This article summarizes the latest clinical applications of PD‐1/PD‐L1 blockade therapy in advanced non‐small cell lung cancer (NSCLC) worldwide and in China, reporting the bottlenecks related to the use of this therapy in clinic. An exploration of the underlying mechanism of PD‐1/PD‐L1 blockade therapy and biomarker identification will maximize the application of immune checkpoint inhibitors in advanced NSCLC and facilitate bedside‐to‐bench studies in cancer immunotherapy.
Keywords: Immune checkpoint inhibitors, PD‐1/PD‐L1, Non‐small‐cell lung cancer, Clinical trials, Combination therapy
Abstract
The use of immune checkpoint inhibitors (ICIs) has become one of the most promising approaches in the field of cancer therapy. Unlike the current therapies that target tumor cells, such as chemotherapy, radiotherapy, or targeted therapy, ICIs directly restore the exhausted host antitumor immune responses mediated by the tumors. Among multiple immune modulators identified, the programmed cell death protein 1 (PD‐1)/programmed cell death protein ligand 1 (PD‐L1) axis leading to the exhaustion of T‐cell immunity in chronic infections and tumors has been widely investigated. Therefore, blocking antibodies targeting PD‐1 or PD‐L1 have been developed and approved for the treatment of various advanced cancers, including non‐small‐cell lung cancer (NSCLC), making them the most successful ICIs. Compared with chemotherapy or radiotherapy, PD‐1/PD‐L1 blockade therapy significantly improves the durable response rate and prolongs long‐term survival with limited adverse effects in both monotherapy and combination therapy for advanced NSCLC. However, extensive challenges exist for further clinical applications, such as a small fraction of benefit population, primary and acquired resistance, the lack of predictive and prognostic biomarkers, and treatment‐related adverse effects. In this article, we summarize the latest clinical applications of PD‐1/PD‐L1 blockade therapy in advanced NSCLC worldwide, as well as in China, and discuss the bottlenecks related to the use of this therapy in clinical practice. An exploration of the underlying mechanism of PD‐1/PD‐L1 blockade therapy and biomarker identification will maximize the application of ICIs in advanced NSCLC and facilitate bedside‐to‐bench studies in cancer immunotherapy as well.
Implications for Practice.
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD‐1) and programmed cell death protein ligand 1 (PD‐L1) display apparent benefits for the treatment of advanced non‐small‐cell lung cancer (NSCLC). However, the clinical applications of these therapies are challenged by the limited benefit population with additional high economic burden and adverse events. This review discusses the bottlenecks of ICI therapy in clinical practice and provides appropriate guidance in the development of predictive biomarkers, the establishment of the criteria for combining PD‐1/PD‐L1 blockade therapy with the existing therapies, and the management of adverse events observed both in monotherapy and combination therapy, which will help maximize the applications of ICIs in advanced NSCLC.
摘要
免疫检查点抑制剂 (ICI) 的使用已成为癌症治疗领域最具前景的方法之一。与靶向肿瘤细胞的现有疗法(如化疗、放疗或靶向治疗)不同,ICI 可直接恢复由肿瘤介导的耗竭的宿主抗肿瘤免疫应答。在已确定的多种免疫调节剂中,已经广泛研究了导致慢性感染和肿瘤中 T 细胞免疫耗竭的程序性细胞死亡蛋白 1 (PD‐1)/程序性细胞死亡蛋白配体 1 (PD‐L1) 轴。因此,已经开发了靶向 PD‐1 或 PD‐L1 的阻断抗体,并批准用于治疗各种晚期癌症,包括非小细胞肺癌 (NSCLC),这使其成为最成功的 ICI。在晚期 NSCLC,与化疗或放疗相比,PD‐1/PD‐L1 阻断治疗无论单药和联合治疗均明显提高了持久缓解率并延长了长期生存,而且不良反应很少。然而,对于进一步的临床应用却存在大量挑战,例如,仅有小部分群体受益,存在原发性和获得性耐药,缺乏预测和预后生物标志物,以及存在治疗相关不良反应。在本文中,我们总结了中国及全球范围内 PD‐1/PD‐L1 阻断治疗在晚期 NSCLC 中的最新临床应用,并讨论了在临床实践中使用该疗法的瓶颈。对 PD‐1/PD‐L1 阻断治疗和生物标志物鉴定的潜在机制的探索将最大化 ICI 在晚期 NSCLC 中的应用,同时,也将促进癌症免疫治疗“从临床到实验室”的研究。
实践意义:在晚期非小细胞肺癌 (NSCLC) 的治疗中,靶向程序性细胞死亡蛋白 1 (PD‐1) 和程序性细胞死亡蛋白配体 1 (PD‐L1) 的免疫检查点抑制剂 (ICI) 呈现出明显益处。然而,这些治疗的临床应用却面临着受益群体有限、额外的高昂经济负担和不良事件等挑战。本篇综述讨论了 ICI 疗法在临床实践中的瓶颈,并为预测生物标志物的形成、将 PD‐1/PD‐L1 阻断治疗与现有治疗相结合的标准确立、以及在单药和联合治疗中观察到的不良事件的管理提供了适当的指导,从而有助于最大化 ICI 在晚期 NSCLC 中的应用。
Introduction
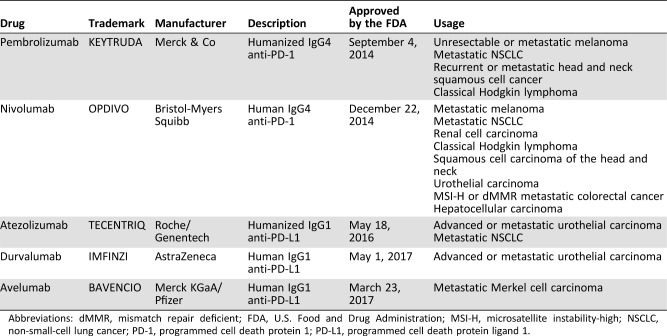
The development of immune checkpoint inhibitor (ICI) agents targeting cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4), programmed cell death protein 1 (PD‐1) or programmed cell death protein ligand 1 (PD‐L1) has garnered tremendous interests in the field of immuno‐oncology because of the recent successful applications in multiple advanced cancers [1], [2], [3]. Although CTLA‐4 is the first immune checkpoint molecule identified in 1987 [4], the PD‐1/PD‐L1 axis has been widely investigated because of the role in the exhaustion of CD8+ T cells [5]. Immuno‐oncologists extended the concept to antitumor immunity, making PD‐1/PD‐L1 the most promising targets for drug development [6]. Therapeutic monoclonal antibodies targeting PD‐1 or PD‐L1 have demonstrated notable clinical efficacy in the treatment of various advanced cancers [6], [7]. Up to the end of 2017, five monoclonal antibodies targeting PD‐1 or PD‐L1 have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of various advanced cancers (Table 1), including melanoma [8], non‐small‐cell lung cancer (NSCLC) [9], head and neck squamous cell cancer [10], classical Hodgkin lymphoma [11], urothelial carcinoma [12], hepatocellular carcinoma [8], Merkel cell carcinoma [13], renal cell carcinoma [14], and colorectal cancer [15]. Immune checkpoint therapy, which was first approved as second‐line treatment and has been extended to first‐line treatment [16], [17], becomes an alternative option for cancer therapy. In this review, we introduce the development of PD‐1/PD‐L1 blockade therapy and its clinical applications in advanced NSCLC. The ongoing clinical trials of PD‐1 and PD‐L1 inhibitors in China are also introduced, which might contribute to a better understanding of ICI therapy in China.
Table 1. Overview of anti‐PD‐1/PD‐L1 antibodies approved by the FDA as of October 2017.
Abbreviations: dMMR, mismatch repair deficient; FDA, U.S. Food and Drug Administration; MSI‐H, microsatellite instability‐high; NSCLC, non‐small‐cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death protein ligand 1.
Materials and Methods
We searched in the PubMed database using the terms “PD‐1, PD‐L1, NSCLC, phase III trial, combination therapy” for articles published by August 2018. Original reports and systematic review articles were also reviewed to identify additional publications. Reports from recent conferences on cancer immunotherapy were included as well. Information regarding the relevant clinical trials was obtained from online databases (https://www.chinadrugtrials.org.cn; https://www.clinicaltrials.gov).
A Brief History of Cancer Immunotherapy
The successful treatment of an inoperable sarcoma using bacterial toxins reported by William Coley in 1910 [18] is recognized as the first example of immunotherapy. After Coley's success, researchers since the late 1990s have made great efforts to manipulate host immune responses for cancer immunotherapy, such as interleukin‐2 (IL‐2) [19], lymphokine‐induced killer cells [20], tumor‐infiltrating lymphocytes (TILs) [21], and the first therapeutic prostate cancer vaccine [22]. However, the clinical applications of these agents remain limited, mainly because of the low therapeutic effectiveness, high toxicity when used at a large dosage, and the cost of the treatments.
The successes of immune checkpoint blockade reagents and chimeric antigen receptor T‐cells against multiple cancers made cancer immunotherapy the scientific breakthrough in 2013 according to Science journal [23]. CTLA‐4 and PD‐1/PD‐L1 are among the targets that draw great attention in the field of cancer immunotherapy. CTLA‐4 was first identified by screening mouse cytolytic‐T‐cell‐derived cDNA libraries and is mainly expressed on activated T cells and regulatory T cells (Treg) [4]. CTLA‐4 inhibits T‐cell proliferation and IL‐2 secretion by competing with CD28 for the B7 ligands [24], [25]. The blockade of CTLA‐4 has been shown to potentiate T‐cell responses in vitro [26] and cause tumor rejection in vivo in murine models [27]. The therapeutic CTLA‐4‐blocking antibody ipilimumab has been developed since 1999 and was approved in 2011 for the treatment of advanced melanoma [28], [29]. The development of CTLA‐4 blocking antibody thus became the milestone of ICIs for cancer immunotherapy. Subsequently, ICIs targeting PD‐1 and PD‐L1, which were cloned in 1992 and 1999, respectively [30], [31], were developed. The antitumor efficacy of these ICIs observed in clinical trials is also encouraging for multiple advanced cancers [7], [32]. At present, five ICIs targeting PD‐1 or PD‐L1 have been approved by the FDA for the treatment of various cancers (Table 1), propelling cancer therapy into a new era.
Mechanisms of PD‐1/PD‐L1 Blockade in Immunotherapy
It is widely accepted that activated T cells are key players in restraining cancer cells initiated by T‐cell receptor (TCR) recognition of peptides presented by major histocompatibility complex molecule. PD‐1 is mainly expressed on activated T cells and functions as a brake of T‐cell activation through binding to the PD‐1 ligands PD‐L1 and PD‐L2 [30], [33]. Upon binding with PD‐L1 and PD‐L2, PD‐1 is phosphorylated by the protein tyrosine kinase Lck, leading to the recruitment of the tyrosine phosphatase Shp2 and the subsequent dephosphorylation of CD28, which in turn inhibits TCR/CD28 signaling and subsequent T‐cell activation signal [34], [35], [36], [37]. The PD‐1 ligand PD‐L1 is expressed on multiple normal tissues and malignant cells [38]. The expression of PD‐L1 is upregulated on tumor cells when exposed to interferon‐γ and other cytokines that are released by local activated T cells, resulting in the resistance of tumor cells to T‐cell immunity, especially within the tumor microenvironment (TME) [39], [40]. After long exposure to tumor antigens in the TME, T cells infiltrated in the TME (named TILs) become exhausted, with characteristics of high expression of PD‐1 and low antitumor function [40]. Therefore, antibodies blocking PD‐1/PD‐L1 interaction largely rescue the function of these exhausted T cells and result in enhanced antitumor immunity [41]. With high expression of PD‐1 on Tregs, which play inhibitory roles in antitumor immunity [42], [43], interruption of PD‐1/PD‐L interaction can release antitumor responses by impairing the suppressive activity of Tregs [44]. In addition to T‐cell immunity, antitumor effects can also be enhanced by redirecting the function of tumor‐associated macrophages [45] and the natural killer cell‐dendritic cell axis in the TME [46].
PD‐1/PD‐L1 Blockade Therapy in Advanced NSCLC
Lung cancer is the leading cause of cancer mortality in China and worldwide [47], [48], [49]. Despite the availability of surgical resection, radiotherapy, platinum‐based chemotherapy, and targeted therapies, the overall efficacies of the present therapies are still limited, with the 5‐year survival rate at approximately 17.4% in NSCLC [50] accounting for approximately 80%–85% of lung cancer cases [49]. Therefore, there is still an urgent need for more clinical approaches with less toxicity and improved efficacy. Several ICIs targeting PD‐1 or PD‐L1 have been approved by the FDA for the clinical treatment of advanced NSCLC, demonstrating notable efficacy in clinical practice.
PD‐1/PD‐L1 Blockade as Second‐Line Treatment in Advanced NSCLC
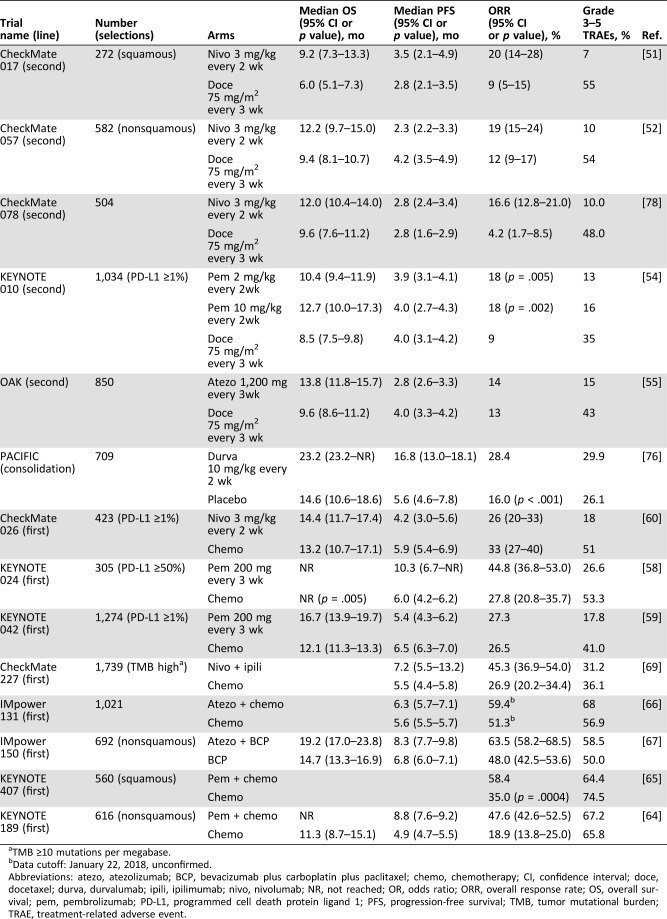
Nivolumab, a human anti‐PD‐1 monoclonal antibody, was the first PD‐1/PD‐L blockade agent for second‐line treatment of advanced NSCLC. A series of international, open‐label, randomized phase III trials have been undertaken [51]. The results from the CheckMate 017 clinical trial demonstrated that nivolumab significantly improved the overall survival (OS), the overall response rate (ORR), and progression‐free survival (PFS) with acceptable safety profiles such as treatment‐related adverse events (TRAEs) and mortality in patients with previously treated advanced squamous NSCLC in comparison with docetaxel (Table 2) [51]. In nonsquamous NSCLC, although the median PFS did not favor nivolumab over docetaxel (2.3 months for nivolumab vs. 4.2 months for docetaxel), the OS and ORR were significantly improved for the patients treated with nivolumab according to the results from the CheckMate 057 trial [52]. Nivolumab thus became the first ICI targeting PD‐1/PD‐L1 approved by the FDA for metastatic NSCLC therapy in 2015 [9]. Importantly, a pooled analysis of the two studies revealed better 2‐year OS rates (23% for nivolumab vs. 8% for docetaxel in squamous NSCLC and 29% with nivolumab vs. 16% with docetaxel in nonsquamous NSCLC), indicating that nivolumab provides a long‐term clinical benefit and a favorable tolerability profile compared with docetaxel in advanced NSCLC [53]. Likewise, the results of the phase II/III clinical trial study KEYNOTE 010 demonstrated that compared with docetaxel, pembrolizumab, another anti‐PD‐1 antibody, significantly improved the OS, PFS, and ORR of the patients with advanced NSCLC who had PD‐L1 expression on ≥50% of tumor cells (Table 2) [54]. Furthermore, ICIs targeting PD‐L1 also exhibit impressive benefits in the treatment of advanced NSCLC. Atezolizumab, a humanized anti‐PD‐L1 monoclonal antibody, significantly prolonged the OS of previously treated patients with advanced NSCLC with a favorable safety profile when compared with docetaxel treatment in both phase II (POPLAR) and phase III (OAK) clinical trials (Table 2) [55], [56].
Table 2. Overview of phase III trials of PD‐1/PD‐L1 blockade therapy in advanced non‐small‐cell lung cancer as of August 2018.
TMB ≥10 mutations per megabase.
Data cutoff: January 22, 2018, unconfirmed.
Abbreviations: atezo, atezolizumab; BCP, bevacizumab plus carboplatin plus paclitaxel; chemo, chemotherapy; CI, confidence interval; doce, docetaxel; durva, durvalumab; ipili, ipilimumab; nivo, nivolumab; NR, not reached; OR, odds ratio; ORR, overall response rate; OS, overall survival; pem, pembrolizumab; PD‐L1, programmed cell death protein ligand 1; PFS, progression‐free survival; TMB, tumor mutational burden; TRAE, treatment‐related adverse event.
Collectively, these results demonstrate that ICIs targeting PD‐1 and PD‐L1 significantly improve clinical efficacy in patients with advanced NSCLC with a favorable safety profile compared with chemotherapy [57], making anti‐PD‐1 and PD‐L1 therapeutic antibodies a new option as second‐line treatment in patients with advanced NSCLC.
PD‐1/PD‐L1 Blockade as First‐Line Treatment in Advanced NSCLC
Based on the promising results of second‐line treatment, the clinical efficacy of PD‐1/PD‐L1 blockade as first‐line treatment for advanced NSCLC has been investigated. Results from the KEYNOTE 024 phase III trial demonstrated that compared with chemotherapy, the OS, PFS, and ORR were significantly improved by pembrolizumab in patients with PD‐L1 expression on ≥50% of tumor cells (Table 2) [58]. In 2016, these data led the FDA to permit pembrolizumab as a single agent for first‐line treatment of patients with metastatic NSCLC [16]. Recently, results from the KEYNOTE 042 phase III trial showed that the OS of patients treated with pembrolizumab was significantly improved even in patients with PD‐L1 expression on ≥1% of tumor cells (Table 2) [59], indicating that more patients might benefit from pembrolizumab treatment. However, despite the favorable safety profile, nivolumab treatment exhibited no significant effects on improving the OS (median, 14.4 months for nivolumab vs. 13.2 months for chemotherapy) or PFS (median, 4.2 months for nivolumab vs. 5.9 months for chemotherapy) in previously untreated patients at stage IV or recurrent patients with PD‐L1 expression on at least 5% of tumor cells [60]. Even in patients with high PD‐L1 positivity (PD‐L1 expression on ≥50% of tumor cells), no difference was demonstrated for nivolumab treatment compared with chemotherapy [60]. A retrospective analysis of the tumor mutational burden (TMB) in these studies showed that patients with a high TMB (≥243 missense mutations) had a higher ORR (47% vs. 28%) and longer PFS (median, 9.7 vs. 5.8 months) with nivolumab treatment. However, the OS was similar between two groups regardless of the TMB level [60]. The differences in patient characteristics, such as PD‐L1 positivity, gender ratio, or TMB, might contribute to the conflicting results for the different efficacy of PD‐1 blockade treatments [60]. The application of nivolumab as first‐line treatment for advanced NSCLC warrants more supporting data before moving into clinical practice, even in combination therapy approaches. Similar to pembrolizumab, atezolizumab also achieved a high ORR (19%) with good tolerability in patients with advanced NSCLC in a phase II trial (BIRCH) [61]. Consequently, the efficacy of atezolizumab as first‐line treatment in NSCLC will be tested in a phase III trial (IMpower110) through recruiting more patients.
Although ICIs targeting PD‐1 and PD‐L1 have been demonstrated with impressive benefits for advanced NSCLC in first‐line treatment trials, there still exists a certain population of patients who do not respond to the therapy. To increase the response rate, combinations of PD‐1 or PD‐L1 blockade with other treatments, such as chemotherapy, targeted therapies, and other ICIs, have been investigated and show impressive improvements in first‐line treatments. For instance, in the KEYNOTE 021 phase II study, the ORR for chemotherapy plus pembrolizumab (55%) was significantly higher than that for chemotherapy alone (29%), even in patients with PD‐L1 expression levels less than 1% (57% vs. 13%) [62]. The incidence of grade 3 or 4 TRAEs was 39% in combination therapy and 26% in chemotherapy, indicating a tolerated safety profile for combination therapy [62]. Considering the promising efficacy and safety profiles of combination therapy, pembrolizumab plus chemotherapy is under an accelerated approval process by the FDA as first‐line treatment for patients with metastatic nonsquamous NSCLC [63]. In the following phase III studies, pembrolizumab plus chemotherapy showed a higher response rate in both nonsquamous and squamous NSCLC (Table 2) [64], [65]. In addition, atezolizumab combined with chemotherapy got a longer median PFS (6.3 months vs. 5.6 months) compared with chemotherapy alone in the phase III trial IMpower 131 (Table 2) [66]. Combined with bevacizumab (a VEGF inhibitor) and chemotherapy, atezolizumab significantly improved PFS, OS, and ORR among patients with metastatic nonsquamous NSCLC, regardless of PD‐L1 expression and EGFR or ALK mutations (Table 2) [67]. In addition, the combination of ICIs targeting two or more immune checkpoint molecules also leads to a synergetic response in clinical practice. In the CheckMate 227 phase III study, the combination of nivolumab and the anti‐CTLA‐4 antibody ipilimumab, which regulate immune responses at different stages and through different mechanisms [68], demonstrated a longer PFS, higher ORR, and comparable adverse events compared with chemotherapy in advanced NSCLC with a high TMB (Table 2) [69]. In a phase Ib trial, the combination of durvalumab (an anti‐PD‐L1 blocking antibody) and the anti‐CTLA‐4 antibody tremelimumab has been used in a dose‐escalation manner among 102 patients with advanced or metastatic NSCLC [70]. The ORR was 23% in durvalumab (10–20 mg/kg every 2 weeks or 4 weeks) treatment combined with tremelimumab (1 mg/kg) regardless of PD‐L1 expression [70]. However, 36% of the 102 patients had TRAEs, and 28% discontinued treatment because of severe TRAEs, among which three deaths were related to the treatment [70]. The assessment of the safety and clinical activity of the combination of durvalumab and tremelimumab versus platinum‐based chemotherapy is still ongoing [71], [72].
PD‐1/PD‐L1 Blockade as Third‐Line Treatment in Advanced NSCLC
The BIRCH phase II trial investigated the clinical benefits of atezolizumab in first‐line, second‐line, and third‐line treatments simultaneously in 667 patients with preselected advanced NSCLC [61]. The median OS was 23.5, 15.5, and 13.2 months in the first‐line, second‐line, and third‐line cohorts, respectively [61]. The median PFS and ORR was comparable between the third‐line (2.8 months, 18%) and second‐line (2.8 months, 19%) cohorts [61]. These results suggested the efficacy of atezolizumab in the third‐line treatment for patients with advanced NSCLC. In another phase II trial (ATLANTIC), the ORR of durvalumab in patients with NSCLC with EGFR and ALK positivity and ≥25% of tumor cells expressing PD‐L1 was 12.2%, which was lower than those with EGFR and ALK negativity (16.4%) or those with ≥90% PD‐L1 expression of tumor cells (30.9%) [73]. Because the clinical trials in this stage are limited, the efficacy of PD‐1/PD‐L1 blockade therapy might be warranted with more phase III trials.
PD‐1/PD‐L1 Blockade as Neoadjuvant and Consolidation Treatment in Advanced NSCLC
In patients with untreated and resectable early (stage I, II, or IIIA) NSCLC, nivolumab treatment exhibited few side effects (TRAE rate of any grade was 23%; of grade 3 or higher was 4.5%) and induced a major pathological response in 45% of resected tumors, demonstrating a good safety and feasibility of neoadjuvant role in early‐stage NSCLC [74]. In advanced NSCLC, platinum‐based doublet chemotherapy concurrent with radiotherapy is the standardized cure strategy. However, its efficacy is still poor [75]. PACIFIC is a phase III trial to compare the efficacy of durvalumab as consolidation treatment and placebo in patients with stage III NSCLC who did not had disease progression after two or more cycles of platinum‐based chemoradiotherapy. The results indicated that the median OS (durvalumab, 23.2 months vs. placebo, 14.6 months), PFS (durvalumab, 16.8 months vs. placebo, 5.6 months), and ORR (durvalumab, 28.4% vs. placebo, 16.0%) were significantly improved in patients receiving durvalumab treatment [76]. Grade 3 or 4 adverse events occurred in 29.9% of patients treated with durvalumab versus 26.1% with placebo, indicating a safety profile for durvalumab (Table 2) [76]. The result suggests that durvalumab may become an effective adjuvant therapy in patients with stage III NSCLC after standard treatment. In addition, anti‐PD‐1/PD‐L1 therapies are also under investigation in the neoadjuvant and consolidation settings for stage III NSCLC, which may provide treatment options in the management of stage III NSCLC [77].
PD‐1/PD‐L1 Blockade Therapy for Advanced NSCLC in China
Lung cancer is the most common cancer and the leading cause of cancer death in China [48] with a great need to improve clinical efficacy. Registered clinical trials of PD‐1/PD‐L1 blockade inhibitors against advanced NSCLC have been initiated since December 2015. CheckMate 078 is a randomized, open‐label, and multinational phase III trial for nivolumab treatment in patients with advanced or metastatic NSCLC for whom platinum‐based doublet chemotherapy has failed. This is the first Chinese study on PD‐1/PD‐L1 inhibitors in NSCLC. Results of CheckMate 078 showed that nivolumab significantly improved OS, ORR, and safety profiles in patients with advanced NSCLC without EGFR or ALK mutations in comparison with docetaxel (Table 2) [78]. The efficacy of nivolumab in CheckMate 078 is comparable to that in CheckMate 017 and CheckMate 057, indicating that Chinese patients with NSCLC benefit from nivolumab treatment similarly. Accordingly, nivolumab was approved by the Chinese Food and Drug Administration (CFDA) on June 15, 2018, as second‐line treatment for advanced NSCLC without EGFR or ALK mutations and became the first commercialized ICI in China on August 28, 2018.
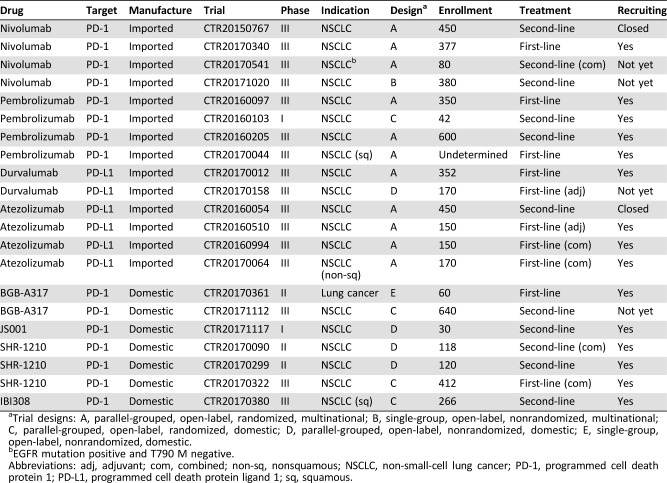
At present, several clinical trials of PD‐1/PD‐L1 inhibitors have been carried out to investigate the clinical efficacy in China [79]. Based on the Chinese drug trial registration website (www://chinadrugtrials.org.cn), 14 clinical trials concerning four FDA‐approved drugs, nivolumab, pembrolizumab, durvalumab, and atezolizumab, are ongoing in China as of October 2017, particularly for patients with advanced NSCLC (Table 3). These include eight trials for first‐line treatment (CTR20170340 for nivolumab, CTR20170044 and CTR20160097 for pembrolizumab, CTR20170012 and CTR20170158 for durvalumab, CTR20160510, CTR20160994, and CTR20170064 for atezolizumab) and six for second‐line treatment (CTR20150767, CTR20170541, and CTR20171020 for nivolumab, CTR20160103 and CTR20160205 for pembrolizumab, CTR20160054 for atezolizumab; Table 3). Combination therapy is designed in 3 of 14 trials (nivolumab plus ipilimumab or chemotherapy in CTR20170541, atezolizumab plus chemotherapy in CTR20170064 and CTR20160994). Thirteen of 14 clinical trials are phase III trials, among which two trials have finished recruitment, three trials have no recruitment, and eight trials are currently recruiting patients in China (as of October 2017; Table 3).
Table 3. Summary of the registered clinical trials of PD‐1/PD‐L1 blockade therapy for lung cancer in China as of October 2017.
Trial designs: A, parallel‐grouped, open‐label, randomized, multinational; B, single‐group, open‐label, nonrandomized, multinational; C, parallel‐grouped, open‐label, randomized, domestic; D, parallel‐grouped, open‐label, nonrandomized, domestic; E, single‐group, open‐label, nonrandomized, domestic.
EGFR mutation positive and T790 M negative.
Abbreviations: adj, adjuvant; com, combined; non‐sq, nonsquamous; NSCLC, non‐small‐cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death protein ligand 1; sq, squamous.
The apparent clinical benefits of ICIs in cancer therapy have drawn great attention from Chinese pharmaceutical companies. Enormous efforts have been made to develop ICIs domestically. As indicated in the Chinese drug trial registration database, eight anti‐PD‐1/PD‐L1 antibodies (BGB‐A317, JS001, SHR‐1210, IBI308, GB226, and GLS‐010 targeting PD‐1; KN035 and CS1001 targeting PD‐L1) developed by Chinese pharmaceutical companies have been approved by the CFDA for clinical trials up to October 2017. Four of these antibodies (BGB‐A317 from BeiGene, Beijing, China; JS001 from Junshi Biosciences, Shanghai, China; SHR‐1210 from Hengrui Medicine, Shanghai, China; IBI308 from Innovent Biologics, Jiangsu, China) are undergoing efficacy evaluation in seven clinical trials for patients with advanced NSCLC in particular, including two trials as first‐line treatment (CTR20170322 for SHR‐1210, CTR20170361 for BGB‐A317) and five trials as second‐line treatment (Table 3). SHR‐1210 plus chemotherapy is designed as combination therapy in CTR20170322 for first‐line treatment and in CTR20170090 for second‐line treatment (Table 3). Among seven clinical trials, three are phase III studies (CTR20170380 for IBI308, CTR20170322 for SHR‐1210, and CTR20171112 for BGB‐A317). The trials CTR20170380 and CTR20170322 are currently recruiting patients in China.
Challenges for PD‐1/PD‐L1 Blockade Therapy in Advanced NSCLC
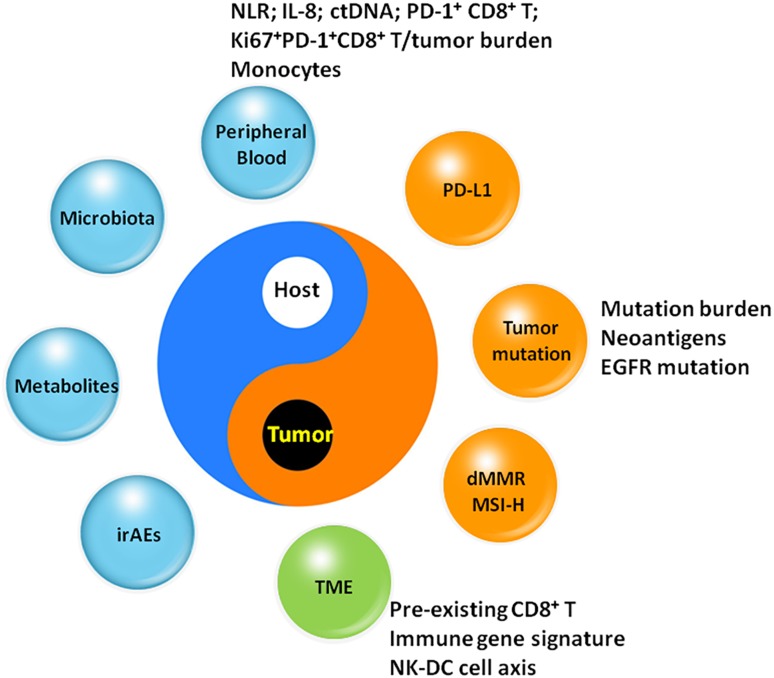
ICI therapy using antibodies targeting PD‐1 or PD‐L1 has demonstrated profound clinical efficacy for advanced NSCLC. However, the clinical applications of these antibodies are still limited because of certain unsolved challenges. First, there are few predictive biomarkers to identify patients who can benefit from ICI therapy. Tissue‐based PD‐L1 expression is the first criterion for the prediction of ICI treatment. The expression of PD‐L1 on tumor cells has been demonstrated to be associated with the efficacy of PD‐1/PD‐L1 blockade therapy in NSCLC [54], [80], [81]. Nevertheless, the association is quite variable among different ICIs. For example, the improvement of the OS by nivolumab for squamous NSCLC and by durvalumab for NSCLC occurs regardless of PD‐L1 expression [52], [76]. The expression levels of PD‐L1 are heterogeneous and dynamic in immunohistochemistry assays with different detecting antibodies, as well as various scoring cutoffs, complicating the interpretation of the results [82], [83], [84]. Panels have been investigated to explore predictive biomarkers (Fig. 1), including nonsynonymous mutation burden and neoantigen prediction [85], [86], [87], [88], [89], defects of mismatch repair genes or high microsatellite instability [90], [91], microbiota [92], [93], and metabolic profiles [94]. However, these predictive biomarkers must be validated in more clinical samples. Importantly, the predictive models integrating multiplex factors show potential in predicting the clinical responses of ICI therapy [89], [95]. Considering the multifactorial properties of cancer‐immune cross‐talk [96], the determination of theoretical predictive models based on comprehensive biomarkers might be more feasible in future applications.
Figure 1.
Biomarkers associated with the efficacy of PD‐1/PD‐L1 blockade therapy.
Abbreviations and sources: ctDNA, circulating tumor DNA [116]; dMMR, mismatch repair deficient; IL‐8, interleukin‐8 [117]; irAE, immune‐related adverse event [118]; Ki67+PD‐1+CD8+T/tumor burden [119]; monocytes, CD14+CD16−HLA‐DRhi monocytes [105]; MSI‐H, microsatellite instability‐high; NK‐DC axis, natural killer‐dendritic cell axis [46]; NLR, neutrophil‐to‐lymphocyte ratio [120]; PD‐1, programmed cell death protein 1; PD‐1+CD8+T [121]; PD‐L1, programmed cell death ligand 1; TME, tumor microenvironment [56], [122].
The second challenge is the occurrence of TRAEs. The incidence of grade 3 or 4 TRAEs ranges from 7% to 29.9% in anti‐PD‐1/PD‐L1 monotherapy for advanced NSCLC, whereas the percentage ranges from 26.1% to 55% in the chemotherapy group, displaying the promising safety profiles of ICI treatments (Table 2). However, the TRAE incidence is much higher in combination therapy (Table 2). For example, 68% of patients receiving atezolizumab plus chemotherapy treatment developed TRAEs, compared with 56.9% in the chemotherapy‐alone group [66]. In the pembrolizumab‐plus‐chemotherapy treatment group, 67.2% of the patients developed TRAEs, which is comparable to chemotherapy [64]. In the combination of durvalumab and tremelimumab, 36% of the patients developed TRAEs, resulting in the discontinuation of the treatment in 28% of the patients and three deaths [70]. So TRAEs are still a great challenge for PD‐1/PD‐L1 blockade therapy, and efforts should be made to decrease and predict severe TRAEs in advance in the future.
The third challenge lies in how to choose optimal combination therapy for advanced NSCLC. Combination therapy of diverse ICIs has been demonstrated to be of great potential in increasing the response rates (Table 2) [69]. Apart from chemotherapy, inhibitors targeting IDO [97], VEGF [98], CTLA‐4 [70], [99], lymphocyte activating 3 [100], [101], or T‐cell immunoglobulin mucin 3 [102], [103] may provide more options for combination strategies in the future. Hence, with the improvement in combination therapy, establishing guiding principles to identify the optimal combination strategy for advanced NSCLC from these combination approaches will greatly extend the clinical applications of anti‐PD‐1/PD‐L1 antibodies in the future.
Conclusion
In recent decades, the conceptual dissection of immunooncology has highlighted the important roles of immune checkpoints in the regulation of T‐cell immunity. This understanding, in turn, has facilitated the development of ICIs for clinical applications against cancers. With the approval of ICIs as both second‐line and first‐line treatments in advanced NSCLC, PD‐1/PD‐L1‐based immune checkpoint therapy has provided more options for the treatment of advanced NSCLC. However, considering the fact that the response rates of these ICIs range from 14% to 20% in unselected patients, it is crucial to identify predictive biomarkers for the selection of patients who are likely to benefit from the ICI treatments. The limited size of the population with clinical benefit also raises an interest in exploring the mechanism involved, especially concerning the immunological properties of the TME. In fact, a concept of “hot” and “cold” TME has been introduced with different levels of immune checkpoint expression in different local regions [104]. The immunological outcomes related to immune checkpoint expression in the TME are still difficult to investigate, although diverse treatment efficacy has been observed [83], [84]. Therefore, new techniques, such as single‐cell sequencing and multiplex immunological imaging in the TME, can be adapted to better understand the complexity and dynamics of the local immune status [105], [106]. This might in turn guide the selection of patients for precise therapy by ICIs.
In addition, combination therapy of ICIs with conventional treatments must be optimized on a case‐by‐case principle. Combined with conventional chemotherapy or radiotherapy, ICI treatment might exhibit synergized clinical efficacy because of the enhanced cytotoxicity of T cells. This can be mediated by antigen release and presentation under a lower tumor burden. In addition, combination therapy of ICIs with other immunological activators such as TLR agonists [107] or supporting nutrition [108] are worthy of exploration when considering the complexity of antitumor immunity. Therefore, enriching the reservoirs of ICIs and defining the immune properties of patients with cancer will help realize the individualized treatment in advanced NSCLC.
In China, the clinical applications of ICI therapy started very recently and remain at an early stage. Clinical trials are ongoing to evaluate the effects of anti‐PD‐1/PD‐L1 antibodies in Chinese patients. Importantly, genetic, biochemical, and microbiota‐associated characteristics of Chinese populations must be considered in the evaluation of clinical efficacy. For instance, Chinese patients with NSCLC have high EGFR mutation rates (50% in the Chinese population vs. 20% in Western populations) [109], [110], [111]. EGFR mutations were previously considered to be associated with low response rates in anti‐PD‐1/PD‐L1 treatments [57], [112], [113]. The Chinese population also harbors different microbiota and microbiomes [114], as well as different biochemical and metabolic profiles [115], which may affect the efficacy of ICI treatments and biomarker profiles for the prediction and prognosis of the diseases. Therefore, together with the ongoing clinical trials, more retrospective or prospective investigations need to be carried out for the validation of treatment efficacy and exploration of biomarker determination, which will finally lead to durable control of the disease.
Acknowledgments
This work was supported by a grant from the National Key R&D Program of China (2016YFC1303300), grants from the National Natural Science Foundation of China (81602573 and 81472875), and a grant from the China Postdoctoral Science Foundation (2017M611582). Editorial service was provided by Wiley Editing Services.
Contributed equally
Author Contributions
Conception/design: Liliang Xia, Yuanyong Liu, Ying Wang
Manuscript writing: Liliang Xia, Yuanyong Liu, Ying Wang
Final approval of manuscript: Liliang Xia, Yuanyong Liu, Ying Wang
Disclosures
The authors indicated no financial relationships.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brower V. Checkpoint blockade immunotherapy for cancer comes of age. J Natl Cancer Inst 2015;107:djv069. [DOI] [PubMed] [Google Scholar]
- 3.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: What's here, what's next? Curr Opin Immunol 2015;33:23–35. [DOI] [PubMed] [Google Scholar]
- 4.Brunet JF, Denizot F, Luciani MF et al. A new member of the immunoglobulin superfamily‐‐CTLA‐4. Nature 1987;328:267–270. [DOI] [PubMed] [Google Scholar]
- 5.Barber DL, Wherry EJ, Masopust D et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–687. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Song Y, Liu D. Recent development in clinical applications of PD‐1 and PD‐L1 antibodies for cancer immunotherapy. J Hematol Oncol 2017;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs 2010;11:1354–1359. [PubMed] [Google Scholar]
- 8.Bhatnagar V, Gormley NJ, Luo L et al. FDA approval summary: Daratumumab for treatment of multiple myeloma after one prior therapy. The Oncologist 2017;22:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazandjian D, Suzman DL, Blumenthal G et al. FDA approval summary: Nivolumab for the treatment of metastatic non‐small cell lung cancer with progression on or after platinum‐based chemotherapy. The Oncologist 2016;21:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkins E, Blumenthal GM, Yuan W et al. FDA approval summary: Pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum‐containing chemotherapy. The Oncologist 2017;22:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasamon YL, de Claro RA, Wang Y et al. FDA approval summary: Nivolumab for the treatment of relapsed or progressive classical hodgkin lymphoma. The Oncologist 2017;22:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning YM, Suzman D, Maher VE et al. FDA approval summary: Atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum‐containing chemotherapy. The Oncologist 2017;22:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ES. Avelumab: First global approval. Drugs 2017;77:929–937. [DOI] [PubMed] [Google Scholar]
- 14.Xu JX, Maher VE, Zhang L et al. FDA approval summary: Nivolumab in advanced renal cell carcinoma after anti‐angiogenic therapy and exploratory predictive biomarker analysis. The Oncologist 2017;22:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sclafani F. PD‐1 inhibition in metastatic dMMR/MSI‐H colorectal cancer. Lancet Oncol 2017;18:1141–1142. [DOI] [PubMed] [Google Scholar]
- 16.Pai‐Scherf L, Blumenthal GM, Li H et al. FDA approval summary: Pembrolizumab for treatment of metastatic non‐small cell lung cancer: First‐line therapy and beyond. The Oncologist 2017;22:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sul J, Blumenthal GM, Jiang X et al. FDA approval summary: Pembrolizumab for the treatment of patients with metastatic non‐small cell lung cancer whose tumors express programmed death‐ligand 1. The Oncologist 2016;21:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the streptococcus erysipelas and the bacillus prodigiosus). Proc R Soc Med 1910;3:1–48. [PMC free article] [PubMed] [Google Scholar]
- 19.Dillman RO. The clinical experience with interleukin‐2 in cancer therapy. Cancer Biother 1994;9:183–209. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Lotze MT, Yang JC et al. Prospective randomized trial of high‐dose interleukin‐2 alone or in conjunction with lymphokine‐activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 1993;85:622–632. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yannelli JR, Yang JC et al. Treatment of patients with metastatic melanoma with autologous tumor‐infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 1994;86:1159–1166. [DOI] [PubMed] [Google Scholar]
- 22.Cheever MA, Higano CS. PROVENGE (sipuleucel‐T) in prostate cancer: The first FDA‐approved therapeutic cancer vaccine. Clin Cancer Res 2011;17:3520–3526. [DOI] [PubMed] [Google Scholar]
- 23.Couzin‐Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432–1433. [DOI] [PubMed] [Google Scholar]
- 24.Krummel MF, Allison JP. CD28 and CTLA‐4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummel MF, Allison JP. CTLA‐4 engagement inhibits IL‐2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 1996;183:2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linsley PS, Greene JL, Tan P et al. Coexpression and functional cooperation of CTLA‐4 and CD28 on activated T lymphocytes. J Exp Med 1992;176:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA‐4 blockade. Science 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 28.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traynor K. Ipilimumab approved for metastatic melanoma. Am J Health System Pharm 2011;68:768. [DOI] [PubMed] [Google Scholar]
- 30.Ishida Y, Agata Y, Shibahara K et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong H, Zhu G, Tamada K et al. B7‐H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 32.Brahmer JR, Tykodi SS, Chow LQ et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keir ME, Butte MJ, Freeman GJ et al. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui E, Cheung J, Zhu J et al. T cell costimulatory receptor CD28 is a primary target for PD‐1‐mediated inhibition. Science 2017;355:1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamphorst AO, Wieland A, Nasti T et al. Rescue of exhausted CD8 t cells by PD‐1‐targeted therapies is CD28‐dependent. Science 2017;355:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokosuka T, Takamatsu M, Kobayashi‐Imanishi W et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012;209:1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman GJ, Long AJ, Iwai Y et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki T, Honjo T. PD‐1 and pD‐1 ligands: From discovery to clinical application. Int Immunol 2007;19:813–824. [DOI] [PubMed] [Google Scholar]
- 39.Dong H, Strome SE, Salomao DR et al. Tumor‐associated B7‐H1 promotes t‐cell apoptosis: A potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 40.Spranger S, Spaapen RM, Zha Y et al. Up‐regulation of PD‐L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Ahn E, Kissick HT et al. Reinvigorating exhausted T cells by blockade of the PD‐1 pathway. For Immunopathol Dis Therap 2015;6:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei T, Zhang J, Qin Y et al. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non‐small‐cell lung cancer patients. Am J Cancer Res 2015;5:2190–2201. [PMC free article] [PubMed] [Google Scholar]
- 43.van de Ven R, Niemeijer AN, Stam AGM et al. High PD‐1 expression on regulatory and effector T‐cells in lung cancer draining lymph nodes. ERJ Open Res 2017;3:0110‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overacre‐Delgoffe AE, Chikina M, Dadey RE et al. Interferon‐γ drives Treg fragility to promote anti‐tumor immunity. Cell 2017;169:1130–1141.e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon SR, Maute RL, Dulken BW et al. PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barry KC, Hsu J, Broz ML et al. A natural killer‐dendritic cell axis defines checkpoint therapy‐responsive tumor microenvironments. Nat Med 2018;24:1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 49.Siegel R, Ma J, Zou Z et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 50.Khanna P, Blais N, Gaudreau PO et al. Immunotherapy comes of age in lung cancer. Clin Lung Cancer 2017;18:13–22. [DOI] [PubMed] [Google Scholar]
- 51.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horn L, Spigel DR, Vokes EE et al. Nivolumab versus docetaxel in previously treated patients with advanced non‐small‐cell lung cancer: Two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 55.Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 57.Ramos‐Esquivel A, van der Laat A, Rojas‐Vigott R et al. Anti‐PD‐1/anti‐PD‐L1 immunotherapy versus docetaxel for previously treated advanced non‐small cell lung cancer: A systematic review and meta‐analysis of randomised clinical trials. ESMO Open 2017;2:e000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reck M, Rodriguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 59.Lopes G, Wu YL, Kudaba I et al. Pembrolizumab (pembro) versus platinum‐based chemotherapy (chemo) as first‐line therapy for advanced/metastatic NSCLC with a PD‐L1 tumor proportion score (TPS) ≥1%: Open‐label, phase 3 KEYNOTE‐042 study. J Clin Oncol 2018; 36(suppl 18):LBA4. [Google Scholar]
- 60.Carbone DP, Reck M, Paz‐Ares L et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017;376:2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters S, Gettinger S, Johnson ML et al. Phase II trial of atezolizumab as first‐line or subsequent therapy for patients with programmed death‐ligand 1‐selected advanced non‐small‐cell lung cancer (BIRCH). J Clin Oncol 2017;35:2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langer CJ, Gadgeel SM, Borghaei H et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: A randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol 2016;17:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reck M. Pembrolizumab as first‐line therapy for metastatic non‐small‐cell lung cancer. Immunotherapy 2018;10:93–105. [DOI] [PubMed] [Google Scholar]
- 64.Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 65.Paz‐Ares LG, Luft A, Tafreshi A et al. Phase 3 study of carboplatin‐paclitaxel/nab‐paclitaxel (chemo) with or without pembrolizumab (pembro) for patients (pts) with metastatic squamous (sq) non‐small cell lung cancer (NSCLC). J Clin Oncol 2018;36(suppl 15):105A. [Google Scholar]
- 66.Jotte RM, Cappuzzo F, Vynnychenko I et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab‐paclitaxel vs carboplatin + nab‐paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36(suppl 18):LBA9000. [Google Scholar]
- 67.Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 68.Wei SC, Levine JH, Cogdill AP et al. Distinct cellular mechanisms underlie anti‐CTLA‐4 and anti‐PD‐1 checkpoint blockade. Cell 2017;170:1120–1133.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonia S, Goldberg SB, Balmanoukian A et al. Safety and antitumour activity of durvalumab plus tremelimumab in non‐small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol 2016;17:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mok T, Schmid P, Aren O et al. 192TiP: NEPTUNE: A global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy versus standard of care (SOC) platinum‐based chemotherapy in the first‐line treatment of patients (pts) with advanced or metastatic NSCLC. J Thorac Oncol 2016;11:S140–S141. [Google Scholar]
- 72.Planchard D, Yokoi T, McCleod MJ et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: Rationale and protocol design of the ARCTIC study. Clin Lung Cancer 2016;17:232–236.e231. [DOI] [PubMed] [Google Scholar]
- 73.Garassino MC, Cho BC, Kim JH et al. Durvalumab as third‐line or later treatment for advanced non‐small‐cell lung cancer (ATLANTIC): An open‐label, single‐arm, phase 2 study. Lancet Oncol 2018;19:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forde PM, Chaft JE, Smith KN et al. Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn JS, Ahn YC, Kim JH et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non‐small‐cell lung cancer: KCSG‐LU05‐04. J Clin Oncol 2015;33:2660–2666. [DOI] [PubMed] [Google Scholar]
- 76.Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 77.Yeh J, Marrone KA, Forde PM. Neoadjuvant and consolidation immuno‐oncology therapy in stage III non‐small cell lung cancer. J Thorac Dis 2018;10:S451–S459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu YL, Lu S, Cheng Y et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non‐small cell lung cancer (NSCLC): Results of the phase 3 CheckMate 078 study. Cancer Res 2018;78(suppl 13):CT114A. [Google Scholar]
- 79.Liu SY, Wu YL. Ongoing clinical trials of PD‐1 and PD‐L1 inhibitors for lung cancer in China. J Hematol Oncol 2017;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gandini S, Massi D, Mandala M. PD‐L1 expression in cancer patients receiving anti PD‐1/PD‐L1 antibodies: A systematic review and meta‐analysis. Crit Rev Oncol Hematol 2016;100:88–98. [DOI] [PubMed] [Google Scholar]
- 81.Aguiar PN Jr, De Mello RA, Hall P et al. PD‐L1 expression as a predictive biomarker in advanced non‐small‐cell lung cancer: Updated survival data. Immunotherapy 2017;9:499–506. [DOI] [PubMed] [Google Scholar]
- 82.Scognamiglio G, De Chiara A, Di Bonito M et al. Variability in immunohistochemical detection of programmed death ligand 1 (PD‐L1) in cancer tissue types. Int J Mol Sci 2016;17:E790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinato DJ, Shiner RJ, White SD et al. Intra‐tumoral heterogeneity in the expression of programmed‐death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology 2016;5:e1213934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hofman P. PD‐L1 immunohistochemistry for non‐small cell lung carcinoma: Which strategy should be adopted? Expert Rev Mol Diagn 2017;17:1097–1108. [DOI] [PubMed] [Google Scholar]
- 85.Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGranahan N, Furness AJ, Rosenthal R et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roszik J, Haydu LE, Hess KR et al. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC Med 2016;14:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luksza M, Riaz N, Makarov V et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 2017;551:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aguiar PN Jr, Tadokoro H, Forones NM et al. MMR deficiency may lead to a high immunogenicity and then an improvement in anti‐PD‐1 efficacy for metastatic colorectal cancer. Immunotherapy 2015;7:1133–1134. [DOI] [PubMed] [Google Scholar]
- 92.Gopalakrishnan V, Spencer CN, Nezi L et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science 2017;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Routy B, Le Chatelier E, Derosa L et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science 2018;359:91–97. [DOI] [PubMed] [Google Scholar]
- 94.Frankel AE, Coughlin LA, Kim J et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017;19:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balachandran VP, Luksza M, Zhao JN et al. Identification of unique neoantigen qualities in long‐term survivors of pancreatic cancer. Nature 2017;551:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blank CU, Haanen JB, Ribas A et al. Cancer immunology. The “cancer immunogram.” Science 2016;352:658–660. [DOI] [PubMed] [Google Scholar]
- 97.Epacadostat shows value in two SCCHN trials. Cancer Discov 2017;7:OF2. [DOI] [PubMed] [Google Scholar]
- 98.Einstein DJ, McDermott DF. Combined blockade of vascular endothelial growth factor and programmed death 1 pathways in advanced kidney cancer. Clin Adv Hematol Oncol 2017;15:478–488. [PubMed] [Google Scholar]
- 99.Hellmann MD, Rizvi NA, Goldman JW et al. Nivolumab plus ipilimumab as first‐line treatment for advanced non‐small‐cell lung cancer (CheckMate 012): Results of an open‐label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Y, Yu H, Rozeboom L et al. LAG‐3 protein expression in non‐small cell lung cancer and its relationship with PD‐1/PD‐L1 and tumor‐infiltrating lymphocytes. J Thorac Oncol 2017;12:814–823. [DOI] [PubMed] [Google Scholar]
- 101.Andrews LP, Marciscano AE, Drake CG et al. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017;276:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu JF, Ma SR, Mao L et al. T‐cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol Oncol 2017;11:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sakuishi K, Apetoh L, Sullivan JM et al. Targeting TIM‐3 and PD‐1 pathways to reverse T cell exhaustion and restore anti‐tumor immunity. J Exp Med 2010;207:2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teng MW, Ngiow SF, Ribas A et al. Classifying cancers based on T‐cell infiltration and PD‐L1. Cancer Res 2015;75:2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krieg C, Nowicka M, Guglietta S et al. High‐dimensional single‐cell analysis predicts response to anti‐PD‐1 immunotherapy. Nat Med 2018;24:144–153. [DOI] [PubMed] [Google Scholar]
- 106.Thommen DS, Koelzer VH, Herzig P et al. A transcriptionally and functionally distinct PD‐1+ CD8+ T cell pool with predictive potential in non‐small‐cell lung cancer treated with PD‐1 blockade. Nat Med 2018;24:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sato‐Kaneko F, Yao S, Ahmadi A et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017;2:93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Triplett TA, Garrison KC, Marshall N et al. Reversal of indoleamine 2,3‐dioxygenase‐mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol 2018;36:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosell R, Moran T, Queralt C et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–967. [DOI] [PubMed] [Google Scholar]
- 110.D'Angelo SP, Pietanza MC, Johnson ML et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi Y, Au JS, Thongprasert S et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nishio M, Hida T, Atagi S et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non‐squamous non‐small cell lung cancer. ESMO Open 2016;1:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gainor JF, Shaw AT, Sequist LV et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer: A retrospective analysis. Clin Cancer Res 2016;22:4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence‐specific variations in human microbiome composition and diversity. Front Microbiol 2017;8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wulan SN, Westerterp KR, Plasqui G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas 2010;65:315–319. [DOI] [PubMed] [Google Scholar]
- 116.Cabel L, Riva F, Servois V et al. Circulating tumor DNA changes for early monitoring of anti‐PD1 immunotherapy: A proof‐of‐concept study. Ann Oncol 2017;28:1996–2001. [DOI] [PubMed] [Google Scholar]
- 117.Sanmamed MF, Perez‐Gracia JL, Schalper KA et al. Changes in serum interleukin‐8 (IL‐8) levels reflect and predict response to anti‐PD‐1 treatment in melanoma and non‐small‐cell lung cancer patients. Ann Oncol 2017;28:1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teraoka S, Fujimoto D, Morimoto T et al. Early immune‐related adverse events and association with outcome in advfanced non‐small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol 2017;12:1798–1805. [DOI] [PubMed] [Google Scholar]
- 119.Huang AC, Postow MA, Orlowski RJ et al. T‐cell invigoration to tumour burden ratio associated with anti‐PD‐1 response. Nature 2017;545:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bagley SJ, Kothari S, Aggarwal C et al. Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017;106:1–7. [DOI] [PubMed] [Google Scholar]
- 121.Kamphorst AO, Pillai RN, Yang S et al. Proliferation of PD‐1+ CD8 T cells in peripheral blood after PD‐1‐targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 2017;114:4993–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geng Y, Shao Y, He W et al. Prognostic role of tumor‐infiltrating lymphocytes in lung cancer: A meta‐analysis. Cell Physiol Biochem 2015;37:1560–1571. [DOI] [PubMed] [Google Scholar]