Abstract
Background
Infliximab is a chimeric anti-tumor necrosis factor alpha (TNF-α) monoclonal antibody that ameliorates inflammation when it binds to and neutralizes TNF-α. It is often used in patients with Crohn’s disease and ulcerative colitis to reduce the severity of disease symptoms and induce disease remission. Infusions are generally administered in the hospital setting due to concerns over patient safety, and limited data exist regarding the incidence and management of infusion reactions (IRs) in an alternate care setting without direct physician oversight.
Aims
The aim of this study was to evaluate the incidence of IRs following administration of infliximab and associated management approaches in an alternate care setting.
Methods
A retrospective chart review of 796 patients with Crohn’s disease or ulcerative colitis that received a combined 5581 infusions with one home infusion provider between January 2014 and November 2016 was conducted. Timing, severity, management approach, and outcomes of IRs were abstracted and analyzed.
Results
A total of 109 infusion reactions (2.0% of all infusions) were recorded in 62 patients (7.8% of all patients). The majority of these reactions were acute and mild or moderate in severity and resolved with rate adjustments and/or medication. Emergency room visits were required in 0.1% of all infusions, and 0.3% of all infusions were not completed due to a reaction.
Conclusions
IRs to infliximab were uncommon and mostly mild or moderate in severity. Resolution of the IR and continuation of therapy was achieved in most patients through a management approach that included prompt recognition and initial treatment via rate adjustments and medications according to physician’s orders.
Keywords: Infliximab, Infusion reaction, Home healthcare, Inflammatory bowel disease
Introduction
Infliximab is a chimeric (human–murine) IgG1 monoclonal antibody with a high affinity for the pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α), which has been implicated in the pathogenesis of a variety of inflammatory diseases. Currently, infliximab is approved by the U.S. Food and Drug Administration for the treatment of Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis [1]. Mechanistically, infliximab neutralizes and blocks the biological activity of TNF-α. In inflammatory bowel disease (IBD), prevention of this TNF-α-mediated pro-inflammatory cascade reduces the activation and proliferation of intestinal mucosal T cells, although the exact mechanism of intestinal inflammatory suppression remains debated [2]. Translational studies have shown that infliximab reduces lamina propria infiltrating CD68 macrophages and downregulates interleukin 17A [3], TNF, and IFN-γ mRNA in the colonic mucosa of patients with IBD [4]. Further, blockade of TNF-α may preserve intestinal epithelial barrier integrity through prevention of TNF-mediated initiation of enterocyte apoptosis [5].
The clinical efficacy of infliximab has been demonstrated through induction and maintenance of remission in Crohn’s disease and ulcerative colitis [6]. Infliximab is administered intravenously, typically over a 2 h time period, with infusion intervals at the induction phase occurring at weeks 0, 2, and 6 followed by maintenance doses every 4–8 weeks. These repeated infusions often necessitate travel back and forth to the hospital and missed days at work and school. Though significant burden has been demonstrated with infliximab infusions administered in the hospital setting [7], concerns associated with safety of an infused-biologic have delayed extensive evaluation of infliximab administration in alternate care sites that do not have on-site physicians administering or overseeing the infusion.
One of the most common adverse events associated with infusion of infliximab is an infusion reaction (IR). A reported overall incidence of both acute and delayed IRs in Crohn’s disease patients in a hospital setting was 6.1%, occurring in 9.7% of all patients, with severe acute reactions accounting for 1.0% of all infusions [8]. Long-term registry and retrospective studies that have addressed the incidence of IRs across many indications, including IBD in the community clinic setting, demonstrated a lower per-infusion incidence (1.3–3.7% all infusions) [9–11]. It is thought that the per-infusion incidence may be higher in short-term studies due to the high frequency of acute reactions that occur during initial infusions [12]. Despite a low reported incidence of IRs in these controlled environments, there remains a paucity of data on the safety of infliximab infusion in alternate care settings including the home. To date, two studies have addressed the benefits and safety of home infusion of infliximab. Both studies demonstrated significant cost savings, patient satisfaction, and no serious adverse events with home infusion of infliximab in adult [13] and pediatric [14] Crohn’s disease patients. However, these studies were limited by study population size and exclusion criteria that included active disease and a history of infliximab-related adverse events [13, 14].
In the current study, we retrospectively evaluated IRs to infliximab administered in the home and AIS and associated outcomes in patients with a primary diagnosis of Crohn’s disease or ulcerative colitis. To the best of our knowledge, this is the largest report to date evaluating IRs in an alternate care setting without direct physician oversight.
Materials and Methods
Study Design
A retrospective chart review was conducted of all IBD patients receiving infliximab in the home setting or ambulatory infusion suite (AIS) via one home infusion company during the period from January 1 2014 to November 23 2016 to assess the severity and incidence of infliximab-related IRs and document management approaches. In both the home and AIS, patients were monitored and received care from experienced nurses and clinical pharmacists. All patients were referred by their physician to receive care in either the patient’s home or the home infusion provider’s infusion suite. Standardized policies and procedures for the infusion and clinical support of patients receiving infliximab via all infusion services locations were followed. All infusions were administered and monitored by a trained nurse. Use of premedication (e.g., antihistamines, acetaminophen, and/or intravenous steroids) was determined by the referring physician. Patients initiating infliximab therapy received 3 loading doses at weeks 0, 2, and 6 followed by maintenance dose infusion intervals every 4–8 weeks as determined by referring physician. The majority of study patients were already receiving infliximab at the start of data collection. Per company policy, patients who were either naïve to infliximab or had a lapse in therapy of greater than 2 dosing intervals received initial doses of infliximab in a controlled environment that included the AIS under the supervision of an infusion nurse and clinical pharmacist. Infliximab was typically infused over a 2 h period, but duration could be shortened on a case-by-case basis as determined by patient request and prescriber agreement if the patient tolerated previous doses with no adverse events. Patients’ vital signs (pulse, blood pressure, and body temperature) were obtained prior to, during, and at completion of infusion. Patients were monitored by an experienced nurse for a minimum of 60 min following the first 3 infusions and a minimum of 30 min following each maintenance infusion for any infusion-related adverse events and were advised to seek medical attention if any side effect occurred. All nurses were trained in proper preparation and administration of infliximab as well as appropriate monitoring, assessment, and management of any adverse infusion-related events and were required to pass a competency test demonstrating these skills.
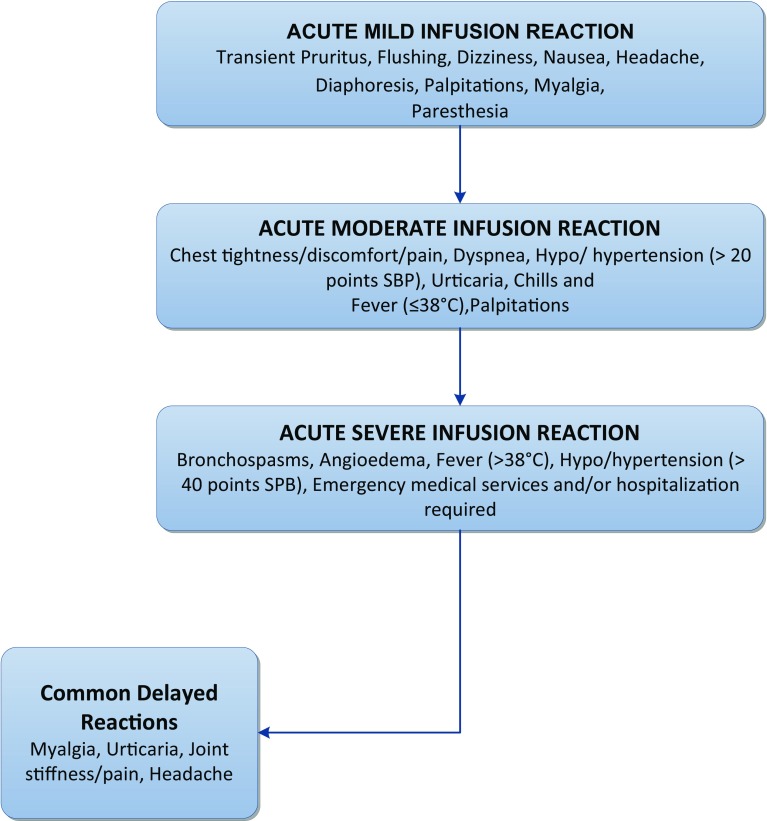
Patient gender, age, and weight were obtained from an electronic database. Diagnosis, infusion intervals, infliximab dosage, occurrence of IRs, and management approaches were abstracted from digital copies of nursing visit reports (NVRs). If an IR was noted on an NVR, the type and severity of the reaction was determined and classified according to the criteria in Fig. 1 which are based on those previously described by Cheifetz and Mayer [15]. Acute reactions were defined as anything occurring during the infusion or within 24 h of the infusion. Acute reactions that occurred after the patient left the AIS or nurse left the patient’s home were captured on the next visit report. Delayed reactions were defined as any reaction occurring greater than 24 h after the infusion but within 14 days of the infusion. Though both delayed and acute IRs can be classified according to severity, we limited our analysis of severity to acute reactions due to the voluntary nature of patient reporting and the difficulty of distinguishing delayed reactions from symptoms of diagnoses.
Fig. 1.
Stratification of infusion reactions by severity. Symptoms associated with acute infusion reactions were sorted and classified as either mild, moderate, or severe according to the outlined criteria. In addition, the most common delayed reactions were identified
Prior to therapy initiation in the patient’s home or AIS, patient-specific acute infusion reaction orders were obtained from their physician. Acute IR emergency drugs typically included epinephrine and diphenhydramine per physician order. For mild acute IRs, infliximab infusion was stopped, and the patient was evaluated for signs and symptoms of an acute reaction followed by administration of oral diphenhydramine per physician orders. For moderate-to-severe reactions, infusion was stopped and epinephrine and/or intravenous diphenhydramine was administered per physician orders followed by notification of physician. If applicable, EMS was called. If reaction subsided, infusion was resumed at one-half previous rate and increased gradually to a rate no greater than previous rate. If reaction did not subside, prescriber was notified for additional medical management while awaiting EMS.
Statistical Analysis
All patient information was anonymized and de-identified. All data were analyzed utilizing GraphPad Prism 7.0 statistical software (La Jolla, CA). Demographics, IR rates (per-infusion and per-patient), and reaction severity were assessed using descriptive statistics. The Chi-square test was used to compare rates of IRs between maintenance and induction infusions, gender, and administration site. All analyses were two-tailed, and significance was set at less than or equal to 0.05.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki, and all data were collected in accordance with the Health Insurance Portability and Accountability Act (HIPAA).
Results
During the study period, 796 patients, 69% with a diagnosis of Crohn’s disease and 31% with a diagnosis of ulcerative colitis, received a total of 5581 infliximab infusions. While most of the patients were already receiving maintenance infliximab infusions at the start of data collection, 105 new patients (13.2%) received an induction infusion during the study period accounting for 251 infusions (4.5% of all infusions administered). A slight majority of infusions were administered in the patient’s home (54.7%, n = 3052) versus an AIS (45.3%, n = 2529). Patients had a mean age of 36 at the start of the data collection period and ranged in age from 6 to 88 years. Females comprised 50.3% of the population (n = 400). The mean weight of female patients was 73.5 kg (median 70.5 kg), and the mean weight of male patients was 77.0 kg (median 74.1 kg). Patients received a mean 7.0 infusions during the study period and a mean infliximab dose of 531 mg (median 500 mg). The mean infliximab maintenance interval was 7.1 weeks.
In total, 109 IRs (2.0% of all infusions) were recorded in 62 patients (7.8% of all patients). Table 1 displays the IRs and proportion for each age range. Of these reactions, 87 (79.8%) were acute, the majority of which were classified as mild (57.5%) or moderate in severity (31.0%). Ten infusions were associated with a severe reaction (11.5%, 0.2% of all infusions), and of these, 8 (9.2%, 0.1% of all infusions) resulted in an emergency room visit. The most common acute reaction was headache which occurred in 23.0% of all acute IRs followed by pruritus which occurred in 14.9% of all acute IRs. Other common acute reactions included dyspnea (13.8%), flushing (13.8%), chest tightness/discomfort (11.5%), and nausea and/or vomiting (10.3%). Table 2 shows the type and frequency of all acute reactions that occurred in at least 2.0% of reactions and a comprehensive list of type and frequency of delayed reactions. There were 22 (20.2%, 0.4% of all infusions) recorded delayed reactions, the most common of which were headache (18.2%), myalgia (18.2%), urticaria (18.2%), and nausea and/or vomiting (18.2%).
Table 1.
Infusion reactions and proportion for each age range
| Age group (years) | n (%) |
|---|---|
| 18 and under | 6 (6.8) |
| 19–29 | 14 (5.8) |
| 30–39 | 12 (7.2) |
| 40–49 | 14 (9.9) |
| 50–59 | 11 (10.4) |
| 60 and over | 5 (9.6) |
| Overall | 62 (7.8) |
Table 2.
Type and frequency of acute and delayed infusion reactions to infliximab
| Reaction | n | % |
|---|---|---|
| Acute infusion reactions (≥ 2.0% of all IRs) | ||
| Headache | 20 | 23.0 |
| Pruritus | 13 | 14.9 |
| Dyspnea | 12 | 13.8 |
| Flushing | 12 | 13.8 |
| Chest tightness/discomfort | 10 | 11.5 |
| Nausea/vomiting | 9 | 10.3 |
| Dizziness | 7 | 8.0 |
| Urticaria/rash | 5 | 5.7 |
| Fever (≤ 38 °C) | 5 | 5.7 |
| Blood pressure increase (> 20 points SBP) | 4 | 4.6 |
| Paresthesia | 2 | 2.3 |
| Delayed infusion reactions | ||
| Headache | 4 | 18.2 |
| Nausea/vomiting | 4 | 18.2 |
| Urticaria/rash | 4 | 18.2 |
| Myalgia | 4 | 18.2 |
| Back pain | 3 | 13.6 |
| Chills | 2 | 9.1 |
| Pruritus | 1 | 4.5 |
| Dizziness | 1 | 4.5 |
| Flushing | 1 | 4.5 |
| Chest tightness/discomfort | 1 | 4.5 |
| Chest pain | 1 | 4.5 |
| Paresthesia | 1 | 4.5 |
| Blood pressure increase (> 20 points SBP) | 1 | 4.5 |
| Fever (≤ 38 °C) | 1 | 4.5 |
| Joint pain | 1 | 4.5 |
SBP systolic blood pressure
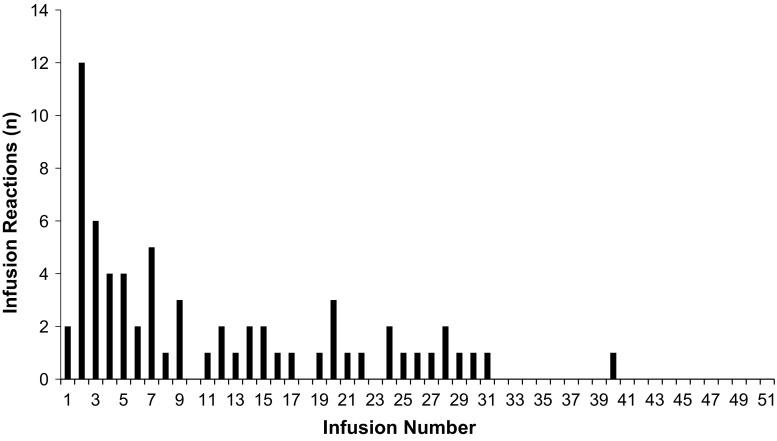
We were able to verify the infusion number that a reaction occurred in patients that received their entirety of care with this home infusion company which is presented in Fig. 2 (66 of 109 IRs in 33 patients). The precise infusion number at which the remaining IRs occurred could not be verified, as they developed in patients on maintenance therapy that had received infliximab infusions prior to start of care with this home infusion company. Twenty IRs (18.3%; 20 of 109 IRs) occurred during or after one of the first 3 infusions (induction dosing). Two of the 109 IRs (1.8%) occurred with the first infusion, and 12 of 109 IRs (11.0%) occurred with the second infusion. Induction infusions were associated with a higher reaction rate than maintenance infusions. At least one IR occurred in 14.3% of patients receiving induction doses versus 6.8% in patients receiving infliximab as part of maintenance therapy (χ2 = 6.1, df = 1, P = 0.0135, n = 796).
Fig. 2.
Distribution of infusion reaction by infusion number. The data are presented as the total number of infusion reactions that occurred at each infusion number
We also examined the incidence of IRs by additional factors including diagnosis, gender, and location of administration (AIS versus home). The overall per-infusion incidence of IRs was 2.0% in Crohn’s disease and 1.9% in ulcerative colitis which is shown in Table 3 (χ2 = 0.009, df = 1, P = 0.9224, n = 5581). Women were more likely to experience an IR than men (χ2 = 20.6, df = 1, P < 0.0001, n = 5581) with a per-infusion incidence of 2.8% versus 1.1% in men. Infusions administered in a patient’s home were associated with a lower per-infusion reaction rate than those administered in the AIS (χ2 = 4.658, df = 1, P = 0.03, n = 5581; 1.6% IR rate per-infusion versus 2.4% in the AIS).
Table 3.
Infusion reactions according to diagnosis
| Total | CD | UC | P value (CD vs UC) | |
|---|---|---|---|---|
| Patients (n) | 796 | 550 | 246 | – |
| Total infusions (n) | 5581 | 3971 | 1610 | – |
| Infusion reactions n (%) | 109 (2.0) | 78 (2.0) | 31 (1.9) | 0.9224 |
| Acute | 87 (1.6) | 64 (1.6) | 23 (1.4) | 0.7032 |
| Delayed | 22 (0.4) | 14 (0.4) | 8 (0.5) | 0.5865 |
| Patients with at least 1 infusion reaction n (%) | 66 (8.3) | 40 (7.3) | 22 (8.9) | 0.5032 |
CD Crohn’s disease, UC ulcerative colitis
Post-infusion reaction management approaches implemented by the infusion nurse are outlined in Table 4. Administration of a medication or another intervention such as stopping and restarting or slowing the infusion occurred in 70.1% of IRs. The IRs that did not receive an intervention were mild in severity and consisted of either headache, pruritus around the infusion site, and/or flushing. These reactions resolved following the completion of the infusion. Antihistamines were administered in 26.4% of IRs and acetaminophen in 10.3% of IRs. Epinephrine was only administered during two infusion reactions. Rate adjustments including slowing the infusion or pausing and restarting the infusion were made in 40.3% of IRs. Resolution of acute IRs was significant enough to warrant continuation of the infusion in most cases. There were 14 reactions (16.1% of 87 acute IRs; 0.3% of all infusions) in 14 patients in which the infusion was stopped and not continued. Five of the 14 patients were able to maintain their scheduled treatment plan and completed their next scheduled infusion, while 9 did not return for any additional infusions in the home or AIS. Overall, 69.4% of patients (43/62) that experienced an IR returned for their next scheduled infusion in the home or AIS. Of the patients that experienced an IR and chose to continue therapy, 69.8% (30/43) did not experience any subsequent reactions. The majority of the remaining patients (10/13, 77.0%) experienced between 1 and 5 additional reactions. There were 2 patients that experienced 10 IRs and 1 patient that experienced 16 IRs, but these reactions were mild in nature and consisted of repeated headache and pruritus following infliximab administration.
Table 4.
Management approaches to acute infusion reactions
| Management of infusion reactions | n | % of Infusion reactions |
|---|---|---|
| Medications | ||
| Antihistamines | 23 | 26.4 |
| Acetaminophen | 9 | 10.3 |
| Ibuprofen | 2 | 2.3 |
| Steroids | 2 | 2.3 |
| Epinephrine | 2 | 2.3 |
| Ondansetron | 1 | 1.2 |
| Omeprazole | 1 | 1.2 |
| Other | ||
| Infusion paused and restarted | 28 | 32.2 |
| Infusion stopped | 14 | 16.1 |
| Infusion slowed | 7 | 8.1 |
| EMS activated | 8 | 9.2 |
| Increased monitoring | 5 | 5.8 |
| Hydration | 4 | 4.6 |
| Any treatment | 61 | 70.1 |
Discussion
Our experience indicates that both acute and delayed infusion reactions to infliximab in the home or ambulatory care setting have a low rate of occurrence. This was true for both the per-infusion reaction rate as well as the per-patient reaction rate. Further, the majority of acute IRs were considered to be mild or moderate in severity and resolved with rate adjustments or administration of antihistamines, steroids, or acetaminophen.
As with all infused-biologics, the immunomodulatory properties of infliximab have prompted careful evaluation of its safety profile. In the majority of studies to date, infliximab has been administered in a controlled setting under physician supervision due to concerns over the development of adverse events, including infusion reactions. Thus, most data have been limited to that obtained from clinical trials or a hospital setting, and there is a paucity of data related to infusions administered in an alternate care setting. Despite these concerns, the reported per-infusion incidence of IRs is low. In the TREAT registry, a multicenter, prospective, observational registry of Crohn’s disease patients in the USA receiving treatment in both community-based and academic practice settings, the per-infusion reaction rate to infliximab was 3.0%, with 0.05% of infusions classified as serious (53,003 total infusions in 3322 patients) [16]. A retrospective, single-site study of Crohn’s disease patients infused with infliximab at a hospital infusion center reported a per-infusion acute reaction rate of 5.4% and a per-patient rate of 8.4% (479 total infusions in 165 patients) [8]. Similar rates have also been demonstrated in patients with other diagnoses. Kelsall et al. [17] reported a per-infusion acute reaction rate of 5.8% (4399 total infusions in 200 patients) in patients with inflammatory arthritis. Over 80% of these reactions were classified as either mild or moderate in severity [17].
A few studies have addressed the safety of infliximab in a community treatment setting. The RemiTRAC Canadian observational patient registry which is primarily comprised of patients with rheumatologic conditions, found 12.3% of 1632 patients reported a least one infusion reaction with a per-infusion reaction rate of 1.3% in 24,852 infliximab infusions [9]. Over 95% of these reactions were classified as mild or moderate in severity [9]. In addition, a multicenter retrospective chart review of Crohn’s disease patients receiving infusions from community-based and academic gastroenterology practices found the per-infusion reaction rate to be 3.5% and the per-patient reaction rate to be 19.1% (6468 infusions in 447 patients) with less than 0.1% of all infusions associated with a serious reaction [11]. Similar reaction rates were demonstrated in a retrospective study of all patient types in a Canadian community clinic setting with an acute reaction per-infusion incidence of 2.5% (20,976 infusions in 3161 patients) [10]. Collectively, these findings suggest that concerns over home or ambulatory infusion of infliximab may not be warranted.
Though infliximab safety is well demonstrated in long-term studies in hospital and community clinic settings, very few studies have addressed infliximab administration in the home. To date, one study has been conducted in the USA in which the cost, safety, and patient satisfaction of home-based infliximab infusions were evaluated in 10 pediatric Crohn’s disease patients [14]. A high level of patient satisfaction and cost savings was reported at the study conclusion [14]. Importantly, no serious adverse events were recorded over 59 infusions during the 2-year study period [14]. Nonetheless, the study population was carefully selected and limited to patients that were in remission, had no previously reported adverse events, were compliant with hospital-based infliximab infusions, and had access to experienced homecare pediatric nursing [14]. Similarly, Kuin and colleagues selected adult Crohn’s disease patients in remission with no previous history of reactions to infliximab to participate in a 1-year home infusion program. Participants reported high levels of satisfaction similar to levels reported in the hospital, and no serious adverse events occurred [13]. A total of 13 of 29 invited patients agreed to participate in the study, though it was noted that a 70% participation rate would have been achieved if infusions had been offered outside of office hours and on weekends [13]. Both of these studies provide encouraging findings regarding safety and patient satisfaction with home administration of infliximab but are limited by small patient and infusion numbers.
To address this gap in knowledge, our study retrospectively evaluated the rate and severity of IRs to infliximab in the largest cohort to date of patients infused in an alternate care setting that included the patient’s home (3052 total home infusions) and company AIS (2529 total AIS infusions). Reaction rates from home and AIS infusions were analyzed and presented together because the available resources and personnel in the AIS are more similar to the home setting than that of a hospital or physician managed clinic setting as both do not have direct physician oversight. Across all of our infusions, the per-infusion reaction rate was 2.0% with a per-patient reaction rate of 7.8% which is consistent with or lower than previously reported IR rates.
We maintained a reporting and stratification system consistent with the previous reports. In the current literature, there is some variation in reporting of infliximab reactions and discrepancies in associated definitions. While this undoubtedly creates a barrier in determining true incidence, we have mitigated this limitation by using a classification system (reaction severity and timing) similar to that recommended by Cheifetz and Mayer [15]. Despite these challenges with reporting, studies consistently report a low rate of reactions requiring EMS and report that the majority of patients continue regularly scheduled infusions. In this study, over 80.0% of the recorded IRs were acute, and most were considered mild or moderate in severity. Importantly, most IRs were easily managed with infusion rate adjustments and/or administration of antihistamines, acetaminophen or steroids based on physician’s orders that were obtained and in place prior to the start of any infusion. There were 10 infusions (0.2% of all infusions) associated with a serious IR, and 8 IRs (0.1% of all infusions) required a visit to the emergency room. The majority of IRs were not severe enough to preclude further infusion of infliximab, and in accordance, the majority of patients that experienced an IR chose to return for their next scheduled infusion.
While we present a robust retrospective data set that demonstrates a low rate of IRs to infliximab in the home and AIS that are successfully managed, the retrospective nature of the study design presents several limitations. The depth of the collected information was limited to what was routinely obtained as part of standardized patient care. Reporting of IRs was limited to chart descriptions that did not allow for further follow-up if the patient received care that required emergency medical services. Documentation of reactions that occurred in the 24 h following the infusion after the patient left the AIS or nurse left the patient’s home was dependent on patient reporting during the next follow-up visit. Further, there are several infusion-related factors that may affect the risk of an IR that were not available as part of this chart review, some of which include concomitant immunosuppressant use, location and severity of disease, and occurrence of a previous infusion reaction. A few studies have demonstrated the influence of one or more of these factors on the incidence of infusion reactions [18, 19].
The nature of this study design includes the risk of selection bias as the majority of infusions during the study period were administered as part of therapy maintenance. Patients who had experienced severe reactions prior to the study collection period may have no longer been receiving infliximab for therapy or no longer receiving therapy in an alternate care setting. In accordance, we also separately analyzed IR rates in patients receiving induction infusions and those receiving maintenance infusions. Rates of IRs among patients induced were higher than those in patients maintained on infliximab during the study period which is consistent with previous reports of IRs in infliximab-naïve and infliximab-experienced patients [9]. Though there was a higher rate of IRs in patients undergoing induction infusions, the reaction rate was consistent with those previously reported [9, 11].
In conclusion, we report that infusion reactions in the home and AIS are uncommon, mild to moderate in severity, and can be successfully managed by the healthcare professionals in place in an alternate care setting. Most reactions did not result in premature discontinuation of therapy out of patient concern, and most patients with a mild or moderate reaction chose to continue infusions in an alternate setting.
Funding
This study received no outside funding and was supported in its entirety by Coram CVS Specialty Infusion Services.
Conflict of interest
L. Allyson Checkley, Loretta Kristofek, Samantha Kile, and William Bolgar are employees of Coram CVS Specialty Infusion Services.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Contributor Information
L. Allyson Checkley, Phone: 303.672.8888, Email: allyson.checkley@coramhc.com.
Loretta Kristofek, Email: loretta.kristofek@coramhc.com.
Samantha Kile, Email: samantha.kile@coramhc.com.
William Bolgar, Email: bill.bolgar@coramhc.com.
References
- 1.Silva LC, Ortigosa LC, Benard G. Anti-TNF-alpha agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2:817–833. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Lu N, Bai A. Clinical use and mechanisms of infliximab treatment on inflammatory bowel disease: a recent update. Biomed Res Int. 2013;2013:581631. doi: 10.1155/2013/581631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caprioli F, Bose F, Rossi RL, et al. Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy. Inflamm Bowel Dis. 2013;19:729–739. doi: 10.1097/MIB.0b013e318280292b. [DOI] [PubMed] [Google Scholar]
- 4.Olsen T, Cui G, Goll R, Husebekk A, Florholmen J. Infliximab therapy decreases the levels of TNF-alpha and IFN-gamma mRNA in colonic mucosa of ulcerative colitis. Scand J Gastroenterol. 2009;44:727–735. doi: 10.1080/00365520902803507. [DOI] [PubMed] [Google Scholar]
- 5.Zeissig S, Bojarski C, Buergel N, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology. 2007;133:312–339. doi: 10.1053/j.gastro.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Buisson A, Seigne AL, D’Huart MC, Bigard MA, Peyrin-Biroulet L. The extra burden of infliximab infusions in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2464–2467. doi: 10.1097/MIB.0b013e3182a19268. [DOI] [PubMed] [Google Scholar]
- 8.Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 9.Choquette D, Faraawi R, Chow A, Rodrigues J, Bensen WJ, Nantel F. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105–1111. doi: 10.3899/jrheum.140538. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme J, Pelletier C, Zacharias R. The safety of infliximab infusions in the community setting. Can J Gastroenterol. 2010;24:307–311. doi: 10.1155/2010/138456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keshavarzian A, Mayer L, Salzberg B, et al. A multicenter retrospective experience of infliximab in Crohn’s disease patients: infusion reaction rates and treatment persistency. Gastroenterol Hepatol (NY) 2007;3:381–390. [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtenstein L, Ron Y, Kivity S, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. 2015;9:806–815. doi: 10.1093/ecco-jcc/jjv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuin S, Stolte SB, van den Brink GR, et al. Short article: Remicade infusions at home: an alternative setting of infliximab therapy for patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2016;28:222–225. doi: 10.1097/MEG.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 14.Condino AA, Fidanza S, Hoffenberg EJ. A home infliximab infusion program. J Pediatr Gastroenterol Nutr. 2005;40:67–69. doi: 10.1097/00005176-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med. 2005;72:250–256. [PubMed] [Google Scholar]
- 16.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelsall J, Rogers P, Galindo G, De Vera MA. Safety of infliximab treatment in patients with rheumatoid arthritis in a real-world clinical setting: description and evaluation of infusion reactions. J Rheumatol. 2012;39:1539–1545. doi: 10.3899/jrheum.110956. [DOI] [PubMed] [Google Scholar]
- 18.Lee TW, Singh R, Fedorak RN. A one-hour infusion of infliximab during maintenance therapy is safe and well tolerated: a prospective cohort study. Aliment Pharmacol Ther. 2011;34:181–187. doi: 10.1111/j.1365-2036.2011.04699.x. [DOI] [PubMed] [Google Scholar]
- 19.Duron C, Goutte M, Pereira B, Bommelaer G, Buisson A. Factors influencing acute infusion reactions in inflammatory bowel disease patients treated with infliximab in the era of scheduled maintenance therapy. Eur J Gastroenterol Hepatol. 2015;27:705–711. doi: 10.1097/MEG.0000000000000354. [DOI] [PubMed] [Google Scholar]