PubMed] [Google Scholar]
- 70.Whittle S, Simmons JG, Byrne ML, Strikwerda-Brown C, Kerestes R, Seal ML, Olsson CA, Dudgeon P, Mundy LK, Patton GC, Allen NB. Associations between early adrenarche, affective brain function and mental health in children. Social cognitive and affective neuroscience. 2015; 10(9): 1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Goozen SHM, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JHH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. J Am Acad Child Psy. 2000; 39(11): 1446–51. [DOI] [PubMed] [Google Scholar]
- 72.van Goozen SHM, Matthys W, Cohen-Kettenis PT, Thijssen JHH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biological psychiatry. 1998; 43(2): 156–8. [DOI] [PubMed] [Google Scholar]
- 73.Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006; 31(10): 1245–56. [DOI] [PubMed] [Google Scholar]
- 74.Dorn LD, Rose SR, Rotenstein D, Susman EJ, Huang B, Loucks TL, Berga SL. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. Journal of pediatric endocrinology & metabolism : JPEM. 2008; 21(5): 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013; 54(1): 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boes AD, Tranel D, Anderson SW, Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behav Neurosci. 2008; 122(3): 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thijssen S, Ringoot AP, Wildeboer A, Bakermans-Kranenburg MJ, El Marroun H, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H, van IMH, White T. Brain morphology of childhood aggressive behavior: A multi-informant study in school-age children. Cogn Affect Behav Neurosci. 2015; 15(3): 564–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmeck K [Personality disorders in adolescence: conceptual issues and treatment approaches]. Prax Kinderpsychol Kinderpsychiatr. 2008; 57(8–9): 625–40. [DOI] [PubMed] [Google Scholar]
- 79.Muller C, Hennebert O, Morfin R. The native anti-glucocorticoid paradigm. J Steroid Biochem Mol Biol. 2006; 100(1–3): 95–105. [DOI] [PubMed] [Google Scholar]
- 80.Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012; 62(3): 210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA. Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav. 2018; 97145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci. 2013; 33(26): 10840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev Psychobiol. 2015; 57(6): 688–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saczawa ME, Graber JA, Brooks-Gunn J, Warren MP. Methodological considerations in use of the cortisol/DHEA(S) ratio in adolescent populations. Psychoneuroendocrino. 2013; 38(11): 2815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


