Abstract
Aims
To investigate cardiovascular disease and mortality trends in control arm participants of diabetes cardiovascular outcome trials (CVOTs).
Methods
We electronically searched CVOTs published before October 2017. Data on all-cause mortality, cardiovascular mortality and events, and baseline characteristics were collected, along with study calendar years. Trends were estimated using negative binomial regressions and reported as rate ratio (RR) per 5-year intervals.
Results
26 CVOTs, conducted from 1961 to 2015, included 86788 participants with 6543 all-cause deaths, 3265 cardiovascular deaths, and 7657 3-point major adverse cardiovascular events (3-P MACE; combined endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke). In unadjusted analysis, there was an increasing trend for 3-P MACE rates over time (5-year RR 1.57; 95% CI 1.34, 1.84); a small increasing trend for cardiovascular disease mortality rates (1.13; 1.01, 1.26); and stable rates for all-cause death. Adjusting for age, sex, previous myocardial infarction, and diabetes duration, there was no evidence of trends for 3-P MACE or cardiovascular disease mortality rates, while reducing rates were observed for nonfatal myocardial infarction (5-year RR: 0.72; 0.54, 0.96), total stroke (0.76; 0.66, 0.88), and nonfatal stroke (0.60; 0.43, 0.82).
Conclusions
In contrast to real-world data, there was no evidence of an improvement in all-cause and cardiovascular mortality in type 2 diabetes participants included in control arms of randomised clinical trials across 5 decades. Further studies should investigate whether and how dissimilarities in populations, procedures, and assessments of exposures and outcomes explain the differences between real-world setting and clinical trials.
Electronic supplementary material
The online version of this article (10.1007/s00592-018-1253-5) contains supplementary material, which is available to authorized users.
Keywords: Cardiovascular, Type 2 diabetes, Randomised trials, Mortality, Trend, Systematic review
Introduction
Type 2 diabetes mellitus (T2DM) is a complex cardiometabolic disorder affecting approximately 8.5% of the global population [1]. Subjects with T2DM have 2–3-times higher risk of cardiovascular disease and death and [2–4], at 40 years of age, have an estimated 8 years shorter life expectancy than subjects without diabetes [3].
Elevated plasma glucose concentration is consistently and directly associated with cardiovascular complications and mortality in multiple, large epidemiological studies in people with T2DM; yet, whether treatment of hyperglycaemia and in particular intensive glucose control translates into a lower risk of cardiovascular disease and mortality remains uncertain. Along with the possible benefit of glucose reduction, a better “control” of other cardiovascular risk factors such as dyslipidaemia and hypertension and an earlier identification of diabetes through screening have likely contributed to the declining rates of diabetes-related complications and mortality in the last two decades [5], as shown in large observational studies from Sweden [6], US [7], and Australia [8].
In contrast with real-life settings, most participants of RCTs have a single or few medical conditions, are younger, and are possibly more adherent to medications (Hawthorne effect) [9]. To assure high internal validity and reduce the variation in baseline risk factors, RCTs use strict inclusion criteria and commonly exclude very ill patients. These factors may potentially contribute to differences between real-world and RCTs in terms of both treatment effects and absolute risk of disease-related outcomes. However, evidence from RCTs is likewise relevant as it complements observations from other sources and it is considerably less prone to bias arising from outcomes definition and assessment, incomplete data collection, and observational confounding [10]. In contrast to “real-world” evidence, a systematic assessment of trends of diabetes-related outcomes from randomised controlled trials (RCTs) is lacking [11–13]. Indeed, recent systematic evaluations included only RCTs published up to March 2011 [13], while the number of available RCTs reporting cardiovascular outcomes has increased significantly since 2008, when the US Food and Drug Administration (FDA) mandated inclusion of cardiovascular outcomes trials (CVOTs) in safety assessments of newer glucose-lowering drugs [14].
In this view, we aimed to systematically investigate trends over the last five decades in cardiovascular events and mortality rates in T2DM patients enrolled in the control arm of RCTs assessing effectiveness of various interventions, including glucose-lowering therapies. We estimated trends in the control arm as the management of glucose and other risk factors in these participants had to follow the best-standard-of-care at the time when RCTs were conducted; therefore, the risk of cardiovascular and all-cause death in control arm participants is closer to the “real-world” risk, particularly if the treatment is associated with important absolute effects.
Materials and methods
Data sources and searches
This study was conducted according to a pre-specified protocol and followed standard guidelines for conducting and reporting systematic reviews (PRISMA checklist reported in the Supplemental Material) [15]. We searched PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) for RCTs published in English from inception until 21 October 2017.
Study selection
Following the PICOS (population, intervention, comparator, outcome, study design) framework, we included RCTs (study design) of any duration in adult patients with T2DM (population) randomised to a specific treatment or strategy (intervention and comparators) and reporting cardiovascular outcomes or mortality (outcome); details on the search strategy are reported in the Supplemental Material. Reference lists of retrieved articles were also manually scanned for all relevant additional studies and reviews. Studies were included if: (1) outcome-specific or mortality number of events and person-years were reported; (2) it was possible to calculate them from the mean/median follow-up, rates, or the total number of participants. When multiple observational follow-ups of the same RCTs were available, we included only the main study (shorter duration) to have a more precise estimate of the rates related to the specific calendar year (Supplemental Table S1).
Data extraction and quality assessment
We used standardised, pre-defined forms for data extraction and quality assessment. Three authors extracted the data independently on: first author name; RCT acronym; year of journal publication; ClinicalTrials.gov (NCT) and PubMed ID (PMID) identifier number; follow-up duration; RCT calendar years (start and end); randomisation treatments; population type and baseline characteristics; mortality and cardiovascular outcomes data. Study quality was assessed using the Cochrane risk of bias tool and disagreement at any stage was solved by consensus or arbitration [16].
Data synthesis and analysis
For each study and outcome, we extracted the number of events (Ev) and exposure time (person-years, PY) in the control (placebo) arm. If Ev or PY were not reported, they were estimated using the following formulae: Ev = rate × PY and PY = mean (or median) follow-up × number of participants. We estimated trends using negative binomial regression with as numerator, as denominator, and calendar time (defined as the mid-point between start and end of the RCT) as continuous variable; trends of incident rates [with 95% confidence intervals (CI)] were displayed in forest plots as rate ratio per 5-year intervals (i.e., ratio comparing 5-year increments of calendar time). To account for possible study-level differences, outcome-specific regressions were progressively adjusted for baseline age, sex, prevalence of myocardial infarction, and diabetes duration.
We performed three sensitivity analyses. First, we estimated adjusted trends excluding studies with patients recruited before year 2000 (mid-point); this post-hoc analysis was decided at the writing stage of the discussion paragraph of the manuscript, to facilitate a temporal comparison of our findings with trends reported in observational studies. Second, at revision stage, we estimated adjusted rate ratios including baseline HbA1c. Lastly, three RCTs (HEART 2D, TOSCA and DEVOTE; references of studies are reported in the Supplemental Material) were included in the main analysis although there were no control arms: for these studies, we pre-planned to use Ev and PY of all participants and to assess the consistency of these results with those obtained after their exclusion.
We used STATA v. 15.0 (Stata Corp, College Station, TX, USA) for data manipulation, analyses, and graphs; p value < 0.05 was considered statistically significant.
Results
Study characteristics
After duplicates exclusion and selection of articles by title and abstract, 46 reports underwent full-text assessment and 26 were included in the quantitative analysis (Supplemental Figure S1); reasons for exclusion of the remaining 20 studies are reported in Table S1: for some studies, it was not possible to estimate person-years of follow-up while others did not include T2DM patients; five studies reported longer observational follow-up after the main RCT.
The characteristics of the included RCTs are shown in Table 1: they span 5 decades, from 1966 to 2015, and enrolled a total of 86,788 (median 2656; range 80–8212) participants with T2DM; most studies (21/26, 80.7%) were conducted after year 2000. Baseline age, HbA1c, and disease duration weighted means were 61.7 years, 7.8% (62.3 mmol/mol), and 9.9 years, respectively, and 63.7% were males. Four RCTs included only subjects with prevalent myocardial infarction while a single RCT only patients without; in the remaining RCTs, the prevalence of myocardial infarction ranged from 2 to 52.7% (with higher prevalence in more recent RCTs); follow-up ranged from 1.5 to 10 years.
Table 1.
Characteristics of included randomised controlled trials
| Randomised Controlled Trial (RCT) | PubMed ID | No. of participants | Male (%) | Follow-up (years)a | Age (years)a | HbA1c (%)a | HbA1c (mmol/mol)a | Diabetes duration (years)a | Prevalent myocardial infarction (%) | RCT calendar year (start–end) | RCT typeb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UGDP | 4926376 | 205 | 30.7 | 7.0 | 55.1 | – | – | 0 | 3.0 | 1961–1966 | SD |
| UKPDS 33 | 9742976 | 1138 | 62.0 | 10.0 | 53.4 | 7.1 | 54.1 | 0 | 2.0 | 1977–1991 | SD |
| DCGPc | 23549519 | 620 | 53.1 | 5.8 | 65.4 | 10.2 | 88.0 | 0 | 7.7 | 1989–1991 | SD |
| STENO-2 | 12556541 | 80 | 70.0 | 7.8 | 55.2 | 8.8 | 72.7 | 6.0 | 2.5 | 1993–1993 | SD |
| JDCS | 20054522 | 1016 | 53.0 | 7.8 | 58.6 | 7.9 | 62.8 | 10.9 | 0 | 1995–1996 | SD |
| VADT | 19092145 | 899 | 97.1 | 5.6 | 60.3 | 9.4 | 79.2 | 11.5 | 19.0 | 2000–2003 | SD |
| PROactive | 16214598 | 2633 | 66.0 | 2.9 | 61.6 | 7.9 | 62.8 | 8.0 | 46.1 | 2001–2002 | PS |
| LOOK AHEAD | 23796131 | 2575 | 40.3 | 9.6 | 58.9 | 7.3 | 56.3 | 5.0 | 6.1 | 2001–2003 | SD |
| RECORD | 19501900 | 2227 | 51.7 | 5.5 | 58.5 | 7.9 | 62.8 | 7.1 | 5.1 | 2001–2003 | PS |
| ADVANCE | 18539916 | 5569 | 57.7 | 5.0 | 66.0 | 7.5 | 58.5 | 8.0 | 12.0 | 2001–2003 | SD |
| ACCORD | 18539917 | 5123 | 61.6 | 3.5 | 62.2 | 8.3 | 67.2 | 10.0 | 18.1 | 2001–2005 | SD |
| ADDITION | 21705063 | 1379 | 57.3 | 5.3 | 60.2 | 7.0 | 53.0 | 0 | 6.1 | 2001–2006 | SD |
| HEART2D | 19246588 | 1115 | 63.3 | 2.7 | 61.0 | 8.3 | 67.2 | 9.1 | 100 | 2002–2005 | SD |
| ORIGINd | 22686416 | 6273 | 66.8 | 6.2 | 63.5 | 6.4 | 46.4 | 5.3 | 35.2 | 2003–2005 | PS |
| TECOS | 26052984 | 7339 | 70.5 | 3.0 | 65.5 | 7.2 | 55.2 | 11.6 | 42.5 | 2008–2012 | PS |
| TOSCA | 28917544 | 3028 | 58.5 | 4.8 | 62.3 | 7.7 | 60.6 | 8.4 | 6.4 | 2008–2014 | PS |
| EXAMINE | 23992602 | 2679 | 68.0 | 1.5 | 61.0 | 8.0 | 63.9 | 7.3 | 100 | 2009–2013 | PS |
| CANVAS | 28605608 | 4347 | 63.3 | 3.6 | 63.4 | 8.2 | 66.1 | 13.7 | 50.8 | 2009–2015 | PS |
| SAVOR-TIMI | 23992601 | 8212 | 66.6 | 2.0 | 65.0 | 8.0 | 63.9 | 10.3 | 37.6 | 2010–2011 | PS |
| LEADER | 27295427 | 4672 | 64.0 | 3.8 | 64.4 | 8.7 | 71.6 | 12.9 | 30.0 | 2010–2012 | PS |
| ALECARDIO | 24682069 | 3610 | 72.5 | 2.0 | 61.0 | 7.8 | 61.7 | 8.6 | 100 | 2010–2012 | PS |
| ELIXA | 26630143 | 3034 | 69.1 | 2.1 | 60.6 | 7.6 | 59.6 | 9.4 | 100 | 2010–2013 | PS |
| EMPAREG | 26378978 | 2333 | 72.0 | 2.9 | 63.2 | 8.1 | 65.0 | 9.5 | 46.4 | 2010–2013 | PS |
| EXSCEL | 28910237 | 7396 | 62.0 | 3.2 | 62.0 | 8.0 | 63.9 | 12.0 | 52.7 | 2010–2015 | PS |
| SUSTAIN-6 | 27633186 | 1649 | 60.0 | 2.1 | 64.6 | 8.7 | 71.6 | 13.6 | 32.9 | 2013–2013 | PS |
| DEVOTE | 28605603 | 7637 | 62.6 | 2.0 | 50.0 | 8.4 | 68.3 | 16.4 | 34.1 | 2013–2014 | PS |
Trials are listed by starting calendar year (older to newer) and number of participants (largest to smallest); their references are reported in the supplementary material. Data shown for control arm (except DEVOTE, TOSCA, HEART2D where arms were combined)
aMean/median; bSD strategy-driven study, PS product-specific study; c563 total participants for total myocardial infarction outcome and 591 for total stroke; din ORIGIN, 11.15% of the population had impaired glucose tolerance or impaired fasting glucose at baseline
– Not available
RCTs reported several outcomes (Table 2 and Table S2): data were complete for all-cause mortality (26 RCTs, 86,788 participants, 6543 events), followed by cardiovascular disease mortality (19 RCTs, 71,405 participants, 3265 events), total stroke (i.e., any type; 16 RCTs, 53,157 participants, 1948 events), and 3-point major adverse cardiovascular events (3-P MACE definitions are reported in Table S3; 15 RCTs, 71,641 participants, 7657 events).
Table 2.
Number of events and rates of included randomised controlled trials
| Randomised controlled trial (RCT) | All-cause mortality | Cardiovascular disease mortality | Major adverse cardiovascular eventsa | Total myocardial infarction | Nonfatal myocardial infarction | Total stroke | Nonfatal stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | |
| UGDP | 21 | 14.6 | 10 | 7.0 | – | – | – | – | – | – | – | – | – | – |
| UKPDS 33 | 213 | 18.9 | – | – | – | – | 186 | 17.4 | 101 | 9.5 | 55 | 5.0 | 44 | 4.0 |
| DCGP | 147 | 48.0 | – | – | – | – | 75 | 27.3 | – | – | 50 | 17.4 | – | – |
| STENO-2 | 15 | 24.0 | 7 | 11.2 | – | – | – | – | 17 | 27.2 | – | – | 20 | 32.1 |
| JDCS | 43 | 5.4 | – | – | – | – | – | – | – | – | 75 | 9.5 | – | – |
| VADT | 95 | 18.9 | 29 | 5.8 | – | – | 78 | 15.5 | – | – | 36 | 7.2 | – | – |
| PROactive | 186 | 24.5 | – | – | – | – | – | – | 144 | 19.0 | 107 | 14.1 | – | – |
| LOOK AHEAD | 202 | 8.6 | 57 | 2.4 | 283 | 12.5 | 191 | 8.4 | 183 | 8.0 | 80 | 3.4 | – | – |
| RECORD | 157 | 12.8 | 71 | 5.8 | 165 | 13.5 | 56 | 4.6 | – | – | 63 | 5.1 | – | – |
| ADVANCE | 533 | 19.1 | – | – | 590 | 21.2 | 337 | 12.1 | – | – | 246 | 8.8 | – | – |
| ACCORD | 203 | 11.4 | 94 | 5.6 | 371 | 22.9 | – | – | 235 | 14.5 | – | – | 61 | 3.7 |
| ADDITION | 92 | 12.5 | – | – | – | – | – | – | – | – | – | – | – | – |
| HEART2D | 102 | 33.9 | 86 | 28.6 | – | – | 126 | 41.9 | 103 | 34.2 | 37 | 12.3 | 35 | 11.6 |
| ORIGIN | 965 | 26.0 | 576 | 15.5 | 1013 | 28.5 | 326 | 9.0 | – | – | 319 | 8.8 | – | – |
| TECOS | 537 | 24.5 | 366 | 16.7 | 746 | 36.2 | 316 | 15.1 | – | – | 183 | 8.7 | – | – |
| TOSCA | 105 | 7.5 | – | – | – | – | – | – | 45 | 3.0 | – | – | 40 | 2.5 |
| EXAMINE | 173 | 43.1 | 130 | 32.4 | 316 | 78.6 | – | – | – | – | – | – | – | – |
| CANVAS | 307 | 19.5 | 201 | 12.8 | 496 | 31.5 | 198 | 12.6 | 183 | 11.6 | 151 | 9.6 | 132 | 8.4 |
| SAVOR-TIMI | 378 | 21.0 | 260 | 14.5 | 609 | 36.0 | 278 | 17.0 | – | – | – | – | – | – |
| LEADER | 447 | 25.0 | 278 | 16.0 | 694 | 39.0 | 339 | 19.0 | 317 | 18.0 | 199 | 11.0 | 177 | 10.0 |
| ALECARDIO | 138 | 19.1 | 98 | 13.6 | 360 | 49.9 | – | – | 239 | 33.1 | – | – | 50 | 6.9 |
| ELIXA | 223 | 33.0 | 158 | 24.0 | – | – | 261 | 41.0 | – | – | 60 | 9.0 | – | – |
| EMPAREG | 194 | 28.6 | 137 | 20.2 | 282 | 43.9 | – | – | – | – | 69 | 10.5 | 60 | 9.1 |
| EXSCEL | 584 | 23.0 | 383 | 15.0 | 905 | 40.0 | 493 | 21.0 | – | – | 218 | 9.0 | – | – |
| SUSTAIN-6 | 60 | 17.6 | 46 | 13.5 | 146 | 44.0 | – | – | 64 | 19.2 | – | – | 44 | 13.1 |
| DEVOTE | 423 | 28.0 | 278 | 18.4 | 681 | 45.0 | – | – | 313 | 23.7 | – | – | 150 | 10.7 |
Trials are listed by starting calendar year (older to newer) and number of participants (largest to smallest); their references are reported in the supplementary material. Data shown for control arm (except DEVOTE, TOSCA, HEART2D where arms were combined)
n number of events; rate are per 1000 person-years
a3-Point major adverse cardiovascular events: cardiovascular death, nonfatal myocardial infarction, nonfatal stroke (details are reported in Supplementary Table S3)
The overall risk of bias was considered low. For all items and RCTs, it was low, high, and unclear in 86.3%, 7.7%, and 6.0% of the cases, respectively (Table S4). The highest domain-specific bias was observed for “blinding of participants and researchers” (nine RCTs, 34.6%), followed by “binding of outcome assessment” (four RCTs, 15.4%) and “incomplete outcome data” (one RCT, 3.8%).
Trends
In view of the number of events, participants, and outcomes reported (Table S2), we estimated formal trends for all-cause and cardiovascular disease mortality, total and nonfatal stroke, total and nonfatal myocardial infarction, and 3-P MACE. Unadjusted temporal trends are depicted in Fig. 1 and 5-year rate ratios are shown in Fig. 2. With the exception of increasing trend in 3-P MACE (unadjusted 5-year rate ratio 1.57; 95% CI 1.34, 1.84), there was no clear trend for other outcomes while a small increase for cardiovascular disease mortality (unadjusted 5-year rate ratio 1.13; 1.01, 1.26) was observed (Fig. 2). These estimates translated into around 6 and 45 more unadjusted cardiovascular disease deaths and 3-P MACE, respectively, per 1000 person-years comparing 2015 to 2000 (Table S5). All-cause mortality, cardiovascular disease mortality, and 3-P MACE rates were positively related to the baseline prevalence of myocardial infarction and males in included participants, while the relationship with HbA1c (Figure S2) was less clear. Accounting for baseline participants’ characteristics, in the fully adjusted models there was no evidence of trends for all-cause mortality (adjusted 5-year rate ratio 0.96; 95% CI 0.84, 1.09), cardiovascular death (0.98; 0.82, 1.17), or 3-P MACE (1.27; 1.00, 1.61) while reducing rates were observed for nonfatal myocardial infarction (0.72; 95% CI 0.54, 0.96), total stroke (0.76; 0.66, 0.88), and nonfatal stroke (0.60; 0.43, 0.82) (Fig. 2). Rates were conversely rising for cardiovascular disease mortality when the analysis was limited to studies with consistent 3-P MACE definitions (adjusted 5-year rate ratio 1.76; 95% CI 1.13, 2.73; Table S6).
Fig. 1.
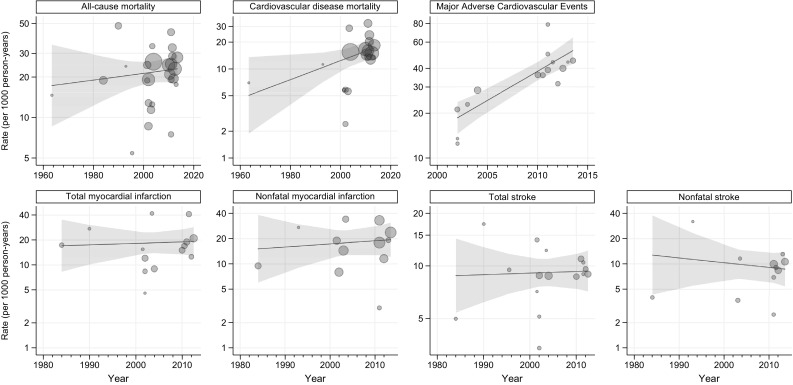
Unadjusted outcome rates by calendar year. Each circle indicates a randomised controlled trial and its size is proportional to the inverse of rate variance. Shadow areas indicate 95% confidence interval
Fig. 2.
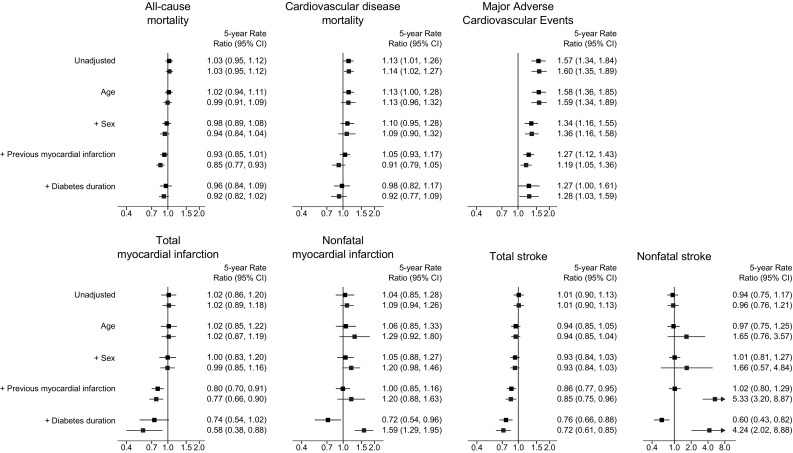
Outcome-specific rate ratios. Black and blue estimates indicate 5-year rate ratios for main and sensitivity analysis (excluding DEVOTE, TOSCA, HEART 2D), respectively. Major adverse cardiovascular events definitions are reported in Supplemental Table S3
Sensitivity analyses
Adjusted rate ratios for trends in various outcomes did not materially change when the analysis: was restricted to studies conducted after year 2000, with the exception of nonfatal stroke for which the rate reduction was not significant (adjusted 5-year rate ratio 0.97; 95% CI 0.71, 1.31) (Table S7); or accounted for baseline HbA1c (Table S8). For most outcomes, the results were also consistent excluding HEART 2D, TOSCA and DEVOTE; conversely, there were increasing trends for nonfatal myocardial infarction (adjusted 5-year rate ratio 1.59; 95% CI 1.29, 1.95) and nonfatal stroke (4.24; 2.02, 8.88) (Fig. 2).
Discussion
In this study, we systematically searched RCTs reporting mortality and cardiovascular events in patients with T2DM randomised to a specific glucose-lowering strategy or to a specific drug, to quantify the rates of these outcomes in control arms and describe their trends. As RCTs included participants from mid-1960 to 2015, it was possible to quantify outcome trends across 5 decades. We found no important changes over the observed years in most of the relevant diabetes-related outcomes, including death from cardiovascular causes, myocardial infarction, or death from any cause; 3-P MACE, a combined cardiovascular endpoint commonly used in RCTs (particularly recent ones), showed an increasing trend which, however, was less evident when accounting for participants’ baseline characteristics across RCTs. Notable exceptions were the declining trends for the individual outcome total stroke, nonfatal stroke, and nonfatal myocardial infarction, and a possible reduction for total myocardial infarction.
Recent decades have been characterised by significant improvements in the diagnosis and treatment of cardiovascular disease risk factors. As a result, declining trends of major cardiovascular disease have been repeatedly reported in observational studies from several countries, both in the general population and in people with T2DM [7] (Table S9). As cardiovascular diseases represent the main cause of death in patients with T2DM, such reduction also translates in a lower mortality risk [2], albeit with wide variation in absolute mortality rates across different countries. The reasons behind such heterogeneity in mortality rates are likely related to clinical (including access to healthcare; screening, early detection and management of T2DM and its complications; proactive ongoing management of hyperglycaemia and other risk factors; patient education and self-management; and prevalent comorbidities), biological/genetic, and socioeconomic differences. Along with the multifaceted syndemic interplay between these elements [17], differences in the processes of measuring (data quality, exposure definitions and assessment, outcome ascertainment) and synthesising (study design and analysis) information could also have contributed. Such heterogeneity in mortality rates was also observed in RCTs included in our analysis; however, in this situation it is more likely attributable to clinical differences rather than study design and analysis.
Variations in rates of single and combined cardiovascular outcomes comparing observational studies and RCTs are more difficult to interpret than mortality. Differences in the definitions and ascertainment of outcomes are well recognised in observational studies (i.e., physician vs self-reported T2DM or cardiovascular outcome), particularly for fatal events, where there are spatiotemporal differences in the definition and reporting of the underlying cause of death [18, 19]. In an attempt to limit heterogeneous comparisons, efforts have been made to standardise definitions of cardiovascular outcomes and their composites in RCTs, thus making geographical and temporal comparisons more reliable. With this in mind, our results indicate a nonsignificant 30% increased risk of major adverse cardiovascular events every 5 years, accounting for differences in demographic and clinical characteristics of RCTs’ participants. These results are possibly linked to rising trends of cardiovascular mortality seen in the analysis of RCTs reporting 3-P MACE, while the contribution of nonfatal myocardial infarction and nonfatal stroke to this trend is uncertain. In fact, there are only seven studies with stratified data for these two outcomes among the RCTs reporting 3-P MACE.
When including all available RCTs, however, we found declining trends for both nonfatal myocardial infarction and stroke. The divergent trends between cardiovascular death and nonfatal cardiovascular events have several possible explanations. More intensive glucose control in recent years (change in glycemic targets), coupled with an increased prevalence of diabetes in multimorbid elderly patients, may have resulted in increasing rates of hypoglycaemia which has been associated with a higher risk of cardiovascular death in post-hoc analysis of RCTs, observational, and experimental studies [20–23]. There is also a possibility that other cardiovascular phenotypes are increasingly contributing to the risk of cardiovascular death. The reduction of cardiovascular death attributable to fatal atherothrombosis due to a widespread use of statin and aspirin, along with the increased risk of heart failure associated with aging [24], could have changed the phenotype “responsible” for the majority of cardiovascular complications and cardiovascular deaths in patients with T2DM, with a shift from myocardial infarction and stroke to chronic heart failure [25–27]. The recent suggestion to include heart failure in CVOTs as a pre-specified component of MACE would help in reducing the misclassification of outcomes and clarify whether and how changes in the cardiovascular death phenotype explain the contrasting trends between fatal and nonfatal events observed in this analysis [24]. Further insights will also be provided by several ongoing CVOTs which included only T2DM with heart failure or were specifically designed to assess the risk of heart failure [14, 24].
Notably, in the sensitivity analysis excluding HEART 2D, TOSCA and DEVOTE, there was an inversion of trends with rising rates for nonfatal myocardial infarction and nonfatal stroke. These findings are likely related to the very low rates for both outcomes reported in TOSCA (3 per 1000 person-years for nonfatal myocardial infarction and 2.5 for nonfatal stroke) when compared to those observed in other RCTs. The reasons for such a striking difference are unknown although, as pointed out by the investigators of this study, they could be attributable to the ubiquitous use of statins, anti-hypertensive and antiplatelet agents [28].
To our knowledge, this is the first study to assess trends of key diabetes-related outcomes including all CVOTs studies conducted after the 2008 FDA guidance on CVOTs. This resulted in a much larger number of studies compared to previous systematic investigations and therefore in a substantial increase in the statistical reliability of the findings [11–13]. We also extracted data simultaneously on several outcomes and baseline characteristics of included participants, to give as clear a picture as possible of cardiovascular complications adjusted for potential confounders associated with outcomes’ rates. The study has also some limitations. We had no access to patient-level data which would have allowed a more detailed assessment of the contribution of confounders (including cardioprotective drugs, such as β-receptor antagonists, ACE-inhibitors, aldosterone antagonists, statins, and anti-hypertensive treatments) on trends and of a possible presence of ecological (aggregation) bias [29]. Moreover, we were not able to extract information across all studies for other potential study-level confounders; however, we adjusted for key covariates strongly related to the risk of cardiovascular disease and death, namely age, sex, duration of diabetes and, more importantly, prevalent cardiovascular disease [3]. Among other possible cardiovascular diseases at baseline, we selected myocardial infarction because it was the only confounder reported in all studies. The adjustment for prevalent myocardial infarction lessens the impact on the estimates of the different baseline risk of outcomes, particularly when comparing RCTs published after vs before 2008.
In contrast to observational data, in this study there was no evidence from RCTs of reducing rates of all-cause and cardiovascular mortality in patients with T2DM. For both RCTs and observational studies, more homogenous definitions of exposure and outcomes, the inclusion of heart failure among pre-specified endpoints, and an easier access to individual participant data will help quantify the differences between experimental and real-world evidence and further elucidate the reasons behind such divergences. Moreover, as prediction models for cardiovascular disease and mortality risk are instrumental in defining treatment strategies, targets, and clinical guidelines, health care decisions should consider that models’ performance could be highly influenced by the nature of the data, as the absolute risk of events is highly heterogeneous comparing RCTs and “real-world” patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
FZ, MJD, SS and KK acknowledge the National Institute for Health Research (NIHR)—Collaboration for Leadership in Applied Health Research (CLAHRC) and Care East Midlands and the NIHR Leicester Biomedical Research Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. LVM conducted this work during his internship as visiting researcher at the Leicester Diabetes Centre.
Author contributions
Study idea, design, and protocol: KK, FZ; literature search: LMV, FZ, NNG; data preparation: LMV, FZ; data analysis: LMV, FZ; first draft: LMV, FZ, KK; study critical revision and manuscript draft: all authors. All authors provided final approval of the version to publish. The corresponding author (FZ) had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Funding
FZ is funded with an unrestricted educational grant from the NIHR CLAHRC East Midlands to the University of Leicester.
Data access and sharing
Data and statistical codes are available on request from the corresponding author (FZ).
Conflict of interest
KK has acted as a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp and Dohme. He has received grants in support of investigator and investigator initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Pfizer, Boehringer Ingelheim and Merck Sharp and Dohme. KK has received funds for research, honoraria for speaking at meetings and has served on advisory boards for Lilly, Sanofi-Aventis, Merck Sharp and Dohme and Novo Nordisk. MJD has acted as consultant, advisory board member and speaker for Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp and Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, an advisory board member for Servier and as a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. She has received grants in support of investigator and investigator initiated trials from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim and Janssen. DRW has received grants in support of investigator-initiated studies and honoraria from Sanofi-Aventis and Novo Nordisk. SS has received honoraria for speaking at meetings and serving on Advisory Boards for Novartis, Novo Nordisk, Janssen, MSD, Lilly, and Boehringer Ingelheim. FZ has received honoraria for speaking at meetings for NAPP Pharmaceuticals. LMV, NNG, DP: no relevant conflict of interest.
Human and animal rights statement
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 5.Simmons RK, Griffin SJ, Lauritzen T, Sandbaek A. Effect of screening for type 2 diabetes on risk of cardiovascular disease and mortality: a controlled trial among 139,075 individuals diagnosed with diabetes in Denmark between 2001 and 2009. Diabetologia. 2017;60(11):2192–2199. doi: 10.1007/s00125-017-4299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawshani A, Rawshani A, Franzen S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 8.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000 to 2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care. 2016;39(6):1018–1026. doi: 10.2337/dc15-2308. [DOI] [PubMed] [Google Scholar]
- 9.Franke RH, Kaul JD. The Hawthorne experiments: first statistical interpretation. Am Sociol Rev. 1978;43:623–643. doi: 10.2307/2094540. [DOI] [Google Scholar]
- 10.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 11.Psaty BM, Prentice RL. Variation in event rates in trials of patients with type 2 diabetes. JAMA. 2009;302(15):1698–1700. doi: 10.1001/jama.2009.1497. [DOI] [PubMed] [Google Scholar]
- 12.Preiss D, Sattar N, McMurray JJ. A systematic review of event rates in clinical trials in diabetes mellitus: the importance of quantifying baseline cardiovascular disease history and proteinuria and implications for clinical trial design. Am Heart J. 2011;161(1):210–219 e211. doi: 10.1016/j.ahj.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1(1):8–15. doi: 10.1161/xJAHA.111.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2018;41(1):14–31. doi: 10.2337/dci17-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389(10072):941–950. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 18.Zaccardi F, Dhalwani NN, Webb DR, Davies MJ, Khunti K. Global burden of hypoglycaemia-related mortality in 109 countries, from 2000 to 2014: an analysis of death certificates. Diabetologia. 2018;61(7):1592–1602. doi: 10.1007/s00125-018-4626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization ICD-10. International Statistical Classification of Diseases and Related Health Problems, vol 2. http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf?ua=1. Accessed 14 June 2018
- 20.Zaccardi F, Davies MJ, Dhalwani NN, et al. Trends in hospital admissions for hypoglycaemia in England: a retrospective, observational study. Lancet Diabetes Endocrinol. 2016;4(8):677–685. doi: 10.1016/S2213-8587(16)30091-2. [DOI] [PubMed] [Google Scholar]
- 21.Khunti K, Davies M, Majeed A, et al. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38(2):316–322. doi: 10.2337/dc14-0920. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick C, Chatterjee S, Seidu S, et al. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(9):2169–2178. doi: 10.1111/dom.13348. [DOI] [PubMed] [Google Scholar]
- 23.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2(10):843–851. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 25.Echouffo-Tcheugui JB, Xu H, DeVore AD, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure registry. Am Heart J. 2016;182:9–20. doi: 10.1016/j.ahj.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Juhaeri J, Gao S, Dai WS. Incidence rates of heart failure, stroke, and acute myocardial infarction among Type 2 diabetic patients using insulin glargine and other insulin. Pharmacoepidemiol Drug Saf. 2009;18(6):497–503. doi: 10.1002/pds.1741. [DOI] [PubMed] [Google Scholar]
- 27.Khan JN, Wilmot EG, Leggate M, et al. Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging. 2014;15(11):1263–1269. doi: 10.1093/ehjci/jeu121. [DOI] [PubMed] [Google Scholar]
- 28.Vaccaro O, Masulli M, Riccardi G. Glucose lowering strategies and cardiovascular disease in type 2 diabetes—teachings from the TOSCA.IT study. Nutr Metab Cardiovasc Dis. 2018;28(7):722–726. doi: 10.1016/j.numecd.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


