Abstract
Purpose
Initial dose of chemotherapy is planned based on body surface area, which does not take body composition into account. We studied the association between fat mass (kg and relative to total body weight) as well as lean mass (kg and relative to total body weight) and toxicity-induced modifications of treatment in breast cancer patients receiving chemotherapy.
Methods
In an observational study among 172 breast cancer patients (stage I–IIIB) in the Netherlands, we assessed body composition using dual-energy X-ray scans. Information on toxicity-induced modifications of treatment, defined as dose reductions, cycle delays, regimen switches, or premature termination of chemotherapy, was abstracted from medical records. Adjusted hazard ratios and 95% confidence intervals (95% CI) were calculated to assess associations between body composition and the risk of toxicity-induced modifications of treatment.
Results
In total, 95 out of 172 (55%) patients experienced toxicity-induced modifications of treatment. Higher absolute and relative fat mass were associated with higher risk of these modifications (HR 1.14 per 5 kg; 95% CI 1.04–1.25 and HR 1.21 per 5%; 95% CI 1.05–1.38, respectively). A higher relative lean mass was associated with a lower risk of modifications (HR 0.83 per 5%; 95% CI 0.72–0.96). There was no association between absolute lean mass and risk of toxicity-induced modifications of treatment.
Conclusions
A higher absolute and a higher relative fat mass was associated with an increased risk of toxicity-induced modifications of treatment. Absolute lean mass was not associated with risk of these treatment modifications, while higher relative lean mass associated with lower risk of modifications. These data suggest that total fat mass importantly determines the risk of toxicities during chemotherapy in breast cancer patients.
Electronic supplementary material
The online version of this article (10.1007/s10549-018-5014-5) contains supplementary material, which is available to authorized users.
Keywords: Chemotherapy, Toxicity, Breast cancer, Body composition, Fat mass
Introduction
Breast cancer patients are often treated with chemotherapy, which usually consist of a combination of anthracyclines (e.g., doxorubicin, epirubicin) and taxanes (e.g., paclitaxel, docetaxel) with or without targeted therapy [1, 2]. Severe side effects during chemotherapy can lead to a dose reduction, cycle delay, or premature termination of treatment. These toxicity-induced modifications of treatment may eventually lead to a reduced dose intensity, and worse outcome [3].
In clinical practice, the administered dose of the chemotherapy is based on the body surface area. Body surface area is usually calculated using the Mosteller formula based on height and weight [4]. However, it does not distinguish lean mass from fat mass or other characteristics of body composition. It has been suggested that body composition may be more important than body surface area for calculating the administered dose of chemotherapy, since previous studies in other cancer types showed that patients with low lean mass have a higher risk of toxicities as a result of treatment [5–9].
In patients with metastatic breast cancer, sarcopenia as well as low muscle mass or low lean mass were associated with increased risk of toxicities during chemotherapy [10–12]. Body composition of metastatic breast cancer patients generally differs from early stage breast cancer patients, because of disease-related sarcopenia and/or cachexia. Therefore, findings in metastatic breast cancer may not be generalizable to early stage breast cancer [10, 12, 13].
So far, only two studies on body composition in association with treatment-related toxicities in early breast cancer patients were published. Both studies suggested that a lower lean mass was associated with an increased risk of toxicities [14, 15].
The goal of this paper was to focus not only on lean mass, but also to study associations between fat mass and risk of toxicities. Thus, we aimed to assess the association between fat mass, lean mass, and the risk of toxicity-induced modifications of treatment [16] in women with stage I-IIIB breast cancer receiving (neo)adjuvant chemotherapy.
Methods
Participants
This study is part of the COBRA-study, an observational multi-centre study among breast cancer patients receiving (neo)adjuvant chemotherapy [17]. Eligible patients were recruited by the medical staff from 11 academic and peripheral hospitals in the Netherlands prior to commencement of chemotherapy. Women were eligible if they were newly diagnosed with operable stage I–IIIB breast cancer, and scheduled to receive (neo)adjuvant chemotherapy. Participants needed to be at least 18 years old and be able to communicate in Dutch. Exclusion criteria were history of cancer, previous treatment with chemotherapy, (intended) pregnancy during the study period, dementia, or other mental conditions that made it impossible to comply with the study procedures.
For the current analyses, data were available for 176 breast cancer patients recruited between May 2013 and September 2016. Four patients had to be excluded, because they had no dual-energy X-ray absorption (DEXA)-scan available. In total, we considered 172 patients for the analyses for this study.
The study protocol was approved by the Medical Ethical Committee of Wageningen University & Research, the Netherlands. All participants provided written informed consent.
Data collection
Body composition
Body composition was assessed using a dual energy X-ray absorptiometry (DEXA) scan. Participants were measured in the hospitals by trained technicians using a total body scan protocol prior to start of chemotherapy (n = 86) or during the first cycle of chemotherapy (n = 86). Based on the total body DEXA-scan body weight (kg), fat mass (kg and relative to total body weight), and lean mass (kg and relative to total body weight) were assessed. Body composition data were included in the models as continuous variables. In addition, we categorized patients into tertiles of lean mass, and tertiles of fat mass (Supplementary Tables 2 and 3). Using this categorization, we defined the lowest tertile of lean mass as “low lean mass,” and the upper two tertiles as “normal lean mass;” in addition, the highest tertile of fat mass was defined as “high fat mass” and the lower two tertiles as “normal fat mass.” Based on this categorization, we created four groups: (1) patients with a normal lean mass and normal fat mass, (2) patients with a normal lean mass and high fat mass, (3) patients with low lean mass and normal fat mass, or (4) patients with low lean mass and high fat mass.
Toxicity-induced modifications of treatment
Information regarding chemotherapy and toxicity was abstracted from medical records using a standardized form. Treatment information included detailed information on type and dose of chemotherapy, number of cycles planned, and start dates of each cycle. Furthermore, information on actual administered dose, toxicities, and reasons for treatment modifications were collected per cycle. Toxicity-induced modifications of treatment were defined as dose reductions, cycle delays, or premature termination of chemotherapy [16]. Also, if a planned cytotoxic regime was changed to another regime because of toxicities, this was reported as a treatment modification. If the reason for a dose reduction or cycle delay was unknown (n = 4), we included them as toxicity-induced modifications of treatment. Logistical or other non-medical reasons for cycle delays were not classified as toxicity-induced modification of treatment.
Patient and clinical characteristics
Information about tumour stage at diagnosis and timing of chemotherapy (adjuvant versus neo-adjuvant) was collected from medical records. Information regarding age at cancer diagnosis and height was collected using a general questionnaire. Based on body weight of the DEXA-scan and self-reported height, BMI at diagnosis was calculated. Chemotherapy regimens were categorized as combined or sequential regimes (Supplementary Table 1); combined regimes included schemes with different components administered together during all cycles, and included TAC, FEC, DOC-CYCLO, CDT(P) PT, and CTP. Sequential regimes included schemes with different components that were administered in different cycles and included AC/P(T), FEC/DOC, and AC/DOC/(T).
Data analysis
Population characteristics are presented as median with interquartile range (IQR) or number with percentage for the total study population, and participants experiencing a toxicity-induced modification of treatment (yes vs. no) separately.
Hazard ratios (HRs) and 95% CI were calculated to assess the association between body composition and time to treatment modification using a Cox proportional hazard regression model; time was expressed as the number of completed cycles of chemotherapy until there was treatment modification. For example, if a patient had 6 planned cycles of chemotherapy and did not experience any toxicity-induced modification of treatment, time was censored at 6 cycles. The proportional hazard assumption was checked and was not violated, as evaluated by the goodness-of-fit test using Schoenfeld residuals (p > 0.05).
For all analyses, we constructed a separate model for each body composition parameter, i.e., BMI (kg/m2), fat mass per 5%, fat mass per 5 kg, lean mass per 5%, and lean mass per 5 kg. In addition, we assessed HRs for the following four groups: (1) patients with a normal lean mass and normal fat mass versus, (2) patients with a normal lean mass and high fat mass, (3) patients with low lean mass and normal fat mass, or (4) low lean mass and high fat mass. Stratified analyses were conducted for patients receiving a sequential regime versus a combined regime, and for patients receiving adjuvant chemotherapy versus neo-adjuvant chemotherapy. A sensitivity analysis was conducted for toxicity-induced treatment modifications occurring within the first 6 cycles of chemotherapy. This was done to account for the fact that patients with a higher number of cycles planned have higher odds of experiencing toxicities as they go through more cycles. In this sensitivity analysis, only toxicity-induced treatment modifications occurring within the first 6 cycles were considered.
Analyses were adjusted for age, since older women have an increased risk of experiencing toxicities and age is associated with specific body composition characteristics [18]. Based on literature, BSA was considered as potential covariate, but not included in the analyses since BSA was strongly related with the body composition parameters (multicollinearity). All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
The median age of the 172 women included was 51.8 years (Table 1). More than half of the patients were overweight or obese at diagnosis. Most patients had a stage II tumour, received adjuvant chemotherapy with a sequential regime consisting of 6 or less planned cycles. Table 1 shows that women experiencing a toxicity-induced modification of treatment were more often treated with a sequential regime of adjuvant chemotherapy compared to the women not experiencing a treatment modification. In addition, women experiencing a toxicity-induced modification of treatment had a higher body weight, were more often overweight or obese, had a higher fat mass and lower percentage of lean mass compared to the women not experiencing a modification.
Table 1.
Demographic, clinical, and body composition characteristics of breast cancer patients treated with chemotherapy
| Total (n = 172) | Toxicity-induced modification of treatment: yes (n = 95) | Toxicity-induced modification of treatment: no (n = 77) | P value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (median, IQR) | 51.8 (46.8; 59.1) | 52.1 (47.4; 60.8) | 51.5 (46.4; 54.6) | 0.16 |
| Medical profile | ||||
| Stage (n, %) | 0.82 | |||
| I | 44 (25.6) | 26 (27.4) | 18 (23.4) | |
| II | 105 (61.1) | 57 (60.0) | 48 (62.3) | |
| III | 23 (13.4) | 12 (12.6) | 11 (14.3) | |
| Chemotherapy (n, %) | 0.68 | |||
| Adjuvant | 111 (64.5) | 67 (70.5) | 44 (57.1) | |
| Neo-adjuvant | 61 (35.5) | 28 (29.5) | 33 (42.9) | |
| Type of chemotherapy (n, %) | 0.03 | |||
| Combined regime | 78 (45.3) | 36 (37.9) | 42 (54.6) | |
| Sequential regime | 94 (54.7) | 59 (62.1) | 35 (45.5) | |
| Number of cycles chemotherapy (n, %) | < 0.01 | |||
| 6 or less | 119 (69.2) | 57 (60.0) | 62 (80.5) | |
| More than 6 | 53 (30.8) | 38 (40.0) | 15 (19.5) | |
| Anthropometry and body composition | ||||
| Body weight, kg (median, IQR) | 70.5 (63.9; 81.7) | 74.1 (64.4; 84.6) | 68.2 (63.1; 76.1) | 0.04 |
| Height, cm (median, IQR) | 168 (164; 173) | 168 (162; 173) | 168 (164; 173) | 0.30 |
| Body surface area (BSA), (median, IQR) | 1.8 (1.7; 2.0) | 1.8 (1.7; 2.0) | 1.8 (1.7; 1.9) | 0.08 |
| Body mass index (BMI) kg/m2 (median, IQR) | 25.5 (22.5; 29.1) | 26.5 (23.9; 29.8) | 24.5 (21.7; 27.2) | < 0.01 |
| Fat mass, percentage (median, IQR) | 36.7 (31.4; 42.2) | 38.6 (33.7; 44.8) | 35.0 (29.7; 39.9) | < 0.01 |
| Fat mass, kg (median, IQR) | 26.0 (20.2; 34.2) | 27.6 (20.8; 36.3) | 23.1 (18.4; 31.1) | 0.01 |
| Lean mass, percentage (median, IQR) | 60.2 (33.2; 65.1) | 58.5 (53.1; 63.5) | 61.8 (57.2; 66.7) | < 0.01 |
| Lean mass, kg (median, IQR) | 43.1 (29.4; 46.8) | 43.1 (39.5; 47.5) | 42.8 (39.3; 46.6) | 0.76 |
| Appendicular skeletal mass, kg (median, IQR) | 18.2 (16.8; 20.2) | 18.2 (16.6; 20.2) | 18.2 (16.9; 20.1) | 0.83 |
| Skeletal muscle index, kg/m2 (median, IQR) | 6.5 (6.0; 7.2) | 6.5 (6.0; 7.3) | 6.4 (6.; 7.1) | 0.56 |
Data are given for the total group and stratified by “Toxicity-induced modification of treatment” Yes or No
*Mann-Witney U for continuous variables, χ2 for categorical variables
Table 2 specifies how often the specific types of treatment modifications occurred. During chemotherapy, more than half of the patients experienced an adjustment in relative dose intensity (95 out of 172 patients, 55%). Of these 95 patients, 14% (n = 13) stopped prematurely, 53% (n = 50) had a dose reduction, and 34% (n = 32) had a cycle delay as their first toxicity-induced modification of treatment. In total, 48% (n = 57) of the women receiving 6 or less planned cycles experienced a toxicity-induced modification versus 72% (n = 38) of the women receiving more than 6 planned cycles.
Table 2.
Distribution of the three types of treatment modifications as experienced by breast cancer patients in the COBRA study among patients who had no more than 6 planned chemotherapy cycles versus more than 6 cycles
| Toxicity | All patients (n = 172) | 6 planned cycles or less (n = 119) | More than 6 planned cycles (n = 53) |
|---|---|---|---|
| Any modification, n (%) | 95 (55.2) | 57 (47.9) | 38 (71.7) |
| Cycle delay | 32 (33.7) | 17 (29.8) | 15 (39.5) |
| Dose reduction | 50 (52.6) | 36 (63.2) | 14 (36.8) |
| Premature termination | 13 (13.7) | 4 (7.0) | 9 (23.7) |
A higher BMI was associated with a higher risk of toxicity-induced modifications of treatment (HR 1.06 per kg/m2 (95% CI 1.02–1.11). Higher absolute fat mass was associated with higher risk of modifications [HR per 5 kg fat mass: 1.14 (95%CI 1.04–1.25)]. Higher relative fat mass was also associated with a higher risk of modifications [HR per 5% increase in fat mass: 1.21 (95% CI 1.05–1.38)], see Table 3. A higher percentage of relative lean mass was associated with a lower risk of toxicity-induced modification of treatment [HR per 5 percent increase in lean mass: 0.83 (95% CI 0.72–0.96)]. Absolute lean mass in kg was not associated with treatment modifications (Table 3).
Table 3.
Association between body composition parameters and risk of toxicity-induced modifications of treatment in early stage breast cancer patients
| Variable | Total/cases | HR | 95% CI |
|---|---|---|---|
| All patients | |||
| BMI per kg/m2 | 172/95 | 1.06 | 1.02; 1.11 |
| Fat mass per 5% | 172/95 | 1.21 | 1.05; 1.38 |
| Fat mass per 5 kg | 172/95 | 1.14 | 1.04; 1.25 |
| Lean mass per 5% | 172/95 | 0.83 | 0.72; 0.96 |
| Lean mass per 5 kg | 172/95 | 1.07 | 0.92; 1.27 |
| Appendicular skeletal mass per kg | 172/95 | 1.01 | 0.95; 1.08 |
| Skeletal muscle index per kg/m2 | 172/95 | 1.07 | 0.87; 1.32 |
| Sensitivity analysis: modifications in the first 6 cycles | |||
| BMI per kg/m2 | 172/73 | 1.06 | 1.01; 1.11 |
| Fat mass per 5% | 172/73 | 1.18 | 1.01; 1.37 |
| Fat mass per 5 kg | 172/73 | 1.13 | 1.02; 1.25 |
| Lean mass per 5% | 172/73 | 0.85 | 0.72; 1.00 |
| Lean mass per 5 kg | 172/73 | 1.08 | 0.91; 1.30 |
| Appendicular skeletal mass per kg | 172/73 | 1.02 | 0.95; 1.09 |
| Skeletal muscle index per kg/m2 | 172/73 | 1.10 | 0.88; 1.38 |
HRs were adjusted for age
Bold values are statistically significant
A total of 54 patients had a low lean mass in combination with high fat mass (Table 4). Having a low lean mass in combination with high fat mass was associated with a higher risk of toxicity-induced modifications of treatment versus having a normal lean mass in combination with normal fat mass (HR 1.33, 95% CI 1.01–1.75).
Table 4.
Categorization of patients into body composition groups and association of body composition groups with risk of toxicity-induced modifications of treatment in early stage breast cancer patients
| Group | Total | Toxicity-induced modification of treatment: yes | Toxicity-induced modification of treatment: no | HR |
|---|---|---|---|---|
| Normal lean and normal fat | 111 | 55 | 56 | Ref |
| Normal lean and high fat* | 4 | 2 | 2 | – |
| Low lean and normal fat* | 3 | 1 | 2 | – |
| Low lean and high fat | 54 | 37 | 17 | 1.33 (1.01, 1.75) |
Categorization is based on tertiles with “normal lean mass” defined as the upper two tertiles of lean mass, “normal fat mass” as the lowest two tertiles of fat mass, “low lean mass” as the lowest tertile of lean mass and “high fat mass” as the upper tertile of fat mass
*Not included in Cox Proportional hazard analysis, because of low numbers. HR adjusted for age
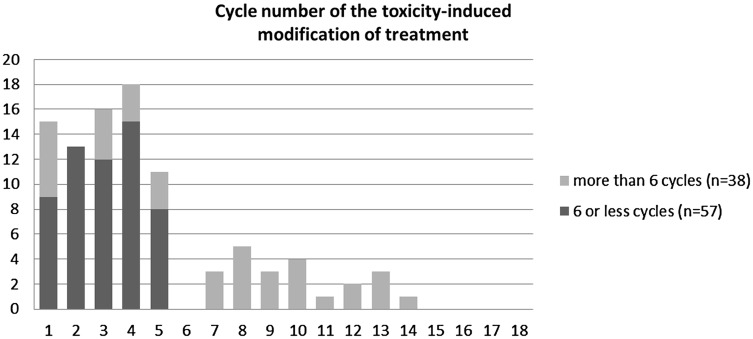
Figure 1 shows the time in cycle numbers until the occurrence of the first toxicity-induced modification of treatment. In total, 73 of the 95 women (77%) experienced their first modification within the first 6 cycles of chemotherapy. Sensitivity analyses including only modifications occurring within the first 6 cycles showed similar results compared to the analysis in which we included modifications in all cycles (Table 3).
Fig. 1.
Cycle number in which the first toxicity-induced modification of treatment occurred, stratified for patients receiving a chemotherapy regime consisting of 6 or less planned cycles and patients receiving a chemotherapy regime consisting of more than 6 planned cycles
Stratified results did not suggest that the associations between body composition and toxicity-induced modifications of treatment were different for combined versus sequential regimes, nor for neo-adjuvant versus adjuvant chemotherapy (data not shown). We had insufficient power to stratify by specific chemotherapy regimens, but none of the regimens appeared more related to toxicity profiles than others (Supplementary Table 1).
Discussion
This study showed that a higher absolute or relative fat mass and a lower percentage of lean mass were associated with an increased risk of toxicity-induced modifications of treatment in stage I-IIIB breast cancer patients treated with chemotherapy, while absolute lean mass in kg was not associated with these modifications. Our results suggest that fat mass strongly determines the risk of treatment modifications during chemotherapy in breast cancer patients.
Two earlier studies [14, 15] stressed the importance of total lean mass in the association with chemotherapy-induced toxicities. However, those studies did not assess chemotherapy-induced toxicities in association with relative lean mass or total fat mass (kg or percentage). The first study (n = 151) used CT-scans to assess body composition and concluded that lower total lean mass and skeletal muscle gauge—a composite endpoint of muscle mass and muscle radio density—were associated with increased risk of treatment-related grade 3–4 toxicities in patients receiving doxorubicin-cyclophosphamide (AC)-taxane-based cytotoxic regimens [14]. The second study (n = 24) concluded that a lower total lean mass was associated with higher incidence of dose-limiting toxicities during the first cycle of FEC100 [15], again with CT-scan as measurement of body composition. Both studies extrapolated total lean mass from skeletal muscle cross-sectional area of a CT-scan at the level of the third vertebrae, but did not report results of toxicity associations with percentage of total lean mass or fat mass. Moreover, the study populations of both studies differed from our population, making it challenging to compare the results. For example, in the study of Prado 22 out of 24 patients experienced an adjustment in relative dose during the first cycle of chemotherapy [15], which is considerably more than our study where 15 out of 172 patients experienced a toxicity-induced modification of treatment after the first cycle. This suggests that the selection process of participants eligible for their study led to a group of patients at high risk of toxicities which may impact the generalizability of those findings. In the study by Shachar et al, the average BMI was 2–3 kg/m2 higher than in our study, while lean mass was slightly lower [14]. Thus, it is plausible that fat mass was higher in that study, and that patients with lower lean, and/or higher fat mass experienced the highest risk of toxicities, in line with the results of our current study. Yet, baseline differences in body composition between studies plus different outcome measures to assess toxicities obstruct direct comparison between studies. We could not differentiate between visceral and subcutaneous abdominal adiposity with our DEXA data. New algorithms are emerging that enable this distinction, which opens up avenues for further study [19].
Possible mechanisms for the observed association between body composition and toxicity-induced modifications of treatment are unclear, but could be either biological or clinical. Depending on the type of cytotoxic agent, drugs may be more hydrophilic or hydrophobic which will affect the clearance and volume of distribution of the drugs. For hydrophilic drugs, it has been hypothesized that patients with a relatively lower lean mass may be overdosed when using body surface area to calculate dosage, and may present with higher rates of toxicity-induced modifications of treatment. In our study, stratified results based on type of chemotherapy did not suggest that associations between body composition and toxicity-induced modifications of treatment were different for combined versus sequential regimes, but we did not have sufficient statistical power for further stratifications.
A more clinical, although speculative, explanation for a higher risk of toxicities in patients with low lean mass could be that clinicians treat patient with a lower percentage of lean mass differently than patients with a higher percentage lean mass, although lean mass is seldom formally assessed in clinical practice. Nevertheless, patients with a low lean mass may be frailer, and may generally experience other comorbidities, which could prompt the medical oncologist to adapt the chemotherapy protocol earlier than patients with a better physical condition.
In conclusion, a higher BMI and a higher fat mass (kg and percentage) are associated with an increased risk of toxicity-induced modifications of treatment, while absolute lean mass was not associated with risk of these modifications. This suggests that total fat mass strongly determines the risk of toxicity-induced modifications of treatment during chemotherapy in breast cancer patients. These findings highlight the importance of fat mass in relation to toxicities and provide unique leads for further clinical studies focussing on implementation of body composition measures during planning of chemotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants for their time to participate in the study. Furthermore, we thank the staff of the following hospitals that helped recruiting the participants: Ziekenhuis Gelderse Vallei, Maxima Medisch Centrum, Reinier de Graaf Ziekenhuis, Onze Lieve Vrouwen Gasthuis, Amphia Ziekenhuis, Canisius Wilhelmina Ziekenhuis, Radboud Universitair Medisch Centrum, Alexander Monro Ziekenhuis, St. Antonius Ziekenhuis, St. Anna Ziekenhuis and Flevoziekenhuis. We would like to thank Merel Snellen for her help during data collection.
Funding
This study was funded by the Dutch Cancer Society (Grant Numbers UW2011-4987 and UW2011-5268).
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval
All procedures performed in the study described in this manuscript were in accordance with the ethical standards of the institutional and/or national research committees.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Richtlijnen oncologische zorg mammacarcinoom [Clinical guidelines breast cancer care] [https://richtlijnendatabase.nl/en/richtlijn/breast_cancer/locoregional_treatment/regional_treatment_for_breast_cancer.html. Accessed 5 Sept 2018
- 2.Denduluri N, Somerfield MR, Eisen A, Holloway JN, Hurria A, King TA, Lyman GH, Partridge AH, Telli ML, Trudeau ME, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2) -negative and adjuvant targeted therapy for HER2-positive breast cancers: an American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol. 2016;34(20):2416–2427. doi: 10.1200/JCO.2016.67.0182. [DOI] [PubMed] [Google Scholar]
- 3.Hebert-Croteau N, Brisson J, Latreille J, Rivard M, Abdelaziz N, Martin G. Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. J Clin Oncol. 2004;22(18):3685–3693. doi: 10.1200/JCO.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 5.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66(4):583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 6.Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, Lee KW, Kim DW, Kang SB, Kim KI, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015;23(3):687–694. doi: 10.1007/s00520-014-2418-6. [DOI] [PubMed] [Google Scholar]
- 7.Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13(11):3264–3268. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 8.Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, Mollevi C, Senesse P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5(4):607–616. doi: 10.1002/cam4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anandavadivelan P, Brismar TB, Nilsson M, Johar AM, Martin L. Sarcopenic obesity: a probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. 2016;35(3):724–730. doi: 10.1016/j.clnu.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 11.Wong AL, Seng KY, Ong EM, Wang LZ, Oscar H, Cordero MT, Copones R, Fan L, Tan SH, Goh BC, et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat. 2014;144(1):143–152. doi: 10.1007/s10549-014-2843-8. [DOI] [PubMed] [Google Scholar]
- 12.Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, Benbow JM, Muss HB. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;23(3):658–665. doi: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care. 2013;7(4):383–389. doi: 10.1097/SPC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 14.Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, Choi SK, Muss HB. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane–based chemotherapy for early-stage breast cancer. Clin Cancer Res. 2017;23(14):3537–3543. doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, Mackey JR, Kuzma M, Damaraju VL, Sawyer MB. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67(1):93–101. doi: 10.1007/s00280-010-1288-y. [DOI] [PubMed] [Google Scholar]
- 16.Kok DE, Winkels RM, van Herpen CML, Kampman E (2018) Toxicity-induced modification of treatment (TIMT): what’s in a name? Eur J Cancer [DOI] [PubMed]
- 17.de Vries YC, van den Berg MM, de Vries JH, Boesveldt S, de Kruif JT, Buist N, Haringhuizen A, Los M, Sommeijer DW, Timmer-Bonte JH, et al. Differences in dietary intake during chemotherapy in breast cancer patients compared to women without cancer. Support Care Cancer. 2017;25(8):2581–2591. doi: 10.1007/s00520-017-3668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, Norton L, Winer EP, Hudis CA, Cancer, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25(24):3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 19.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20(5):1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.