Summary
Bladder augmentation is a demanding surgical procedure and exclusively offered for selected children and has only a small spectrum of indications. Paediatric bladder voiding dysfunction occurs either on a basis of neurological dysfunction caused by congenital neural tube defects or on a basis of rare congenital anatomic malformations. Neurogenic bladder dysfunction often responds well to a combination of specific drugs and/or intermittent self-catheterization. However, selected patients with spinal dysraphism and children with congenital malformations like bladder exstrophy and resulting small bladder capacity might require bladder augmentation. Ileocystoplasty is the preferred method of bladder augmentation to date. Because of the substantial long-and short-term morbidity of augmentation cystoplasty, recent studies have tried to incorporate new techniques and technologies, such as the use of biomaterials to overcome or reduce the adverse effects. In this regard, homografts and allografts have been implemented in bladder augmentation with varying results, but recent studies have shown promising data in terms of proliferation of urothelium and muscle cells by using biological silk grafts.
Keywords: Urinary bladder dysfunction, Neurogenic bladder, Bladder augmentation, Ileocystoplasty, Biomaterials
Zusammenfassung
Die Harnblasenaugmentation ist eine anspruchsvolle chirurgische Operation, die betroffenen Kindern unter bestimmten Voraussetzungen vorbehalten ist. Die kindliche Harnblasenentleerungsstörung basiert überwiegend auf angeborenen Neuralrohrdefekten und seltener auf angeborenen Defekten mit fehlentwickelter Harnblase. Die Dysfunktion bei neurogener Blase ist sowohl gut medikamentös als auch mit der sog. intermittierenden Selbstkatheterisierung behandelbar. Jedoch kann die Blasenaugmentation für ausgewählte Patienten mit spinaler Dysraphie und Kinder mit angeborenen Fehlbildungen wie Blasenekstrophie zur Vergrößerung der Blasenkapazität erforderlich sein. Die derzeitig am häufigsten verwendete Ileozystoplastie hat erhebliche unerwünschte postoperative Nebeneffekte. Zur Reduzierung dieser substanziellen Lang- und Kurzzeit-Komorbiditäten wird derzeit an neuen Techniken unter Verwendung von Homografts und Allografts geforscht, wobei aktuell auch zunehmend die Verwendung von Biomaterialien wie biologischen Transplantaten aus Seide untersucht wird, die eine Einsprossung von körpereigenem Urothel und Muskelzellen erlauben könnten.
Schlüsselwörter: Harnblasendysfunktion, Neurogene Harnblase, Blasenaugmentation, Ileozystoplastie, Biomaterialien
Introduction
Loss or malfunction of the lower urinary tract may cause urinary incontinence and chronic renal failure. The most common underlying conditions are spinal dysraphism (spina bifida), congenital malformations (exstrophy-epispadias complex, cloacal malformations) and trauma. Modern treatment of lower urinary tract dysfunctions consists of clean intermittent catheterization (as proposed by Lapides in 1972 [1]), medical treatment (anticholinergic medication and botulinum toxin A [2, 3]) and surgical reconstruction (augmentation cystoplasty, creation of a catheterizable conduit [4, 5]).
In this article we review various conditions and surgical options, and highlight new concepts for the use of biomaterials and tissue engineering in the field of urinary bladder reconstruction.
Clinical presentation and issues
Neuropathic bladder
Neural tube defects represent one of the most common birth defects (33–52/100,000 live births [6, 7]) as well as the most common cause of neurogenic bladder dysfunction [8]. In this regard, there is high accuracy and precision for obtaining the diagnosis by antenatal ultrasound [9].
Clinical presentation of neuropathic bladder includes incontinence, recurrent urinary tract infection and, if left untreated, chronic renal failure and end-stage renal disease [10]. Bladder dysfunction is caused by detrusor and/or sphincter over- and underactivity (detrusor sphincter dyssynergy). A high-pressure and low-compliance bladder causes destruction of the bladder architecture, leading to diverticulation and loss of contractility, subsequently to vesicoureteral reflux, chronic renal failure and incontinence [10, 11].
Congenital malformations
Several rare anatomic malformations of the urogenital tract can cause bladder dysfunction as well, and are often diagnosed via prenatal ultrasound or magnetic resonance imaging (MRI) [12, 13]. Urogenital malformations that might require bladder augmentation include cloacal exstrophy (~0.19/100,000 live births [14, 15]) and bladder exstrophy (~3.3/100,000 live births [12]). In both entities, the volume of the urinary bladder is compromised, as is the compliance of the bladder wall. Again, insufficient treatment can lead to renal impairment [13].
Therapeutic options
Modern treatment of lower urinary tract dysfunctions consists of
Clean intermittent catheterization (CIC)
Medical treatment (anticholinergic medication and botulinum toxin A)
Surgical reconstruction
Clean intermittent catheterization
Clean intermittent self-catheterization (CIC) was introduced in 1972 and revolutionized the treatment of bladder dysfunction [1, 16]. CIC effectively lowers the intravesical pressure, provides urinary continence and consequently acts as protection against renal failure. It is the baseline treatment of bladder dysfunction and is also used in children with malformations of the exstrophy complex in addition to surgical management [17].
Pharmacological non-surgical treatment
Anticholinergic oral medication (i. e. oxybutynin) and muscle relaxation drugs in combination with intermittent self-catheterization poses an excellent option for long-term treatment in cases with neurogenic bladder dysfunction [18–20]. Side effects of the medical treatment include anticholinergic symptoms like drowsiness, flushes and palpitations. Additionally, a high compliance is needed, but in 75–90% [2, 20] of all patients with neurogenic bladder dysfunction, this non-surgical treatment shows good results. In case of persistent high intravesical pressure, submucosal injection of botulinum toxin A is implemented [21, 22].
Surgical treatment
Bladder augmentation
If medical treatment and/or interventional methods have failed, and high intravesical pressure and urinary incontinence or recurring urinary tract infections persist combined with present vesicoureteral reflux and impaired renal function, surgical treatment in terms of bladder augmentation is indicated [8, 23]. Urinary bladder augmentation-reconstruction includes and simplifies:
- Augmentation of the bladder capacity
- via enterocystoplasty
- or autoaugmentation
treatment of incontinence
catheterizable conduit (Mitrofanoff appendicovesicostomy)
The median age of children with neurogenic bladder dysfunction who undergo bladder augmentation is 12 years and more than half of these patients have spina bifida as the underlying disease [5]. In anatomical malformations, bladder augmentation is considered earlier than in children with neurogenic bladder dysfunction; the median age of those children is 6.4 years [15, 24]. The success rate of bladder augmentation with regard to the increase in bladder capacity as well as reduced intravesical pressure is high. In addition, the progression or the occurrence of kidney dysfunction can be avoided. Nonetheless, bladder augmentation requires close monitoring and further treatment of incontinence, and, thus, creation of a catheterizable Mitrofanoff conduit by use of the appendix vermiformis or small bowel is often part of the augmentation procedure [13]. In this regard, a catherizable urinary stoma may be crucial in the upkeep of the patient’s compliance to prevent short- and long-term complications such as mucous plugging and chronic renal disease [25].
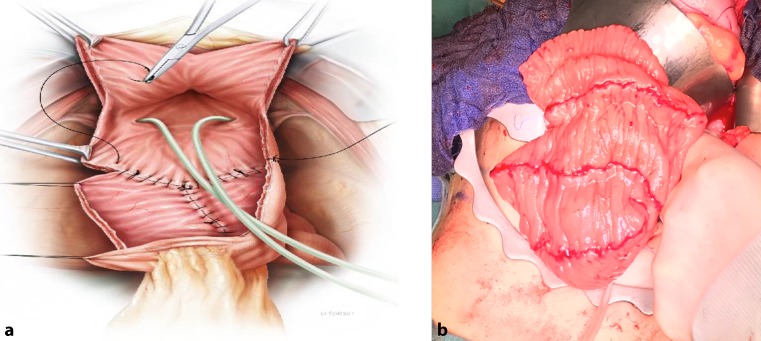
Ileum is most commonly used to perform an augmentation cystoplasty (Fig. 1), followed by colon and stomach [5, 15]. Complications include small bowel obstruction, haematuria-dysuria syndrome, and an increase of urothelial cell carcinoma [5, 26]. In rare cases, the ureter can also be used for cystoplasty. However, ureteral dilatation with ipsilateral impaired renal function with no vesicoureteral reflux are prerequisites [5]. In even less cases, autoaugmentation (vesicomyectomy or vesicomyotomy) can be performed, but only in patients with a preoperative bladder volume of 75–80% compared to normal sized bladders of healthy individuals. Fibrosis and regrowth of the detrusor muscle with need for surgical revision have been described [5].
Fig. 1.
Scheme (a) and intraoperative view (b) of Ileocystoplasty. (Painting in a with Courtesy of Stephan Spitzer [http://www.spitzer-illustration.com/], Stephan Spitzer© all rights reserved)
Although the incidence of short-and long-term complications is high, patients report a significant improvement in their quality of life [13].
Current risk factors and morbidity
Current risk factors and morbidity associated with bladder augmentation by non-urothelial tissue are shown in Table 1.
Table 1.
Complication profile in bladder augmentation using gastrointestinal tissue
| Complication | Incidence (%) |
|---|---|
| Bowel obstruction | 3.2–10.3 |
| Bladder calculi | 15–40 |
| Bladder perforation | 2–8.6 |
| Excessive mucous production | −100 |
| Metabolic acidosis, metabolic deterioration | −100 |
| Malignant transformation of bowel/tumour formation | 0.5–10 |
Exposing non-urothelial tissue to urine causes a complex of problems. Through the constant contact of intestinal mucosa with urine, the accumulation of nitrosamines and infections can lead to premalignant and malignant degeneration in the long-term with incidences of 1.2–10.3% [5, 27–33]. In a recent review, Husmann et al. suggested that the primary bladder dysfunction as opposed to the augmentation cystoplasty is responsible for the development of intravesical neoplasia [25]. Moreover, bladder calculi with 15–40% [5, 34] as well as metabolic dysfunction with up to a 100% incidence rate can occur [34–36]. Enterocystoplasty (gastrocystoplasties and ileocystoplasties) increases the risk of small bowel obstruction with a rate up to a 3.2–10.3% [5, 37, 38]. Bladder perforation rate ranges from 2 to 8.6% [5]; additionally, spontaneous bladder rupture can occur in 3% and is connected to poor catherization compliance [25]. Excessive mucous production seems to occur more frequently after colocystoplasties and less frequently after ileocystoplasties and gastrocystoplasties [34, 39, 40].
Renal scarring and chronic renal failure in patients who undergo augmentation cystoplasty poses a long-term complication that seems to be linked to incompliance with self-catherization as well as catherization per urethra [25].
The implementation of an urothelium-like tissue might avoid such short-and long-term adverse effects (Table 1). Moreover, a high level of elasticity, a good compliance to high and rapid changes of the affecting pressure would be essential requirements to the used tissue. In addition, the potential development of a malignant degeneration of the used tissue should be excluded.
Animal testing in experimental bladder augmentation
Naturally derived biomaterials
-
Fascia and muscle grafts (Table 2)
Experimental bladder augmentation with muscle or muscle-backed flaps has been done as early as the beginning of the last century. In 1917, Neuhof performed bladder augmentation in dogs utilizing free fascia grafts [41]; details on the outcome are not available.
Several experimental animal studies have since been performed on muscle-backed peritoneum, as well as rectus abdominis muscle flaps in rats [42]. Weingarten et al. found an increase in bladder volume in dogs after myoperitoneocystoplasty [43]. Manzoni et al. performed augmentation cystoplasty in thirty rats using rectus abdominis grafts, but bladder stones, chronic inflammatory response at sutures sites as well as undesirable residual muscle contractility occurred [42].
-
Demucosalized intestinal tissue (Table 3)
Since the 1980s, animal studies (rats, calves, rabbits and pigs) focussing on using demucosalized colonic tissue and small intestine have been performed. Weingarten et al. found increased bladder volumes as well as urothelial growth [43].
Oesch et al.. [44] performed augmentation cystoplasty using stripped coecum and reported urothelial growth in only approximately half of the test rats after 4 months [44]. Motley et al. described urothelial growth in 10 out of 11 calves after sigmoidocystoplasty, but residual intestinal mucosa and graft diverticulation occurred [45]. Niku et al. showed incomplete urothelial growth in rabbits after colocystoplasty, leading to postoperative demise of several test animals due to inflammation [46]. Clementson Kockum et al. stated that de-epithelialized colocystoplasty leads to graft contraction, fibrosis and metaplasia in a study done on 21 piglets [47].
Burgu et al. tried to overcome the side effects of ileocystoplasty by either adding gastric tissue or by performing reversed in situ ileocystoplasty in rats. There was no improvement in terms of metabolic imbalances and stone formation following gastroileocystoplasty and ileocystoplasty [48].
-
Other autologous tissue (Table 4)
Thangappan et al. successfully performed augmentation cystoplasty in 12 rats using de-epithelialized bladder wall grafts, although chronic inflammation as well as residual donor urothelial cells were found [49].
Although augmentation with live-related-donor bladder grafts in rats showed promising results, transferring this particular two-step procedure to humans appeared difficult, as stated by Yamataka et al. [50].
Ureterocystaugmentation was performed successfully in pigs, but only after iatrogenic creation of megaureters as reported by Ikeguchi et al. [51].
Human dura mater, stomach and de-epithelialized small intestine tissue were used in augmentation cystoplasty in rabbits performed by Cranidis et al. The grafts covered iatrogenic bladder diverticuli following dertrusorectomy. The best results were obtained by using small intestine, although remnant intestinal mucosa was described. Graft contraction as well as bladder stones and fibrosis occurred in the groups with use of de-epithelialized gastric tissue and dura mater [52].
To bypass risks and complications affiliated with gastric or intestinal resection, pedicled gastrocystoplasty was performed in a rodent model in 2004. Unfortunately, this method showed a number of side effects such as bladder calculi, metaplasia, passing of the test animal as well as scarring [53, 54].
Dapena et al. demonstrated that hysterocystoplasty entails less adverse effects in animal studies than conventional enterocystoplasties in terms of metabolic imbalances and bladder calculi. However, smooth muscle cells were found to be sparse and there was evidence of fibrosis leading to obstruction [55–57].
In a rat model, human amniotic membranes were successfully tested as hypoallergenic grafts, but the bladder capacity did not increase after application of amniotic membranes for augmentation because of the small size of the defect in the bladder wall and graft [58].
Table 2.
Fascia and muscle grafts in experimental bladder augmentation
Table 3.
Demucosalized intestinal tissue in experimental bladder augmentation
| Year | Author | Animal | Graft material | Adverse effect |
|---|---|---|---|---|
| 1988 | Oesch et al. [44] | Rats | De-epithelialized coecum | Incomplete urothelial coverage |
| 1990 | Motley et al. [45] | Calves | Sigmoid | Graft diverticulation, residual intestinal mucosa |
| 1995 | Niku et al. [46] | Rabbits | Colon | Postoperative mortality, inflammation |
| 1999 | Clementson Kockum et al. [47] | Piglets | De-epithelialized colon | Graft contraction, fibrosis, metaplasia |
| 2011 | Burgu et al. [48] | Rats | Ileum, gastric tissue | Metabolic imbalances, bladder stones |
Table 4.
Various types of tissue used in experimental bladder augmentation
| Year | Author | Animal | Graft material | Adverse effect |
|---|---|---|---|---|
| 2012 | Thangappan et al. [49] | Rats | De-epithelialized bladder wall grafts | Chronic inflammation, residual donor cells |
| 2003 | Yamataka et al. [50] | Rats | Bladder wall grafts | Two-step procedure and immunosuppressants may be required |
| 1998 | Ikeguchi et al. [51] | Pigs | Ureteral tissue | Megaureter required |
| 1998 | Cranidis et al. [52] | Rabbits | Human dura mater, de-epithelialized small intestine and gastric tissue | Residual intestinal mucosa, stomach perforation, graft contraction |
| 2004 | Aslan et al. [53] | Rat | Pedicled gastric tissue | Bladder stones, metaplasia, postoperative mortality, scarring |
| 2012, 2013 | Dapena et al. [55, 56] | Rat | Uterus | Fibrosis |
| 2017 | Barski et al. [58] | Rat | Human amniotic membrane | No increase in capacity |
Cell-seeded biological grafts
Studies investigating cell-seeded biological grafts are outlined in Table 5.
Table 5.
Seeded de-epithelialized intestinal tissue in experimental bladder augmentation
| Year | Author | Animal | Graft material | Adverse effect |
|---|---|---|---|---|
| 2001 | Blanco Bruned et al. [59] | Rats | Seeded intestinal grafts | No increase in capacity |
| 2005 | Hafez et al. [60] | Pigs | Seeded demucosalized colon | No information on results with neuropathic bladder cells |
| 2015 | Hidas et al. [61] | Pigs | Seeded demucosalized colon | No information on results with neuropathic bladder cells |
| 2004 | Fraser et al. [62] | Minipigs | Seeded de-epithelialized uterine tissue/colon | Incomplete urothelial coverage, graft contraction, fibrosis |
| 2011 | Turner et al. [63] | Pigs | De-epithelialized colon, urothelium sheets | Graft shrinkage |
Schaefer et al. succeeded in transferring urothelial cells to colon and gastric grafts in vitro. However, they did not perform augmentation cystoplasty in vivo [64].
No increase in postoperative bladder volume was found following intestinal grafts seeded with urothelium in rats as demonstrated by Blanco Bruned et al., which resulted in a high mortality rate of 63.3% [59].
Seeding colonic grafts with urothelial cells and smooth muscle cells with an aerosol spraying technique was developed in 2003 [65]. Two ensuing studies showed the effectiveness of this bladder augmentation method in terms of cell adhesion and confluent epithelial coverage, although smooth muscle cell growth occurred only after an additional adding of detrusor cells to the urothelial cells [60, 61]. Hafez et al. compared aerosol transfer of smooth muscle cells onto demucosalized colon grafts to conventional colocystoplasty and found complete urothelial coverage only in the animals that underwent the aerosol graft cystoplasty [60]. Hidas et al. showed similar results, with no fibrosis or inflammation in porcine cystoplasty using the aerosol transfer technique. However, Hidas et al. stated that further studies in animals with neuropathic bladder should be done, as the results in a neuropathic bladder population might deviate [61].
Incomplete urothelial covering of seeded de-epithelialized uterine tissue derived from minipigs was reported in an experimental cystoplasty study done by Fraser et al., as well as graft contraction and fibrosis in de-epithelialized colonic tissue. [62].
Turner et al. successfully combined urothelium sheets with de-epithelialized colon tissue in a porcine model, but described graft shrinkage [63].
-
Acellular matrix grafts (Table 6)
Biomaterials such as acellular matrix grafts and bladder submucosa collagen matrix have been used successfully in animal trials because of their good biocompatibility [66]. However, acellular bladder matrix grafts do not promote the ingrowth of smooth muscle cells and there is therefore no structural integration. Postoperative urinoma and urinary tract infection can occur as well, and antigenicity cannot be precluded completely [67–70].
Kropp et al. described complete urothelial graft overgrowth in 22 rats after cystoplasty with porcine-derived small intestinal submucosa (SIS); however, there were bladder calculi, leakage, inflammation and incomplete smooth muscle cell growth noticed [71].
Parshotam Kumar et al. reported on the evidence of fibrosis with SIS in augmentation cystoplasty in lambs [72].
Sharma et al. seeded stem cells unto de-epithelialized small intestine tissue and used the graft to perform augmentation cystoplasty in primates, showing urothelial and smooth muscle growth but no increase in postoperative bladder volume [73].
By managing to perform a bladder augmentation using only smooth-muscle cells sheets, Talab et al. showed that neovascularization and epithelialization can be achieved without the use of a scaffold. However, there was no testing regarding the postoperative bladder volume. [74].
Muscle cell migration could also be enhanced by seeding acellular bladder grafts with adipose-derived stem cells, as demonstrated by Zhe et al., although bladder calculi and insufficient smooth muscle cell growth occurred [75].
Smooth muscle cells harvested from neuropathic bladders showed similar results to matrices seeded with normal cells when seeded unto matrices in vitro and then transplanted in vivo [76, 77]. This adds a new perspective to the findings of Subramaniam et al., who found that urothelial cells harvested from patients with bladder dysfunction showed reduced proliferation and differentiation [78].
-
Polymers, collagen grafts, glycosaminoglycans (Table 7)
Nano-structured polymers have been tested since 2007, providing adequate surface properties for smooth muscle and urothelium proliferation. However, death as a consequence of bladder leak was reported as well as untimely biodegradation, sparse smooth muscle cell growth, fibrosis as well as no increase of the bladder capacity [79, 80]. However, seeded polymer grafts did not show these adverse effects as shown by Kwon et al. [80]. Parshotam et al. found that augmentation cystoplasty using INTEGRA® (INTEGRA LIFE SCIENCE CORPORATION, Plainsboro, New Jersey, USA) collagen matrix showed better results than SURGISIS® (COOK, Spencer, Indiana, USA) collagen matrix or demucosalized enterocystoplasty in lambs. Mucous cysts as well as intestinal obstruction, fibrosis and graft shrinkage were described in the enterocystplasty as well as in the SURGISIS® (COOK, Spencer, Indiana, USA) groups [72].
Zhou et al. advanced the use of tissue-engineered grafts by applying vascular endothelial growth factor and platelet-derived growth factor onto bladder acellular matrices to enhance muscle and vascular ingrowth. While the bioactive factors did promote smooth muscle cell regeneration and neovascularization, urinary leakage and bladder stone formation occurred, as well as graft shrinkage, scarring and graft calcification [81].
Further experimentation by Vardar et al. on collagen–fibrin scaffolds showed improved urothelialization and smooth muscle cell growth by adding insulin-like growth factor. Nonetheless, there was hypertrophy of the constructed urothelium which could lead to outlet obstruction [82].
In a recent study from 2017, unseeded and seeded cystoplasty collagen grafts were compared, with inconsistent results regarding the ingrowth of urothelial and smooth muscle cells [83].
Table 6.
Acellular matrix grafts in experimental bladder augmentation
| Year | Author | Animal | Graft material | Adverse effect |
|---|---|---|---|---|
| 1995 | Kropp et al. [71] | Rats | SIS | Bladder stones, inflammation, leakage, inflammation, incomplete smooths muscle cell growth |
| 2010 | Parshotam Kumar et al. [72] | Sheep | SIS | Fibrosis |
| 2011 | Sharma et al. [73] | Primates | Seeded SIS with stem cells | No increase in capacity |
| 2014 | Talab et al. [74] | Rabbits | Smooth-muscle cell sheets | Fibrosis, no information on postoperative bladder capacity |
| 2016 | Zhe et al. [75] | Rats | ACS-seeded acellular bladder grafts | Bladder stones, insufficient cell growth |
SIS small intestinal submucosa, ACS adipose-derived stem cells
Table 7.
Polymers, collagen grafts, glycosaminoglycans in experimental bladder augmentation
| Year | Author | Animal | Material | Adverse effect |
|---|---|---|---|---|
| 2007 | Pattison et al. [79] | Rats | Polymer scaffolds | Bladder leak, bladder stones postoperative mortality, slow biodegradation, mechanical difficulties |
| 2008 | Kwon et al. [80] | Dogs | Polymer scaffold | Chronic inflammation, rapid scaffold degradation |
| 2010 | Parshotam Kumar et al. [72] | Lambs | Collagen scaffolds (INTEGRA® [INTEGRA LIFE SCIENCE CORPORATION, Plainsboro, New Jersey, USA], SURGISIS® [COOK, Spencer, Indiana, USA]) | Fibrosis, graft contraction |
| 2013 | Zhou et al. [81] | Rabbits | Acellular bladder matric and growth factors | Bladder stones, graft shrinkage/calcification/scarring, urinary leakage |
| 2016 | Vardar et al. [82] | – | Collagen–fibrin scaffold and IGF-1 | Possible outlet obstruction because of tissue hypertrophy |
| 2017 | Leonhäuser et al. [83] | Minipigs | Unseeded and seeded collagen scaffolds | Inconsistent cell ingrowth, risk of leakage |
Synthetic materials
Synthetic materials which have been employed in experimental bladder augmentation are presented in Table 8.
Table 8.
Synthetic materials in experimental bladder augmentation
| Year | Author | Animal | Material | Adverse effect |
|---|---|---|---|---|
| 1957 | Kudish [84] | Dogs | Polyvinyl sponges | Foreign body reaction |
| 1970 | Kelâmi et al. [85] | Dogs | Teflon® (BARD INC., Murray Hill, New Jersey, USA) felt | Fibrosis, incomplete urothelial coverage, no smooth muscle cell growth, graft collapse |
| 1994 | Virseda Chamorro et al. [86] | Dogs | Gore-Tex® (W.L. GORE and ASSOCIATES, INC., Flagstaff, Arizona, USA) | No increase in capacity |
Polyvinyl sponges [84], Teflon® (BARD INC., Murray Hill, New Jersey, USA; [85]), as well as Gore-Tex® (W.L. GORE and ASSOCIATES, INC., Flagstaff, Arizona, USA); patches [86] have been used in experimental animal studies, too. However, there was no increase in capacity due to the stiffness of the material and no ingrowth of muscle cells [86]. Moreover, foreign body reactions, fibrosis, incomplete urothelial growth as well as no smooth muscle cell ingrowth have been described [84, 85].
Silk-based scaffolds
Silk-based scaffolds which have been used in experimental bladder augmentation are shown in Table 9.
Table 9.
Silk scaffolds in experimental bladder augmentation
| Year | Author | Animal | Material | Adverse effect |
|---|---|---|---|---|
| 2013 | Seth et al. [87] | Rat | Silkworm silk scaffold/combined with SIS | Foreign body reaction |
| 2014 | Chung et al. [88] | Rat | Silkworm silk fibroin scaffolds combined with SIS | Bladder stones, bladder rupture, chronic inflammation, residual silk |
| 2015 | Zhao et al. [66] | Rat | Silkworm silk combined with acellular bladder matrix graft | No increase in capacity |
| 2013 | Tu et al. [89] | Pigs | Acellular silkworm silk scaffolds | Urinary leakage, bladder calculi, graft contraction |
SIS small intestinal submucosa
To facilitate the growth of urothelial and smooth muscle cells, grafts have been lined with silkworm silk (Bombyx mori). However, Seth et al. described a high incidence of foreign body reaction in either silk matrices or small intestinal submucosa combined with silkworm silk [87]. Chung et al. found regrowth of smooth muscle cells by combining small intestinal submucosa and silkworm silk in a rat cystoplasty model, but again, bladder stones and bladder rupture as well as chronic inflammation and residual silk were present [88]. Zhao et al. used silkworm silk to line a bladder acellular matrix graft in rats. Although there was ingrowth of smooth muscle cells present, bladder stones as well as graft perforation and chronic inflammatory response occurred [66]. Tu et al. tested acellular silkworm silk scaffolds in pigs, and although smooth muscle cells as well as nerve cells and neovascularisation were successful, urinary leakage as well as urinary calculi and graft contraction still ensued [89].
Due to the fact of current inflammation, stone production, leakage and persistence of silk graft remnants, this particular method for bladder augmentation needs to be further investigated. Presumably, a more suitable silk donor has to be found to eliminate these adverse effects such as antigenicity and formation of calculi.
Conclusion and perspective
In conclusion, the implementation of tissue and neo-organs fabricated in vitro seems feasible in partial or total organ reconstruction. Seeded biomaterials (collagen, keratin, alginate, acellular tissue matrices, synthetic polymers) surpass non-biomaterials (Teflon® [BARD INC., Murray Hill, New Jersey, USA], silicone, Vicryl® [ETHICON INC., Cincinetti, Ohio, USA], polyvinyl, unseeded collagen matrices) in terms of biocompatibility, degradation, cell adhesion substrate, tissue development, mechanical and physical properties as well as plasticity.
The best results in seeding the grafts are obtained by using autologous urothelial and smooth muscle cells.
Research concerning the use of stem cells, amniotic fluid and progenitor cells from urine as well as “printing” 3D scaffolds in vitro is still ongoing [76, 77, 90].
Minimizing or alleviating the ailments of congenital malformations or traumatic injuries of the urinary tract by operative means remains a challenge for paediatric surgeons as well as for interdisciplinary carers.
New techniques and possibilities in the operating field of bladder augmentation and ureteral reconstruction give new future perspectives in terms of reducing side effects and maximizing the quality of life of the afflicted patients.
Abbreviations
- CIC
Clean intermittent self-catheterization
- MRI
Magnetic resonance imaging
- SIS
Small intestinal submucosa
Funding
Open access funding provided by Medical University of Vienna.
Conflict of interest
S. Langer, C. Radtke, E. Györi, A. Springer and M.L. Metzelder declare that they have no competing interests.
References
- 1.Lapides J, Diokno AC, Gould FR, Lowe BS. Further observations on self-catheterization. J Urol. 1976;116:169–171. doi: 10.1016/s0022-5347(17)58730-3. [DOI] [PubMed] [Google Scholar]
- 2.Altaweel W, Jednack R, Bilodeau C, Corcos J. Repeated intradetrusor botulinum toxin type A in children with neurogenic bladder due to myelomeningocele. J Urol. 2006;175:1102–1105. doi: 10.1016/S0022-5347(05)00400-3. [DOI] [PubMed] [Google Scholar]
- 3.Tudor KI, Sakakibara R, Panicker JN. Neurogenic lower urinary tract dysfunction: evaluation and management. J Neurol. 2016;263:2555–2564. doi: 10.1007/s00415-016-8212-2. [DOI] [PubMed] [Google Scholar]
- 4.Gor RA, Elliott SP. Surgical management of neurogenic lower urinary tract dysfunction. Urol Clin North Am. 2017;44:475–490. doi: 10.1016/j.ucl.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Lee B, Featherstone N, Nagappan P, McCarthy L, O’Toole S. British Association of Paediatric Urologists consensus statement on the management of the neuropathic bladder. J Pediatr Urol. 2016;12:76–87. doi: 10.1016/j.jpurol.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Atta CAM, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, et al. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am J Public Health. 2016;106:e24–e34. doi: 10.2105/AJPH.2015.302902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks JD, Khoshnood B. Epidemiology of common neurosurgical diseases in the neonate. Neurosurg Clin N Am. 1998;9:63–72. [PubMed] [Google Scholar]
- 8.Diamond DA, Chan IHY, Holland AJA, Kurtz MP, Nelson C, Estrada CR, et al. Advances in paediatric urology. Lancet. 2017;390:1061–1071. doi: 10.1016/S0140-6736(17)32282-1. [DOI] [PubMed] [Google Scholar]
- 9.Roberts CJ, Evans KT, Hibbard BM, Laurence KM, Roberts EE, Robertson IB. Diagnostic effectiveness of ultrasound in detection of neural tube defect. The South Wales experience of 2509 scans (1977–1982) in high-risk mothers. Lancet. 1983;2:1068–1069. doi: 10.1016/s0140-6736(83)91049-8. [DOI] [PubMed] [Google Scholar]
- 10.Sturm RM, Cheng EY. The management of the pediatric neurogenic bladder. Curr Bladder Dysfunct Rep. 2016;11:225–233. doi: 10.1007/s11884-016-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nseyo U, Santiago-Lastra Y. Long-term complications of the neurogenic bladder. Urol Clin North Am. 2017;44:355–366. doi: 10.1016/j.ucl.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Siffel C, Correa A, Amar E, Bakker MK, Bermejo-Sánchez E, Bianca S, et al. Bladder exstrophy: an epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research, and an overview of the literature. Am J Med Genet. 2011;157:321–332. doi: 10.1002/ajmg.c.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.‘t Hoen L, Ecclestone H, Blok BFM, Karsenty G, Phé V, Bossier R, et al. Long-term effectiveness and complication rates of bladder augmentation in patients with neurogenic bladder dysfunction: a systematic review. Neurourol Urodyn. 2017;36:1685–1702. doi: 10.1002/nau.23205. [DOI] [PubMed] [Google Scholar]
- 14.Cuschieri A. Descriptive epidemiology of isolated anal anomalies: a survey of 4.6 million births in europe. Am J Med Genet. 2001;103:207–215. doi: 10.1002/ajmg.1532.abs. [DOI] [PubMed] [Google Scholar]
- 15.Casey JT, Chan KH, Hasegawa Y, Large T, Judge B, Kaefer M, et al. Long-term follow-up of composite bladder augmentation incorporating stomach in a multi-institutional cohort of patients with cloacal exstrophy. J Pediatr Urol. 2017;13(1):43.e1–43.e6. doi: 10.1016/j.jpurol.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Lapides J, Diokno AC, Silber SJ, Lowe BS. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458–461. doi: 10.1016/s0022-5347(17)61055-3. [DOI] [PubMed] [Google Scholar]
- 17.De Castro R, Pavanello P, Dòmini R. Indications for bladder augmentation in the exstrophy-epispadias complex. Br J Urol. 1994;73:303–307. doi: 10.1111/j.1464-410x.1994.tb07523.x. [DOI] [PubMed] [Google Scholar]
- 18.Dos Santos J, Lopes RI, Koyle MA. Bladder and bowel dysfunction in children: an update on the diagnosis and treatment of a common, but underdiagnosed pediatric problem. Can Urol Assoc J. 2017;11:64. doi: 10.5489/cuaj.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stothers L, Tsang B, Nigro M, Lazare D, Macnab A. An integrative review of standardized clinical evaluation tool utilization in anticholinergic drug trials for neurogenic lower urinary tract dysfunction. Spinal Cord. 2016;54:1114–1120. doi: 10.1038/sc.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verpoorten C, Buyse GM. The neurogenic bladder: medical treatment. Pediatr Nephrol. 2008;23:717–725. doi: 10.1007/s00467-007-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apostolidis A, Dasgupta P, Denys P, Elneil S, Fowler CJ, Giannantoni A, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55:100–119. doi: 10.1016/j.eururo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Khan MK, VanderBrink BA, DeFoor WR, Minevich E, Jackson E, Noh P, et al. Botulinum toxin injection in the pediatric population with medically refractory neuropathic bladder. J Pediatr Urol. 2016;12:104.e1–104.e6. doi: 10.1016/j.jpurol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, ’t Hoen L, Blok B. Summary of European Association of Urology (EAU) guidelines on neuro-urology. Eur Urol. 2016;69:324–333. doi: 10.1016/j.eururo.2015.07.071. [DOI] [PubMed] [Google Scholar]
- 24.Gearhart JP, Albertsen PC, Marshall FF, Jeffs RD. Pediatric applications of augmentation cystoplasty: the Johns Hopkins experience. J Urol. 1986;136:430–432. doi: 10.1016/s0022-5347(17)44893-2. [DOI] [PubMed] [Google Scholar]
- 25.Husmann DA. Mortality following augmentation cystoplasty: a transitional urologist’s viewpoint. J Pediatr Urol. 2017;13:358–364. doi: 10.1016/j.jpurol.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Mingin GC, Stock JA, Hanna MK. Gastrocystoplasty: long-term complications in 22 patients. J Urol. 1999;162:1122–1125. doi: 10.1016/S0022-5347(01)68092-3. [DOI] [PubMed] [Google Scholar]
- 27.Biardeau X, Chartier-Kastler E, Rouprêt M, Phé V. Risk of malignancy after augmentation cystoplasty: a systematic review. Neurourol Urodyn. 2016;35:675–682. doi: 10.1002/nau.22775. [DOI] [PubMed] [Google Scholar]
- 28.Boissier R, Di Crocco E, Faure A, Hery G, Delaporte V, Lechevallier E, et al. What is the outcome of paediatric gastrocystoplasty when the patients reach adulthood? BJU Int. 2016;118:980–986. doi: 10.1111/bju.13558. [DOI] [PubMed] [Google Scholar]
- 29.Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004;172(4):1649–1652. doi: 10.1097/01.ju.0000140194.87974.56. [DOI] [PubMed] [Google Scholar]
- 30.Esquena Fernández S, Abascal JM, Tremps E, Morote J. Gastric cancer in augmentation gastrocystoplasty. Urol Int. 2005;74:368–370. doi: 10.1159/000084441. [DOI] [PubMed] [Google Scholar]
- 31.Kälble T, Hofmann I, Thüroff JW, Stein R, Hautmann R, Riedmiller H, et al. Sekundärmalignome in Harnableitungen. Urologe. 2012;51:500–506. doi: 10.1007/s00120-012-2815-8. [DOI] [PubMed] [Google Scholar]
- 32.Kispal ZF, Kardos D, Jilling T, Kereskai L, Isaacs M, Balogh DL, et al. Long-term histological and mucin alterations in the neobladder mucosa following urinary bladder augmentation or substitution with gastrointestinal segment. J Pediatr Urol. 2015;11:349.e1–349.e6. doi: 10.1016/j.jpurol.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Kono Y, Terada N, Takashima Y, Hikami K, Hida T, Goto S, et al. Advanced adenocarcinoma of the bladder after augmentation gastrocystoplasty. Hinyokika Kiyo. 2016;62:33–37. [PubMed] [Google Scholar]
- 34.Greenwell TJ, Venn SN, Mundy AR. Augmentation cystoplasty. BJU Int. 2001;88:511–525. doi: 10.1046/j.1464-4096.2001.001206. [DOI] [PubMed] [Google Scholar]
- 35.Odeh RI, Farhat WA, Penna FJ, Koyle MA, Lee LC, Butt H, et al. Outcomes of seromuscular bladder augmentation versus standard ileocystoplasty: a single institution experience over 14 years. J Pediatr Urol. 2017;13:200.e1–200.e5. doi: 10.1016/j.jpurol.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 36.Escudero RM, Patiño GE, Fernández ER, Gil MJC, García EL, Alonso AH, et al. Bladder augmentation using the gastrointestinal tract. Indication, follow up and complications. Arch Esp Urol. 2011;64:953–959. [PubMed] [Google Scholar]
- 37.Schlomer BJ, Copp HL. Cumulative incidence of outcomes and urologic procedures after augmentation cystoplasty. J Pediatr Urol. 2014;10:1043–1050. doi: 10.1016/j.jpurol.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metcalfe PD, Cain MP, Kaefer M, Gilley DA, Meldrum KK, Misseri R, et al. What is the need for additional bladder surgery after bladder augmentation in childhood? J Urol. 2006;176:1801–1805. doi: 10.1016/j.juro.2006.03.126. [DOI] [PubMed] [Google Scholar]
- 39.Gough DC. Enterocystoplasty. BJU Int. 2001;88:739–743. doi: 10.1046/j.1464-4096.2001.gough.2464.x. [DOI] [PubMed] [Google Scholar]
- 40.Murray K, Nurse DE, Mundy AR. Secreto-motor function of intestinal segments used in lower urinary tract reconstruction. Br J Urol. 1987;60:532–535. doi: 10.1111/j.1464-410x.1987.tb05037.x. [DOI] [PubMed] [Google Scholar]
- 41.Neuhof H. Fascial transplantation into visceral defects: an experimental and clinical study. Surg. Gynecol. Obstet. 1917;25:383–392. [Google Scholar]
- 42.Manzoni C, Grottesi A, D’Urzo C, Pintus C, Fadda G, Perrelli L. An original technique for bladder autoaugmentation with protective abdominal rectus muscle flaps: an experimental study in rats. J Surg Res. 2001;99:169–174. doi: 10.1006/jsre.2001.6098. [DOI] [PubMed] [Google Scholar]
- 43.Weingarten JL, Cromie WJ, Paty RJ. Augmentation myoperitoneocystoplasty. J Urol. 1990;144:156–158. doi: 10.1016/s0022-5347(17)39400-4. [DOI] [PubMed] [Google Scholar]
- 44.Oesch I. Neourothelium in bladder augmentation. An experimental study in rats. Eur Urol. 1988;14:328–329. doi: 10.1159/000472971. [DOI] [PubMed] [Google Scholar]
- 45.Motley RC, Montgomery BT, Zollman PE, Holley KE, Kramer SA. Augmentation cystoplasty utilizing de-epithelialized sigmoid colon: a preliminary study. J Urol. 1990;143:1257–1260. doi: 10.1016/s0022-5347(17)40249-7. [DOI] [PubMed] [Google Scholar]
- 46.Niku SD, Scherz HC, Stein PC, Parsons CL. Intestinal de-epithelialization and augmentation cystoplasty: an animal model. Urology. 1995;46:36–39. doi: 10.1016/S0090-4295(99)80155-1. [DOI] [PubMed] [Google Scholar]
- 47.Clementson Kockum C, Willén R, Malmfors G. Bladder augmentation with different forms of intestinal grafts: an experimental study in the pig. BJU Int. 1999;83(3):305–311. doi: 10.1046/j.1464-410x.1999.00895.x. [DOI] [PubMed] [Google Scholar]
- 48.Burgu B, Gökce Mİ, Aydoğdu Ö, Süer E, Kankaya D, Soygür T. Combining gastric and ileal segments, does it overcome segment-related complications? An experimental study on rats. Urol Res. 2011;39:39–44. doi: 10.1007/s00240-010-0283-4. [DOI] [PubMed] [Google Scholar]
- 49.Thangappan R, Eandi JA, Modi J, Kurzrock EA. Epithelium-free bladder wall graft: epithelial Ingrowth and regeneration—clinical implications for partial cystectomy. J Urol. 2012;187:1450–1457. doi: 10.1016/j.juro.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Yamataka A, Wang K, Okada Y, Kobayashi H, Lane GJ, Yanai T, et al. Living-related partial bladder transplantation for bladder augmentation in rats: an experimental study. J Pediatr Surg. 2003;38:913–915. doi: 10.1016/s0022-3468(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 51.Ikeguchi EF, Stifleman MD, Hensle TW. Ureteral tissue expansion for bladder augmentation. J Urol. 1998;159:1665–1668. doi: 10.1097/00005392-199805000-00086. [DOI] [PubMed] [Google Scholar]
- 52.Cranidis A, Nestoridis G, Delakas D, Lumbakis P, Kanavaros P. Bladder autoaugmentation in the rabbit using de-epithelialized segments of small intestine, stomach and lyophilized human dura mater. Br J Urol. 1998;81:62–67. doi: 10.1046/j.1464-410x.1998.00475.x. [DOI] [PubMed] [Google Scholar]
- 53.Aslan A, Akkaya B, Karagüzel G, Karpuzoglu G, Melikoglu M. Bladder augmentation with an omental pedicled gastric seromuscular flap without the necessity of gastric resection. Urol Res. 2004;32:298–303. doi: 10.1007/s00240-004-0417-7. [DOI] [PubMed] [Google Scholar]
- 54.Close CE, Anderson PD, Edwards GA, Mitchell ME, Dewan PA. Autoaugmentation gastrocystoplasty: further studies of the sheep model. BJU Int. 2004;94:658–662. doi: 10.1111/j.1464-410X.2004.05018.x. [DOI] [PubMed] [Google Scholar]
- 55.Dapena L, Dapena I, Regadera J, Gaspar MJ, González-Peramato P. Histerocystoplasty: a novel surgical procedure in the rat. J Surg Res. 2012;175:157–162. doi: 10.1016/j.jss.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Dapena L, Dapena I, Regadera J, Silva-Mato A, González-Peramato P. Bladder autoaugmentation with protective autologous uterine flap. Experimental study in the rat. Int J Surg. 2013;11:270–274. doi: 10.1016/j.ijsu.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Kotecha R, Toledo-Pereyra LH. Hysterocystoplasty: a new surgical technique for bladder reconstruction. J Surg Res. 2012;176:397–399. doi: 10.1016/j.jss.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Barski D, Gerullis H, Ecke T, Yang J, Varga G, Boros M, et al. Bladder reconstruction with human amniotic membrane in a xenograft rat model: a preclinical study. Int J Med Sci. 2017;14:310–318. doi: 10.7150/ijms.18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanco Bruned JL, Alvarez Díaz JF, Sáez López A, Oliver Llinares F, Prado Fernández C, González Landa G. Seromuscular colocystoplasty lined by urothelium. Experimental study in rats. Cir Pediatr. 2001;14:162–167. [PubMed] [Google Scholar]
- 60.Hafez AT, Afshar K, Bägli DJ, Bahoric A, Aitken K, Smith CR, et al. Aerosol transfer of bladder urothelial and smooth muscle cells onto demucosalized colonic segments for porcine bladder augmentation in vivo: a 6-week experimental study. J Urol. 2005;174(4):1663–1668. doi: 10.1097/01.ju.0000177727.56790.98. [DOI] [PubMed] [Google Scholar]
- 61.Hidas G, Lee HJ, Bahoric A, Kelly MS, Watts B, Liu Z, et al. Aerosol transfer of bladder urothelial and smooth muscle cells onto demucosalized colonic segments for bladder augmentation: In vivo, long term, and functional pilot study. J Pediatr Urol. 2015;11:260.e1–260.e6. doi: 10.1016/j.jpurol.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraser M, Thomas DF, Pitt E, Harnden P, Trejdosiewicz LK, Southgate J. A surgical model of composite cystoplasty with cultured urothelial cells: a controlled study of gross outcome and urothelial phenotype. BJU Int. 2004;93:609–616. doi: 10.1111/j.1464-410x.2003.04675.x. [DOI] [PubMed] [Google Scholar]
- 63.Turner A, Subramanian R, Thomas DFM, Hinley J, Abbas SK, Stahlschmidt J, et al. Transplantation of autologous differentiated urothelium in an experimental model of composite cystoplasty. Eur Urol. 2011;59:447–454. doi: 10.1016/j.eururo.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaefer BM, Lorenz C, Back W, Moll R, Sun TT, Schober C, et al. Autologous transplantation of urothelium into demucosalized gastrointestinal segments: evidence for epithelialization and differentiation of in vitro expanded and transplanted urothelial cells. J Urol. 1998;159:284–290. doi: 10.1016/s0022-5347(01)64083-7. [DOI] [PubMed] [Google Scholar]
- 65.Hafez AT, Bägli DJ, Bahoric A, Aitken K, Smith CR, Herz D, et al. Aerosol transfer of bladder urothelial and smooth muscle cells onto demucosalized colonic segments: a pilot study. J Urol. 2003;169:2316–2320. doi: 10.1097/01.ju.0000067485.51252.f5. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y, He Y, Guo J, Wu J, Zhou Z, Zhang M, et al. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater. 2015;23:91–102. doi: 10.1016/j.actbio.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 67.Probst M, Piechota HJ, Dahiya R, Tanagho EA. Homologous bladder augmentation in dog with the bladder acellular matrix graft. BJU Int. 2000;85:362–371. doi: 10.1046/j.1464-410x.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 68.Wefer J, Sievert KD, Schlote N, Wefer AE, Nunes L, Dahiya R, et al. Time dependent smooth muscle regeneration and maturation in a bladder acellular matrix graft: histological studies and in vivo functional evaluation. J Urol. 2001;165:1755–1759. [PubMed] [Google Scholar]
- 69.Falke G, Caffaratti J, Atala A. Tissue engineering of the bladder. World J Urol. 2000;18:36–43. doi: 10.1007/s003450050007. [DOI] [PubMed] [Google Scholar]
- 70.Cayan S, Chermansky C, Schlote N, Sekido N, Nunes L, Dahiya R, et al. The bladder acellular matrix graft in a rat chemical cystitis model: functional and histologic evaluation. J Urol. 2002;168:798–804. doi: 10.1016/s0022-5347(05)64746-5. [DOI] [PubMed] [Google Scholar]
- 71.Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46:396–400. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- 72.Parshotam Kumar G, Barker A, Ahmed S, Gerath J, Orford J. Urinary bladder auto augmentation using INTEGRA® and SURGISIS®: an experimental model. Pediatr Surg Int. 2010;26:275–280. doi: 10.1007/s00383-009-2521-9. [DOI] [PubMed] [Google Scholar]
- 73.Sharma AK, Bury MI, Marks AJ, Fuller NJ, Meisner JW, Tapaskar N, et al. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells. 2011;29:241–250. doi: 10.1002/stem.568. [DOI] [PubMed] [Google Scholar]
- 74.Talab SS, Kajbafzadeh A-M, Elmi A, Tourchi A, Sabetkish S, Sabetkish N, et al. Bladder reconstruction using scaffold-less autologous smooth muscle cell sheet engineering: early histological outcomes for autoaugmentation cystoplasty. BJU Int. 2014;114:937–945. doi: 10.1111/bju.12685. [DOI] [PubMed] [Google Scholar]
- 75.Zhe Z, Jun D, Yang Z, Mingxi X, Ke Z, Ming Z, et al. Bladder acellular matrix grafts seeded with adipose-derived stem cells and incubated intraperitoneally promote the regeneration of bladder smooth muscle and nerve in a rat model of bladder augmentation. Stem Cells Dev. 2016;25:405–414. doi: 10.1089/scd.2015.0246. [DOI] [PubMed] [Google Scholar]
- 76.Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81–104. doi: 10.1093/bmb/ldr003. [DOI] [PubMed] [Google Scholar]
- 77.Yoo JJ, Olson J, Atala A, Kim B. Regenerative medicine strategies for treating neurogenic bladder. Int Neurourol J. 2011;15:109–119. doi: 10.5213/inj.2011.15.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–2020. doi: 10.1016/j.juro.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 79.Pattison M, Webster TJ, Leslie J, Kaefer M, Haberstroh KM. Evaluating the in vitro and in vivo efficacy of nano-structured polymers for bladder tissue replacement applications. Macromol Biosci. 2007;7:690–700. doi: 10.1002/mabi.200600297. [DOI] [PubMed] [Google Scholar]
- 80.Kwon TG, Yoo JJ, Atala A. Local and systemic effects of a tissue engineered neobladder in a canine cystoplasty model. J Urol. 2008;179:2035–2041. doi: 10.1016/j.juro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Zhou L, Yang B, Sun C, Qiu X, Sun Z, Chen Y, et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A. 2013;19:264–276. doi: 10.1089/ten.tea.2011.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vardar E, Larsson HM, Engelhardt EM, Pinnagoda K, Briquez PS, Hubbell JA, et al. IGF-1-containing multi-layered collagen-fibrin hybrid scaffolds for bladder tissue engineering. Acta Biomater. 2016;41:75–85. doi: 10.1016/j.actbio.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Leonhäuser D, Stollenwerk K, Seifarth V, Zraik IM, Vogt M, Srinivasan PK, et al. Two differentially structured collagen scaffolds for potential urinary bladder augmentation: proof of concept study in a Göttingen minipig model. J Transl Med. 2017;15:3. doi: 10.1186/s12967-016-1112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kudish HG. The use of polyvinyl sponge for experimental cystoplasty. J Urol. 1957;78:232–235. doi: 10.1016/S0022-5347(17)66428-0. [DOI] [PubMed] [Google Scholar]
- 85.Kelâmi A, Dustmann HO, Lüdtke-Handjery A, Cárcamo V, Herlld G. Experimental investigations of bladder regeneration using teflon-felt as a bladder wall substitute. J Urol. 1970;104:693–698. doi: 10.1016/s0022-5347(17)61813-5. [DOI] [PubMed] [Google Scholar]
- 86.Virseda Chamorro M, González Meli B, Salinas Casado J, Mellado F, Galán Torres JA, García Marcos J, et al. Experimental bladder augmentation with Gore-tex amine: biomechanical, biochemical and biostructural aspects. Arch Esp Urol. 1994;47:958–966. [PubMed] [Google Scholar]
- 87.Seth A, Chung YG, Gil ES, Tu D, Franck D, Di Vizio D, et al. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials. 2013;34:4758–4765. doi: 10.1016/j.biomaterials.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung YG, Algarrahi K, Franck D, Tu DD, Adam RM, Kaplan DL, et al. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials. 2014;35:7452–7459. doi: 10.1016/j.biomaterials.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tu DD, Chung YG, Gil ES, Seth A, Franck D, Cristofaro V, et al. Bladder tissue regeneration using acellular bi-layer silk scaffolds in a large animal model of augmentation cystoplasty. Biomaterials. 2013;34:8681–8689. doi: 10.1016/j.biomaterials.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garriboli M, Radford A, Southgate J. Regenerative medicine in urology. Eur J Pediatr Surg. 2014;24:227–236. doi: 10.1055/s-0034-1382259. [DOI] [PubMed] [Google Scholar]