Abstract
Objective:
To investigate associations in extremely preterm (<28wks; EPT) toddlers between neonatal neuroimaging and 18–22 month developmental and behavioral outcomes.
Study design:
Cohort analysis from the NICHD Neonatal Research Network (NRN) SUPPORT Neuroimaging and Neurodevelopmental Outcomes Study of EPT infants. Subjects underwent cranial ultrasound (CUS) and near-term magnetic resonance imaging (MRI). At 18–22 months of corrected age, the assessment included the Brief Infant Toddler Social Emotional Assessment (BITSEA) Problem and Competence Scale scores and the Bayley Scales of Infant Development, 3rd edition (Bayley-III). The BITSEA Problem Scale assesses dysregulation; the Competence Scale assesses social-emotional competence. We examined associations of Problem and Competence scores and positive screen rates with CUS and near-term MRI. Mean BITSEA and Bayley-III scores were compared using ANOVA and Positive Screen rates with chi-square. We computed correlations between BITSEA and Bayley-III scores.
Results:
Of the 397 children, Positive BITSEA Screens were found in 34% for the Problem score and 26% for the Competence score. Presence of lesions on near-term MRI that included cerebellar lesions were significantly associated with lower BITSEA Competence but not with Problem scores; Competence scores were inversely related to the presence/significance of lesions. Positive Screens on Competence scores and on both Competence and Problem scores were significantly associated with Bayley-III Cognitive and Language scores <85 (P < .001).
Conclusion:
Social-emotional competence contributes to deficits in cognitive and language development. Presence of injury on near-term MRI that includes cerebellar lesions is associated with later social-emotional competence and may be a useful predictor to guide early assessment and intervention.
Keywords: Extremely preterm, neuroimaging, behavior
Children born preterm are at high risk of motor, cognitive and behavioral deficits.1–3 Though most studies have focused on motor and cognitive abnormalities, behavioral deficits are particularly common in children born preterm. Indeed, compared with children born at term, children born preterm are up to 4 times more likely to have behavioral problems,4 including problems with attention, behavioral organization, social-emotional function, and selfmonitoring.5 These behavioral issues may adversely impact early cognitive and language development.
Although most studies of behavioral outcomes in children born preterm evaluate children at school age or adolescence, poor self-regulation and social functioning have been described as early as 2 years of age.6 There is limited evidence that deficits found at preschool age remain stable from early childhood through school age,7 though longitudinal studies of behavior in preterm children are rare.8 Although the etiology of behavioral abnormalities in preterm children is unknown and likely to be multifactorial, perinatal brain injury has been implicated as a contributing cause. Children born preterm are at high risk for brain injury, particularly white matter injury.9, 10 Abnormalities on cranial ultrasound (CUS) and conventional brain magnetic resonance imaging (MRI) at term equivalent age have been associated with cognitive, psychomotor and neurosensory delays at 18 to 24 months of age.11, 12 Abnormalities in specific brain regions may have a role in behavioral outcomes. Limperopoulos et al demonstrated that early cerebellar injury noted on CUS and confirmed with MRI was associated with behavioral, cognitive, language and motor deficits in children born very preterm at 34 months of age.13
The purpose of this study was to investigate the associations between abnormalities on CUS and near-term conventional brain MRI and behavioral, cognitive and language outcomes in extremely preterm (<28 weeks of gestation; EPT) toddlers at 18–22 months. We hypothesized that composite adverse findings on early and late CUS and severity of white matter abnormalities (WMA) and significant cerebellar lesions on near-term MRI would be associated with Brief Infant Toddler Social Emotional Assessment (BITSEA)14 Problem and Competence Scale scores and Positive Screen rates. A second hypothesis was that associations with scale scores would remain after controlling for other demographic, perinatal and in-hospital variables and that significant cerebellar lesions on near-term MRI would be associated with standardized BITSEA Problem and Competence scale scores and Positive Screen rates for each scale after controlling for such variables. Our third hypothesis was that Positive Screen rates would be associated with significantly lower Bayley Scales of Infant Development, 3rd edition (Bayley-III) Cognitive and Language Composite scores.
Methods
This study was a secondary analysis of data from the prospective Eunice Kennedy Shriver National Institute of Child Health and Development Neonatal Research Network (NRN) Surfactant Positive Airway Pressure and Pulse Oximetry Trial (SUPPORT) Neuroimaging and Neurodevelopmental Outcomes (NEURO) Study of EPT infants (ClinicalTrials.gov: NCT00063063 and NCT00233324).11 Children eligible for inclusion were enrolled in the NEURO study and seen for 18–22-month follow-up examination at 1 of the 16 participating centers. The follow-up examination included the BITSEA and the Bayley-III. Bayley-III testers were unaware of neuroimaging and pre-discharge morbidities. The NEURO study enrolled children born February 2005 to February 2009, and follow-up assessments took place from 2006–2011. The NEURO protocol required an “early CUS” at 4–14 days, “late CUS” at 35–42 weeks postmenstrual age (PMA), and conventional brain MRI at 35–42 weeks PMA within 2 weeks of the late CUS. Cranial US and near-term MRI studies were interpreted by separate, masked, central readers. ‘Composite adverse findings’ on early and late CUS, severity of white matter abnormalities on near-term MRI,9, 12 and ‘significant cerebellar lesions’ on near-term MRI were assessed.11 A composite adverse finding on early CUS was defined as presence of grade III or IV intraventricular hemorrhage (IVH) or cystic periventricular leukomalacia (PVL) on either or both sides. A composite adverse finding on late CUS was defined as cystic PVL, porencephalic cyst, or moderate-to-severe ventricular enlargement (defined as a ventricular-to-brain ratio of 1:3 to 2:3 and >2:3, respectively, on either or both sides), or shunt. Significant cerebellar lesions were defined as lesions that were bilateral, cystic (cystic spaces including volume loss or atrophy), and/or ≥4 mm in size. Adverse findings on near-term MRI were defined as moderate or severe WMA and/or significant cerebellar lesions.
BITSEA and Bayley-III.
The BITSEA is a parent-completed rating scale for children 12 months, 0 days to 35 months, 30 days.14 It includes 42 items and yields standardized scores for the Problem and Competence Scales based on age and sex. The Problem Scale assesses for externalizing problems (ie, disruptive or aggressive behaviors), internalizing problems (ie, anxiety, withdrawal), dysregulation and maladaptive and atypical behaviors. Higher Problem Scale scores indicate more difficulties in these areas. The Competence Scale assesses the social emotional competencies that emerge at this age, such as symbolic and imitative play and cooperation. Lower Competence Scale scores indicate lower social emotional competencies. Total standardized scores and Positive Screen scores were assessed for each of the scales. Competence Scale scores of ≤ 13 or ≤ 15 (for boys and girls, respectively) and a Problem Scale ≥ 15 indicate positive screens for children aged 18–23 months.14
Using the NRN definition, infants were considered to have neurodevelopmental impairment if they have at least one of the following conditions: moderate or severe cerebral palsy, Gross Motor Function Classification System level of at least 2 (on a scale of 1 to 5, with 5 indicating the most severe impairment), profound hearing loss requiring amplification in both ears, profound visual impairment with visual acuity of less than 20/200 in both eyes, or cognitive impairment. Cognitive impairment was defined as a Bayley-III Cognitive Composite score of <85 (one standard deviation below the mean ± SD score of 100 ± 15). Bayley-III scores range from 55 to 145; lower scores indicate a greater degree of developmental delay. We selected 85 as a cutoff point to adjust for the difference between the Bayley-II and Bayley-III in estimating cognitive performance on the basis of data showing 97% agreement between a Bayley-II Mental Developmental Index score lower than 70 and a Bayley- III Cognitive Composite score lower than 85.15, 16 A Bayley-III Cognitive Composite score <85 indicates moderate cognitive delay and neurodevelopmental impairment, per current NRN definitions.15
Statistical Analyses.
We compared BITSEA Problem Scale and Competence Scale scores and Positive Screen rates at 18–22 months corrected age across demographic, perinatal, and neonatal characteristics. In addition, we examined the relationship between BITSEA scores, Positive screen rates and Bayley-III language and/or cognitive composite scores <85. Analysis of variance was used to examine associations between impairments on the Bayley-III and mean BITSEA Problem and Competence Scale scores, and chi-square tests to examine associations of these impairments with Positive Screen rates.
To further explore the relationship between BITSEA scores and Bayley-III cognitive and language scores, we categorized children into four groups based on Positive Screens on the 2 BITSEA scales: (1) did not screen positive on either the Problem Scale or Competence Scale, (2) Positive Screen based on Competence Scale score only, (3) Positive Screen based on Problem Scale score only, and (4) Positive Screens based on both Problem Scale and Competence Scale scores. Using chi-square tests, we compared the number of children with Bayley-III cognitive or language scores <85 who fell into each of those four groups.
Next, we tested for differences in BITSEA Problem and Competence Scale scores based on neuroimaging findings on the early CUS, late CUS, and near-term MRI, described in Methods, above. In addition, we compared BITSEA scores by whether near-term MRI indicated any cerebellar lesions, any non-cerebellar lesions only (those who had any WMA and/or lesion outside of the cerebellum only), or no lesions. There was no overlap in categories, however it should be noted that the “cerebellar lesions” category includes those who have cerebellar lesions regardless of whether they also have supratentorial non-cerebellar lesions. Linear trends were tested using the Cochrane-Armitage test for Positive Screens and contrasts in ANOVA for mean Problem and Competence Scale scores. Finally, we fit generalized linear mixed effect models of BITSEA scores by neuroimaging findings. Analysis included center as a random effect and controlled for the following variables, using previously described definitions:11 birthweight, gestational age, multiple gestation, race/ethnicity, sex, antenatal steroids, Medicaid status, cesarean delivery, late-onset sepsis, surgery for retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), patent ductus arteriosus (PDA), postnatal steroids, and bronchopulmonary dysplasia (BPD).
Results
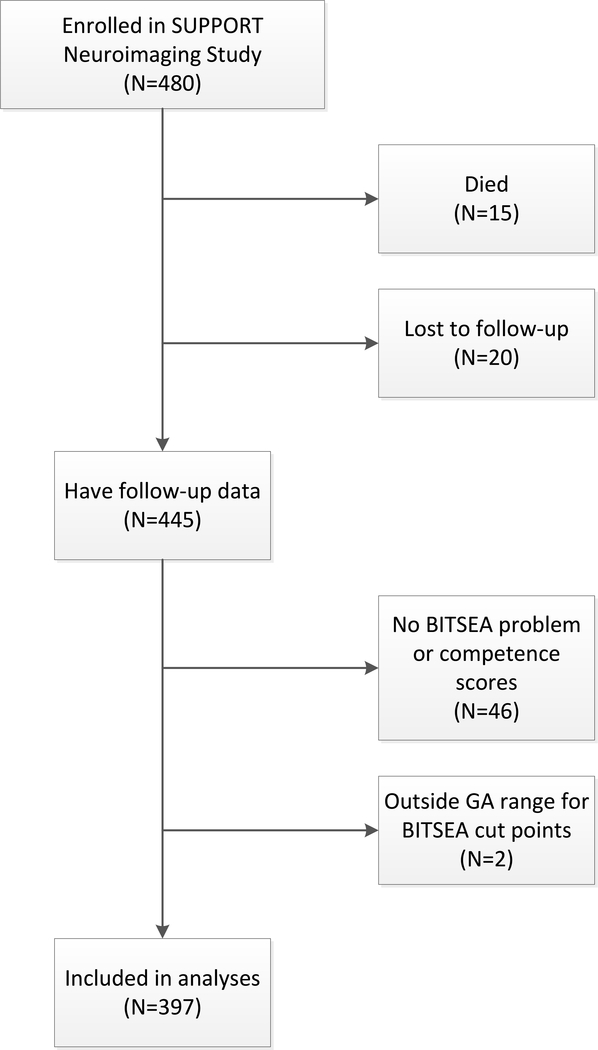
Three hundred ninety-seven children were included in the study. Children were enrolled from February 2005 to February 2009. The sample selection process is illustrated in Figure 1 (available at www.jpeds.com) and the demographic, perinatal, and neonatal characteristics of the 397 children included in the present analyses are shown in Table 1. We compared the characteristics of the 46 children not included in the analyses due to missing BITSEA scores to the 397 included. Those included in our analyses were less likely to be male (54% vs 69%, p=0.047) and more likely to have had surgery for ROP, NEC, or PDA (19% vs. 7%, p=0.041).
Legend for Online Figure 1:
Sample Selection Flowchart
Table 1.
Demographic, Perinatal and Neonatal Cohort Characteristics (N=397)
| Characteristic | N (%) |
|---|---|
| Demographic and perinatal characteristics | |
| Birth weight (g)…mean (SD) | 843.16 (188.42) |
| Estimated gestational age (wk)…mean (SD) | 25.78 (1.02) |
| Multiple gestation | 90 (23) |
| Race/ethnicity | |
| Non-Hispanic black | 122 (31) |
| Non-Hispanic white | 176 (44) |
| Hispanic | 85 (21) |
| Other | 14 (4) |
| Male | 213 (54) |
| Antenatal steroids | 380 (96) |
| Cesarean delivery | 275 (69) |
| Neonatal characteristics | |
| PDA | 201 (51) |
| Late sepsis | 130 (33) |
| NEC | 28 (7) |
| Severe ROP | 36 (9) |
| Surgery for PDA, NEC, and/or ROP | 74 (19) |
| Postnatal steroids | 36 (9) |
| BPD (traditional definition) | 152 (38) |
| Neonatal Neuroimaging | |
| Early CUS adverse finding | 39 (10) |
| Late CUS adverse finding | 22 (6) |
| Moderate or severe WMA on ntMRI | 76 (19) |
| Any cerebellar lesions on ntMRI | 65 (16) |
| Significant cerebellar lesions on ntMRI | 40 (10) |
Note: Data is missing for 5 participants on severe ROP and 4 participants on postnatal steroids.
BITSEA Positive Screens Rates.
Overall rates of Positive Screens on the BITSEA Problem and Competence Scales were 34% and 26%, respectively. As shown in Table 2, there were significant race/ethnicity differences in Positive Screen rates on the Problem Scale (p=0.028) with black children having the highest number of Positive Screens (44%) and children in the ‘other’ race category having the least (21%). In addition, Medicaid enrollment was associated with higher Problem Scale Positive Screen rates (p<0.001). Positive Screens on the Competence Scale occurred more frequently among children with lower birth weight (<750 g) (p=0.045), who had severe ROP (p<0.001) or who had surgery for PDA, NEC, and/or ROP (p=0.005). In addition, children with Bayley-III Cognitive or Language Composite scores <85 were significantly more likely to have Positive Screens on the Competence Scale (p<0.001).
Table 2.
BITSEA Problem and Competence Scales by Demographic Characteristics, Bayley Scales, and Neuroimaging Findings
| Characteristic | N | Problem |
Competence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) with positive screen | Mean (SD) score | N (%) with positive screen | Mean (SD) score | ||||||||
| Demographic characteristics | |||||||||||
| Birth weight | |||||||||||
| < 750 g | 137 | 44 (32) | 12.6 (7.2) | 45 (33)* | 15.7 (3.8)** | ||||||
| 750–999 g | 174 | 63 (36) | 13.1 (7.3) | 40 (23) | 16.6 (3.6) | ||||||
| ≥ 1000 g | 86 | 29 (34) | 12.0 (6.8) | 17 (20) | 17.4 (3.4) | ||||||
| Estimated gestational age | |||||||||||
| 24 weeks | 49 | 20 (41) | 13.2 (7.2) | 16 (33) | 15.3 (4.5)* | ||||||
| 25 weeks | 113 | 38 (34) | 13.0 (7.1) | 29 (26) | 16.3 (3.6) | ||||||
| 26 weeks | 111 | 35 (32) | 12.3 (7.1) | 29 (26) | 16.6 (3.3) | ||||||
| 27 weeks | 124 | 43 (35) | 12.6 (7.4) | 28 (23) | 17.0 (3.7) | ||||||
| Multiple gestation | |||||||||||
| Yes | 90 | 24 (27) | 10.6 (6.4)** | 19 (21) | 16.6 (3.5) | ||||||
| No | 307 | 112 (36) | 13.3 (7.3) | 83 (27) | 16.4 (3.7) | ||||||
| Race/ethnicity | |||||||||||
| Non-Hispanic black | 122 | 54 (44)* | 14.9 (8.7)*** | 40 (33) | 16.9 (3.6) | ||||||
| Non-Hispanic white | 176 | 50 (28) | 11.2 (6.0) | 37 (21) | 15.8 (4.1) | ||||||
| Hispanic | 85 | 29 (34) | 12.9 (6.5) | 24 (28) | 16.5 (3.2) | ||||||
| Other | 14 | 3 (21) | 11.9 (6.2) | 1 (7) | 16.9 (2.8) | ||||||
| Medicaid enrollment | |||||||||||
| Yes | 199 | 84 (42)*** | 14.2 (7.6)*** | 57 (29) | 16.1 (3.7) | ||||||
| No | 198 | 52 (26) | 11.2 (6.4) | 45 (23) | 16.8 (3.6) | ||||||
| Gender | |||||||||||
| Male | 213 | 75 (35) | 12.8 (7.0) | 53 (25) | 15.8 (3.8)*** | ||||||
| Female | 184 | 61 (33) | 12.6 (7.4) | 49 (27) | 17.2 (3.3) | ||||||
| Antenatal steroids | |||||||||||
| Yes | 380 | 129 (34) | 12.7 (7.2) | 98 (26) | 16.5 (3.7) | ||||||
| No | 17 | 7 (41) | 12.6 (6.0) | 4 (24) | 16.4 (4.1) | ||||||
| Cesarean delivery | |||||||||||
| Yes | 275 | 92 (33) | 12.3 (6.9) | 70 (26) | 16.3 (3.7) | ||||||
| No | 122 | 44 (36) | 13.7 (7.6) | 32 (26) | 16.7 (3.7) | ||||||
| Neonatal characteristics | |||||||||||
| PDA | |||||||||||
| Yes | 201 | 65 (32) | 12.2 (6.7) | 55 (28) | 16.1 (3.7) | ||||||
| No | 196 | 71 (36) | 13.2 (7.6) | 47 (24) | 16.8 (3.6) | ||||||
| Late sepsis | |||||||||||
| Yes | 130 | 44 (34) | 12.7 (7.3) | 38 (29) | 16.4 (3.6) | ||||||
| No | 267 | 92 (34) | 12.7 (7.1) | 64 (24) | 16.5 (3.7) | ||||||
| Proven NEC | |||||||||||
| Yes | 28 | 12 (43) | 13.5 (8.6) | 8 (29) | 16.2 (3.2) | ||||||
| No | 369 | 124 (34) | 12.7 (7.1) | 94 (26) | 16.5 (3.7) | ||||||
| Severe ROP | |||||||||||
| Yes | 36 | 14 (39) | 13.7 (7.6) | 17 (50)*** | 14.2 (3.1)*** | ||||||
| No | 356 | 119 (33) | 12.5 (7.0) | 82 (23) | 16.7 (3.7) | ||||||
| Surgery for PDA, NEC, and/or ROP | |||||||||||
| Yes | 74 | 27 (36) | 12.6 (6.8) | 28 (39)** | 15.2 (3.2)** | ||||||
| No | 323 | 109 (34) | 12.7 (7.3) | 74 (23) | 16.7 (3.7) | ||||||
| Postnatal steroids | |||||||||||
| Yes | 36 | 14 (39) | 13.0 (6.7) | 12 (34) | 15.2 (4.7)* | ||||||
| No | 357 | 121 (34) | 12.7 (7.2) | 89 (25) | 16.6 (3.5) | ||||||
| BPD (traditional definition) | |||||||||||
| Yes | 152 | 49 (32) | 12.3 (7.1) | 41 (28) | 16.2 (4.0) | ||||||
| No | 245 | 87 (36) | 13.0 (7.2) | 61 (25) | 16.6 (3.4) | ||||||
| Vision impairment (blind in both eyes) | |||||||||||
| Yes | 1 | 0 (0) | 7.0 (NA) | 1 (100) | 13.0 (NA) | ||||||
| No | 396 | 136 (34) | 12.7 (7.2) | 101 (26) | 16.5 (3.7) | ||||||
| Hearing impairment | |||||||||||
| Yes | 6 | 1 (17) | 12.7 (7.2) | 3 (50) | 14.8 (2.6) | ||||||
| No | 391 | 135 (35) | 11.8 (6.6) | 99 (26) | 16.5 (3.7) | ||||||
| BSID-III Cognitive and Language Scores | |||||||||||
| BSID-III Cognitive Composite | |||||||||||
| < 85 | 88 | 30 (34) | 12.7 (6.0) | 40 (47)*** | 14.3 (4.2)*** | ||||||
| ≥ 85 | 307 | 106 (35) | 12.7 (7.5) | 62 (20) | 17.1 (3.2) | ||||||
| BSID-III Language Composite | |||||||||||
| < 85 | 163 | 64 (39) | 13.4 (7.1) | 67 (42)*** | 14.8 (3.9)*** | ||||||
| ≥ 85 | 226 | 70 (31) | 12.3 (7.3) | 35 (16) | 17.6 (3.0) | ||||||
| Neuroimaging Findings | |||||||||||
| Early CUS | |||||||||||
| Normal | 283 | 96 (34) | 12.3 (6.3) | 72 (25) | 16.9 (3.5) | ||||||
| No IVH grade 3–4/PVL | 75 | 27 (36) | 12.7 (7.2) | 21 (29) | 16.4 (3.7) | ||||||
| IVH grade 3–4/PVL | 39 | 13 (33) | 12.9 (7.5) | 9 (23) | 16.3 (3.8) | ||||||
| Late CUS | |||||||||||
| Normal | 287 | 101 (35) | 11.0 (3.8) | 69 (24) | 16.9 (3.4) | ||||||
| No IVH grade 3–4/PVL | 88 | 30 (34) | 13.0 (7.4) | 27 (31) | 16.6 (3.6) | ||||||
| IVH grade 3–4/PVL | 22 | 5 (23) | 12.3 (6.9) | 6 (27) | 15.8 (4.0) | ||||||
| Severity of WMA | |||||||||||
| Normal | 88 | 32 (36) | 12.7 (7.6) | 19 (22) | 16.9 (3.6) | ||||||
| Mild | 233 | 79 (34) | 13.0 (7.3) | 63 (27) | 16.2 (3.8) | ||||||
| Moderate | 61 | 21 (34) | 11.9 (6.7) | 15 (25) | 16.6 (3.5) | ||||||
| Severe | 15 | 4 (27) | 11.9 (3.3) | 5 (33) | 16.3 (3.4) | ||||||
| Lesions | |||||||||||
| No lesions | 215 | 73 (34) | 12.7 (7.5) | 52 (24) | 16.8 (3.6)* | ||||||
| Non-cerebellar lesions only | 117 | 37 (32) | 12.2 (7.2) | 31 (27) | 16.4 (3.5) | ||||||
| Cerebellar lesions | 65 | 26 (40) | 13.6 (6.2) | 19 (30) | 15.3 (4.0) | ||||||
| Significance of cerebellar lesions | |||||||||||
| No cerebellar lesions | 332 | 110 (33) | 12.5 (7.4) | 83 (25) | 16.7 (3.6)*** | ||||||
| Non-significant lesions | 25 | 10 (40) | 14.3 (6.7) | 6 (24) | 15.6 (4.2) | ||||||
| Significant lesions | 40 | 16 (40) | 13.1 (5.8) | 13 (33) | 15.2 (4.0) | ||||||
p< 0.05
p < 0.01
p < 0.001
Mean BITSEA Scores.
As with Positive Screen rates, there were race/ethnicity and Medicaid enrollment status differences in Problem Scale mean scores, with black children and children enrolled in Medicaid having significantly higher Problem Scale scores than other groups (p<0.001 for both; Table 2). Multiple gestation was also associated with higher Problem Scale scores (p=0.001). Male children, children with birth weight <750 grams and those born at 24 weeks of gestation had significantly lower (worse) Competence Scale scores (p<0.001, p=0.003 and p=0.037, respectively). Children who received postnatal steroids, surgery for PDA, NEC, and/or ROP or a diagnosis of severe ROP also had lower Competence Scale scores (p=0.032, p=0.001 and p<0.001, respectively). Finally, children with Bayley-III Cognitive or Language Composite scores <85 had significantly lower Competence Scale scores (p<0.001).
Bayley-III cognitive and language composite scores <85 and BITSEA positive screen rates.
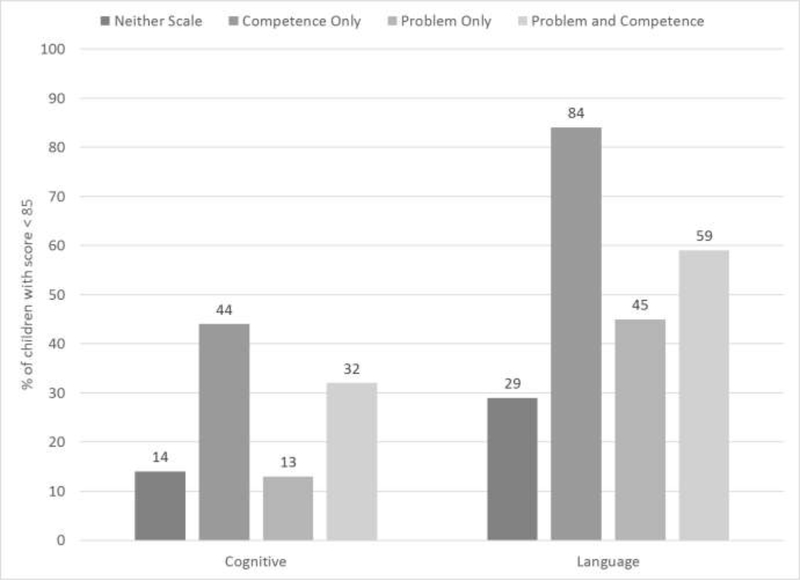
Children with Bayley-III Cognitive or Language Composite scores <85 had significantly lower BITSEA Competence Scale scores (p<0.001) and significantly higher rates of Positive Screens on the Competence Scale (p<0.001; Table 3). Children with Positive Screens on both the Problem and Competence scales were also more likely to have Bayley-III Cognitive and Language Composite scores <85 (p<0.001 for both) (Figure 2; available at www.jpeds.com). Mean BITSEA Problem Scale scores and Positive Screen Rates were not significantly associated with Bayley-III scores <85.
Table 3.
Bayley Cognitive and Language Scores by BITSEA Problem and Competence Scales
| Bayley Cognitive < 85 |
Bayley Language < 85 |
|||
|---|---|---|---|---|
| Adj RR (95% CI) | p-value | Adj RR (95% CI) | p-value | |
| BITSEA Problem Scale | ||||
| Positive Screen (yes vs. no) | 1.05 (0.66, 1.66) | 0.833 | 1.23 (0.89, 1.71) | 0.206 |
| Scale Score | 1.00 (1.00, 1.01) | 0.719 | 1.01 (1.00, 1.01) | 0.096 |
| BITSEA Competence Scale | ||||
| Positive Screen (yes vs. no) | 2.33 (1.49, 3.64) | < 0.001 | 1.94 (1.40, 2.69) | < 0.001 |
| Scale Score | 0.97 (0.96, 0.98) | < 0.001 | 0.95 (0.94, 0.97) | < 0.001 |
| BITSEA Problem and Competence Scales | ||||
| Positive Screen | ||||
| Both Scales | 2.07 (1.13, 3.80) | 0.019 | 1.85 (1.17, 2.92) | 0.009 |
| Problem Only | 0.94 (0.48, 1.85) | 0.860 | 1.48 (0.96, 2.29) | 0.077 |
| Competence Only | 2.51 (1.42, 4.44) | 0.002 | 2.62 (1.71, 4.01) | < 0.001 |
| Neither Scale | REF | REF | ||
Legend for Online Figure 2:
Percentage of Children with BSID-III Cognitive and Language Scores <85 by Positive Screens on BITSEA Problem and Competence Scales
Note: Percentages are adjusted for center, birthweight, gestational age, multiple gestation, race/ethnicity, Medicaid enrollment, sex, antenatal steroids, cesarean delivery, late-onset sepsis, surgery for ROP, NEC, or PDA, postnatal steroids, and BPD
Neuroimaging findings and BITSEA scores.
Table 2 details BITSEA scale scores and Positive Screen rates for each set of neuroimaging findings (IVH Grade 3–4/PVL, cerebellar lesions, significant cerebellar lesions, and non-cerebellar lesions only on MRI, Table 2). Of the 40 children with significant cerebellar lesions, 13 (33%) had IVH grade 3–4/PVL. Of the 39 children with IVH grade 3–4/PVL, 13 (33%) had significant cerebellar lesions. The majority of noncerebellar lesions included isolated abnormalities in cerebral white matter signal. These types of lesions comprised 77% of non-cerebellar lesions in children with cerebellar lesions and 96% of the lesions found in the ‘non-cerebellar lesions only’ group. The ‘non-cerebellar lesions only’ group also included a small number with abnormal signal in the basal ganglia or thalamus (only 1–5% for all). There was no association between Positive Screens on either scale and neuroimaging findings, before or after adjustment for study site, demographic and medical characteristics.
Though cerebellar lesions were associated with greater positive screen rates on the Problem and Competence Scales, these findings did not reach statistical significance (Table V; available at www.jpeds.com). The presence of cerebellar lesions on near-term MRI was significantly associated with lower mean BITSEA Competence Scale scores (p=0.001). Competence Scale scores were highest (best) for children with no lesions, next highest for those with non-cerebellar lesions only and lowest (worst) for those with cerebellar lesions. Mean Competence Scale scores were also significantly lower for children with significant cerebellar lesions compared with those with non-significant lesions and no lesions (p<0.001). The differences in Competence Scale scores for children with any cerebellar lesions (vs. no lesions) remained significant after controlling for study site, demographic and medical characteristics (p=0.04; Table 4). However, in evaluating the differences between children with significant cerebellar lesions on near-term MRI compared with those without, the findings did not reach statistical significance after controlling for other factors (p=0.092; Table 4). There was no association between the presence of cerebellar lesions ≥4mm in size and BITSEA scores, which is consistent with other studies.17, 18 Of the 65 infants with cerebellar lesions, 50 also had non-cerebellar lesions and 15 did not have non-cerebellar lesions. When we compared the BITSEA scores of the children with cerebellar and non-cerebellar lesions to those with cerebellar lesions only, the mean scores were nearly identical and therefore, considering the small sample sizes, we collapsed the two categories. For example, the adjusted mean competence scores for children with cerebellar and non-cerebellar lesions were 15.74 compared with 15.75 for children with cerebellar lesions but no noncerebellar lesions (p=0.990).
Table 5.
Model-Adjusted Proportions of Positive Screens on BITSEA by Neuroimaging Findings at 18- to 22-Months
| Neuroimaging Findings | Problem |
Competence |
||
|---|---|---|---|---|
| Adjusted % (95% CI) | p-value | Adjusted % (95% CI) | p-value | |
| Early CUS | ||||
| Normal | 0.31 (0.23, 0.40) | REF | 0.19 (0.11, 0.30) | REF |
| No IVH grade 3–4/cPVL | 0.33 (0.21, 0.47) | 0.753 | 0.21 (0.11, 0.36) | 0.718 |
| IVH grade 3–4/cPVL | 0.28 (0.15, 0.46) | 0.754 | 0.15 (0.06, 0.31) | 0.518 |
| Late CUS | ||||
| Normal | 0.32 (0.24, 0.42) | REF | 0.17 (0.10, 0.28) | REF |
| No IVH grade 3–4/cPVL | 0.29 (0.19, 0.41) | 0.521 | 0.23 (0.13, 0.38) | 0.237 |
| IVH grade 3–4/cPVL | 0.21 (0.08, 0.44) | 0.267 | 0.18 (0.07, 0.41) | 0.908 |
| Severity of WMA | ||||
| Normal | 0.34 (0.23, 0.48) | REF | 0.15 (0.08, 0.28) | REF |
| Mild | 0.30 (0.22, 0.39) | 0.415 | 0.20 (0.12, 0.31) | 0.352 |
| Moderate | 0.31 (0.19, 0.46) | 0.649 | 0.18 (0.09, 0.34) | 0.640 |
| Severe | 0.26 (0.09, 0.54) | 0.518 | 0.27 (0.10, 0.56) | 0.282 |
| Lesions | ||||
| No lesions | 0.31 (0.23, 0.40) | REF | 0.18 (0.11, 0.29) | REF |
| Non-cerebellar lesions only | 0.27 (0.18, 0.38) | 0.446 | 0.20 (0.11, 0.34) | 0.638 |
| Cerebellar lesions | 0.37 (0.24, 0.53) | 0.356 | 0.20 (0.10, 0.35) | |
| Significance of cerebellar lesions | ||||
| No cerebellar lesions | 0.29 (0.22, 0.38) | REF | 0.19 (0.11, 0.29) | REF |
| Non-significant lesions | 0.37 (0.20, 0.59) | 0.423 | 0.17 (0.06, 0.38) | 0.783 |
| Significant lesions | 0.38 (0.22, 0.56) | 0.317 | 0.21 (0.10, 0.40) | 0.688 |
Note: REF=reference category; means are adjusted for center, birthweight, gestational age, multiple gestation, race/ethnicity, Medicaid enrollment, gender, antenatal steroids, C-section, late-onset sepsis, surgery for ROP, NEC, or PDA, postnatal steroids, and BPD.
Table 4.
Model-Adjusted Mean BITSEA Scores by Neuroimaging Findings at 18- to 22-Months
| Neuroimaging Findings | Problem |
Competence |
||
|---|---|---|---|---|
| Adjusted Mean (95% CI) | p-value | Adjusted Mean (95% CI) | p-value | |
| Early CUS | ||||
| Normal | 12.6 (11.4, 13.7) | REF | 16.5 (15.8, 17.3) | REF |
| No IVH grade 3–4/cPVL | 12.8 (10.9, 14.6) | 0.798 | 16.5 (15.4, 17.5) | 0.883 |
| IVH grade 3–4/cPVL | 12.3 (9.9, 14.6) | 0.801 | 17.2 (15.9, 18.5) | 0.283 |
| Late CUS | ||||
| Normal | 12.9 (11.7, 14.1) | REF | 16.8 (16.0, 17.5) | REF |
| No IVH grade 3–4/cPVL | 11.9 (10.2, 13.6) | 0.230 | 16.0 (15.0, 17.0) | 0.091 |
| IVH grade 3–4/cPVL | 10.9 (7.8, 14.0) | 0.200 | 17.0 (15.3, 18.6) | 0.799 |
| Severity of WMA | ||||
| Normal | 12.7 (11.0, 14.4) | REF | 16.9 (16.0, 17.9) | REF |
| Mild | 12.7 (11.4, 14.0) | 0.982 | 16.4 (15.7, 17.2) | 0.272 |
| Moderate | 12.0 (9.9, 14.0) | 0.534 | 16.8 (15.7, 17.9) | 0.865 |
| Severe | 12.4 (8.7, 16.0) | 0.857 | 16.1 (14.2, 18.1) | 0.436 |
| Lesions | ||||
| No lesions | 12.6 (11.3, 13.8) | REF | 16.8 (16.1, 17.6) | REF |
| Non-cerebellar lesions only | 12.1 (10.5, 13.6) | 0.551 | 16.5 (15.6, 17.4) | 0.452 |
| Cerebellar lesions | 13.5 (11.6, 15.5) | 0.339 | 15.7 (14.6, 16.8) | 0.040 |
| Significance of cerebellar lesions | ||||
| No cerebellar lesions | 12.4 (11.2, 13.5) | REF | 16.7 (16.0, 17.4) | REF |
| Non-significant lesions | 14.2 (11.3, 17.1) | 0.230 | 15.9 (14.4, 17.4) | 0.261 |
| Significant lesions | 13.2 (10.7, 15.6) | 0.517 | 15.7 (14.4, 17.0) | 0.092 |
Note: REF=reference category; means are adjusted for center, birthweight, gestational age, multiple gestation, race/ethnicity, Medicaid enrollment, gender, antenatal steroids, C-section, late-onset sepsis, surgery for ROP, NEC, or PDA, postnatal steroids, and BPD.
The mean (standard deviation) PMA at the time of near-term MRI was 37.8 (2.2) weeks, and the median (interquartile range) was 37.4 (36.1–39.0) weeks. When we examined PMA at the time of MRI in the models of the relationship between MRI results and BITSEA problem and competence scores, the results did not change.
Discussion
In this extremely preterm cohort, we have demonstrated that the presence of cerebellar lesions on near-term MRI is significantly associated with lower BITSEA Competence Scale scores but not worse Problem Scale scores, and that Competence Scale scores were inversely related to the presence and significance of cerebellar lesions on near-term MRI. Those with cerebellar lesions had lower social emotional competencies compared with those without these lesions. This relationship remained significant even after controlling for study site, demographic and medical characteristics. BITSEA Problem Scale scores and Positive Screens were not associated with any CUS or near-term MRI findings. Positive Screens on the Competence Scale were significantly associated with Bayley-III Language and Cognitive Composite scores <85, indicating that children who screened positive for difficulties with social emotional competencies were more likely to have moderate deficits in language and cognition.
Cranial ultrasound and MRI at term equivalent age have been associated with cognitive, psychomotor and neurosensory delays at 18–24 months of age11, 12, 19 and at school age.20 In unadjusted analyses, early CUS, late CUS abnormalities and moderate or severe WMA on nearterm MRI were associated with adverse cognitive and neuromotor outcomes at 18–22 months corrected age in the primary NEURO study.11 In addition, significant cerebellar lesions on nearterm MRI and late CUS adverse findings were both independently associated with neurodevelopmental impairment or death and with significant gross motor impairment or death, even after controlling for demographic and medical variables. Further, we recently found that cerebellar lesions on near-term MRI were associated with lower mean full scale intelligence quotients (FSIQs), higher rates of FSIQs <70 and <85, and higher rates of moderateto-severe neuromotor disability (defined as a FSIQ <70, cerebral palsy with a Gross Motor Function Classification System level ≥2, severe hearing impairment or severe vision impairment) at 6–7 years of age in the NEURO cohort.20 However, apart from this study, there is limited data describing early behavioral correlates to imaging findings.13, 21 The current findings demonstrate that cerebellar abnormalities on near-term MRI are also associated with early behavioral abnormalities, which are in turn, associated with moderate cognitive and language delay at 18–22 months. Though cerebellar injury has been linked to motor, visuospatial, cognitive and language outcomes in children born preterm,22–25 there are few studies linking cerebellar abnormalities on near-term MRI with early behavioral outcomes in EPT children.13, 25, 26 It is notable that no other neuroimaging findings were associated with behavioral outcomes, and that the behavioral abnormalities seen primarily involved deficits in social-emotional competence, rather than problem behaviors. Cerebellar abnormalities may result from altered neurogenesis, even in the absence of cerebral injury in infants born preterm,23 which could partially explain the lack of association of behavioral outcomes with neuroimaging abnormalities outside the cerebellum. In addition, the cerebellum is critically involved in visuomotor functioning via a cerebello-cerebral network. In this network, afferent connections from the posterior parietal cortex provide input to the cerebellum via the pontine nuclei, and output from the cerebellum returns to the posterior parietal cortex via the thalamus.23 Although the posterior parietal cortex plays a critical role in visuomotor functioning, the cerebello-cerebral network is also critically important for visuomotor and visuospatial processing.13, 23 Through cerebellar interconnectedness with the cerebral hemispheres, the cerebellum is also vital in cognitive and emotional functioning.27
The BITSEA Competence Scale assesses competencies that emerge in infancy and the early toddler years, such as curiosity, interest in new things, mastery motivation, awareness of others’ feelings, attention skills, social relatedness, symbolic and imitative play, play with peers, cooperation, and compliance with adult requests.14 Visuomotor adeptness is critical to developing the skills necessary to demonstrate social-emotional competence, such as interacting with and exploring the world, recognizing and mimicking facial expressions, nonverbal communication and recognizing and manipulating symbols. Visuomotor deficits are extremely common in children born preterm at school age and are evident even after controlling for other neurologic abnormalities.28 It may be that the early behavioral competence deficits that EPT children demonstrate at 18–22 months result from visuomotor disabilities that contribute more to behavioral competence than to behavioral problems measured by the BITSEA Problem Scale. This could explain why cerebellar abnormalities were not associated with Problem Scale results.
Although cerebellar function has been shown to be important in the long-term neurodevelopment of children born EPT, near-term MRI is not currently routinely recommended in the management of children born EPT.29 Cranial ultrasound is instead the routine neuroimaging method for identifying cranial abnormalities, though without appropriate views, cerebellar lesions are rarely visualized using this method.11, 30 The present findings suggest that assessment of cerebellar findings on near-term MRI could prove a useful tool for prognostication for families of children born EPT, as cerebellar abnormalities on near-term MRI could serve as early neuroanatomic biomarkers of later behavioral competence. Consistent with our findings at 18–22 months, studies of EPT children at school age have demonstrated that behavioral deficits are commonly associated with cognitive and language deficits found at that time. This finding has led some researchers to theorize that behavioral abnormalities underlie the deficits found in cognition, language and executive functioning in children born preterm at school age.5 Recently, researchers have posited a ‘preterm behavioral phenotype’ consisting of inattention, anxiety, and social difficulties.6 A study by Scott et al demonstrated that kindergarten children who were born EPT had deficits in competence skills including behavioral organization and self-monitoring.5 These findings remained significant even when children with neurosensory disorders or cognitive deficits were excluded, indicating that these children remain at risk for behavior problems even in the absence of global deficits.
Though most studies of behavioral outcomes in children born preterm report on children at school age or adolescence, a few other studies have demonstrated problems in behavioral competence in preterm children, including poor self-regulation and social functioning, as early as 2 years of age.6 There is also evidence that deficits found at preschool age remain stable from early childhood through school age.7 Lower social competence in the preschool years is associated with more externalizing and internalizing behavior problems at 10–14 years of age.31 It is possible that precursors of these school-age problems are present in very early childhood. Early social-emotional competence is vital for societal interaction and the development of the higher order executive processes critical to academic success.32 Trials of parenting interventions in early childhood have demonstrated improvement in behavioral problems and improved behavioral competence among typically developing infants and children as well as preterm children.33, 34 Thus, this delay in recognition of behavioral deficits may result in a missed opportunity for interventions aimed at improving this modifiable outcome.
Strengths of the study include the large sample size, multiple neuroimaging modalities assessed, and central reading of all neuroimaging. The study also had several notable limitations. First, though the BITSEA has been well-validated across multiple settings, this is an observational parent-report measure, and direct assessments of behavior were not utilized in the study. In addition, though Bayley-III cognitive and language scores were related to BITSEA social Competence, aspects of cognition that may mediate the effects of brain abnormalities on behavior, such as visuomotor skills, were not targeted for assessment. Further, a limited number of the CUS studies included mastoid views. Cerebellar injury may have been seen better by CUS in addition to near-term MRI had those views been obtained. It is also unknown whether findings at 18–22 months are predictive of findings at later ages, though follow-up of the NEURO cohort to school age will permit more comprehensive evaluation of associations of early brain abnormalities with neurodevelopmental outcomes. Also, the near-term MRI for this study called for a conventional, qualitative MRI only, with a goal of generalizability. Structured central reader evaluation was generally focused on white matter abnormalities according to a widely used scoring instrument at that time9, 12 which has since been expanded.35 It is possible that advanced MRI such as diffusion tensor imaging measures and volumetric measures may have further detailed injury-outcome associations. Finally, language delays could account for the Competence Scale deficits seen, as language determines much social interaction at this age.
Our findings indicate that deficits in social-emotional competence are associated with cognitive and language deficits seen in toddlers born EPT and should be considered in early outcome studies of EPT children. In addition, cerebellar injury on near-term MRI may be a marker for early behavioral challenges. Further study is needed to assess whether early neuroimaging findings are associated with behavioral challenges through later childhood and whether early institution of interventions aimed at enhancing social-emotional competence may provide a means for improving developmental outcomes in EPT children.
Acknowledgments
Supported by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies. The authors declare no conflicts of interest.
Abbreviations:
- EPT
Extremely Preterm
- BITSEA
Brief Infant Toddler Social Emotional Assessment
- PS
Problem Scale
- CS
Competence Scale
- Bayley-III
Bayley Scales of Infant Development, 3rd ed.
- NRN
Eunice Kennedy Shriver National Institute of Child Health and Development Neonatal Research Network
- NEURO
Neonatal Research Network SUPPORT Neuroimaging and Neurodevelopmental Outcomes Study
- WMA
white matter abnormalities
- FSIQ
Full Scale IQ
List of additional members of the Eunice Kennedy Shriver National Institute of Child Health and Development Neonatal Research Network
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network’s SUPPORT Trial Neuroimaging Secondary Protocol through cooperative agreements. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Drs. Abhik Das (DCC Principal Investigator), Marie Gantz, Lisa Wrage, and Helen Cheng (DCC Statisticians) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Betty R. Vohr, MD; William Oh, MD; Angelita M. Hensman, RN BSN; Barbara Alksninis, PNP; Dawn Andrews, RN; Kristen Angela, RN; Susan Barnett, RRT; Bill Cashore, MD; Melinda Caskey, MD; Kim Francis, RN; Dan Gingras, RRT; Katharine Johnson, MD; Theresa M. Leach, MEd CAES; Bonnie E. Stephens, MD; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Nancy S. Newman, RN; Deanne E. Wilson-Costello, MD; Bonnie S. Siner, RN; Arlene Zadell, RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD.
Children’s National Medical Center, Washington DC – Dorothy Bulas, MD.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Patricia Ashley, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sharon F. Freedman, MD; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; William F. Malcolm, MD; David K. Wallace, MD MPH.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, RR25008, M01 RR39) – David P. Carlton, MD; Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Susie Buchter, MD; Anthony J. Piazza, MD; Sheena Carter, PhD; Sobha Fritz, PhD; Ellen C. Hale, RN BS CCRC; Amy K. Hutchinson, MD; Maureen Mulligan LaRossa, RN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Gregory M. Sokol, MD; Brenda B. Poindexter, MD MS; Anna M. Dusick, MD FAAP; James A. Lemons, MD; Leslie D. Wilson, BSN CCRC; Faithe Hamer, BS; Ann B. Cook, MS; Dianne E. Herron, RN; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP.
National Heart, Lung, and Blood Institute – Mary Anne Berberich, PhD; Carol J. Blaisdell, MD; Dorothy B. Gail, PhD; James P. Kiley, PhD.
RTI International (U10 HD36790) – Abhik Das, PhD; Marie G. Gantz, PhD; Jamie E. Newman, PhD MPH; Helen Cheng, MS; Betty K. Hastings; Elizabeth M. McClure, Med; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; W. Kenneth Poole, PhD; James W. Pickett II, BS; Dennis Wallace, PhD; Lisa A. Wrage, MPH; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University and Lucile Packard Children’s Hospital (U10 HD27880, UL1 RR25744, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Patrick D. Barnes, MD; Barbara Bentley, PsychD MSEd; Elizabeth F. Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP; Jean G. Kohn, MD MPH; Melinda S. Proud, RCP; Renee P. Pyle, PhD; Hali E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Elisabeth C. McGowan, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN. Vivien A. Phillips, RN BSN; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Maria Hopkins, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Richard V. Rector, PhD; Leslie Rodriguez, PhD; Amanda Soong, MD; Sally Whitley, MA OTR-L FAOTA; Sheree York, PT DPT MS PCS.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) – Neil N. Finer, MD; Maynard R. Rasmussen, MD; Paul R. Wozniak, MD; Yvonne E. Vaucher, MD MPH; Wade Rich, RRT; Kathy Arnell, RNC; Rene Barbieri-Welge; Ayala Ben-Tall; Renee Bridge, RN; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Elaine Ito; Meghan Lukasik; Deborah Pontillo; Donna Posin, OTR/L MPA; Cheryl Runyan; James Wilkes; Paul Zlotnik.
University of Iowa Children’s Hospital (U10 HD53109, UL1 RR24979, M01 RR59) – Edward F. Bell, MD; John A. Widness, MD; Michael J. Acarregui, MD; Jonathan M. Klein, MD; Tarah T. Colaizy, MD MPH; Karen J. Johnson, RN BSN; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Maria Calejo, MEd; Alexis N. Diaz, BA; Silvia M. Frade Eguaras, BA; Andrea Garcia, MA; Kasey Hamlin-Smith, PhD; Michelle Harwood Berkowits, PhD; Sylvia Hiriart-Fajardo, MD; Elaine O. Mathews, RN; Helina Pierre, BA; Arielle Riguard, MD; Alexandra Stroerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Robin K. Ohls, MD; Janell Fuller, MD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN; Jean Lowe, PhD; Rebecca Montman, BSN; Sandra Brown, RN BSN.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44) – Nirupama Laroia, MD; Dale L. Phelps, MD; Gary J. Myers, MD; Gary D. Markowitz, MD; Linda J. Reubens, RN CCRC; Diane Hust, MS RN CS; Lisa Augostino; Julie Babish Johnson, MSW; Erica Burnell, RN; Harris Gelbard, MD PhD; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Jonathan Mink, MD PhD; Carlos Torres, MD; David Wang, MD; Kelley Yost, PhD.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Roy J. Heyne, MD; Sally S. Adams, MS RN CPNP; James Allen, RRT; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, PsyD PA-C; Jackie F. Hickman, RN; Melissa H. Leps, RN; Linda A. Madden, RN CPNP; Melissa Martin, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Araceli Solis, RRT; Lizette E. Torres, RN; Catherine Twell Boatman, MS CIMI; Diana M Vasil, RNC-NIC.
University of Texas Health Science Center at Houston Medical School and Children’s Memorial Hermann Hospital (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Patricia W. Evans, MD; Esther G. Akpa, RN BSN; Nora I. Alaniz, BS; Beverly Foley Harris, RN BSN; Charles Green, PhD; Margarita Jiminez, MD MPH; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; Margaret L. Poundstone, RN BSN; Stacy Reddoch, BA; Saba Siddiki, MD; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64) – Bradley A. Yoder, MD; Roger G. Faix, MD; Shawna Baker, RN; Karie Bird, RN BSN; Anna E. Bullwinkle, RN; Jill Burnett, RNC BSN; Laura Cole, RN; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC BSN; R. Edison Steele, RN; Michael Steffen, PhD; Kimberlee Weaver-Lewis, MS RN.
Wake Forest University, Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Nancy J. Peters, RN CCRP; Barbara G. Jackson, RN BSN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Beena G. Sood, MD MS; Thomas L. Slovis, MD; Athina Pappas, MD; Rebecca Bara, RN BSN; Elizabeth Billian, RN MBA; Laura A. Goldston, MA; Mary Johnson, RN BSN.
*List of additional members of the Eunice Kennedy Shriver National Institute of Child Health and Development Neonatal Research Network is available at www.jpeds.com (Appendix).
Trial registration ClinicalTrials.gov NCT00063063 and NCT0000
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Conrad AL, Richman L, Lindgren S, Nopoulos P. Biological and Environmental Predictors of Behavioral Sequelae in Children Born Preterm. Pediatrics. 2010;125:e83–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peralta-Carcelen M, Carlo WA, Pappas A, Vaucher YE, Yeates KO, Phillips VA, et al. Behavioral Problems and Socioemotional Competence at 18 to 22 Months of Extremely Premature Children. Pediatrics. 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peralta-Carcelen M, Bailey K, Rector R, Gantz M. Behavioral and socioemotional competence problems of extremely low birth weight children. Journal of perinatology : official journal of the California Perinatal Association. 2013;33:887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. Journal of developmental and behavioral pediatrics : JDBP. 2009;30:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, et al. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. Journal of developmental and behavioral pediatrics : JDBP. 2012;33:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, et al. Early emergence of behavior and social-emotional problems in very preterm infants. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:909–18. [DOI] [PubMed] [Google Scholar]
- [7].Gray RF, Indurkhya A, McCormick MC. Prevalence, Stability, and Predictors of Clinically Significant Behavior Problems in Low Birth Weight Children at 3, 5, and 8 Years of Age. Pediatrics. 2004;114:736–43. [DOI] [PubMed] [Google Scholar]
- [8].Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatric research. 2011;69:11R–8R. [DOI] [PubMed] [Google Scholar]
- [9].Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. The Journal of Pediatrics. 2003;143:171–9. [DOI] [PubMed] [Google Scholar]
- [10].Volpe JJ. The Encephalopathy of Prematurity—Brain Injury and Impaired Brain Development Inextricably Intertwined. Seminars in Pediatric Neurology. 2009;16:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135:e32–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to Predict Neurodevelopmental Outcomes in Preterm Infants. The New England Journal of Medicine. 2006;355:685–94. [DOI] [PubMed] [Google Scholar]
- [13].Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cerebral cortex (New York, NY : 1991). 2014;24:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DV. The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. Journal of pediatric psychology. 2004;29:143–55. [DOI] [PubMed] [Google Scholar]
- [15].Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. The New England journal of medicine. 2017;376:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatric research. 2014;75:670. [DOI] [PubMed] [Google Scholar]
- [17].Steggerda SJ, De Bruïne FT, van den Berg-Huysmans AA, Rijken M, Leijser LM, Walther FJ, et al. Small Cerebellar Hemorrhage in Preterm Infants: Perinatal and Postnatal Factors and Outcome. The Cerebellum. 2013;12:794–801. [DOI] [PubMed] [Google Scholar]
- [18].Brossard-Racine M, du Plessis AJ, Limperopoulos C. Developmental Cerebellar Cognitive Affective Syndrome in Ex-preterm Survivors Following Cerebellar Injury. Cerebellum (London, England). 2015;14:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Skiöld B, Vollmer B, Böhm B, Hallberg B, Horsch S, Mosskin M, et al. Neonatal Magnetic Resonance Imaging and Outcome at Age 30 Months in Extremely Preterm Infants. The Journal of Pediatrics. 2012;160:559–66.e1. [DOI] [PubMed] [Google Scholar]
- [20].Hintz SR, Vohr BR, Bann CM, Taylor HG, Das A, Gustafson KE, et al. Preterm Neuroimaging and School-Age Cognitive Outcomes. Pediatrics. 2018;142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Clark CAC, Woodward LJ, Horwood LJ, Moor S. Development of Emotional and Behavioral Regulation in Children Born Extremely Preterm and Very Preterm: Biological and Social Influences. Child Development. 2008;79:1444–62. [DOI] [PubMed] [Google Scholar]
- [22].Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Jr., Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–93. [DOI] [PubMed] [Google Scholar]
- [23].Van Braeckel KNJA Taylor HG. Visuospatial and visuomotor deficits in preterm children: The involvement of cerebellar dysfunctioning. Developmental Medicine and Child Neurology. 2013;55:19–22. [DOI] [PubMed] [Google Scholar]
- [24].Zayek MM, Benjamin JT, Maertens P, Trimm RF, Lal CV, Eyal FG. Cerebellar hemorrhage: a major morbidity in extremely preterm infants. Journal of perinatology : official journal of the California Perinatal Association. 2012;32:699. [DOI] [PubMed] [Google Scholar]
- [25].Stoodley CJ, Limperopoulos C. Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Seminars in fetal & neonatal medicine. 2016;21:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pieterman K, Batalle D, Dudink J, Tournier JD, Hughes EJ, Barnett M, et al. Cerebellocerebral connectivity in the developing brain. Brain Structure & Function. 2017;222:1625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Panigrahy A, Wisnowski JL, Furtado A, Lepore N, Paquette L, Bluml S. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatric Radiology. 2012;42:33–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Butcher PR, Bouma A, Stremmelaar EF, Bos AF, Smithson M, Van Braeckel KN. Visuospatial perception in children born preterm with no major neurological disorders. Neuropsychology. 2012;26:723–34. [DOI] [PubMed] [Google Scholar]
- [29].Ho T, Dukhovny D, Zupancic JA, Goldmann DA, Horbar JD, Pursley DM. Choosing Wisely in Newborn Medicine: Five Opportunities to Increase Value. Pediatrics. 2015;136:e482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–38. [DOI] [PubMed] [Google Scholar]
- [31].Bornstein MH, Hahn CS, Haynes OM. Social competence, externalizing, and internalizing behavioral adjustment from early childhood through early adolescence: developmental cascades. Development and psychopathology. 2010;22:717–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sokol B, Muller U, Carpendale J, Young A, Iarocci G. Self- and Social-Regulation: The Development of Social Interaction, Social Understanding, and Executive Functions: Oxford University Press; 2010. [Google Scholar]
- [33].Gardner F, Shaw DS, Dishion TJ, Burton J, Supplee L. Randomized prevention trial for early conduct problems: effects on proactive parenting and links to toddler disruptive behavior. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association (Division 43). 2007;21:398. [DOI] [PubMed] [Google Scholar]
- [34].Landry SH, Smith KE, Swank PR, Guttentag C. A responsive parenting intervention: the optimal timing across early childhood for impacting maternal behaviors and child outcomes. Developmental psychology. 2008;44:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR American journal of neuroradiology. 2013;34:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]