Pediatric Nephrology
10.1007/s00467-018-4091-3
The original version of this article unfortunately contained two mistakes. The presentation of Table 1 and Fig. 1 was incorrect. The corrected versions are given below.
Table 1.
Eculizumab dosage regimen, standard therapy according to EMA/FDA
| Weight category | Induction phase | Maintenance phase |
|---|---|---|
| Above 40 kg | 900 mg, every week, for 4 weeks | 1200 mg, in fifth week, every 14 days thereafter |
| 30 to < 40 kg | 600 mg, every week, for 2 weeks | 900 mg, in third week, every 14 days thereafter |
| 20 to < 30 kg | 600 mg every week, for 2 weeks | 600 mg, in third week, every 14 days thereafter |
| 10 to < 20 kg | 300 mg once | 300 mg, in second week, every 14 days thereafter |
| 5 to < 10 kg | 300 mg once | 300 mg, in second week, every 21 days thereafter |
Eculizumab has to be administrated intravenously
EMA European Medicines Agency, FDA Food and Drug Administration
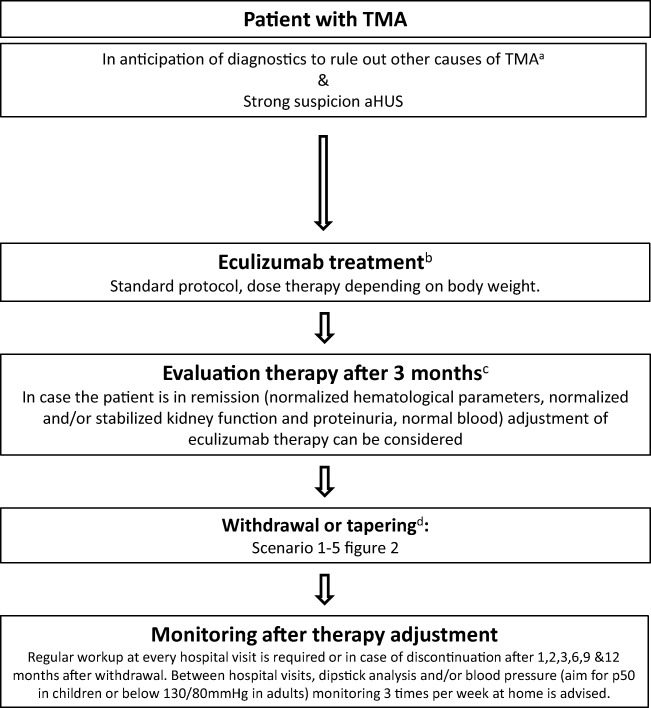
Fig. 1.
Treatment algorithm. After adequate exclusion of other causes of thrombotic microangiopathy (TMA) such as thrombocytopenic purpura (TTP), Shiga toxin-producing Escherichia coli-hemolytic uremic syndrome (STEC-HUS), or secondary TMA and in patients with strong suspicion of atypical hemolytic uremic syndrome (aHUS), eculizumab treatment should be started within 24 h after presentation. When the patient is stable and in remission, withdrawal or tapering can be considered, depending on patient characteristics (see Fig. 2). After therapy adjustment, strict monitoring is essential. NB in case of antibodies against complement factor H, a different treatment protocol has to be initiated as described by Loirat et al. [1]. a, For extensive overview of practical diagnostics approach for TMA, see Fakhouri et al. [3]. b, Treatment should preferably be started within 24 h after presentation. In adults with first episode of aHUS in native kidney, treatment with plasma exchange (PE) for 4 days (high volume PE with 1.5 plasma volume) is advised to allow diagnosis of secondary causes of aHUS. Adolescents may be considered adults [33]. After exclusion of secondary causes of aHUS and if the patient does not show a favorable response after 4 days of PE, treatment should be switched to eculizumab. Starting treatment with eculizumab within 7 days after presentation in PE-resistant patients was effective in the clinical trials [32]. In case the patient is PE sensitive, PE should be tapered and discontinued in the course of 1 month [9, 10]. c, Improvement of platelets and lactate dehydrogenase (LDH) is expected within 2–4 weeks. If no response, consider alternative diagnosis or inefficacy of eculizumab (C5 polymorphism p.Arg885His) [102]. d, See Fig. 2 for the different scenarios to withdraw or taper eculizumab, depending on patient characteristics
Footnotes
The online version of the original article can be found at 10.1007/s00467-018-4091-3
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.