This review summarizes the developmental process of immunotherapy in lung cancer, ongoing international and domestic clinical trials in the field, and the challenges related to immunotherapy in the lung cancer patient population in China. The goal is to provide detailed information for future immunotherapy‐related clinical trials in China.
Keywords: Biogenetics, Checkpoint inhibitors, Immunotherapy, Lung cancer
Abstract
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer‐related deaths in China. The recent emergence of immunotherapy treatment options, such as the use of programmed cell death protein 1 (PD‐1)/programmed death‐ligand 1 (PD‐L1) checkpoint inhibitors, has also led to a paradigm shift in the treatment of non‐small cell lung cancer, and has provided promising directions for the treatment of small cell lung cancer. This review provides a summary of the developmental process of immunotherapy, especially immune checkpoint inhibitors in lung cancer, ongoing international and domestic clinical trials in this field, and the challenges and considerations related to the use of immunotherapy in Chinese patients with lung cancer, with the aim of providing detailed information for future immunotherapy‐related clinical trials in China. Research regarding immune checkpoint inhibitors in China is several years behind similar research in several developed countries. However, although PD‐1/PD‐L1 inhibitor‐related clinical trials remain in their early stages in China, increased efforts by Chinese clinicians, researchers, and government staff have been directed toward trying to introduce novel drugs into the clinical setting. Because of the specific characteristics of Chinese patients with lung cancer (such as high epidermal growth factor receptor mutation rates, later disease stages, and different toxicity profiles), large‐scale clinical trials targeting the Chinese population or Chinese participation in multinational trials should be promoted.
Implications for Practice.
As the leading cause of cancer‐related morbidity and mortality, lung cancer is a major public health problem in China. Immunotherapy based on programmed cell death protein 1/programmed death‐ligand 1 checkpoint inhibitors may result in new treatment directions and a paradigm shift for Chinese patients with lung cancer. Although checkpoint inhibitor‐related clinical trials remain in their early stages in China, increased efforts by Chinese clinicians, researchers, and government staff have been directed toward trying to introduce novel drugs into the clinical setting by encouraging the development of large‐scale clinical trials targeting the Chinese population and promoting Chinese patients with lung cancer to participate in international trials.
摘要
肺癌是最常被诊断出的癌症,也是中国癌症相关死亡的主要原因。最近出现的免疫治疗方案,如程序性细胞死亡蛋白 1 (PD‐1)/程序性死亡 ‐ 配体 1 (PD‐L1) 检查点抑制剂的使用,致使非小细胞肺癌的治疗发生了范式转变,同时也为小细胞肺癌的治疗提供了颇具前景的发展方向。本篇综述概述了免疫治疗的发展历程,特别是免疫检查点抑制剂在肺癌治疗中的使用,该领域正在进行的国际和国内临床试验,以及与中国肺癌患者使用免疫治疗相关的挑战和注意事项, 旨在为中国未来的免疫治疗相关临床试验提供详细信息。中国关于免疫检查点抑制剂的研究较若干发达国家的类似研究落后了几年的时间。然而,尽管在中国,与 PD‐1/PD‐L1 抑制剂相关的临床试验仍处于早期阶段,但中国的临床医生、研究人员和政府工作人员的不懈努力旨在尝试将新药引入临床环境中。鉴于中国肺癌患者的具体特征(如较高的表皮生长因子受体突变率、疾病分期较晚以及不同的毒性特征),应大力推广针对中国人群的大规模临床试验或鼓励中国参与者参与跨国试验。
实践意义:作为癌症相关发病率和死亡率的主要原因,肺癌是中国的一个重大公共卫生问题。基于程序性细胞死亡蛋白 1 /程序性死亡 ‐ 配体 1 检查点抑制剂的免疫治疗可能会为中国肺癌患者带来新的治疗方向和范式转变。尽管在中国,与检查点抑制剂相关的临床试验仍处于早期阶段,但中国的临床医生、研究人员和政府工作人员的不懈努力旨在尝试通过鼓励开发针对中国人群的大规模临床试验并鼓励中国肺癌患者参与国际试验,将新药引入临床环境中。
Introduction
Immunotherapy has been one of the breakthrough technologies in cancer therapy during the last decade. According to the antitumor mechanism, immune‐oncology (I‐O) is divided into three classes: active immunity (including vaccines, cytokines, and checkpoint inhibitors), passive immunity (adoptive cell infusion and targeted monoclonal antibodies), and hybrid immunity (combination of active and passive methods). Vaccines stimulate a patient's own immune system to mount a response to tumor antigens, and generate an active immune response against tumor antigens. Cytokines are considered nonspecific immune stimulation, and boost activity of immune effector cells and stromal cells at the tumor sites. Immune checkpoints exist to dampen or terminate immune activity to guard against autoimmunity and allow for self‐tolerance. However, tumors can take advantage of these immune checkpoint pathways to evade destruction. Checkpoint inhibitors have demonstrated delayed tumor growth and increased survival. Compared with the difficult and convoluted development pathways of other antitumor classes, such as vaccines and cytokines, checkpoint inhibitors received great attention when they first appeared. Inhibitors for many checkpoints, including cytotoxic T‐lymphocyte associated protein 4 (CTLA‐4), programmed cell death protein 1 (PD‐1 [and its ligand programmed death‐ligand 1 (PD‐L1)]), B7‐H3, B7x, lymphocyte‐activation gene 3, mucin domain‐containing molecule‐3, V‐domain Ig suppressor of T‐cell activation, and B‐ and T‐cell lymphocyte attenuator, have been described and studied [1]. To date, the best characterized and most clinically studied checkpoints are CTLA‐4, PD‐1, and PD‐L1 [2].
Lung cancer, a leading cause of death worldwide, is classified histologically as either non‐small cell lung cancer (NSCLC, accounting for approximately 85% of lung cancer, including squamous [SQCC] or non‐squamous cell carcinoma [non‐SQCC]) or small cell lung cancer (SCLC, accounting for approximately 15% of lung cancer). In China, the situation is particularly urgent. In contrast to lung cancer incidence rates in most Western countries, where lung cancer death rates are decreasing, the rates in China are still increasing, and lung cancer remains the most commonly diagnosed cancer and the leading cause of cancer‐related death [2]. More than 50% of patients with lung cancer present with metastases at the time of diagnosis. The prognosis for patients with metastatic or stage IV disease is extremely poor, with 5‐year survival rates reported as less than 5%. Limited treatment options are available for both advanced NSCLC and SCLC, and response rates to standard chemotherapy range between approximately 15% and 30%.
Recently, as has been experienced with other solid tumors, the emergence of PD‐1/PD‐L1 inhibitors has led to a paradigm shift in the treatment of NSCLC and has provided promising directions for the treatment of SCLC. In this article, we summarize the developmental process of immunotherapy in lung cancer, ongoing international and domestic clinical trials in this field, and the challenges relating to immunotherapy in the Chinese lung cancer patient population in the hope of providing detailed information for future immunotherapy‐related clinical trials in China.
Current Status of Lung Cancer Management in China
Epidemiology
Lung cancer has been the leading cause of cancer‐related morbidity and mortality for many years in China, accounting for 35.78% of all newly diagnosed lung cancer cases and 37.56% of lung cancer deaths worldwide [3]. In 2015, 733,300 patients were diagnosed, and 610,200 patients died from lung cancer [4], with a mortality rate that has increased 464.84% in the past several decades [5]. More than 50% patients were estimated to harbor distant metastases at initial diagnosis. As a leading risk factor, approximately 52.4% and 19.4% of lung cancer can be attributed to smoking in men and women, respectively [3]. Other risk factors include air pollution (environmental exposure to asbestos, nickel, chromium, and arsenic and indoor exposure to radon, unventilated coal‐fueled stoves, and cooking fumes), chronic pulmonary disease (such as pulmonary tuberculosis, chronic bronchitis, and emphysema), and genetic mutations [6].
Histologically, NSCLC accounts for approximately 85% of lung cancer. Comparing SQCC with non‐SQCC such as adenocarcinoma and other histological types, adenocarcinoma has replaced SQCC as the most predominant histological lung cancer subtype in China. In the past several years, the proportion of adenocarcinoma has increased to 43.36%–46.80%, while the proportion of SQCC has decreased to 24.16%–32.23% [6]. The change in predominant histological type has also influenced the molecular epidemiology of Chinese patients with NSCLC. Patients with adenocarcinoma and those of East Asian ethnicity have the highest risk of harboring epidermal growth factor receptor (EGFR) mutations; the prevalence of EGFR mutations in those patients is relatively higher than that in patients from Western countries, accounting for approximately 28.4% of the unselected NSCLC Chinese population, 40.3%–64.5% of patients with adenocarcinoma, and 75% of certain clinically enriched populations (i.e., patients who were nonsmokers with adenocarcinoma), although accounting for only approximately 2.1%–8.0% of patients with SQCC [5]. Other documented gene variations included anaplastic lymphoma kinase (ALK) rearrangements (1.5%–10%), ROS proto‐oncogene 1 (ROS1) fusions (1%–2%), RET rearrangements (1.3%–1.9%), mesenchymal‐epithelial transition factor (c‐MET) gene amplification (4.5%), v‐raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations (1.5%–3.0%), human epidermal growth factor receptor 2 (HER2) mutations (2.4%–5.94%), fibroblast growth factor receptor (FGFR) mutations (0.6%–3.5%), and discoidin domain receptor tyrosine kinase 2 (DDR2) mutations (0.5%–4.6%) [6]. With a deepening understanding of the molecular mechanisms, targeted therapies and the concomitant molecular tests have played an increasingly important role in the road map of lung cancer management [6].
Standard of Treatment
In China, the standard for advanced NSCLC treatment is comprehensive treatment, mainly using systemic therapy [7]. According to the China Experts Consensus on the Diagnosis and Treatment of Advanced Stage Primary Lung Cancer (2016 Version) [8], EGFR tyrosine kinase inhibitors (TKIs; drugs approved by the China Food and Drug Administration [CFDA] include gefitinib [2010], erlotinib [2017], afatinib [2017], and icotinib [2014]) could be used as the first‐line systemic therapy in patients with sensitizing EGFR mutations that are documented before the application of first‐line therapy. For patients with locally advanced or metastatic NSCLC who have ALK or ROS1 rearrangements, crizotinib (approved in 2013) is recommended as the first‐line therapy. For patients without driving genes, such as EGFR mutations or ALK rearrangement, platinum‐based regimens remain the mainstay of first‐line systemic therapy. In China, gemcitabine (27.4%), docetaxel (16.2%), paclitaxel (13.5%), and pemetrexed (9.2%) are the most common choices in platinum‐based doublet chemotherapy regimens for first‐line chemotherapy [7]. For patients with unresectable, locally advanced, metastatic or recurrent non‐SQCC, bevacizumab (a recombinant monoclonal antibody that inhibits the vascular endothelial growth factor pathway, approved in 2015) is an option in combination with chemotherapy. Second‐line choices for systematic therapy include docetaxel, pemetrexed, and EGFR‐TKIs (drugs approved by the CFDA include gefitinib [2005], erlotinib [2006], afatinib [2017], icotinib [2011], and osimertinib [only for EGFR T790M mutation‐positive patients, 2017]); third‐line choices include clinical trials or the best supporting treatment. Recently, PD‐1 inhibitor nivolumab (approved by the CFDA in June 2018) became a new second‐line choice for patients with locally advanced or metastatic NSCLC with intolerance to or progression after previous platinum‐based chemotherapy.
For patients with extensive‐stage SCLC (accounting for two thirds of patients with SCLC) in China, chemotherapy is the most important and standard first‐line treatment. The recommended first‐line chemotherapy regimens for patients with an Eastern Cooperative Oncology Group performance score (ECOG PS) of 0–2 include etoposide + cisplatin, etoposide + carboplatin, irinotecan + cisplatin, or irinotecan + carboplatin. If treatment fails, patients with recurrence or progression within 3 months are encouraged to participate in clinical trials; topotecan, irinotecan, gemcitabine, or paclitaxel are considered for patients with recurrence within 3–6 months [8].
Dilemmas and Challenges
Treatment Disparity.
Large disparities in the treatment of lung cancer in China may affect the clinical outcomes of patients. According to recent studies, 20.6% of Chinese patients with stage I NSCLC are overtreated, and 20.1% of stage II patients are undertreated. Although China has adopted the National Comprehensive Cancer Network clinical practice guidelines for lung cancer and a Chinese‐specific version is available, only 19.6% of stage IIIA patients and 30.7% of stage IIIB patients receive the recommended systematic therapy [9]. Treatment disparities can be attributed to socioeconomic factors, including high medical costs, insufficient insurance coverage, or the lack of oncologist and patient compliance with advanced therapies [9]. In real‐world settings, treatment disparities between different areas, or between the domestic consensus and global guidelines, may hinder the introduction, or obscure the authentic efficacy, of globally emerging novel drugs.
Standstill in the Outcome Improvement of SCLC.
Systemic treatment options for patients with SCLC have not improved significantly over the last few decades. Most patients relapse (approximately 80% of limited‐stage patients and almost all extensive‐stage patients) within the first year after initial therapy. Patients with refractory or drug‐resistant relapse are less responsive to most drugs (response rate ≤10%), and the response rate for patients with sensitive relapse is only 25% [10]. Treatment limitations encourage the research and development of novel agents, such as immune checkpoint inhibitors. Unfortunately, in China, there is still a long way to go for those drugs to be incorporated into clinical practice [11].
Advances in Immunotherapy in China
Global Situation
NSCLC.
NSCLC is a remarkably heterogeneous tumor that presents a large mutational load, encoding numerous potential neo‐antigens. Thus, agents that activate the host immune system and impede the immune escape of cancer cells in the tumor microenvironment are expected to improve clinical antitumor efficacy. Immunotherapy, represented by PD‐1/PD‐L1 checkpoint inhibitors, has dramatically changed the landscape for the first‐ and second‐line treatment of NSCLC and has offered promising potential for the treatment of SCLC. To date, PD‐1/PD‐L1 inhibitors have been used widely or studied intensively in many clinical settings, such as monotherapy or combination therapy (including combinations between two different checkpoint pathways, with chemotherapy, with targeted drugs, with antiangiogenesis drugs, with surgery as neoadjuvant therapy, and with radiotherapy), as first‐line or subsequent‐line therapy, in the general population and in specific subgroups.
Approved PD‐1/PD‐L1 inhibitors for NSCLC include nivolumab (2015, second line), pembrolizumab (2015, second line in patients with PD‐L1 expression ≥1%; 2016, first line in patients with PD‐L1 ≥50%), atezolizumab (2016, second line), and durvalumab (2018, second line). Checkmate‐017 and Checkmate‐057 have shown significantly better efficacy and safety profiles with nivolumab than with docetaxel when treating patients with advanced, previously treated SQCC (i.e., objective response rate [ORR]: 20% vs. 9%; p = .008) [12] and non‐SQCC patients [13], which led to the approval of nivolumab as a second‐line treatment of NSCLC. Based on the positive efficacy and safety profiles demonstrated by pembrolizumab (KEYNOTE‐010) and atezolizumab (OAK), they were successively approved as second‐line drugs for NSCLC. The KEYNOTE‐024 study showed that pembrolizumab was associated with significantly longer progression‐free survival (PFS) and overall survival (OS) and with fewer adverse events than platinum‐based chemotherapy in patients with PD‐L1 expression ≥50% advanced NSCLC (median PFS: 10.3 months vs. 6.0 months; p < .001), which led to the 2016 approval of pembrolizumab as a first‐line therapy for patients with previously untreated, advanced NSCLC with high PD‐L1 expression (≥50%). In the 2018 AACR annual meeting, the OS of KEYNOTE‐024 was reported. Pembrolizumab showed OS benefit over chemotherapy as first‐line therapy for advanced NSCLC with PD‐L1 tumor proportion score ≥50% (median OS of 30.0 months vs. 14.2 months, p = .002). The results will further enhance the role of pembrolizumab as a new standard of care for first‐line therapy for advanced NSCLC that express high levels of PD‐L1.
Currently, a series of randomized clinical trials of PD‐1/PD‐L1 inhibitors as first‐line therapies are ongoing, including Checkmate‐012 (nivolumab monotherapy or combined with platinum‐based doublet chemotherapy, erlotinib, bevacizumab, or ipilimumab), CheckMate‐816 (nivolumab and ipilimumab as neoadjuvant therapy), NCT02309177 (nivolumab with nab‐P and carboplatin), KEYNOTE‐001/021/042 (pembrolizumab monotherapy, combined with chemotherapy or targeted therapy), MYSTIC (durvalumab combined with tremelimumab), BIRCH (atezolizumab monotherapy), IMpower 130/131/132 (atezolizumab with chemotherapy), and NCT02088112 (durvalumab with gefitinib) [14].
The latest articles and abstracts published in the 2018 ASCO annual meeting have reported a series of recent results on the advances of immune checkpoint inhibitors in lung cancer. Focusing on immune checkpoint inhibitor monotherapy, 5‐year follow‐up results from the CA209‐003 study (n = 129) showed promising long‐term OS and durable responses in a proportion of patients with pretreated advanced NSCLC receiving nivolumab treatment [15]. The estimated 5‐year OS rate was 16% for all nivolumab‐treated patients. Seventy‐five percent of the 5‐year survivors received no further therapy after nivolumab and were without evidence of progressive disease at last follow‐up before the data cutoff. Similarly, 4‐year follow‐up results from the KEYNOTE‐001 study (n = 550, 101 treatment‐naive; 449 previously treated) demonstrated long‐term OS benefit for both treatment‐naïve and previously treated advanced NSCLC with positive PD‐L1 expression. ORR was 41.6% and 22.9%, median OS was 22.3 months and 10.5 months, and estimated 4‐year OS rate was 27.2% and 16.4% for treatment‐naïve and previously treated patients, respectively. Kaplan‐Meier curves for OS appeared to plateau after 42 months for treatment‐naïve patients and 36 months for previously treated patients [16]. In the latest results from KEYNOTE‐042, the benefit of first‐line pembrolizumab treatment has expanded to the previously untreated advanced/metastatic NSCLC population with PD‐L1 expression ≥1% and without sensitizing EGFR or ALK alterations, but not only limited to high PD‐L1‐expressing population (PD‐L1 ≥50%) [17]. Compared with standard first‐line chemotherapy, pembrolizumab significantly improved OS in patients with PD‐L1 ≥50% (hazard ratio [HR]: 0.69; p = .0003), PD‐L1 ≥20% (HR: 0.77; p = .002), and PD‐L1 ≥1% (HR: 0.81; p = .0018).
As data presented in the ATLANTI study (n = 444) showed, even patients with heavily pretreated, EGFR/ALK mutation‐positive advanced NSCLC may also benefit from greater than or equal to third‐line PD‐1/PD‐L1 inhibitors treatment, with durable efficacy and a promising effect on OS [18]. For high PD‐L1 expressing (≥25%) and EGFR/ALK (+) patients, median OS after anti‐PD‐L1 antibody durvalumab treatment was 13.3 months, which was similar to the median OS of extremely high PD‐L1 expressing (≥90%) and EGFR/ALK (−) patients (13.2 months), and with a 1‐year OS rate of up to 53%. In contrast, the median OS of PD‐L1 <25% and EGFR/ALK (+) patients was only 9.9 months. Avelumab is another emerging PD‐L1 inhibitor. Two‐and‐a‐half‐year follow‐up from the JAVELIN Solid Tumor trial (n = 154) showed that avelumab as sequential‐line therapy was associated with durable responses and long‐term OS in patients with platinum‐treated advanced NSCLC [19]. The ORR was 14.1%, median duration of response was 17.5 months, PFS rates at 6 months and 1 year were 24.2% and 16.5%, and OS rates at 1 and 2 years were 42.5% and 25.0%, respectively, during a median follow‐up period of 33.9 months, and increased PD‐L1 expression seemed to be associated with higher ORR and improved OS.
Focusing on the combination therapy of immune checkpoint inhibitors, recent results were also remarkable. In the Checkmate‐012 study, first‐line combination therapy with nivolumab and CTLA‐4 inhibitor ipilimumab demonstrated a manageable safety profile and promising, durable efficacy in advanced NSCLC [20]. The pooled ORR was 43%, median PFS was 8.0 months, and 1‐ and 2‐year OS rate was 76% and 49%, respectively. Efficacy was enhanced in patients with PD‐L1 tumor expression ≥1%. Encouraging activity and improved OS was also observed for first‐line nivolumab plus platinum‐based doublet chemotherapy in patients with advanced NSCLC, showing an ORR of 46%, disease control rate of 89%, median OS of 19.2 months, and 1‐, 2‐, and 3‐year OS rate of 71%, 37%, and 25%, respectively [21]. Based on the results from Checkmate‐012 and considering tumor mutational burden (TMB) an emerging predictive biomarker, phase III study Checkmate‐227 further examined survival benefit with first‐line nivolumab plus ipilimumab among patients with advanced NSCLC with a high TMB (≥10 mutations/Mb) [22]. Median PFS was significantly longer with first‐line nivolumab plus ipilimumab than with chemotherapy among patients with NSCLC and a high tumor mutational burden (7.2 months vs. 5.5 months; HR: 0.58; p < .001), irrespective of PD‐L1 expression level. It is noteworthy that the Checkmate‐227 study is also being conducted in China.
Based on the solid evidence from a series of randomized controlled trials, pembrolizumab plus platinum‐containing chemotherapy has been approved by the U.S. FDA for first‐line treatment in advanced, nonsquamous NSCLC. The latest results from the KEYNOTE 021 G cohort demonstrated that in patients with previously untreated stage IIIB/IV non‐squamous NSCLC without EGFR mutations/ALK translocations, the risk of death for the pembrolizumab plus chemotherapy arm was reduced by nearly half compared with chemotherapy alone (HR: 0.56; nominal p = .0151) after a median follow‐up period of 24 months [23]. The combination of pembrolizumab and standard first‐line chemotherapy (carboplatin + pemetrexed) significantly improved the treatment response and survival prognosis in patients with non‐squamous NSCLC (pembrolizumab + chemotherapy arm vs. chemotherapy arm: ORR: 57% vs. 30%; p = .0016; median PFS: 24.0 months vs. 9.3 months; p = .0049; median OS: not reached vs. 21.1 months; p = .0151). The larger‐scale phase III study KEYNOTE‐189 further confirmed the conclusion [24] that in patients with previously untreated metastatic non‐squamous NSCLC, the addition of pembrolizumab to standard chemotherapy resulted in significantly better response and longer overall survival and progression‐free survival than chemotherapy alone (p < .001 for each comparison). In patients with previously untreated metastatic squamous NSCLC (KEYNOTE‐407), the addition of pembrolizumab to standard chemotherapy also significantly improved treatment response (ORR almost doubled in the pembrolizumab + chemotherapy arm: 58.4% vs. 35.0%; p = .0004), with a tolerable safety profile [25].
Other combination regimens containing immune checkpoint inhibitors such as durvalumab plus CTLA‐4 inhibitor tremelimumab [26], durvalumab plus gefitinib (NCT02088112) [27], atezolizumab plus chemotherapy with or without bevacizumab (IMpower 131 and 150) [28], [29], and atezolizumab plus alectinib (in ALK‐positive patients) [30] also showed positive efficacy and acceptable toxicity, which may provide more options for combination therapy with immune checkpoint inhibitors in the future.
Identifying optimal populations that have the greatest potential to benefit from I‐O optimizes therapeutic efficacy; therefore, the investigation of potential predictive biomarkers has become a hot spot in I‐O‐related fields [31]. PD‐L1 has been widely accepted as a predictive biomarker of the anti‐PD‐1/L1 therapy responses of many cancers; nevertheless, immunogenic mutation loads, such as microsatellite instability‐high and TMB, also show significant response to checkpoint blockade, which is probably due to PD‐1/L1 status and tumor‐infiltrating lymphocyte content [32]. In the Checkmate‐026 study (n = 541), the PFS benefit was more obvious in patients with high TMB when comparing the efficacy of nivolumab and standard chemotherapy (median PFS of 9.7 vs. 5.8 months; ORR: 46.8% vs. 28.3%), which was consistent with the improved survival data shown in Checkmate‐227 [33].
SCLC.
PD‐1/PD‐L1 inhibitors have also shown promising results in patients with SCLC. Nivolumab monotherapy showed favorable efficacy for patients with recurrent SCLC in the phase I/II Checkmate‐032 trial (n = 98; ORR: 10%; median OS: 4.4 months; 1‐year OS rate: 33%; median PFS: 1.4 months; treatment‐related adverse events: 13%) [34]. Preliminary results from the KEYNOTE‐028 study were also remarkable. Patients with PD‐L1‐positive, chemotherapy‐treated, extensive‐stage SCLC benefitted from pembrolizumab treatment (n = 24; ORR: 33%; median OS: 9.7 months; 1‐year OS rate: 35.7%; median PFS: 1.9 months), although the small sample size might affect the potency of the statistical data [35]. Recently, combination regimens, including nivolumab and ipilimumab (Checkmate‐032), and ipilimumab and chemotherapy, have also been under evaluation.
Ongoing International Clinical Trials in China
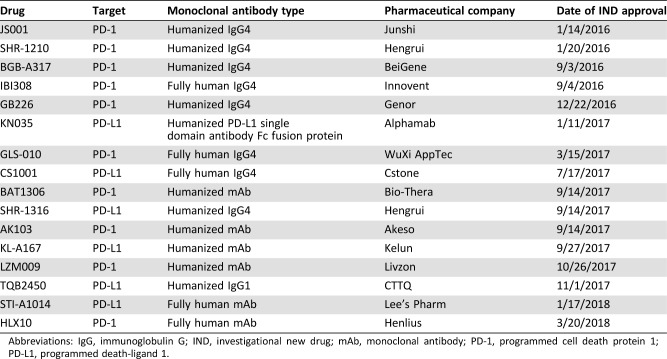
Research regarding immune checkpoint inhibitors in China is several years behind similar research in developed countries (e.g., U.S.). However, greater efforts by Chinese clinicians, researchers, and government staff have encouraged Chinese patients with lung cancer to participate in global randomized clinical trials. As of April 25, 2018, a total of six checkpoint inhibitors made by foreign companies have applied for new drug clinical trial application in China (Table 1).
Table 1. Checkpoint inhibitors made by foreign companies that applied for new drug clinical trial application in China (until April 25, 2018).
Abbreviation: IND, investigational new drug.
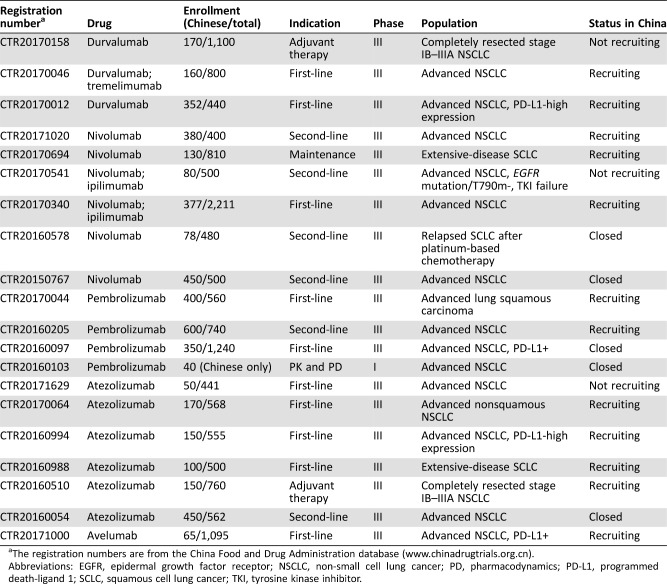
To date, Chinese sites are involved in 20 global clinical trials, including 10 first‐line trials, 6 second‐line trials, 2 adjuvant therapy trials, 1 maintenance therapy trial, and 1 trial for study of pharmacokinetics and pharmacodynamics (Table 2). Representative international trials enrolling patients from Chinese medical centers include KEYNOTE‐042, NCT03003962, NEPTUNE, IMpower 133, IMpower 210, MK‐3475‐033, and CheckMate‐078 (conducted by Chinese sites and Chinese hospitals accounted for >70% of the centers), etc. Thus, the latest advances in those global trials as previously described may also reflect the situation of Chinese patients to a certain extent.
Table 2. Ongoing international clinical trials of programmed cell death protein 1 and PD‐L1 inhibitors on lung cancer with participating Chinese centers (until April 25, 2018).
The registration numbers are from the China Food and Drug Administration database (www.chinadrugtrials.org.cn).
Abbreviations: EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; PD, pharmacodynamics; PD‐L1, programmed death‐ligand 1; SCLC, squamous cell lung cancer; TKI, tyrosine kinase inhibitor.
CheckMate 078 (CTR20150767) was the third phase III study to demonstrate improved OS with nivolumab compared with docetaxel in patients with advanced, previously treated NSCLC (median OS of 12.0 months vs. 9.6 months; p = .0006). More importantly, CheckMate 078 was the first phase III study to show benefit of a checkpoint inhibitor in an East Asian (predominantly Chinese, 90%) patient population. Overall, the safety and efficacy of nivolumab were consistent with the global CheckMate 017 and 057 trials. In June 2018, nivolumab became the first immune checkpoint inhibitor to be approved as second‐line therapy for locally advanced or metastatic NSCLC in China.
Domestic Drugs
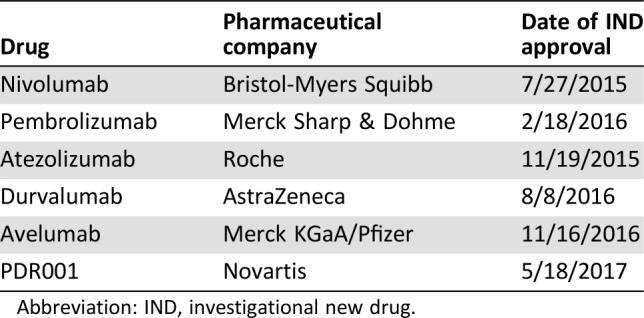
During the last few years, the overall clinical research environment in China has significantly improved, leading to an increase in the development of emerging domestic drugs. As of April 25, 2018, a total of 16 checkpoint inhibitors made by domestic companies have applied for new drug clinical trial application in China (Table 3).
Table 3. Checkpoint inhibitors made by domestic companies that applied for new drug clinical trial application in China (until April 25, 2018).
Abbreviations: IgG, immunoglobulin G; IND, investigational new drug; mAb, monoclonal antibody; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1.
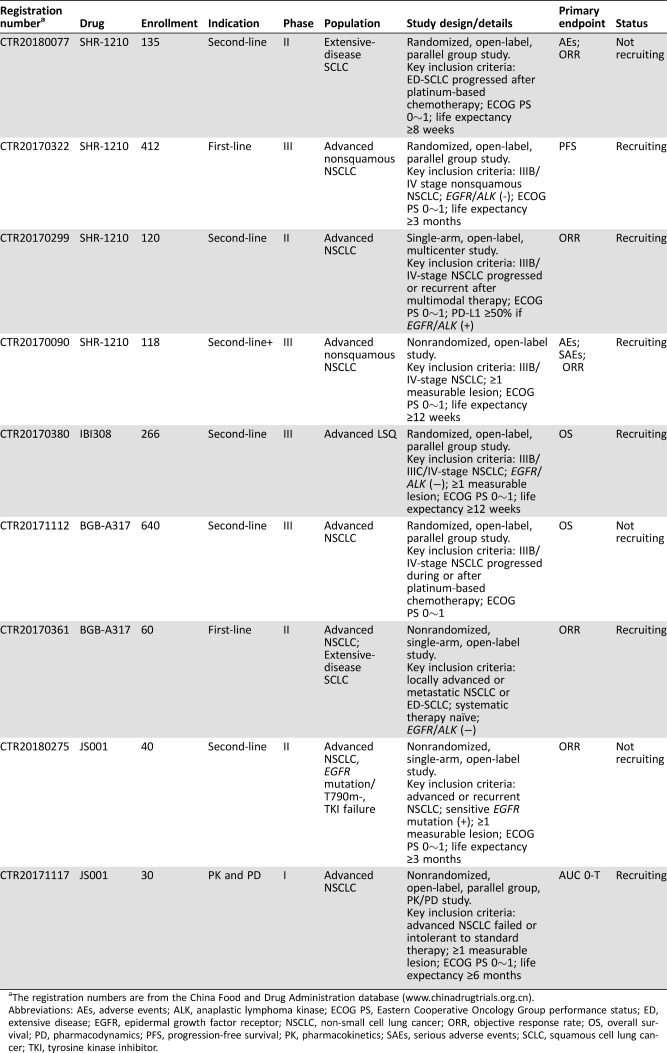
JS‐001 (CTR20180275, CTR20171117), SHR1210 (CTR20180077, CTR20170322, CTR20170299, CTR20170090), IBI308 (CTR20170380), and BGB‐A317 (CTR20171112, CTR20170361) are the front‐runners in the competition and have been approved by the CFDA for clinical trials among patients with lung cancer (Table 4). Moreover, other drugs (GB226, KN035, GLS‐010, CS1001, BAT1306, etc.) are also approved by the CFDA for clinical trial.
Table 4. Ongoing clinical trials with domestic programmed cell death protein 1 or programmed death‐ligand 1 inhibitors on advanced lung cancer (until April 25, 2018).
The registration numbers are from the China Food and Drug Administration database (www.chinadrugtrials.org.cn).
Abbreviations: AEs, adverse events; ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive disease; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; ORR, objective response rate; OS, overall survival; PD, pharmacodynamics; PFS, progression‐free survival; PK, pharmacokinetics; SAEs, serious adverse events; SCLC, squamous cell lung cancer; TKI, tyrosine kinase inhibitor.
In the 2018 ASCO annual meeting, results from a phase Ib study of SHR‐1210 plus apatinib for previously heavily treated advanced nonsquamous NSCLC patients were presented [36]. Twenty‐seven patients after failure of ≥2 lines of systemic therapies were treated with SHR‐1210 in combination with apatinib (250 mg or 375 mg) up to disease progression or intolerable toxicity. The pooled ORR and disease control rate was 41.2% and 94.1% in the two arms with different apatinib dosage, and the median PFS was 24 weeks in the apatinib 250 mg arm and not reached in the apatinib 375 mg arm, respectively. Although higher ORR seemed to be observed in the apatinib 375 mg arm, dose‐limiting toxicity resulted in dose reduction of apatinib to 250 mg in a portion of patients (nearly 50%). Thus, considering the balance of efficacy and safety, SHR‐1210 in combination with apatinib at a dose of 250 mg was well tolerated with promising antitumor effect, even in previously heavily treated NSCLC patients. A further phase II trial based on SHR‐1210 plus apatinib 250 mg regimen is ongoing.
Exploring Biomarkers
Although none of the four global PD‐L1‐detecting antibodies (28‐8, 22C3, SP142, and SP263) have been listed in China yet, pathologists in most Chinese hospitals have spontaneously conducted PD‐L1 expression studies and consensus studies (for detecting antibodies). In China, 28‐8, 22C3, SP142, SP263, E1L3N, and BP6001 are commonly used research‐oriented antibodies. At present, the expert opinion on PD‐L1 detection in China is being developed, which may provide authoritative guidance for the exploration of PD‐L1 biomarkers in the Chinese population [37].
Special Focus: Differences Between China and Other Countries
Ethnicity
The characteristics of Chinese patients are different from those of patients from Western countries, and specific ethnic features should be considered factors in trial designs (i.e., the Chinese population has relatively high rates of hepatitis B virus infection and traditional medicine use). Furthermore, Chinese patients with NSCLC may have more driver gene mutations, different gene profiles, better clinical responses to chemotherapy, and different toxicity profiles. Although the JapicCTI‐132072 trial, conducted in Japan, showed that the clinical efficacy of the PD‐1 inhibitor nivolumab as a second‐line regimen in Asian NSCLC patients is consistent with that in Western patients (Independent Review Committee assessed ORR of 25.7%, median OS of 16.3 months, and median PFS of 4.2 months) [38], more direct and robust evidence is still necessary for the clinical application of novel immunotherapy drugs.
Biogenetics
EGFR Mutation.
The prevalence of EGFR mutations is approximately 50% among Chinese patients with NSCLC, which is significantly higher than that in Western countries. The presence of EGFR mutations and ALK rearrangements (alterations typically associated with a lack of tobacco exposure) have been suggested to be associated with lower ORR to PD‐1 inhibitors. Therefore, trials to test whether the ORR to immunotherapy can be improved by administering concurrent TKI therapy in EGFR‐positive patients and large‐scale clinical trials specifically targeting the Chinese population should be conducted.
PD‐L1 Expression.
Results from a single‐center study showed that the PD‐L1 expression rate in Chinese NSCLC patients might be different from that in Western countries. International studies showed that PD‐L1 expression profiles were similar in different sample storage states (fresh or archived specimens), histological types (SQCC or adenocarcinoma), patient groups (treated or untreated), and tumor sites (primary or metastatic). Regardless of whether patients had SQCC or non‐SQCC such as adenocarcinoma, the proportion of patients with PD‐L1 <1%, 1%–49%, or ≥50% was 1:2:2, which meant that 40% of patients had positive PD‐L1 expression (≥50%) [39]. However, a single‐center study (n > 1,000) conducted by the Pathology Department of Sun Yat‐sen Hospital in China showed that PD‐L1 expression in Chinese NSCLC patients is related to histological type (Yuan Li, private data). A higher proportion of patients with adenocarcinoma expressed negative PD‐L1 levels; patients with PD‐L1 <1% accounted for 79% of all adenocarcinoma patients, whereas patients with PD‐L1 ≥50% accounted for only 7.6%. In SQCC patients, the proportions of patients with PD‐L1 <1%, 1%–49%, or ≥50% was similar to those in patients from Western countries (1:1:1). The reason for the different PD‐L1 expression profiles between Chinese and foreign populations remains unclear. Therefore, it has been suggested that it would be prudent to carry out a nationwide, multicenter study to clarify the PD‐L1 expression profiles in Chinese NSCLC patients, to confirm whether there are ethnic differences and whether the results of international studies are applicable to the Chinese population [40].
Unmet Demands
Limited Access to Novel Drugs and Promising Directions.
Ongoing international clinical trials rarely target, or even consider, the characteristics of the Chinese population, and according to the Chinese drug introduction policy, drug‐related research and approval in China is always several years behind that in developed countries of the world. Thus, Chinese patients always have limited access to promising, novel drugs [37].
Conclusion
As the leading cause of cancer‐related morbidity and mortality, lung cancer is a major public health problem in China, and immunotherapy based on PD‐1/PD‐L1 inhibitors may result in new treatment directions and a paradigm shift for the Chinese lung cancer population. PD‐1/PD‐L1 inhibitor‐related clinical trials remain in their early stages in China, although greater efforts by personnel of various departments have been directed toward trying to introduce novel drugs into the clinic. Considering the specific characteristics of the Chinese population with lung cancer (such as high EGFR mutation rates, later disease stages, and different toxicity profiles), large‐scale clinical trials targeting the Chinese population, or participation in multinational trials, should be promoted.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2016YFC1303300), the National Natural Science Foundation of China (81672272), the Key Project of Shanghai Health & Family Planning Commission (201540365), the Shanghai Municipal Science & Technology Commission Research Project (17431906103), and the Shanghai Scientific Research Projects (14140902800). Editorial/language assistance was provided by David P. Figgitt, Ph.D., Content Ed Net, with funding from BMS China.
Author Contributions
Conception/design: Shun Lu
Provision of study material or patients: Shun Lu, Yongfeng Yu, Yi Yang
Collection and/or assembly of data: Shun Lu, Yongfeng Yu, Yi Yang
Data analysis and interpretation: Shun Lu
Manuscript writing: Shun Lu, Yongfeng Yu
Final approval of manuscript: Shun Lu, Yongfeng Yu, Yi Yang
Disclosures
The authors indicated no financial relationships.
References
- 1.Assal A, Kaner J, Pendurti G et al. Emerging targets in cancer immunotherapy: Beyond CTLA‐4 and PD‐1. Immunotherapy 2015;7:1169–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostou VK, Brahmer JR. Cancer immunotherapy: A future paradigm shift in the treatment of non‐small cell lung cancer. Clin Cancer Res 2015;21:976–984. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zeng H et al. Epidemiology of lung cancer in China. Thorac Cancer 2015;6:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 5.She J, Yang P, Hong Q et al. Lung cancer in China: Challenges and interventions. Chest 2013;143:1117–1126. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C. Lung cancer molecular epidemiology in China: Recent trends. Transl Lung Cancer Res 2014;3:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Sun Y. Medical management of lung cancer: Experience in China. Thorac Cancer 2015;6:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Sun Y, Yu J et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Asia Pac J Clin Oncol 2017;13:87–103. [DOI] [PubMed] [Google Scholar]
- 9.Yang LL, Zhang XC, Yang XN et al. Lung cancer treatment disparities in China: A question in need of an answer. The Oncologist 2014;19:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurwitz JL, McCoy F, Scullin P et al. New advances in the second‐line treatment of small cell lung cancer. The Oncologist 2009;14:986–994. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Xing P, Fan Y et al. Current small cell lung cancer treatment in China. Thorac Cancer 2015;6:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X, Liu Y, Zhang J et al. PD‐1/PD‐L1 checkpoint blockades in non‐small cell lung cancer: New development and challenges. Cancer Lett 2017;405:29–37. [DOI] [PubMed] [Google Scholar]
- 15.Gettinger S, Horn L, Jackman D et al. Five‐year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: Results from the CA209‐003 study. J Clin Oncol 2018;36:1675–1684. [DOI] [PubMed] [Google Scholar]
- 16.Felip E, Hellmann M, Hui R et al. 4‐year overall survival for patients with advanced NSCLC treated with pembrolizumab: Results from KEYNOTE‐001. J Clin Oncol 2018;36(suppl 15):9030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes G, Wu YL, Kudaba I et al. Pembrolizumab (pembro) versus platinum‐based chemotherapy (chemo) as first‐line therapy for advanced/metastatic NSCLC with a PD‐L1 tumor proportion score (TPS) ≥1%: Open‐label, phase 3 KEYNOTE‐ 042 study. J Clin Oncol 2018;36(suppl 18):LBA4a. [Google Scholar]
- 18.Garassino M, Cho B, Kim JH et al. Durvalumab in ≥ 3rd‐line advanced NSCLC: Updated results from the phase 2 ATLANTIC study. J Clin Oncol 2018;36(suppl 15):9058a. [Google Scholar]
- 19.Rajan A, Gulley JL, Spigel DR et al. Avelumab (anti–PD‐L1) in patients with platinum‐treated advanced NSCLC: 2.5‐year follow‐up from the JAVELIN Solid Tumor trial. J Clin Oncol 2018;36(suppl 15):9090a. [Google Scholar]
- 20.Goldman JW, Antonia SJ, Gettinger SN et al. Nivolumab (N) plus ipilimumab (I) as first‐line (1L) treatment for advanced (adv) NSCLC: 2‐yr OS and long‐term outcomes from CheckMate 012. J Clin Oncol 2017;35(suppl 15):9093a. [Google Scholar]
- 21.Juergens R, Hellmann M, Brahmer J et al. OA 17.03 first‐line nivolumab plus platinum‐based doublet chemotherapy for advanced NSCLC: CheckMate 012 3‐year update. J Thorac Oncol 2017;12(11 suppl 2):S1792–S1793. [Google Scholar]
- 22.Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentzler RD, Langer CJ, Borghaei H et al. 24‐month overall survival from KEYNOTE‐021 cohort G: Pemetrexed‐carboplatin plus pembrolizumab as first‐line therapy for advanced nonsquamous NSCLC. J Clin Oncol 2018;36(suppl 15):9026a. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 25.LA Paz‐Ares LG, Tafreshi A et al. Phase 3 study of carboplatin‐paclitaxel/nab‐paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non‐small cell lung cancer (NSCLC). J Clin Oncol 2018;36(suppl 15):105a. [Google Scholar]
- 26.Antonia S, Goldberg SB, Balmanoukian A et al. Safety and antitumour activity of durvalumab plus tremelimumab in non‐small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol 2016;17:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons DL, Chow LQ, Kim DW et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti‐programmed cell death‐ligand‐1 (PD‐L1) antibody, combined with gefitinib (G): A phase I expansion in TKI‐naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11(4 suppl):S79. [Google Scholar]
- 28.Jotte RM, Cappuzzo F, Vynnychenko I et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab‐paclitaxel vs carboplatin + nab‐paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36(suppl 18):LBA9000a. [Google Scholar]
- 29.Reck M, Socinski MA, Cappuzzo F et al. Primary PFS and safety analyses of a randomized Phase III study of carboplatin + paclitaxel +/− bevacizumab, with or without atezolizumab in 1L non‐squamous metastatic NSCLC (IMpower150). Ann Oncol 2017;28(suppl 11):LBA1_PRa. [Google Scholar]
- 30.Kim DW, Gadgeel SM, Gettinger SN et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). J Clin Oncol 2018;36(suppl):9009a. [Google Scholar]
- 31.Manson G, Norwood J, Marabelle A et al. Biomarkers associated with checkpoint inhibitors. Ann Oncol 2016;27:1199–1206. [DOI] [PubMed] [Google Scholar]
- 32.Dong ZY, Wu SP, Liao RQ et al. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumour Biol 2016;37:4251–4261. [DOI] [PubMed] [Google Scholar]
- 33.Peters S, Creelan B, Hellmann MD et al. Impact of tumor mutation burden on the efficacy of first‐line nivolumab in stage iv or recurrent non‐small cell lung cancer: An exploratory analysis of CheckMate 026. Cancer Res 2017;77:CT082a. [Google Scholar]
- 34.Antonia SJ, Lopez‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 35.Ott PA, Elez E, Hiret S et al. Pembrolizumab in patients with extensive‐stage small‐cell lung cancer: Results from the phase Ib KEYNOTE‐028 study. J Clin Oncol 2017;35:3823–3829. [DOI] [PubMed] [Google Scholar]
- 36.Zhou CC, Gao GH, Wu FY et al. A phase Ib study of SHR‐1210 plus apatinib for heavily previously treated advanced non‐squamous non‐small cell lung cancer (NSCLC) patients. J Clin Oncol 2018;36(suppl 15):e21017a. [Google Scholar]
- 37.Liu SY, Wu YL. Ongoing clinical trials of PD‐1 and PD‐L1 inhibitors for lung cancer in China. J Hematol Oncol 2017;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hida T, Nishio M, Nogami N et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non‐small cell lung cancer. Cancer Sci 2017;108:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal CR, Abreu DR, Felip E et al. Prevalence of PD‐L1 expression in patients with non‐small cell lung cancer screened for enrollment in KEYNOTE‐001, ‐010, and ‐024. Ann Oncol 2016;27:1060P. [Google Scholar]
- 40.Jiang L, Su X, Zhang T et al. PD‐L1 expression and its relationship with oncogenic drivers in non‐small cell lung cancer (NSCLC). Oncotarget 2017;8:26845–26857. [DOI] [PMC free article] [PubMed] [Google Scholar]