Abstract
Purpose
Previous studies have shown that > 50% of colorectal cancer (CRC) patients treated with adjuvant chemotherapy gain weight after diagnosis. This may affect long-term health. Therefore, prevention of weight gain has been incorporated in oncological guidelines for CRC with a focus on patients that undergo adjuvant chemotherapy treatment. It is, however, unknown how changes in weight after diagnosis relate to weight before diagnosis and whether weight changes from pre-to-post diagnosis are restricted to chemotherapy treatment. We therefore examined pre-to-post diagnosis weight trajectories and compared them between those treated with and without adjuvant chemotherapy.
Methods
We included 1184 patients diagnosed with stages I–III CRC between 2010 and 2015 from an ongoing observational prospective study. At diagnosis, patients reported current weight and usual weight 2 years before diagnosis. In the 2 years following diagnosis, weight was self-reported repeatedly. We used linear mixed models to analyse weight trajectories.
Results
Mean pre-to-post diagnosis weight change was −0.8 (95% CI −1.1, −0.4) kg. Post-diagnosis weight gain was + 3.5 (95% CI 2.7, 4.3) kg in patients who had lost ≥ 5% weight before diagnosis, while on average clinically relevant weight gain after diagnosis was absent in the groups without pre-diagnosis weight loss. Pre-to-post diagnosis weight change was similar in patients treated with (−0.1 kg (95%CI −0.8, 0.6)) and without adjuvant chemotherapy (−0.9 kg (95%CI −1.4, −0.5)).
Conclusions
Overall, hardly any pre-to-post diagnosis weight change was observed among CRC patients, because post-diagnosis weight gain was mainly observed in patients who lost weight before diagnosis. This was observed independent of treatment with adjuvant chemotherapy.
Keywords: Colorectal cancer, Weight change, Weight gain, Chemotherapy
Introduction
Survival of colorectal cancer (CRC) has markedly improved over recent decades, which underlines the importance to study factors that can affect long-term health and quality of life of CRC survivors. One of the factors that may affect health and quality of life is body weight. Weight loss, either before diagnosis or during cancer treatment, is an important negative prognostic marker [1–4]. Therefore, in the hospital nutritional advice to cancer patients is mainly focused on prevention and/or treatment of unintentional weight loss. However, overweight and obesity are also affecting long-term health and quality of life among patients with non-metastatic disease. Therefore, prevention of weight gain after CRC diagnosis has recently been incorporated in the Dutch oncological nutritional therapy guidelines [5].
Many CRC patients are overweight or obese at diagnosis, as excess body weight is a risk factor for CRC [6]. Overweight/obese CRC survivors have an elevated risk of co-morbid disease, such as cardiovascular disease and diabetes, both at diagnosis and in the years following a diagnosis [7–9]. Weight gain after diagnosis might exacerbate existing co-morbid disease progression and further increase the risk of developing such diseases. Several studies reported that weight gain after diagnosis is common among CRC patients [1–3, 10, 11]. All these studies showed that weight gain after diagnosis was more common than weight loss after diagnosis [1–3, 10, 11]. The proportion of weight gain after diagnosis typically ranged from 25% to over 50% of patients [1–3, 10, 11]. In these studies, weight gain was defined as either a weight gain of ≥ 5 kg [1, 10] or ≥ 5% [2, 3, 11].
Although body weight may increase after CRC diagnosis, studies so far did not assess how body weight changed relative to usual body weight before diagnosis. Weight loss before CRC diagnosis is common [4, 12] as unintended weight loss could be one of the reasons for patients to see a physician, leading to the diagnosis of CRC. Thus, it is possible that patients catch up for this pre-diagnostic weight loss in the period during and after treatment. It is currently unknown if post-diagnosis weight change is different for patients with pre-diagnosis weight change compared to patients who were weight stable before diagnosis. Post-diagnosis weight gain might be more problematic in terms of long-term health if it results in overall weight gain compared to usual weight than when it reflects catching up for pre-diagnostic weight loss.
Weight gain is a common side-effect of chemotherapy in breast cancer patients [13], but weight gain is also common among non-metastatic CRC patients during and after chemotherapy. Two studies that both included > 500 colon cancer patients with stage III disease treated with adjuvant chemotherapy reported that the majority (51–65%) of patients experienced weight gain [3, 10]. Weight gain is observed both during and after adjuvant chemotherapy [11]. Therefore, prevention of weight gain in oncological guidelines has a focus on patients treated with adjuvant chemotherapy [5]. However, there is only indirect evidence that weight gain after diagnosis is more prevalent among patients treated with adjuvant chemotherapy than among patients treated without adjuvant chemotherapy. Studies that included non-metastatic CRC patients irrespective of chemotherapy treatment reported lower proportions (28%) of weight gain [1, 2] than studies among CRC patients treated with adjuvant chemotherapy (51–65%) [3, 10]. There are no studies that directly compared weight changes between patients treated with or without adjuvant chemotherapy.
Weight trajectories should ideally include data on weight at multiple time points, both before and after diagnosis, to fully capture weight changes among CRC patients. This information is currently lacking and therefore it remains unclear whether post-diagnosis weight eventually surpasses usual pre-diagnosis weight. Our aim was to examine pre-to-post diagnosis weight trajectories in CRC patients with non-metastatic disease and to compare these weight trajectories among patients treated with and without adjuvant chemotherapy.
Methods
Study population
We used data of the COLON study, an ongoing prospective multicentre cohort study among CRC patients in the Netherlands [14]. Eligible participants with newly diagnosed colon or rectal cancer were invited by hospital staff to participate in the study during a routine clinical visit before scheduled surgery. Data were collected shortly after diagnosis, before treatment started, and at two or three time points in the first 2 years after diagnosis (see “Assessment of body weight”). Follow-up data were available until January 2018. All study participants provided written informed consent and the study was approved by the local review board.
This study was performed among all participants diagnosed with stage I–III CRC between 2010 and 2015 who had a surgical resection (n = 1225). We excluded 70 participants who had information on weight available for < 2 time points. Thus, data of 1152 participants remained for analyses. Of these participants, 16 (1%) had missing self-reported weight before diagnosis and 217 (19%) did not complete 2 years of follow-up. We chose to exclude patients with stage IV disease a priori, because survival for these patients is generally poor and weight loss and cachexia are common at the end of life.
Assessment of body weight
At diagnosis, participants completed a survey with questions on body weight 2 years prior to diagnosis, and current weight. Participants repetitively answered surveys about their current body weight at 6 months, 1 year (only for the subsample treated with adjuvant chemotherapy), and 2 years after diagnosis.
Assessment of covariates
We obtained information on clinical factors, including disease stage, tumour site, receipt of neo-adjuvant treatment, type of surgery, stoma placement after surgery, complications within 30 days after surgery, receipt of adjuvant chemotherapy, type of chemotherapy, and presence of comorbidities from the Dutch ColoRectal Audit [15]. At diagnosis, all participants completed a questionnaire on demographic and lifestyle information, including education, smoking behaviour, and height. Body mass index (BMI) at diagnosis was computed in kg/m2.
Statistical analyses
We calculated pre-diagnosis, post-diagnosis, and pre-to-post diagnosis weight changes as weight at the end of the period minus weight at the start of the period, so negative differences indicate weight loss and positive differences indicate weight gain. Pre-diagnostic weight changes were grouped in three pre-defined categories: weight loss ≥ 5%, weight stable −5 to +5%, and weight gain ≥ 5%. Characteristics of the study population were compared across pre-diagnosis weight change groups and across adjuvant chemotherapy treatment. Differences in categorical variables were assessed by using a chi-squared test, and differences in means of continuous variables were tested by using analysis of variance or a t-test.
We fitted linear mixed models to examine weight trajectories over 4 years (2 years pre-diagnosis to 2 years post-diagnosis). Linear mixed models take into account both the individual trajectories of change (random effects) and population averages (fixed effects) by using all available measurements and including participants with incomplete data [16]. Time was scaled in years (continuous) with the date of study enrolment (shortly after diagnosis) defined as time is zero. Time for each post-diagnosis weight was calculated as date of self-reported weight collection minus the date of study enrolment. Time for pre-diagnosis weight was set at −2 years for all subjects.
The final model included a random intercept, a random slope for time, and a random curvature for time (i.e. taking into account each participant’s weight at diagnosis and the linear and quadratic slope). Using a step-up model building strategy, the random curvature model had much better fit than a random intercept model and a random slope model.
As fixed factors, we included baseline demographic determinants (sex, age, height, education, and smoking) and clinical factors (stage, tumour site, neo-adjuvant treatment, stoma, type of surgery, complications after surgery, and comorbidities). Age and height were centred to aid the interpretability of intercepts. The clinical factors neo-adjuvant treatment, stoma, and surgical complications were coded as not present before and at diagnosis. All fixed effects were included in the model as an interaction term with time. Only significant covariates and/or interactions were retained. Including additional interactions with time*time for the remaining covariates did not improve the model. The final model used in all analyses included the following fixed factors: time, sex, age, height, education, smoking, complications, stoma, type of surgery, comorbidities, time*time, education*time, and complications*time. The coefficient for time represents average annual linear change and the coefficient for time*time captures additional quadratic (curvilinear) change in weight in kilogrammes.
Additionally, we performed several stratified weight trajectory analyses. First, we stratified by pre-diagnosis weight change category (≥ 5% loss, stable, ≥ 5% gain) to further explore if weight gain after diagnosis differed by pre-diagnosis weight change. Second, we stratified by receipt of chemotherapy to compare weight trajectories among those treated with and without adjuvant chemotherapy. Third, as an exploratory analysis, we stratified by BMI at diagnosis to compare weight trajectories among survivors with a healthy BMI (18.5–25 kg/m2) and those with overweight or obesity (BMI ≥ 25 kg/m2). Weight trajectories were depicted based on predicted values by using the average study population, except for type of surgery in which laparoscopic surgery served as reference category. Two sensitivity analyses were performed to reduce heterogeneity between patients in the analyses stratified by chemotherapy. First by excluding patients with other adjuvant chemotherapy regimens than capecitabine combined with oxaliplatin and second by excluding patients with rectal tumours from the analyses. In the Netherlands, rectal tumours are generally not treated with adjuvant chemotherapy, which is in line with the Dutch oncological guidelines.
In all analyses, a p value < 0.05 was considered statistically significant. Statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of the study population according to pre-diagnosis weight change and adjuvant chemotherapy are shown in Table 1. Participants with ≥ 5% weight gain before diagnosis were on average slightly younger, more commonly female, obese at diagnosis (BMI ≥ 30 kg/m2), and presenting with one or more comorbidities compared to those with either stable weight or ≥ 5% weight loss before diagnosis. Participants with ≥ 5% weight loss before diagnosis had more often a tumour located in the colon compared to those with stable weight or weight gain. Patients treated with adjuvant chemotherapy were slightly younger and had unfavourable clinical characteristics compared to patients not treated with chemotherapy; other characteristics, such as BMI, were similar between the two groups.
Table 1.
Clinical and personal characteristics of 1152 non-metastatic colorectal cancer patients according to pre-diagnosis weight change and adjuvant chemotherapy1
| Weight change in the 2 years before diagnosis | Adjuvant chemotherapy | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall2 | Loss (≥ 5%) | Stable (−5% to 5%) | Gain (≥ 5%) | p value weight change group | No | Yes | p value chemo therapy | |
| N (%) | 1152 (100%) | 279 (25%) | 788 (69%) | 69 (6%) | 844 (75%) | 282 (25%) | ||
| Sex | < 0.001 | 0.21 | ||||||
| Men | 737 (64%) | 179 (64%) | 527 (67%) | 19 (28%) | 547 (65%) | 171 (61%) | ||
| Age at diagnosis (mean ± SD), years | 66 ± 9 | 66 ± 9 | 66 ± 8 | 63 ± 11 | 0.029 | 67 ± 9 | 63 ± 8 | < 0.001 |
| BMI at diagnosis (mean ± SD), kg/m2 | 26.5 ± 4.0 | 26.0 ± 4.0 | 26.5 ± 3.9 | 29.2 ± 4.1 | < 0.001 | 26.5 ± 3.9 | 26.6 ± 4.3 | 0.66 |
| BMI at diagnosis, kg/m2 | < 0.001 | 0.64 | ||||||
| < 18.5 | 10 (1%) | 3 (1%) | 6 (1%) | 0 (0%) | 6 (1%) | 4 (1%) | ||
| 18.5–25 | 447 (39%) | 130 (47%) | 301 (38%) | 13 (19%) | 331 (39%) | 107 (38%) | ||
| 25–30 | 497 (43%) | 110 (39%) | 352 (45%) | 27 (39%) | 362 (43%) | 122 (43%) | ||
| 30–35 | 161 (14%) | 27 (10%) | 107 (14%) | 23 (33%) | 120 (14%) | 37 (13%) | ||
| > 35 | 37 (3%) | 9 (3%) | 22 (3%) | 6 (9%) | 25 (3%) | 12 (4%) | ||
| Education level | 0.061 | 0.37 | ||||||
| Low | 505 (44%) | 133 (48%) | 330 (42%) | 36 (52%) | 383 (45%) | 115 (41%) | ||
| Medium | 277 (24%) | 58 (21%) | 198 (25%) | 20 (29%) | 195 (23%) | 72 (26%) | ||
| High | 365 (32%) | 87 (31%) | 260 (33%) | 13 (19%) | 262 (31%) | 95 (34%) | ||
| Smoking at diagnosis | 0.005 | 0.27 | ||||||
| Yes | 133 (11%) | 47 (17%) | 73 (9%) | 11 (16%) | 102 (12%) | 25 (9%) | ||
| Former | 682 (59%) | 166 (60%) | 468 (59%) | 40 (58%) | 498 (59%) | 168 (59%) | ||
| Never | 334 (29%) | 67 (24%) | 247 (32%) | 18 (26%) | 240 (28%) | 89 (32%) | ||
| Tumour stage | 0.26 | < 0.001 | ||||||
| I | 299 (26%) | 60 (22%) | 218 (28%) | 19 (28%) | 297 (35%) | – | ||
| II | 350 (30%) | 96 (34%) | 229 (29%) | 19 (28%) | 311 (37%) | 27 (10%) | ||
| III | 503 (44%) | 123 (44%) | 341 (43%) | 31 (45%) | 236 (28%) | 255 (90%) | ||
| Tumour location | 0.038 | < 0.001 | ||||||
| Colon | 778 (68%) | 206 (74%) | 517 (66%) | 45 (65%) | 499 (59%) | 259 (92%) | ||
| Rectum | 374 (32%) | 73 (26%) | 271 (34%) | 24 (35%) | 345 (41%) | 23 (8%) | ||
| Adjuvant chemotherapy | 0.18 | – | ||||||
| Yes | 282 (24%) | 80 (29%) | 184 (23%) | 16 (23%) | – | 282 (100%) | ||
| No | 844 (73%) | 192 (69%) | 587 (74%) | 52 (75%) | 844 (100%) | – | ||
| Adjuvant chemotherapy regimen | 0.94 | – | ||||||
| Capecitabine + oxaliplatin | 214 (19%) | 61 (22%) | 140 (18%) | 12 (17%) | – | 214 (76%) | ||
| Capecitabine | 37 (3%) | 12 (4%) | 22 (3%) | 2 (3%) | – | 37 (13%) | ||
| Other | 7 (1%) | 2 (1%) | 5 (1%) | 0 (0%) | – | 7 (2%) | ||
| Neo-adjuvant treatment | 0.34 | < 0.001 | ||||||
| Yes | 270 (23%) | 57 (20%) | 189 (24%) | 19 (28%) | 250 (30%) | 15 (5%) | ||
| No | 882 (77%) | 222 (79%) | 599 (76%) | 50 (72%) | 594 (70%) | 267 (95%) | ||
| Stoma | 0.061 | < 0.001 | ||||||
| Yes | 340 (30%) | 67 (24%) | 245 (31%) | 22 (32%) | 309 (37%) | 25 (9%) | ||
| No | 783 (68%) | 207 (74%) | 521 (66%) | 46 (67%) | 508 (60%) | 255 (90%) | ||
| Surgery | 0.054 | 0.67 | ||||||
| Laparoscopic | 725 (63%) | 162 (58%) | 512 (65%) | 38 (55%) | 538 (64%) | 177 (62%) | ||
| Conversion | 73 (6%) | 18 (6%) | 46 (6%) | 9 (13%) | 51 (6%) | 21 (7%) | ||
| Open | 303 (26%) | 84 (30%) | 198 (25%) | 19 (28%) | 213 (25%) | 76 (27%) | ||
| Complications after surgery | < 0.001 | < 0.001 | ||||||
| Yes | 323 (28%) | 96 (35%) | 195 (25%) | 26 (37%) | 257 (30%) | 55 (20%) | ||
| No | 787 (68%) | 170 (61%) | 569 (72%) | 40 (58%) | 553 (66%) | 220 (78%) | ||
| Comorbidity | 0.023 | 0.018 | ||||||
| Yes | 773 (67%) | 182 (65%) | 524 (67%) | 56 (81%) | 581 (69%) | 173 (61%) | ||
| No | 370 (32%) | 97 (35%) | 256 (32%) | 12 (17%) | 256 (30%) | 107 (38%) | ||
1Some counts do not add to totals because of missing data
2Includes 16 participants with missing pre-diagnosis weight and 26 with missing chemotherapy status
Compared to pre-diagnosis weight, mean weight change was −0.8 (95% CI −1.1, −0.4) kg over the 4-year period (Table 2). Over this total period, weight change was < 5% for the majority of people (66%), while 14% of all patients experienced pre-to-post diagnosis weight gain of ≥ 5% and 20% experienced weight loss of ≥ 5%. When only the 2 years post-diagnosis were taken into account, mean weight change in the 2 years after diagnosis was + 1.2 (95%CI 0.9, 1.5) kg.
Table 2.
Two-year post-diagnosis and 4-year pre-to-post diagnosis weight changes by pre-diagnosis weight change groups and by adjuvant chemotherapy1
| Weight change during 2 years before diagnosis | Adjuvant chemotherapy | |||||
|---|---|---|---|---|---|---|
| Overall | Loss (≥ 5%) | Stable (−5% to 5%) | Gain (≥ 5%) | No | Yes | |
| N (%) | 922 (100%) | 221 (24%) | 644 (70%) | 57 (6%) | 687 (75%) | 215 (23%) |
| Pre-diagnosis weight change (mean (95% CI)), kg | −1.9 (−2.3, −1.6) | −8.3 (−9.1, −7,5) | −0.5 (−0.6, −0.3) | +6.2 (5.5, 6.8) | −1.9 (−2.2, −1.5) | −2.2 (−2.8, −1.5) |
| Pre-to-post diagnosis weight change (4 years) | ||||||
| Absolute weight change (mean (95% CI)), kg | −0.8 (−1.1, −0.4) | −4.8 (−5.7, −3.9) | +0.0 (−0.3, 0.4) | +6.1 (4.5, 7.6) | −0.9 (−1.4, −0.5) | −0.1 (−0.8, 0.6) |
| Weight loss (%) | 20 | 48 | 11 | 4 | 21 | 16 |
| Weight stable (%) | 66 | 47 | 76 | 30 | 65 | 69 |
| Weight gain (%) | 14 | 5 | 12 | 67 | 14 | 15 |
| Post-diagnosis weight change (2 years) | ||||||
| Absolute weight change (mean (95% CI)), kg | +1.2 (0.9, 1.5) | +3.5 (2.7, 4.3) | +0.5 (0.2, 0.8) | −0.1 (−1.7, 1.5) | +0.9 (0.5, 1.3) | +2.1 (1.5, 2.7) |
| Weight loss (%) | 9 | 5 | 9 | 26 | 10 | 6 |
| Weight stable (%) | 70 | 54 | 77 | 55 | 71 | 67 |
| Weight gain (%) | 21 | 42 | 14 | 19 | 19 | 27 |
1230 participants with pre-diagnosis weight and/or 2-year post-diagnosis weight information missing omitted from table (n = 16 and n = 217, respectively)
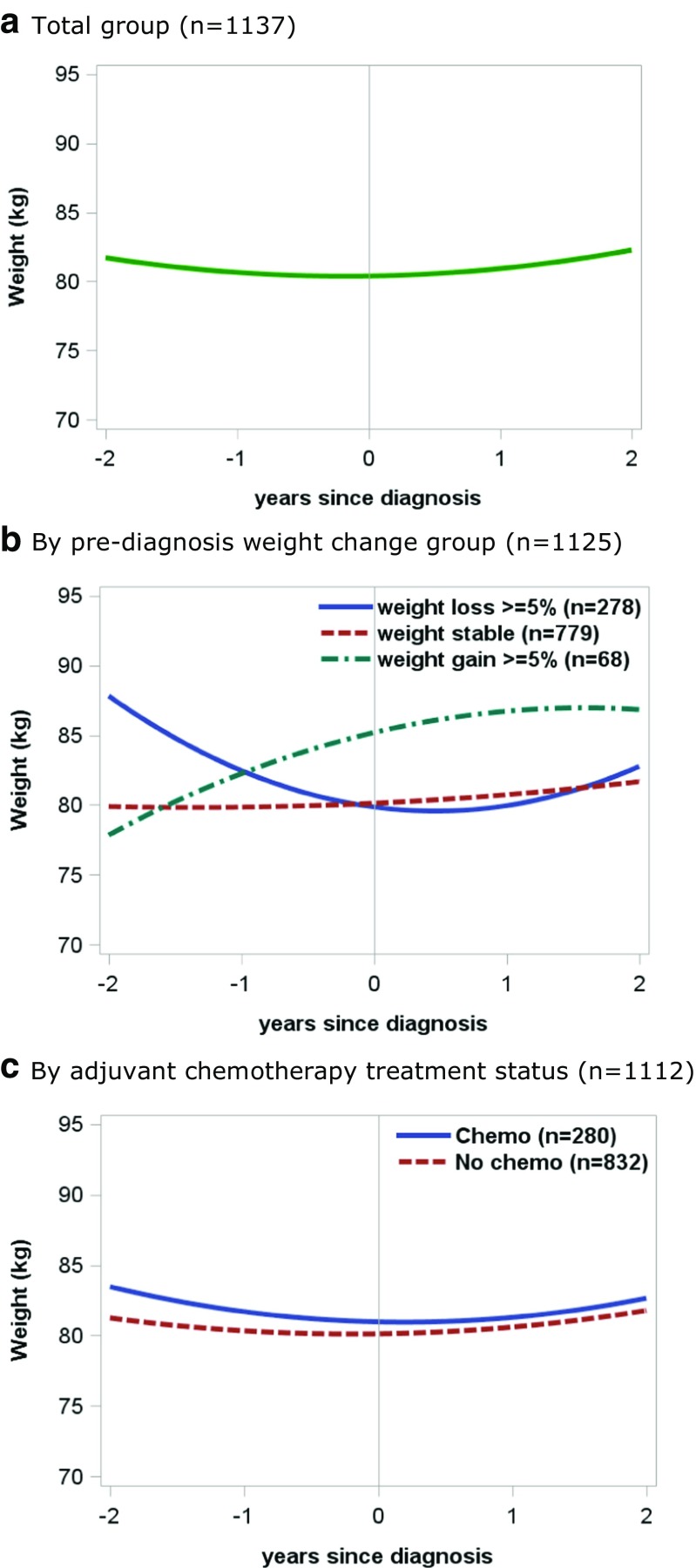
The estimated 4-year weight trajectory in the entire cohort is presented in Fig. 1A. The full model showed a clear positive quadratic relationship of weight changes in the entire cohort (p < 0.001), but no linear effect was present (+ 0.04 kg annual weight gain, p = 0.68). In other words, weight decreased before diagnosis while weight increased after diagnosis. Overall, weight 2 years after diagnosis was similar to weight 2 years before diagnosis.
Fig. 1.
Weight trajectories from 2 years before diagnosis to 2 years after diagnosis in colorectal cancer patients (weight trajectories were based on predicted values from mixed models for a population with laparoscopic surgery and of average age, height, education, sex, smoking status, complications, stoma, type of surgery, and comorbidities). A. Total group (n = 1137) B. By pre-diagnosis weight change group (n = 1125) C. By adjuvant chemotherapy treatment status (n = 1112)
To explore if post-diagnosis weight trajectories differed by pre-diagnosis weight change, we stratified the weight trajectory analyses by pre-diagnosis weight change. A mean gain in body weight after diagnosis was most prominent in the group that had lost weight before diagnosis (Fig. 1B; Table 2). In this group, 42% gained weight after diagnosis and this proportion was much larger than that seen for the pre-diagnosis weight stable and weight gain groups (14% and 19%, respectively; Table 2). In absolute numbers, post-diagnosis weight gain was on average + 3.5 (95% CI 2.7, 4.3) kg in the group that had lost weight pre-diagnosis. However, taking the 2 years before diagnosis into account, mean weight change was −4.8 (95% CI −5.7, −3.9) kg in this group. On average, clinically relevant weight change after diagnosis was absent when pre-diagnosis weight was stable or when pre-diagnosis weight gain ≥ 5% was present.
Weight trajectories were similar for those treated with and without adjuvant chemotherapy (Fig. 1C; Table 2). In both groups, overall weight 2 years after diagnosis was similar to overall weight 2 years before diagnosis. Sensitivity analyses excluding patients with other adjuvant chemotherapy regimens than capecitabine combined with oxaliplatin or excluding patients with rectal tumours did not change the results (data not shown). Weight trajectories were similar for those with a BMI of 18.5–25 kg/m2 and a BMI ≥ 25 kg/m2 at diagnosis (data not shown).
Discussion
We examined pre-to-post diagnosis weight trajectories among patients with non-metastatic CRC. Overall, hardly any pre-to-post diagnosis weight change was observed among CRC patients, because post-diagnosis weight gain was mainly observed in patients who lost weight before diagnosis. This was observed independent of treatment with adjuvant chemotherapy.
This was the first study that examined pre-to-post diagnosis weight changes in CRC patients, therefore we can only compare our results on post-diagnosis weight changes with previous studies. All previous studies on post-diagnosis weight change in CRC patients with non-metastatic disease showed that weight gain was more common than weight loss [1–3, 10, 11, 17], which is in line with our study. We found that 21% of patients with non-metastatic CRC experienced ≥ 5% weight gain in the first 2 years after diagnosis, which is slightly lower than the 28% reported in previous studies [1, 2]. Among patients treated with adjuvant chemotherapy, 27% of patients experienced ≥ 5% weight gain in our study. Although the proportion of patients treated with chemotherapy who experienced weight gain in the current study was lower compared with other studies (36–65%) [3, 10, 11], the mean post-diagnosis weight gain of + 2.1 kg in patients treated with chemotherapy was similar to the mean weight gain of + 2.0 kg reported in a previous study based on body weights retrieved from medical records [11]. Weight gain was seen both during and after adjuvant chemotherapy [11], although in this study we were not able to make this distinction. While previous studies focussed on post-diagnosis weight changes, the current study also included usual weight pre-diagnosis into the analysis of weight changes. Our analyses revealed that post-diagnosis weight gain was most prominent in patients who lost ≥ 5% weight before diagnosis and therefore mean pre-to-post diagnosis weight gain was absent in the overall population.
The current study was the first that compared weight changes between CRC patients treated with and without adjuvant chemotherapy. By including weight data at multiple time points during the course of the disease, both before and after diagnosis, we showed that weight trajectories were similar for those treated with and without chemotherapy. In both groups, weight 2 years post-diagnosis diagnosis did on average not surpass usual pre-diagnosis weight. However, in both groups, about 15% experienced pre-to-post diagnosis weight gain of ≥ 5%. It was unexpected that weight trajectories over the course of CRC were independent of adjuvant chemotherapy treatment. Previous studies showed that post-diagnosis weight gain was more common in studies among patients treated with adjuvant therapy than in studies that included patients irrespective of adjuvant chemotherapy (36–65% versus 28%, respectively) [1–3, 10, 11]. Our results imply that weight gain is not a common side-effect of adjuvant chemotherapy in CRC patients with non-metastatic disease.
A limitation of this study is that body weight was self-reported at each time point, perhaps leading to measurement error with regard to weight change. Cross-sectional data show that self-reported weight values are typically slightly lower than directly measured values [18], although bias may differ by weight status and gender [18, 19]. However, good-to-excellent agreement was reported for self-reported and directly measured values of body weight in studies with similar demographic characteristics to this study [20, 21]. Participants are also likely to have internal consistency in their reporting, such that the degree of underreporting will be similar each time [19]. Therefore, changes in weight may be less prone to such bias than individual weight measurements. In our study, weight 2 years prior to diagnosis was recalled while post-diagnosis weights were collected prospectively, which may decrease internal consistency. However, good-to-excellent agreement was also reported for pre-diagnosis weight recalled shortly after diagnosis and directly measured values of pre-diagnosis body weight [22]. We assume that weight 2 years before CRC diagnosis reflects usual pre-diagnosis weight, since the median time from onset of symptoms (such as weight loss) until the start of treatment is usually 4 to 5 months [23, 24]. Another limitation is that we did not have information on changes in body composition. Even when pre-to-post diagnosis weight gain is not present, post-diagnosis weight gain may still lead to an increase in fat mass with a loss in muscle mass. Future research should be done to determine how post-diagnosis weight gain affects body composition.
This study has several strengths. First, the COLON study provided an opportunity to explore weight trajectories over the course of the disease in a large group of CRC patients, since we prospectively collected weight several times after diagnosis and also had pre-diagnosis weight available. We used mixed models to examine weight trajectories over 4 years. An advantage of mixed models is that participants with incomplete weight data were still included in the analyses. Second, we had detailed treatment information available so we were able to compare weight trajectories between those treated with and without adjuvant chemotherapy. Third, we were able to adjust for many covariates that could potentially affect weight change. Although other factors, such as physical activity and physical functioning, not included in the multivariate analyses could also affect weight change. However, both the adjusted weight trajectories (Fig. 1) and the crude weight changes (Table 2) showed similar results. Lastly, the study population was representative of the total population of Dutch stage I–III CRC survivors with respect to stage of disease and location of the tumour (colon or rectum), but the proportion of females and the mean age were slightly lower as compared to the total population of CRC survivors [25, 26]. Although not perfectly comparable, we believe our findings are generalizable to the total Dutch population of stage I–III CRC survivors, but they cannot be generalised to stage IV CRC survivors.
In clinical practice, not only weight loss, but also weight gain should receive attention as is stated in the Dutch Dieticians Oncology Group guidelines for bowel cancer therapy [5]. Based on our results, weight changes should be monitored over the course of the disease in all patients, taking pre-diagnosis weight change into account. A previous study suggested that pre-to-post diagnosis weight change, weight loss as well as weight gain, may be associated with a higher mortality risk among CRC patients with non-metastatic disease [1]. In contrast, post-diagnosis weight gain did not seem to be associated with mortality risk [1, 2, 10]. Our results, together with these other studies [1, 2, 10], emphasise the importance of taking pre-diagnosis weight into account when examining weight changes in CRC patients. Our study showed that 14% of all patients experienced pre-to-post diagnosis weight gain and pre-to-post diagnosis weight gain was equally prevalent among patients treated with and without adjuvant chemotherapy. Therefore, weight gain prevention should not only be targeted at patients receiving adjuvant chemotherapy, but at all CRC patients with non-metastatic disease.
In conclusion, pre-to-post diagnosis weight change was largely absent among CRC patients with non-metastatic disease, because post-diagnosis weight gain was mainly observed in patients who lost weight before diagnosis. This was observed independent of treatment with adjuvant chemotherapy. Future studies are needed to confirm our findings and to assess how weight change relates to survival and the development of co-morbidities to provide a solid basis for future recommendations directed towards managing weight during the course of CRC.
Acknowledgements
The authors thank all participants for their time to participate in the study. Furthermore, we would like to thank the co-workers from the following hospitals for their involvement in recruitment for the COLON study: Hospital Gelderse Vallei, Ede; RadboudUMC, Nijmegen; Slingeland Hospital, Doetinchem; Canisius Wilhelmina Hospital, Nijmegen; Rijnstate Hospital, Arnhem; Gelre Hospitals, Apeldoorn/Zutphen; Hospital Bernhoven, Uden; Isala, Zwolle; Hospital Group Twente ZGT, Almelo; Martini Hospital, Groningen; and Admiraal de Ruyter Hospital, Goes/Vlissingen, all in the Netherlands. Also we would like to thank Joeri Kalter for his help with data management.
Funding
The COLON study is sponsored by Wereld Kanker Onderzoek Fonds, including funds from grant 2014/1179 as part of the World Cancer Research Fund International Regular Grant Programme; Alpe d’Huzes/Dutch Cancer Society (UM 2012-5653, UW 2013-5927, UW 2015-7946); and ERA-NET on Translational Cancer Research (TRANSCAN/Dutch Cancer Society: UW2013-6397, UW2014-6877).
Conflict of interest
The authors declare that they have no conflict of interest. P.C. van de Meeberg is also affiliated to the Dutch Obesity Clinic as a paid advisor. We have full control of all primary data and we agree to allow the journal to review our data if requested.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomark Prev. 2011;20(7):1410–1420. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, Cespedes Feliciano EM, Xiao J, Caan BJ. Association of Weight Change after Colorectal Cancer Diagnosis and Outcomes in the Kaiser Permanente Northern California Population. Cancer Epidemiol Biomark Prev. 2017;26(1):30–37. doi: 10.1158/1055-9965.EPI-16-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergidis J, Gresham G, Lim HJ, Renouf DJ, Kennecke HF, Ruan JY, Chang JT, Cheung WY. Impact of weight changes after the diagnosis of stage III colon cancer on survival outcomes. Clin Colorectal Cancer. 2016;15(1):16–23. doi: 10.1016/j.clcc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Walter V, Jansen L, Hoffmeister M, Ulrich A, Roth W, Blaker H, Chang-Claude J, Brenner H. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am J Clin Nutr. 2016;104(4):1110–1120. doi: 10.3945/ajcn.116.136531. [DOI] [PubMed] [Google Scholar]
- 5.Dutch Dieticians Oncology Group (2017) Bowel cancer, nation-wide guideline, version 3.0. https://www.oncoline.nl/bowel-cancer. Accessed 23 Apr 2018
- 6.World Cancer Research Fund/American Institute for Cancer Research (2017) Continuous update project report: diet, nutrition, physical activity and colorectal cancer. Available at: wcrf.org/colorectal-cancer-2017
- 7.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011;47(2):267–276. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, Forsythe LP, Rowland JH. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. Journal of Cancer Survivorship: Research and Practice. 2015;9(2):239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 9.De Marco MF, Janssen-Heijnen ML, van der Heijden LH, Coebergh JW (2000) Comorbidity and colorectal cancer according to subsite and stage: a population-based study. Eur J Cancer 36 (1):95–99. doi:10.1016/s0959-8049(99)00221-x [DOI] [PubMed]
- 10.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS, Cancer, Leukemia Group B Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and leukemia group B 89803. J Clin Oncol. 2008;26(25):4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkels RM, Snetselaar T, Adriaans A, van Warmerdam LJC, Vreugdenhil A, Slooter GD, Straathof JW, Kampman E, van Lieshout R, Beijer S. Changes in body weight in patients with colorectal cancer treated with surgery and adjuvant chemotherapy: an observational study. Cancer Treat Res Commun. 2016;9:111–115. doi: 10.1016/j.ctarc.2016.09.002. [DOI] [Google Scholar]
- 12.Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan ML, Prado CM. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study) Cancer Epidemiol Biomark Prev. 2017;26(7):1008–1015. doi: 10.1158/1055-9965.EPI-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Berg MM, Winkels RM, de Kruif JT, van Laarhoven HW, Visser M, de Vries JH, de Vries YC, Kampman E. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer. 2017;17(1):259. doi: 10.1186/s12885-017-3242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkels RM, Heine-Broring RC, van Zutphen M, van Harten-Gerritsen S, Kok DE, van Duijnhoven FJ, Kampman E. The COLON study: colorectal cancer: longitudinal, observational study on nutritional and lifestyle factors that may influence colorectal tumour recurrence, survival and quality of life. BMC Cancer. 2014;14(1):374. doi: 10.1186/1471-2407-14-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Leersum NJ, Snijders HS, Henneman D, Kolfschoten NE, Gooiker GA, ten Berge MG, Eddes EH, Wouters MW, Tollenaar RA, Dutch Surgical Colorectal Cancer Audit G. Bemelman WA, van Dam RM, Elferink MA, Karsten TM, van Krieken JH, Lemmens VE, Rutten HJ, Manusama ER, van de Velde CJ, Meijerink WJ, Wiggers T, van der Harst E, Dekker JW, Boerma D. The Dutch surgical colorectal audit. Eur J Surg Oncol. 2013;39(10):1063–1070. doi: 10.1016/j.ejso.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000. [Google Scholar]
- 17.Lee DW, Han SW, Cha Y, Lee KH, Kim TY, Oh DY, Im SA, Bang YJ, Park JW, Ryoo SB, Jeong SY, Kang GH, Park KJ, Kim TY. Prognostic influence of body mass index and body weight gain during adjuvant FOLFOX chemotherapy in Korean colorectal cancer patients. BMC Cancer. 2015;15:690. doi: 10.1186/s12885-015-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8(4):307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Hattori A, Sturm R. The obesity epidemic and changes in self-report biases in BMI. Obesity. 2013;21(4):856–860. doi: 10.1002/oby.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 21.Yoong SL, Carey ML, D'Este C, Sanson-Fisher RW. Agreement between self-reported and measured weight and height collected in general practice patients: a prospective study. BMC Med Res Methodol. 2013;13:38. doi: 10.1186/1471-2288-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivarius NF, Andreasen AH, Loken J. Accuracy of 1-, 5- and 10-year body weight recall given in a standard questionnaire. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1997;21(1):67–71. doi: 10.1038/sj.ijo.0800365. [DOI] [PubMed] [Google Scholar]
- 23.Van Hout AM, de Wit NJ, Rutten FH, Peeters PH. Determinants of patient’s and doctor’s delay in diagnosis and treatment of colorectal cancer. Eur J Gastroenterol Hepatol. 2011;23(11):1056–1063. doi: 10.1097/MEG.0b013e32834c4839. [DOI] [PubMed] [Google Scholar]
- 24.Langenbach MR, Sauerland S, Krobel KW, Zirngibl H. Why so late?!–delay in treatment of colorectal cancer is socially determined. Langenbeck’s Arch Surg. 2010;395(8):1017–1024. doi: 10.1007/s00423-010-0664-8. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer NPM, Bos A, Lemmens V, Tanis PJ, Hugen N, Nagtegaal ID, de Wilt JHW, Verhoeven RHA. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758–2766. doi: 10.1002/ijc.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netherlands Cancer Registry (2018) Dutch cancer figures. https://www.cijfersoverkanker.nl. Accessed 24 Sept 2018