Abstract
Purpose
The safety and clinical efficacy of WT1 human leukocyte antigen (HLA) class I peptide vaccine have been established, but the safety of a cocktail vaccine of WT1 HLA class I and II peptides has not. To verify its safety, we performed a phase I clinical trial for patients with recurrent malignant gliomas and assessed the immunological responses and survival data.
Patients and methods
Fourteen HLA-A*24:02-positive patients with recurrent malignant glioma (2 with grade 3, 12 with grade 4) were enrolled. Every week, the patients received alternately a vaccine containing 3 mg of WT1 HLA-A*24:02-restricted (HLA class I) peptide and a cocktail vaccine of the HLA class I peptide and one of 0.75, 1.5 or 3 mg of the WT1 HLA class II peptide. For patients who showed no significant adverse effects within 6 weeks, the WT1 vaccine was continued at 2–4-week intervals.
Results
Eleven of the 14 patients completed WT1 vaccination for 6 weeks, while 3 patients dropped out earlier due to disease progression. All patients showed grade I level of skin disorders at the injection sites. No grade III/IV toxicity or dose-limiting toxicity was observed for any dose of WT1 HLA class II peptide. Six of the 14 patients had stable disease at 6 weeks. Median OS and 1-year OS rates were 24.7 weeks and 36%, respectively.
Conclusion
The safety of a cocktail vaccine of WT1 HLA class I and II peptides for malignant gliomas was verified. This vaccine is, therefore, considered promising for patients with recurrent malignant glioma.
Keywords: WT1, Peptide vaccine, Cancer vaccine, Cancer immunotherapy, HLA class II peptide, Malignant glioma
Introduction
Prognosis of recurrent glioblastoma multiforme (GBM) is extremely poor and treatment options are limited. Extensive research to develop new treatments for recurrent GBM is ongoing [1–3], and immunotherapies have proven to be promising.
Immunotherapies for malignant gliomas have been tried in the form of several procedures such as monoclonal antibody therapy, tumor-associated antigen (TAA) peptide-based dendritic cell or vaccine immunotherapies, and checkpoint inhibitors [4–10]. Advancements in tumor immunology have resulted in the identification of TAAs that can be used for cancer vaccines, in which the TAA-derived epitopes are combined with HLA class I molecules, followed by recognition by CD8+ cytotoxic T lymphocytes (CTLs). One of the identified TAAs was the product of Wilms’ tumor gene 1(WT1) [11–14].
The wild-type WT1 gene has been shown to be overexpressed in hematological malignancies and almost all solid tumors [15, 16], while gliomas, as well as other solid tumors, have been found to express the WT1 protein [17]. A definite correlation has been established between WT1 expression and cellular proliferation activity as assessed by WHO grade [18]. The wild-type WT1 gene functions as an oncogene [15, 16], which suggests that tumor escape via downregulation of WT1 is unlikely to occur.
We previously performed a phase I/II clinical trial to examine the safety of as well as the clinical and immunological responses to a WT1 HLA class I peptide vaccine for HLA-A*24:02-positive patients with myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and breast and lung cancers [19, 20]. We were able to demonstrate that the WT1 peptide vaccine emulsified with Montanide ISA51 adjuvant was safe for patients with normal hematopoiesis, and that the induction of WT1 peptide-specific CD8+ CTLs was associated with clinical response. Furthermore, we performed a phase II clinical trial of the same vaccine for HLA-A*24:02-positive patients with recurrent glioblastoma multiforme (GBM) and again showed that it was safe with more favorable clinical outcomes than obtained with previously reported approaches for recurrent GBM [21].
CD4+ T cells, which recognize antigenic peptides in the context of major histocompatibility complex (MHC) class II molecules, have a helper function for priming, regulating, and maintaining CD8+ CTLs [22], and for guiding them to tumor sites, where they can directly kill tumor cells in an MHC class II-restricted manner [23]. One of the reasons for the transient clinical effect of HLA class I peptide vaccine may be that the CD8+ CTLs elicited by the HLA class I peptide could not be sufficiently stimulated by the peptide-specific CD4+ helper T cells and thus failed to become memory-type CTLs [22]. Simultaneous induction of the TAA-specific CTLs and CD4+ helper T cells is, therefore, thought to be crucial for the achievement of sufficient clinical effect.
We previously identified a WT1 protein-derived 16-mer HLA class II peptide, WT1332 (KRYFKLSHLQMHSRKH), which can elicit WT1332-specific CD4+ T-cell responses, and demonstrated that this WT1332 peptide was endogenously generated from the WT1 protein in WT1-expressing tumor cells. Dendritic cells were found to take up apoptotic WT1-expressing tumor cells, and present the WT1332 peptide on their cell surface in association with HLA class II molecules [24–26]. Furthermore, the WT1332 peptide could induce WT1332-specific T-helper cell type 1(Th1)-type CD4+ T cells, resulting in an increase in WT1-specific CD8+ CTLs in vitro. Importantly, WT1332-specific CD4+ T cells could not only aid the expansion of WT1-specific CD8+ CTLs but also directly kill HLA class II-positive WT1-expressing tumor cells [26]. In a recent phase I clinical study of the WT1332 peptide vaccine (OCV-501), OCV-501 containing 0.3, 1.0, or 3.0 mg of the peptide was subcutaneously administered on a weekly basis to elderly patients with AML in complete remission for 4 weeks. This study demonstrated that OCV-501 was safe and well tolerated, and that WT1332 peptide-specific delayed-type hypersensitivity occurred as an immunological response [27].
We designed a phase I clinical study to investigate the dose-limiting toxicity of the WT1 class II peptide in a cocktail vaccine of WT1 HLA class I and class II peptides. In this article, we report on the safety of this cocktail vaccine for patients with recurrent malignant glioma, as well as on WT1-specific immunological responses and clinical outcomes.
Clinical materials and methods
Patient population
Patients from 20 to 80 years old with malignant glioma were eligible for enrolment if they were resistant to conventional chemotherapy. Other inclusion criteria were: (1) immunohistochemically determined positive WT1 expression in glioma cells; (2) the presence of HLA-A*24:02 and at least one of HLA-DRB1*04:05, *15:01, *15:02, *08:03, HLA-DPB1*09:01, and *05:01; (3) performance status of 0–2 (Eastern Cooperative Oncology Group); (4) no severely impaired organ function; (5) no administration of chemotherapy, immunotherapy, immunosuppressive therapy, or radiotherapy within 4 weeks prior to WT1 vaccination.
WT1 peptides
A cocktail vaccine consisting of a mixture of HLA-A*24:02-restricted modified WT1-peptide (WT1235: amino acids 235–243; CYTWNQMNL, abbreviated as WT1 HLA class I peptide) [28] and HLA class II-restricted WT1 peptide (WT1332: amino acids 332–347; KRYFKLSHLQMHSRKH, abbreviated as WT1 HLA class II peptide) emulsified with Montanide ISA 51 adjuvant was used as the WT1 peptide vaccine (Fig. 1).
Fig. 1.
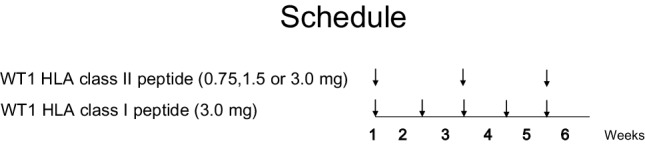
Schedule of WT1 peptide vaccination
HLA-A*2402-restricted, modified 9-mer WT1-peptide was substituted for methionine (M) to tyrosine (Y) at amino acid position 2 (the anchor position) of the natural WT1 peptide (CMTWNQMNL). This modification resulted in a much stronger CTL activity against WT1-expressing tumor cells than that of the natural peptide [28]. The WT1 peptides (GMP grade) were purchased from Neo MPS Inc. (San Diego, CA, USA) or the Peptide Institute (Osaka, Japan) in the form of lyophilized peptides.
Vaccination design
The WT1 HLA class II peptide was dose escalated from 0.75 to 1.5 and then to 3.0 mg/body while the doses of the WT1 HLA class I peptide remained at 3.0 mg. The three plus-three cohort design was used. Patients received an intradermal administration of a cocktail vaccine comprising 3 mg of WT1 HLA class I peptide and one of 0.75, 1.5 or 3.0 mg of WT1 HLA class II peptide as the vaccine, followed by a vaccine containing 3 mg of WT1 HLA class I peptide in the second week. Administration of the cocktail vaccine and the WT1 HLA class I peptide vaccine was alternated every week (Fig. 1).
The observation period for determination of safety was set at 6 weeks from the first administration of the WT1 vaccine. If the safety was confirmed during this period, WT1 vaccination was continued at 2–4-week intervals with the patients’ informed consent.
Endpoints
The primary endpoint was safety. Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Dose-limiting toxicity (DLT) was defined as any of the following adverse events occurring during the 6-week period from the start of the vaccination: grade 4 hematological toxicity lasting more than 7 days, grade 3 or worse neutropenia accompanied by high fever (> 38 °C) (febrile neutropenia), and any non-hematological toxicity of grade 3 or worse in other organ systems, including reactions at the vaccine injection sites. These adverse events were then judged as possibly, probably, or definitely related to treatment.
Secondary endpoints were overall survival (OS) time and WT1-specific immunological responses.
WT1-specific immunological response
DTH
For immunological monitoring, delayed-type hypersensitivity (DTH) to either WT1 HLA class I or II peptides was examined by intradermally injecting 0.05 ml of a saline solution of either WT1 HLA class I or II peptide (200 µg/ml). A positive test result was defined as > 2 mm induration and/or eruption within 48 h.
Tetramer assay
Frozen peripheral blood mononuclear cells (PBMCs) from patients were thawed, incubated for 1 h at 37 °C in X-VIVO 15 medium (Lonza Inc., Walkersville, MD) supplemented with 10% AB serum (Gemini Bio-Products, West Sacramento, CA), and then passed through 40-µm nylon mesh to remove debris.
The cells were then incubated with Clear Back (MBL Co. Ltd., Nagoya, Japan) in phosphate buffered saline containing 5% FBS and 0.02% sodium azide (fluorescence-activated cell sorting (FACS) buffer) at room temperature for 5 min, stained with PE-labeled HLA-A*24:02/WT1235 mutant tetramer (WT1235 tetramer) (MBL) for 1 h at 4 °C, and then further stained with anti-CD3, -CD8 and -CD4 antibodies for 25 min at 4 °C in the dark. Next, the cells were washed three times, re-suspended in an appropriate volume of FACS buffer, incubated with 7-AAD (eBioscience, San Diego, CA) for 5 min before analysis, and analyzed with FACSAria (BD Biosciences, Franklin Lakes, NJ). The data were analyzed with FlowJo software (Tree Star, Ashland, OR). The frequency of WT1-specific T cells was calculated as WT1-tetramer+ CD8+ T cells/CD8+ T cells.
The monoclonal antibodies (mAbs) used for flow cytometric analysis were anti-CD3-Pacific Blue, anti-CD3-V500, anti-CD4-V500, anti-CD4-APC-H7, anti-CD8-FITC (Beckman Coulter, Brea, CA), anti-TNFα-APC and anti-IFNγ-PE (eBioscience).
Tetramer and intracellular cytokine staining assays
PBMCs were suspended in 2 ml of X-VIVO 15 medium supplemented with 10% AB serum, 40 IU/ml of human recombinant IL-2 (kindly provided by Shionogi & Co., Ltd., Osaka, Japan), 1 µg/ml of wild-type WT1235 peptide and 20 µg/ml of WT1332 helper peptide, plated into a 24-well plate, cultured for 1 week and then used for tetramer and intracellular cytokine staining assays. Statistical analysis was performed by means of the Mann–Whitney’s U test.
WT1 peptides-specific intracellular cytokine staining assay
PBMCs were incubated with 1 µg/ml of WT1 HLA class I peptide or 10 µg/ml of WT1 HLA class II peptide in the presence of 2 µg/ml CD28/CD49d Costimulatory Reagent (BD Biosciences) and 10 µg/ml Brefeldin A (Sigma, St Louis, MO) for 5 h. Intracellular cytokine staining was performed using BD Cytofix/Cytoperm Buffer (BD Biosciences) according to the manufacturer’s recommended procedures after surface staining of CD3, CD4 and CD8. The cells were then analyzed with FACSAria and the data with FlowJo software.
Results
Patient characteristics and safety assessment
This study’s enrolment comprised 14 patients with a median age of 48 years, all of whom were WT1 vaccinated (Fig. 1). Twelve of them had glioblastoma multiforme (GBM) and two had anaplastic astrocytoma. These malignant gliomas were refractory to temozolomide. To prevent brain edema, ten patients with neurological symptoms were treated with steroids, while the remaining four patients with PS 0 were not given steroids.
Three of the patients were vaccinated with 0.75 mg, four with 1.5 mg, and four with 3.0 mg of WT1 HLA class II peptide, as well as with 3.0 mg of WT1 HLA class I peptide for all 14 subjects. All vaccinations were completed in 6 weeks (Table 1). Evaluation for safety showed that 11 patients did not have DLT as the primary endpoint, while three patients dropped out within 6 weeks due to disease progression. Grade I skin eruption was observed at the injection sites in all patients, but no grade III/IV hematological or non-hematological toxicities were detected for any dosage of WT1 HLA class II peptide during the 6-week period.
Table 1.
Patients’ characteristics
| Patient no. | Age | Sex | Diseases | Operation | MGMT methylation | Pretreatment | Status before WT1 vaccine | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CR/relapse | Dissemination | Steroid | PS | |||||||
| 1 | 45 | F | GBM | Resection | N.D | RT/TMZ, CARE | Relapse | (−) | (−) | 1 |
| 2 | 39 | F | GBM | Resection | N.D | RT/TMZ, ACNU | Relapse | (−) | (+) | 1 |
| 3 | 61 | M | GBM | Resection | N.D | RT/TMZ | Relapse | (−) | (+) | 1 |
| 4 | 64 | F | GBM | Resection | N.D | RT/TMZ, IFN | Relapse | (−) | (−) | 1 |
| 5 | 55 | F | GBM | Resection | N.D | RT/TMZ, PAV | Relapse | (−) | (−) | 0 |
| 6 | 58 | M | GBM | Resection | (+) | RT/TMZ | Relapse | (−) | (−) | 0 |
| 7 | 31 | M | A.A | – | N.D | RT/TMZ | Relapse | (−) | (−) | 0 |
| 8 | 55 | F | GBM | Resection | N.D | RT/TMZ | Relapse | (−) | (+) | 1 |
| 9 | 38 | M | A.A | – | (−) | RT/TMZ, CARE | Relapse | (−) | (−) | 1 |
| 10 | 44 | M | GBM | Resection | N.D | RT/TMZ | Relapse | (−) | (+) | 1 |
| 11 | 53 | M | GBM | Resection | (−) | RT/TMZ | Relapse | (−) | (−) | 0 |
GBM glioblastoma multiforme, AA anaplastic astrocytoma, MGMT O6-methylguanine-DNA methyltransferase, N.D not determined, RT radiation therapy, TMZ temozolomide, IFN interferon, ACNU nimustine hydrochloride, CARE carboplatin and etoposide, PAV procarbazine, ACNU and vincristine, PS ECOG performance score
Clinical outcomes
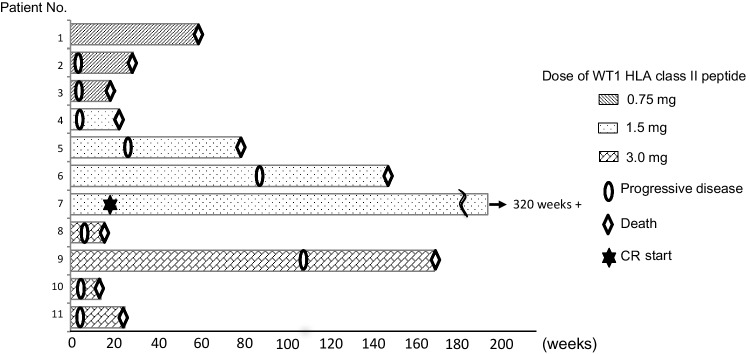
Six of the 14 patients had stable disease 6 weeks after WT1 vaccination. The median OS time and 1-year OS rate were 24.7 weeks and 36%, respectively (Table 2). Figure 2a shows swimmer plot of clinical outcomes for each patient.
Table 2.
WT1-specific immune and clinical responses
| Patient no. | Doses (mg) | Times | AE | DTH (mm) 235/332 | WT1 tetramer+CD8* T cells/ CD8*T cells (%)a | IFN-y-producing CD8*T cells/ CD8*T cells (%)a | TNF-a-producing CD4+T cells/CD4*T cells (%)a | Clinical responses | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 4–8 weeks | 10 months–1 year 3 months | Pre | 4–8 weeks | 10 months–1 year 3 months | Pre | 4–8 weeks | 10 months–1 year 3 months | 6 weeks | 12 weeks | PFS (weeks) | OS (weeks) | |||||
| 1 | 0.75 | 30 | (−) | –/3 | 0.03 | 1.25 | 1.3 | 1.05 | 0.33 | 6.72 | SD | SD | 58.4 | 58.4 | |||
| 2 | 0.75 | 21 | (−) | –/– | 0.76 | 43.9 | 0 | 39.8 | 0 | 48.3 | PD | PD | 4 | 28.5 | |||
| 3 | 0.75 | 11 | (−) | 4/6 | 0.09 | 2.55 | 0 | 2.2 | 0 | 26.7 | PD | PD | 4 | 18.4 | |||
| 4 | 1.5 | 10 | (−) | –/– | 0.29 | 0.31 | 0 | 0.03 | 0.33 | 0.39 | PD | PD | 4 | 22.2 | |||
| 5 | 1.5 | 35 | (−) | 3/– | 0.05 | 1.06 | 0.14 | 0 | 2 | 0.68 | 0.87 | 3.7 | 1.96 | SD | SD | 23.7 | 77.1 |
| 6 | 1.5 | 49 | (−) | –/3 | 0.02 | 2.50 | 0.36 | 0.25 | 12.11 | 2.18 | 0.16 | 12.69 | 8.73 | SD | SD | 84.5 | 144.5 |
| 7 | 1.5 | 63 | (−) | –/– | 0.04 | 11.6 | 28.00 | 0.16 | 4.79 | 21.33 | 0.04 | 41.4 | 41.5 | SD | SD | > 320.1 | > 320.1 |
| 8 | 3 | g | (−) | –/– | 0.03 | 0.27 | 0 | SD | PD | 8.8 | 15.7 | ||||||
| 9 | 3 | 49 | (−) | –/– | 0.09 | 0.50 | 0.14 | 0 | 0.15 | 0.2 | 0.17 | 2.34 | 10.23 | SD | SD | 105.3 | 165.8 |
| 10 | 3 | 7 | (−) | 4/– | 0.1 | 5.42 | 0.23 | 6.08 | 0.01 | 17.21 | PD | PD | 4 | 13.9 | |||
| 11 | 3 | 25 | (−) | 3/3 | 0.08 | 2.03 | 0 | 0.53 | 0.04 | 7.52 | PD | PD | 4.2 | 24.7 | |||
DTH delayed-type hypersensitivity, Doses doses of WT1 class II peptide, Times times of WT1 vaccine, AE adverse effect, SD stable disease, PD progressive disease
aFrequencies of WT1 tetramer+ CD8+ T cells, IFN-γ-producing CD8+ T cells, and IFN-α-producing CD4+ T cells were analyzed after 1-week culture of PBMCs
Fig. 2.
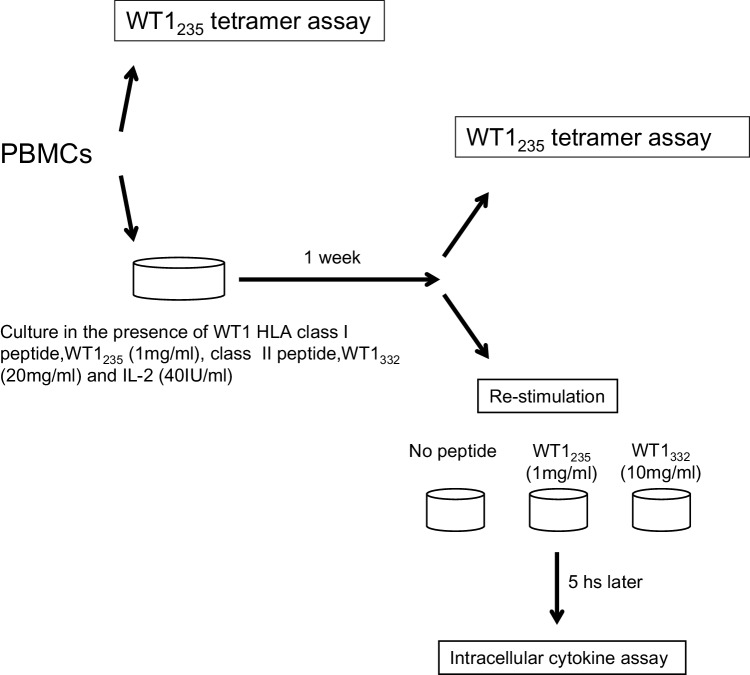
Schema for WT1 tetramer and intracellular cytokine assays. PBMC samples were divided in two, and one part was used for the WT1 tetramer assay. The other part was cultured in the presence of WT1 HLA class I and II peptides, and IL-2 for 1 week and then used for the WT1 tetramer and intracellular cytokine assays after re-stimulation with each of the WT1 peptides
Immunological responses and their possible associations with clinical outcomes
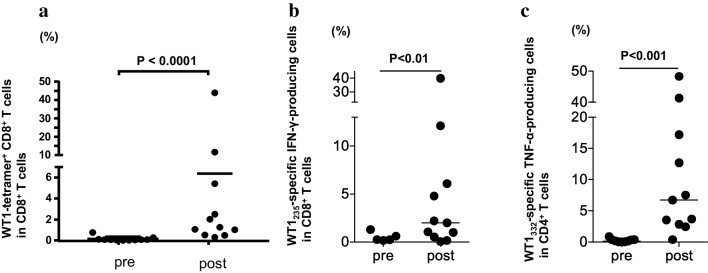
Frequency of WT1-specific CD8+ T cells in PBMCs was assayed using the WT1 tetramer, once just after sampling and again after the culture for 1 week, in the presence of WT1 HLA class I and II peptides, and of IL-2 (Fig. 3). Frequency of WT1-specific CD8+ T cells after the culture for 1 week is shown in Fig. 4a and Table 2 for each patient both before WT1 vaccination and 4–8 weeks after WT1 vaccination. WT1 tetramer+ CD8+ T cells had markedly increased 4–8 weeks after WT1 vaccination in 7 of the 11 patients who had completed the 6-week WT1 vaccination schedule.
Fig. 3.
A swimmer plot of clinical outcomes for each patient
Fig. 4.
Induction of WT1-specific T cells by WT1 peptide vaccination. a Induction of WT1 tetramer+ CD8+ T cells by WT1 peptide vaccination. The WT1 tetramer assay was performed after culture for 1 week in the presence of WT1 HLA class I and II peptides, and IL-2. Frequencies of WT1 tetramer+ CD8+ T cells in CD8+ T cells after culture for 1 week are shown for each patient pre- and 4–8 weeks after WT1 vaccination. b, c Analysis of WT1 HLA class I-specific cytokine-producing CD8+ T cells and class II peptide-specific cytokine-producing CD4+ T cells. Frequencies of WT1 HLA class I (WT1235)-specific IFN-γ-producing CD8+ T cells (left) and WT1 HLA class II (WT1332)-specific TNF-α-producing CD4+ T cells (right) were examined by means of re-stimulation with each WT1 peptide after a 1-week culture in the presence of WT1 HLA class I (WT1235) and II (WT1332) peptides, and IL-2. The frequencies are shown for each patient pre- and 4–8 weeks post-WT1 vaccination
WT1 HLA class I peptide (WT1235)-specific IFN-γ-producing CD8+ T cells had increased markedly in 6 of the 11 patients (Fig. 4b), while WT1 HLA class II peptide (WT1332)-specific TNF-α-producing CD4+ T cells showed a marked increase in 10 of 11 patients 4–8 weeks after WT1 vaccination (Fig. 4c).
These results demonstrated that both WT1 HLA class I peptide (WT1 235)-specific CD8+ T cells and WT1 HLA class II peptide (WT1332)-specific CD4+ T cells were induced by the cocktail vaccine of WT1 HLA class I and II peptides.
DTH of all patients for WT1 HLA class I and II peptides was negative before WT1 peptide vaccination, but in 6 weeks had become positive for WT1 HLA class I peptide alone in two, for WT1 class II peptide alone in two, and for both WT1 HLA class I and II peptides in two among 11 patients (Table 2).
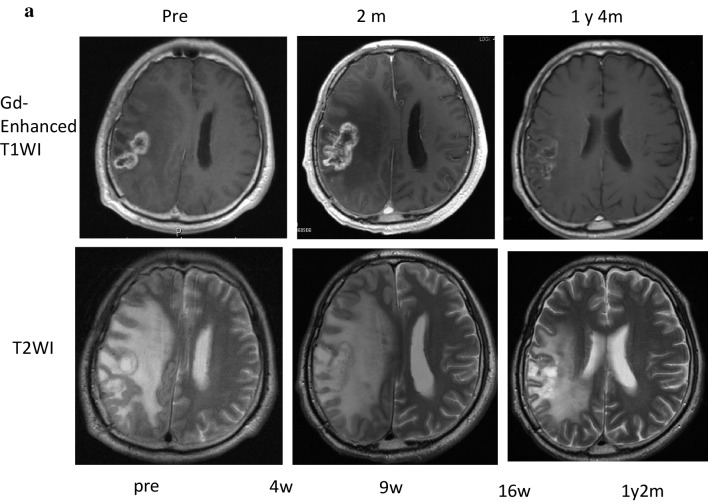
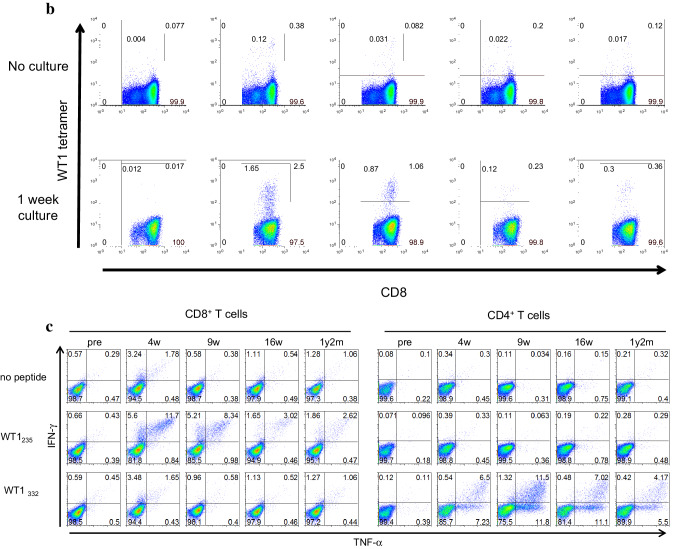
The clinical course and immune-monitoring of patient 6 with GBM in the right parietal lobe are shown in Fig. 5. The patient received partial resection of the GBM, followed by combination therapy of radiation and temozolomide (TMZ), and two subsequent courses of maintenance therapy with TMZ. However, since this patient was suffering from a progressive disease, he was enrolled in this clinical study and received a total of 49 WT1 vaccinations over 1 year and 9 months. His disease was kept stable for about 1 year, and after that reduction in the Gd-enhanced lesion and improvement in the midline shift on MRI were observed (Fig. 5a). After that, however, he had recurrence near the original tumor 1 year and 9 months after the WT1 vaccination. Frequency of WT1 tetramer+ CD8+ T cells had notably increased from 0.017 to 2.5% 4 weeks after the WT1 vaccination and a high frequency of 0.36% remained for 1 year and 2 months after the vaccination (Fig. 5b).
Fig. 5.
Clinical course and immune-monitoring of patient 6. a Magnetic resonance imaging (MRI) results are shown at three time points: pre-, 2 months (2 m) and 1 year and 4 months (1 y 4 m) post-WT1 vaccination. Upper and lower columns show Gadolinium (Gd)-enhanced weighted imaging (Gd-enhanced WI) and T2 weighted imaging (T2WI), respectively. FACS analysis for WT1 tetramer FACS analysis for WT1 tetramer+ CD8+ T cells. Dot blots of WT1 tetramer+ CD8+ T cells are shown pre- and at 4 weeks, 9 weeks, 16 weeks, and 1 year and 2 months post-WT1 vaccination. Upper and lower columns show tetramer assay without and with 1-week culture of PBMCs, respectively. c FACS analysis of cytokine-producing CD8+ and CD4+ T cells. Dot blots of IFN-γ- and/or -TNF-α-producing CD8+ and CD4+ T cells are shown pre- and at 4 weeks, 9 weeks, 16 weeks, and 1 year and 2 months post-WT1 vaccination. PBMCs were not re-stimulated or re-stimulated with either WT1235 or WT1332 peptide and analyzed
CD8+ and CD4+ T cells of patient 6 produced both interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) in response to WT1 HLA class I (WT1235) and II (WT1332) peptides, respectively, both time at 4 weeks, and 1 year and 2 months post-WT1 vaccination (Fig. 5c).
All four patients who survived over 1 year maintained high frequencies of WT1-specific CD8+ T cells and Th1-typed CD4+ T cells during about 1 year of WT1 vaccination (Table 2).
Discussion
This is the first report of use of a cocktail vaccine of WT1 HLA class I and II peptides for the treatment of malignancies. In this phase I study, we evaluated first the safety and second the efficacy of WT1 peptide vaccine for patients with recurrent malignant glioma. Adverse events of grade III/IV were not observed and this therapy was well tolerated at any dose of the WT1 HLA class II peptide. Thus, the safety of the cocktail vaccine of WT1 HLA class I and II peptides was confirmed.
We previously treated hematopoietic malignancies including leukemia and various kinds of solid tumors with WT1 HLA class I peptide vaccine and reported that the vaccination elicited WT1-specific immunological response, followed by clinical responses without severe adverse effects [29]. One of the most important problems of WT1 vaccination with WT1 HLA class I peptide alone was that WT1-specific CD8+ T cells generally increased at 4–8 weeks post-WT1 vaccination, but afterwards decreased gradually, and their frequency could not be maintained for a long time (data not shown). It should be noted that, compared with WT1 vaccination with the WT1 HLA class I peptide alone, a cocktail vaccine with WT1 HLA class I and II peptides tended to result in a powerful induction of WT1-specific CD8+ T cells 4–8 weeks after WT1 vaccination and in long-term maintenance of a high frequency of WT1-specific CD8+ T cells (> 0.1%) and Th1-typed WT1-specific CD4+ T cells for about 1 year, as observed in four long-term survivors who were treated with the cocktail vaccine (Table 2).
We previously reported that WT1332 peptide in vitro could induce WT1332-specific Th1-type CD4+ T cells which caused an increase in WT1-specific CD8+ CTLs [24]. In the study reported here, the cocktail vaccine of WT1 HLA class I and II peptides in vivo induced, as expected, WT1 HLA class I peptide (WT1235)-specific CD8+ CTLs and class II peptide (WT1332)-specific IFN-γ-producing Th1-type CD4+ T cells. We assume that the induction of WT1 class II peptide-specific Th1-type CD4+ T cells generated the strong and long-term maintenance of WT1-specific CD8+ T cells.
Dendritic cell (DC) vaccines using WT1 HLA class I and II peptides (DC/WT1-I/II) have recently been administered by several institutes, and it was reported that they were well tolerated and induced WT1-specific immune responses and clinical effects [30–33]. Homma et al. performed a phase I clinical study (DC/WT1-I/II) of treatment for patients with pancreatic ductal adenocarcinoma using DCs pulsed with a mixture of the same WT1 HLA class I and II peptides as those used in our study, combined with chemotherapy [30]. They demonstrated that the combination therapy was well tolerated and WT1-specific IFN-γ-producing CD4+ T cells increased significantly following treatment with DC/WT1-I/II. WT1 peptide-specific DTH was detected in four of the seven patients vaccinated with DC/WT1-I/II while the WT1-specific DTH-positive patients showed a significant improvement in OS and progression-free survival (PFS) compared with the historical control (chemotherapy only) patients [30]. Safety, enhanced immunological responses, and favorable clinical outcomes observed in our study seem to be consistent with the results of previous studies of these DC vaccine therapies.
The data accumulated so far demonstrate that WT1 vaccine induce clinical effects in association with the induction of WT1-specific CTLs, but do not cause damage to WT1-expressing normal tissues. The question of why the WT1 vaccine does not damage WT1-expressing normal cells is very important. Two possible explanations are that, first, normal WT1-expressing cells are ignored because the expression of WT1 peptide/MHC complexes on the cell surface is too little to be recognized by WT1-specific CTLs. The second explanation is that normal WT1-expressing cells possess as yet unknown protective mechanisms against WT1-specific CTL-mediated cytotoxicity. Although the cocktail vaccine used in our study induced stronger WT1-specific CTL responses than those induced by WT1 HLA class I peptide alone, no toxicity for normal tissue was observed, which is an important point for the use of this vaccine in clinical settings.
Abbreviations
- AML
Acute myeloid leukemia
- CTL
Cytotoxic T lymphocyte
- DLT
Dose-limiting toxicity
- DTH
Delayed-type hypersensitivity
- FACS
Fluorescence-activated cell sorting
- GBM
Glioblastoma multiforme
- HLA
Human leukocyte antigen
- IFN
Interferon
- mAbs
Monoclonal antibodies
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
- TAA
Tumor-associated antigen
- Th1
T-helper cell type 1
- TMZ
Temozolomide
- TNF
Tumor necrosis factor
- WT1
Wilms’ tumor gene 1
Author contributions
AT and HS organized this study and prepared this manuscript. NH and NK recruited the patients and evaluated clinical responses. FF, SM and HN analyzed WT1-specific immune responses. NH, SN, JN, YO and YO supported this study and gave the useful suggestions. SM and JS advised about statistical analysis.
Funding
This study was supported by the Grants-in-Aid for Scientific Research of Japan Society for the promotion of Science, Grant number 26462179.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was registered on University Hospital Medical Information Network (UMIN) Center and trial number was UMIN000003506. This clinical study was approved in 2010 by the Ethical Review Board of the Faculty of Medicine, Osaka University and approval number was 09248. This study was performed according to Helsinki declaration.
Informed consent
Informed consent was obtained from each enrolled patient.
References
- 1.Lau D, Magill ST, Aghi MK. Molecularly targeted therapies for recurrent glioblastoma: current and future targets. Neurosurg Focus. 2014;37(6):E15. doi: 10.3171/2014.9.FOCUS14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selznick LA, Shamji MF, Fecci P, Gromeier M, Friedman AH, Sampson J. Molecular strategies for the treatment of malignant glioma—genes, viruses, and vaccines. Neurosurg Rev. 2008;31(2):141–155. doi: 10.1007/s10143-008-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin VA, Mendelssohn ND, Chan J, Stovall MC, Peak SJ, Yee JL, Hui RL, Chen DM. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol. 2015;11:145–150. doi: 10.1007/s11060-014-1693-x. [DOI] [PubMed] [Google Scholar]
- 4.Weathers SP, Gilbert MR. Current challenges in designing GBM trials for immunotherapy. J Neurooncol. 2015;11:331–337. doi: 10.1007/s11060-015-1716-2. [DOI] [PubMed] [Google Scholar]
- 5.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AA, Fisher JL, Ernstoff MS, Fadul CE. Vaccine-based immunotherapy for glioblastoma. CNS Oncol. 2013;2(4):331–349. doi: 10.2217/cns.13.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polyzoidis S, Tuazon J, Brazil L, Beaney R, Al-Sarraj ST, Doey L, Logan J, Hurwitz V, Jarosz J, Bhangoo R, Gullan R, Mijovic A, Richardson M, Farzaneh F, Ashkan K. Active dendritic cell immunotherapy for glioblastoma: current status and challenges. Br J Neurosurg. 2015;29:197–205. doi: 10.3109/02688697.2014.994473. [DOI] [PubMed] [Google Scholar]
- 8.Yu JS, Liu G, Ying H, Yong WH, Black KL, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, et al. Clinical evaluation of personalized peptide vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 10.Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen-A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol. 2010;29:337–344. doi: 10.1200/JCO.2010.29.7499. [DOI] [PubMed] [Google Scholar]
- 11.Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H, et al. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol. 2000;164:1873–1880. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 12.Oka Y, Tsuboi A, Elisseeva OA, Udaka K, Sugiyama H. WT1 as a novel target antigen for cancer immunotherapy. Curr Cancer Drug Targets. 2002;2:45–54. doi: 10.2174/1568009023334088. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Bellantuono I, Elsässer A, Marley SB, Gordon MY, Goldman JM, Stauss HJ. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95(7):2198–2203. [PubMed] [Google Scholar]
- 14.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95(1):286–293. [PubMed] [Google Scholar]
- 15.Sugiyama H. Wilms tumor gene WT1 as a tumor marker for leukemic blast cells and its role in leukemogenesis. Methods Mol Med. 2002;68:223–237. [PubMed] [Google Scholar]
- 16.Oji Y, Ogawa H, Tamaki H, Oka Y, Tsuboi A, Kim EH, Soma T, Tatekawa T, Kawakami M, Asada M, Kishimoto T, Sugiyama H. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90(2):194–204. doi: 10.1111/j.1349-7006.1999.tb00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S, et al. Overexpression of the Wilms’ tumor gene WT1 in primary astrocytic tumors. Cancer Sci. 2004;95:822–827. doi: 10.1111/j.1349-7006.2004.tb02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba Y, Hashimoto N, Tsuboi A, Rabo C, Oka Y, Kinoshita M, Kagawa N, Oji Y, Sugiyama H, Yoshimine T Prognostic value of WT1 protein expression level and MIB-1 staining index as predictor of response to WT1 immunotherapy in glioblastoma patients. Brain Tumor Pathol. 2010;27(1):29–34. doi: 10.1007/s10014-010-0265-9. [DOI] [PubMed] [Google Scholar]
- 19.Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita S, Oka Y, Tsuboi A, Kawakami M, Maruno M, Izumoto S, Osaki T, Taguchi T, Ueda T, Myoui A, Nishida S, Shirakata T, Ohno S, Oji Y, Aozasa K, Hatazawa J, Udaka K, Yoshikawa H, Yoshimine T, Noguchi S, Kawase I, Nakatsuka S, Sugiyama H, Sakamoto J. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Jpn J Clin Oncol. 2006;36(4):231–236. doi: 10.1093/jjco/hyl005. [DOI] [PubMed] [Google Scholar]
- 21.Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Yang MC, Knoff J, Wu TC, Hung CF. Cancer immunotherapy employing an innovative strategy to enhance CD4+ T cell help in the tumor microenvironment. PLoS One. 2014;9(12):e115711. doi: 10.1371/journal.pone.0115711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong TD, Clements VK, Ostrand-Rosenberg S. MHC class II-transfected tumor cells directly present antigen to tumor-specific CD4+ T lymphocytes. J Immunol. 1998;160:661–666. [PubMed] [Google Scholar]
- 24.Fujiki F, Oka Y, Tsuboi A, Kawakami M, Kawakatsu M, Nakajima H, Elisseeva OA, Harada Y, Ito K, Li Z, Tatsumi N, Sakaguchi N, Fujioka T, Masuda T, Yasukawa M, Udaka K, Kawase I, Oji Y, Sugiyama H. Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother. 2007;30(3):282–293. doi: 10.1097/01.cji.0000211337.91513.94. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Niiya H, Azuma T, Uchida N, Yakushijin Y, Sakai I, Hato T, Takahashi M, Senju S, Nishimura Y, Yasukawa M. Direct recognition and lysis of leukemia cells by WT1-specific CD4+ T lymphocytes in an HLA class II-restricted manner. Blood. 2005;106(4):1415–1418. doi: 10.1182/blood-2005-01-0413. [DOI] [PubMed] [Google Scholar]
- 26.Fujiki F, Oka Y, Kawakatsu M, Tsuboi A, Nakajima H, Elisseeva OA, Harada Y, Li Z, Tatsumi N, Kamino E, Shirakata T, Nishida S, Taniguchi Y, Kawase I, Oji Y, Sugiyama H. A WT1 protein-derived, naturally processed 16-mer peptide, WT1(332), is a promiscuous helper peptide for induction of WT1-specific Th1-type CD4(+) T cells. Microbiol Immunol. 2008;52(12):591–600. doi: 10.1111/j.1348-0421.2008.00080.x. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Sakura T, Miyawaki S, Toga K, Sogo S, Heike Y. A new peptide vaccine OCV-501: in vitro pharmacology and phase 1 study in patients with acute myeloid leukemia. Cancer Immunol Immunother. 2017;66(7):851–863. doi: 10.1007/s00262-017-1981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuboi A, Oka Y, Udaka K, Murakami M, Masuda T, et al. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol Immunother. 2002;51:614–62030. doi: 10.1007/s00262-002-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oka Y, Tsuboi A, Nakata J, Nishida S, Hosen N, Kumanogoh A, Oji Y, Sugiyama H. Wilms’ Tumor Gene 1 (WT1) peptide vaccine therapy for hematological malignancies: from CTL epitope identification to recent progress in clinical studies including a cure-oriented strategy. Oncol Res Treat. 2017;18(11):682–69031. doi: 10.1159/000481353. [DOI] [PubMed] [Google Scholar]
- 30.Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, Uchiyama K, Kajihara M, Arakawa H, Misawa T, Toyama Y, Yanagisawa S, Ikegami M, Kan S, Hayashi K, Komita H, Kamata Y, Ito M, Ishidao T, Yusa S, Shimodaira S, Gong J, Sugiyama H, Ohkusa T, Tajiri H. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20(16):4228–4239. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 31.Dagvadorj N, Deuretzbacher A, Weisenberger D, Baumeister E, Trebing J, Lang I, Köchel C, Kapp M, Kapp K, Beilhack A, Hünig T, Einsele H, Wajant H, Grigoleit GU. Targeting of the WT1 91-138 fragment to human dendritic cells improves leukemia-specific T-cell responses providing an alternative approach to WT1-based vaccination. Cancer Immunol Immunother. 2017;66(3):319–332. doi: 10.1007/s00262-016-1938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NN, Loeb DM, Khuu H, Stroncek D, Ariyo T, Raffeld M, Delbrook C, Mackall CL, Wayne AS, Fry TJ. Induction of immune response after allogeneic Wilms’ Tumor 1 dendritic cell vaccination and donor lymphocyte infusion in patients with hematologic malignancies and post-transplantation relapse. Biol Blood Marrow Transplant. 2016;22(12):2149–2154. doi: 10.1016/j.bbmt.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimodaira S, Sano K, Hirabayashi K, Koya T, Higuchi Y, Mizuno Y, Yamaoka N, Yuzawa M, Kobayashi T, Ito K, Koizumi T. Dendritic cell-based adjuvant vaccination targeting Wilms’ Tumor 1 in patients with advanced colorectal cancer. Vaccines (Basel) 2015;11(4):3. doi: 10.3390/vaccines3041004. [DOI] [PMC free article] [PubMed] [Google Scholar]