Abstract
Background
Many studies have examined the effects of exclusive enteral nutrition (EEN) in children with Crohn’s disease (CD), but corticosteroids are considered a superior therapy and are frequently used in China. This meta-analysis aims to compare the efficacy of EEN with corticosteroids in treating pediatric CD.
Methods
A comprehensive retrieval from medical databases, including PubMed, EMBASE, MEDLINE, Web of Science, Wanfang data, VIP and CNKI, was performed using the search terms “diet therapy”, “exclusive enteral nutrition”, “Crohn’s disease”, “inflammatory bowel diseases”, “child” and “pediatrics” from January 1990 to April 2017.
Results
We included 18 studies from 1329 identified sources in this meta-analysis. EEN was as effective as corticosteroids in inducing remission rate of children suffering from CD (OR = 1.35; 95% CI 0.90, 2.10; P = 0.14). Nevertheless, patients who received EEN were more likely to achieve both endoscopic mucosal healing (OR = 5.24; 95% CI 2.06, 13.37; P = 0.0005) and histological mucosal healing (OR = 4.78; 95% CI 1.89, 12.08; P = 0.0009) than those who received corticosteroids; the Pediatric Crohn’s Disease Activity Index was lower [mean difference (MD) = − 3.67; 95% CI − 4.91, − 2.43] and weight gain was higher (MD = 1.92; 95% CI 0.02, 3.83; P = 0.05) in those patients who received EEN than in those who received corticosteroids. No difference was found in relapse rate (OR = 0.57; 95% CI 0.25, 1.29; P = 0.18), height for age or body mass index between the patients treated with EEN and corticosteroids at the 1-year end point.
Conclusions
This meta-analysis reveals that there is no significant difference between EEN and corticosteroids in the efficacy of inducing remission rate of CD in a pediatric population, but EEN is superior to corticosteroids in improving short-term mucosal inflammation and reducing the PCDAI index.
Electronic supplementary material
The online version of this article (10.1007/s12519-018-0204-0) contains supplementary material, which is available to authorized users.
Keywords: Children, Corticosteroids, Crohn’s disease, Exclusive enteral nutrition, Inflammatory bowel disease, Nutrition
Introduction
Crohn’s disease (CD) is a chronic relapsing inflammatory condition of the gastrointestinal tract. Causes of CD still remain unclear, but it is thought to result from an interaction of individuals’ genetics factors, epigenetic factors, microbial exposure, immune response and environment factors [1–4]. Approximately, 25% of patients are diagnosed with CD before the age of 18 years [5], and the incidence of pediatric CD is increasing in both developed and developing nations [6, 7]. The goals of treatment in pediatric CD are to induce and maintain remission, relieve symptoms and optimize growth, while minimizing side effects [8].
After an accidental discovery in the 1970s that exclusive enteral nutrition (EEN) could induce remission of CD, EEN has provided an innovative way to induce remission and optimize nutrition following diagnosis [9]. In 2014, the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Crohn’s and Colitis Organization (ECCO) issued revised consensus guidelines, recommending that EEN be considered as the first-line induction therapy for children with CD [10]. For induction of remission, patients were treated with EEN via the oral route or nasogastric tube feeding for approximately 6–8 weeks. Additionally, only chewing gum and water were allowed [11–13]. The dietary sources of protein included elemental, semi-elemental and polymeric diets, but various levels of proteins or lipids seem to not have much impact on efficacy [14–16].
Corticosteroids have been considered a major therapeutic option to induce remission in patients with active CD, achieving a clinical remission in 60–91% of treated patients [1]. However, side effects of corticosteroids, such as Cushing appearance, bone demineralization and severe growth retardation, can be harmful to children’s natural physical development [17]. In addition, evidence-based medicine supports that EEN therapy has fewer adverse events and lower side effect rates than corticosteroids [18]. EEN therapy was also suggested to be more effective in children than adults [19].
Two meta-analyses [18, 20] concluded that EEN, as a primary therapeutic approach for CD, showed no significant difference from corticosteroids in inducing clinical remission. In contrast, two other meta-analyses [21, 22] concluded that patients’ remission rates with corticosteroids were statistically superior to that with EEN. Although many studies have compared short-term remission rates between patients treated with corticosteroids and EEN, few studies have focused on the relapse rate in long-term follow-up, especially in children. Endoscopic sustained mucosal healing in Crohn’s disease was reported to be associated with longer remission time, less inflammatory activity and decreased hospitalization rates [23, 24]; corticosteroids were thought to be associated with poor mucosal healing [25], while EEN was effective in inducing mucosal remission [26]. Few meta-analyses have been performed comparing the differences in efficacy of these two therapies on mucosal healing, and we still do not know whether EEN or corticosteroids will prolong the time that pediatric patients with CD remain in remission.
EEN versus placebo-controlled experiments cannot be carried out in pediatric patients with CD because of difficulty in passing ethical review, and any “placebo” which was nutritionally complete to sustain nutrition during treatment would be regarded as EEN. As a result, most studies have chosen corticosteroid therapy as the control group. In this meta-analysis, we compared the remission rates between two therapy groups at 8 weeks and relapse rates at 1 year. We also included more high-quality studies and further evaluated the effects of two therapeutic strategies on mucosal healing, nutritional status and growth patterns.
Methods
Data sources and search strategy
We conducted literature retrieval on PubMed, EMBASE and MEDLINE, Web of Science from January 1990 to April 2017. The search terms “diet therapy” and “exclusive enteral nutrition” were combined using “OR”, the search terms “inflammatory bowel diseases” and “Crohn’s disease” were combined using “OR”, and the search terms “pediatric” and “child” were combined using “OR”. These three groups were then combined using “AND”. For example, searching in PubMed: ((((pediatric[MeSH]) OR child[MeSH])) AND ((exclusive enteral nutrition[MeSH]) OR diet therapy[MeSH])) AND ((Crohn disease[MeSH]) OR inflammatory bowel diseases[MeSH]). Filters: publication date from 1990/01/01 to 2017/04/01. Language restrictions were not imposed. We also searched the literature in Wanfang data, VIP and China National Knowledge Internet with Chinese words for the same keywords and the same time span.
Selection criteria
Two investigators (YY and CK) independently screened the titles, abstracts and full texts using the search strategy mentioned above. We included studies that: (1) were RCTs or observational; (2) enrolled pediatric patients (under the age of 18 years) with CD; and (3) compared systemic corticosteroid drugs (prednisone, prednisolone) with EEN (polymeric formula, semi-elemental formula or elemental formula). Patients in the EEN arm were not to receive any other medication, and patients receiving corticosteroids were to be treated only with corticosteroids, or the same treatments were to be taken in both comparator arms in similar ways. Studies were excluded in any case when articles (1) did not give a precise definition of remission, (2) provided insufficient data for the outcomes of interest or (3) did not contain at least one clearly defined corticosteroids comparator arm. If a literature result was reported several times, we included the study that corresponded to the longest duration or had the largest sample size.
Outcome measures
The primary outcome measures were induced remission rate (percentage of subjects achieving remission after 6–8 weeks of treatment) and relapse rate (percentage of subjects relapsing at 1-year end point of follow-up). The secondary outcomes were collected at baseline and after the induced treatment, e.g., inflammation index including the Pediatric Crohn’s Disease Activity Index (PCDAI) [27], C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR); growth parameters such as weight and length; mucosal healing [endoscopic lesions were assessed according to a validated score standard (Crohn’s Disease Endoscopic Index of Severity, CDEIS) [28] and histological lesions were assessed according to a scoring system previously validated [29]. The data on height for age and body mass index (BMI) were collected both at baseline and at the 1-year end point. Endoscopic mucosal healing or histological mucosal healing was separately defined as a decrease in endoscopic or histological scores by 50% or more when compared with baseline values.
Quality assessment
We abstracted the following data from each study: first author, year of publication, origin, interventions and control groups (drug and dosage), participants’ characteristics (number of each group, age) and underlying condition. We used the Newcastle–Ottawa scale to assess the quality of observational studies. This assessment had three sections (selection, comparability and exposure, respectively) and altogether eight items. Studies with a score less than 5 in the present study were excluded from the final analysis [30]. We also evaluated the bias in randomized controlled trials using the Jadad scale, which included the evaluation of randomization, blinding of outcome assessment and description of withdrawals and dropouts. Studies with a Jadad score more than 3 were regarded as high quality and would be included in the final analysis [31].
We analyzed the results of the RCTs, prospective cohort studies and retrospective cohort studies separately to determine whether the results from the non-RCTs affected our calculation of obvious heterogeneity or produced a different outcome from the more robust RCTs. Because there was a mild heterogeneous effect across strata when we compared each study type, respectively, with our main result, we concluded that it was appropriate to combine study types as hypothesis generating rather than confirmatory analysis.
Statistical analysis
All the meta-analyses were conducted using Review Manager 5.3. The odds ratio (OR) [32] with 95% confidence intervals (CI) [33] was applied to analyze dichotomous variables, and mean difference (MD) [34] with a 95% CI was used to analyze continuous ones. If continuous data were presented in mean and standard deviation (SD) [35] of base and final, we would use statistical algorithms to calculate the difference value’s mean and SD.
The Chi-square test was used to evaluate the heterogeneity with significance set at the P < 0.05 level, and the I2 statistic value was interpreted as three separate levels: 25% (low heterogeneity), 50% (moderate heterogeneity) and 75% (high heterogeneity) [36]. The random-effect model was conducted when the heterogeneity between studies was too high; otherwise, the fixed-effect model was used [37].
Results
Literature research and characteristic of studies
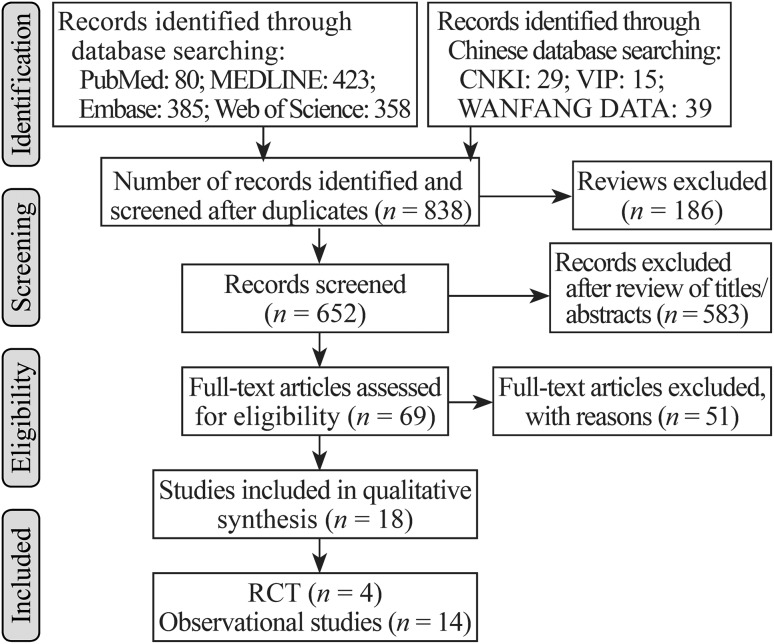
A total of 1329 publications retrieved from the database were scanned for relevance. After reviewing, we included 69 articles that potentially met inclusion criteria. After these full texts were studied, the bibliographies were checked. Overall, 4 RCTs [38–41] and 14 observational studies [42–55] that reached our inclusion criteria constituted the base of our analysis. Three of the observational studies were abstracts that provided necessary data for inclusion in the meta-analysis (Fig. 1, flow chart). The characteristics of the included studies are shown in Table 1.
Fig. 1.
Flow diagram of trials for inclusion in the systematic review. n number of records
Table 1.
Characteristics of included studies
| References | Origin | Study groups, dose, number of patients | Age at diagnosis (y) | Patients | Treatment time (wk) | Clinical remission | Quality score |
|---|---|---|---|---|---|---|---|
| Randomized controlled trail | |||||||
| Terrin et al. [38] | Italy | EEN: extensively hydrolyzed formula 50–60 kcal/kg/d (n = 10). CS: prednisolone 1.6 mg/kg/d and mesalazine (n = 10) |
12.4 (7–17) | Children with active CD | 8 | PCDAI < 10 | 5 |
| Thomas et al. [39] | England | EEN: elemental formula (n = 12) CS: prednisolone 2 mg/kg/d (maximum 60 mg/d) and sulphasalazine 25 mg/kg/d (n = 12) |
12.9 (5.7–17.2) | Children with active CD | 4 | Activity was graded According to the Lloyd–Still activity index |
3 |
| Ruuska et al. [40] | Finland | EEN: whole-protein based on weight (n = 10) CS: prednisolone 1.5 mg/kg/d up to a maximum of 60 mg (n = 9) |
8.5–18.6 | Children with new onset or relapsing CD | 8 | PCDAI < 10 | 3 |
| Borrelli et al. [41] | Italy | EEN: polymeric formula 120% to 130% of the recommended daily requirement (n = 19) CS: methylprednisolone 1.6 mg/kg/d (up to maximum 60 mg) for 4 wk, then tapered by 5–10 mg/kg, each week over 6 wk (n = 18) |
4–17 | Diagnosis of CD within 12 wk | 10 | PCDAI < 10 | 3 |
| Retrospective analysis of records | |||||||
| Canani et al. [42] | Italy | EEN: polymeric formula (n = 12); semi-elemental diet (n = 13); elemental diet (n = 12). 50–70 kcal/kg CS: methylprednisolone (1–2 mg/kg/d, maximal dose 40 mg/d) for 4 wk with subsequent gradual tapering over at least 4 wk (n = 10) |
Polymeric: 11.5 (9–17) Semi-elemental: 11.8 (8–15) Elemental: 12.1 (7–16) CS: 12.4 (8–17) |
Children with newly diagnosed CD | 8 | PCDAI < 10 | 8 |
| Papadopoulou et al. [43] | Greece | EEN: elemental diet. The daily intake of the elemental diet ranged between 60 and 135 mL/kg/d, calculated to provide 140% of the recommended daily allowance of energy (n = 30) CS: prednisolone, 2 mg/kg/d up to a maximal dose of 60 mg/d, the dose was halved every 2–4 wk until a dose of 5–10 mg was achieved (n = 28) |
EEN: 12.6 ± 3.1 CS: 12 ± 3.1 |
Children with diagnosed CD | 8 | Both the absence of clinical symptoms referable to Crohn’s disease (i.e., diarrhea, abdominal pain, fever) and achievement of a disease activity score > 80 | 8 |
| Lambert et al. [44] | Australia | EEN: polymeric formula (n = 31). After completion of EEN, normal diet was reintroduced gradually and children were encouraged to continue supplementary volumes of enteral formula (500–1000 mL daily) CS: corticosteroids (n = 26). |
EEN: 9.9 ± 4 CS: 9.97 ± 4.7 |
Children with newly diagnosed CD | 6–8 | PCDAI < 15 | 9 |
| Soo et al. [45] | Canada | EEN: polymeric formula (n = 33) or semi-elemental formula (n = 3) for 6 wk and then partially over the next 2 wk depending on patient compliance CS: prednisone, 1 mg/kg/d to a maximum dose of 50 mg/d (n = 69) |
EEN: 12.9 (7.4–16.2) CS: 11.2 (2.4–16.8) |
Children with newly diagnosed CD | 6–8 | PCDAI < 10 | 9 |
| Levine et al. [46] | Israel | EEN: polymeric formula (n = 43) CS: prednisone, 1–2 mg/kg up to 60 mg equivalent of prednisone (n = 114) |
EEN: 12.3 ± 3.9 CS: 13.3 ± 3.1 |
Children with newly diagnosed CD | 6–8 | PCDAI < 10 | 8 |
| Wang et al. [47] | China | EEN: polymer formula, according to the normal age of the required amount of 120–130% (n = 25) CS: oral methylprednisolone at an initial dose of 1.6 mg/kg/d (maximal dose ≤ 60 mg/d). After 4 wk of treatment, the dose was reduced (n = 23) |
EEN: 9.3 ± 2.6 CS: 10.2 ± 3.1 |
Children with diagnosed CD | 12 | CDAI < 150 points or lower than the baseline value of at least 100 points and CRP normal | 9 |
| Hojsak et al. [48] | Croatia | EEN: polymeric formula, taken exclusively for 6–8 wk either through nasogastric tube or orally (n = 57) CS: corticosteroids (n = 17). |
13.4 (1–17.9) | Children with newly diagnosed CD | 6–8 | PCDAI < 10 | 8 |
| Grover et al. [49] | Australia | EEN: polymeric formula, Nutrison (1 kcal/mL, Nutricia, UK, 4 g protein, 3.9 g fat/100 mL) through nasogastric tube (NGT) or resource protein (1.25 kcal/mL, Nestle, 9.4 g protein, 3.5 g fat/100 mL) orally based on children preference and dietetic consultation (n = 43) CS: corticosteroids: 10 mg/d prednisolone (n = 46) |
EEN: 13 (11.35–14) CS: 11.5 (9.5–13) |
Children with newly diagnosed CD | 6 | PCDAI < 10 | 9 |
| Luo et al. [50] | China | EEN: polymeric formula, the average caloric intake in EEN group was 117.9 ± 4.2 kcal (n = 10) CS: the average dosage for prednisone was 1.1 ± 0.4 mg/kg (n = 15). The average dosage for hydrocortisone was 8.7 ± 2.3 mg/kg (n = 3) |
EEN: 11.1 (5–15) CS: 11.6 (1–16) |
Children with active CD (PCDAI > 10) | 8 | PCDAI < 10 | 8 |
| Hradsky et al. [51] | Czech Republic | EEN: polymeric formula (n = 29) CS: prednisone 1–2 mg/kg/d (up to 40 mg/d, exceptionally 60 mg/d) approximately 2 mont (n = 36) |
EEN: 13.91 (12.3–15.02) CS: 14.85 (11.25–15.57) |
Children with newly diagnosed CD | 6–10 | NG | 7 |
| Goncalves et al. [52] (abstract) | Portuguese | EEN: polymeric formula for 8 wk (1500–2000 mL/d) (n = 11) CS: the steroid dose was 1 mg/kg/d (n = 19) |
There was no difference in age | Children with newly diagnosed CD | 8 | NG | 7 |
| Gavin et al. [53] (abstract) | UK | EEN: exclusive enteral nutrition (n = 43) CS: steroids (n = 19) |
13 (2–16) | Children with newly diagnosed CD | NG | Clinical remission was determined using a physician global assessment and blood biochemistry | 6 |
| Scarpato et al. [54] (abstract) | Italy | EEN: receiving exclusive enteral nutrition for 8 wk, followed by a gradual introduction of foods during the subsequent 4 wk (n = 33). CS: treated with oral corticosteroids with tapering off by week 11 (n = 11). |
EEN: 10.4 ± 3.5 CS: 11.7 ± 4.6 |
Children with newly diagnosed CD | 8 | NG | 7 |
| Prospective analysis of records | |||||||
| Kierkus et al. [55] | Poland | EEN: each infused 1400–2200 mL. This provided approximately 50 kcal/kg/d (n = 20). CS: corticosteroids (n = 24) |
EEN: 13.4 ± 5.18 CS: 13.8 ± 4.34 |
Children with moderate to severe CD (PCDAI > 30) | 6 | PCDAI < 10 | 9 |
EEN exclusive enteral nutrition group, CS corticosteroid group, PCDAI Pediatric Crohn’s Disease Activity Index, CD Crohn’s disease, n number, NG not given
Quality assessment
The details of quality assessment based on the NOS are shown in Supplementary Table 1. The Newcastle–Ottawa scale revealed that study qualities varied from 7 to 9. The qualities of the included studies were good and fair. The quality of the four RCTs we included showed a moderate level. All articles displayed similar baselines and they were then grouped randomly. Patients included in the RCTs had complete follow-up information. The number of withdrawals and the reasons for withdrawal were described in the articles. However, only one RCT adopted the single-blind method, with three articles for three points and one article for five points.
Effects of interventions
Induced remission rate of exclusive enteral nutrition vs. corticosteroids
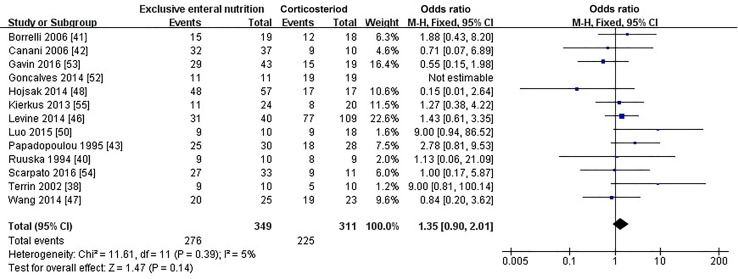
Three RCTs [38, 40, 41] and ten observational studies [42, 43, 46–48, 50, 52–55] provided data on the induced remission rate after 6–8 weeks of treatment. Soo et al. [45] calculated the remission rate after 3 months treatment, so data from their paper were excluded. Overall, we analyzed 13 papers including 349 pediatric patients treated with exclusive elemental diet and 311 pediatric patients treated with corticosteroids. The heterogeneity test showed I2 = 5%, suggesting that there was a mild heterogeneity between the studies, so we performed the fixed-effect model in our study. We pooled all the results of these trials and found no evidence for a significant difference in the percentage of children achieving remission between those treated with EEN and those with corticosteroids (OR = 1.35; 95% CI 0.90, 2.10; P = 0.14; Fig. 2). Meta-analysis of only RCTs showed that EEN was more effective than corticosteroids (OR = 2.62; 95% CI 0.86, 7.94; P = 0.09, Supplementary Table 2).
Fig. 2.
Forest plot for the comparison of induced remission rates between EEN versus corticosteroids for pediatric Crohn’s disease. No significant difference in induced remission rates was found between the EEN and corticosteroid groups (OR = 1.35; 95% CI 0.90, 2.10; P = 0.14). EEN exclusive enteral nutrition, OR odds ratio, CI confidence interval, df degree of freedom
Influence of exclusive enteral nutrition vs. corticosteroids on 1-year relapse rate
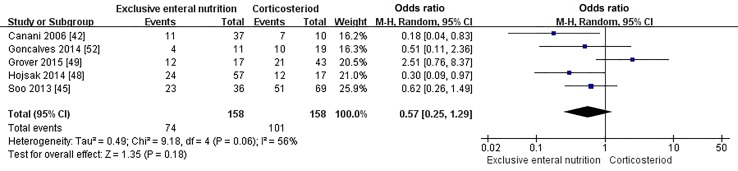
Patients in five observational studies [42, 45, 48, 49, 52] were induced into remission with EEN or corticosteroids, and all of them used thiopurine and/or mesalamine as maintenance therapy. One study [44] that used maintenance enteral nutrition as maintenance therapy was excluded from the meta-analysis. There was only one RCT article, which was excluded as well. Furthermore, we included and analyzed five retrospective cohort studies (158 cases in the EEN group, 154 cases in the corticosteroids group). The meta-analysis of the five retrospective cohort studies also showed that no significant difference existed in the proportions of 1-year relapse rates between the EEN and corticosteroids groups (OR = 0.57; 95% CI 0.25, 1.29; P = 0.18, Fig. 3).
Fig. 3.
Forest plot for the comparison of 1-year relapse rates between EEN versus corticosteroids for pediatric Crohn’s disease. No significant difference in 1-year relapse rates was found between the EEN and corticosteroids groups (OR = 0.57; 95% CI 0.25, 1.29; P = 0.18). EEN exclusive enteral nutrition, OR odds ratio, CI confidence interval, df degree of freedom
Mucosal healing
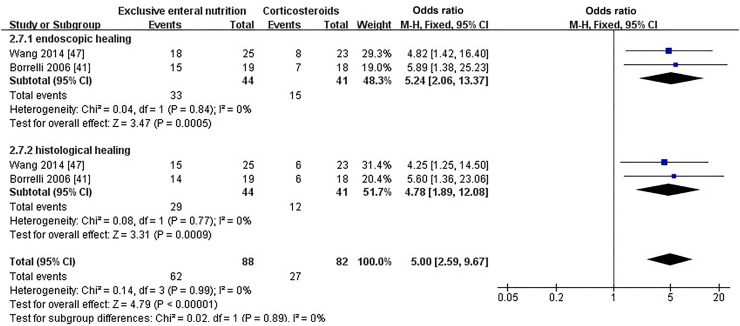
In total, 3 of 18 articles [41, 42, 47] provided information on mucosal healing of patients at the end of induction. The method of Canani et al. [42] defining mucosal healing was different from other articles, so data from the research were excluded. Overall, patients who received EEN were more likely to achieve both endoscopic mucosal healing (OR = 5.24, 95% CI 2.06, 13.37; P = 0.0005, Fig. 4) and histological mucosal healing (OR = 4.78, 95% CI 1.89, 12.08; P = 0.0009, Fig. 4) than those who received corticosteroids.
Fig. 4.
Forest plot for the comparison of mucosal healing between EEN versus corticosteroids for pediatric Crohn’s disease. A significant difference both in endoscopic and histological mucosal healing was found between the EEN and corticosteroid groups (OR = 5.24 0, 95% CI 2.06, 13.37; P = 0.0005 and OR = 4.78 0, 95% CI 1.89, 12.08; P = 0.0009, respectively). EEN exclusive enteral nutrition, OR odds ratio, CI confidence interval, df degree of freedom
Effects of exclusive enteral nutrition vs. corticosteroids on inflammation
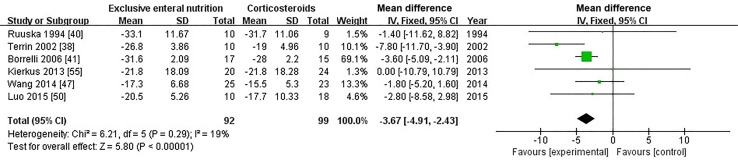
The data were abstracted from studies that measured PCDAI, CRP and ESR at baseline and at the end of induction in patients receiving EEN and corticosteroids. Then, we used statistical algorithms to calculate the difference value’s mean and SD. There was a distinct decline of PCDAI in patients who received EEN compared with those who received corticosteroids (MD = − 3.67; 95% CI − 4.91, − 2.43; P < 0.0001, Fig. 5). However, we found no significant difference in terms of CRP and ESR between patients who received corticosteroids or EEN (MD = 1.07; 95% CI 0.18, 1.96; P = 0.02, on CRP, and MD = 0.60; 95% CI − 1.82, 3.03; P = 0.63, on ESR, Supplementary Figs. 1–2).
Fig. 5.
Forest plot for the comparison of PCDAI between EEN versus corticosteroids for pediatric Crohn’s disease. A significant difference in PCDAI was found between the EEN and corticosteroid groups with a standard mean difference of − 3.67 (95% CI − 4.91, − 2.43; P < 0.00001). PCDAI Pediatric Crohn’s Disease Activity Index, EEN exclusive enteral nutrition, CI confidence interval, df degree of freedom
Effects of exclusive enteral nutrition vs. corticosteroids on growth
After induced therapy, patients who received EEN seemed to gain more weight than those who received corticosteroids (MD = 1.92; 95% CI = 0.02, 3.83; P = 0.05, Supplementary Fig. 3), but height showed no significant difference (MD = 0.24; 95% CI = − 1.98, 2.45; P = 0.83, Supplementary Fig. 4). We also found no significant difference at the 1-year end point in either height for age (MD = 0.45; 95% CI − 0.11, 1.02; P = 0.11, Supplementary Fig. 5) or BMI (MD = 0.10; 95% CI − 0.34, 0.54; P = 0.66, Supplementary Fig. 6).
Discussion
Crohn’s disease is a chronic, progressive disease characterized by repeated relapses after remissions in most cases. The chronic intestinal inflammation that occurs in CD can result in intestinal complications such as strictures, fistulas, and abscesses during the whole period [56, 57]. Medical treatment of CD includes two major parts: induction and maintenance therapy. These phases of treatment involve achieving control of inflammation in a relatively short time and then sustaining that control to prevent patients from relapse. A number of investigators provided much evidence to illustrate the mechanism behind EEN. The primary components underlying the actions of EEN were as follows: inhibiting the expression of inflammatory factors, such as tumor necrosis factor-α, interleukin (IL)-6 and IL-1β [58–60], increasing the release of vascular endothelial growth factor and transforming growth factor-β (TGF-β) to improve intestinal endometrial repairment [61, 62], providing essential amino acids which can promote intestinal mucosal barrier formation [63, 64] and activating mucosal immunity, resulting in the maintenance of intestinal homeostasis [65].
Treatments for induced remission in CD patients include corticosteroids, EEN and biologic agents. Recently, people in many countries, such as the Japanese, Europeans, British, and North Americans, have highlighted the remission induction efficacy of EEN on pediatric CD [66–69]. There were two meta-analyses [15, 16] hinting that CS was superior to EEN in achieving control of inflammation in acute CD. These two meta-analyses were published in 1995 and 1996, and the retrieval times were between the 1980s and 1990s. The studies included in those two meta-analyses usually used EEN as remission induction therapy for fewer than 4 weeks, whereas the induction time of the studies we included mostly lasted for 6–8 weeks, with only one study lasting 4 weeks. In addition, those two meta-analyses included patients of all ages, and our meta-analyses focused on pediatric trials. Two other pediatric meta-analyses [17, 18] also determined that EEN and corticosteroids were equally effective, similar to our study’s conclusions, suggesting that the benefits of EEN may differ between children and adults.
No significant difference was found in 1-year relapse rates between EEN and corticosteroids. In our subgroup meta-analysis of the three articles [15–17], choosing thiopurine as single maintenance therapy had a similar result (EEN vs. CS: OR = 0.93, 95% CI 0.36, 2.43; P = 0.13). The use of thiopurine or mesalamine will impact the results, but it is also unethical to observe relapse rates without any maintenance therapy in child patients.
Recently, many researchers have regarded mucosal healing as a promising therapeutic target, and mucosal healing may substantially modify the course of CD. Mucosal remission was reported to be possibly associated with a sustained remission rate [26, 70]. Treatment with steroids was thought to have no positive impacts on mucosal healing [71]. In contrast, some studies reported approximately 19–75% of children treated with EEN achieved mucosal healing, but these results were unreliable since the definition of mucosal healing among the different studies varied [13, 72–74]. In our meta-analyses, there were two articles that used the CDEIS index to evaluate endoscopic mucosal healing, and the EEN group showed better outcomes than the CS group. A similar outcome was found for histological mucosal healing. A prospective longitudinal cohort study revealed that only complete mucosal healing (SES-CD = 0) post-EEN induction correlated with a lower sustained remission rate, and the sustained remission rate of near-complete mucosal healing patients (SES 1–3) was similar to that of patients with more active endoscopic disease [70]. Therefore, there is an urgent need to enlarge sample sizes, achieve cooperation between multiple centers and adopt uniform criteria to prospectively validate whether EEN is more effective in mucosal healing than CS and whether mucosal healing induced by EEN is conducive to sustained remission.
We also found that, after achieving induced remission, patients who received EEN treatment seemed to gain more weight than those who received corticosteroids. This result might be associated with nutritional support from EEN. In addition, we found that inflammation markers, such as CRP and ESR, showed no significant difference. However, patients who received EEN were 3.7 times more likely to have a decline of PCDAI score than those who received corticosteroids. The PCDAI is an index of severity of pediatric CD including physical examination, growth parameters and commonly performed laboratory tests [27]. The weight gain in the EEN group may be one factor contributing to PCDAI score decline. Despite the well-described adverse effects that corticosteroids can have on development, growth, and pubertal maturation, particularly if there has been a clinical course of frequent relapses resulting in inadequate nutrition and associated with repeated courses of steroid treatment [75], using corticosteroids as inducing treatment shows similar effects with EEN on the change of height for age and BMI at 1 year. This result may suggest that applying corticosteroids as short-term induction therapy does not have such an adverse impact on long-term growth and development in pediatric patients as expected. However, other growth and development indicators, such as bone age, bone density and gonad development, need to be further evaluated. Most importantly, doctors must still be very prudent in choosing appropriate and effective inducing therapy for CD pediatric patients to reduce repeated and long-term use of corticosteroids.
Our meta-analysis has several limitations. The observational studies were not designed randomly, and the influence of subjectivity among doctors or parents on the experimental results could not be ruled out; RCT experiments with multicenter, large-scale, strict double-blind and randomly allocated trials should be conducted in the future.
In conclusion, our study suggests the induction remission rate of EEN is similar to that of corticosteroids and that EEN and corticosteroid therapy had no significantly different effects on 1-year relapse rate. However, EEN is superior to corticosteroids in terms of positive effects on short-term mucosal healing and reduction of PCDAI. For these reasons, we recommend EEN as an effective and viable first-line treatment for induction of CD remission.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
YY conceptualized and designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. KCC designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. JC conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No external funding for this manuscript.
Ethical approval
None.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
References
- 1.Rutgeerts P, Lofberg R, Malchow H, Lamers C, Olaison G, Jewell D, et al. A comparison of budesonide with prednisolone for active Crohn’s disease. N Engl J Med. 1994;331:842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. doi: 10.1053/j.gastro.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees CW, Satsangi J. Genetics of inflammatory bowel disease: implications for disease pathogenesis and natural history. Expert Rev Gastroenterol Hepatol. 2009;3:513–534. doi: 10.1586/egh.09.45. [DOI] [PubMed] [Google Scholar]
- 5.Turunen P, Ashorn M, Auvinen A, Iltanen S, Huhtala H, Kolho KL. Long-term health outcomes in pediatric inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2009;15:56–62. doi: 10.1002/ibd.20558. [DOI] [PubMed] [Google Scholar]
- 6.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 7.Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112:1120–1134. doi: 10.1038/ajg.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Kim Y, Cho JM, Oh SH, Kim KM. Therapeutic efficacy of oral enteral nutrition in pediatric Crohn’s disease: a single center non-comparative retrospective study. Yonsei Med J. 2016;57:1185–1191. doi: 10.3349/ymj.2016.57.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voitk AJ, Echave V, Feller JH, Brown RA, Gurd FN. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Arch Surg. 1973;107:329–333. doi: 10.1001/archsurg.1973.01350200189039. [DOI] [PubMed] [Google Scholar]
- 10.Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Whitten KE, Rogers P, Ooi CY, Day AS. International survey of enteral nutrition protocols used in children with Crohn’s disease. J Dig Dis. 2012;13:107–112. doi: 10.1111/j.1751-2980.2011.00558.x. [DOI] [PubMed] [Google Scholar]
- 12.Frivolt K, Schwerd T, Werkstetter KJ, Schwarzer A, Schatz SB, Bufler P, et al. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: predictors of efficacy and outcome. Aliment Pharmacol Ther. 2014;39:1398–1407. doi: 10.1111/apt.12770. [DOI] [PubMed] [Google Scholar]
- 13.Rubio A, Pigneur B, Garnier-Lengliné H, Talbotec C, Schmitz J, Canioni D, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther. 2011;33:1332–1339. doi: 10.1111/j.1365-2036.2011.04662.x. [DOI] [PubMed] [Google Scholar]
- 14.Khoshoo V, Reifen R, Neuman MG, Griffiths A, Pencharz PB. Effect of low- and high-fat, peptide-based diets on body composition and disease activity in adolescents with active Crohn’s disease. JPEN J Parenter Enteral Nutr. 1996;20:401–405. doi: 10.1177/0148607196020006401. [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Krantz M, Bodin L, Stenhammar L, Lindquist B. Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: a multicentre randomized controlled trial. Acta Paediatr. 2004;93:327–335. doi: 10.1111/j.1651-2227.2004.tb02956.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues AF, Johnson T, Davies P, Murphy MS. Does polymeric formula improve adherence to liquid diet therapy in children with active Crohn’s disease? Arch Dis Child. 2007;92:767–770. doi: 10.1136/adc.2006.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker-Smith JA. Dietary treatment of active Crohn’s disease. Dietary treatment is best for children. BMJ. 1997;314:1827–1828. doi: 10.1136/bmj.314.7097.1827a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 2000;31:8–15. doi: 10.1097/00005176-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Banares F, Cabre E, Esteve-Comas M, Gassull MA. How effective is enteral nutrition in inducing clinical remission in active Crohn’s disease? A meta-analysis of the randomized clinical trials. JPEN J Parenter Enteral Nutr. 1995;19:356–364. doi: 10.1177/0148607195019005356. [DOI] [PubMed] [Google Scholar]
- 20.Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther. 2007;26:795–806. doi: 10.1111/j.1365-2036.2007.03431.x. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths AM, Ohlsson A, Sherman PM, Sutherland LR. Meta-analysis of enteral nutrition as a primary treatment of active Crohn’s disease. Gastroenterology. 1995;108:1056–1067. doi: 10.1016/0016-5085(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 22.Messori A, Trallori G, D’Albasio G, Milla M, Vannozzi G, Pacini F. Defined-formula diets versus steroids in the treatment of active Crohn’s disease: a meta-analysis. Scand J Gastroenterol. 1996;31:267–272. doi: 10.3109/00365529609004877. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, et al. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2014;59:516–521. doi: 10.1097/MPG.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 24.Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 25.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 26.Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol. 2014;49:638–645. doi: 10.1007/s00535-013-0815-0. [DOI] [PubMed] [Google Scholar]
- 27.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. doi: 10.1097/00005176-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/S0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2003. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 5 May 2012.
- 31.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Wirtz MA. Basic statistical measures for dichotomous data formats: risk, odds, logits, relative risk. odds ratio. Die Rehabil. 2017;56:264–271. doi: 10.1055/s-0043-109930. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe R, Abramson MJ. Modern statistical methods in respiratory medicine. Respirology. 2014;19:9–13. doi: 10.1111/resp.12223. [DOI] [PubMed] [Google Scholar]
- 34.Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14:30. doi: 10.1186/1471-2288-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DK, In J, Lee S. Standard deviation and standard error of the mean. Korean J Anesthesiol. 2015;68:220–223. doi: 10.4097/kjae.2015.68.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 38.Terrin G, Canani RB, Ambrosini A, Viola F, De Mesquita MB, Di Nardo G, et al. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn’s disease. Ital J Pediatr. 2002;28:401–405. [Google Scholar]
- 39.Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn’s disease. J Pediatr Gastroenterol Nutr. 1993;17:75–81. doi: 10.1097/00005176-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Ruuska T, Savilahti E, Maki M, Ormala T, Visakorpi JK. Exclusive whole protein enteral diet versus prednisolone in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 1994;19:175–180. doi: 10.1097/00005176-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4:744–753. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. 2006;38:381–387. doi: 10.1016/j.dld.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulou A, Rawashdeh M, Brown G, McNeish A, Booth I. Remission following an elemental diet or prednisolone in Crohn’s disease. Acta Paediatr. 1995;84:79–83. doi: 10.1111/j.1651-2227.1995.tb13490.x. [DOI] [PubMed] [Google Scholar]
- 44.Lambert B, Lemberg DA, Leach ST, Day AS. Longer-term outcomes of nutritional management of Crohn’s disease in children. Dig Dis Sci. 2012;57:2171–2177. doi: 10.1007/s10620-012-2232-2. [DOI] [PubMed] [Google Scholar]
- 45.Soo J, Malik BA, Turner JM, Persad R, Wine E, Siminoski K, et al. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn’s disease. Dig Dis Sci. 2013;58:3584–3591. doi: 10.1007/s10620-013-2855-y. [DOI] [PubMed] [Google Scholar]
- 46.Levine A, Turner D, Pfeffer Gik T, Amil Dias J, Veres G, Shaoul R, et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: evaluation of the porto IBD group “growth relapse and outcomes with therapy” (GROWTH CD) study. Inflamm Bowel Dis. 2014;20:278–285. doi: 10.1097/01.MIB.0000437735.11953.68. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Jin X, Zhao H, Liu S, Gong Y, Wu J, et al. Enteral nutrition versus corticosteroids in treatment of active pediatric Crohn’s disease. Clin J Med Off. 2014;12:1227–1230. [Google Scholar]
- 48.Hojsak I, Pavic AM, Misak Z, Kolacek S. Risk factors for relapse and surgery rate in children with Crohn’s disease. Eur J Pediatr. 2014;173:617–621. doi: 10.1007/s00431-013-2230-1. [DOI] [PubMed] [Google Scholar]
- 49.Grover Z, Lewindon P. Two-year outcomes after exclusive enteral nutrition induction are superior to corticosteroids in pediatric Crohn’s disease treated early with thiopurines. Dig Dis Sci. 2015;60:3069–3074. doi: 10.1007/s10620-015-3722-9. [DOI] [PubMed] [Google Scholar]
- 50.Luo Y, Yu J, Zhao H, Lou J, Chen F, Peng K, et al. Short-term efficacy of exclusive enteral nutrition in pediatric Crohn’s disease: practice in China. Gastroenterol Res Pract. 2015;2015:428354. doi: 10.1155/2015/428354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hradsky O, Copova I, Zarubova K, Nevoral J, Bronsky J. Time to relapse in children with Crohn’s disease treated with azathioprine and nutritional therapy or corticosteroids. Dig Dis Sci. 2016;61:2041–2050. doi: 10.1007/s10620-016-4103-8. [DOI] [PubMed] [Google Scholar]
- 52.Goncalves JP, Tavares M, Quaresma L, Rego H, Ratola A, Trindade E, et al. Impaired remission in pediatric Cronh’s disease-are steroids to blame? Gastroenterology. 2014;146:S–782. doi: 10.1016/S0016-5085(14)62821-3. [DOI] [Google Scholar]
- 53.Gavin J, Ashton JJ, Heather N, Marino LV, Beattie RM. Crohns disease: initial treatment and outcomes at 12 months. Arch Dis Child. 2016;101:A24. doi: 10.1136/archdischild-2016-311535.12. [DOI] [Google Scholar]
- 54.Scarpato E, Strisciuglio C, Martinelli M, Tortora C, Cenni S, Serra MR, et al. Does exclusive enteral nutrition affect the clinical course in paediatric Crohn’s disease patients? J Pediatr Gastroenterol Nutr. 2016;62:702. [Google Scholar]
- 55.Kierkus J, Szymanska S, Szczepanski M, Wiernicka A, Szymanska E, Matuszczyk M, et al. The efficacy of total enteral nutrition in inducing remission and improving nutritional status in children with moderate to severe Crohn’s disease. J Crohns Colitis. 2013;8:57–61. [Google Scholar]
- 56.Kim SC, Ferry GD. Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology. 2004;126:1550–1560. doi: 10.1053/j.gastro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106–1113. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 58.DiFedele LM, He J, Bonkowski EL, Han X, Held MA, Bohan A, et al. Tumor necrosis factor alpha blockade restores growth hormone signaling in murine colitis. Gastroenterology. 2005;128:1278–1291. doi: 10.1053/j.gastro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Beattie RM, Camacho-Hubner C, Wacharasindhu S, Cotterill AM, Walker-Smith JA, Savage MO. Responsiveness of IGF-I and IGFBP-3 to therapeutic intervention in children and adolescents with Crohn’s disease. Clin Endocrinol (Oxf) 1998;49:483–489. doi: 10.1046/j.1365-2265.1998.00562.x. [DOI] [PubMed] [Google Scholar]
- 60.Bannerjee K, Camacho-Hubner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, et al. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38:270–275. doi: 10.1097/00005176-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Wedrychowicz A, Kowalska-Duplaga K, Jedynak-Wasowicz U, Pieczarkowski S, Sladek M, Tomasik P, et al. Serum concentrations of VEGF and TGF-β1 during exclusive enteral nutrition in IBD. J Pediatr Gastroenterol Nutr. 2011;53:150–155. doi: 10.1097/MPG.0b013e3182144c74. [DOI] [PubMed] [Google Scholar]
- 62.Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- 63.Blum S, Brito F. Current state of the art of medical foods. Nestle Nutr Inst Workshop Ser. 2014;79:141–151. doi: 10.1159/000362800. [DOI] [PubMed] [Google Scholar]
- 64.Nichols NL, Bertolo RF. Luminal threonine concentration acutely affects intestinal mucosal protein and mucin synthesis in piglets. J Nutr. 2008;138:1298–1303. doi: 10.1093/jn/138.7.1298. [DOI] [PubMed] [Google Scholar]
- 65.Keenan JI, Hooper EM, Tyrer PC, Day AS. Influences of enteral nutrition upon CEACAM6 expression by intestinal epithelial cells. Innate immun. 2014;20:848–856. doi: 10.1177/1753425913513309. [DOI] [PubMed] [Google Scholar]
- 66.Konno M, Kobayashi A, Tomomasa T, Kaneko H, Toyoda S, Nakazato Y, et al. Guidelines for the treatment of Crohn’s disease in children. Pediatr Int. 2006;48:349–352. doi: 10.1111/j.1442-200X.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 67.Sandhu BK, Fell JM, Beattie RM, Mitton SG, Wilson DC, Jenkins H. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010;50(Suppl 1):S1–S13. doi: 10.1097/MPG.0b013e3181c92c53. [DOI] [PubMed] [Google Scholar]
- 68.Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schutz T, et al. ESPEN guidelines on enteral nutrition: gastroenterology. Clin Nutr. 2006;25:260–274. doi: 10.1016/j.clnu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Critch J, Day AS, Otley A, King-Moore C, Teitelbaum JE, Shashidhar H. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;54:298–305. doi: 10.1097/MPG.0b013e318235b397. [DOI] [PubMed] [Google Scholar]
- 70.Grover Z, Burgess C, Muir R, Reilly C, Lewindon PJ. Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn’s disease. J Crohns Colitis. 2016;10:1159–1164. doi: 10.1093/ecco-jcc/jjw075. [DOI] [PubMed] [Google Scholar]
- 71.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 72.Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier F, Davies S, Murch S, Derkx B, et al. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther. 2004;20:167–172. doi: 10.1111/j.1365-2036.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 73.Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281–289. doi: 10.1046/j.1365-2036.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- 74.Beattie RM, Schiffrin EJ, Donnet-Hughes A, Huggett AC, Domizio P, MacDonald TT, et al. Polymeric nutrition as the primary therapy in children with small bowel Crohn’s disease. Aliment Pharmacol Ther. 1994;8:609–615. doi: 10.1111/j.1365-2036.1994.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 75.Brain CE, Savage MO. Growth and puberty in chronic inflammatory bowel disease. Bailliere’s Clin Gastroenterol. 1994;8:83–100. doi: 10.1016/S0950-3528(06)80020-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.