Abstract
Exposure of polar bears (Ursus maritimus) to persistent organic pollutants was discovered in the 1970s, but recent evidence suggests the presence of unknown toxic chemicals in their blood. Protein and phospholipid depleted serum was stirred with polyethersulfone capillaries to extract a broad range of analytes, and nontarget mass spectrometry with “fragmentation flagging” was used for detection. Hundreds of analytes were discovered belonging to 13 classes, including novel polychlorinated biphenyl (PCB) metabolites and many fluorinated or chlorinated substances not previously detected. All analytes were detected in the oldest (mid-1980s) archived polar bear serum from Hudson Bay and Beaufort Sea, and all fluorinated classes showed increasing trends. A mouse experiment confirmed the novel PCB metabolites, suggesting that these could be widespread in mammals. Historical exposure and toxic risk has been underestimated, and emerging contaminants pose uncertain risks to this threatened species.
Keywords: environmental chemistry, halogenated contaminants, mass spectrometry, nontarget discovery, polar bear
Graphical Abstract

High resolution mass spectrometry revealed hundreds of previously unknown halogenated contaminants in polar bear serum. A mouse study confirmed the 5 new PCB metabolite classes. Stable time-trends of PCB metabolites and increasing fluoroalkyl contaminants in polar bear serum between the mid-1980s and 2016 make toxicological studies on these new contaminants warranted
Polar bears (Ursus maritimus) are apex predators of the Arctic marine foodweb, making them vulnerable to bioaccumulative and persistent chemicals transported long-range from mid-latitudes. Since the 1970s, many halogenated contaminants have been identified in polar bears[1], including polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane (DDE) and perfluoroalkyl acids. These problematic discoveries in the Arctic led to classification of such substances as Persistent Organic Pollutants (POPs), and have enabled international regulatory bans or restrictions on these chemicals under the Stockholm Convention[2]. However, in polar bear blood there are still unidentified toxic contaminants. In an attempt to identify all chemicals in polar bear plasma that disrupt thyroid hormone binding, it was concluded that 25% of the biological activity was from unidentified chemicals[3]. Known contaminants and certain metabolites are linked to adverse health effects in polar bears, such as immune-suppression and endocrine disruption[4]. Contaminants in polar bears are also a health concern to indigenous people who depend on marine mammals for subsistence. Discovering the unknown environmental pollutants in polar bears is therefore of great importance for the conservation and management of polar bears, and the health of the Arctic ecosystem.
Here we analyzed pooled polar bear serum from each of the Hudson Bay and Beaufort Sea subpopulations in the Canadian Arctic with the aim of detecting unknown fluorinated or chlorinated global contaminants of biological relevance. A polyethersulfone stir bar-assisted sorptive method was developed for sample extraction and a non-target high resolution mass spectrometry (Nt-HRMS) method[6] coupled to HPLC was used for unknown discovery. A controlled mouse study, where mice were exposed to a PCB-mixture or vehicle control[7], was then performed to confirm the novel PCB metabolites discovered in polar bears. Finally, temporal trends of all discovered halogenated contaminants were analyzed in archived samples from the two locations dating to the mid-1980s. Relevant experimental details can be found in the Supporting information (SI). Our study suggests that historical exposure and toxic risk in polar bears has been underestimated, and health risks posed by these emerging halogenated pollutants should be evaluated.
For organohalogen discovery, representative pooled serum from each of the Hudson Bay and Beaufort Sea subpopulations (Table S3) was extracted and analyzed. In-source fragmentation flagging in the two extracts showed hundreds of chromatographic peaks (e.g. [35Cl]− and [C2F5]−) corresponding to organochlorine and organofluorine analytes (Figure 1). Complete Nt-HRMS analyses over these peaks revealed 13 classes of new halogenated contaminants, including 5 classes of PCB Metabolites (Class 1.1–1.5, 181 analytes), 4 classes of Perfluoroalkyl Sulfonates (PFSAs, Class 2.1–2.4, 35 analytes), and 4 classes of Other Polychlorinated Compounds (Class 3.1–3.4, 8 analytes). Detailed discovery and characterization of these contaminants are presented below. For the PCB metabolites, confirmation in experimental mice is also discussed.
Figure 1.

Extracted chromatograms of in-source fluorine- and chlorine-related fragments in pooled polar bear serum from Hudson Bay (blue traces) and Beaufort Sea (red traces).
Metabolism of PCBs has been extensively studied and reviewed[5a, 5b](Figure 2). In addition to OH-PCBs (Figure S3) and dihydroxylated PCBs (DiOH-PCBs, Figure S4), known contaminants in polar bears[5a, 8], we detected 5 more classes of metabolites (Table 1). The first two classes are PCB-Sulfates (Class 1.1, SO4-PCBs) and OH-PCB-Sulfates (Class 1.2, OH-SO4-PCBs). Both classes had diagnostic MS/MS spectra showing neutral loss of SO3 from the molecular ion (Figures S5.1 and S6), and two SO4-PCBs were confirmed by authentic standards. Benefiting from the HPLC separation, in total, >50 SO4-PCBs and >40 OH-SO4-PCBs were detected, and this is their first report in polar bears. We confirmed the dose-response production of Cl2- to Cl7-SO4-PCBs and of Cl1- to Cl8-OH-SO4-PCBs in mice exposed to a PCB mixture (Figure S2).
Figure 2.

Summary scheme of established[5] and newly proposed PCB biotransformation pathways in polar bears. Example representative structures shown, positions of functional groups and Cl-substitution are uncertain
Table 1.
Non-target chemical classes detected in polar bear serum
| group | Class No. | name | molecular formula | total analytes detected | CL[a] |
|---|---|---|---|---|---|
|
Polychlorinated Biphenyl (PCB) Metabolites |
1.1 | SO4-PCB | C12ClnH9-nSO4− n=2–8 |
51 | 1&3[b] |
| 1.2 | OH-SO4-PCB | C12ClnH9-nSO5− n=3–7 |
42 | 3 | |
| 1.3 | OH-SO2CH3-PCB | C13ClnH11-nSO3− n=3–8 |
45 | 3 | |
| 1.4 | DiOH-SO2CH3-PCB | C13ClnH11-nSO4− n=2–7 |
22 | 3 | |
| 1.5 | SO3-PCBs | C12ClnH11-nSO3− n=3–6 |
21 | 3 | |
|
Perfluoroalkyl Sulfonates (PFSAs) |
2.1 | Cyclic or Unsaturated PFSAs |
CnF2n-1SO3− n=8–10 |
8 | 3 |
| 2.2 | Ether PFSAs | CnF2n+1SO4− n=6– |
13 | 3 | |
| 2.3 | Unsaturated Ether-, Cyclic Ether- or Carbonyl PFSAs |
CnF2n-1SO4− n=7–9 |
11 | 3 | |
| 2.4 | x:2 Chlorinated Perfluoroalkyl Ether Sulfonates |
ClCnF2nSO4− n=6–8 |
3 | 1&2[b] | |
|
Other Polychlorinated Compounds |
3.1 | Chlorinated Aromatics |
C8ClnH7-nO− n=6–7 |
2 | 4 |
| 3.2 | Tetrachloro Aromatic Sulfate |
C14Cl4H7SO4− | 1 | 4 | |
| 3.3 | Heptachlorinated Hydroxylated Nitroaromatics |
C14Cl7H5NO6− C14Cl7H7NO6− |
1 1 |
4 | |
| 3.4 | Hexachlorinated Compounds |
C15Cl6H13O5− C15Cl6H15O3− |
2 1 |
4 |
CL” confidence level of the proposed structure, assigned based on criteria proposed by Schymanski et al[26].
CL=1 was assigned to only some congeners or isomers due to the availability of authentic standards.
The next two classes of PCB metabolites are derivatives of methylsulfone-PCBs (SO2CH3-PCBs)[5b] (Figure 2), specifically Hydroxylated SO2CH3-PCBs (Class 1.3, OH-SO2CH3-PCBs, >40 congeners) and Dihydroxylated SO2CH3-PCBs (Class 1.4, DiOH-SO2CH3-PCBs, >20 congeners). To our knowledge, the DiOH-SO2CH3-PCBs have never been reported, and the only previous report of OH-SO2CH3-PCBs was in people accidentally poisoned by cooking oil contaminated with PCBs[9]. The MS/MS spectra of OH-SO2CH3-PCBs were relatively complex, but most congeners showed an initial diagnostic loss of SO2CH2 (Figure 3), demonstrating the methylsulfone functionality. The third oxygen atom was typically lost as CO, consistent with a core OH-PCB structure. Similarly, the DiOH-SO2CH3-PCBs also had highly diagnostic loss of the methylsulfone moiety and subsequent neutral losses of HCl and CO (Figure S7). This class could be formed in mammals by sequential hydroxylation, or epoxidation and hydrolysis of SO2CH3-PCBs (Figure 2). We confirmed the dose-response production of Cl2- to Cl5-OH-SO2CH3-PCBs in experimental mice, and several Cl3- and Cl4- DiOH-SO2CH3PCB congeners were detected in high-dose mice (Figure S2).
Figure 3.

Extracted chromatograms of Class 1.3 OH-SO2CH3-PCB congeners in the Hudson Bay pooled polar bear serum, MS/MS spectra of selected Cl6- congeners and proposed fragmentation mechanisms for each product ion
The final class of PCB metabolites discovered was the PCB-Sulfonates (Class 1.5, SO3-PCBs, 21 congeners). These have never been reported in any samples. MS/MS experiments showed characteristic neutral loss of SO2 (Figure S8), analogous to fragmentation of alkylbenzenesulfonates[10]. For the most abundant congeners, diagnostic [SO3]− and [SO3Cl]− fragment ions were also observed, confirming the presence of a sulfonate functional group. The pathway leading to SO3-PCBs is uncertain, however, sulfonic acid metabolites were reported for some compounds[11] whereby the SO3 moiety is either added to a double bond[11b] or can replace a chlorine atom[11a]. We therefore propose pathways (Figure 2) via oxidation of PCB thiols, intermediates in SO2CH3-PCB formation, or via gut microflora, which reduce SO42− to SO3H− and add SO3H− to parent PCBs. We also observed the dose-response production of Cl2- to Cl7-SO3-PCBs in experimental mice.
Perfluoroalkyl acids such as perfluorooctanesulfonate (PFOS, C8F17SO3−) have been detected in polar bears since 2001[12], and only yield saturated perfluoroalkyl fragments during fragmentation (i.e. [CnF2n+1]−). The observation of unique in-source fragments such as [C3F5]− and [C2F5O]− in pooled polar bear serum (Figure 1) was therefore attributable to new classes fluoroalkyl contaminants. The first class are the Cyclic or Unsaturated PFSAs (Class 2.1). These substances share the general chemical formula CnF2n-1SO3− (n=8–10, ≥ 8 analytes) and are analogous to PFOS but with 2 fewer F atoms. In MS/MS spectra, [SO3]− and [SO3F]− ions were consistent with PFOS (Figure S9), but diagnostic perfluoroalkenyl ions (e.g. C5F9−) indicated a ring or double-bond in the core structures. Neither unsaturated nor cyclic PFSAs has been reported in polar bears.
The second class of new PFSAs are the Ether-PFSAs (Class 2.2, CnF2n+1SO4−, n=6–9, ≥ 13 analytes), flagged by unique [C2F5O]− ions and characterized by the simultaneous detection of PFSA-specific ions (e.g. [SO3]− and [SO3F]−) and perfluoroalkoxy fragments (e.g. [C2F5O]−, Figure 4). We previously detected C7-C14 ether-PFSAs in fish from China[13]. The current discovery in polar bears is the first evidence that ether-PFSAs are global contaminants.
Figure 4.
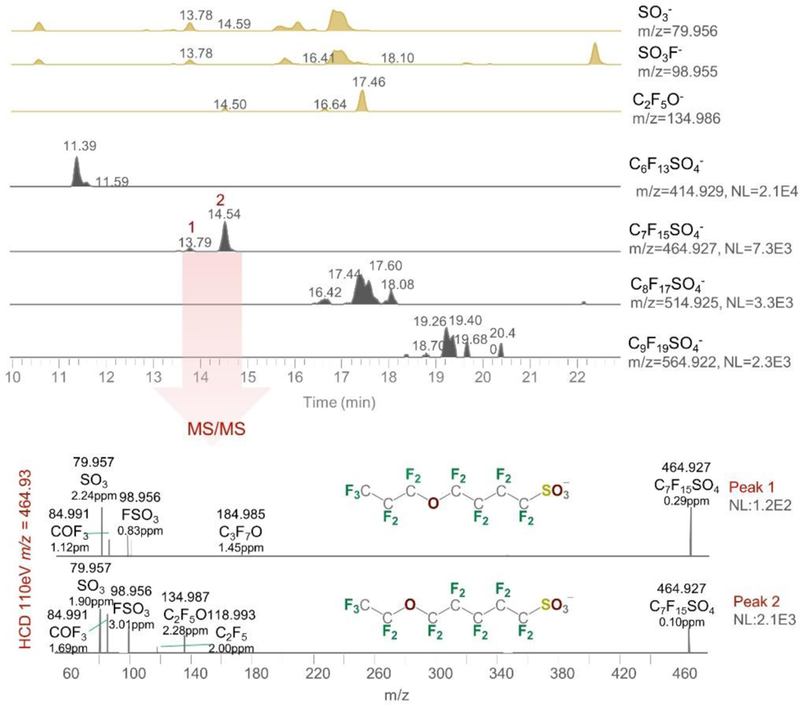
Extracted chromatograms of in-source [SO3]−, [SO3F]− and [C2F5O]− fragments (brown peaks) and Class 2.2 Ether-PFSA homologs (grey peaks) in the Hudson Bay pooled polar bear serum, and MS/MS of the C7-homolog Proposed structures for two C7-homolog isomers are shown.
The third PFSA class detected is structurally ambiguous, and generally termed Unsaturated Ether-, Cyclic Ether-, or Carbonyl-PFSAs (Class 2.3, CnF2n-1SO4−, n=7–9, ≥ 11 analytes). MS/MS analyses revealed typical PFSA fragments, perfluoroalkenyl fragments, and neutral loss of perfluoroalkoxy ions (i.e. CnF2nO) for all isomers (Figure S10). Homologous compounds matching this class were reported in aqueous film-forming foam (AFFF)-impacted concrete[14] and in fish from China[13]. The current discovery in polar bears is also the first evidence of a new global contaminant class.
The final PFSA class, flagged by in-source [Cl]− and confirmed by authentic F-53B standard (CClF2C5F10OC2F4SO3−, Table S1), are the x:2 Chlorine Substituted Perfluoroalkyl Ether Sulfonates (Class 2.4, ClCnF2nSO4−, n=6–8, 3 analytes). F-53B is a mist suppressant used only in China since the late 1970s[15], and C7-C12 homologs have been detected in samples from China[13, 15]. F-53B was recently report in Greenland polar bears[16], but our study is the first report of the C6 and C7-homologs in polar bears, and the first report of C6 in the environment.
Four classes of other new polychlorinated compounds were also detected and assigned unambiguous empirical formulas; albeit the core structures remain uncertain (Table S1). The first 3 classes were chlorinated aromatics with OH- (Class 3.1 and 3.3), SO4- (Class 3.2) or NO2- functionality (Class 3.3). The Cl7 homolog in Class 3.1 (C8Cl7O−) shares the same molecular formula with 4-hydroxy-heptachlorostyrene (4-OH-HpCS, Figure S12), a suspect octachlorostyrene metabolite only once reported in polar bears[17]. Based on MSn experiments, the single Class 3.2 analyte (C14Cl4H7SO4−) was consistent with a sulfated metabolite of dichlorodiphenyldichloroethylene (Figure S13), which could possibly be formed by sulfation of hydroxylated DDE, a metabolite has been detected in seals[18]. One possible structure for the C14Cl7H5NO6− in Class 3.3 is a hydroxylated nitro-polychlorinated diphenyl ether (OH-NO2-PCDE, Figure S14). Some herbicides are nitro-PCDEs (e.g. nitrofen[19] and oxyfluorfen[20]), and could be oxidized to OH-NO2-PCDEs.
To examine the time trends of these new halogenated contaminants, we further analyzed pooled male serum from the archive going back to the mid-1980s for the Hudson Bay and Beaufort Sea subpopulations (Table S4). Two pooled samples were generated for each year examined (SI), and linear regression was run for internal standard corrected total response within each class to test for significant temporal trends. All 5 classes of PCB metabolites had no trends at either location (Figure S16) but significant increasing trends were detected for all 4 PFSA classes (Class 2.1–2.4) in Beaufort Sea (1985–2006) and for Class 2.4 in Hudson Bay (1985–2006, Figure 5). Class 2.1–2.3 had doubling times between 7.0–9.0 years in Beaufort Sea (Figure S17). The distinctly lower doubling time for Class 2.4 in the Beaufort Sea subpopulation (3.7 years, 95% confidence interval 3.2–4.5) than in Hudson Bay (8.1 years, 95% confidence interval 6.1–11.9) is consistent with the source of this class coming from China[15], as the Beaufort Sea receives inflow from the North Pacific Ocean through the Bering Strait. For classes showing increasing trends, all individual homologues within the class were tested and had similar increasing trends (Figure 5) and comparable doubling times (Figure S17).
Figure 5.
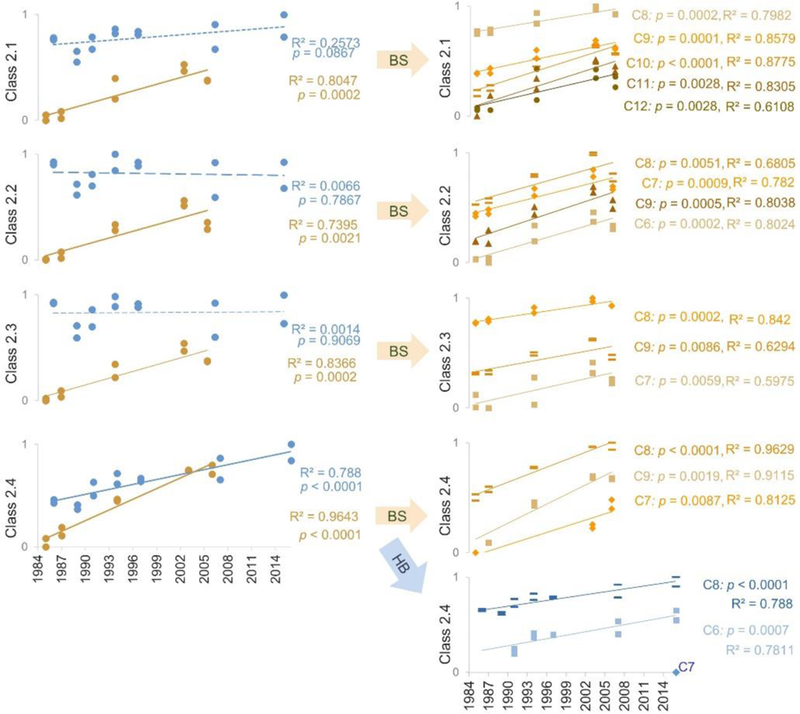
Temporal trends of Class 2.1–2.3 PFSA classes in polar bear serum from Hudson Bay (HB, blue data) and Beaufort Sea (BS, brown data). Solid, dashed and dotted lines indicate simple linear regressions with p < 0.05, 0.05 < p < 0.1 and p > 0.1, respectively. The y-axis represents normalized log10(IS-corrected peak area) per gram dry serum.
By removal of major protein and phospholipid interferences and using a sensitive stir bar-assisted extraction step (SI), our Nt-HRMS methods detected more than 200 new organohalogen compounds in polar bear serum. As shown for most classes of substances discovered, the method benefited tremendously from HPLC to separate isomeric homologues or congeners prior to mass spectral characterization. Their detection in two distinct and distant subpopulations, and in archived samples dating to the mid-1980s indicates long and wide contamination of the Arctic with these previously unknown persistent and bioaccumulative chemicals whose toxicity remains unknown. The contamination of top predator polar bears suggests exposure to the same contaminants in the underlying marine foodweb, and the mouse study provides evidence that other mammals, including people, may be exposed to the 5 novel classes of PCB metabolites. The novel PCB metabolites described here may have been missed in previous studies of serum due to a historic reliance on GC-based techniques that require silica gel clean-up steps, derivatization, and use of strong acids or bases for sample preparation[21]. Considering regulatory restrictions and controls implemented for the past 1–2 decades, the detected stable PCB metabolite trends and increasing PFSAs for the past 3 decades in polar bears are worth further study. The presence of these substances in blood means they could disrupt normal physiology, as related OH-PCBs[22], PFSAs[23] and 4-OH-HpCS[17] bind to serum proteins and can thereby disrupt hormone homeostasis. For example, SO4-PCBs bind to estrogen and androgen receptors[24]. The new chlorinated aromatics compounds (Class 3.1–3.4) may also be bioactivated to reactive intermediates (e.g. epoxides), just as PCBs[5b]. While challenging, understanding the individual- and population-level effects of these new contaminants raises additional concerns for the conservation and management of polar bears in a changing climate[25].
Supplementary Material
Acknowledgements
Funding for this project was from that Natural Sciences and Engineering Research Council of Canada (NSERC, Martin PI). The synthesis of PCB metabolite standards and the mouse study were respectively supported by grants ES013661 (Lehmler PI) and ES019487, ES025708 and GM111381 (Cui PI) from the National Institutes of Health of the United States (NIH). Additional research support was provided by the Environmental Health Sciences Research Center (NIH ES005605) and the Center for Ecogenetics and Environmental Health (NIH ES0007033). Financial support to Yanna Liu was the Chinese Scholarship Council, and Killam Trusts. Dr. Amila O De Silva (Environment and Climate Change Canada) provided the 4-PFECHS standard. We thank Drs Derek Muir (Environment and Climate Change Canada), Shira Joudan (University of Toronto) and Lisa A. D’Agostino (Stockholm University) for comments on a draft, and Drs Lillemor Asplund and Dennis Lindqvist (Stockholm University) for comments on GC-based cleanup and sample preparation.
References
- [1].Letcher RJ, Morris AD, Dyck M, Sverko E, Reiner EJ, Blair DAD, Chu SG, Shen L, Sci Total Environ 2018, 610–611, 121–136. [DOI] [PubMed] [Google Scholar]
- [2].Stockholm Convention., Stockholm Convention, Geneva, Switzerland, 2009. [Google Scholar]
- [3].Simon E, van Velzen M, Brandsma SH, Lie E, Loken K, de Boer J, Bytingsvik J, Jenssen BM, Aars J, Hamers T, Lamoree MH, Environ Sci Technol 2013, 47, 8902–8912. [DOI] [PubMed] [Google Scholar]
- [4].Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, Sonne C, Verreault J, Vijayan MM, Gabrielsen GW, Sci Total Environ 2010, 408, 2995–3043. [DOI] [PubMed] [Google Scholar]
- [5].a) Letcher RJ, Klasson-Wehler E, Bergman A, in The Handbook of Environmental Chemistry Vol. 3 Part K New Types of Persistent Halogenated Compounds, Vol. 3 (Ed.: Paasivirta J), pringer-Verlag Berlin; 2000 [Google Scholar]; b) Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW, Crit Rev Toxicol 2015, 45, 245–272 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dhakal K, He X, Lehmler HJ, Teesch LM, Duffel MW, Robertson LW, Chem Res Toxicol 2012, 25, 2796–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu Y, P. A.S., Martin JW, Anal Chem 2015, 87, 4260–4268. [DOI] [PubMed] [Google Scholar]
- [7].Cheng SL, Li X, Lehmler H-J, Phillips B, Shen D, Cui JY, Toxicol Sci 2018, kfy208. [DOI] [PMC free article] [PubMed]
- [8].Sandau CD, Carleton University (Ottawa, ON, Canada: ), 2000. [Google Scholar]
- [9].Haraguchi K, Kuroki H, Masuda Y, Chemosphere 1987, 16, 2033–2038. [Google Scholar]
- [10].Andreu V, Picó Y, Anal Chem 2004, 76, 2878–2885. [DOI] [PubMed] [Google Scholar]
- [11].a) Kalkhoff SJ, Kolpin DW, Thurman EM, Ferrer I, Barcelo D, Environ Sci Technol 1998, 32, 1738–1740 [Google Scholar]; b) He X, Li J, Gao H, Qiu F, Hu K, Cui X, Yao X, Drug Metab Dispos 2003, 31, 983–985. [DOI] [PubMed] [Google Scholar]
- [12].Giesy JP, Kannan K, Environ Sci Technol 2001, 35, 1339–1342. [DOI] [PubMed] [Google Scholar]
- [13].Liu Y, Qian M, Ma X, Zhu L, Martin JW, Environ Sci Technol 2018, 52, 5830–5840. [DOI] [PubMed] [Google Scholar]
- [14].Baduel C, Mueller JF, Rotander A, Corfield J, Gomez-Ramos MJ, Chemosphere 2017, 185, 1030–1038. [DOI] [PubMed] [Google Scholar]
- [15].Wang S, Huang J, Yang Y, Hui Y, Ge Y, Larssen T, Yu G, Deng S, Wang B, Harman C, Environ Sci Technol 2013, 47, 10163–10170. [DOI] [PubMed] [Google Scholar]
- [16].Gebbink WA, Bossi R, Riget FF, Rosing-Asvid A, Sonne C, Dietz R, Chemosphere 2016, 144, 2384–2391. [DOI] [PubMed] [Google Scholar]
- [17].Sandau CD, Meerts IATM, Environ Sci Technol 2000, 34, 3871. [Google Scholar]
- [18].Jansson B, Jensen S, Olsson M, Renberg L, Sundstrom G, Vaz R, Ambio 1975, 4, 93–97. [Google Scholar]
- [19].Brown TN, Wania F, Environ Sci Technol 2008, 42, 5202–5209. [DOI] [PubMed] [Google Scholar]
- [20].National Water-Qaulity Assessment Project, 2016, URL: https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2016&map=OXYFLUORFEN&hilo=L&disp=Oxyfluorfen (Accessed on October 2, 2018)
- [21].Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman A, Wehler EK, Arch Environ Contam Toxicol 2002, 42, 105–117. [DOI] [PubMed] [Google Scholar]
- [22].Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP, Environ Toxicol Chem 2003, 22, 2639–2649. [DOI] [PubMed] [Google Scholar]
- [23].Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T, Toxicol Sci 2009, 109, 206–216. [DOI] [PubMed] [Google Scholar]
- [24].Flor S, He X, Lehmler HJ, Ludewig G, Environ Sci Pollut Res Int 2016, 23, 2186–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) McKinney MA, Peacock E, Letcher RJ, Environ Sci Technol 2009, 43, 4334–4339 [DOI] [PubMed] [Google Scholar]; b) Bromaghin JF, McDonald TL, Stirling I, Derocher AE, Richardson ES, Regehr EV, Douglas DC, Durner GM, Atwood T, Amstrup SC, Ecol Appl 2015, 25, 634–651. [DOI] [PubMed] [Google Scholar]
- [26].Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J, Environ Sci Technol 2014, 48, 2097–2098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


