Abstract
Background Medication-related hospital admissions (MRAs) are frequently used to measure outcomes in studies involving medication reviews. The process of identifying MRAs is subjective and time-consuming, and practical, validated alternatives are required. Objective The aim of this study was to develop and validate a practical tool to identify MRAs. Setting Uppsala University Hospital, Sweden. Method We reviewed existing literature on methods to identify MRAs. The tool AT-HARM10 was developed using an iterative process including content validity and feasibility testing. The tool’s inter-rater reliability (IRR) and criterion-related validity (CRV) were assessed: four pairs of either final-year undergraduate or postgraduate pharmacy students applied the tool to one of two batches of 50 older patients’ hospital admissions. Assessment of the same 100 admissions by two experienced clinicians acted as gold standard. Main outcome measure Cohen’s and Fleiss’ kappa for IRR, and sensitivity, specificity, and positive and negative predictive value for CRV. Results AT-HARM10 consists of ten closed questions to distinguish between admissions that are unlikely to be and those that are possibly medication-related. The IRR was moderate to substantial (Cohen’s kappa values were 0.45–0.75 and Fleiss’ kappa values were 0.46 and 0.58). The sensitivity and specificity values were 70/86% and 74/70%, positive and negative predictive values were 73/74% and 71/83% respectively. Both AT-HARM10 and the gold standard identified approximately 50% of the admissions as MRAs. Conclusion AT-HARM10 has been developed as a practical tool to identify MRAs and the tool is valid for use in older patients by final-year undergraduate and postgraduate pharmacy students.
Electronic supplementary material
The online version of this article (10.1007/s11096-018-0768-8) contains supplementary material, which is available to authorized users.
Keywords: Assessment tool, Drug-related problems, Elderly, Hospital admissions, Medication-related admissions, Sweden
Impacts on practice
The tool, ATHARM10, can be used to identify medication-related hospital admissions in older patients as a valid outcome in clinical research.
The ATHARM10 tool potentially decreases research costs as it can be used by final-year undergraduate or postgraduate pharmacy students with little involvement of clinical experts.
Introduction
Medication-related problems (MRPs) are highly prevalent among older patients taking multiple medications, and can lead to a negative impact on health outcomes and increasing healthcare costs [1]. MRPs are defined here as “undesirable patient experiences that involve medication therapy and that actually or potentially interfere with desired patient outcomes” [2]. These not only involve adverse drug reactions (ADRs) to prescribed medication, but can also involve problems such as inappropriate prescribing and non-compliance, and problems related to over-the-counter (OTC) medications. Up to 30% of older patients’ hospital admissions can be attributed to MRPs [3, 4]. Over half of these medication-related admissions (MRAs) are possibly or definitely preventable [5–8]. Healthcare interventions, such as medication reviews, have been introduced to promote appropriate prescribing, increase the correct use of medications and decrease the incidence of MRAs [9–11]. As such interventions primarily target medications and medication-related issues, MRAs are consequently the main admissions that they can impact. The use of the incidence of MRAs as an outcome measure has therefore recently been proposed as part of a standard core-outcome set for studies involving medication reviews in older patients taking multiple medications [12].
However, there is no validated method of identifying MRAs. The most common method is for an expert panel to assess the patient’s medical record and reach a consensus [11, 13, 14]. The use of an expert panel is often viewed as the “gold standard”. In medicine, a gold standard refers to a test, a treatment, or a benchmark that is the best available under reasonable conditions [15]. An expert panel assessment involves the use of senior clinicians or researchers and is often time-consuming, which makes it a costly method. To lower research costs, involvement of under- or postgraduate students should be considered in academic research [16]. However, the identification of MRAs by either an expert panel or students will inevitably introduce a degree of subjectivism. The use of standardized methods that have been tested for reliability and validity can minimize this subjectivism [15, 17, 18]. Validated tools for determining the association between ADRs and hospital admissions, such as the Naranjo algorithm, are often used [19–23]. Unfortunately, none of these tools have been developed to identify the full range of MRPs that can result in hospital admissions, leading to the risk of underestimating the incidence of MRAs. In fact, non-compliance has been found to be one of the largest contributors to MRAs [5, 24, 25]. One standardized method to identify the full range of MRAs in older people has recently been developed [26]. However, the performance of this method in terms of predictive validity, sensitivity and specificity has not yet been validated and it also uses an expert panel for the assessment.
Our research group is currently carrying out a cluster-randomized controlled trial [the Medication Reviews Bridging Healthcare (MedBridge) study] which aims to evaluate the effects of comprehensive medication reviews in hospitalized older patients [27]. One of the outcome measures is the incidence of MRAs, and more than 5000 readmissions are expected during the 12-month follow-up period. There is thus a need for a practical assessment tool, defined as being possible to use by under- or postgraduate pharmacy students, within a limited time frame and without the need for an expert panel.
Aim of the study
The aim of this study was to develop and validate a practical tool to identify MRAs.
Ethics approval
This study is part of the MedBridge study, which has received ethical approval from the Swedish Central Ethical Review Board (CEPN Ö21-2016). Additional approval for the medical record screening of non-MedBridge study participants was received from Uppsala University Hospital, in compliance with local regulations.
Method
Development of the tool
The first part of the study was an unstructured review of the available literature to find existing methods or tools used to identify MRAs. Relevant articles related to MRAs were obtained using the Medline database in February 2016. Articles in languages other than English or Swedish and abstract-only articles were excluded. The literature search identified several articles using various methods to identify MRAs [3, 4, 6, 11, 13, 25, 28–40]. Some studies included a variety of MRPs [3, 4, 11, 25, 31, 32, 34, 35, 37–39] as causes of MRAs, often referring to existing MRP classification systems like the eight categories by Strand et al. [2]. Some studies only included ADRs [6, 13, 29, 30, 33, 36, 40]. When assessing causality, one study [28] used completely implicit methodology while all the others [3, 4, 6, 11, 13, 26, 29–38] used established criteria such as the Naranjo probability scale [21], the Hallas criteria [22] or the Kramer algorithm [20] to guide an expert panel (consisting of physicians or pharmacists with various degrees of seniority).
The results of the literature search were used to develop the preliminary version of our tool. We defined an MRA as a hospital admission of which an MRP is either the main cause for admission or a significantly contributing cause for admission (i.e. without the MRP, the patient would not have been admitted). Elements from established tools and previously published studies were listed to include all relevant categories of MRPs with the potential to cause, or contribute to, a hospital admission, and to include reasons for classifying admissions as non-medication-related [2, 5, 11, 19–21, 25, 41–46]. Overlapping elements were combined and reformulated. The tool was designed in the form of a questionnaire with yes/no answers, and it consisted of ten questions in the final version (Table 1). Explicit lists with medication-specific triggers or clinical rules were excluded to make the tool less time-consuming. The rationale for choosing the questionnaire format was that it would be easy to use. Questions 1–3 in the tool are used to identify admissions that are unlikely to be medication-related (U1–3), while questions 4–10 are used to identify possibly MRAs (P4–10). The assessment is finished as soon as the answer is “yes” to any of the questions. Only if all the questions are answered “no”, the assessment is indecisive and should still be examined by an expert panel. The terms unlikely and possibly are in line with the causality terminology of the UMC-WHO system [23]. The reason for not distinguishing between other degrees of certainty or preventability was to make the assessment not too complex and time-consuming. For the same reason, we decided that only a limited amount of data would be available for the assessments (admission notes, medication list upon admission, laboratory data during hospital stay, and discharge summary). Instructions for use, including examples, were developed for the tool (“Supplementary material”).
Table 1.
The final version of the assessment tool for identifying hospital admissions related to medications (AT-HARM10)
| Question | References | MRP category [2] |
|---|---|---|
| U1. Was the admission caused by an infection or a previously undiagnosed disease (e.g. diabetes or heart failure) that is not medication-related? | [44, 45] | n.a. |
| U2. Was the admission caused by progression of a previously diagnosed disease that is not medication-related? | [19, 21, 41, 46] | n.a. |
| U3. Was the admission caused by physical trauma, substance intoxication, social circumstances or allergies that are not medication-related? | [19, 21, 41, 46] | n.a. |
| P4. Is it hinted or stated in the medical record that the admission was medication-related (including non-compliance)? | [21] | Any MRP category |
| P5. Might (side) effects of the medications the patient was taking (prescribed or not prescribed) prior to hospitalization have caused the admission (including over-treatment)? | [5, 19–21, 25, 41] | 5. Overdosage 6. Adverse drug reaction 8. Drug use without indication |
| P6. Are there abnormal laboratory results or vital signs that could be medication-related and might have caused the admission? | [5, 20, 21, 42] | 2. Improper drug selection 5. Overdosage |
| P7. Was there any drug-drug interaction or drug-disease interaction (i.e. a contraindication) that might have caused the admission? | [11, 25, 43] | 2. Improper drug selection 7. Drug interaction |
| P8. Did the patient have any previously diagnosed untreated or sub-optimally treated (e.g. dose too low) indications that might have caused the admission? | [5, 25, 43] | 1. Untreated indication 2. Improper drug selection 3. Subtherapeutic dosage |
| P9. Was the patient admitted because of a problem with the dosage form or pharmaceutical formulation (i.e. failure to receive the medication)? | [5, 11, 25] | 4. Failure to receive drug |
| P10. Is the cause of the admission a response to cessation or withdrawal of medication therapy? | [47] | 6. Adverse drug reaction |
Three questions are used to identify admissions that are unlikely to be medication-related (U1-U3) and seven questions (P4-P10) to identify possible medication-related admissions. References to criteria used in existing tools or former studies that were identified in the literature search, and the corresponding eight medication-related problem (MRP) categories by Strand et al. [2], are listed for each question (1–8)
n.a. not applicable, MRP medication-related problem
Content validity
The concept of content validity relies on the assumption that a tool is intrinsically valid if all relevant aspects and no irrelevant aspects are included [17]. The content validity of the preliminary version of the tool was assessed using a questionnaire to score each question for relevance, understandability and completeness. Seven clinical pharmacists were asked to fill in the questionnaire and were encouraged to suggest additions or modifications. The questions that gained low scores were deleted or changed. Two questions (P9–10; Table 1) were added and seven questions were rephrased. The number of questions was eventually set at ten in the final version: Assessment Tool for identifying Hospital Admissions Related to Medications (AT-HARM10).
One hundred admissions of patients aged 65 years or older, discharged from two internal medicine wards at Uppsala University Hospital between January and April 2016, were randomly selected to test the content validity of the final version of the tool. Data were obtained from the hospital’s electronic medical record system (Cosmic®). Seven pharmacy students (five final-year undergraduates, one doctoral student and one postgraduate clinical pharmacy student) received information about the tool (“Supplementary material”) and a 1-h training by two researchers (YA-S and UG) on how to use the tool. One of the researchers (UG) was an experienced clinical pharmacist and researcher. The students then applied the tool to five admissions (none of which were subsequently used in the study). The results of the assessments were discussed in plenum. Each student assessed 50 or 100 of these admissions (see “Inter-rater reliability” section), resulting in a total of 400 assessments. The number of the specific question within AT-HARM10, that was used to classify each admission as unlikely to be or possibly medication-related, was recorded for these 400 assessments, to determine whether all questions were relevant and which questions were used the most.
Clinical utility
An assessment tool must be practical and it should be possible to use it within a reasonable time frame [48]. Fifteen clinical pharmacists each applied the tool to 10 randomly selected admissions of patients aged 65 years or older, discharged from one internal medicine ward and one geriatric ward at Uppsala University Hospital (Sweden) between December 2015 and January 2016. Data were obtained from the hospital’s electronic medical record system (Cosmic®). The pharmacists evaluated whether the limited patient data provided for the assessments were sufficient to satisfactorily answer the questions. They also discussed the tool’s appropriateness and user-friendliness. Time spent on assessing the admissions was measured and averaged to determine the acceptability of the time taken.
Inter-rater reliability
The inter-rater reliability (IRR) refers to the degree of consistency among the assessors when assessing the same set of samples [15]. The seven participants of the content validity test (five undergraduate and two postgraduate pharmacy students) were divided into four pairs (one student was part of both pair 1 and 4 to enable the formation of four pairs). The pairs consisted of either only postgraduate students or only undergraduate students (Table 2).
Table 2.
Grouping for the inter-rater reliability test
| Pair | Admission number |
|---|---|
| 1 | 1–50 |
| 2a | 1–50 |
| 3 | 51–100 |
| 4 | 51–100 |
aPostgraduate students
The tool, instructions for use and examples (“Supplementary material”), and anonymized patient data from 50 admissions (either 1–50 or 51–100 of the 100 admissions used for the content validity test, see “Content validity” section) were sent to each assessor. These patient data consisted of copies of the electronic medical records: admission notes, medication list upon admission, laboratory data during hospital stay and discharge summary.
Each assessor then independently applied AT-HARM10 to their assigned 50 hospital admissions, classifying them as either unlikely to be or possibly MRAs. After assessing the admissions separately, each pair of assessors discussed the admissions that they disagreed on to reach consensus. Cohen’s kappa within each couple and Fleiss’ kappa between pairs assessing the same admissions were calculated to determine the IRR. Cohen’s kappa measures the agreement between two assessors and Fleiss’ kappa is used in cases with more than two assessors [15, 49]. The kappa values were then interpreted according to Hammond et al. [15] (Text Box 1).
Text Box 1.
Interpretation of kappa values for inferring strength of agreement [15]
| Kappa | Strength of agreement |
|---|---|
| 0 | None |
| 0–0.2 | Slight |
| 0.21–0.4 | Fair |
| 0.41–0.6 | Moderate |
| 0.61–0.8 | Substantial |
| 0.81–1.0 | Almost perfect |
Criterion-related validity
The traditional definition of criterion-related validity (CRV) is a measure of the validity of a tool by correlating the results with those from some other measure, ideally a gold standard, which has been used and accepted in the field [17]. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), as defined in Text Box 2, were used to measure the CRV.
Text Box 2.
Criterion-related validity definitions for AT-HARM10 (based on definitions in Hammond et al. [15])
| Sensitivity: The probability that the tool will detect possible medication-related admissions (MRAs) among the admissions that are truly related to medication according to the gold standard |
| Specificity: The probability that the tool will detect unlikely to be MRAs among admissions that are truly not related to medication according to the gold standard |
| Positive predictive value (PPV): The percentage of admissions identified by the tool as possibly MRAs that are truly related to medication according to the gold standard |
| Negative predictive value (NPV): The percentage of admissions identified by the tool as unlikely to be MRAs that are truly not related to medication according to the gold standard |
We defined the gold standard as an expert panel of experienced clinicians. One consultant physician (geriatrics and primary care specialist) and one senior clinical pharmacist and researcher (UG) assessed the same 100 admissions as the four pairs of study assessors, see “Inter-rater reliability” section. The experts had access to all patient data in the electronic medical journal. These data included medical notes from all healthcare professionals, medication histories, and laboratory results from both hospital and primary care facilities. They did not use AT-HARM10 or any other tool for the evaluations. The experts assessed the admissions individually, classifying them as either unlikely to be or possibly an MRA. They then discussed the cases on which they disagreed to reach consensus and hence created the gold standard for the 100 admissions. In cases where consensus could not be reached, a third expert was available for a decisive vote.
The sensitivity, specificity, PPV and NPV were calculated according to the formula in Table 3. The primary outcomes for the CRV were the consensus results of the AT-HARM10 assessments of all 100 patient admissions, performed by couples 1 + 3 and 2 + 4, compared to the gold standard.
Table 3.
Formula used to calculate sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) as outcome measures for the criterion-related validity
| Gold standard unlikely MRA |
Gold standard possibly MRA |
Total | |
|---|---|---|---|
| ATHARM10 unlikely MRA |
A (true negative) | B (false negative) | A + B |
| ATHARM10 possibly MRA |
C (false positive) | D (true positive) | C + D |
| Total | A + C | B + D | |
MRA medication-related admission
Results
Clarification: all results in this section relate to the testing of the final tool, AT-HARM10, not to the work done on earlier versions of the tool as this was covered in the Methods section above.
Content validity
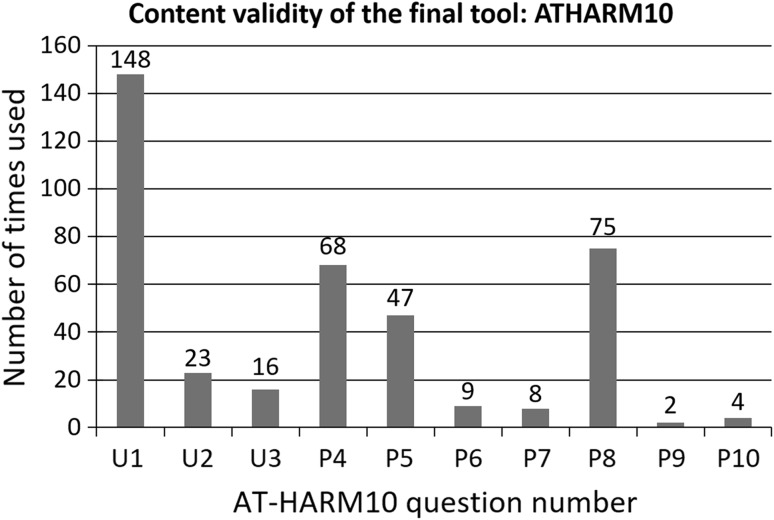
After the development phase resulting in the final tool, AT-HARM10, the question that was used most often when assessing the admissions was question U1 (148/400), followed by questions P8 (75/400), P4 (68/400) and P5 (47/400), see Fig. 1. All the questions were used at least twice. In all cases, at least one question was answered with “Yes”, hence no expert panel was needed.
Fig. 1.
The number of times each of the questions (U1–3 and P4–10) were used by within 400 assessments of in total 100 admissions
Clinical utility
According to the fifteen assessing clinical pharmacists, AT-HARM10 was sufficiently relevant and user-friendly and was easy to use; no further changes were suggested. The limited data obtained from the example patients were deemed sufficient to answer the questions in the tool. The time used to assess each admission was on average 5.7 (range 2.5–14) min.
Inter-rater reliability
The strength of agreement was substantial for one pair of study assessors (Cohen’s kappa 0.75) and moderate for the other three (Cohen’s kappa 0.45–0.57), see Table 4. The strength of agreement among all assessors was moderate (Fleiss’ kappa 0.58 and 0.46 for the two batches of data).
Table 4.
Inter-rater reliability between assessors using AT-HARM10
| Pairs | Admissions assessed | Cohen’s kappa | Fleiss’ kappa |
|---|---|---|---|
| 1 | 1–50 | 0.75 | 0.58 |
| 2a | 1–50 | 0.45 | |
| 3 | 51–100 | 0.52 | 0.46 |
| 4 | 51–100 | 0.57 |
aPostgraduate students
Criterion-related validity
The gold standard experts reached consensus for all 100 assessments, which resulted in 50% of the 100 admissions being classified as unlikely to be and 50% as possibly a MRA. Pairs 1 + 3 and 2 + 4 assigned 52% and 42%, respectively, to unlikely, and 48% and 58%, respectively, to possibly. The sensitivity was 70% and 86% for pairs 1 + 3 and 2 + 4, respectively. The specificity was 70% and 74% for 1 + 3 and 2 + 4, respectively. The PPV and NPV were 73%/74% for 1 + 3/2 + 4 and 71%/83% for 1 + 3/2 + 4, respectively (Table 5).
Table 5.
Criterion-related validity of AT-HARM10
| Gold standard | Total pairs | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | ||
|---|---|---|---|---|---|---|---|
| Unlikely | Possibly | ||||||
| Pair 1 + 3 | |||||||
| Unlikely | 37 | 15 | 52 (52%) | 70 | 74 | 73 | 71 |
| Possibly | 13 | 35 | 48 (48%) | ||||
| Pair 2 + 4 | |||||||
| Unlikely | 35 | 7 | 42 (42%) | 86 | 70 | 74 | 83 |
| Possibly | 15 | 33 | 58 (58%) | ||||
| Total gold standard | 50 (50%) | 50 (50%) | |||||
The number of unlikely to be and possibly medication-related admissions, as classified by the gold standard and the study pairs, are provided
Sens. sensitivity, Spec. specificity, PPV positive predictive value, NPV negative predictive value
Discussion
We have developed a tool for identifying MRAs, AT-HARM10, and validated the tool for use in older patients by final-year undergraduate or postgraduate pharmacy students. The IRR, with Cohen’s kappa values ranging from 0.45 to 0.75 and Fleiss’ kappa values of 0.46 and 0.58, was moderate to substantial, and the CRV, with values ranging from 70 to 86%, was moderate to high [15]. No expert panel was needed for the assessments using AT-HARM10. An assessment took on average 6 (range 2.5–14) min, meaning that the expected 5000 readmissions in the MedBridge study can be assessed in 500 h. The recently published method by Thevelin et al. [26] had similar IRR (Cohen’s kappa values from 0.33 to 0.86 and a Fleiss’ kappa of 0.41), but the mean time needed to assess an admission was considerably longer (23 ± 6 min) and the method involves the use of experts in geriatric medicine.
Approximately half of all admissions in this study were considered possibly medication-related, according to both the gold standard experts (50%) and the AT-HARM10 assessments (48–58%). These figures are higher than anything we have identified in the literature (the highest was 30% [3, 4]). There are several possible explanations for this: we included all types of MRPs in our assessments and we did not assess the degree of certainty (i.e. we had the pragmatic view that for some chronic diseases, typically diabetes mellitus and congestive heart failure, it is hard to rule out the possibility that suboptimal therapy had contributed to the admission).
Another way of looking at the results is that, with ATHARM10, researchers can quickly rule out 50% of the admissions as unlikely to be medication-related. Hence, should one wish to elaborate on preventability or degree of certainty, for example using an expert panel, only half of the hospital admissions need to be evaluated in more depth.
The tool was developed in close collaboration with the intended future users, which helped to keep the process on track. Also, since AT-HARM10 has been developed and tested over a period of nearly 2 years, we feel that all possible aspects of the tool and its use have been carefully considered. All ten questions were based on criteria from previous studies or existing tools, and the tool takes into consideration all possible MRP categories. A broad range of relevant validation parameters have successfully been tested and all of these are standard parameters in validation studies [15, 17, 48, 49].
One aspect that was deemed important throughout the study was the care taken to thoroughly introduce and demonstrate the use of the tool before starting any assessments. The tool is therefore not just the ten questions, it also includes the whole package of instructions and examples (“Supplementary material”), relying on thorough elaboration and group discussion before starting the assessments.
There are several limitations to this study. First, AT-HARM10 does not assess the degree of certainty or the preventability of MRAs. This was deliberate, as we wanted to keep the tool simple and straightforward, specifically for measuring the incidence of MRAs as a research outcome. Second, the study would perhaps have benefitted from more input from the medical profession; i.e. by including experts in diagnostics. The gold standard may not necessarily be totally reliable; however, an expert panel with full access to the patient data is the best we currently have. Third, we did not perform a comprehensive systematic review of the literature. We may have missed relevant studies in our literature review. However, no validated methods were found in a recent more structured literature review either [26]. We consider AT-HARM10 a valid and practical tool to identify MRAs in older patients and a valuable addition to already existing research methods. It is however, at this moment, unclear if the tool can be used as it is in patients younger than 65 years, by other healthcare students (e.g. medical students) and in other countries. The information sources used for the assessment may need to be adjusted for the local situation. To confirm the results of this study, AT-HARM10 would benefit from further validation performed by independent, national and international research groups with different patient populations.
Conclusion
AT-HARM10 has been developed as a practical tool to identify MRAs and the tool is valid for use in older patients by final-year undergraduate and postgraduate pharmacy students.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are sincerely grateful to Dr. Christina Grzechnik Mörk for her contribution as one of the gold standard experts. We would also like to thank all the participating final-year pharmacy students at Uppsala University and the clinical pharmacists at Uppsala University Hospital for their time and valuable input in the development and validation of AT-HARM10.
Funding
This study is part of the MedBridge study which has received governmental research funding from the Uppsala-Örebro Regional Research Council [Grant Numbers RFR-555601, RFR-641791 and RFR-735911], Region Uppsala [Grant Numbers LUL-527721, LUL-614061 and LUL-716201], Region Gävleborg [Grant Numbers CFUG-658451 and CFUG-698771] and Region Västmanland [Grant Numbers LTV-675921, LTV-712341 and LTV-736641]. The study has also received funding from the Swedish Pharmacists Association [Sveriges Farmaceuter], the Thuréus Fund for Geriatric Research [Thuréus stiftelse för främjande av geriatrisk forskning], the Geriatric Fund [Geriatriska fonden] and the Swedish Heart and Lung Association [Riksförbundet HjärtLung; Grant Number FA 2017:38].
Conflicts of interest
All authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koh Y, Kutty FBM, Li SC. Drug-related problems in hospitalized patients on polypharmacy: the influence of age and gender. Ther Clin Risk Manag. 2005;1(1):39–48. doi: 10.2147/tcrm.1.1.39.53597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strand L, Morely P, Cipolle R, Ramsey R, Lamsam G. Drug-related problems: their structure and function. Ann Pharmacother. 1990;24(11):1093–1097. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 3.Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J. 2001;31(4):199–205. doi: 10.1046/j.1445-5994.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan CYL, Yi M, Ling A, Jardine DL. Adverse drug events are a major cause of acute medical admission. Intern Med J. 2014;44(7):633–638. doi: 10.1111/imj.12455. [DOI] [PubMed] [Google Scholar]
- 5.Howard RL. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Heal Care. 2003;12(4):280–285. doi: 10.1136/qhc.12.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahern F, Sahm LJ, Lynch D, McCarthy S. Determining the frequency and preventability of adverse drug reaction-related admissions to an Irish University Hospital: a cross-sectional study. Emerg Med J. 2014;31(1):24–29. doi: 10.1136/emermed-2012-201945. [DOI] [PubMed] [Google Scholar]
- 7.Winterstein AG, Sauer BC, Hepler CD, Poole C, Suárez EC, Kaiser JM. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36(7–8):1238–1248. doi: 10.1345/aph.1A225. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham G, Dodd TR, Grant DJ, McMurdo ME, Richards RM. Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing. 1997;26(5):375–382. doi: 10.1093/ageing/26.5.375. [DOI] [PubMed] [Google Scholar]
- 9.Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol. 2013;112(6):359–373. doi: 10.1111/bcpt.12062. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900. doi: 10.1001/archinternmed.2009.71. [DOI] [PubMed] [Google Scholar]
- 11.Hellström LM, Bondesson Å, Höglund P, Midlöv P, Holmdahl L, Rickhag E, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol. 2011;67(7):741–752. doi: 10.1007/s00228-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 12.Beuscart JB, Knol W, Cullinan S, Schneider C, Dalleur O, Boland B, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16(1):21. doi: 10.1186/s12916-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mjörndal T, Boman MD, Hägg S, Bäckström M, Wiholm B-E, Wahlin A, et al. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11(1):65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson M, Sjölander M, Pfister B, Jonsson J, Schneede J, Lövheim H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. Eur J Clin Pharmacol. 2017;73(7):827–835. doi: 10.1007/s00228-017-2249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond F, Malec J, Buschbader R. Handbook for clinical research: design, statistics, and implementation. New York: Demos Medical Publishing; 2014. pp. 180–191. [Google Scholar]
- 16.Hoonpongsimanont W, Sahota PK, Ng NN, Farooqui MJ, Chakravarthy B, Patel BLS. Research Associates Program: expanding clinical research productivity with undergraduate students. SAGE Open Med. 2017;5:1–7. doi: 10.1177/2050312117730245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streiner D, Norman G. Health measurement scales: a practical guide to their development and use. Oxford: Oxford Scholarship Online; 2008. pp. 1–37. [Google Scholar]
- 18.Peat J, Barton B, Elliot E. Statistics workbook for evidence-based health care. 1. Hoboken: Wiley; 2009. pp. 147–153. [Google Scholar]
- 19.Gallagher RM, Kirkham JJ, Mason JR, Bird KA, Williamson PR, Nunn AJ, et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE. 2011;6(12):e28096. doi: 10.1371/journal.pone.0028096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions: I. Background, description, and instructions for use. JAMA. 1979;242(7):623–632. [PubMed] [Google Scholar]
- 21.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 22.Hallas J, Gram L, Grodum E, Damsbo N, Brosen K, Haghfelt T, et al. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol. 1992;33(1):61–68. doi: 10.1111/j.1365-2125.1992.tb04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. 2016 [cited 2017 Dec 20]. http://www.who.int/medicines%0A/areas/quality_safety/safety_efficacy/WHOcausality%0A_assessment.pdf.
- 24.Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M, et al. Adverse drug reactions as a cause of hospital admissions: a 6-month experience in a single center in Greece. Eur J Intern Med. 2008;19(7):505–510. doi: 10.1016/j.ejim.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Singh H, Kumar BN, Sinha T, Dulhani N. The incidence and nature of drug-related hospital admission: a 6-month observational study in a tertiary health care hospital. J Pharmacol Pharmacother. 2011;2(1):17–20. doi: 10.4103/0976-500X.77095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thevelin S, Spinewine A, Beuscart J, Boland B, Marien S, Vaillant F, et al. Development of a standardized chart review method to identify drug-related hospital admissions in older people. Br J Clin Pharmacol. 2018;84(11):2600–2614. doi: 10.1111/bcp.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempen TGH, Bertilsson M, Lindner K-J, Sulku J, Nielsen EI, Högberg A, et al. Medication Reviews Bridging Healthcare (MedBridge): study protocol for a pragmatic cluster-randomised crossover trial. Contemp Clin Trials. 2017;61:126–132. doi: 10.1016/j.cct.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Hasford J, Göttler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol. 2002;58(4):285–291. doi: 10.1007/s00228-002-0467-0. [DOI] [PubMed] [Google Scholar]
- 29.Marcum ZA, Pugh MJV, Amuan ME, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of potentially preventable unplanned hospitalizations caused by therapeutic failures and adverse drug withdrawal events among older veterans. J Gerontol Ser A Biol Sci Med Sci. 2012;67 A(8):867–874. doi: 10.1093/gerona/gls001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers S, Wilson D, Wan S, Griffin M, Rai G, Farrell J. Medication-related admissions in older people. Drugs Aging. 2012;26(11):951–961. doi: 10.2165/11316750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Meier F, Maas R, Sonst A, Patapovas A, Muller F, Plank-Kiegele B, et al. Adverse drug events in patients admitted to an emergency department: an analysis of direct costs. Pharmacoepidemiol Drug Saf. 2014;24(2):176–186. doi: 10.1002/pds.3663. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet-Zamponi D, D’Arailh L, Konrat C, Delpierre S, Lieberherr D, Lemaire A, et al. Drug-related readmissions to medical units of older adults discharged from acute geriatric units: results of the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2013;61(1):113–121. doi: 10.1111/jgs.12037. [DOI] [PubMed] [Google Scholar]
- 33.Davies EC, Green CF, Mottram DR, Rowe PH, Pirmohamed M. Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol. 2010;70(5):749–755. doi: 10.1111/j.1365-2125.2010.03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juntti-Patinen L, Kuitunen T, Pere P, Neuvonen PJ. Drug-related visits to a district hospital emergency room. Basic Clin Pharmacol Toxicol. 2006;98(2):212–217. doi: 10.1111/j.1742-7843.2006.pto_264.x. [DOI] [PubMed] [Google Scholar]
- 35.Dormann H, Sonst A, Müller F, Vogler R, Patapovas A, Pfistermeister B, et al. Adverse drug events in older patients admitted as an emergency: the role of potentially inappropriate medication in elderly people (PRISCUS) Dtsch Arztebl Int. 2013;110(13):213–219. doi: 10.3238/arztebl.2013.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kongkaew C, Hann M, Mandal J, Williams SD, Metcalfe D, Noyce PR, et al. Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy. 2013;33(8):827–837. doi: 10.1002/phar.1287. [DOI] [PubMed] [Google Scholar]
- 38.Leendertse AJ, Egberts ACG, Stoker LJ, van den Bemt PMLA, HARM Study Group Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890–1896. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- 39.Somers A, Robays H, Vander Stichele R, Van Maele G, Bogaert M, Petrovic M. Contribution of drug related problems to hospital admission in the elderly. J Nutr Heal Aging. 2010;14(6):477–482. doi: 10.1007/s12603-009-0237-0. [DOI] [PubMed] [Google Scholar]
- 40.Dormann H, Neubert A, Criegee-Rieck M, Egger T, Radespiel-Tröger M, Azaz-Livshits T, et al. Readmissions and adverse drug reactions in internal medicine: the economic impact. J Intern Med. 2004;255(6):653–663. doi: 10.1111/j.1365-2796.2004.01326.x. [DOI] [PubMed] [Google Scholar]
- 41.Leendertse AJ, De Koning FHP, Goudswaard AN, Jonkhoff AR, Van Den Bogert SCA, De Gier HJ, et al. Preventing hospital admissions by reviewing medication (PHARM) in primary care: design of the cluster randomised, controlled, multi-centre PHARM-study. BMC Health Serv Res. 2011;11:4. doi: 10.1186/1472-6963-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilbur K, Hazi H, El-Bedawi A. Drug-related hospital visits and admissions associated with laboratory or physiologic abnormalities—a systematic-review. PLoS ONE. 2013;8(6):e66803. doi: 10.1371/journal.pone.0066803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bürkle T, Müller F, Patapovas A, Sonst A, Pfistermeister B, Plank-Kiegele B, et al. A new approach to identify, classify and count drug-related events. Br J Clin Pharmacol. 2013;76(S1):56–68. doi: 10.1111/bcp.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warlé-van Herwaarden MF, Kramers C, Sturkenboom MC, van den Bemt PMLA, De Smet PAGM. Targeting outpatient drug safety: recommendations of the Dutch HARM-Wrestling Task Force. Drug Saf. 2012;35(3):245–259. doi: 10.2165/11596000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Nivya K, Sri Sai Kiran V, Ragoo N, Jayaprakash B, Sonal Sekhar M. Systemic review on drug related hospital admissions—a pubmed based search. Saudi Pharm J. 2015;23(1):1–8. doi: 10.1016/j.jsps.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63(2):136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60(1):34–41. doi: 10.1111/j.1532-5415.2011.03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowyer P, Lee J, Kramer J, Taylor R, Kielhofner G. Determining the clinical utility of the Short Child Occupational Profile (SCOPE) Br J Occup Ther. 2012;1:19–26. [Google Scholar]
- 49.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76(5):378–382. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.