Abstract
Cell polarity, manifested by the localization of proteins to distinct polar plasma membrane domains, is a key prerequisite of multicellular life. In plants, PIN auxin transporters are prominent polarity markers crucial for a plethora of developmental processes. Cell polarity mechanisms in plants are distinct from other eukaryotes and still largely elusive. In particular, how the cell polarities are propagated and maintained following cell division remains unknown. Plant cytokinesis is orchestrated by the cell plate – a transient centrifugally growing endomembrane compartment ultimately forming the cross wall1. Trafficking of polar membrane proteins is typically redirected to the cell plate, and these will consequently have opposite polarity at least in one of the daughter cells2–5. Here, we provide mechanistic insights into post-cytokinetic re-establishment of cell polarity as manifested by the apical, polar localization of PIN2. We show that the apical domain is defined in a cell-intrinsic manner and that re-establishment of PIN2 localization to this domain requires de novo protein secretion and endocytosis, but not transcytosis. Furthermore, we identify a PINOID-related kinase WAG1, which phosphorylates PIN2 in vitro6 and is transcriptionally upregulated specifically in dividing cells, as a crucial regulator of post-cytokinetic PIN2 polarity re-establishment.
Cells in the Arabidopsis root meristem are a perfect model to study cell polarity as they possess at least 4 distinct plasma membrane (PM) domains marked by the asymmetric accumulation of different cargoes7 (Fig. 1a). Among these, PIN2 in the epidermis is particularly interesting for its pronounced apical polarity, which is crucial for shootward auxin transport and root gravitropism8,9. It has been reported that during cytokinesis, PM cargoes, including polarly localized ones, localize to the cell plate (CP)2–5,10. To dissect which trafficking pathways were responsible for CP cargo delivery we utilized the Dendra photoconvertible marker11. When we photoconverted functional (Fig. S1) PIN2-Dendra or Dendra-PIP1;4 from green to red prior to cytokinesis, we observed the contribution of both de novo secretion and endocytic recycling to the CP, (Fig. 1b-c, Fig. S1, Movie S1), consistently with previous findings10. It was reported that in newly divided cells, PIN2 is localized at both sides of the cross wall4. Thus, the rerouting of endomembrane trafficking during cytokinesis results in apolar localization of the otherwise strictly polar PIN24,12.
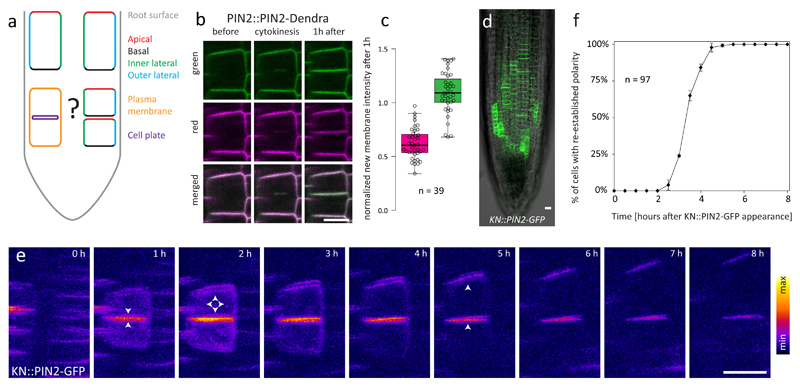
Figure 1. Cell polarity needs to be re-established after cytokinesis.
a: Cells in the Arabidopsis root possess at least 4 distinct PM domains: apical, basal, inner lateral and outer lateral7. During cytokinesis, virtually all membrane traffic is redirected to the cell plate12, implying that the daughter cells must have a mechanism to re-establish polarity afterwards. b: Both pre-existing (magenta) ad newly synthesized (green) pools of PIN2-Dendra localize to the CP and newly formed PM in cells that were photoconverted prior to the onset of cytokinesis. c: Quantification of b. The graph shows signal intensity at the newly formed PM 1h after cytokinesis normalized to the intensity of the neighbouring old PMs in both red and green channels; higher relative intensity of the green channel confirms predominant contribution of de novo secretion to the CP protein pool. The box-plot represents median, 1st and 3rd quartile; the whiskers extend to data points <1,5 interquartile range away from the 1st/3rd quartile; all data points are shown as circles. n indicates the number of cells from 8 roots and 3 independent experiments d: Expression pattern of the KN::PIN2-GFP construct in the root meristem. The experiment was repeated independently >3 times with similar results. e: A time-series of a single newly-divided cell pair expressing KN::PIN2-GFP. Up to 1 hour after cytokinesis, signal is almost exclusively at the CP. 1-3 hours after cytokinesis, signal appears at all PM domains, and typical apical polar distribution pattern is re-established 2-5 hours after cytokinesis. Arrowheads indicate predominant signal localization. f: Quantitative analysis of the dynamics of KN::PIN2-GFP polarity re-establishment. The timepoint at which both daughter cells had clearly apically localized KN::PIN2-GFP signal (between 4 and 5 h in case of the cell pair shown in e) was scored for each cell pair, and the percentage of cell pairs with re-established polarity was plotted against time. The graph shows mean +/- SD of 3 independent experiments, n indicates the total number of cell pairs. The number of roots/cell pairs analysed in each experiment was 5/47, 3/30 and 2/20, respectively. Scale bars = 10 μm
When is apical PIN2 localization subsequently re-established, and which cellular mechanisms are required for this process? There was no difference in PIN2 promoter activity between the daughter cells (Fig. S1), implying post-transcriptional regulation. To address the dynamic changes of subcellular PIN2 localization, live-cell imaging is indispensable since time is the decisive variable. However, exact determination of PIN2 polar localization dynamics is difficult using PIN2::PIN2-XFP reporter lines, since the original apical domain is pre-occupied with molecules inherited from the mother cell, conventional microscopy cannot distinguish between adjacent newly formed PMs due to the diffraction limit, and super-resolution techniques are limited in time-lapse potentiality and throughput. To overcome these limitations, we expressed PIN2-GFP from the cytokinesis-specific KNOLLE promoter13 and performed time-lapse imaging. This approach enabled us to observe the trafficking fate of molecules synthesized in a narrow time window during and immediately after the cytokinetic event (Fig. 1d-e, Movie S2). In recently divided cells, the KN::PIN2-GFP signal could be observed almost exclusively at the CP/new PM, further confirming redirection of secretion to the CP during cytokinesis12, but also afterwards (Fig. 1d-e). 1-2 hours after cytokinesis, KN::PIN2-GFP signal was still strongest at the newly formed membrane pair but started to appear also at the apical and lateral domains of the upper cell. By 3-5 hours after cytokinesis, the signal clearly localized to the apical domains of both daughter cells, marking completed polarity re-establishment (Fig. 1e-f). KN::PIN2-GFP only non-significantly rescued root gravitropism in the pin2 mutant, did not affect the phenotype of Col-0, and its signal intensities at newly formed PMs were comparable to those in a complementing PIN2::PIN2-GFP line (Fig. S1), suggesting that the observed localization was not an overexpression artefact.
Taken together, these results confirm that during and immediately after cytokinesis, virtually all membrane traffic of both daughter cells is re-directed to the CP12, creating a situation in which the lower cell has a correctly, i.e. apically localized PIN2, but the upper daughter cell has an ectopic basally localized pool of PIN2 in addition to the apical pool inherited from the mother cell4. Therefore, the cell must possess a mechanism to re-establish proper localization of PIN2 and other polar proteins (Fig. 1a).
In plants, cell fate is determined by positional information conveyed by numerous intercellular signalling molecules14. We therefore tested whether tissue context and cell-to-cell signalling is required for re-establishment of cell polarity after cytokinesis. In other developmental contexts, for example during organogenesis or vascularization, auxin itself serves as polarizing cue for the localization of PIN proteins15,16; therefore, we first tested whether post-cytokinetic polarity re-establishment is regulated by auxin levels in cells or its directional flow across the tissue. Nonetheless, PIN2 polarity developed normally in KN::PIN2-GFP roots when we exogenously applied the natural auxin Indole-3-Acetic Acid (IAA) (Fig. S2,S6). In plants treated with the auxin transport inhibitor Naphtyl Phtalamic Acid (NPA)17, apical polarity was established correctly, but with a significant delay (Fig. 2a-b,e, S6).
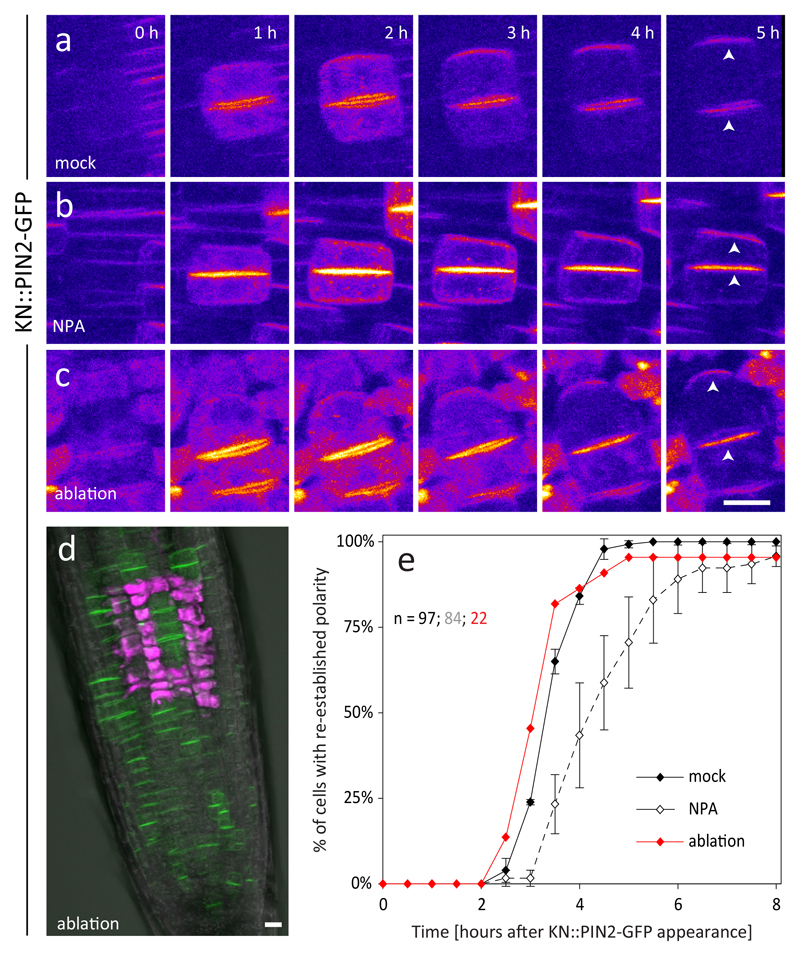
Figure 2. Apical-basal polarity of newly divided cells is established in a cell-intrinsic manner.
a-b: Re-establishment of apical PIN2 polarity in KN::PIN2-GFP plants treated with the auxin transport inhibitor NPA (b) is qualitatively not altered compared to the mock control (a). c: Laser ablation of surrounding cells does not prevent the re-establishment of apical PIN2 polarity. d: KN::PIN2-GFP expressing root in which laser ablated cells are marked by the uptake of PI (magenta). The experiment was repeated independently 3 times with similar results. e: quantitative analysis of the dynamics of KN::PIN2-GFP polarity re-establishment in a-c. NPA treatment causes a delay of polarity re-establishment compared to the control, while laser ablation of surrounding cells has no effect. The control is the same as in 1f. The graph shows mean +/- SD of 3 independent experiments, n indicates the total number of cell pairs. The number of roots/cell pairs analysed in each experiment was 4/20, 4/35 and 3/29 in b and 3/9, 1/5 and 4/8 in c, respectively. Due to the smaller sample size of c caused by technical limitations, values from all experiments were pooled and analysed together. Arrowheads indicate apical polar localization of PIN2-GFP. Scale bars = 10 μm
To test more generally for a requirement of tissue context, we separated the KN::PIN2-GFP root meristematic and transition zone from (i) the root tip, (ii) the differentiation zone and the rest of the plant, or (iii) both. The pattern of cell division occurrence and orientation was disturbed as described before18 but PIN2 polarity re-establishment remained unaffected (Fig. S2, S6), suggesting that this process does not depend on long-range cell-to-cell signalling. To address the importance of short-range signalling, we isolated small patches of cells from their neighbours by laser ablation. We could still observe normal re-establishment of apical PIN2 polarity in isolated patches of as few as three cells (Fig. 2c-e, S6), arguing against the influence of short-range signalling.
Together, these results indicate that while auxin transport and/or signalling can affect PIN trafficking and polarity, presumably through transcriptional reprograming16, neither polarized auxin flow nor other cell-to-cell signalling pathways are primary cues defining apical-basal polarity in newly divided root cells. Therefore, post-cytokinetic polarity re-establishment must be governed by an unknown cell-intrinsic mechanism instead.
We next addressed the cellular machinery that executes PIN2 polarity re-establishment. To test whether the ectopic basal PIN2 molecules were delivered to the apical domain by transcytosis (trafficking-based protein translocation between polar domains)19, we created a KN::PIN2-Dendra line, photoconverted newly divided cell pairs and followed them during polarity re-establishment. The apical signal distribution pattern developed in both channels showing contribution of both pre-existing PIN2 molecules (in magenta) and the de novo synthesized PIN2 (in green) (Fig. 3a-b). However, when we, after photoconversion, photobleached the red signal from the endosomes and all cell sides except the new, CP-derived PMs, we did not observe a significant apical signal in the red channel (Fig. 3c-d) arguing against significant contribution of basal-to-apical PIN2 transcytosis to polarity establishment in this context. Furthermore, only weak, presumably lateral-diffusion-based redistribution of red signal was observed after photoconversion of the new PM domain in PIN2::PIN2-Dendra, and apical signal was not restored in KN::PIN2-GFP cells treated with the translation inhibitor cycloheximide (CHX) (Fig. S3, S6). Finally, treatment with the ARF-GEF inhibitor Brefeldin A (BFA) prevented PIN2 polarity re-establishment of KN::PIN2-GFP in the big3 mutant, where it inhibits secretion12, but not in the wild type (Fig. S3, S6). Taken together, these data demonstrate that re-establishment of PIN2 apical polarity depends on secretion of de novo synthesized PIN2 molecules but not on their basal-to-apical transcytosis.
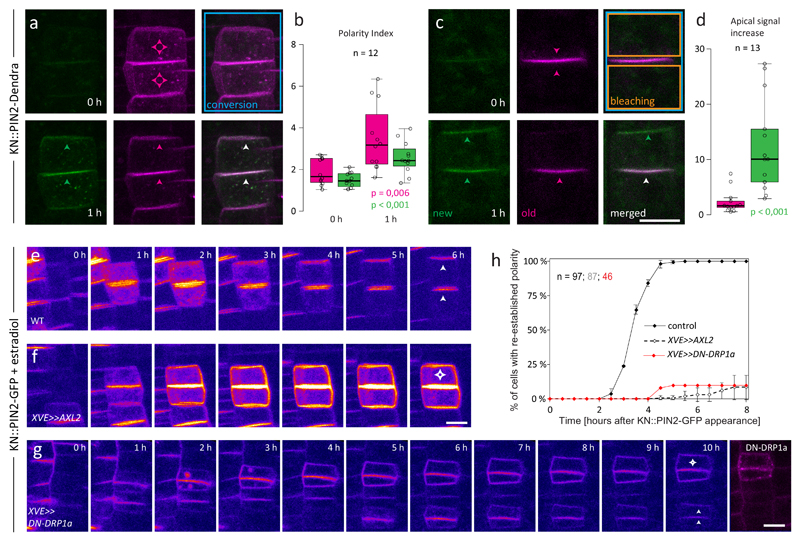
Figure 3. PIN2 apical polarity re-establishment requires secretion and endocytosis, but not transcytosis.
a: A newly divided cell pair of KN::PIN2-Dendra that was photoconverted before polarity re-establishment. Within 1 hour after photoconversion, apical polar distribution of both newly synthesized (green) and pre-existing (magenta) PIN2-Dendra pools can be observed. b: quantitative analysis of a. The value of Polarity index (ratio of signal intensity at the apical PM/lateral PMs of the upper cell) significantly increased in both channels within 1 hour after photoconversion. n = 12 cell pairs from 11 roots in 4 independent experiments. c: A similar experiment as in a with an additional step where all signal except that at the new membrane pair was photobleached immediately after photoconversion. In this case, apical polarity develops only in the green channel. d: quantification of c. The graph shows the increase in red and green signal intensity at the apical PM of the upper cell within 1 hour after photoconversion and photobleaching. Significantly larger signal increase in the green channel indicates predominant contribution of de novo secretion to polarity re-establishment. n = 13 cells from 9 roots in 3 independent experiments. Arrowheads indicate predominant localization of PIN2-Dendra in the corresponding channel; white = both channels. e-f: Inhibition of endocytosis by estradiol-inducible overexpression of Auxilin-like2 in KN::PIN2-GFP cells caused prolonged residence of signal at the PM and abolished the cells’ ability to re-establish apical PIN2 polarity. g: Apical polarity re-establishment in KN::PIN2 x XVE>>DN-DRP1a-mRFP after estradiol treatment. Thanks to patchy expression of the construct (see the last frame), cells with high levels of DN-DRP1a-mRFP that fail to re-establish apical polarity could be seen alongside cells with low levels, where polarity re-establishment proceeded normally. h: quantitative analysis of e-g. The control is the same as in 1f. The graph shows mean +/- SD of 3 (f), resp. mean of 2 (g) independent experiments, n indicates the total number of cell pairs. The number of roots/cell pairs analysed in each experiment was 4/29, 6/38 and 3/20 in f and 3/21 and 4/25, in g, respectively. Arrowheads indicate predominant localization of PIN2-GFP. Box-plots represent median, 1st and 3rd quartile; the whiskers extend to data points <1,5 interquartile range away from the 1st/3rd quartile; all data points are shown as circles. P-values were calculated using two-tailed two-sample t-test with unequal variance. Scale bars = 10 μm
Ectopic basal and lateral PIN2 molecules need to be removed from the PM during polarity re-establishment. Polarity of PINs has been linked to their slower lateral diffusion within the PM4,20–22, we therefore speculated that higher PIN2 mobility in the new PM domain might contribute to PIN2 removal and polarity re-establishment and tested this with Fluorescence Recovery After Photobleaching (FRAP) experiments. We did observe slightly higher PIN2 FRAP rates in the newly formed membranes compared to the old ones, however, the differences were significant only in PIN2::PIN2-GFP, but not in PIN2::PIN2-mCherry (Fig. S4). We therefore conclude that they reflected differential properties of the fluorophores rather than of the cargo, and that specific lateral diffusion properties probably do not play a major role in PIN2 polarity re-establishment.
A link between clathrin-mediated endocytosis (CME) and PIN polarity23 as well as post-cytokinetic PIN2 polarity re-establishment has previously been proposed3,4. However, strong pleiotropic defects of the mutants used in these studies make it difficult to dissect direct and indirect effects. To re-examine the role of CME in PIN2 polarity re-establishment, we conditionally inhibited endocytosis by inducible overexpression of the putative clathrin-uncoating factor Auxilin-like224. In KN::PIN2-GFP x XVE>>AXL2 plants treated with estradiol, most cells completely lost the ability to polarize PIN2 (Fig. 3f,h, S6, Movie S3), while polarity re-establishment proceeded normally in estradiol-treated KN::PIN2-GFP (Fig. 3e) and mock-treated KN::PIN2-GFP x XVE>>AXL2 (data not shown) controls. Inducible overexpression of a dominant-negative version of Dynamin-related protein 1A (DRP1a)5 led to the same result (Fig. 3g,h, S6), confirming the direct requirement of functional CME for post-cytokinetic PIN2 polarity re-establishment.
Multiple approaches have confirmed that (de)phosphorylation of PIN proteins by PINOID (PID) and its homologues WAG1 and WAG2 regulates their apical-basal localization6,25–28. PIN2 polarity re-establishment in the KN::PIN2S3A-GFP with three PID-phosphorylated serines S237, S258 and S310 mutated to alanines6 showed a delay in polarity re-establishment compared to the KN::PIN2-GFP control (Fig. S5, S6); however, introducing KN::PIN2-GFP into the pid wag1 wag2 loss-of-function mutant6 led to complete PIN2 polarity re-establishment failure (Fig. 4a-c, S6, Movie S4). Therefore, PID and WAG kinases mediate PIN2 polarity re-establishment by phosphorylating S237, S258 and S310, but also other PIN2 residues or additional polarity regulators28–30. Notably, analysis of the expression dynamics revealed that the abundance of WAG1 was strongly upregulated specifically in dividing epidermal cells (Fig. 4d-e, Movie S5).
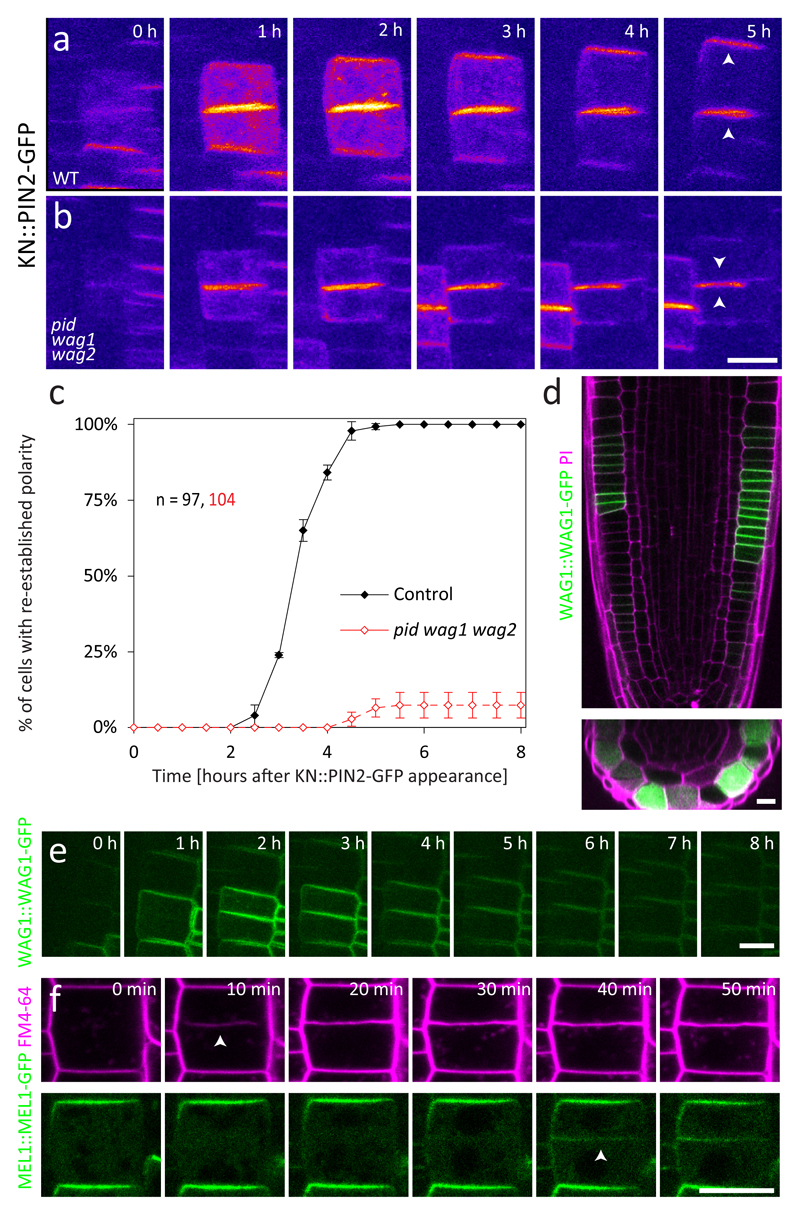
Figure 4. PIN apical polarity re-establishment is mediated by the AGCVIII kinase WAG1.
a-b: KN::PIN2-GFP cells failed to re-establish apical polarity in the pid wag1 wag2 triple mutant background. Arrowheads indicate predominant localization of PIN2-GFP. c: quantitative analysis of a-b. The control is the same as in 1f. The graph shows mean +/- SD of 3 independent experiments, n indicates the total number of cell pairs. The number of roots/cell pairs analysed in each experiment was 4/30, 4/38 and 4/36. d-e: Expression analysis of WAG1::WAG1-GFP. The signal is strongly and specifically increased in dividing cells exclusively in the epidermis. XY and XZ sections through the same root (d) and a time-lapse of a single dividing cell from a different root (e) are shown. n > 100 cells from 16 roots in 4 independent experiments. f: Subcellular localization of MEL1::MEL1-GFP in dividing cells stained with FM4-64. MEL1-GFP never appears at the cell plate and can be detected only at the newly formed apical PM > 30 minutes after cell division. Arrowheads indicate the first appearance of the cell plate and of MEL1-GFP at the new PM, respectively. n = 80 cells from 18 roots in 3 independent experiments. Scale bars = 10 μm
Together, these results show that the cell cycle-regulated WAG1 and its homologs PID and WAG2 play a key role in PIN2 post-cytokinetic polarity re-establishment, explaining the previously reported pronounced PIN2 polarity defect in the pid wag1 wag2 roots6.
PID/WAG kinases have been shown to interact genetically with MEL genes encoding NPH3-like proteins of unknown function31. Notably, the membrane-associated MEL proteins co-localize with PINs and polarity of PINs is reduced in higher order mel mutants31. This prompted us to analyse the dynamic localization of the MEL1-GFP reporter in dividing epidermal cells. We detected MEL1-GFP localization at the apical PM of the mother cell and in the cytoplasm, but not at the cell plate. MEL1-GFP first appeared at the newly formed apical domain not before 30 min. after cell division (Fig. 4f), presumably after it had lost CP and acquired PM identity. This localization pattern is a manifestation of the cell-intrinsic polarity cue inherited from the mother cell during cytokinesis and defining the apical domain of the daughter cell.
Maintenance of individual cell polarities over repeated rounds of cell division is crucial for tissue polarity and proper development in multicellular organisms. We show here that re-definition of the root epidermal cell apical-basal polarity after cell division is achieved in a cell-intrinsic manner. Subsequent PIN2 polarity re-establishment requires de novo protein secretion, CME and the activity of cell cycle-regulated WAG1 and related AGCVIII kinases. On the other hand, our detailed analysis does not support a major contribution of basal-to-apical transcytosis to re-establishment of PIN2 polarity. Based on our findings, we propose the following model: 1) During cytokinesis and in the first hour thereafter, PIN2 targeting is redirected to the cell plate along with most endomembrane traffic12; 2) PIN2 molecules localized ectopically to the basal side of the upper cell must be endocytosed, but are gradually turned over rather than being transcytosed to the apical side; and 3) During cytokinesis, WAG1 kinase is transcriptionally upregulated and together with its homologues required for re-establishment of PIN2 localization to the apical PM, which is marked by the presence of MEL proteins. It remains a challenge for future investigations to elucidate the precise molecular machinery responsible for differential endocytosis rates between the apical and other PM domains, and to uncover the nature of the cell-intrinsic polarity cue responsible for proper re-definition of apical-basal polarity of newly divided cells.
Methods
Plant material and growth conditions
Seeds were surface-sterilized by chlorine vapor, sown on ½ Murashige-Skoog medium supplemented with 1% sucrose and 1% agar and grown in vitro under long day conditions. The transgenic lines PIN2::PIN2-Dendra32, PIN2::nls-GFP32, PIN2::PIN2-GFP8, PIN2::PIN2-mCherry32 and WAG1::WAG1-GFP6 were described previously. The lines PIN2::Dendra-PIP1;4, KN::PIN2-GFP, KN::PIN2-Dendra, KN::PIN2S3A-GFP and MEL1::MEL1-GFP were generated by transformation of the respective constructs into Col-0 by the floral dip method33. KN::PIN2-GFP was introduced into the eir1-134, XVE>>AXL224, pid wag1 wag26, big312 and XVE>>DN-DRP1a-mRFP5 backgrounds by genetic crossing. In case of KN::PIN2-GFP x pid wag1 wag2, pid+/- wag1 wag2 plants were used for the cross. pid+/- plants were identified by PCR-based genotyping in F1 and F2 generations, and seedlings were selected based on the no cotyledon phenotype from an F3 KN::PIN2-GFP+ x pid+/-wag1-/-wag2-/- line for the experiments. Due to high level of XVE>>DN-DRP1a-mRFP silencing, the construct was re-transformed in our laboratory, T1 plants were used for the cross and the resulting F1/T2 plants were used for the experiments.
Molecular cloning
All cloning was performed using the Gateway technology (Invitrogen). To generate KN::PIN2-GFP, a promoter fragment 1kb upstream of the KNOLLE (At1g08560) start codon was cloned into pDONR P4-P1r, PIN2-GFP coding sequence was cloned into pDONR 221 and both entry clones were recombined into the binary vector pB7m24GW,3. KN::PIN2-Dendra was generated analogically. To introduce S3A mutations into the PIN2-GFP sequence, an N-terminal PIN2 fragment containing the three mutations was amplified from the PIN:: PIN2S3A-Venus line gDNA6, fused to a C-terminal fragment containing the GFP tag by overlap PCR, and cloned into pDONR221 which was used to generate KN::PIN2S3A-GFP. For MEL1::MEL1-GFP, a 2,9kb fragment upstream of MEL1 (At4g37590) start codon was cloned into pDONR P4-P1r, MEL1 coding sequence without a stop codon into pDONR 221 and EGFP coding sequence into pDONR P2r-P3, all three entry clones were then recombined into pH7m34GW,0. PIN2::Dendra-PIP1;4 was generated analogically by recombining the 1397bp PIN2 promoter in pDONR P4-P1r, Dendra coding sequence without a stop codon in pDONR 221 and PIP1;4 coding sequence in pDONR P2r-P3 into pB7m34GW,0. Sequences of all primers used can be found in Supplementary table 1.
Imaging and image analysis
4-day-old seedlings were mounted on a slice of growth medium, containing the respective chemicals in case of pharmacological experiments, placed into a chambered coverslip (Lab-Tek) and imaged with Zeiss LSM700, LSM800 or LSM880 inverted confocal microscopes; long time-lapse imaging was performed using a vertically oriented LSM700 microscope as described previously35. To apply chemical treatments, the respective amount of compound stock solution was pre-dissolved in 100μl H2O, pipetted onto a slice of growth medium and incubated 1-2 hours at room temperature to diffuse. The seedlings were then transferred into a chambered coverslip (Lab-Tek), covered with the treatment-including medium and imaged, in case of inhibitors together with a mock control containing only the solvent. The drugs (abbreviation; manufacturer; stock concentration and solvent; final concentration) were as follows: Naphtyl-phtalamic Acid (NPA; Duchefa; 10mM DMSO; 10μM), Indole-3-acetic acid (IAA; Sigma-Aldrich, 10mM ethanol; 100nM), β-Estradiol (EST; Sigma-Aldrich; 10mM DMSO; 10μM), Brefeldin A (BFA; Sigma-Aldrich; 50mM DMSO; 25μM) Cycloheximide (CHX; Sigma-Aldrich; 50mM DMSO; 25μM), Propidium Iodide (PI; Sigma-Aldrich; 1mg/ml H2O; 50μg/ml), FM4-64 (Invitrogen; 2mM H2O; 2μM). In tissue context disruption experiments, roots mounted on growth medium were manually cut with a razor blade under a stereomicroscope. Individual cells were ablated with a 355nm pulsed laser at a Zeiss Observer inverted microscope equipped with Andor iXon 897 Spinning Disk system, PI was used to mark the ablated cells. Dendra photoconversion was performed as described previously36. Images were handled and analysed with FIJI37 and Adobe Photoshop. All KN::PIN2-GFP time lapse data is presented as maximum intensity projections of a Z-stack.
Phenotypic analysis
Plates with 4-day old light-grown seedlings were scanned on an Epson Perfection V700 flatbed scanner and root Vertical Growth Index was measured as described previously38.
Reproducibility and statistics
The number of independent repetitions of experiments, as well as exact sample sizes, is described in the figure legends. Tukey boxplots were generated with BoxPlotR (http://shiny.chemgrid.org/boxplotr/). Statistical significance was tested as described in the figure legends. For the purpose of statistical analysis of KNOLLE::PIN2-GFP experiments, any cell pair that failed to repolarize in the time course of the experiment was considered to have repolarized after 10 hours.
Supplementary Material
Acknowledgements
We are thankful to Niko Geldner, Christian Luschnig, Gerd Jürgens, Remko Offringa and Yunpei Takano for sharing published material. We would also like to acknowledge Maciek Adamowski, Urszula Kania and Candela Cuesta for providing entry clones, and the Biomaging Facility at IST Austria for providing excellent imaging service and assistance. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme / ERC grant agreement n° 742985. Additionally, funding was received from Ministry of Education of the Czech Republic/MŠMT proj. NPUI - LO1417.
Footnotes
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Author contributions
M.G. designed experiments, performed experiments, analysed data and wrote the manuscript. M.F. designed experiments, analysed data and edited the manuscript. J.F. initiated the project, acquired funding, designed experiments and wrote the manuscript.
Competing interests
The authors have no competing financial or non-financial interests.
References
- 1.Smertenko A, et al. Plant Cytokinesis: Terminology for Structures and Processes. Trends Cell Biol. 2017;27:885–894. doi: 10.1016/j.tcb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Geldner N, et al. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 3.Mravec J, et al. Cell Plate Restricted Association of DRP1A and PIN Proteins Is Required for Cell Polarity Establishment in Arabidopsis. Curr Biol. 2011;21:1055–1060. doi: 10.1016/j.cub.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Men S, et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinari A, et al. DRP1-Dependent Endocytosis is Essential for Polar Localization and Boron-Induced Degradation of the Borate Transporter BOR1 in Arabidopsis thaliana. Plant Cell Physiol. 2016;57:1985–2000. doi: 10.1093/pcp/pcw121. [DOI] [PubMed] [Google Scholar]
- 6.Dhonukshe P, et al. Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development. 2010;137:3245–55. doi: 10.1242/dev.052456. [DOI] [PubMed] [Google Scholar]
- 7.Kania U, et al. Polar delivery in plants; commonalities and differences to animal epithelial cells. Open Biol. 2014;4 doi: 10.1098/rsob.140017. 140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abas L, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–56. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 9.Baster P, et al. SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 2013;32:260–74. doi: 10.1038/emboj.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhonukshe P, et al. Endocytosis of Cell Surface Material Mediates Cell Plate Formation during Plant Cytokinesis. Dev Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Gurskaya NG, et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol. 2006;24:461–465. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- 12.Richter S, et al. Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. Elife. 2014;2014:1–16. doi: 10.7554/eLife.02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauber MH, et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol. 1997;139:1485–93. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparks E, et al. Spatiotemporal signalling in plant development. Nat Rev Genet. 2013;14:631–44. doi: 10.1038/nrg3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazur E, et al. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci Rep. 2016;6 doi: 10.1038/srep33754. 33754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prát T, et al. WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity. PLOS Genet. 2018;14:e1007177. doi: 10.1371/journal.pgen.1007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon S, et al. Defining the selectivity of processes along the auxin response chain: a study using auxin analogues. New Phytol. 2013;200:1034–1048. doi: 10.1111/nph.12437. [DOI] [PubMed] [Google Scholar]
- 18.Sena G, et al. Organ regeneration does not require a functional stem cell niche in plants. Nature. 2009;457:1150–1153. doi: 10.1038/nature07597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleine-Vehn J, et al. Cellular and molecular requirements for polar PIN targeting and transcytosis in plants. Mol Plant. 2008;1:1056–66. doi: 10.1093/mp/ssn062. [DOI] [PubMed] [Google Scholar]
- 20.Kleine-Vehn J, et al. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol Syst Biol. 2011;7:540. doi: 10.1038/msb.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feraru E, et al. PIN Polarity Maintenance by the Cell Wall in Arabidopsis. Curr Biol. 2011;21:338–343. doi: 10.1016/j.cub.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Martinière A, et al. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc Natl Acad Sci U S A. 2012;109:12805–10. doi: 10.1073/pnas.1202040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23:1920–31. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamowski M, et al. A Functional Study of AUXILIN-LIKE1 and 2, Two Putative Clathrin Uncoating Factors in Arabidopsis. Plant Cell. 2018;30:700–716. doi: 10.1105/tpc.17.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friml J, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 26.Michniewicz M, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–56. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Kleine-Vehn J, et al. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell. 2009;21:3839–49. doi: 10.1105/tpc.109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci U S A. 2010;107:918–22. doi: 10.1073/pnas.0909460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang F, et al. Phosphorylation of Conserved PIN Motifs Directs Arabidopsis PIN1 Polarity and Auxin Transport. Plant Cell. 2010;22:1129–1142. doi: 10.1105/tpc.109.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weller B, et al. Dynamic PIN-FORMED auxin efflux carrier phosphorylation at the plasma membrane controls auxin efflux-dependent growth. Proc Natl Acad Sci U S A. 2017;114:E887–E896. doi: 10.1073/pnas.1614380114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furutani M, et al. Polar-localized NPH3-like proteins regulate polarity and endocytosis of PIN-FORMED auxin efflux carriers. Development. 2011;138:2069–78. doi: 10.1242/dev.057745. [DOI] [PubMed] [Google Scholar]
- 32.Salanenka Y, et al. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc Natl Acad Sci. 2018;115:3716–3721. doi: 10.1073/pnas.1721760115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 34.Luschnig C, et al. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–87. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Wangenheim D, et al. Live tracking of moving samples in confocal microscopy for vertically grown roots. Elife. 2017;6:e26792. doi: 10.7554/eLife.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jásik J, et al. PIN2 Turnover in Arabidopsis Root Epidermal Cells Explored by the Photoconvertible Protein Dendra2. PLoS One. 2013;8:e61403. doi: 10.1371/journal.pone.0061403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grabov a, et al. Morphometric analysis of root shape. New Phytol. 2005;165:641–651. doi: 10.1111/j.1469-8137.2004.01258.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.