Abstract
Previous studies in our laboratory have shown that nicotine exposure decreases glucose transport across the blood–brain barrier in ischemia-reperfusion conditions. We hypothesize that nicotine can also dysregulate brain parenchymal glucose utilization by altering glucose transporters with effects on sensitivity to ischemic stroke. In this study, we investigated the effects of nicotine exposure on neuronal glucose utilization using an in vitro ischemic stroke model. We also tested the effects of e-Cig vaping on ischemic brain glucose utilization using an acute brain slice technique. Primary cortical neurons and brain slices were subjected to oxygen-glucose deprivation followed by reoxygenation to mimic ischemia-reperfusion injury. We estimated brain cell glucose utilization by measuring the uptake of [3H] deoxy-D-glucose. Immunofluorescence and western blotting were done to characterize glucose transporters (GLUTs) and α7 nicotinic acetylcholine receptor (nAChR) expression. Furthermore, we used a glycolytic stress test to measure the effects of nicotine exposure on neuronal glucose metabolism. We observed that short- and long-term nicotine/cotinine exposure significantly decreased neuronal glucose utilization in ischemic conditions and the non-specific nAChR antagonist, mecamylamine reversed this effect. Nicotine/cotinine exposure also decreased neuronal GLUT1 and up-regulated α7 nAChR expression and decreased glycolysis. Exposure of mice to e-Cig vapor for 7 days likewise decreases brain glucose uptake under normoxic and ischemic conditions along with down-regulation of GLUT1 and GLUT3 expressions. These data support, from a cerebrovascular perspective, that nicotine and/or e-Cig vaping induce a state of glucose deprivation at the neurovascular unit which could lead to enhanced ischemic brain injury and/or stroke risk.
Keywords: brain, E-cigarette, glucose utilization, ischemic stroke, neuron, nicotine
The smoking of tobacco is a well-known risk factor for stroke (Alberg et al. 2014), which is the fifth leading cause of mortality in the US (Writing Group Members et al. 2016). Electronic cigarettes (e-Cigs) are a newer form of smoking device, which contain liquid nicotine as the principal component along with other additives including propylene glycol, glycerol, and flavoring agents (Rahman et al. 2014). Since their first introduction in the US market in 2007, e-Cigs have been rapidly gaining popularity among adults and especially, adolescents (McMillen et al. 2015). Although e-Cigs are considered as a safer alternative to tobacco smoking, little is known about the short- and long-term health effects of this type of exposure, which could be significant considering their extensive use in adolescents (Kaisar et al. 2016). Recent studies found that e-Cig use, commonly known as vaping, has been associated with increased carcinogenic potential (Lee et al. 2018), oxidative stress, and inflammatory activity (Muthumalage et al. 2017), as well as altered blood–brain barrier (BBB) permeability and worsened ischemic brain injury (Kaisar et al. 2017). Up to this date, there have not been many investigative studies into the direct effects of e-Cig use on ischemic stroke. Previous studies in our laboratory have demonstrated a number of deleterious effects of nicotine exposure, the principal component of e-Cigs, on ischemic stroke outcome which includes altered BBB permeability (Abbruscato et al. 2002), inhibited ion transporter function at the BBB (Abbruscato et al. 2004; Paulson et al. 2006), and increased vasogenic and cytotoxic brain edema after ischemia and ischemia-reperfusion (Paulson et al. 2010).
The brain uses glucose as a primary fuel for energy generation. The major portion of glucose (25%) used by the body is consumed by the brain although it represents only 2% of total body mass (Belanger et al. 2011). In the brain, glucose is utilized primarily by neurons (Lundgaard et al. 2015) through different glucose transporters including the facilitative glucose transporter 1 (GLUT1) (Maher et al. 1994) and GLUT3 (Vannucci et al. 1997) to maintain brain activity. The transport of glucose across the BBB and entry into brain parenchyma play a vital role in maintaining normal brain physiology and energy metabolism (Shah et al. 2012). In ischemic stroke, occlusion of a major cerebral artery causes severe reduction in cerebral blood flow which disrupts the supply of oxygen, glucose, and other nutrients to the brain (Sims and Muyderman 2010) leading to increased energy demand of the ischemic brain. After stroke, there is an initial decrease in brain glucose metabolism followed by a significant increase in brain glucose utilization as well as increased glucose transporter function and expression (McCall et al. 1996; Vannucci et al. 1996). Factors adversely affecting the brain and neuronal glucose utilization after ischemic stroke could prove detrimental to stroke induced brain injury and long-term functional outcome.
Nicotine can also adversely impact ischemic brain glucose transport and utilization which have not been fully investigated yet. Previous studies in our laboratory have shown that chronic nicotine treatment significantly decreased glucose transport across the BBB along with a significant reduction in brain endothelial GLUT1 expression (Shah et al. 2015). Nicotine could also alter cerebral glucose utilization beyond the BBB in ischemic conditions further aggravating the glucose-deprived state of the ischemic brain. Review of existing literature showed inconsistent reports on the effects of nicotine on brain glucose utilization. Some studies have shown increased cerebral glucose utilization with nicotine mostly in the nicotine binding regions of the brain in partially immobilized rats (London et al. 1988; Marenco et al. 2000). Another study reported tolerance to nicotine challenge in some brain regions of subchronically nicotine-exposed rats (London et al. 1990). However, Marenco et al. (2000) demonstrated that acute nicotine treatment decreased overall brain glucose metabolism in freely moving rats. A reduction in global glucose metabolism was also observed in a human study with acute nicotine administration (Stapleton et al. 2003). A later study showed that nicotine treatment also decreased glucose oxidation in the brains of freely moving rats (Wang et al. 2010). However, no studies to date have investigated the effects of acute and chronic nicotine treatment on brain glucose utilization in ischemic stroke.
We hypothesize that nicotine could affect brain glucose utilization differently in a pathophysiological state like cerebral ischemia, whereby a glucose-deprived state could make the brain vulnerable to ischemic brain injury. In this study, we sought to assess the effects of both the short-term and long-term exposure of nicotine on ischemic brain glucose utilization along with the expression of the major brain glucose transporters (GLUT1, GLUT3). We used an in vitro ischemic stroke model, oxygen-glucose deprivation (OGD), of mouse primary cortical neurons, since neurons are the most involved members of the neurovascular unit in glucose utilization and brain activity (Lundgaard et al. 2015). We also utilized a more translational in vivo model to study the effects of e-Cig vaping, which contains vaporized nicotine, on ischemic brain glucose utilization using an acute brain slices technique, which allowed us to observe the effects of nicotine in intact brain parenchyma.
Materials and methods
Mouse primary cortical neuronal cultures
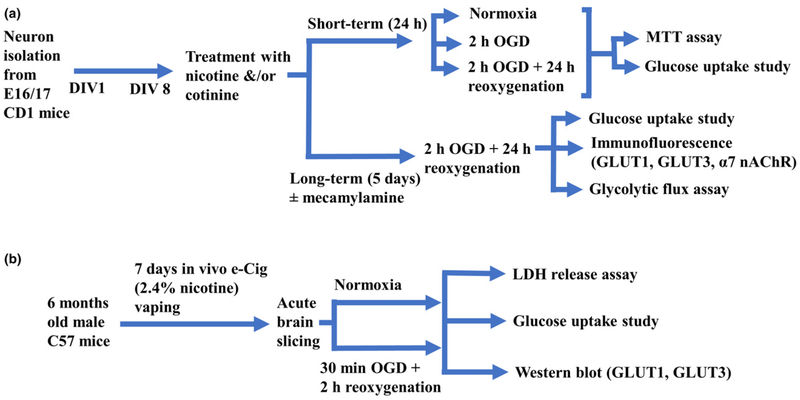
Mouse primary cortical neurons were isolated and cultured as previously described (Islam et al. 2016) with a slight modification. This protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Texas Tech University Health Sciences Center, Lubbock, Texas (Protocol# 08017). In brief, cerebral cortices were isolated from E16 or E17 embryos (CD-1 mice; Charles River Laboratories, Inc., Wilmington, MA, USA; Cat# CRL:22, RRID: IMSR_CRL:22) and dissected in Hank’s balanced salt solution without Ca2+ and Mg2+ supplemented with 250 μg/mL gentamycin (Fisher Scientific, Hampton, NH, USA; Cat# MT30005CR). Dissected pieces of meninges-free cortices were digested in 0.25% trypsin for 15 min at 37°C followed by neutralization with 10% fetal bovine serum. Dissociated cell suspensions were seeded (0.75 × 104per cm2 surface area) into poly-D-lysine (Sigma-Aldrich, St. Louis, MO, USA; Cat# P6407)-coated 6- or 96-well plates and cultured in Neurobasal medium (Thermo Fisher Scientific, Waltham, MA, USA; Cat# 21103049) supplemented with 0.5 mM Glutamax (Thermo Fisher; Cat# 35050061), 25 μg/mL gentamicin, and 2% B27 (Thermo Fisher; Cat# 17504044) at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was totally replaced with fresh Neurobasal medium after overnight incubation and half of the media was refreshed every 2 days onwards. Study design to use primary cortical neurons in the in vitro studies is summarized in Fig. 1(a).
Fig. 1.
Flow diagram of the study designs. (a) In vitro studies, (b) In vivo/ex vivo studies; DIV, day in vitro; OGD, oxygen-glucose deprivation; MTT, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; GLUT, glucose transporter; nAChR, nicotinic acetylcholine receptor; e-Cig, electronic cigarettes; LDH, lactate dehydrogenase.
OGD studies to model ischemic stroke
After 8 days of culture, mature neurons were treated with 10 μM nicotine (Sigma-Aldrich; Cat# N3876) and 5 μM cotinine (Sigma-Aldrich; Cat# C5923) for 1 or 5 days, followed by exposure to 2 h of OGD treatment. Neurons were exposed to the Earle’ s balanced salt solution (140 mM NaCl, 5.36 mM KCl, 0.83 mM MgSO4, 1.8 mM CaCl2, 1.02 mM NaH2PO4, and 6.19 mM NaHCO3) to create an aglycemic condition. Hypoxia (1% oxygen) was induced as described previously (Yang et al. 2011) by placing the cells in a custom-made hypoxic polymer glove box (Coy Laboratories, Grass Lake, MI, USA), which was infused with 95% N2 and 5% CO2, and the temperature was maintained at 37°C. Nicotine and cotinine were added to the Earle’s balanced salt solution at the start of OGD. After OGD, the medium was replaced by neurobasal medium containing the same nicotine/cotinine concentrations noted above, and cells were returned to cell culture incubator for 24 h for reoxygenation (OGD/R). Primary neurons grown in normal conditions (specified above) served as normoxic controls.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
The neuronal cell viability was measured using previously reported 3-(45-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide (MTT) assay (Marutani et al. 2012), with a slight modification. In brief, 3 h before the end of experiment, MTT (Fisher Scientific; Cat# AC158990010) stock solution was added to the cells to achieve a final concentration of 0.5 mg/mL and incubated at 37°C for 3 h in the dark. After the incubation period, the cells were washed with Hank’s balanced salt solution, followed by addition of dimethyl sulfoxide to dissolve the formed blue dye and absorbance was measured at 570 nm together with reference absorbance at 670 nm (BioTek, Winooski, VT, USA). Cell viability is presented as ratio to control cells by the absorbance at 570 nm.
Radioactive deoxy-D-glucose uptake assay
Glucose uptake of neurons was the primary endpoint of this study which was determined by the radio isotopic evaluation method described previously (O’Donnell 1993), using [3H] deoxy-D-glucose (Perkin Elmer, Waltham, MA, USA; Cat# NET328A250UC) as a radioactive tracer. In brief, neuronal cells cultured on 6-well plates were equilibrated with a HEPES-based physiological buffer (136 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2, 0.4 mM k2HPO4, 25 mM HEPES) at 37°C for 10 min in a shaking water bath. The cells were then incubated with the same buffer containing [3H] deoxy-D-glucose (0.5 μCi/mL) for 5 min at 37°C, followed by aspiration of the medium and rapid triple rinse by ice-cold buffer. Next, the cells were lysed with 0.5 mL of 1% Triton X-100 in 10 mM NaOH, and 100-μl lysates in duplicates were used to measure cell-associated radioactivity by a liquid scintillation counter (Perkin Elmer). The total protein level in each well was measured using the bicinchoninic acid assay (Thermo Fisher; Cat# 23223, 23224) and was used to normalize the glucose uptake data.
Immunofluorescence
Primary cortical neuronal cells were seeded in Lab-Tek II four-well CC2 chamber slides (Thermo Fisher; Cat# 154917), grown and treated as mentioned earlier; then fixed (using 4% paraformaldehyde in 1 × phosphate-buffered saline, PBS), washed, and permeabilized using 0.5% Triton X-100. The cells were then blocked with 2% goat serum and 1% bovine serum albumin in PBS (blocking buffer) for 1 h at room temperature followed by overnight incubation at 4°C with mouse monoclonal anti-GLUT1 antibody (1 : 100; Abcam, Cambridge, UK; Cat# ab40084, RRID: AB_2190927), mouse monoclonal anti-GLUT3 antibody (1 : 250; Santa Cruz Biotechnology, Santa Cruz, Dallas, TX, USA; Cat# sc-74497, RRID: AB_1124974), rabbit monoclonal anti-NeuN antibody (1 : 500; Cell Signaling Technology, Danvers, MA, USA; Cat# 12943, RRID: AB_2630395), rat monoclonal anti-alpha-7 antibody (1 : 50; Santa Cruz Biotechnology; Cat# sc-58607, RRID: AB_784835). After incubation, cells were washed three times with PBS for 5 min each and incubated with donkey anti-rabbit Alexa 594 (1 : 1000; Thermo Fisher; Cat# A-11059, RRID: AB_142495), donkey anti-mouse Alexa 488 (1 : 1000, Cat# A-21207, RRID: AB_141637), and goat anti-rat Alexa 488 (1 : 1000; Cell Signaling; Cat# 4416S, RRID: AB_10693769) for 2 h at 22°C. After washing it three times with PBS, cells were incubated with 4′,6-diamidino-2-phenylindole, dihydrochloride (Thermo Fisher; Cat# D1306) for 10 min at 22°C followed by again washing it three times with PBS and one time with de-ionized water for 5 min. Slides were then air-dried for 5 min. Next, cells were carefully coverslipped with mounting media. All specimens were then observed for immunofluorescence using Nikon Eclipse Ti-E Epi-Fluorescence microscope (Nikon, Minato, Tokyo, Japan) with 20× objective lens. Images were captured by Photometrics CoolSNAP HQ2 (PHOTOMETRICS, Tucson, AZ, USA) camera and mean total fluorescence intensity was calculated for each color channel using NIS elements AR software and each intensity was expressed relative to the neuronal marker, NeuN. Six images were arbitrarily captured and analyzed from each group for each isolation.
Glycolytic flux assay
Glycolysis stress test was done to assess extracellular acidification rates (ECAR) and oxygen consumption rate (OCR) in neurons by a Seahorse XFe24 bioanalyzer (Seahorse Bioscience, North Billerica, MA, USA). Cells were seeded at a density of 1.5 × 105 cells/well in 24-well plates (Seahorse; Cat# 100777-004) and were treated with nicotine/cotinine for 5 days as described previously followed by OGD/R experiments. The glycolysis stress test was performed according to the Seahorse Bioscience protocol. Briefly, sensor cartridges (Seahorse, Cat# 100867-100) were soaked in XF calibrant to hydrate overnight at 37°C without CO2. On the day of the experiment, cells were washed twice and incubated with seahorse XF base medium (Seahorse, Cat# 103334-100) (glucose-free, with addition of 2 mM glutamax, 2% B27, pH adjusted to 7.4 with NaOH) for 1 h at 37°C in the absence of CO2 prior to the beginning of the assay. The glycolysis stress test employed in this study used sequential injections of glucose (Sigma-Aldrich; Cat# G8270) (10 mM, port A), oligomycin (Fisher Scientific; Cat# ICN15178605) (1 μM, port B), and 2-deoxyglucose (Sigma-Aldrich; Cat# D8375) (50 mM, port C). Response to the saturating concentration of glucose (10 mM) indicates glycolysis in basal condition. Oligomycin inhibits mitochondrial ATP production causing an increase in ECAR corresponding to the cellular maximum glycolytic capacity. 2-deoxyglucose inhibits glycolysis resulting in a decrease in ECAR which concludes that the ECAR produced is because of glycolysis. Three measurements were performed in each session at 3-min intervals. From the glycolysis assay, we determined the following parameters: the glycolysis rate (average ECAR of three measurements between glucose and oligomycin injection), the glycolytic capacity (average ECAR of three measurements between oligomycin and 2-deoxyglucose injection), and glycolytic reserve (the difference between glycolysis rate and glycolytic capacity). The total protein level in each well was measured using bicinchoninic acid assay (Thermo Fisher) and was used to normalize the flux data.
Animals and surgical procedures
All studies were approved by the IACUC of Texas Tech University Health Sciences Center, Lubbock, Texas (IACUC protocol 15004). This study was not pre-registered and no randomization/blinding was performed. A total of 18 C57BL/6J male mice (6 months old) (Charles River; Cat# CRL:27, RRID: IMSR_CRL:27) were kept under standardized light and dark conditions (12 h), humidity (70%), and temperature (22°C). Mice were singly housed and given ad libitum access to food and water. Study design for the in vivo studies is summarized in Fig. 1(b).
The behavior of the animals was monitored every day to minimize animal suffering. We applied the following exclusion criteria: severe weight loss, infections, or significant behavioral deficits (decreased mobility, seizures, lethargy). No medication was given and no animal met the exclusion criteria during the study.
In vivo e-Cig vaping
Mice were exposed (via direct inhalation) to e-Cig vapor (Blu™, 24 mg/mL nicotine) mixed with oxygenated air or oxygenated air alone, six times/day; one cartridge/day, 6–8 h/day, 7 days/week for 1 week following a modified International Organization for Standardization/Federal Trade Commission (ISO/FTC) standard smoking protocol (35 mL puff depth volume, 4 s puff duration, one puff per 60 s, 16 puffs/session) in the laboratory. E-Cig vapor was generated using a Single Cigarette Smoking Machines (SCSM, CH Technologies Inc., Westwood, NJ, USA) following a previously published method (Kaisar et al. 2017).
Acute brain slicing and glucose uptake study
Acute brain slicing was done in mice exposed to e-Cig vapor according to previously published protocols with slight modifications in the laboratory (Nishi et al. 1997; Meyer et al. 2014). Mice were killed by an overdose of isoflurane which is an IACUC-approved method of painless killing of rodents. Death was confirmed by cervical dislocation. Brain was extracted quickly and placed in oxygenated HEPES-based physiological buffer (136 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2, 0.4 mM k2HPO4, 25 mM HEPES, 10 mM glucose, 0.4 mM ascorbic acid, gentamycin sulfate 50x μg/mL). Meninges were removed, and cerebellum was cut and discarded. Then, the brain was glued to the mounting chamber and was divided into two hemispheres by a sharp blade. Using a Leica VT1000 S Vibrating blade microtome (Leica Biosystems, Wetzlar, Germany; Cat# 14047235613), 200-μm thin coronal slices were cut from each hemisphere after discarding 1.2 mm brain area rostrally. Slices were kept in a brain slice keeper (Scientific Systems Design Inc., Mississauga, Ontario, Canada; Cat# S-BSK4) immersed in HEPES buffer with continuous oxygen perfusion at 37°C. Brain slices were kept for a recovery period of 45 min followed by 30 min of OGD treatment in the hypoxia chamber described above in HEPES buffer without glucose. NetwellsTM inserts (Fisher Scientific; Cat# 07-200-213) were utilized which facilitated washing and other experimental procedures of different brain slices samples. After OGD, slices were reoxygenated with glucose containing buffer for 2 h at a tissue-culture incubator at 37°C, 5% CO2, and 20% O2. Fresh oxygenated buffer with glucose was given to brain slices every 30 min. After that, slices were washed followed by incubation with [3H] deoxy-D-glucose (0.1 μCi/mL) and [14C] Sucrose (0.02 μCi/mL) at 37°C for 5 min in a shaking water bath to measure glucose uptake and extracellular volume, respectively, followed by washing the slices for three times with ice-cold buffer utilizing the netwell inserts. The slices were collected and then digested in 1 mL tissue solubilizer SolvableTM (Perkin Elmer; Cat# 6NE9100) at 55°C overnight, cooled, and mixed with 5 mL scintillation fluid. For each sample, dual label counting was performed simultaneously in a scintillation counter for 10 min. Lactate dehydrogenase (LDH) release assay was also done to measure brain slices viability using a commercial LDH assay kit (Sigma-Aldrich; Cat# 11644793001 ROCHE).
Western blot of brain slices
Four slices from each brain slices sample were separated and treated with normoxia or OGD/R in the same manner described above. After that, they were collected and stored at − 80°C until further analysis. Membrane protein lysates were isolated from each sample using a commercial kit (Thermo Fisher; Cat# 89842). Protein concentrations of isolated protein lysates were determined using bicinchoninic acid assay. Exactly 45 μg of protein from each sample was loaded and separated using a 10% Tris-glycine polyacrylamide precast gel (Bio-Rad Laboratories, Hercules, CA, USA; Cat# 4568034). This method has been used previously for the analysis of western blot immunoreactivity (Yang et al. 2006). Protein samples were then transferred to a polyvinylidene difluoride membrane (Thermo Fisher; Cat# IPVH00010) and then membranes were incubated in blocking buffer (1% Tween-20 containing Trisbuffered saline (TBST) with 5% non-fat dry milk) to block the nonspecific protein bands for 1 h at 22°C. Membranes were incubated with mouse monoclonal anti-GLUT1 antibody (1 : 3000; Abcam Cat# ab40084, RRID: AB_2190927), goat polyclonal anti-GLUT3 antibody (1 : 200; Santa Cruz Biotechnology Cat# sc-7582, RRID: AB_641074) in TBST with 0.5% non-fat dry milk at 4°C overnight. After three times washing with TBST for 10 min each, membranes were incubated with anti-mouse (Sigma Aldrich; Cat# GENXA931-1ML, RRID: AB_772209) and anti-goat (Santa Cruz; Cat# sc-2354, RRID: AB_628490) Ig G-horseradish peroxidase secondary antibody (1 : 5000) in TBST with 0.5% non-fat dry milk for 2 h at 22°C. After three times 10 min wash with TBST, the protein signals were detected by enhanced chemiluminescence-detecting reagents (Thermo Fisher; Cat# 34577) and visualized in X-ray films in the dark. The protein bands were quantified relative to beta-actin in Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
The sample size for the animal study was estimated based on previous work (Shah et al. 2015) with nicotine and ischemic stroke in our laboratory. No sample size calculation was performed and there were no sample size differences between the beginning and end of the experiments. All experimental data passed the Shapiro–Wilk normality test (p > 0.05) and no outliers were removed from the analysis. All data are expressed as the mean ± SEM. All in vitro data represent 3–4 independent primary neuronal isolations with 3–6 replicate treatments per isolation (n = 3–4). The values were analyzed by one-way analysis of variance and Tukey’s post hoc multiple comparison for all the experimental data except for the glycolysis stress test where two-way analysis of variance and Sidak’s post hoc multiple comparison was used for ECAR and OCR data and Student’s t-test (two-tailed) was used for the other parameters (Prism, version 7.0; GraphPad Software Inc., San Diego, CA, USA). p values less than 0.05 were considered statistically significant.
Results
Nicotine only at a very high dose is toxic to cortical neurons
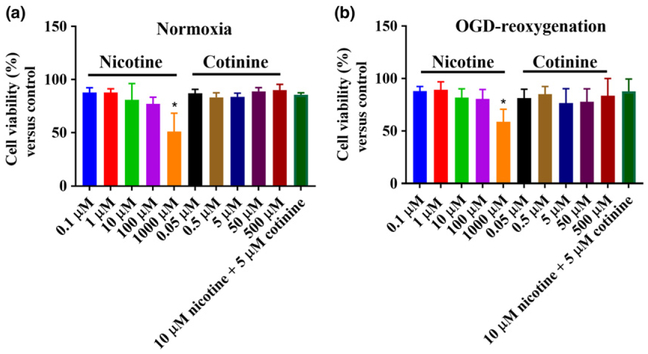
Nicotine at a dose of 1000 μM was toxic to neurons in both normoxic and OGD/R (OGD-reoxygenation; 2 h OGD followed by 24 h reoxygenation) conditions compared to control (p < 0.05) as observed in MTT assay (Fig. 2). Cotinine did not show any toxicity to neurons at any dose level up to 500 μM. We chose nicotine and cotinine concentrations (10 and 5 μM, respectively) for further experiments which have been widely studied in in vitro conditions and they also reflect the brain levels observed in an in vivo model of chronic nicotine treatment (Doura et al. 2008). These nicotine and cotinine doses were not toxic to the neurons either used alone or in combination (Fig. 2) as observed in this study.
Fig. 2.
Effects of nicotine (0.1–1000 μM) and cotinine (0.5–500 μM) on neuronal viability by 3-(45-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide (MTT) assay in normoxic (a) and oxygen-glucose deprivation/reoxygenation (OGD/R) conditions (b). In normoxic condition, nicotine at a concentration of 1000 μM significantly decreased neuronal viability compared to no-treatment group. No other nicotine or cotinine concentration caused any significant change in neuronal viability. In OGD/R conditions, nicotine and cotinine, like the normoxic condition, did not cause any significant change in neuronal viability except for 1000 μM nicotine which was significantly toxic to the neurons compared to control. 10 μM nicotine and 5 μM cotinine in combination treatment also did not cause neuronal toxicity in normoxia and OGD/R. *p < 0.05.
Nicotine/cotinine decreases neuronal glucose uptake in ischemic conditions with both short-term and long-term treatment
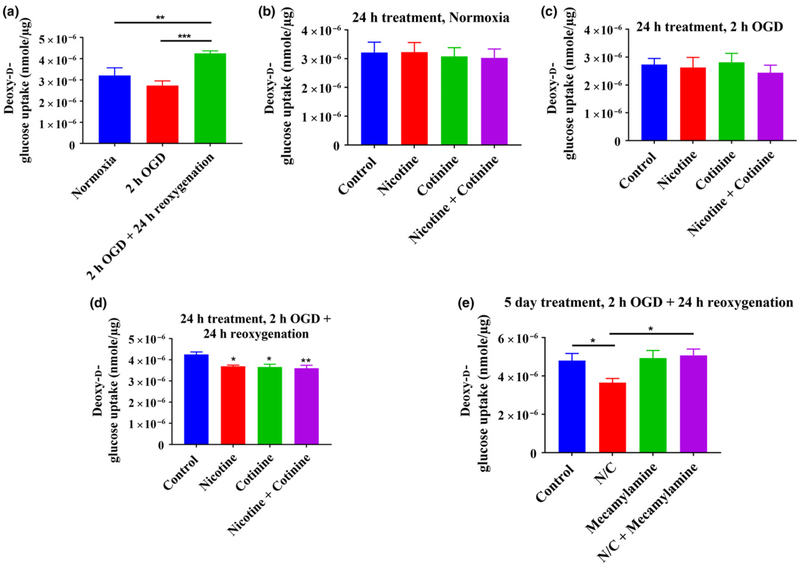
Radioactive deoxy-D-glucose uptake studies revealed that glucose uptake in the cortical neurons were reduced after 2 h of OGD compared to normoxia (3.2 × 10−6 ± 3.6 × 10−7 vs. 2.73 × 10−6 ± 2.2 × 10−7 nmol/μg for normoxia vs. OGD) (Fig. 3a). Reoxygenation after OGD for 24 h, however, increased neuronal glucose uptake (p < 0.01 vs. normoxia and p < 0.001 vs. OGD) (Fig. 3a). The exposure of neurons with nicotine (10 μM) and cotinine (5 μM), single agent as well as in combination, for 24 h did not change the neuronal glucose uptake in normoxic (Fig. 3b) and OGD conditions (Fig. 3c). However, glucose uptake was decreased significantly (p < 0.05 for both nicotine and cotinine and p < 0.01 when in combination vs. control) in OGD/R conditions (Fig. 3d). As nicotine and cotinine had more significant effects on glucose uptake in combination, we selected the combination of these two (10 μM nicotine and 5 μM cotinine) for long-term exposure and also for all further studies. This combination more accurately mimicked in vivo conditions and what would be expected as estimated levels of nicotine and cotinine in the brain (Lockman et al. 2005; Doura et al. 2008). The combination of nicotine (10 μM) and cotinine (5 μM) is represented as nicotine/ cotinine in this manuscript. Results of the study showed that 5 days of exposure of neurons with nicotine/cotinine significantly decreased glucose uptake after OGD/R (p < 0.05 vs. control) (Fig. 3e). Furthermore, it was also found that neuronal glucose uptake was brought back to normal when neurons were simultaneously exposed with 20 μM mecamylamine, a non-selective and non-competitive nicotinic acetylcholine receptor (nAChR) antagonist (p < 0.05 for nicotine/cotinine + mecamylamine vs. nicotine/cotinine) (Fig. 3e). This indicated that the effects of nicotine/cotinine on neuronal glucose uptake were mediated by nAChRs.
Fig. 3.
Effects of oxygen-glucose deprivation (OGD) and OGD/R on neuronal glucose uptake (a). Two hours of OGD decreased glucose uptake compared to normoxia which was not statistically significant. However, 24 h reoxygenation following 2-h OGD significantly increased neuronal glucose uptake compared to normoxia and OGD. (b–d) represent effects of short-term (24 h) nicotine (10 μM) and cotinine (5 μM) on neuronal glucose uptake in normoxic, OGD, and OGD/R conditions, respectively: Nicotine and/or cotinine did not cause any significant change in neuronal glucose uptake compared to control both in normoxic and OGD conditions. However, in OGD/R conditions, nicotine and cotinine both caused statistically significant decrease in neuronal glucose uptake compared to control. Combination of nicotine and cotinine resulted in a more significant decrease in glucose uptake compared to control. (e) After long-term (5 days) treatment, nicotine/cotinine also significantly decreased neuronal glucose uptake compared to control in OGD/R conditions. Simultaneous exposure with 20 μM mecamylamine, a non-selective nAChR antagonist, could reverse the effects of nicotine/cotinine treatment on neuronal glucose uptake. Mecamylamine alone did not cause any significant change in neuronal glucose uptake compared to control. N/C, nicotine/cotinine; *p < 0.05, **p < 0.01, ***p < 0.001.
Long-term nicotine/cotinine treatment decreases neuronal GLUT1 expression in ischemic conditions
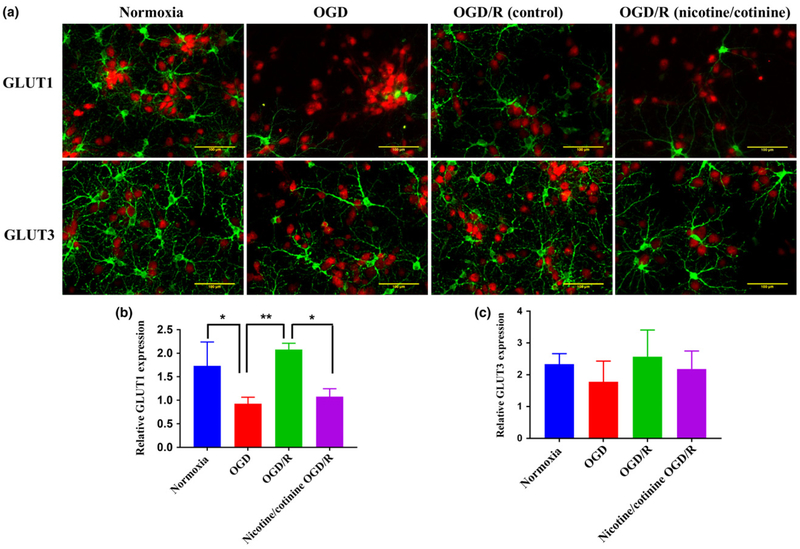
GLUT3 is the most widely expressed glucose transporter in neurons along with GLUT1 (Maher 1995; Choeiri et al. 2002; Shah et al. 2012). We measured the expression of these transporters by immunofluorescence in cultured cortical neurons after 5 days of nicotine/cotinine treatment followed by OGD/R as described previously. After OGD, neuronal GLUT1 expression was reduced compared to normoxia which was again significantly increased after OGD/R (p < 0.01 for OGD/R vs. OGD) (Fig. 4a and b). However, 5 days of nicotine/cotinine treatment significantly decreased GLUT1 expression in OGD/R condition (Fig. 4a and b), while no significant effect on GLUT3 was observed (Fig. 4a and c). These results indicate that GLUT1 could play a crucial role in nicotine-induced aberrant neuronal and brain glucose metabolism in ischemic stroke.
Fig. 4.
Effects of long-term (5 days) combined nicotine (10 μM) and cotinine (5 μM) treatment on neuronal GLUT1 and GLUT3 expression by immunofluorescence studies. (a) Immunofluorescence images of neurons where green color represents GLUT1 or GLUT3, while red color represents the neuronal marker (NeuN). Quantification of GLUT1 (b) and GLUT3 (c) expression relative to NeuN. oxygen-glucose deprivation (OGD) significantly decreases GLUT1 expression compared to normoxia, while OGD/R caused a significant increase in neuronal GLUT1 expression compared to OGD. Five days of nicotine/cotinine treatment significantly decreased GLUT1 expression after OGD/R in comparison with OGD/R control. There was a slight decrease in GLUT3 expression after OGD compared to control. OGD/R demonstrated an increase in expression compared to OGD and nicotine/cotinine treatment did not cause any change in GLUT3 expression in OGD/R compared to control. No statistically significant change was observed in any condition. Normoxia, OGD, and OGD/R groups are without any nicotine/cotinine exposure, nicotine/cotinine OGD/R group is with 5 days of nicotine/cotinine exposure. GLUT, glucose transporter. *p < 0.05, **p < 0.01.
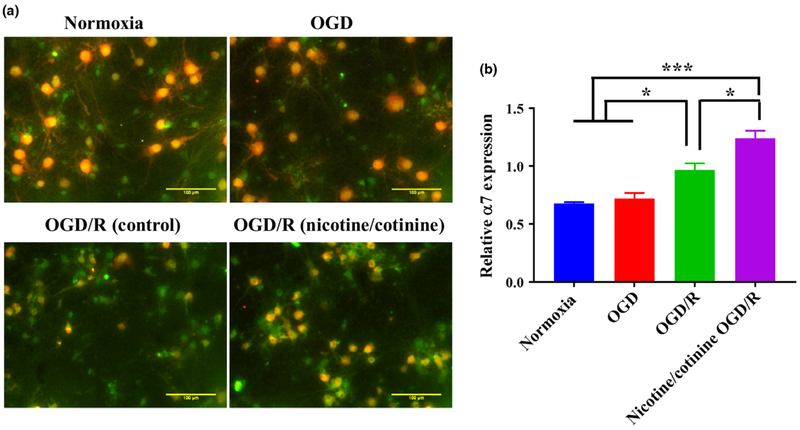
Long-term nicotine/cotinine treatment up-regulates alpha7 nicotinic acetylcholine receptor in ischemic conditions
In our glucose uptake studies, we have demonstrated that the effects of nicotine/cotinine on neuronal glucose uptake in ischemic conditions were mediated by nAChRs. α7 nAChR is one of the most abundantly expressed nAChR subtypes in brain which has been demonstrated to play a neuroprotective role in ischemic stroke in several studies by modulating neuroinflammation and oxidative stress (Han et al. 2014a,b). The long-term modulation of α7 nAChR with nicotine/cotinine, however, could affect ischemic brain metabolism and injury in a different manner. We observed that in primary cortical neurons, OGD did not cause any change in α7 nAChR expression while it was significantly increased (p < 0.05 vs. OGD) in OGD/R conditions (Fig. 5a and b). In OGD/R conditions, 5 days of nicotine/cotinine treatment further significantly increased α7 nAChR expression (p < 0.05) (Fig. 5a and b). This receptor subtype thus could be associated with nicotine/cotinine-induced diminished brain glucose utilization in ischemic stroke.
Fig. 5.
Effects of long-term (5 days) combined nicotine (10 μM) and cotinine (5 μM) treatment on neuronal α7 nAChR expression by immunofluorescence studies. (a) Immunofluorescence images of neurons where green color represents α7 and red color represents the neuronal marker (NeuN). (b) Quantification of α7 expression relative to NeuN. Oxygen-glucose deprivation (OGD) did not cause any change in α7 expression compared to control. OGD/R significantly increased receptor expression compared to normoxia and OGD. Nicotine/cotinine treatment further significantly increased α7 expression in OGD/R condition. Normoxia, OGD, and OGD/R groups are without any nicotine/cotinine exposure, nicotine/cotinine OGD/R group is with 5 days of nicotine/cotinine exposure. *p < 0.05, ***p < 0.001.
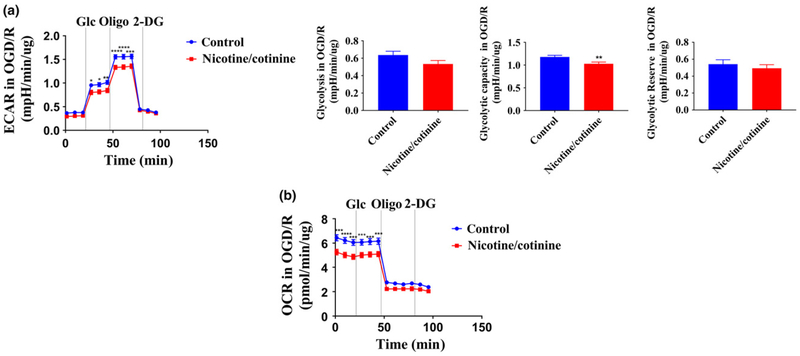
Long-term nicotine/cotinine treatment decreases neuronal glycolytic capacity and oxygen consumption in ischemic conditions
After 5 days of nicotine/cotinine treatment followed by OGD/R, neuronal ECAR (indicating glycolytic flux) and OCR (indicating mitochondrial respiration) were analyzed by Seahorse XFe24 bioanalyzer. Nicotine/cotinine treatment significantly decreased neuronal ECAR at 27 (p < 0.05), 35 (p < 0.05), 44 (p < 0.01), 52 (p < 0.0001), 61 (p < 0.0001), and 69 (p < 0.001) minutes in the glycolysis stress test (Fig. 6a). Neuronal glycolytic capacity was also significantly reduced (p < 0.01) with a non-significant decrease in glycolysis rate (Fig. 6a). Nicotine/cotinine treatment also significantly decreases neuronal OCR at 1 (p < 0.001), 9 (p < 0.0001), 18 (p < 0.001), 27 (p < 0.001), 35 (p < 0.001), and 44 (p < 0.001) minutes indicating possible mitochondrial dysfunctions (Fig. 6b).
Fig. 6.
Effects of long-term (5 days) combined nicotine (10 αM) and cotinine (5 αM) treatment on neuronal glycolytic flux (ECAR) (a) and mitochondrial respiration (OCR) (b) in oxygen-glucose deprivation/reoxygenation (OGD/R) condition. Nicotine/cotinine treatment decreases neuronal ECAR at 27, 35, 44, 52, 61, and 69 min. Neuronal glycolytic capacity was also significantly reduced along with a non-significant decrease in glycolysis rate. Nicotine/cotinine treatment also significantly decreases neuronal OCR at 1, 9, 18, 27, 35, and 44 min. Glc, Glucose; Oligo, Oligomycin; 2-DG, 2-deoxy-D-glucose; ECAR, extracellular acidification rate; OCR, oxygen consumption rate; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
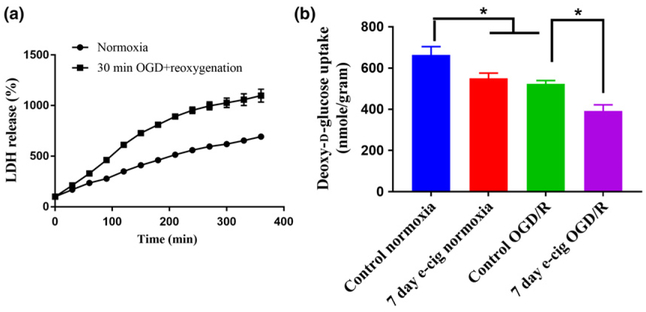
E-Cig vaping decreases glucose utilization in brain slices in normoxic and ischemic conditions
Radioactive deoxy-D-glucose uptake was measured for 5 min in acute brain slices, since this time point was in the linear range of time-dependent glucose uptake curve (0–15 min). We also chose to use 30 min OGD + 2 h reoxygenation to mimic ischemia-reperfusion conditions, which was based on LDH release assay where we observed this timepoint to be in the linear range of the LDH release curve that plateaus at 180 min, indicating death of most of the brain slice cells (Fig. 7a). This study revealed that brain glucose uptake after OGD/R was significantly decreased (p < 0.05) compared to normoxia (Fig. 7b). Glucose utilization was also significantly decreased in brain slices of mice exposed to 7 days of e-Cig vaping in normoxia (p < 0.05 vs. control) and OGD/R (p < 0.05 vs. control) (Fig. 7b).
Fig. 7.
(a) Brain slices viability study by lactate dehydrogenase (LDH) release assay (n = 2) as a percentage of the baseline, which is after 30 min of normoxia/oxygen-glucose deprivation (OGD). LDH release increased with time for both normoxia and reoxygenation groups which indicated the cell death of brain slices over time. The reoxygenated slices’ viability was less than those of normoxia. For normoxic slices, LDH release was increased almost linearly wherein, the reoxygenated slices’ LDH release reached a plateau after 180 min. (b) Effects of 7 days of e-Cig vaping on brain slices glucose uptake in normoxic and OGD/R conditions. OGD/R significantly decreases brain glucose uptake compared to normoxia. Seven days of exposure to e-Cig vapor significantly decreases brain glucose uptake in normoxic conditions compared to control animals (not exposed to e-Cig vapor). Similarly, 7 days of e-Cig vaping significantly decreased brain glucose uptake compared to control animals in OGD/R condition. Control normoxia: brain slices from no treatment animals; 7 days of e-Cig normoxia: brain slices from 7 days of e-Cig-exposed animals; Control OGD/R: brain slices from no treatment animals after 30 min OGD and 2 h of reoxygenation; 7 days of e-Cig OGD/R: brain slices from 7 days of e-Cig exposed animals after 30 min OGD and 2 h of reoxygenation. *p < 0.05; n = 6–8 mice for each group.
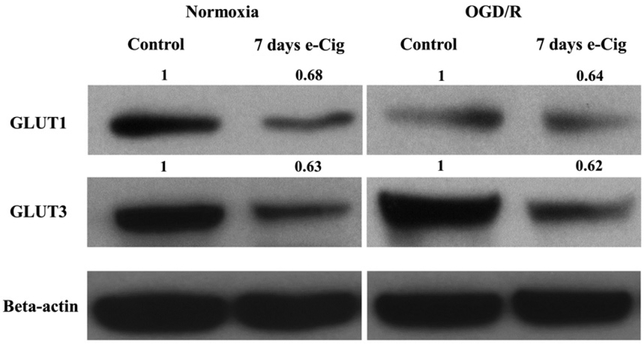
E-Cig vaping decreases GLUT1 and GLUT3 expression in brain slices in normoxic and ischemic conditions
Western blotting was completed in brain slices to investigate changes in glucose transporter expression with e-Cig exposure in normoxic and ischemic conditions. Results of the study (Fig. 8) showed that e-Cig exposure for 7 days resulted in decreased expression of both GLUT1 and GLUT3 in normoxic brain slices. Furthermore, it has been found that 7-day e-Cig exposure decreased the expression of both GLUT1 and GLUT3 in the brain slices after OGD/R (Fig. 8).
Fig. 8.
Effects of 7 days of e-Cig vaping on brain slices GLUT1 and GLUT3 expression in normoxic and OGD/R conditions by western blot. Protein bands were quantified relative to beta-actin. The values on the bands are relative to respective controls. Seven days of e-Cig vaping causes a decrease in both GLUT1 and GLUT3 expression compared to control in normoxic slices. E-Cig vaping for 7 days similarly decreases both GLUT1 and GLUT3 expression compared to control in OGD/R slices. Control normoxia: brain slices from no treatment animals; 7 days of e-Cig normoxia: brain slices from 7 days of e-Cig-exposed animals; Control OGD/R: brain slices from no treatment animals after 30 min OGDand 2 h of reoxygenation;7 days of e-Cig OGD/R: brain slices from 7 days of e-Cig-exposed animals after 30 min OGD and 2 h of reoxygenation; n = 3 mice for each group. GLUT, glucose transporter; OGD, oxygen-glucose deprivation/reoxygenation.
Discussion
The rising popularity of e-Cigs and their widespread use call for more research into their short-term and long-term brain health effects. These devices contain nicotine which can create a long-term addiction in the users (Rahman et al. 2014; Selya et al. 2017). A few recent studies suggest some toxic effects of e-Cig exposure on vital organs, such as the lung and heart (Lee et al. 2018). Kaisar et al. (2017) also demonstrated that e-Cig vaping could alter BBB permeability and worsen ischemic brain injury. The injurious effects of nicotine alone on ischemic stroke outcome have also been shown previously (Abbruscato et al. 2004; Paulson et al. 2010). Glucose utilization and metabolism during ischemic stroke is a crucial factor which could affect stroke outcome and long-term recovery because of the increased energy demand of the ischemic brain (McCall et al. 1996; Vannucci et al. 1996). Glucose is transported across the BBB to the brain parenchyma followed by uptake into individual brain cells by glucose transporters (Vannucci et al. 1997). Previous study in our laboratory has shown that chronic nicotine exposure decreases glucose transport across the BBB in an in vivo ischemic stroke model (Shah et al. 2015). This result indicates that nicotine can decrease glucose availability in the ischemic brain. Furthermore, nicotine could negatively affect ischemic brain parenchymal glucose uptake which has not been adequately studied under these conditions. Combined with the reported effects of nicotine on reduced BBB glucose transport, nicotine could also significantly alter ischemic brain glucose metabolism leading to increased brain damage. In this study, we aimed to observe the effects of short-term and long-term nicotine exposure on neuronal glucose uptake in ischemic conditions utilizing an in vitro ischemic stroke model. We also utilized an ex vivo brain slice technique to look at the effects of in vivo e-Cig vaping on glucose utilization in brain slices subjected to in vitro ischemic conditions. We hypothesized that nicotine and e-Cig exposure would alter ischemic brain glucose utilization resulting in an aggravated energy tension of the ischemic brain, explaining some of the observed damaging effects of nicotine exposure on stroke outcome (Abbruscato et al. 2002, 2004; Paulson et al. 2006, 2010).
First, we looked at the effects of nicotine and its principal metabolite cotinine on neuronal viability by MTT assay. Both in normoxic and OGD/R conditions, only 1000 μM nicotine exerted significant toxicity compared to control, which is a very high dose and not commonly used with in vitro studies. We chose 10 μM nicotine and 5 μM cotinine for our in vitro studies based on the brain level of nicotine and cotinine as observed in an in vivo chronic nicotine treatment study using osmotic minipump (Doura et al. 2008; Lomazzo et al. 2011). These concentrations, either alone or in combination, also did not cause any significant change in neuronal viability, as observed in this study. We then studied the effects of nicotine and cotinine on glucose uptake in primary cortical neurons. We observed that nicotine/cotinine treatment decreased neuronal glucose uptake in OGD/R conditions after both short- and long-term treatment. Cotinine alone caused similar changes in glucose uptake to that of nicotine, which supported the recent findings of significant biological activities of cotinine (Echeverria et al. 2017; Grizzell et al. 2017; Mendoza et al. 2018). Although no direct evidence of detrimental effects of cotinine on ischemic stroke injury or brain glucose metabolism has been observed till now, it has shown similar BBB disrupting activity compared to nicotine (Abbruscato et al. 2002) indicating a potential role in exacerbation of stroke pathology, since BBB disruption is a crucial event in the evolution of ischemic stroke brain damage. In our ex vivo brain slicing studies, we found that 7-day exposure to e-Cig vaping in mice significantly decreased glucose uptake in brain slices both in normoxic and OGD/R conditions. In this study, we used 6-month-old mice to more accurately mimic the aged stroke population as this animal group represents human adult age of more than 30 years (Flurkey et al. 2007). Consideration of age as a biological determinant is essential in the design of preclinical stroke studies and provides additional translational relevance to the disease condition. The smoking strategy we used also mimicked the smoking behavior of a chronic smoker since using subcutaneous osmotic pumps fails to mimic the peak/troughs observed with human smoking cycles (Benowitz 2009). In the brain slicing studies, we also found effects of e-Cig vaping in normoxic conditions which could be attributed to the difference between in vitro and in vivo/ex vivo conditions. Although nicotine in e-Cig vapor might be primarily responsible for the observed effects, the effects of non-nicotine constituents (glycerin, propylene glycol, and flavoring chemicals) should also be considered. A recent study demonstrated inflammatory and oxidative responses in human monocytic cell lines by commonly used e-Cig flavors and flavored e-liquids without nicotine (Muthumalage et al. 2017). The toxic effects of different e-Cig flavoring agents have been discussed by Kaur et al. (2018). Another study found no toxic effects of glycerin and propylene glycol in a subchronic inhalation study in rats (Phillips et al. 2017). Primary cortical neurons were cultured from developing mice, while adult mice were used in the in vivo/ex vivo study, which could further explain the observed differences between the two systems. The results of study suggest that long-term nicotine and e-Cig use could create an enhanced glucose-deprived state in the ischemic brain leading to increased ischemic brain injury. Although there have been no such studies to date regarding cerebral ischemia, some studies reported conflicting results about the acute effects of nicotine on brain glucose utilization. London et al. (1988) reported that acute nicotine treatment increased local cerebral glucose utilization mostly in the nicotine binding regions. Rats were used in this study, which were partially immobilized and regional brain glucose utilization was measured after femoral vein and artery catheterization. They used 0.1, 0.3, and 1.0 mg/kg dosages of nicotine given by intraperitoneal injections. In a later study, nicotine’s effects were measured in both partially immobilized and freely moving rats (Marenco et al. 2000). In the partially immobilized rats, nicotine significantly increased glucose utilization in regions with high nAChR density with non-significant effects on the other regions. But the freely moving rats which were given 0.4 mg/kg of nicotine showed a significant reduction in the global brain glucose utilization, although the nicotine binding regions showed an increase in glucose utilization. London et al. (1990) further reported tolerance to nicotine challenge in some brain regions of subchronically nicotine-exposed rats. Stapleton et al. (2003) studied the effects of acute nicotine treatment at a dose of 1.5 mg by a 10 s intravenous infusion in humans. They found that nicotine significantly decreased glucose utilization in most of the brain regions. In a later preclinical study, 0.7 mg/kg nicotine significantly decreased regional brain glucose oxidation similar to the results found by Wang et al. (2010) using freely moving rats. A more recent study investigated brain energy metabolism in humans using positron emission tomography. Overnight abstinence from smoking resulted in a significant reduction in global cerebral blood flow and metabolic oxygen rate in smokers compared to non-smokers, this impairment was acutely restored by renewed smoking (Vafaee et al. 2015). These results indicate that a period of smoking-abstinence in chronic smokers could dysregulate brain energy metabolism. Furthermore, the immobilization stress could partially explain the higher brain glucose utilization observed in some preclinical studies as stress has been shown to significantly increase cerebral glucose utilization (Carlsson et al. 1977). These results support our findings of diminished glucose utilizations in nicotine-exposed neurons and brain slices. The results of our study indicate that nicotine and e-Cig could negatively affect ischemic brain glucose utilization and availability, leading to enhanced brain injury after ischemic stroke.
The facilitative glucose transporters, GLUT1 and GLUT3 are the most abundant brain glucose transporters which play crucial role in different physiological and pathophysiological conditions (Shah et al. 2012). The primary GLUT isoform in neuron is GLUT3 (Vannucci et al. 1997). The highly glycosylated 55 kDa GLUT1 is present primarily at the BBB, while the less glycosylated 45 kDa GLUT1 has been observed in neurons and glia (Maher et al. 1994; Vannucci et al. 1997; Nakamura et al. 2017). We found that OGD decreases GLUT1 expression in primary cortical neurons compared to control. After reoxygenation, GLUT1 expression is significantly increased indicating an enhanced energy demand of the ischemic brain which is consistent with previous research findings showing that experimental ischemic conditions elevated GLUT1 and GLUT3 levels in immature rodent brains (Vannucci et al. 1996, 1998; Li et al. 2013). Five days of nicotine/cotinine treatment decreases neuronal GLUT1 expression in ischemic conditions. This is the first study investigating the effects of long-term nicotine treatment on glucose transporter expression in an in vitro ischemic stroke model. Although no significant change in GLUT3 was observed in the in vitro studies, our in vivo studies with e-Cig vaping revealed that both GLUT1 and GLUT3 expression was reduced in mouse brain slices in normoxia and OGD/R, accounting for the diminished glucose uptake observed in radioactive uptake studies. The difference between in vitro and in vivo results could be explained by some possible limitations inherent with in vitro experiments; not completely recapitulating ischemic stroke conditions, and the focus on subset cortical neuronal population. Based on our previous finding of decreased BBB expression of GLUT1 (Shah et al. 2015) and the recent findings of decreased neuronal and brain parenchymal GLUT1 expression in ischemic stroke, the facilitative glucose transporter isoform GLUT1 appears to be a key player in modulating the effects of nicotine on ischemic brain glucose utilization. Although the observed changes in the glucose transporters expression suggest that nicotine and e-Cig pre-exposure could enhance brain injury after an acute ischemic stroke, a possibility exists that these effects could be analogous to ischemic pre-conditioning. Heitmeier et al. (2018) showed that chronic inhibition of GLUTs could have beneficial effects in myocardial ischemia-reperfusion injury. Hypoxic pre-conditioning also caused up-regulation of GLUT1 and GLUT3 in neuronal culture after anoxic exposure (Yu et al. 2008). Espinoza-Rojo et al. (2010) have discussed the possible role of GLUTs regulation in cerebral ischemia which can be utilized in stroke therapeutics.
Glucose taken up by a cell is first phosphorylated by hexokinases to produce glucose-6-phosphate, which is trapped inside the cell. This metabolic intermediate can either produce pyruvate after a series of reactions or enter the pentose phosphate pathway. Pyruvate can either be metabolized to lactate-producing two ATPs, a process known as glycolysis; or can enter the mitochondrial tricarboxylic acid (TCA) cycle which generates a total of 38 ATPs (Bouche et al. 2004; Gatenby and Gillies 2004). We found that 5 days of nicotine/cotinine treatment decreases neuronal glycolysis and glycolytic capacity in ischemic condition. Nicotine/cotinine also decreased neuronal mitochondrial respiration after OGD/R. These data support our findings of nicotine-induced reduced neuronal glucose utilization in ischemia and suggest that nicotine may affect two major pathways of glucose utilization.
The physiological and pathological effects of nicotine are primarily mediated by nAChRs (Albuquerque et al. 2009). α4β2 and α7 are the most widely expressed nicotinic receptor subtypes in the brain (Paterson and Nordberg 2000). Although, studies have shown a neuroprotective role of α7 activation in cerebral ischemia (Han et al. 2014a,b) and also alleviates myocardial ischemia-reperfusion injury (Hou et al. 2018), this receptor subtype could also play a significant role in nicotine-induced abnormalities in ischemic brain glucose utilization. We have shown that the effects of nicotine and cotinine on neuronal glucose utilization in OGD/R were mediated by nAChRs as their inhibitory effect on glucose uptake was reversed by mecamylamine, a non-selective nAChR antagonist. Immunofluorescent studies further revealed that α7 nAChR was increased in OGD/R condition compared with OGD. This result is consistent with the study by Utsugisawa et al. (2000) who observed increased α7 nAChR expression after OGD/R in PC12 cells. In our study, 5 days of nicotine/cotinine treatment further significantly increased α7 nAChR expression after OGD/R. Chronic nicotine exposure has been previously shown to up-regulate neuronal α7 nAChR expression (Nuutinen et al. 2006). Cotinine was also demonstrated to be a weak agonist and a positive allosteric modulator of α7 nAChR (Briggs and McKenna 1998; Echeverria and Zeitlin 2012). Another study surprisingly showed down-regulation of α7 nAChRs with chronic cotinine treatment (Terry et al. 2005). But the effects of nicotine/cotinine on this receptor subtype in ischemic conditions have not been reported yet. Our results suggest that α7 nAChR could also be involved with the effects of nicotine on glucose utilization in the ischemic brain and further studies should be done to investigate a possible mechanistic link between the activity of this receptor and glucose utilization.
There were some limitations in this study. One of these is that there was no control group with exposure of e-Cig vapor without any nicotine. Also, the primary neurons were isolated and cultured from mice fetus which might not perfectly represent the adult nervous system.
Conclusion
This study was the first to investigate the effects of nicotine and e-Cig vaping on ischemic brain glucose utilization with a combination of in vitro and in vivo/ex vivo experiments. We have shown that nicotine/cotinine and nicotine-containing e-Cig vapor could significantly decrease brain glucose utilization in ischemic stroke. Brain parenchymal GLUT1 inhibition by nicotine could be a major contributory factor to this effect as observed in our study. Along with the inhibitory effect on GLUT1 at the ischemic BBB, chronic e-Cig vaping could create a significantly glucose-deprived state at the ischemic brain which may lead to worsened stroke outcome and recovery.
Acknowledgments and conflict of interest disclosure
The author(s) acknowledge Heidi Villalba and Thamer Albekairi for their help in e-Cig vaping studies. The author(s) declare(s) that there is no conflict of interest. This work was supported by the National Institute of Health [National Institute of Neurological Disorders and Stroke (NINDS) grant # R01NS076012 to TJA and National Institute on Drug Abuse (NIDA) grant # R01DA8292121 to LC and TJA].
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations used:
- BBB
blood–brain barrier
- ECAR
extracellular acidification rate
- e-Cig
electronic cigarette
- GLUT
glucose transporter
- LDH
lactate dehydrogenase
- MTT
3-(45-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide
- nAChR
nicotinic acetylcholine receptor
- NVU
neurovascular unit
- OCR
oxygen consumption rate
- OGD/R
oxygen-glucose deprivation/reoxygenation
Footnotes
Open science badges
This article has received a badge for *Open Materials* because it provided all relevant information to reproduce the study in the manuscript. The complete Open Science Disclosure form for this article can be found at the end of the article. More information about the Open Practices badges can be found at https://cos.io/our-services/open-science-badges/.
References
- Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT and Davis TP (2002) Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J. Pharm. Sci 91, 2525–2538. [DOI] [PubMed] [Google Scholar]
- Abbruscato TJ, Lopez SP, Roder K and Paulson JR (2004) Regulation of blood-brain barrier Na, K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J. Pharmacol. Exp. Ther 310, 459–468. [DOI] [PubMed] [Google Scholar]
- Alberg AJ, Shopland DR and Cummings KM (2014) The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am. J. Epidemiol 179, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M and Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I and Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 14, 724–738. [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2009) Pharmacology of nicotine: addiction, smokinginduced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol 49, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche C, Serdy S, Kahn CR and Goldfine AB (2004) The cellular fate of glucose and its relevance in type 2 diabetes. Endocr. Rev 25, 807–830. [DOI] [PubMed] [Google Scholar]
- Briggs CA and McKenna DG (1998) Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology 37, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Carlsson C, Hagerdal M, Kaasik AE and Siesjo BK (1977) A catecholamine-mediated increase in cerebral oxygen uptake during immobilisation stress in rats. Brain Res. 119, 223–231. [DOI] [PubMed] [Google Scholar]
- Choeiri C, Staines W and Messier C (2002) Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience 111, 19–34. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB and Perry DC (2008) Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 1215, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V and Zeitlin R (2012) Cotinine: a potential new therapeutic agent against Alzheimer’s disease. CNS Neurosci. Ther 18, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Barreto GE, Avila-Rodriguezc M, Tarasov VV and Aliev G (2017) Is VEGF a key target of cotinine and other potential therapies against alzheimer disease? Curr. Alzheimer Res 14, 1155–1163. [DOI] [PubMed] [Google Scholar]
- Espinoza-Rojo M, Iturralde-Rodriguez KI, Chanez-Cardenas ME, Ruiz-Tachiquin ME and Aguilera P (2010) Glucose transporters regulation on ischemic brain: possible role as therapeutic target. Cent. Nerv. Syst. Agents Med. Chem 10, 317–325. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM and Harrison D (2007) Mouse models in aging research In: The Mouse in Biomedical Research (2nd edn, Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL eds.), pp. 637–672. Elsevier, New York City, NY. [Google Scholar]
- Gatenby RA and Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899. [DOI] [PubMed] [Google Scholar]
- Grizzell JA, Patel S, Barreto GE and Echeverria V (2017) Cotinine improves visual recognition memory and decreases cortical Tau phosphorylation in the Tg6799 mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 78, 75–81. [DOI] [PubMed] [Google Scholar]
- Han Z, Li L, Wang L, Degos V, Maze M and Su H (2014a) Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J. Neurochem 131, 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Shen F, He Y, Degos V, Camus M, Maze M, Young WL and Su H (2014b) Activation of alpha-7 nicotinic acetylcholine receptor reduces ischemic stroke injury through reduction of proinflammatory macrophages and oxidative stress. PLoS ONE 9, e105711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmeier MR, Payne MA, Weinheimer C, Kovacs A, Hresko RC, Jay PY and Hruz PW (2018) Metabolic and cardiac adaptation to chronic pharmacologic blockade of facilitative glucose transport in murine dilated cardiomyopathy and myocardial ischemia. Sci. Rep 8, 6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Zhou Y, Yang H, Liu Y, Mao X, Qin X, Li X, Zhang X and Hu Y (2018) Alpha7 nicotinic acetylcholine receptor activation protects against myocardial reperfusion injury through modulation of autophagy. Biochem. Biophys. Res. Commun 500, 357–364. [DOI] [PubMed] [Google Scholar]
- Islam MR, Yang L, Lee YS, Hruby VJ, Karamyan VT and Abbruscato TJ (2016) Enkephalin-fentanyl multifunctional opioids as potential neuroprotectants for ischemic stroke treatment. Curr. Pharm. Des 22, 6459–6468. [DOI] [PubMed] [Google Scholar]
- Kaisar MA, Prasad S, Liles T and Cucullo L (2016) A decade of e-cigarettes: limited research & unresolved safety concerns. Toxicology 365, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Villalba H, Prasad S, Liles T, Sifat AE, Sajja RK, Abbruscato TJ and Cucullo L (2017) Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is Metformin a viable countermeasure? Redox. Biol 13, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Muthumalage T and Rahman I (2018) Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett 288, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Park SH, Weng MW, Wang HT, Huang WC, Lepor H, Wu XR, Chen LC and Tang MS (2018) E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl Acad. Sci. USA 115, E1560–E1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Han H, Hou R, Wei L, Wang G, Li C and Li D (2013) Progesterone treatment before experimental hypoxia-ischemia enhances the expression of glucose transporter proteins GLUT1 and GLUT3 in neonatal rats. Neurosci. Bull 29, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman PR, McAfee G, Geldenhuys WJ, Van der Schyf CJ, Abbruscato TJ and Allen DD (2005) Brain uptake kinetics of nicotine and cotinine after chronic nicotine exposure. J. Pharmacol. Exp. Ther 314, 636–642. [DOI] [PubMed] [Google Scholar]
- Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC and Kellar KJ (2011) Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J. Neurochem 119, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Connolly RJ, Szikszay M, Wamsley JK and Dam M (1988) Effects of nicotine on local cerebral glucose utilization in the rat. J. Neurosci 8, 3920–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Fanelli RJ, Kimes AS and Moses RL (1990) Effects of chronic nicotine on cerebral glucose utilization in the rat. Brain Res. 520, 208–214. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Li B, Xie L et al. (2015) Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun 6, 6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher F (1995) Immunolocalization of GLUT1 and GLUT3 glucose transporters in primary cultured neurons and glia. J. Neurosci. Res 42, 459–469. [DOI] [PubMed] [Google Scholar]
- Maher F, Vannucci SJ and Simpson IA (1994) Glucose transporter proteins in brain. FASEB J. 8, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Marenco T, Bernstein S, Cumming P and Clarke PB (2000) Effects of nicotine and chlorisondamine on cerebral glucose utilization in immobilized and freely-moving rats. Br. J. Pharmacol 129, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutani E, Kosugi S, Tokuda K et al. (2012) A novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonist prevents ischemic neuronal death. J. Biol. Chem 287, 32124–32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall AL, Van Bueren AM, Nipper V, Moholt-Siebert M, Downes H and Lessov N (1996) Forebrain ischemia increases GLUT1 protein in brain microvessels and parenchyma. J. Cereb. Blood Flow Metab 16, 69–76. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP and Klein JD (2015) Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob. Res 17, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Mendoza C, Barreto GE, Iarkov A, Tarasov VV, Aliev G and Echeverria V (2018) Cotinine: a therapy for memory extinction in post-traumatic stress disorder. Mol. Neurobiol 55, 6700–6711. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Torres-Altoro MI, Tan Z et al. (2014) Ischemic stroke injury is mediated by aberrant Cdk5. J. Neurosci 34, 8259–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK and Rahman I (2017) Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol 8, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Osaka H, Muramatsu SI et al. (2017) Gene therapy for a mouse model of glucose transporter-1 deficiency syndrome. Mol. Genet. Metab. Rep 10, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL and Greengard P (1997) Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J. Neurosci 17, 8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen S, Ekokoski E, Lahdensuo E and Tuominen RK (2006) Nicotine-induced upregulation of human neuronal nicotinic alpha7-receptors is potentiated by modulation of cAMP and PKC in SH-EP1-halpha7 cells. Eur. J. Pharmacol 544, 21–30. [DOI] [PubMed] [Google Scholar]
- O’Donnell ME (1993) Role of Na-K-Cl cotransport in vascular endothelial cell volume regulation. Am. J. Physiol 264, C1316–C1326. [DOI] [PubMed] [Google Scholar]
- Paterson D and Nordberg A (2000) Neuronal nicotinic receptors in the human brain. Prog. Neurobiol 61, 75–111. [DOI] [PubMed] [Google Scholar]
- Paulson JR, Roder KE, McAfee G, Allen DD, Van der Schyf CJ and Abbruscato TJ (2006) Tobacco smoke chemicals attenuate brain-to-blood potassium transport mediated by the Na, K,2Cl-cotransporter during hypoxia-reoxygenation. J. Pharmacol. Exp. Ther 316, 248–254. [DOI] [PubMed] [Google Scholar]
- Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, Bickel U and Abbruscato TJ (2010) Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J. Pharmacol. Exp. Ther 332, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, Titz B, Kogel U et al. (2017) Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food Chem. Toxicol 109, 315–332. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Hann N, Wilson A and Worrall-Carter L (2014) Electronic cigarettes: patterns of use, health effects, use in smoking cessation and regulatory issues. Tob. Induc. Dis 12, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Dierker L, Rose JS, Hedeker D and Mermelstein RJ (2017) The role of nicotine dependence in e-cigarettes’ potential for smoking reduction. Nicotine Tob. Res 10.1093/ntr/ntx160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Desilva S and Abbruscato T (2012) The role of glucose transporters in brain disease: diabetes and Alzheimer’s disease. Int. J. Mol. Sci 13, 12629–12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KK, Boreddy PR and Abbruscato TJ (2015) Nicotine preexposure reduces stroke-induced glucose transporter-1 activity at the blood-brain barrier in mice. Fluids Barriers CNS 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NR and Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim. Biophys. Acta 1802, 80–91. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE and London ED (2003) Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology 28, 765–772. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr, Hernandez CM, Hohnadel EJ, Bouchard KP and Buccafusco JJ (2005) Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 11, 229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugisawa K, Nagane Y, Obara D and Tohgi H (2000) Increased expression of alpha7 nAChR after transient hypoxia in PC12 cells. NeuroReport 11, 2209–2212. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A, Imamirad N, Vang K, Chakravarty MM, Lerch JP and Cumming P (2015) Smoking normalizes cerebral blood flow and oxygen consumption after 12-hour abstention. J. Cereb. Blood Flow Metab 35, 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Seaman LB and Vannucci RC (1996) Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J. Cereb. Blood Flow Metab 16, 77–81. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F and Simpson IA (1997) Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia 21, 2–21. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Reinhart R, Maher F, Bondy CA, Lee WH, Vannucci RC and Simpson IA (1998) Alterations in GLUT1 and GLUT3 glucose transporter gene expression following unilateral hypoxia-ischemia in the immature rat brain. Brain Res. Dev. Brain Res 107, 255–264. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang L, Jiang Y, Ma X, Chowdhury GM and Mason GF (2010) Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. J. Neurochem 113, 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group Members, Mozaffarian D, Benjamin EJ et al. (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e360. [DOI] [PubMed] [Google Scholar]
- Yang T, Roder KE, Bhat GJ, Thekkumkara TJ and Abbruscato TJ (2006) Protein kinase C family members as a target for regulation of blood-brain barrier Na, K,2Cl-cotransporter during in vitro stroke conditions and nicotine exposure. Pharm. Res 23, 291–302. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang H, Shah K, Karamyan VT and Abbruscato TJ (2011) Opioid receptor agonists reduce brain edema in stroke. Brain Res. 1383, 307–316. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhao T, Guo M, Fang H, Ma J, Ding A, Wang F, Chan P and Fan M (2008) Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 1211, 22–29. [DOI] [PubMed] [Google Scholar]