Abstract
Diabetic nephropathy (DN) is a research priority for scientists around the world because of its high prevalence and poor prognosis. Although several mechanisms have been shown to be involved in its pathogenesis and many useful drugs have been developed, the management of DN remains challenging. Increasing amounts of evidence show that silent information regulator 2 homolog 1 (sirtuin-1), a nicotinamide adenine dinucleotide (NAD+)–dependent protein deacetylase, plays a crucial role in the pathogenesis and development of DN. Clinical data show that gene polymorphisms of sirtuin-1 affect patient vulnerability to DN. In addition, upregulation of sirtuin-1 attenuates DN in various experimental models of diabetes and in renal cells, including podocytes, mesangial cells, and renal proximal tubular cells, incubated with high concentrations of glucose or advanced glycation end products. Mechanistically, sirtuin-1 has its renoprotective effects by modulating metabolic homeostasis and autophagy, resisting apoptosis and oxidative stress, and inhibiting inflammation through deacetylation of histones and the transcription factors p53, forkhead box group O, nuclear factor-κB, hypoxia-inducible factor-1α, and others. Furthermore, some microRNAs have been implicated in the progression of DN because they target sirtuin-1 mRNA. Several synthetic drugs and natural compounds have been identified that upregulate the expression and activity of sirtuin-1, which protects against DN. The present review will summarize advances in knowledge regarding the role of sirtuin-1 in the pathogenesis of DN. The available evidence implies that sirtuin-1 has great potential as a clinical target for the prevention and treatment of diabetes.
Keywords: Sirtuin-1, Deacetylase, Diabetic nephropathy, Signaling pathway, Pathogenesis
Background
Diabetic nephropathy (DN), also referred to as diabetic kidney disease, occurs in 20–40% of all diabetic patients, and therefore affects hundreds of millions of people worldwide [1]. The early stages of DN are characterized by microalbuminuria, which is usually ignored. However, when this albuminuria becomes severe, it is progressive and irreversible, ultimately being associated with renal dysfunction and a high risk of cardiovascular death. Although a lot of progress has been made in the understanding of the mechanisms and appropriate treatment of DN, it remains a substantial clinical problem.
Acetylation and deacetylation regulate cell proliferation, apoptosis, autophagy, energy homeostasis, inflammation, and oxidative stress (OS). Acetylation of histone promotes transcription by opening chromatin binding sites, and acetylation of transcription factors and transcriptional coregulatory proteins regulate their transcriptional activity by modulating their subcellular localization, DNA-binding affinity, and degradation [2]. Deacetylases counteract these effects by removing acetyl groups [3]. Sirtuins are nicotinamide adenine dinucleotide (NAD+)–dependent deacetylases that function as intracellular regulators of transcriptional activity. Sirtuin-1 is the most widely expressed and extensively studied member of the sirtuin family. Six other sirtuins have been identified that have distinct biological functions associated with their distinct localization, substrate specificity, and binding partners [4]. A reduction in the NAD+/NADH ratio in the presence of excess nutrient supply results in lower renal expression of sirtuin-1 in diabetic patients and experimental models of diabetes. Accumulating evidence shows that sirtuin-1 plays a crucial role in the pathogenesis of DN [5–8]. In the present review, we discuss the role of sirtuin-1 in the pathogenesis of DN and the mechanisms involved.
Pathogenic features and molecular mechanisms of diabetic nephropathy
DN is a chronic complication of diabetes, and its clinical and pathological characteristics require many years to develop in humans. The natural history of DN caused by type 1 diabetes mellitus (T1DM; the consequence of the autoimmune destruction of pancreatic islet beta-cells) is further divided into five stages: normoalbuminuria with hyperfiltration, microalbuminuria, macroalbuminuria, decline of the glomerular filtration rate (GFR), and the dialysis-dependent stage [9, 10]. DN caused by type 2 diabetes mellitus (T2DM; the consequence of insulin resistance (IR) and the failure of beta-cell compensation) seems to be more complex and progresses more rapidly due to the presence of IR and other disturbances, such as hypertension. The histological alterations characterizing DN can be identified in glomeruli, tubules, the interstitium, and vessels.
The glomerular defects are the most important lesions, involving a reduction in podocyte number and foot process effacement, glomerular basement membrane thickening, mesangial expansion, nodular sclerosis, and global glomerulosclerosis [11]. The reduction in podocyte number is caused by apoptosis and detachment; podocytes detachment and foot process effacement are due to cytoskeletal changes and podocyte de-differentiation. Because they are a critical component of the mechanism of glomerular filtration, the loss of and phenotypic alterations in podocytes are the principal cause of albuminuria in DN. The mesangial expansion and glomerulosclerosis result from mesangial cell proliferation, hypertrophy, phenotypic changes, and subsequently excessive mesangial matrix accumulation, which ultimately result in lower GFR. Tubular epithelial cells in diabetic kidneys can undergo hypertrophy, apoptosis, and/or transformation into mesenchymal cells. Simultaneously, the interstitium becomes infiltrated with pro-inflammatory cells and fibroblasts. The progression of tubulointerstitial fibrosis and glomerulosclerosis causes a deterioration in renal dysfunction. Furthermore, diabetes induces arteriolar hyalinosis. Thus, during the progression of DN, nearly all the cell types in the kidney demonstrate abnormalities, including proliferation, hypertrophy, de-differentiation, and apoptosis.
Several molecular mechanisms are involved in the pathogenesis of DN, namely activation of the polyol pathway during hyperglycemia, hexosamine pathway, and protein kinase C (PKC); accumulation of intracellular advanced glycation end products (AGEs); glomerular hyperfiltration; and hypertension [12]. The glucose metabolic changes result in the excessive production of free radicals, such as reactive oxygen species (ROS). ROS-induced OS causes DNA damage, specifically strand breakage and base alterations, which activate p53 and its downstream pathway to induce cell cycle arrest or apoptosis [13]. DNA damage in mitochondria results in mitochondrial dysfunction, which in turn generates more ROS. Inflammation develops as a response to OS-induced damage, which promotes repair and remodeling. This involves activation of the nuclear factor kappa B (NF-κB) pathway in renal cells, especially endothelial cells (ECs), which secrete adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion protein-1 (VCAM-1), and chemokines, such as monocyte chemotactic protein-1 (MCP-1) and interleukins. These pro-inflammatory adhesion molecules and chemokines attract monocytes, macrophages, and T lymphocytes, which infiltrate kidney tissue, resulting in activation of tumor necrosis factor-α (TNF-α) signaling, and therefore the aggravation of kidney lesions and fibrosis [12].
In addition, hyperfiltration or/and metabolic changes in diabetic kidneys cause excessive oxygen consumption, which results in hypoxia and the expression of the oxygen sensor hypoxia-inducible factor-1α (HIF-1α) [14]. Activation of HIF-1 signaling also activates the vascular endothelial growth factor (VEGF) signaling pathway to promote angiogenesis. This abnormal angiogenesis induced during DN causes glomerular hypertrophy and plasma leakage, which promotes glomerulosclerosis and arteriolar hyalinosis [15, 16]. Thus, glucose metabolic changes, hemodynamic alterations, OS, inflammation, and hypoxia are the main pathophysiological features of DN. Autophagy, which is a protective mechanism that maintains intracellular homeostasis, is also impaired as a result of the activation of the pathways listed above, which aggravates renal cellular dysfunction and apoptosis.
Evidence for the role of sirtuin-1 in humans and animal models of diabetic nephropathy
There is emerging clinical evidence to suggest that gene polymorphisms of sirtuin-1 affect patient susceptibility to DN (Table 1). Maeda et al. discovered that single nucleotide polymorphisms (SNPs) in the SIRT1 gene (encoding sirtuin-1), but not in the genes encoding other sirtuin family members (sirtuins-2–6), are associated with DN in Japanese T2DM patients [17]. Tang et al. investigated the associations between DN and SNPs in p300 and SIRT1 in Chinese patients with T2DM [18], and demonstrated that the SIRT1 rs4746720 allele C was associated with the urinary albumin/creatinine (Alb/Cre) ratio. P300 allele G and the SIRT1 TC genotype are associated with the development of DN, while the G and TT genotypes are predisposed to more severe DN. Another study of 1066 Han Chinese with T2DM showed that patients with the SIRT1 rs10823108 AA genotype had a lower risk of developing DN [8]. These associations between SIRT1 gene polymorphisms and DN suggest that sirtuin-1 is implicated in the initiation of DN.
Table 1.
The role of sirtuin-1 in clinical studies of diabetic nephropathy
| Race | Case load | Type of diabetes | The role of sirtuin-1 in DN | References |
|---|---|---|---|---|
| Japanese | Study 1: 747 overt proteinuria cases vs.557 controls; | T2DM | SNP rs4746720 in sirtuin-1 is significantly associated with proteinuria, and rs2236319, rs10823108, rs3818292, and rs4746720 are associated with combined phenotypes (proteinuria and ESRD). | [17] |
| Study 2: 455 overt proteinuria cases vs. 965 controls; | ||||
| Study 3: 300 end-stage renal disease cases vs.218 controls | ||||
| Chinese | DM with DN 628 cases vs. DM without DN 388 cases | T2DM | rs20551 G alleles in p300 and rs4746720 C alleles in SIRT1 correlate with an increase in ACR. P300 allele G and the sirtuin-1 TC genotype are associated with the development of DN, while the G and TT genotypes predispose to more severe DN. | [18] |
| Chinese | 653 DM with DN vs. 413 without DN | T2DM | SIRT1 rs10823108 AA genotype is associated with a decreased risk of DN. | [8] |
| Chinese | 495 DM patients: | T2DM | Serum Sirtuin-1 levels of diabetes patients are significantly lower than those in the control group, and decrease with the increase in ACR. | [19] |
| Normoalbuminuric group (ACR < 30 mg/g, n = 186) | ||||
| Microalbuminuric group (ACR 30–300 mg/g, n = 169) | ||||
| Macroalbuminuric group (ACR > 300 mg/g, n = 140) |
SNP, single nucleotide polymorphism; DM, diabetes mellitus; DN, diabetic nephropathy; ACR, urinary albumin to creatinine ratio
A number of models of diabetes have been used to evaluate the renoprotective effects of sirtuin-1, including both T1DM [20, 21] and T2DM models [22, 23], such as the db/db diabetic mouse, streptozotocin (STZ) alone diabetic mouse/rat model, or a combination of STZ- and high-fat diet–induced diabetic mouse/rat models. To identify the role of sirtuin-1 in the kidney, Chuang et al. generated mice with an 80% reduction in sirtuin-1 expression [24], which showed no defect in glomerular function until they were rendered diabetic, when they demonstrated severe albuminuria and mitochondrial dysfunction. In addition, Hasegawa et al. used proximal tubule–specific sirtuin-1 transgenic and sirtuin-1 knockout mice to reveal that sirtuin-1 protects against albuminuria in diabetes [25]. Finally, podocyte-specific knockout of sirtuin-1 reduced the quality and quantity of podocytes and worsened albuminuria in diabetic mice [26].
In vitro, renoprotective effects of sirtuin-1 are studied in primary renal parenchymal cells and cell lines in the presence of high concentrations of glucose (HG) or AGEs.
Podocytes
Apoptosis and the detachment of podocytes, and foot process effacement, cause albuminuria in DN. Multiple studies show that sirtuin-1 is necessary for the maintenance of cytoskeletal integrity and the survival of podocytes [27, 28]. A model of non-diabetic podocyte injury (induced by a nephrotoxic serum) revealed that sirtuin-1 can deacetylate cortactin, which is crucial for the maintenance of the actin cytoskeleton and the structure of the slit diaphragm between podocyte processes [27]. Treatment with AGE-modified bovine serum albumin downregulated sirtuin-1 in cultured podocytes, which increased the acetylation of forkhead box group O (FoxO)4, resulting in apoptosis because of greater expression of the pro-apoptotic gene BCL2 like 11 [29]. Hasegawa et al. referred to “proximal tubule-podocyte communication,” by which sirtuin-1 in tubules downregulates claudin-1 expression in podocytes to protect against diabetes-induced albuminuria [25, 30]. Claudin-1 belongs to the claudin family of proteins that constitutes the tight junction. High levels of claudin-1 have been reported to be associated with podocyte effacement and albuminuria. Sirtuin-1 downregulates the expression of claudin-1 by deacetylating histone H3 and H4. Conversely, diabetes-induced upregulation of claudin-1 in podocytes causes slit diaphragm-tight junction transition and consequently proteinuria [31]. The potential mechanism of this proximal tubule-podocyte communication will be discussed below.
Glomerular mesangial cells
Glomerular mesangial cells (GMCs) secrete numerous cytokines under diabetic conditions, including transforming growth factor-β1 (TGF-β1), leading to the expansion of the mesangial area and glomerular sclerosis. Under these conditions, a reduction in sirtuin-1 of GMCs has been observed, which leads to inflammation and fibrosis [32]. Normally, sirtuin-1 blocks the activation of pro-hypertrophic Akt signaling, as well as augmenting the activity of anti-hypertrophic AMP-activated protein kinase (AMPK) signaling in GMCs [33]. However, sirtuin-1 protein levels and deacetylase activity decline in a dose- and time-dependent manner in GMCs cultured with AGEs [34]. Furthermore, in a rat glomerular mesangial cell line that was exposed to HG for 72–144 h, the expression of sirtuin-1 decreased, while expression of the renal pro-fibrotic factors vimentin and fibronectin (FN) was induced [35].
Renal ECs
Although studies regarding the role of sirtuin-1 in diabetic endothelial injury have been few in number, they do provide evidence that changes in sirtuin-1 expression in ECs also involve DN. Mice with an endothelium-specific deletion of sirtuin-1 show peritubular capillary rarefaction and fibrosis, due to activation of notch-1 signaling [36]. Moreover, the cleavage of sirtuin-1 by cathepsin is involved in stress-induced premature senescence in ECs [37]. Dermal-derived human microvascular ECs incubated in HG medium show early senescence and develop an irregular and hypertrophic phenotype, associated with lower sirtuin-1 mRNA expression [38]. In addition, in human glomerular ECs exposed to HG, AMPK phosphorylation (pAMPK) and sirtuin-1 expression were lower and there was more apoptosis [39].
Proximal tubular cells
Lower sirtuin-1 expression was observed in proximal tubular cells (PTCs), which are fragile parenchymal cells, under HG conditions. Fu et al. reported that the mRNA and protein expression of sirtuin-1 by PTCs incubated in HG decreased to 19% and 36% of the control level, respectively [40]. Xue et al. showed similar time-dependent reductions in HK-2 cells (a PTC cell line) [41]. Studies by Hasegawa et al. revealed that sirtuin-1 expression in PTCs affects glomerular function by influencing podocytes. They found that sirtuin-1 expression in PTCs is low prior to the appearance of albuminuria in STZ-induced or db/db diabetic mice. PTC-specific knockout of sirtuin-1 causes albuminuria in non-diabetic mice and aggravates the albuminuria of diabetic mice. Furthermore, PTC-specific sirtuin-1 transgenic mice are protected against DN [25]. They called this phenomenon “proximal tubule-podocyte communication” and proposed that HG stress triggers a decline in sirtuin-1 expression in proximal tubules, leading to the release of the humoral mediator nicotinamide mononucleotide (NMN), which causes an increase in claudin-1 expression in podocytes [30]. NMN is a NAD+ intermediate, which is found at a lower concentration in the medium of PTCs incubated in HG than in that of PTCs incubated with a normal concentration of glucose. When the medium from PTCs incubated in HG was used to culture podocytes, the podocytes exhibited downregulation of sirtuin-1 and upregulation of claudin-1, whereas the levels of sirtuin-1 and claudin-1 did not change when the podocytes were incubated in HG medium alone. These findings indicate that some factors (NMN, not HG) in the conditioned medium from HG-incubated PTCs affect the expression of sirtuin-1 and claudin-1 in podocytes [25]. Finally, a fluorescence-labeling technique showed that NMN could be released by PTCs and affect podocytes [30].
Roles of sirtuin-1 in signaling
AMPK/sirtuin-1/PGC-1α signaling
Under diabetic conditions, the downregulation of AMPK/sirtuin-1/PGC-1α signaling induces hypertrophy, OS, and mitochondrial and autophagy dysfunction, all which promote the development of DN (Fig. 1). Both AMPK and sirtuin-1 have been identified as intracellular energy sensors, detecting and responding to AMP/ATP and NAD+/NADH ratios, respectively, and therefore being activated under conditions of energy depletion and deactivated in diabetes [42–44]. However, AMPK and sirtuin-1 also regulate one another’s activity [42]. Sirtuin-1 deacetylates lysine residues in liver kinase B1 (LKB1), promoting its migration from the nucleus to the cytoplasm, where it can catalyze the phosphorylation and activation of AMPK [45]. Concurrently, AMPK activates downstream signaling in a sirtuin-1–dependent manner [46]. In addition, AMPK upregulates sirtuin-1 by increasing cellular NAD+ levels [47]. Finally, Fulco et al. showed that glucose restriction–induced activation of AMPK increases sirtuin-1 activity by promoting transcription of the NAD+ biosynthetic enzyme nicotinamide phosphoribosyltransferase (Nampt) [48].
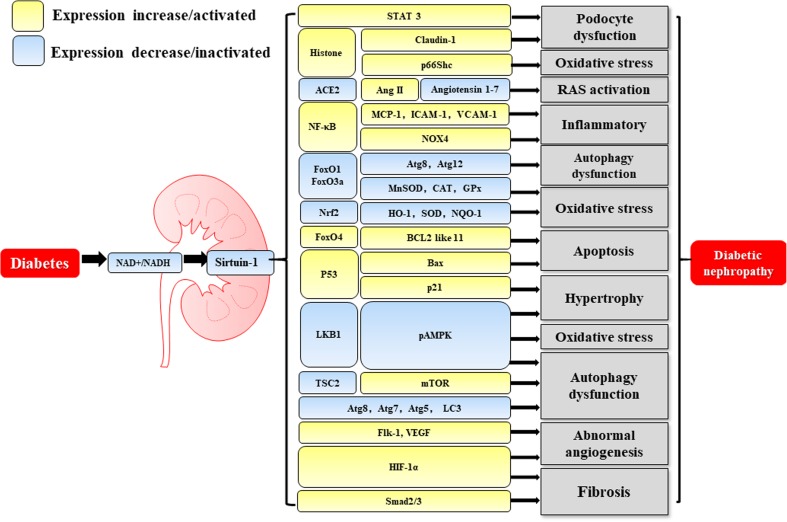
Fig. 1.
The molecular mechanisms of sirtuin-1 involvement in diabetic nephropathy. The changes in glucose metabolism in diabetes are associated with greater production of NADH and a reduction in the NAD+/NADH ratio, resulting in lower expression of sirtuin-1. Downregulation of sirtuin-1 causes greater acetylation of histones and several crucial transcription factors, such as STAT3, NF-κB, FoxO4, p53, HIF-1α, and Smad2/3, which increases their expression levels and transcriptional activation activities. Activation of STAT3 and upregulation of claudin-1 result in podocyte dysfunction. Acetylation of p66Shc facilitates its phosphorylation and translocation to the mitochondria, where it promotes hydrogen peroxide production. Activation of NF-κB signaling promotes the expression of its pro-inflammatory downstream effectors MCP-1, ICAM-1, VCAM-1, and NOX4. Acetylation of FoxO4 promotes expression of the pro-apoptotic gene BCL2 like 11, activating apoptosis. Acetylation of p53 stabilizes and activates it, resulting in target gene transcription, including that of p21 and Bax, inducing cell cycle arrest and apoptosis. Furthermore, the activation of Smad2/3 and HIF-1α induces fibrosis. HIF-1α and Flk-1 activate the VEGF pathway, causing abnormal angiogenesis. Lower expression of sirtuin-1 also leads to lower expression and/or inactivation of ACE2, FoxO1, FoxO3a, Nrf2, LKB1, TSC2, Atg8, Atg7, Atg5, and LC3, either directly (increase in acetylation) or indirectly. The inactivation of ACE2 removes its regulatory effect on Ang II and activates RAS. The inactivation of Nrf2 and the lower activity of FoxO1 and FoxO3a inhibit the expression of anti-oxidants such as Mn-SOD, CAT, GPs, HO-1, SOD, and NQO-1, which aggravates oxidative stress and mitochondrial dysfunction. The inactivation of LKB1 results in downregulation of the AMPK/PGC-1 pathway, which impairs autophagy and mitochondrial function, and promotes hypertrophy. As an inhibitor of the mTOR pathway, inactivation of TSC2 promotes activation of the mTOR pathway, which inhibits autophagy. Lower expression of Atg8, Atg7, Atg5, and LC3 impairs autophagy. Metabolic disturbance, oxidative stress, inflammation, impaired autophagy, hypoxia, abnormal angiogenesis, apoptosis, fibrogenesis, and activation of the RAS combine to cause the kidney lesions in diabetes
Under diabetic conditions, pAMPK was lower in glomeruli and tubules, implying the inactivation of AMPK [49, 50]. Previous studies showed that calorie restriction (CR) has beneficial effects on DN via AMPK and sirtuin-1 [51]. The levels of pAMPK and sirtuin-1 are lower in diabetic kidney, but the phosphodiesterase type 4 (PDE4) inhibitor roflumilast mimics the effects of CR to restore the levels of pAMPK/sirtuin-1 and alleviate DN [52]. AMPK and sirtuin-1 share peroxisome proliferator–activated receptor (PPAR)-γ coactivator 1α (PGC-1α) as a target, which is a transcriptional coactivator that modulates metabolic homeostasis [53], and mitochondrial biogenesis and function [54]. It has been suggested that the phosphorylation of PGC-1α by AMPK makes it more susceptible to deacetylation by sirtuin-1 [47] and enhances its ability to activate its own promoter [55].
Adiponectin is an adipokine that has protective anti-oxidant and anti-inflammatory effects, but its expression is downregulated in IR, obesity, and T2DM. The receptors of adiponectin include adiponectin receptor (AdipoR)1 and AdipoR2, which activate AMPK and PPARα. Resveratrol, an activator of sirtuin-1, promotes the phosphorylation of AMPK and the activation of sirtuin-1/PGC-1α signaling, which prevents apoptosis and OS, and thereby ameliorates DN in db/db mice [56]. In addition, Park et al. reported that HG-induced OS and apoptosis in cultured glomerular ECs are prevented by resveratrol treatment, which activates AMPK/sirtuin-1/PGC-1α and PPARα signaling by increasing the expression of AdipoR1 and AdipoR2 [39].
Furthermore, several natural substances have been identified to ameliorate DN by activating AMPK/sirtuin-1/PGC-1α signaling. In vitro, glycyrrhizic acid attenuates cell proliferation and TGF-β1 overexpression, and ameliorates the low expression and activity of AMPK, sirtuin-1, and manganese superoxide dismutase (Mn-SOD) induced by HG in renal tubular cells [57]. In vivo, glycyrrhizic acid ameliorates DN by inhibiting ROS and activating AMPK/sirtuin-1/PGC-1α signaling in db/db mice [22]. Cai et al. reported that grape seed procyanidin B2 upregulated the expression of AMPK, sirtuin-1, and PGC-1α in podocytes [58], while Bao et al. showed that grape seed proanthocyanidin prevents diabetes-induced OS and mitochondrial dysfunction in podocytes by activating AMPK/sirtuin-1/PGC-1α signaling [59].
Sirtuin-1 and mammalian target of rapamycin
Decreases in sirtuin-1 expression alleviate suppression of the mTOR pathway, which promotes HG-induced renal cell autophagy dysfunction and senescence. Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase and component of the mTOR complex, which is a key modulator of cellular proliferation, autophagy, and lipid metabolism. Tuberous sclerosis complex (TSC)1 and TSC2 are inhibitors of the mTOR signaling pathway, and it is reported that the negative regulation of mTOR signaling by sirtuin-1 is TSC2-dependent. Immunoprecipitation indicated that sirtuin-1 interacts with TSC2, but the exact mechanism is uncertain [60]. HG induces the upregulation of mTOR and downregulation of sirtuin-1 in MCs [61], while rapamycin, an inhibitor of mTORC1, inhibits the downstream pathway and arrests HG-induced MC senescence. However, the silencing of sirtuin-1 completely inhibits the effects of rapamycin in HG-treated MCs. These findings suggest that sirtuin-1 regulates the mTOR pathway, which mediates the induction of MC senescence by HG [61]. Another study confirmed that rapamycin partially inhibits mTORC1 and restores sirtuin-1 activity in palmitate-treated podocytes [62].
Sirtuin-1 and the Keap1/Nrf2/ARE pathway
The activation of the nuclear factor erythroid 2–related factor 2 (Nrf2)/anti-oxidant response element (ARE) pathway is suppressed in DN due to low sirtuin-1 levels, which in turn decreases anti-oxidant capacity. Accumulation of AGEs in diabetic kidney stimulates the generation of ROS, causing OS and promoting the progression of DN. ROS/anti-oxidant homeostasis is disturbed when the production of ROS exceeds the production of anti-oxidants, and diabetic complications result from OS due both to excessive production of ROS and a reduction in anti-oxidant capacity [13].
The Nrf2 is a transcription factor that binds to the ARE of DNA and switches on its target genes, including NAD(P)H quinone dehydrogenase 1 (NQO-1), heme oxygenase 1 (HO-1), and superoxide dismutase (SOD). Kelch-like ECH–associated protein 1 (Keap1) acts as a negative regulator of Nrf2 by binding it, blocking its transfer to the nucleus, and promoting its degradation. The Nrf2/ARE pathway is a critical cellular anti-oxidant mechanism that is inactivated during chronic OS, including that associated with diabetes, and sirtuin-1 has a significant anti-oxidant effect because it activates the Nrf2/ARE pathway [63, 64]. Previous studies demonstrated cross-talk between sirtuin-1 and the Keap1/Nrf2/ARE pathway: sirtuin-1 enhances the activity of the Keap1/Nrf2/ARE pathway by decreasing Keap1 expression, deacetylating and reducing the ubiquitination of Nrf2, and promoting ARE-binding ability in AGE-treated GMCs [65]. In addition, Huang et al. reported that polydatin, a glucoside of resveratrol, ameliorates AGE-induced FN and TGF-β1 expression by activating the sirtuin-1/Nrf2/ARE pathway in rat GMCs [64]. Zhang et al. reported that paeonol, an extract of Cortex moutan, delayed the progression of fibrosis by activating sirtuin-1 and therefore the Nrf2 pathway in DN [66]. Ling Zhou et al. found that HG decreased sirtuin-1 activity in HK-2 cells, enhanced Keap1 expression, and promoted the ubiquitination and degradation of Nrf2 via a pathway involving NF-κB and microRNA (miR)-29 [67]. However, Nrf2 also positively regulates the protein expression of sirtuin-1 [65], and the Nrf2 activator tBHQ increases the expression and deacetylase activity of sirtuin-1, while small interfering (si)RNAs targeting Nrf2 reduce sirtuin-1 expression and activity, leading to greater expression of FN and TGF-β1 in GMCs [65]. Although the underlying mechanism of these effects is unknown, some studies show that Nrf2 promotes sirtuin-1 expression by negatively regulating p53 [68].
Sirtuin-1 and p66Shc
Diabetes-induced downregulation of sirtuin-1 increases OS in mitochondria by promoting histone H3 and p66Shc acetylation. Mitochondria are the primary source of the excess ROS [13]. The adaptor protein 66 kDa Src homology 2 domain–containing protein (p66Shc) is a crucial regulator of mitochondrial ROS production and contributes to DN by promoting OS [69–71]. p66Shc mainly expresses in the renal tubular cells and glomeruli and is a direct target of sirtuin-1. A recent study indicates that HG-induced downregulation of sirtuin-1 promotes the expression of p66Shc by increasing histone H3 and p66shc acetylation [72]. Histone H3 acetylation promotes transcription of p66Shc. The acetylation of p66Shc facilitates its phosphorylation and translocation to mitochondria, where it promotes hydrogen peroxide production [69, 73, 74]. In addition, diabetes, HG, and palmitate stimulate p66Shc, which activates a redox mechanism that upregulates miR-34a expression, targeting sirtuin-1 for degradation, and causing endothelial dysfunction. These effects are prevented when p66shc expression is silenced, while overexpression of p66Shc enhances HG and palmitate-induced miR-34a expression, thus reducing that of sirtuin-1 [75].
Sirtuin-1 and HIF-1
Sirtuin-1 deficiency under diabetic condition leads to activation of HIF-1, which results in abnormal angiogenesis and fibrosis in the kidney. Recent studies demonstrated that hypoxia is also involved in the pathogenesis of DN [14]. Hyperfiltration or/and metabolic changes in diabetic kidneys cause excessive oxygen consumption, resulting in hypoxia and expression of the oxygen sensor HIF-1. HIF-1 is composed of a functional α-subunit and a constitutive β-subunit and promotes epithelial-to-mesenchymal transition (EMT) in vitro, while renal epithelial knockout of HIF-1α prevents tubulointerstitial fibrosis [76]. HIF-1α is a downstream target of sirtuin-1, and during hypoxia downregulation of sirtuin-1 leads to greater acetylation and activation of HIF-1α. When sirtuin-1 is activated by resveratrol, HIF-1α is deacetylated and inactivated, which prevents HG-induced expression of connective tissue growth factor (CTGF), endothelin-1, FN, TGF-β1, and VEGF [32].
Sirtuin-1 and NF-κB
Diabetes-induced downregulation of sirtuin-1 leads to activation of NF-κB signaling, which promotes the activation of downstream pro-inflammatory factors and inhibits anti-oxidative stress Nrf2/ARE pathway involved in the development of DN. NF-κB is a transcription factor that governs the expression of genes involved in inflammation. The canonical pathway of NF-κB activation involves the p65 and p50 subunits [77], and it is now known that sirtuin-1 can deacetylate the p65 subunit of NF-κB and inhibit NF-κB pro-inflammatory signaling and the downstream production of MCP-1, ICAM-1, and VCAM-1 [77–79]. MCP-1 exerts pro-inflammatory effects by regulating the migration and infiltration of monocytes/macrophages, a process that could thus be regulated by a sirtuin-1/NF-κB p65 pathway [80]. Podocyte-specific ablation of sirtuin-1 in db/db mice results in severe proteinuria and kidney injury, which is accompanied by greater acetylation of p65 and STAT3 [26]. NADPH oxidase 4 (NOX4) expression is higher in DN, while podocyte-specific knockout of NOX4 attenuates DN. Previous studies suggest that NF-κB directly regulates NOX4 expression by binding to its promoter, and sirtuin-1 could therefore ameliorate inflammation in DN, at least in part through deacetylation of NF-κB and downregulation of NOX4 [81]. NF-κB could also interact with the Nrf2/ARE pathway via sirtuin-1 and miR-29. In support of this, Zhou et al. found that, in HG-incubated tubular epithelial cells, downregulation of sirtuin-1 increased the acetylation and activity of NF-κB, which directly binds to the promoter and downregulates miR-29 expression, thereby increasing the expression of Keap1 and inhibiting the Nrf2/ARE pathway [67].
Sirtuin-1 and FOXO
Sirtuin-1 deacetylates FOXO, which changes the transcriptional activity of FOXO target genes to prevent OS, inflammation, and apoptosis in DN. FOXO belongs to the forkhead family of transcription factors, which have a conserved DNA-binding domain. In mammals, four family members have been identified: FoxO1, FoxO3a, FoxO4, and FoxO6. The expression of FoxO1 and FoxO3a is ubiquitous, while FoxO4 is highly expressed in the muscle and heart and FoxO6 is expressed in the brain [82]. Acetylation both promotes and reduces FOXO transcriptional activity and mediates various biological functions of FOXO [83].
FoxO1 has been extensively studied and shown to regulate cellular events, such as metabolism, proliferation, redox status, stress resistance, inflammation, aging, and apoptosis [84]. Sirtuin-1 reduces the acetylation of FoxO1, which enhances the DNA-binding affinity of FoxO1 [85]. The sirtuin-1/FoxO1 pathway has an anti-oxidant effect because it increases expression of Mn-SOD and catalase (CAT) [86, 87]. However, the expression of FoxO1 is lower, which is associated with the accumulation of extracellular matrix (ECM) and OS in type 1 diabetic kidney. Xu et al. found that puerarin upregulated the expression of sirtuin-1, FoxO1, and PGC-1α in renal cortex and was therefore protective against DN [87]. In addition, treatment of DN with the sirtuin-1 agonist resveratrol increased SOD activity and reduced MDA, collagen IV, and FN expression by increasing FoxO1 activity [88].
FoxO3a regulates cellular processes, including metabolism, cellular proliferation and differentiation, OS resistance, inflammation, aging, and apoptosis [84]. Sirtuin-1 deacetylates FoxO3a, which enhances FoxO3a-induced autophagy and anti-oxidant effects, while suppressing FoxO3a-induced cell death. Resveratrol deacetylates FoxO3a and attenuates the OS caused by hyperglycemia, both in vivo and in vitro. Silencing sirtuin-1 induces overexpression of acetylated FoxO3a, which aggravates the OS in HG-induced tubular epithelial cells; resveratrol treatment fails to protect against these effects [89]. Sirtuin-1 activates autophagy by deacetylating FoxO1 and FoxO3a in the nucleus [90–92], the mechanisms of which will be described below.
In addition to FoxO1 and FoxO3a, FoxO4 is deacetylated by sirtuin-1. It is involved in cellular proliferation, inflammation, aging, and apoptosis in mammals [84]. AGEs cause podocyte apoptosis by downregulating sirtuin-1 and increasing acetylation of FoxO4, which promotes expression of BCL2 like 11 [29].
Sirtuin-1 and p53
Diabetes-induced decreases in sirtuin-1 expression involve apoptosis via activation of the p53 pathway. Apoptosis of podocytes, ECs, and tubular epithelial cells causes albuminuria and renal dysfunction in DN. Sirtuin-1 exerts anti-apoptotic effects to protect against cellular injury by deacetylating pro-apoptotic proteins. p53 is a tumor suppressor gene that is activated and induces cell cycle arrest or apoptosis under DNA-damaging conditions [93]. Acetylation stabilizes and activates p53, promoting target gene transcription, including that of p21 and Bax [93]. Sirtuin-1 negatively regulates p53 by deacetylating it at the C-terminal lysine-382 residue, and this relationship between sirtuin-1 and p53 is closely associated with aging and diabetes [93]. The sirtuin-1/p53 pathway has also been implicated in HG-induced apoptosis: study of cultured PTCs has shown that incubation in HG reduces sirtuin-1 protein expression and increases the expression of c-caspase-3 and c-PARP, and the acetylation of p53, especially when small interfering RNA against sirtuin-1 are used [94]. In addition, resveratrol prevented increases in expressions of p38 and p53, the dephosphorylation of histone H3, PTC apoptosis, and albuminuria in DN by activating sirtuin-1 [94, 95]. A recent study suggested that a p53/miR-155-5p/sirtuin-1 loop is present in the kidney of animals with DN: p53 promoted the expression of miR-155-5p, which reduced sirtuin-1 expression, in turn downregulating sirtuin-1 and disinhibiting p53 [96].
Sirtuin-1 and autophagy-related proteins
Decreases in sirtuin-1 expression inhibit autophagy under diabetic conditions by suppressing the expression of autophagy-related proteins, FOXO and AMPK, and activating mTOR. Autophagy is a regulated process that disassembles and recycles unnecessary or dysfunctional cellular components. The process of macroautophagy, the most intensively investigated type of autophagy, includes phagophore formation and elongation, autophagosome formation, lysosome fusion, and degradation in autolysosomes [97]. Autophagy-related (Atg)5, Atg7, and Atg8 are required for the formation and elongation of the autophagosomal membrane, while microtubule-associated protein light chain 3 (LC3) and pro-autophagic Bcl2/adenovirusE1 V 19 kDa interacting protein 3 (Bnip3) are necessary for autophagy. Under starvation conditions, sirtuin-1 promotes the activity of FoxO1 and FoxO3a, which are localized to the nucleus and bind to promoter sequences of Atg8 and Atg12 [90]. Deacetylation of LC3 by sirtuin-1 promotes its nucleocytoplasmic transport and the formation of autophagosomes [98]. An increasing body of evidence indicates that a deficiency in autophagy contributes to the pathogenesis of DN [99–102]. Sirtuin-1 may restore autophagy by deacetylating FoxO1 and FoxO3 in the nucleus [90–92] and Atg5, Atg7, and Atg8 in the cytosol [103]. Activation of sirtuin-1 restores the expression of FoxO3, which positively regulates Bnip3, and thus enhances autophagy in the kidneys of db/db mice [7]. Knockdown of sirtuin-1 inhibits Atg7, Atg5, and LC3, and abolishes the resveratrol-mediated amelioration of the defect in autophagy induced by hypoxia in cultured PTCs [7].
Furthermore, both the AMPK and mTOR pathways regulate autophagy. AMPK is a positive regulator of autophagy, while the mTOR pathway is a negative regulator, activating or inhibiting UNC51-like kinase 1 complex, which is essential for the initiation of autophagy [104, 105]. Indeed, the impaired autophagy observed in DN is associated with defects in both the mTOR and AMPK pathways. Thus, sirtuin-1 might regulate autophagy via mTOR and AMPK in diabetic kidneys. Recently, a study showed that a p53/miR-155-5p/sirtuin-1 pathway regulates autophagy in PTCs incubated in HG [96], which will be discussed further below.
Sirtuin-1 and the TGF-β1/smad pathway
Sirtuin-1 prevents diabetic renal fibrogenesis by inhibiting TGF-β1/small mothers against decapentaplegic homolog (smad) 2/3 pathway. Tubulointerstitial fibrosis and renal dysfunction are pathological features of the end stage of various kidney diseases, including DN. There is increasing evidence of the anti-fibrotic effects of sirtuin-1 in experimental models of diabetes. TGF-β1 is a pro-fibrogenic factor that is upregulated in DN; it binds to its receptor and activates the downstream mediators smad 2/3 [106, 107]. Phosphorylation and acetylation of smad2/3 enhance their activity and cause accumulation of ECM, including of collagen and FN [106–109]. However, smad2/3 have also been identified as targets of sirtuin-1. Recent studies report that TGF-β1 promotes smad2/3 acetylation, while resveratrol treatment deacetylates smad3 by activating sirtuin-1 in cultured PTs [110]. In addition, resveratrol administration abolishes TGF-β1/smad3-induced renal fibrosis in a mouse model of unilateral ureteral obstruction. In subtotally or 5/6 nephrectomized rat models, sirtuin-1 activator SRT3025 attenuated TGF-β1 overexpression, GFR decline, and proteinuria [108]. However, studies investigating whether sirtuin-1 can prevent renal fibrogenesis in DN through TGF-β1/smad2/3 deacetylation have not been reported [110]. Another study revealed reduced sirtuin-1 and increased acetylase p300, TGF-β1, and collagen I expression in HG-incubated microvascular ECs [111]. Diabetes caused high TGF-β1 expression in the kidney and albuminuria was blunted in sirtuin-1 overexpressing transgenic mice.
Sirtuin-1 and STAT3
Signal transducer and activator of transcription3 (STAT3) are transcription factors, which are activated under diabetic conditions. The transcriptional activity of STAT3 is negatively regulated by sirtuin-1. A study by Liu et al. revealed that phosphorylation and acetylation of p65 and STAT3 were higher in the glomeruli of db/db mice than in those of db/m mice [26]. They also confirmed that acetylation of p65 and STAT3 is required for their transcriptional activity by mutating the acetylated residues. Knockout of sirtuin-1 in podocytes significantly increased acetylation of p65 and STAT3 and the mice were more susceptible to DN.
Sirtuin-1 and VEGF mediate angiogenesis
Reduced sirtuin-1 expression results in angiopoietin 2, VEGF, and Flk-1 upregulation, which eventually leads to abnormal angiogenesis in DN. Abnormal angiogenesis occurs in the kidneys of patients and animal models with DN. Emerging evidence demonstrates that abnormal angiogenesis is associated with glomerular hypertrophy and plasma leakage, which play a pathological role in DN [112]. VEGF is a pro-angiogenic factor that binds to its receptor VEGFR-2 (KDR/Flk-1) and generates angiogenic signals by dimerization and phosphorylation of VEGFR-2 [113]. Angiopoietins are vascular growth factors, including angiopoietin 1 (effective factor) and angiopoietin 2 (antagonist of angiopoietin 1). Angiopoietin 1 and angiopoietin 2 competitively bind to their receptor tie-2 (tyrosine kinase with Ig and EGF homology domain 2). High concentrations of angiopoietin 2 and VEGF promote endothelial proliferation and angiogenesis, whereas apoptosis is induced when the upregulation of angiopoietin 2 is not paralleled by that of VEGF [114]. It is reported that VEGF, Flk-1, and angiopoietin 2 are upregulated in DN, and the upregulation of sirtuin-1 in cultured ECs reduces Flk-1 expression. In addition, resveratrol has beneficial effects in cultured podocytes and ECs by ameliorating the HG-induced expression of VEGF and Flk-1, but these effects are abolished by knocking down sirtuin-1 [115].These findings suggest that sirtuin-1 can attenuate abnormal angiogenesis in DN by modulating VEGF, Flk-1, and angiopoietin 2.
Sirtuin-1 and the renin-angiotensin system
Downregulation of sirtuin-1 is involved in DN partly via its interacting with RAS. Inhibition of the renin-angiotensin system (RAS) benefits diabetic and DN patients, which indicates that the RAS is involved in the progression of DN. Angiotensin II (Ang II) is the principal effector of the RAS, but angiotensin-converting enzyme 2 (ACE2) counteracts the effects of Ang II by hydrolyzing Ang II to form angiotensin 1–7, which is protective against DN [116]. There is some evidence to suggest that sirtuin-1 interacts with the RAS: sirtuin-1 targets and activates the ACE2 promoter [116, 117], while Ang II regulates sirtuin-1 expression and activity. The expression of sirtuin-1 declines and the acetylation of p53 increases in a time-dependent fashion in Ang II–treated podocytes. Olmesartan, an angiotensin receptor blocker, is reported to increase sirtuin-1 activity, and to reduce p53 acetylation and p38 phosphorylation in the kidneys of diabetic db/db mice [118]. Furthermore, angiotensin 1–7 could protect against DN in db/db mice by increasing sirtuin-1 expression [116].
Endogenous factors regulate sirtuin-1 in diabetic nephropathy
Nampt and sirtuin-1
Metabolism of glucose and fatty acids produces NADH, which results in a reduction in the NAD+/NADH ratio under conditions of nutrient excess, such as diabetes, and is also associated with a reduction in the expression of sirtuin-1. Nampt is a key enzyme catalyzing NAD+ biosynthesis. Endogenous Nampt expression in the kidneys of STZ-induced diabetic rats was reported to be 2.36-fold higher than that of control rats [35], and immunohistochemistry demonstrated that Nampt is localized to the glomerular and tubular cells of diabetic rats [119]. In vitro, progressively higher expression of Nampt, NF-κB p65, FoxO1, and Bax, and progressively lower expression of sirtuin-1, were observed in the glomerular mesangial HBZY-1 cell line when it was exposed to HG conditions, which induced excessive expression of vimentin and FN [35].
Heterogeneous nuclear ribonucleoprotein F and sirtuin-1
Heterogeneous nuclear ribonucleoprotein F (hnRNP F) belongs to the pre-mRNA-binding protein family and regulates gene expression at transcriptional and post-transcriptional levels. It was reported that the expression of hnRNP F, sirtuin-1, and FoxO3a was lower in human type 2 diabetic kidneys than non-diabetic kidneys. Overexpression of hnRNP F leads to higher levels of binding to the sirtuin-1 promoter, activating transcription, and leading to the attenuation of OS in PTCs, tubulointerstitial fibrosis, and apoptosis in db/db mice, because of deacetylation of Foxo3a and p53, and greater expression of CAT [120].
MicroRNAs and sirtuin-1
miRNAs are 21–25 nucleotide–long non-coding RNAs that regulate target gene expression by binding to their 3’-untranslated regions (UTRs), causing degradation or repressing their translation. Accumulating evidence demonstrates that specific miRNAs contribute to the pathogenesis of DN by targeting sirtuin-1 mRNA (Fig. 2).
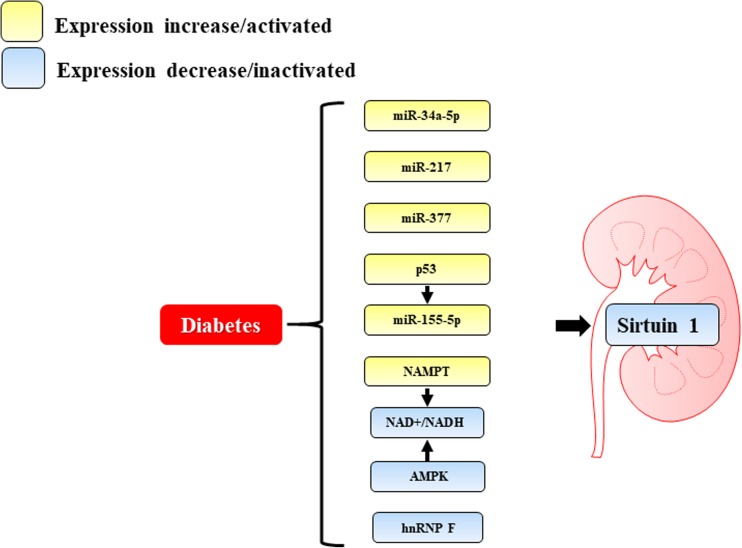
Fig. 2.
Endogenous factors regulating sirtuin-1 in diabetic nephropathy. A reduction in the NAD+/NADH ratio induced by higher Nampt expression and the metabolism of glucose and fatty acids causes a downregulation of sirtuin-1 during diabetes. Low expression of hnRNP F in diabetes downregulates sirtuin-1 transcription. High expression of miR-34a-5p, miR-377, and miR-155-5p suppresses sirtuin-1 levels by directly targeting its 3’-untranslated region. Upregulation of miR-34a-5p and miR-217 expression is negatively correlated with sirtuin-1 expression in diabetic nephropathy. Diabetes-induced DNA damage promotes the expression and activity of p53, which induces miR-155-5p expression and inhibits sirtuin-1 expression. Downregulation of AMPK decreases the NAD+ concentration and consequently the expression of sirtuin-1
miR-34a-5p has been identified to suppress sirtuin-1 by directly targeting its 3’-UTR. When miR-34a-5p expression is high, sirtuin-1 expression falls, increasing the expression of the pro-fibrogenic factor TGF-β1, FN, and collagen type I in the kidneys of high-fat diet and STZ-induced diabetic mice [41]. It is also known that long non-coding RNAs (lncRNAs) can interact with miRNAs. 1700020I14Rik (ENSMUST00000147425) is a lncRNA that directly targets miR-34a-5p and interacts with it in an Ago2-dependent manner. Downregulation of 1700020I14Rik has been shown to promote proliferation and fibrosis via a miR-34a-5p/sirtuin-1 pathway in MCs [121].
Recently, a clinical study suggested that urinary miR-377 could be used as an early biomarker of DN in pediatric T1DM. The urinary miR-377 level is higher in T1DM patients with microalbuminuria, than in both normoalbuminuric patients and healthy controls [122]. HG triggers the upregulation of miR-377 in GMCs, which inhibits PPARγ expression and promotes plasminogen activator inhibitor-1 (PAI-1) and TGF-β1 expression [123]. miR-377 was identified to target the 3’-UTR of sirtuin-1 mRNA directly and reduces sirtuin-1 protein abundance in the adipose tissue of high-fat diet–fed mice [124]. However, further studies regarding the role of the interaction between miR-377 and sirtuin-1 in the pathogenesis and development of DN are required.
Another clinical study showed that miR-217 closely correlates with the severity of DN in patients with T2DM. Serum miR-217 levels positively correlate with the severity of albuminuria and negatively correlate with sirtuin-1 expression in patients with T2DM [19]. In addition, the study by Shao et al. showed that miR-217 expression increases in HG-treated rat GMCs. By contrast, silencing miR-217 or resveratrol treatment promotes the expression of sirtuin-1, which results in lower expression of HIF-1α and ameliorates HG-induced increases in CTGF, endothelin-1, FN, TGF-β1, and VEGF expression in rat GMCs [32].
Recently, miR-155-5p has become of interest due to its effects on renal tubule injury as part of a p53/miR-155-5p/sirtuin-1 pathway. Baker et al. measured the expression of this miRNA in the glomeruli and proximal tubules of 98 patients and discovered that miR-155-5p expression is high in the proximal tubules of individuals with DN, and in membranoproliferative glomerulonephritis and focal segmental glomerulosclerosis [125]. Bioinformatic analysis predicted the existence of binding sites of miR-155-5p on sirtuin-1 mRNA, and Wang et al. confirmed the presence of a p53/miR-155-5p/sirtuin-1 pathway in tubule cells incubated in HG medium [96].
Ubiquitin-specific protease 22 and sirtuin-1
AGEs regulate sirtuin-1 expression by enhancing its ubiquitination and proteasome-mediated degradation. Ubiquitin-specific protease 22 (USP22) reduces the degradation of sirtuin-1 and the expression of FN and TGF-β1 in AGE-treated GMCs, whereas depletion of USP22 promotes sirtuin-1 degradation and the expression of FN and TGF-β1 in this cell model. Therefore, an AGE/USP22/sirtuin-1 pathway may be involved in the pathological progression of DN [126].
In addition to the above, caspase1, Ang II, and cyclin-dependent kinase 5 (CDK5) have been shown to regulate the level and activity of sirtuin-1 in other tissue and models, indicating that they are probably also involved in the development of DN. Caspase 1 is a member of the cysteine protease caspase family, which is activated by the “inflammasome” and required for the cleavage of multiple substrates, such as IL-1β, Il-18, and sirtuin-1; thus, it is probably also involved in atherosclerosis and DN [127, 128]. One study suggested that activation of caspase 1 decreases the level of sirtuin-1 by cleaving sirtuin-1 and promotes pro-inflammatory cytokine activation in the ECs of the aorta of ApoE−/− mice fed a high-fat diet, resulting in the activation of ECs and the initiation of vascular inflammation [127]. Knockout of caspase 1 increased sirtuin-1 levels and decreased aortic monocyte recruitment. Whether caspase-1 involves in DN via regulating sirtuin-1 is required to study. As mentioned in the section of sirtuin-1 and RAS, Ang II regulates sirtuin-1 expression and activity. A study from Huang found that Ang II activated JKN and subsequently led to sirtuin-1 degradation, which enhanced insulin-like growth factor receptor II signaling during Ang II cardiac hypertrophy and apoptosis [129]. Ang II time dependently reduced sirtuin-1 expression and induced apoptosis of cultured podocytes, which was reversed by the AT1 blocker olmesartan [118]. CDK5 is a serine/threonine kinase that phosphorylates sirtuin-1 and inhibits the anti-senescent and anti-inflammatory activity of sitruin-1 [130]. Phosphorylation of sirtuin-1 at S47 by CDK5 prevents sirtuin-1 nuclear exportation and association with the telomeric repeat–binding factor 2–interacting protein 1 in ECs. Recently, the CDK5-dependent ubiquitin-proteasome pathway was reported to mediate the degradation of sirtuin-1 in Parkinson’s disease models. Inhibition of CDK5 blocked sirtuin-1 degradation [131]. Although CDK5 contributes to podocyte apoptosis and renal tubulointerstitial fibrosis in DN [132, 133], it is unknown whether CDK5 suppression reserves DN by blocking sirtuin-1 degradation.
Treatments targeting sirtuin-1 in diabetic nephropathy
There is a growing literature describing the therapeutic effects of synthetic drugs and natural compounds on inflammation, OS, apoptosis, and fibrosis in DN, which are exerted through the upregulation or activation of sirtuin-1.
As shown in Table 2, it is clear that several types of kidney cells are responsive to sirtuin-1 activators such as resveratrol. These findings provide a foundation for preclinical and clinical trials targeting sirtuin-1 in diabetic animals and patients with diabetes.
Table 2.
Synthetic drugs and natural compounds that increase the expression of or activate sirtuin-1 in high glucose or advanced glycation end product–treated renal cells
| Drug or natural substances | Renal cell | Mechanism of renoprotection | Pathway | Reference |
|---|---|---|---|---|
| Glycyrrhizic acid | HG-treated renal tubular epithelial cell line (NRK-52E) | Anti-oxidant, anti-proliferative | AMPK/sirtuin-1/PGC-1α signaling, TGF-β1, Mn-SOD | [57] |
| Glucagon-like peptide-1 | Podocytes cultured in HG medium | Anti-apoptotic, anti-oxidant, anti-inflammatory | Sirtuin-1, IL-1, IL-6 | [134] |
| Grape seed procyanidin B2 | High-dose glucosamine-treated rat mesangial cell line (HBZY-1) | Restore mitochondrial function, anti-apoptotic, anti-oxidant | AMPK/sirtuin-1/PGC-1α | [135] |
| Metformin | HG-treated primary rat podocytes | Improve podocyte insulin resistance and glucose uptake, reduce glomerular filtration barrier permeability | AMPK, sirtuin-1 | [136] |
| Olmesartan | HG-treated conditionally immortalized mouse podocytes | Anti-apoptotic | Angiotensin II/p38/sirtuin-1 | [118] |
| Probucol | HG-treated human proximal tubular epithelial cells (HK-2) | Anti-oxidant, anti-fibrotic | p66Shc, AMPK/sirtuin-1/AcH3 | [137] |
| Puerarin (active compound of radix puerariae) | HG-treated conditionally immortalized murine podocytes | Anti-oxidant | Sirtuin-1, NF-κB, NOX4 | [81] |
| Polydatin (glucoside of resveratrol) | Advanced glycation end product–treated glomerular mesangial cells | Anti-oxidant | Sirtuin-1/Nrf2/ARE | [64] |
| Panax notoginseng saponins | HG-treated rat mesangial cells | Anti-inflammatory, anti-oxidant, anti-fibrotic | Sirtuin-1/NF-κB, PAI-1, TGF-β1, MCP-1, SOD | [80] |
| Resveratrol | HG-treated human endothelial cells | Counteract the other pro-atherosclerotic effects, downregulate endothelial nitric oxide synthase | Sirtuin-1 | [138] |
| HG-treated primary rat mesangial cells | Anti-senescent | mTOR, sirtuin-1 | [61] | |
| HG-treated primary rat mesangial cells | Anti-oxidant, restore mitochondrial function | Sirtuin-1, Mn-SOD | [63] | |
| HG-treated conditionally immortalized mouse podocytes; HG-treated immortalized mouse endothelial cell line | Suppress VEGF expression and secretion in podocytes, suppress Flk-1 expression in glomerular endothelial cells, ameliorate hyperpermeability and cellular junction disruption | Sirtuin-1, VEGF, Flk-1 | [115] | |
| Advanced glycation end product–treated rat primary glomerular mesangial cells | Anti-oxidant, anti-fibrotic | Sirtuin-1, Nrf2/ARE, TGF-β1 | [34] | |
| HG-treated NMS2 mesangial cells | Anti-oxidant, anti-apoptotic | AMPK/sirtuin-1/PGC-1α | [56] | |
| HG-treated human kidney epithelial (HK-2) cells | Anti-oxidant | Sirtuin-1/FOXO3a | [89] | |
| Shenkang injection (composed of Radix Astragali, Rhubarb, Astragalus, Safflower, and Salvia) | HG-treated primary renal proximal tubular epithelial cells | Anti-senescent, anti-oxidant | mTOR, p66Shc, sirtuin-1, PPARγ, P16INK4, cyclin D1, SOD | [40] |
| Theobromine | HG-treated immortalized human mesangial cells | Anti-fibrotic | NOX4, AMPK, sirtuin-1/TGF-β | [139] |
| Tetrahydroxystilbene glucoside (active component of Polygonum multiflorum Thunb) | HG-treated rat mesangial cell line (HBZY-1) | Anti-oxidant | Sirtuin-1/TGF-β1, COX-2 | [140] |
A list of preclinical studies of treatments for DN that target sirtuin-1 is shown in Table 3. These have shown the following effects: (1) a reduction in the urinary Alb/Cre ratio or 24 h urine albumin; (2) an amelioration of renal histopathology; (3) reductions in markers of OS, inflammation, and apoptosis; (4) an improvement in autophagy; and/or (5) prevention of fibrogenesis. Furthermore, we showed that fenofibrate, a PPARα agonist, can stimulate fibroblast growth factor 21/sirtuin-1–dependent autophagy, which can prevent T1DM-induced cardiac damage [145]. We also found that it protects against T1DM-induced nephropathy by activating fibroblast growth factor 21 and Nrf2 pathways, although sirtuin-1 was not implicated in this study [146]. Thus, whether the renoprotective effects of fenofibrate in T1DM are due to the upregulation of sirtuin-1 remains unclear.
Table 3.
Synthetic drugs and natural compounds identified as regulators of sirtuin-1 in preclinical studies of diabetic nephropathy in animal models
| Substance | Animal model | Mechanism of renoprotection | Pathway | Reference |
|---|---|---|---|---|
| 3,5-Diiodo-L-thyronine | T1DM: STZ-induced diabetic rat | Prevent decrease in sirtuin-1 expression and activity; anti-fibrotic transforming growth factor-β1 expression, fibronectin and type IV collagen | Sirtuin-1/NF-κB | [141] |
| Allium sativum (garlic) | T1DM: STZ-induced diabetic rat T2DM: STZ + niacinamide–induced diabetic rat |
Increase sirtuin-1 and sirtuin-2 gene expression in kidney | Sirtuin-1; sirtuin-2 | [21] |
| Glycyrrhizic acid | T2DM: db/db mouse | Anti-oxidant, anti-fibrotic | AMPK/sirtuin-1/PGC-1α signaling | [22] |
| Grape seed proanthocyanidin extracts | T2DM: high-carbohydrate/high-fat diet and STZ-induced diabetic rat | Restore mitochondrial function, anti-apoptotic, anti-oxidant, increase nephrin and podocalyxin | AMPK/sirtuin-1/PGC-1α | [59] |
| Hesperidin and quercetin | T1DM: STZ-induced diabetic rat | Anti-oxidant | NF-κB, sirtuin-1, SOD, CAT | [142] |
| INT-777 (G protein–coupled bile acid receptor TGR5 agonist) | T2DM: db/db mouse | Increase renal mitochondrial biogenesis, decrease oxidative stress, increase fatty acid beta-oxidation | Sirtuin-1, sirtuin-3, Nrf1, SOD2 | [143] |
| Olmesartan | T2DM: db/db mice | Anti-apoptotic, suppress p38 phosphorylation | Angiotensin II/p38/sirtuin-1 | [118] |
| Probucol | T2DM: high-fat, high-cholesterol Western diet and STZ-induced diabetic mice | Anti-oxidant, anti-fibrotic | p66Shc, AMPK/sirtuin-1/AcH3 | [137] |
| Puerarin (active compound of Radix Puerariae) | STZ-induced diabetes in endothelial nitric oxide synthase–null (eNOS−/−) mouse | Anti-oxidant | NF-κB, NOX4 | [81] |
| T1DM: STZ-induced diabetic mouse | Anti-oxidant, anti-inflammatory | Sirtuin-1/FOXO1, TNF-α, NF-κB, IL-6 | [87] | |
| Panax notoginseng saponins | T1DM: alloxan-induced rat | Anti-inflammatory, anti-oxidant, anti-fibrotic | Sirtuin-1/NF-κB, PAI-1, TGF-β1, MCP-1, SOD | [80] |
| Resveratrol | T1DM: STZ-induced diabetic rat | Anti-oxidant, prevent dephosphorylation of histone H3, reduce the expression of p38 and p53 | Sirtuin, p53, p38 | [95] |
| T1DM: STZ-induced diabetic rat | Anti-oxidant | Sirtuin-1/FOXO1 | [88] | |
| T1DM: STZ-induced diabetic rat | Modulate angiogenesis | Sirtuin-1, VEGF, Flk-1, Tie-2 | [115] | |
| T1DM: STZ-induced diabetic rat | Anti-oxidant, anti-fibrotic | Sirtuin-1, Nrf2/ARE, TGF-β1, FN | [34] | |
| T2DM: db/db mouse | Prevent renal lipotoxicity and glucotoxicity, anti-oxidant, anti-apoptotic | AMPK/sirtuin-1/PGC-1α | [56] | |
| T2DM: STZ-induced diabetic rat | Anti-inflammatory; enhance autophagy | NAD+/sirtuin-1, TNF-α, IL-6, IL-1β, IL-10 | [7] | |
| T1DM: STZ-induced diabetic rat | Anti-oxidant | Sirtuin-1/FoxO3a | [89] | |
| Resveratrol and rosuvastatin | T2DM: STZ + niacinamide–induced diabetic rat | Anti-oxidant, anti-fibrotic | TGF-β1, NF-κB/p65, Nrf2, sirtuin-1, FoxO1 | [144] |
| Roflumilast | T1DM: STZ-induced diabetic rat | Anti-oxidant, anti-fibrotic, anti-apoptotic | AMPK/sirtuin-1, FoxO1, HO-1 | [52] |
| Theobromine | Spontaneously hypertensive rat treated with STZ | Anti-fibrotic | NOX4, AMPK, sirtuin-1/TGF-β | [139] |
| Tetrahydroxystilbene glucoside (active component extract of Polygonum multiflorum Thunb) | T1DM: STZ-induced diabetic rat | Anti-oxidant | Sirtuin-1/TGF-β1, COX-2 | [140] |
A number of substances and plant extracts that have been found to restore sirtuin-1 activity and have renoprotective effects in preclinical models of DN have been prescribed clinically. Of these, resveratrol is one of the most extensively studied sirtuin-1 activators. However, although the protective effects of resveratrol have been shown in vitro and in vivo, its clinical benefits are controversial [147]. A randomized double-blind placebo-controlled trial revealed that 6 months of low-dose or high-dose resveratrol supplementation did not improve arterial pressure, blood glucose, uric acid, adiponectin, or IL-6 in patients with T2DM [148]. By contrast, a study of 66 patients with T2DM showed that resveratrol (1 g/day for 45 days) improved systolic blood pressure, blood glucose, and IR, but not kidney function [149]. This study used creatinine, rather than urinary Alb/Cre, as an index of kidney function, which is probably why a renoprotective effect was not identified. Another randomized double-blind clinical trial evaluated the effects of resveratrol on albuminuria in DN. The 60 patients enrolled, who had DN and albuminuria, were divided into two groups: resveratrol- (500 mg/day) and losartan (an angiotensin receptor blocker, 12.5 mg/day)-treated, and placebo- and losartan (12.5 mg/day)-treated. After 90-day treatment, the urinary Alb/Cre ratio was significantly lower in the resveratrol group, although GFR and serum creatinine were not different. Although sirtuin-1 was not measured in this study, serum anti-oxidant enzymes, such as SOD, CAT, and glutathione peroxidase, and nitric oxide, were significantly higher in the resveratrol group [150].
Grape seed extracts have been shown to protect against diabetes-induced kidney lesions by activating sirtuin-1 in renal cell lines and animal models. A double-blind randomized controlled trial demonstrated that grape seed extracts could benefit T2DM patients with high cardiovascular risk by ameliorating inflammation and OS [151], but renoprotective effects and sirtuin-1 levels were not evaluated in this trial.
Inhibition of the RAS benefits patients with DN. Olmesartan, an angiotensin receptor blocker, has been shown to prevent microalbuminuria in T2DM patients [152]. In vitro and in vivo studies showed that one of its renoprotective effects is to reduce podocyte apoptosis by increasing sirtuin-1 expression [118], although the mechanism has not been identified in humans.
So far, sirtuin-1 activators, such as resveratrol, have not been shown definitively to have beneficial effects on DN in clinical trials, although they have been shown to have renoprotective effects in preclinical studies. DN takes several weeks to develop in animal models but many years to develop in humans; thus, the processes involved in the development of DN in human are probably more complicated than those in animal models. This might explain, at least partially, the differences in the effects of sirtuin-1 activators between clinical and preclinical studies. In addition, DN is generally progressive and irreversible at the time it is diagnosed. Therefore, when treatment is initiated will have a profound effect on the therapeutic outcomes of sirtuin-1 activator treatments in DN patients.
Conclusions and perspectives
Metabolic disturbance, OS, inflammation, impairs autophagy, hypoxia, abnormal angiogenesis, apoptosis, and activation of the RAS result in kidney lesions in diabetes. The deacetylase sirtuin-1 is involved in all of these aspects of the pathogenesis of DN. Downregulation of sirtuin-1 in diabetes increases the acetylation of histones and that of crucial transcription factors, including p53, FoxO, NF-κB, and Nrf2, which are involved in numerous feedback loops and networks that promote the development of DN. Its key role in DN makes sirtuin-1 a target for preventive and therapeutic purposes. Here, we summarized the synthetic drugs and natural compounds that are used in the treatment of DN and which target sirtuin-1. In vitro studies have identified the molecular effects of these substances in various types of renal cells, and preclinical studies have shown protective effects against DN and on sirtuin-1 and its downstream target proteins. Although some clinical trials demonstrated that sirtuin-1 activators, such as resveratrol, benefit patients with T2DM and microalbuminuria, others did not show protective effects, possibly due to differences in dose, disease stage, treatment duration, and the characteristics of the patients studied. Because the regulation and effects of sirtuin-1 are complex, further investigation of its molecular interactions, such as with miRNAs and lncRNAs, which may underpin its protective effects, is required. In addition, well-designed clinical trials in patients with T2DM and T1DM are required to assess the renoprotective effects of substances that have been shown to have beneficial effects exerted via sirtuin-1 in vitro and in vivo.
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- AdipoR
adiponectin receptor
- AGE
advanced glycation end product
- AMPK
AMP-dependent protein kinase
- Ang II
angiotensin II
- ARE
anti-oxidant response element
- Atg
autophagy-related gene
- Bnip3
Bcl2/adenovirusE1 V 19 kDa interacting protein 3
- CAT
catalase
- CDK5
cyclin-dependent kinase 5
- CR
calorie restriction
- CTGF
connective tissue growth factor
- DN
diabetic nephropathy
- ECM
extracellular matrix
- EC
endothelial cell
- EMT
epithelial-to-mesenchymal transition
- FN
fibronectin
- FOXO
forkhead box group O
- GFR
glomerular filtration rate
- GMC
glomerular mesangial cell
- HG
high glucose
- HIF-1α
hypoxia-inducible factor-1α
- hnRNP F
heterogeneous nuclear ribonucleoprotein F
- HO-1
heme oxygenase 1
- ICAM-1
intercellular adhesion molecule-1
- IR
insulin resistance
- Keap1
Kelch-like ECH–associated protein 1
- LC3
light chain 3
- LincRNA
long intergenic non-coding RNA
- LKB1
liver kinase B1
- lncRNA
long non-coding RNA
- MCP-1
monocyte chemotactic protein-1
- miRNA
microRNA
- Mn-SOD
manganese superoxide dismutase
- NAD+
nicotinamide adenine dinucleotide
- Nampt
nicotinamide phosphoribosyltransferase
- NF-κB
nuclear factor-κB
- NMN
nicotinamide mononucleotide
- NOX4
NADPH oxidase 4
- NQO-1
NAD(P)H quinone dehydrogenase 1
- Nrf2
nuclear factor erythroid 2–related factor 2
- PAI-1
plasminogen activator inhibitor-1
- p66Shc
66 kDa Src homology 2 domain–containing protein
- PGC-1α
peroxisome proliferator–activated receptor-γ coactivator 1α
- PKC
protein kinase C
- PPAR
peroxisome proliferator–activated receptor
- PTC
proximal tubular cell
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- Smad
small mothers against decapentaplegic homolog
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- STAT3
signal transducer and activator of transcription3
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TNF-α
tumor necrosis factor-α
- TGF-β1
transforming growth factor-β1
- USP22
ubiquitin-specific protease 22
- VCAM-1
vascular cell adhesion protein-1
- VEGF
vascular endothelial growth factor
Authors’ contributions
All authors wrote, revised, and approved the manuscript. All authors read and approved the final manuscript.
Funding information
The authors’ work cited in this review was supported in part by grants from the National Natural Science Foundation of China (81400725 to W.S., 81700635 to Y.C.), the Natural Science Foundation of Jilin Province (20160101030JC to W.S.), the 13th Five-Year scientific research planning program of Jilin province (JJKH20180210KJ to W.S), the National Postdoctoral Program for Innovative Talents (BX201700098 to Y.C.), and the Postdoctoral Science Foundation of China (2017M621216 to Y.C.).
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wanning Wang, Email: 15531030@qq.com.
Weixia Sun, Email: sunweixia_78@163.com.
Yanli Cheng, Email: leoai918@163.com.
Zhonggao Xu, Phone: +8615804303293, Email: renalxu@163.com.
Lu Cai, Phone: 502-852-2214, Email: l0cai001@louisville.edu.
References
- 1.Gheith O, Farouk N, Nampoory N, Halim MA, al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie. 2008;90(2):306–312. doi: 10.1016/j.biochi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Kong L, et al. Sirtuin 1: a target for kidney diseases. Mol Med. 2015;21:87–97. doi: 10.2119/molmed.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16(10):4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J. 2013;37(5):315–325. doi: 10.4093/dmj.2013.37.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakino S, Hasegawa K, Itoh H. Sirtuin and metabolic kidney disease. Kidney Int. 2015;88(4):691–698. doi: 10.1038/ki.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, Lv Z, Chen Z, Han J, Jia L, Wang L. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract. 2016;212(4):310–318. doi: 10.1016/j.prp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Wei J, Hou X, Liu H, Guo F, Zhou Y, Zhang Y, Qu Y, Gu J, Zhou Y, Jia X, Qin G, Feng L. SIRT1 rs10823108 and FOXO1 rs17446614 responsible for genetic susceptibility to diabetic nephropathy. Sci Rep. 2017;7(1):10285. doi: 10.1038/s41598-017-10612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol. 2014;51(6):905–915. doi: 10.1007/s00592-014-0650-7. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Tervaert TW, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 12.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124(3):139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 13.Sifuentes-Franco S, et al. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018;2018:1875870. doi: 10.1155/2018/1875870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takiyama Y, Haneda M. Hypoxia in diabetic kidneys. Biomed Res Int. 2014;2014:837421. doi: 10.1155/2014/837421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58(7):1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanabe K, et al. Antiangiogenic therapy for diabetic nephropathy. Biomed Res Int. 2017;2017:5724069. doi: 10.1155/2017/5724069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda S, Koya D, Araki SI, Babazono T, Umezono T, Toyoda M, Kawai K, Imanishi M, Uzu T, Suzuki D, Maegawa H, Kashiwagi A, Iwamoto Y, Nakamura Y. Association between single nucleotide polymorphisms within genes encoding sirtuin families and diabetic nephropathy in Japanese subjects with type 2 diabetes. Clin Exp Nephrol. 2011;15(3):381–390. doi: 10.1007/s10157-011-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang K, Sun M, Shen J, Zhou B. Transcriptional coactivator p300 and silent information regulator 1 (SIRT1) gene polymorphism associated with diabetic kidney disease in a Chinese cohort. Exp Clin Endocrinol Diabetes. 2017;125(8):530–537. doi: 10.1055/s-0043-103966. [DOI] [PubMed] [Google Scholar]
- 19.Shao Y, Ren H, Lv C, Ma X, Wu C, Wang Q. Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine. 2017;55(1):130–138. doi: 10.1007/s12020-016-1069-4. [DOI] [PubMed] [Google Scholar]
- 20.Tikoo K, Tripathi DN, Kabra DG, Sharma V, Gaikwad AB. Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett. 2007;581(5):1071–1078. doi: 10.1016/j.febslet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Arab Sadeghabadi Z, Ziamajidi N, Abbasalipourkabir R, Mohseni R. Garlic (Allium sativum) increases SIRT1 and SIRT2 gene expressions in the kidney and liver tissues of STZ- and STZ+niacinamide-induced diabetic rats. J Basic Clin Physiol Pharmacol. 2018;29:463–467. doi: 10.1515/jbcpp-2017-0079. [DOI] [PubMed] [Google Scholar]
- 22.Hou S, et al. Glycyrrhizic acid prevents diabetic nephropathy by activating AMPK/SIRT1/PGC-1alpha signaling in db/db mice. J Diabetes Res. 2017;2017:2865912. doi: 10.1155/2017/2865912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Chang B, Zhang Y, Yang P, Liu L. Rhein promotes the expression of SIRT1 in kidney tissues of type 2 diabetic rat. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(5):615–619. [PubMed] [Google Scholar]
- 24.Chuang PY, Xu J, Dai Y, Jia F, Mallipattu SK, Yacoub R, Gu L, Premsrirut PK, He JC. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol. 2014;184(7):1940–1956. doi: 10.1016/j.ajpath.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou MM, Chuang PY, He JC. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63(7):2440–2453. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motonishi S, Nangaku M, Wada T, Ishimoto Y, Ohse T, Matsusaka T, Kubota N, Shimizu A, Kadowaki T, Tobe K, Inagi R. Sirtuin1 maintains actin cytoskeleton by deacetylation of cortactin in injured podocytes. J Am Soc Nephrol. 2015;26(8):1939–1959. doi: 10.1681/ASN.2014030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatani Y, Inagi R. Epigenetic regulation through SIRT1 in podocytes. Curr Hypertens Rev. 2016;12(2):89–94. doi: 10.2174/1573402112666160302102515. [DOI] [PubMed] [Google Scholar]
- 29.Chuang PY, Dai Y, Liu R, He H, Kretzler M, Jim B, Cohen CD, He JC. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One. 2011;6(8):e23566. doi: 10.1371/journal.pone.0023566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa K, Wakino S, Sakamaki Y, Muraoka H, Umino H, Minakuchi H, Yoshifuji A, Naitoh M, Shinozuka K, Futatsugi K, Urai H, Kanda T, Tokuyama H, Hayashi K, Itoh H. Communication from tubular epithelial cells to podocytes through Sirt1 and nicotinic acid metabolism. Curr Hypertens Rev. 2016;12(2):95–104. doi: 10.2174/1573402112666160302102217. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Sunq A, Roth RA, Hou J. Inducible expression of claudin-1 in glomerular podocytes generates aberrant tight junctions and proteinuria through slit diaphragm destabilization. J Am Soc Nephrol. 2017;28(1):106–117. doi: 10.1681/ASN.2015121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao Y, Lv C, Wu C, Zhou Y, Wang Q. Mir-217 promotes inflammation and fibrosis in high glucose cultured rat glomerular mesangial cells via Sirt1/HIF-1alpha signaling pathway. Diabetes Metab Res Rev. 2016;32(6):534–543. doi: 10.1002/dmrr.2788. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, Cui S, Wei R, Chen X, Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell Physiol Biochem. 2011;27(6):681–690. doi: 10.1159/000330077. [DOI] [PubMed] [Google Scholar]
- 34.Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, Liu P, Huang H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–540. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Liang Y, Hu T, Wei R, Cai C, Wang P, Wang L, Qiao W, Feng L. Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-kappaB p65 and Sirt1 pathway; NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt. Exp Ther Med. 2017;14(5):4181–4193. doi: 10.3892/etm.2017.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kida Y, Zullo JA, Goligorsky MS. Endothelial sirtuin 1 inactivation enhances capillary rarefaction and fibrosis following kidney injury through Notch activation. Biochem Biophys Res Commun. 2016;478(3):1074–1079. doi: 10.1016/j.bbrc.2016.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012;180(3):973–983. doi: 10.1016/j.ajpath.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8(1):e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HS, Lim JH, Kim MY, Kim Y, Hong YA, Choi SR, Chung S, Kim HW, Choi BS, Kim YS, Chang YS, Park CW. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J Transl Med. 2016;14(1):176. doi: 10.1186/s12967-016-0922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu B, et al. (2018) Preventive effect of Shenkang injection against high glucose-induced senescence of renal tubular cells. Front Med. 10.1007/s11684-017-0586-8 [DOI] [PubMed]
- 41.Xue M, Li Y, Hu F, Jia YJ, Zheng ZJ, Wang L, Xue YM. High glucose up-regulates microRNA-34a-5p to aggravate fibrosis by targeting SIRT1 in HK-2cells. Biochem Biophys Res Commun. 2018;498(1):38–44. doi: 10.1016/j.bbrc.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 42.Ruderman NB, Julia Xu X, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kume S, Kitada M, Kanasaki K, Maegawa H, Koya D. Anti-aging molecule, Sirt1: a novel therapeutic target for diabetic nephropathy. Arch Pharm Res. 2013;36(2):230–236. doi: 10.1007/s12272-013-0019-4. [DOI] [PubMed] [Google Scholar]
- 44.Kume S, et al. Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int. 2014;2014:315494. doi: 10.1155/2014/315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283(41):27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14(5):661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding DF, You N, Wu XM, Xu JR, Hu AP, Ye XL, Zhu Q, Jiang XQ, Miao H, Liu C, Lu YB. Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am J Nephrol. 2010;31(4):363–374. doi: 10.1159/000300388. [DOI] [PubMed] [Google Scholar]
- 50.Cammisotto PG, Londono I, Gingras D, Bendayan M. Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol. 2008;294(4):F881–F889. doi: 10.1152/ajprenal.00373.2007. [DOI] [PubMed] [Google Scholar]
- 51.Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K, Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res. 2011;2011:1–11. doi: 10.1155/2011/908185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tikoo K, Lodea S, Karpe PA, Kumar S. Calorie restriction mimicking effects of roflumilast prevents diabetic nephropathy. Biochem Biophys Res Commun. 2014;450(4):1581–1586. doi: 10.1016/j.bbrc.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 53.Besseiche A, Riveline JP, Gautier JF, Bréant B, Blondeau B. Metabolic roles of PGC-1alpha and its implications for type 2 diabetes. Diabetes Metab. 2015;41(5):347–357. doi: 10.1016/j.diabet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282(4):647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 55.Jager S, Handschin C, St.-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim MY, Lim JH, Youn HH, Hong YA, Yang KS, Park HS, Chung S, Koh SH, Shin SJ, Choi BS, Kim HW, Kim YS, Lee JH, Chang YS, Park CW. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia. 2013;56(1):204–217. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 57.Hou S, Zheng F, Li Y, Gao L, Zhang J. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int J Mol Sci. 2014;15(9):15026–15043. doi: 10.3390/ijms150915026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai X, Bao L, Ren J, Li Y, Zhang Z. Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1alpha axis in vitro. Food Funct. 2016;7(2):805–815. doi: 10.1039/c5fo01062d. [DOI] [PubMed] [Google Scholar]
- 59.Bao L, Cai X, Dai X, Ding Y, Jiang Y, Li Y, Zhang Z, Li Y. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-gamma coactivator 1alpha in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct. 2014;5(8):1872–1880. doi: 10.1039/c4fo00340c. [DOI] [PubMed] [Google Scholar]
- 60.Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5(2):e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F, Chen X. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev. 2012;133(6):387–400. doi: 10.1016/j.mad.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S, Tikoo K. Independent role of PP2A and mTORc1 in palmitate induced podocyte death. Biochimie. 2015;112:73–84. doi: 10.1016/j.biochi.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Nie L, Yin YG, Tang JL, Zhou JY, Li DD, Zhou SW. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259(3):395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 64.Huang K, Chen C, Hao J, Huang J, Wang S, Liu P, Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol Cell Endocrinol. 2015;399:178–189. doi: 10.1016/j.mce.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Huang K, Gao X, Wei W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-beta1 expressions in rat glomerular mesangial cells. Exp Cell Res. 2017;361(1):63–72. doi: 10.1016/j.yexcr.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Chen Z, Gong W, Zou Y, Xu F, Chen L, Huang H. Paeonol ameliorates diabetic renal fibrosis through promoting the activation of the Nrf2/ARE pathway via up-regulating Sirt1. Front Pharmacol. 2018;9:512. doi: 10.3389/fphar.2018.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou L, Xu DY, Sha WG, Shen L, Lu GY, Yin X, Wang MJ. High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J Transl Med. 2015;13:352. doi: 10.1186/s12967-015-0710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon DS, Choi Y, Lee JW. Cellular localization of NRF2 determines the self-renewal and osteogenic differentiation potential of human MSCs via the P53-SIRT1 axis. Cell Death Dis. 2016;7:e2093. doi: 10.1038/cddis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar S, Kim YR, Vikram A, Naqvi A, Li Q, Kassan M, Kumar V, Bachschmid MM, Jacobs JS, Kumar A, Irani K. Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci U S A. 2017;114(7):1714–1719. doi: 10.1073/pnas.1614112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galimov ER. The role of p66shc in oxidative stress and apoptosis. Acta Nat. 2010;2(4):44–51. [PMC free article] [PubMed] [Google Scholar]
- 71.Xu X, Zhu X, Ma M, Han Y, Hu C, Yuan S, Yang Y, Xiao L, Liu F, Kanwar YS, Sun L. p66Shc: a novel biomarker of tubular oxidative injury in patients with diabetic nephropathy. Sci Rep. 2016;6:29302. doi: 10.1038/srep29302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, Zhang R, Cai H, Liu DP, Liang CC. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109(6):639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 73.Song P, et al. PKCdelta promotes high glucose induced renal tubular oxidative damage via regulating activation and translocation of p66Shc. Oxidative Med Cell Longev. 2014;2014:746531. doi: 10.1155/2014/746531. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Sun L, Xiao L, Nie J, Liu FY, Ling GH, Zhu XJ, Tang WB, Chen WC, Xia YC, Zhan M, Ma MM, Peng YM, Liu H, Liu YH, Kanwar YS. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Ren Physiol. 2010;299(5):F1014–F1025. doi: 10.1152/ajprenal.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q, Kim YR, Vikram A, Kumar S, Kassan M, Gabani M, Lee SK, Jacobs JS, Irani K. P66Shc-induced MicroRNA-34a causes diabetic endothelial dysfunction by downregulating sirtuin1. Arterioscler Thromb Vasc Biol. 2016;36(12):2394–2403. doi: 10.1161/ATVBAHA.116.308321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gasparini C, Feldmann M. NF-kappaB as a target for modulating inflammatory responses. Curr Pharm Des. 2012;18(35):5735–5745. doi: 10.2174/138161212803530763. [DOI] [PubMed] [Google Scholar]
- 78.Du YG, et al. Tangshen formula improves inflammation in renal tissue of diabetic nephropathy through SIRT1/NF-kappaB pathway. Exp Ther Med. 2018;15(2):2156–2164. doi: 10.3892/etm.2017.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab Vasc Dis Res. 2014;11(2):92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 80.Du YG, et al. Panax notoginseng saponins protect kidney from diabetes by up-regulating silent information regulator 1 and activating antioxidant proteins in rats. Chin J Integr Med. 2016;22(12):910–917. doi: 10.1007/s11655-015-2446-1. [DOI] [PubMed] [Google Scholar]
- 81.Li X, Cai W, Lee K, Liu B, Deng Y, Chen Y, Zhang X, He JC, Zhong Y. Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci Rep. 2017;7(1):14603. doi: 10.1038/s41598-017-14906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2011;14(4):579–592. doi: 10.1089/ars.2010.3419. [DOI] [PubMed] [Google Scholar]
- 83.Lalmansingh AS, Karmakar S, Jin Y, Nagaich AK. Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim Biophys Acta. 2012;1819(7):707–715. doi: 10.1016/j.bbagrm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Murtaza G, et al. FOXO transcriptional factors and long-term living. Oxidative Med Cell Longev. 2017;2017:3494289. doi: 10.1155/2017/3494289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 2011;1813(11):1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Carlomosti F, D’Agostino M, Beji S, Torcinaro A, Rizzi R, Zaccagnini G, Maimone B, di Stefano V, de Santa F, Cordisco S, Antonini A, Ciarapica R, Dellambra E, Martelli F, Avitabile D, Capogrossi MC, Magenta A. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid Redox Signal. 2017;27(6):328–344. doi: 10.1089/ars.2016.6643. [DOI] [PubMed] [Google Scholar]
- 87.Xu X, Zheng N, Chen Z, Huang W, Liang T, Kuang H. Puerarin, isolated from Pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene. 2016;591(2):411–416. doi: 10.1016/j.gene.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 88.Wu L, Zhang Y, Ma X, Zhang N, Qin G. The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol Biol Rep. 2012;39(9):9085–9093. doi: 10.1007/s11033-012-1780-z. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Meng L, Zhao L, Wang Z, Liu H, Liu G, Guan G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res Clin Pract. 2017;126:172–181. doi: 10.1016/j.diabres.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 90.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284(41):28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hariharan N, Maejima Y, Nakae J, Paik J, DePinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107(12):1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki SI, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ong ALC, Ramasamy TS. Role of sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev. 2018;43:64–80. doi: 10.1016/j.arr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Wang XL, Wu LY, Zhao L, Sun LN, Liu HY, Liu G, Guan GJ. SIRT1 activator ameliorates the renal tubular injury induced by hyperglycemia in vivo and in vitro via inhibiting apoptosis. Biomed Pharmacother. 2016;83:41–50. doi: 10.1016/j.biopha.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 95.Tikoo K, Singh K, Kabra D, Sharma V, Gaikwad A. Change in histone H3 phosphorylation, MAP kinase p38, SIR 2 and p53 expression by resveratrol in preventing streptozotocin induced type I diabetic nephropathy. Free Radic Res. 2008;42(4):397–404. doi: 10.1080/10715760801998646. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Zheng ZJ, Jia YJ, Yang YL, Xue YM. Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of diabetic kidney disease. J Transl Med. 2018;16(1):146. doi: 10.1186/s12967-018-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang R, Xu Y, Wan W, Shou X, Qian J, You Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J, Liu W. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57(3):456–466. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka Y, et al. Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res. 2012;2012:628978. doi: 10.1155/2012/628978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kume S, Yamahara K, Yasuda M, Maegawa H, Koya D. Autophagy: emerging therapeutic target for diabetic nephropathy. Semin Nephrol. 2014;34(1):9–16. doi: 10.1016/j.semnephrol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Kume S, Koya D. Autophagy: a novel therapeutic target for diabetic nephropathy. Diabetes Metab J. 2015;39(6):451–460. doi: 10.4093/dmj.2015.39.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitada M, Ogura Y, Monno I, Koya D. Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr Diab Rep. 2017;17(7):53. doi: 10.1007/s11892-017-0879-y. [DOI] [PubMed] [Google Scholar]
- 103.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105(9):3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su Y, Lu J, Gong P, Chen X, Liang C, Zhang J. Rapamycin induces autophagy to alleviate acute kidney injury following cerebral ischemia and reperfusion via the mTORC1/ATG13/ULK1 signaling pathway. Mol Med Rep. 2018;18(6):5445–5454. doi: 10.3892/mmr.2018.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224(1):R15–R30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang F, Hao Y, Zhang X, Qin J. Effect of echinacoside on kidney fibrosis by inhibition of TGF-beta1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des Devel Ther. 2017;11:2813–2826. doi: 10.2147/DDDT.S143805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao Y, Li Y, Zeng X, Ye Z, Li X, Zhang L. Losartan alleviates renal fibrosis and inhibits endothelial-to-mesenchymal transition (EMT) under high-fat diet-induced hyperglycemia. Front Pharmacol. 2018;9:1213. doi: 10.3389/fphar.2018.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Connelly KA, Thai K, Wu X, Kapus A, Kepecs D, Gilbert RE. Sirtuin 1 activation reduces transforming growth factor-beta1-induced fibrogenesis and affords organ protection in a model of progressive, experimental kidney and associated cardiac disease. Am J Pathol. 2017;187(1):80–90. doi: 10.1016/j.ajpath.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 109.Bugyei-Twum A, Ford C, Civitarese R, Seegobin J, Advani SL, Desjardins JF, Kabir G, Zhang Y, Mitchell M, Switzer J, Thai K, Shen V, Abadeh A, Singh KK, Billia F, Advani A, Gilbert RE, Connelly KA. Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc Res. 2018;114(12):1629–1641. doi: 10.1093/cvr/cvy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177(3):1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mortuza R, Feng B, Chakrabarti S. SIRT1 reduction causes renal and retinal injury in diabetes through endothelin 1 and transforming growth factor beta1. J Cell Mol Med. 2015;19(8):1857–1867. doi: 10.1111/jcmm.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kanesaki Y, Suzuki D, Uehara G, Toyoda M, Katoh T, Sakai H, Watanabe T. Vascular endothelial growth factor gene expression is correlated with glomerular neovascularization in human diabetic nephropathy. Am J Kidney Dis. 2005;45(2):288–294. doi: 10.1053/j.ajkd.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 113.Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J Biol Chem. 1995;270(12):6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- 114.Davis B, Dei Cas A, Long DA, White KE, Hayward A, Ku CH, Woolf AS, Bilous R, Viberti G, Gnudi L. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol. 2007;18(8):2320–2329. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- 115.Wen D, Huang X, Zhang M, Zhang L, Chen J, Gu Y, Hao CM. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS One. 2013;8(12):e82336. doi: 10.1371/journal.pone.0082336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, Lopaschuk GD, Oudit GY. Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol. 2014;306(8):F812–F821. doi: 10.1152/ajprenal.00655.2013. [DOI] [PubMed] [Google Scholar]
- 117.Clarke NE, Belyaev ND, Lambert DW, Turner AJ. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond) 2014;126(7):507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 118.Gu J, Yang M, Qi N, Mei S, Chen J, Song S, Jing Y, Chen M, He L, Sun L, Hu H, Li L, Wüthrich RP, Wu M, Mei C. Olmesartan prevents microalbuminuria in db/db diabetic mice through inhibition of angiotensin II/p38/SIRT1-induced podocyte apoptosis. Kidney Blood Press Res. 2016;41(6):848–864. doi: 10.1159/000452588. [DOI] [PubMed] [Google Scholar]
- 119.Benito-Martin A, Ucero AC, Izquierdo MC, Santamaria B, Picatoste B, Carrasco S, Lorenzo O, Ruiz-Ortega M, Egido J, Ortiz A. Endogenous NAMPT dampens chemokine expression and apoptotic responses in stressed tubular cells. Biochim Biophys Acta. 2014;1842(2):293–303. doi: 10.1016/j.bbadis.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 120.Lo CS, Shi Y, Chenier I, Ghosh A, Wu CH, Cailhier JF, Ethier J, Lattouf JB, Filep JG, Ingelfinger JR, Zhang SL, Chan JSD. Heterogeneous nuclear ribonucleoprotein F stimulates sirtuin-1 gene expression and attenuates nephropathy progression in diabetic mice. Diabetes. 2017;66(7):1964–1978. doi: 10.2337/db16-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li A, Peng R, Sun Y, Liu H, Peng H, Zhang Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1alpha signaling. Cell Death Dis. 2018;9(5):461. doi: 10.1038/s41419-018-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El-Samahy MH, et al. Urinary miRNA-377 and miRNA-216a as biomarkers of nephropathy and subclinical atherosclerotic risk in pediatric patients with type 1 diabetes. J Diabetes Complicat. 2018;32(2):185–192. doi: 10.1016/j.jdiacomp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 123.Duan LJ, Ding M, Hou LJ, Cui YT, Li CJ, Yu DM. Long noncoding RNA TUG1 alleviates extracellular matrix accumulation via mediating microRNA-377 targeting of PPARgamma in diabetic nephropathy. Biochem Biophys Res Commun. 2017;484(3):598–604. doi: 10.1016/j.bbrc.2017.01.145. [DOI] [PubMed] [Google Scholar]
- 124.Peng J, et al. MiR-377 promotes white adipose tissue inflammation and decreases insulin sensitivity in obesity via suppression of sirtuin-1 (SIRT1) Oncotarget. 2017;8(41):70550–70563. doi: 10.18632/oncotarget.19742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baker MA, Davis SJ, Liu P, Pan X, Williams AM, Iczkowski KA, Gallagher ST, Bishop K, Regner KR, Liu Y, Liang M. Tissue-specific microRNA expression patterns in four types of kidney disease. J Am Soc Nephrol. 2017;28(10):2985–2992. doi: 10.1681/ASN.2016121280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang KP, Chen C, Hao J, Huang JY, Liu PQ, Huang HQ. AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-beta1 expression in male rat glomerular mesangial cells. Endocrinology. 2015;156(1):268–279. doi: 10.1210/en.2014-1381. [DOI] [PubMed] [Google Scholar]
- 127.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, Tilley DG, Monroy MA, Choi ET, Thomas CJ, Jiang X, Wang H, Yang XF. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35(4):804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shahzad K, Bock F, al-Dabet MM, Gadi I, Kohli S, Nazir S, Ghosh S, Ranjan S, Wang H, Madhusudhan T, Nawroth PP, Isermann B. Caspase-1, but not caspase-3, promotes diabetic nephropathy. J Am Soc Nephrol. 2016;27(8):2270–2275. doi: 10.1681/ASN.2015060676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang CY, Kuo WW, Yeh YL, Ho TJ, Lin JY, Lin DY, Chu CH, Tsai FJ, Tsai CH, Huang CY. ANG II promotes IGF-IIR expression and cardiomyocyte apoptosis by inhibiting HSF1 via JNK activation and SIRT1 degradation. Cell Death Differ. 2014;21(8):1262–1274. doi: 10.1038/cdd.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bai B, Liang Y, Xu C, Lee MYK, Xu A, Wu D, Vanhoutte PM, Wang Y. Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation. 2012;126(6):729–740. doi: 10.1161/CIRCULATIONAHA.112.118778. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Q, Zhang P, Qi GJ, Zhang Z, He F, Lv ZX, Peng X, Cai HW, Li TX, Wang XM, Tian B. Cdk5 suppression blocks SIRT1 degradation via the ubiquitin-proteasome pathway in Parkinson’s disease models. Biochim Biophys Acta Gen Subj. 2018;1862(6):1443–1451. doi: 10.1016/j.bbagen.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y, Gao X, Chen S, Zhao M, Chen J, Liu R, Cheng S, Qi M, Wang S, Liu W. Cyclin-dependent kinase 5 contributes to endoplasmic reticulum stress induced podocyte apoptosis via promoting MEKK1 phosphorylation at Ser280 in diabetic nephropathy. Cell Signal. 2017;31:31–40. doi: 10.1016/j.cellsig.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 133.Bai X, et al. CDK5 promotes renal tubulointerstitial fibrosis in diabetic nephropathy via ERK1/2/PPARgamma pathway. Oncotarget. 2016;7(24):36510–36528. doi: 10.18632/oncotarget.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi JX, Huang Q. Glucagon-like peptide-1 protects mouse podocytes against high glucose-induced apoptosis, and suppresses reactive oxygen species production and proinflammatory cytokine secretion, through sirtuin 1 activation in vitro. Mol Med Rep. 2018;18(2):1789–1797. doi: 10.3892/mmr.2018.9085. [DOI] [PubMed] [Google Scholar]
- 135.Bao L, Cai X, Zhang Z, Li Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase-silent mating type information regulation 2 homologue 1-PPARgamma co-activator-1alpha axis in rat mesangial cells under high-dose glucosamine. Br J Nutr. 2015;113(1):35–44. doi: 10.1017/S000711451400347X. [DOI] [PubMed] [Google Scholar]
- 136.Rogacka D, Audzeyenka I, Rychłowski M, Rachubik P, Szrejder M, Angielski S, Piwkowska A. Metformin overcomes high glucose-induced insulin resistance of podocytes by pleiotropic effects on SIRT1 and AMPK. Biochim Biophys Acta. 2018;1864(1):115–125. doi: 10.1016/j.bbadis.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 137.Yang S, Zhao L, Han Y, Liu Y, Chen C, Zhan M, Xiong X, Zhu X, Xiao L, Hu C, Liu F, Zhou Z, Kanwar YS, Sun L. Probucol ameliorates renal injury in diabetic nephropathy by inhibiting the expression of the redox enzyme p66Shc. Redox Biol. 2017;13:482–497. doi: 10.1016/j.redox.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang J, Wang N, Zhu Y, Feng P. Roles of SIRT1 in high glucose-induced endothelial impairment: association with diabetic atherosclerosis. Arch Med Res. 2011;42(5):354–360. doi: 10.1016/j.arcmed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 139.Papadimitriou A, Silva KC, Peixoto EBMI, Borges CM, Lopes de Faria JM, Lopes de Faria JB. Theobromine increases NAD(+)/Sirt-1 activity and protects the kidney under diabetic conditions. Am J Physiol Ren Physiol. 2015;308(3):F209–F225. doi: 10.1152/ajprenal.00252.2014. [DOI] [PubMed] [Google Scholar]
- 140.Li C, Cai F, Yang Y, Zhao X, Wang C, Li J, Jia Y, Tang J, liu Q. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: involvement of SIRT1 and TGF-beta1 pathway. Eur J Pharmacol. 2010;649(1–3):382–389. doi: 10.1016/j.ejphar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 141.Shang G, Gao P, Zhao Z, Chen Q, Jiang T, Zhang N, Li H. 3,5-Diiodo-l-thyronine ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. Biochim Biophys Acta. 2013;1832(5):674–684. doi: 10.1016/j.bbadis.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 142.Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S. The effect of hesperidin and quercetin on oxidative stress, NF-kappaB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed Pharmacother. 2017;90:500–508. doi: 10.1016/j.biopha.2017.03.102. [DOI] [PubMed] [Google Scholar]
- 143.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, Lucia S, Adorini L, DAgati VD, Levi J, Rosenberg A, Kopp JB, Gius DR, Saleem MA, Levi M. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. 2016;27(5):1362–1378. doi: 10.1681/ASN.2014121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hussein MM, Mahfouz MK. Effect of resveratrol and rosuvastatin on experimental diabetic nephropathy in rats. Biomed Pharmacother. 2016;82:685–692. doi: 10.1016/j.biopha.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 145.Zhang J, Cheng Y, Gu J, Wang S, Zhou S, Wang Y, Tan Y, Feng W, Fu Y, Mellen N, Cheng R, Ma J, Zhang C, Li Z, Cai L. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin Sci (Lond) 2016;130(8):625–641. doi: 10.1042/CS20150623. [DOI] [PubMed] [Google Scholar]
- 146.Cheng Y, Zhang J, Guo W, Li F, Sun W, Chen J, Zhang C, Lu X, Tan Y, Feng W, Fu Y, Liu GC, Xu Z, Cai L. Up-regulation of Nrf2 is involved in FGF21-mediated fenofibrate protection against type 1 diabetic nephropathy. Free Radic Biol Med. 2016;93:94–109. doi: 10.1016/j.freeradbiomed.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ozturk E, et al. Resveratrol and diabetes: a critical review of clinical studies. Biomed Pharmacother. 2017;95:230–234. doi: 10.1016/j.biopha.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 148.Bo S, Ponzo V, Ciccone G, Evangelista A, Saba F, Goitre I, Procopio M, Pagano GF, Cassader M, Gambino R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol Res. 2016;111:896–905. doi: 10.1016/j.phrs.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 149.Movahed A, et al. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid Based Complement Alternat Med. 2013;2013:851267. doi: 10.1155/2013/851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, Omrani GR, Shams M (2019) Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double-blind placebo-controlled clinical trial. Diabetes Metab 45(1):53–59 [DOI] [PubMed]
- 151.Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med. 2009;26(5):526–531. doi: 10.1111/j.1464-5491.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 152.Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G, ROADMAP Trial Investigators Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]