Abstract
Maintaining ideal and balanced levels of IL-1β is of extreme importance as a way to avoid host tissue damage. Intranasal infection of Ifnlr1−/− mice with Staphylococcus aureus led to significantly improved bacterial clearance, survival and decrease of proinflammatory cytokines in the airway including IL-1β, which was decreased by 75% (P<0.0001) and 97% (P<0.001) at 4 and 24 h after infection, respectively. Treatment of Ifnlr1−/− mice with mouse recombinant IL-1β led to increased bacterial burden in the airway and lung. Neutrophils purified from lungs of Ifnlr1−/− infected mice displayed a 50% decrease (P<0.05) in IL-1β levels compared to neutrophils isolated from WT mice. Mice lacking NLRP3 and caspase-1 had reduced IL-1β levels 4 h after infection, due to reductions or absence of active caspase-1 respectively, levels at 24 h were comparable to WT infected mice. Ifnlr1−/− infected mice had decreases in both active caspase-1 and neutrophil elastase indicating an important role for the neutrophil serine protease in IL-1β processing. By inhibiting neutrophil elastase, we were able to decrease IL-1β levels by 39% in Nlrp3−/− infected mice when compared to WT mice. These results highlight the crucial role of both proteases in IL-1β processing, via inflammasome dependent and independent mechanisms.
Introduction
The interferon (IFN) family of cytokines is comprised of three groups (type I, II and III IFN) that signal through distinct receptors to regulate hundreds of genes influencing a multitude of cellular functions. The role of interferons in infectious disease is varied and depending upon the microorganism can be protective or inhibitory to pathogen clearance (1, 2). Staphylococcus aureus is an important human pathogen that is major cause of pneumonia, skin and soft tissue infections. Type I and III signaling contributes to the pathogenesis of S. aureus infection in the airway (3–5) and we recently showed that type III IFN signaling contributes to S. aureus colonization of the upper airway in the context of influenza co-infection (6).
Type III IFN, not unlike type I IFN, activates a signaling cascade via the JAK/STAT pathway. The type III IFN receptor (IFNLR) is a heterodimer of IL-28R and IL-10RB that senses the type III ligands IL-29 (IFNL1), IL-28A/B (IFNL2/3) and IFNL4. Mice only express IFNL2 and IFNL3 (7). A unique property of the type III IFN receptor is its tissue distribution, with expression largely restricted to the epithelium and neutrophils (7, 8). The kinetics of type I and III also vary depending upon the infection, while the target genes are overlapping (7, 9, 10). It has been shown in some systems that type I IFNs can influence inflammasome activation (11, 12).
The inflammasome is a multi-protein complex that leads to production of inflammatory cytokines, most notably IL-1β and IL-18, in response to microbial and danger signals. Initial receptor recognition of pathogen associated molecular patterns (PAMPs) leads to production of pro-proteins that are then cleaved by executioner caspases to facilitate secretion of the active form (13). In some circumstances, this cleavage to the active form can occur via caspase-1 independent mechanisms (14). Inflammasome activation contributes to inflammation in the airway (15) and S. aureus has been shown to activate the inflammasome through several of its toxins. Work-to-date has identified the NLRP3 and caspase-1 inflammasome as the major mechanism of activation, with additional contributions from NLRC5, NLRP7 and AIM2 (16, 17). In the airway, activation of the NLRP3 inflammasome contributes to pulmonary pathology (17).
In the experiments detailed in this study we show that type III interferon signaling contributes significantly to inflammatory cytokine production and inflammasome activation in response to S. aureus in the airway. We show that IL-1β production in response to S. aureus is both caspase-1 dependent and independent, with neutrophil elastase contributing to IL-1β production and providing a mechanism for the reduction of IL-1β in the absence of type III IFN signaling. Inactivation of type III IFN improves the ability of mice to clear the infection.
Results
Type III interferon signaling contributes to the pathogenesis of S. aureus pneumonia
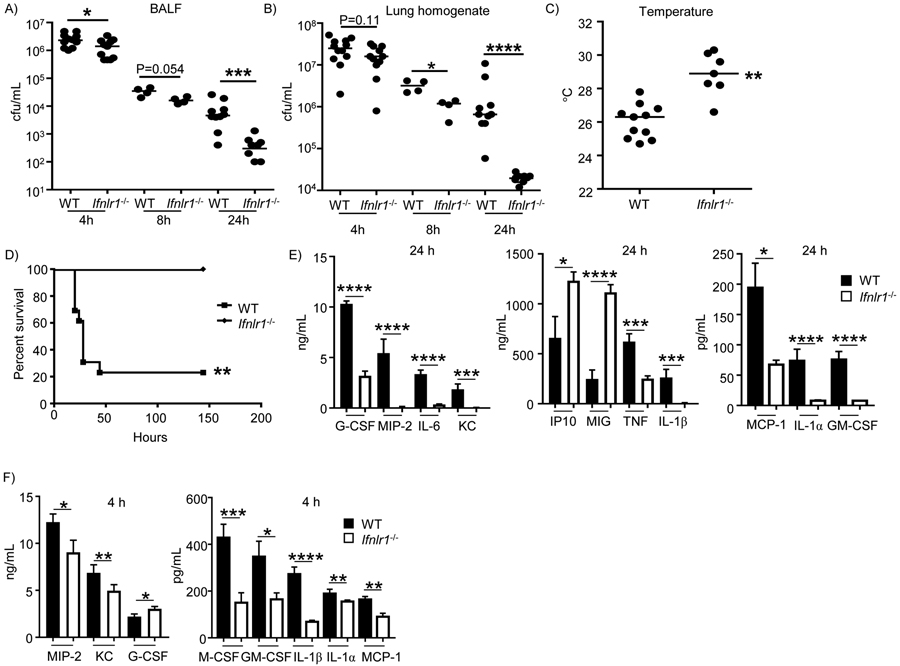
To better understand the role of type III interferon signaling in our model of acute pneumonia, WT and Ifnlr1−/− deficient mice were intranasally infected and analysed at three different time points: 4 h, 8 h and 24 h of infection. Mice lacking the type III interferon receptor displayed significantly improved bacterial clearance. Ifnlr−/− mice had 9-fold less bacteria in the airway (P<0.001), assessed in bronchoalveolar lavage fluid (BALF) and 11-fold less bacteria in lung tissue (P<0.0001) 24 hours after infection (Figure 1A&B) when compared to WT mice. We could already observe a significant decrease in the bacterial burden in the airway (44% decrease; P<0.05; Figure 1A) at 4 h after infection and in the lung tissue 8 h after infection (67% decrease; P<0.05; Figure 1B), indicating an early role for type III interferon signaling in pathogenesis in our acute pneumonia model. Consistent with their reduced bacterial counts, Ifnlr1−/− infected mice had improved body temperatures compared to WT infected mice after 24 hours of infection (Figure 1C; P<0.01). In a mortality model of infection, no death was observed for Ifnlr1−/− mice, whereas 75% of WT mice succumbed to infection (Figure 1D; P<0.01). These data indicate that type III IFN signaling contributes to pathogenesis of S. aureus infection.
Figure 1. Type III interferon signaling contributes to pathogenesis and cytokine production in response to S. aureus in the airway.
C57BL/6J WT and Ifnlr1−/− mice were intranasally infected with 4 × 107 cfu of S. aureus USA300. A) BALF and B) lung homogenate were enumerated for bacterial counts at 4 h, 8 h and 24 h after infection. C) External temperature of WT and Iflnr1−/− mice prior to euthanasia after a 24 h infection. D) WT C57BL/6J and Ifnlr−/− mice were intranasally infected with 108 cfu of S. aureus USA300 and mortality assessed (n=WT-13, Ifnlr-/−−7). E) Cytokine levels in BALF of WT and Ifnlr1−/− mice infected with S. aureus for 24 h (n=WT-10, Ifnlr-/−−10). F) Cytokine levels in BALF of WT and Ifnlr1−/− mice infected with S. aureus for 4 h (n=WT-12, Ifnlr1-/−−11). Each point represents a mouse. Data is from at least two independent experiments except A&B) 4 and 24 hours that are from three independent experiments. Lines display medians. Graphs display means with standard deviation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Mortality data (D) was assessed using a Fishers exact test. For all other analysis a nonparametric Mann-Whitney test was used to assess differences between WT and Ifnlr1−/−.
Type III IFN signaling contributes to inflammatory cytokine production
To better understand the improved phenotype in the absence of type III IFN signaling we conducted multiplex cytokine analysis of bronchoalveolar lavage fluid. Comparison of BALF from WT and Ifnlr1−/− infected mice showed significant differences in several cytokines after 24 h of infection. The proinflammatory cytokines IL-6 (89%; P<0.0001; Figure 1E) and TNF (61%; P<0.001; Figure 1E), were decreased in the type III interferon receptor deficient mice. Significant reductions were also observed for MCP-1 (56%; P<0.05; Figure 1E), IL-1α (89%; P<0.0001; Figure 1E) and GM-CSF (90%; P<0.0001; Figure 1E). IP-10 (P<0.05; Figure 1E) and MIG (P<0.0001; Figure 1E) were observed to be increased in Ifnlr1−/− compared to WT mice at 24 h of infection. No difference was observed on cytokine levels between uninfected WT and Ifnlr1−/− mice (Supplementary Figure 1). Despite a lack of difference in neutrophil recruitment to the airway and the lungs in WT and Ifnlr1−/− mice (Supplementary Figure 2), Ifnlr1−/− infected mice had significant reductions in neutrophil related cytokines at 24 h with MIP-2 (96%; P<0.0001; Figure 1E), KC (97%; P<0.001; Figure 1E) and G-CSF (69%; P<0.0001; Figure 1E) being significantly reduced. As we observed a significant decrease in bacterial numbers at 24 h, the cytokine differences could be a direct reflection of this, therefore we studied an earlier time point. At 4 h of infection and comparable to what we observed at 24 h, similar numbers of neutrophils were being recruited to the airway and (Supplementary Figure 3) despite the decrease of 26% and 28% in MIP-2 (P<0.05; Figure 1F) and KC (P<0.01; Figure 1F) respectively. Interestingly at this early time G-CSF had an increase of 37% (P<0.05; Figure 1F). The early time point (4 h) was however reflective of the later time point (24 h) with significant reductions observed for M-CSF, GM-CSF, IL-1α and MCP-1 (Figure 1F).
IL-1β was the cytokine that displayed one of the most dramatic differences. After 4 h of infection, even though no significant differences were observed with other proinflammatory cytokines, such as TNF or IL-6 (Supplementary Figure 6), a 75% decrease in IL-1β was evident in Ifnlr1−/− infected versus WT infected mice (P<0.0001; Figure 1F). By 24 h of infection Ifnlr1−/− mice had a 97% decrease in IL-1β levels (Figure 1 E).
IL-1β contributes to pathology and is produced by neutrophils
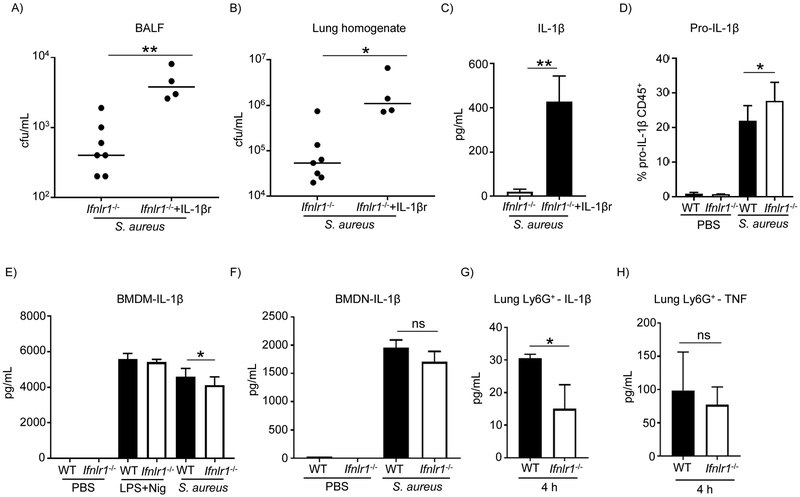
IL-1β is known to contribute to immunopathology in the airway and in response to S. aureus (5, 15, 17). We first assessed its ability to impede bacterial clearance in our model with Ifnlr1−/− mice. Ifnlr1−/− mice were treated with recombinant mouse IL-1β on the day of infection following intranasal infection with S. aureus. Ifnlr1−/− mice treated with IL-1β had increased bacterial loads in the airway and in the lung (P<0.01 and P<0.05, respectively; Figure 2A&B). Higher levels of IL-1β on Ifnlr1−/− mice treated with recombinant mouse IL-1β were confirmed by ELISA (Figure 2C). To assess if the decrease observed in IL-1β levels in Ifnlr1−/− mice (Figure 1E&F) was due to a lack of pro-IL-1β production, we assessed pro-IL-1β levels intracellularly by flow cytometry in cells from BALF. There were higher intracellular levels of pro-IL-1β in the CD45 positive population in Ifnlr1−/− mice when compared to WT mice (P<0.05; Figure 2D) indicating that the difference in IL-1β was not due to production of pro-IL-1β but likely an impairment in the cleavage of pro-IL-1β in the Ifnlr1−/− mice. This was also confirmed at the transcriptional level. qRT-PCR was performed on lung homogenate at 4 h of infection and no differences in Il1b transcript levels were observed between WT and Ifnlr1−/− mice (Supplementary Figure 8). To further assess if the differences in IL-1β levels in BALF were due to an impairment in cleavage and not due to differences in cell death/lysis between WT and Ifnlr1−/− mice, total protein content, cytotoxicity (LDH) and cell viability (DAPI staining) were performed following 4 and 24 h of infection. No differences were observed between WT and knockout mice (Supplementary Figure 9), supporting the hypothesis that there was reduced IL-1β maturation in the Ifnlr1−/− mice.
Figure 2. IL-1β role and regulation in response to S. aureus in the airway.
C57BL/6J WT and Ifnlr1−/− mice were intranasally infected with 4 × 107 cfu of S. aureus USA300. A) BALF and B) lung homogenate were enumerated for bacterial counts at 24 h after infection. C) Quantitation of IL-1β in BALF by ELISA. n=Ifnlr−/−−6, Ifnlr−/−+IL-1β−4. D) Intracellular levels of pro-IL-1β in CD45+ cells from BALF of WT and Ifnlr1−/− mice following a 4 h infection, determined by flow cytometry (n=WT-8, Ifnlr1−/− −8). E) IL-1β levels from purified bone marrow macrophages from WT and Ifnlr1−/− mice determined by ELISA n=5. F) IL-1β levels from purified bone marrow neutrophils from WT and Ifnlr1−/− mice determined by ELISA. n=5. G) IL-1β levels from neutrophils of WT and Ifnlr1−/− mice purified from S. aureus infected lungs following a 4 h infection determined by ELISA. n=5.. H) TNF levels from neutrophils of WT and Ifnlr1−/− mice purified from S. aureus infected lungs following a 4 h infection determined by ELISA. n=5. Each point represents a mouse. In vitro and ex vivo experiments are from two independent experiments. Lines display medians. Graphs display means with standard deviation. *P<0.05, **P<0.01. A nonparametric Mann-Whitney test was used to assess differences between WT and Ifnlr1−/−.
To better understand the effect of type III IFN signaling on IL-1β activation we purified macrophages and neutrophils from bone marrow given their role as IL-1β producing cells in the airway upon infection. Utilizing bone marrow derived macrophages and neutrophils we observed a 10% decreased in IL-1β production from S. aureus stimulated Ifnlr−/− cells, compared to WT infected cells (Figure 2E&F). This minor decrease did not reflect the IL-1β phenotype observed in the airway. To have a better understanding of IL-1β production in vivo, neutrophils were isolated from the lung of WT and Ifnlr1−/− mice that had been infected with S. aureus for 4 h. Neutrophils were chosen due to their early recruitment to the airway and noted responsiveness to type III IFN signaling (8, 18). Neutrophils purified from lungs of Ifnlr1−/− mice displayed a 50% decrease (P<0.05) in IL-1β levels compared to neutrophils isolated from WT mice (P<0.05; Figure 2G), suggesting that type III IFN signaling is involved in modulation of IL-1β levels in response to S. aureus. This was specific to IL-1β as TNF levels were unchanged (Figure 2H), analogous to that observed in the airway at 4 h post infection (Supplementary Figure 6).
IL-1β secretion is partly caspase-1 independent in a S. aureus pneumonia model
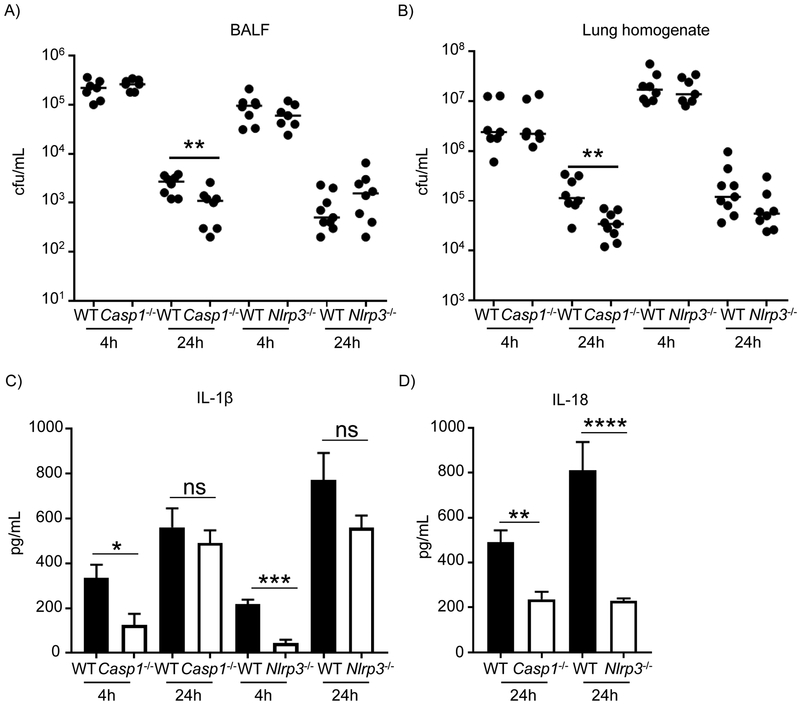
IL-1β is a potent pro-inflammatory cytokine produced as an inactive precursor (31 kDa) that is cleaved to its active form typically by the action of caspase-1 (19). To better understand the reduction of IL-1β in the Ifnlr1−/− mice and given the roles of NLRP3 and caspase-1 in inflammasome activation (20, 21) wild-type, Casp1−/− and Nlrp3−/− mice were intranasally infected with S. aureus and IL-1β was detected by ELISA 4 and 24 h after infection. Bacterial clearance was not different in Nlrp3−/− mice, consistent with previous reports (17), but after 24 hours there was a significant improvement in clearance in Casp1−/− mice (P<0.01; Figure 3A&B). After 4 hours of infection, IL-1β levels were significantly lower in both Casp1−/− and Nlrp3−/− mice relative to WT mice (77%; P<0.05 and 79%; P<0.001, respectively; Figure 3C). However, no significant differences were observed in IL-1β levels in both knockout mice 24 hours after infection (Figure 3C). These results suggest a time dependent role for caspase-1 in IL-1β production in response to S. aureus infection in the lung, whereby it is required early but not later in infection and implicates the action of other IL-1β converting enzymes at later stages of infection.
Figure 3. Caspase-1 independent production of IL-1β.
WT, Casp1−/− and Nlrp3−/− mice were intranasally infected with 4 × 107 cfu of S. aureus USA300 for 4 h and 24 h. A) BALF and B) lung homogenate were enumerated for bacterial counts. C) IL-1β levels in BALF determined by ELISA (4 h n=WT/6NJ-7, Casp1-/−−7, WT-8, Nlrp3-/−−7; 24 h n=WT-9, Casp1-/−−8, WT-9, Nlrp3-/−−8). D) IL-18 levels in BALF, determined by ELISA (n=WT-9, Casp1−/− −8, WT-9, Nlrp3-/−−8). Each point represents a mouse. Data is from two independent experiments. Lines display median. Graphs display means with standard deviation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. A nonparametric Mann-Whitney test was used to assess differences between WT and KO mice.
Similarly, IL-18 is also expressed as an intracellular inactive precursor that requires proteolytic activation. Even though some caspase-1 independent mechanisms have been described, namely through chymase and granzyme B, caspase-1 is still considered to be the main mechanism in the conversion of IL-18 into its active form (22). IL-18 levels were detected by ELISA following a 24 h infection and unlike what was observed for IL-1β, the levels were significantly lower in both Casp1−/− and Nlrp3−/− mice when compared to WT (P<0.01 and P<0.0001, respectively; Figure 3D), demonstrating a major role of caspase-1 and the NLRP3 inflammasome in IL-18 processing in our acute S. aureus pneumonia model. IL-18 levels were also significantly lower in Ifnlr1−/− mice at both 4 h and 24 h of infection (Supplementary Figure 11), suggesting lower active caspase-1 levels in Ifnlr1−/− mice.
Neutrophil elastase is involved in IL-1β processing at later stages of S. aureus lung infection
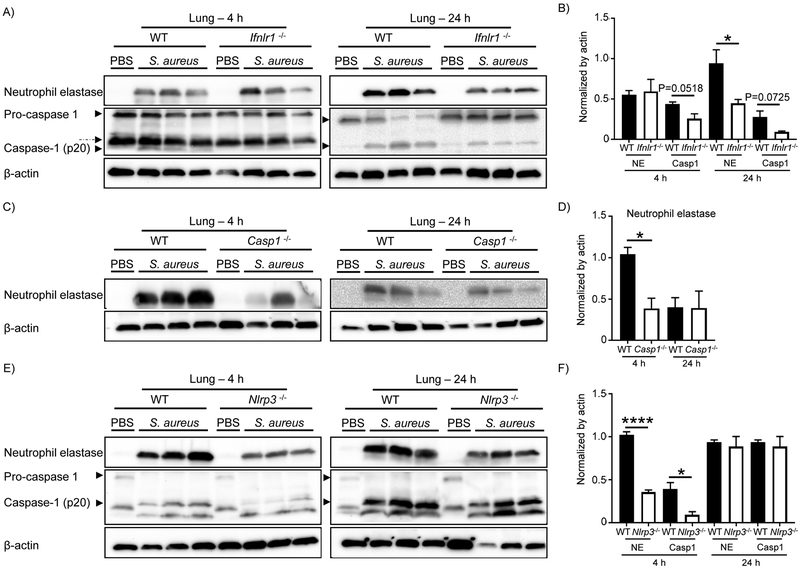
Given the importance of IL-1β during infection as well as its potential to cause tissue damage, it is not surprising that multiple IL-1β activation systems exist. One non-canonical mechanism of IL-1β activation is through the neutrophil serine proteases (NSPs), such as neutrophil elastase (NE). The ability of NE to release mature IL-1β has been reported in response to LPS in the absence of caspase-1 (23). To examine the influence of active caspase-1 and NE in IL-1β activation in our S. aureus pneumonia model we analyzed expression of both proteins in lung homogenates from WT, Ifnlr1−/−, Casp1−/− and Nlrp3−/− mice intranasally infected with S. aureus for 4 h and 24 h. No differences were observed for NE protein levels at 4 h of infection between WT and Ifnlr1−/− mice (Figure 4A&B), however a 52% decrease in NE protein levels was observed at 24 h in Ifnlr1−/− mice (P <0.05; Figure 4A&B). Activation of caspase-1 leads to the formation of its two subunits, p20 and p10. We observed an increase in caspase-1 p20 subunit in the lung homogenate of WT and Ifnlr1−/− mice upon S. aureus infection (Figure 4A). The level of caspase-1 p20 was lower in Ifnlr1−/− mice at both 4 h and 24 h of infection (41%, P=0.0518 and 66%, P= 0.0725, respectively; Figure 4B) when compared to WT. To determine if the reduction in NE levels were not due to impaired induction of neutrophil serine proteases in Ifnlr1−/− mice because of the decreased bacterial levels (Figure 1A&B), protein levels of cathepsin G were also determined. Levels of cathepsin G were increased in Ifnlr−/− mice when compared to WT mice (Supplementary Figure 12), indicating the reduction in NE was not due to impaired stimulation.
Figure 4. Neutrophil elastase contributes to activation of IL-1β.
A) Western blot analysis of caspase-1 and neutrophil elastase expression in lungs of WT and Ifnlr1−/− mice following 4 and 24 hours of infection with S. aureus. Dashed arrow indicates unspecific band. B) Densitometry of NE and caspase-1 (p20) expression from A). C) Western blot analysis of neutrophil elastase expression in lungs of WT and Casp1−/− mice following 4 and 24 hours of infection with S. aureus. D) Densitometry of NE expression from C). E) Western blot analysis of neutrophil elastase and caspase-1 expression in lungs of WT and Nlrp3−/− mice following 4 and 24 hours of infection with S. aureus. F) Densitometry of NE and caspase-1 (p20) expression from E). Data is from two independent experiments except C)-4h from three independent experiments. Each lane is an individual mouse. Representative blots are shown from eight infected mice for each genotype and two mice for each uninfected genotype. Graphs display means with standard deviation. ****P<0.0001 and *P<0.05. An unpaired t-test was used.
In the absence of caspase-1 there was a 63% decrease in NE expression at 4 h of infection compared to WT mice (P <0.05; Figure 4C), consistent with the lower IL-1β levels observed at 4 h in Casp1−/− mice (Figure 3 C). At 24 h of infection NE levels were similar between WT and Casp1-/−. Given that both WT and Casp1−/− mice produced similar levels of active IL-1β at 24 h, our results suggest a role for NE in IL-1β processing and activation in our pneumonia model. In Nlrp3−/− infected mice both NE and active caspase-1 were significantly lower, with a 65% and 76% decrease respectively (P <0.0001 and P <0.05, respectively; Figure 4C&D) after 4 h of infection. The observed difference was not a reflection of a difference in total neutrophil numbers recruited to the airway (Supplementary Figure 13). Levels of both proteins were analogous to WT at 24 h of infection (Figure 4E&F) consistent with the levels of IL-1β (Figure 3C). Even though the NLRP3 inflammasome is required in vitro (24, 25), our data indicates the importance of other inflammasome proteins in the production of IL-1β in vivo. These results suggest that the decrease observed in IL-1β levels in Ifnlr1−/− mice is a result of an impairment in activation of both caspase-1 and neutrophil elastase. We observed that caspase-1 is particularly important early in infection and the role of neutrophil elastase is more apparent later in infection.
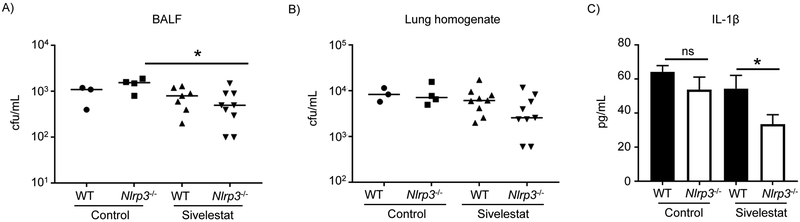
Neutrophil elastase contributes to IL-1β activation in S. aureus lung infection
Neutrophil serine proteases, such as neutrophil elastase are part of the inflammasome-independent known mechanisms for activation of IL-1β (23, 26). To assess the contribution of neutrophil elastase in IL-1β activation in S. aureus lung infection WT and Nlrp3−/− mice were treated with sivelestat, a neutrophil elastase inhibitor, in a 24 h model of acute pneumonia. Mice lacking NLRP3 and treated with the neutrophil elastase inhibitor displayed a slight reduction in bacterial clearance (Figure 5A&B). In the absence of NLRP3 we observed a 39% decrease in IL1-β levels when compared to WT treated mice (P <0.05; Figure 5C). No change was observed in WT mice as they are still producing IL-1β via caspase-1-dependent mechanisms. These data show the important role for neutrophil elastase in IL-1β activation in vivo in addition to the activity of caspase-1.
Figure 5. Neutrophil elastase contribution to IL-1β production in vivo.
C57BL/6J WT and Nlrp3−/− mice treated with sivelestat were intranasally infected with 4 × 107 cfu of S. aureus USA300 for 24 h. A) BALF and B) lung homogenate were enumerated for bacterial counts. C) IL-1β levels in BALF, determined by ELISA. n=WT control-3, Nlrp3−/− control-4; n=9 for sivelestat treated groups. Each point represents a mouse. Data is from two independent experiments. Lines display median. Graphs display means with standard deviation. *P<0.05. ns-not significant. A nonparametric Mann-Whitney test was used to assess differences.
Discussion
Type III IFN signaling has been investigated in the context of viral infections but not to the same extent in bacterial infections. In this report, we show that type III IFN contributes to the pathogenesis of S. aureus in the airway, influencing proinflammatory cytokine production and the inflammasome. We demonstrate that production of IL-1β in response to S. aureus is not entirely dependent on the canonical caspase-1 dependent inflammasome, but also requires neutrophil elastase.
We demonstrate a role for NLRP3/caspase-1 and neutrophil elastase in IL-1β production in response to S. aureus in the airway. In vitro data has shown that activation of the inflammasome and cleavage of IL-1β in response to S. aureus is largely NLRP3 and caspase-1 dependent. This has been shown with purified cells and cell lines using S. aureus lipoproteins and several of the S. aureus toxins (21, 27). Our data suggest there are several systems that contribute to IL-1β activation and that the in vitro data does not fully reflect what occurs in vivo in the airway. We observed IL-1β to be largely NLRP3/caspase-1 dependent early (4 h) during infection, while the inflammasome was dispensable for IL-1β production at 24 h. We saw significant reductions in IL-1β in the absence of IFNLR at 4 and 24 h that correlated with reductions in both caspase-1 and NE, which was not due to a lack of pro-IL-1β production or from changes in cell death. This reduction was also evident in purified neutrophils from WT and Ifnlr−/− infected mice. The reduction in NE was also not the result of reduced neutrophil numbers or their induction of neutrophil proteases, as cathepsin G was still produced in the absence of IFNLR. All these points suggest a requirement for both caspase-1 and NE in the processing of IL-1β in response to S. aureus in the airway, providing signaling redundancy in its maturation. Therefore, only when we inhibited NE in the absence of NLRP3 did we observe an effect on IL-1β levels in the airway. The neutrophil serine proteases are present in neutrophilic granules and have known IL-1β cleavage sites (14). Activation of IL-1β within neutrophils is largely protease dependent (28) but they can also act extracellularly and facilitate IL-1β cleavage in other cells such as macrophages (29, 30). The contribution of neutrophil serine proteases to IL-1β activation has been noted in inflammatory disorders such as arthritis where there is a large neutrophil component (31–33). Given that neutrophils are the most abundant cell recruited to the airway following infection, the contribution of neutrophil elastase to IL-1β activation to supplement the action of caspase-1 is consistent with this. Although in the absence of IFNLR we observe a significant improvement in bacterial clearance, we did not observe any significant cellular changes, such as neutrophil numbers. This clearance phenotype is potentially a result of the reduced cytokine production and its associated immunopathology, the direct effect of a secreted factor or a change in function with one of the professional phagocytes under these conditions.
Production of IL-1β is potentially detrimental in the airway as overproduction contributes to immunopathology (15). The role of the inflammasome in S. aureus pathogenesis is varied. In cutaneous models of skin infection, it plays an important role (34), while in the airway, data for its role is varied. Inhibition of IL-1β with the antagonist drug Anakinra reduces immunopathology (5), while in different mouse strains and in co-infection with influenza it can play an important role (35). Our data with NLRP3 knockout mice is consistent with previous work showing that the inflammasome contributes to excessive inflammation and pathology but not clearance (17). However, we did observe a clearance phenotype in the absence of caspase-1. Multiple receptors are known to utilize caspase-1 for inflammasome activation and this data also suggests that a downstream product of caspase-1 may be inhibitory to bacterial clearance or is suppressed by caspase-1. Moreover, caspase-1 has also been reported to activate the transcription factor NF-kB regardless of its activation and hence lead to increased inflammation in vivo (36). The absence of caspase-1 may also lead to less proinflammatory cytokines, which as we have shown in our Iflnr1−/− mice with the exogenous addition of IL-1β, may contribute to immunopathology and reduced capacity to clear the infection.
In the absence of the type III IFN receptor, we observed improved bacterial clearance of S. aureus, decreased mortality and large reductions in proinflammatory cytokine production. Our novel observation was that the absence of IL-1β was due to both reductions in caspase-1 but also a significant reduction in neutrophil elastase, suggesting a possible crosstalk between type III IFN signaling and NE regulation. While it has been shown that type III IFN is important in viral infection less is known about its role in bacterial infections (9, 37). Most studies so far have generated correlative data that haven’t utilized genetic knockouts. One study that was the initial examination of S. aureus and Pseudomonas aeruginosa reported type III IFN to contribute to pathogenesis and pulmonary pathology in the absence of cellular infiltrate differences (7). Our clearance phenotype is consistent with that study. The contribution of neutrophils to disease has recently garnered significant attention as it was discovered they are responsive to type III IFN. In models where neutrophils play a prominent role, their responses to type III IFN have been shown to reduce inflammation in arthritis and intestinal inflammation as well aid in the clearance of fungal infection (8, 18, 38). As they are a prominent cell in the airway in response to infection and given our observations with neutrophil elastase and IL-1β activation, we see type III IFN signaling in neutrophils playing an important role in the pathogenesis of S. aureus pneumonia.
The inflammatory response to microbial insult is orchestrated by a multitude of receptors and many interconnected signaling pathways. We have observed that the inflammatory response to S. aureus is significantly reduced in the absence of type III IFN signaling and this is connected to the inflammasome. IL-1β is activated by S. aureus not only through a canonical caspase-1 dependent pathway, but also through neutrophil elastase. It is likely that the function of the neutrophil serine proteases does not end with a role in IL-1β signaling and highlights their multiple functions in addition to the contribution type III IFN signaling plays in the pathogenesis of S. aureus infection.
Material and Methods
Animal studies
S. aureus strain USA300 FRP3757 was grown in Luria-Bertani (LB) broth at 37°C to exponential phase (optical density of 1.0 at 600 nm). Six to eight week old C57BL/6J WT, C57BL/6NJ WT, Ifnlr1−/−, Casp1−/− and Nlrp3−/− mice were intranasally infected while under anesthesia with 4 × 107 cfu of S. aureus USA300 for 4 h, 8 h or 24 h as previously described (3).
C57BL/6NJ WT was used as the WT control for Casp1−/− infections. C57BL/6 WT, C57BL/6NJ WT, Casp1−/− and Nlrp3−/− mice were purchased from Jackson Laboratories and Ifnlr1−/− mice were provided by Bristol Myers Squibb. Both sexes of mice were used and co-housed for at least two weeks prior to infection. For mortality studies C57BL/6 WT and Ifnlr1−/− mice were intranasally infected with 108 cfu of S. aureus USA300 and mortality assessed for 7 days. For inhibition of neutrophil elastase C57BL/6 WT and Nlrp3−/− mice were treated intraperitoneally with 10 mg/kg of sivelestat (TOCRIS) or vehicle control 24 h prior to infection as well as on the day of infection and infected with S. aureus as described above. Ifnlr1−/− mice were treated with 300 ng of recombinant IL-1β (Tocris) or PBS intranasally at the time of infection. Temperatures were measured using the TW2 infrared thermometer (Thermoworks).
Bronchoalveolar lavage fluid (BALF) was obtained from three successive washes with 1 ml of PBS. BALF was used to enumerate bacterial counts and quantify cytokine levels. Lung tissue was homogenized and used to enumerate bacterial counts, immunoblotting and transcriptional analysis. Cytokine levels were quantified using multiplex technology (Eve Technologies) and ELISA.
Animal work in this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory animals of the National Institutes of Health, the Animal Welfare Act, and U.S. federal law. Protocols were approved by the Animal Care and Use Committee of Columbia University.
Isolation of mouse bone marrow neutrophils and macrophages
Neutrophils were isolated from the bone marrow of the femurs and tibias of naive WT and Ifnlr1−/− mice by density gradient centrifugation as previously described (39). Briefly, bone marrow cells were overlaid on a Histopaque 1119 and Histopaque 1077 gradient (Sigma) and centrifuged for 30 minutes at 720 x g at room temperature without brake. Thereafter, neutrophils were collected at the interface between the 2 layers. After assessment of cell number and viability, neutrophils were resuspended in HBSS containing Ca2+, Mg2+ and 0.1% gelatin. Macrophages were generated from bone marrow over a period of 7 days in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin and 20 ng/mL macrophage colony stimulating factor (M-CSF).
Bone marrow derived neutrophils were used for experiments on the day of purification and seeded into 96 well plates at a density of 500,000 cell/well. Cells were incubated with S. aureus at a MOI of 10 for 16–18 hours at 37 °C in an atmosphere containing 5% CO2. Bone marrow derived macrophages were previously seeded onto 96 well plates at a density of 100,000/well, washed with antibiotic-free media and incubated with S. aureus at a MOI of 10 for 16–18 hours at 37 °C in an atmosphere containing 5% CO2. LPS (100 ng/mL) and nigericin (5 μM) were used as a positive control. IL-1β levels in cell-free supernatants were analyzed by ELISA.
Isolation and ex vivo culture of lung neutrophils
WT and Ifnlr1−/− mice were intranasally infected with 4 × 107 cfu of S. aureus USA300. Following a 4 h infection, a single-cell suspension of lung cells was prepared through proteolytic digestion of the lungs. Briefly, murine lungs were inflated with 2 mL of Dispase (5U/mL) followed by 0.5 mL of 1% low melting agarose. Lungs were then placed in 3 mL of dispase solution (5U/ml) for 45 min at RT. The lung cell suspensions were filtered through different pore size cell strainers, starting with 100 μm and subsequently with 70 μm and 40 μm. After hypotonic red cell lysis, magnetic-activated cell sorting (MACS) was used to isolate Ly6G+ cells from the lung single-cell suspension using PE microbeads (Miltenyi Biotec) and PE-Ly6G+ antibody, following the manufacturers protocol. All MACS-purified cell preparations were assessed for purity by flow cytometry. Neutrophils were resuspended in RPMI 1640 medium containing 10% fetal bovine serum, 1% PenStrep and 500 μg/mL gentamicin and seeded on to 96-well plates for 16–18 hours at 37 °C in an atmosphere containing 5% CO2. IL-1β levels in cell-free supernatants was analyzed by ELISA.
ELISA and Immunoblotting
BALF was analyzed for IL-1β (Biolegend) and IL-18 (ThermoFisher Scientific) by ELISA according to the manufacturer’s instructions. Lung homogenates were lysed in RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 1% Triton X-100), containing HALT protease and phosphatase inhibitors (Pierce) and used to assess neutrophil elastase (R&D Systems-MAB4517), cathepsin G (MyBioSource-MBS2029904), caspase-1 (pro-form and active form - p20; R&D Systems-MAB6215) and β-actin (Sigma) protein levels. Secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology Inc.) were used. Densitometry was performed using Image J (NIH).
Flow cytometry
Cells in BALF and lung homogenate were stained with fluorescently labeled antibodies: CD11b-AF594 (M1/70), Ly6G-BV605 (1A8), CD11c-PerCP-Cy5.5 (N418), CD45-AF700 (30-F11), BV510-CD103 (2E7), Siglec-F-AF647 (E50–2440; BD Biosciences), MHCII-APC-Cy7 (M5/114.15.2), Ly6C-PE-Texas Red (AL-2; BD Biosciences) and DAPI to assess cell viability. Antibodies were from Biolegend unless otherwise stated. Cells in the airway and lung were classified as before (40, 41). For IL-1β intracellular staining, surface staining was performed as stated above and was followed by fixation and then permeabilization, using the fluorescently labeled antibody pro-IL-1β-APC (NJTEN3) and the two-step protocol for intracellular (cytoplasmic) proteins (eBioscience) according to manufacturers instructions. We have adhered to the EJI recommended guidelines for the use of flow cytometry.
Transcriptional analysis
Total RNA was extracted from lung homogenate using the E.Z.N.A. Total RNA Kit I (OMEGA biotech) according to manufactures instructions and followed by DNase treatment using the DNA-free DNA removal kit (Life Technologies). cDNA was generated using the High Capacity cDNA reverse Transcriptase Kit (Applied Biosystems). Quantitative real-time RT-PCR (QRT-PCR) was performed using Power SYBR Green PCR Master Mix in a Step One Plus Real-Time PCR System (Applied Biosystems). Primers for Il1B were: 5’-TCAACTGTGAAATGCCACCT-3’; 5’-TCCACAGCCACAATGAGTGA and for Actb 5’-AGTGTGACGTTGACATCCGT-3’; 5’-TGCTAGGAGCCAGAGCA-3’.
Statistics
Animal data was assumed to not follow a normal distribution, thus a nonparametric Mann-Whitney test was used. Mortality data was assessed using a Fishers exact test. Unpaired t-test was used for densitometry analysis. Statistics were performed with Prism software (GraphPad, La Jolla LA USA).
Supplementary Material
Acknowledgments
This work was supported through The American Association of Immunologists Careers in Immunology Fellowship Program (to SP) and funding through the NIH (R56HL125653 and R01HL134870 to DP). Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Kovarik P, Castiglia V, Ivin M, and Ebner F. Type I Interferons in Bacterial Infections: A Balancing Act. Front Immunol. 2016;7:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNab F, Mayer-Barber K, Sher A, Wack A, and O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker D, and Prince A. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol. 2012;189(8):4040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker D, Planet PJ, Soong G, Narechania A, and Prince A. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. Plos Pathog. 2014;10(2):e1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen TS, and Prince AS. Bacterial pathogens activate a common inflammatory pathway through IFNlambda regulation of PDCD4. Plos Pathog. 2013;9(10):e1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planet PJ, Parker D, Cohen TS, Smith H, Leon JD, Ryan C, et al. Lambda Interferon Restructures the Nasal Microbiome and Increases Susceptibility to Staphylococcus aureus Superinfection. MBio. 2016;7(1):e01939–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen TS, and Parker D. Microbial pathogenesis and type III interferons. Cytokine Growth Factor Rev. 2016;29:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, et al. IFN-lambda resolves inflammation via suppression of neutrophil infiltration and IL-1beta production. J Exp Med. 2015;212(6):845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, et al. Interferon-lambda Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness. Immunity. 2017;46(5):875–90 e6. [DOI] [PubMed] [Google Scholar]
- 10.Crotta S, Davidson S, Mahlakoiv T, Desmet CJ, Buckwalter MR, Albert ML, et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. Plos Pathog. 2013;9(11):e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopitar-Jerala N The Role of Interferons in Inflammation and Inflammasome Activation. Front Immunol. 2017;8:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BH, Chee JD, Bradfield CJ, Park ES, Kumar P, and MacMicking JD. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat Immunol. 2016;17(5):481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz P, and Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20. [DOI] [PubMed] [Google Scholar]
- 14.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, and Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. [DOI] [PubMed] [Google Scholar]
- 15.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, and Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–8. [DOI] [PubMed] [Google Scholar]
- 16.Melehani JH, and Duncan JA. Inflammasome Activation Can Mediate Tissue-Specific Pathogenesis or Protection in Staphylococcus aureus Infection. Curr Top Microbiol Immunol. 2016;397:257–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis. 2012;205(5):807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espinosa V, Dutta O, McElrath C, Du P, Chang YJ, Cicciarelli B, et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci Immunol. 2017;2(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–74. [DOI] [PubMed] [Google Scholar]
- 20.Afonina IS, Muller C, Martin SJ, and Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42(6):991–1004. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Planillo R, Franchi L, Miller LS, and Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183(6):3942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Veerdonk FLV, Netea MG, Dinarello CA, and Joosten LAB. Inflammasome activation and IL-1 beta and IL-18 processing during infection. Trends Immunol. 2011;32(3):110–6. [DOI] [PubMed] [Google Scholar]
- 23.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, and Corr M. Caspase 1-Independent Activation of Interleukin-1 beta in Neutrophil-Predominant Inflammation. Arthritis Rheum. 2009;60(12):3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gicquel T, Robert S, Loyer P, Victoni T, Bodin A, Ribault C, et al. IL-1 beta production is dependent on the activation of purinergic receptors and NLRP3 pathway in human macrophages. Faseb J. 2015;29(10):4162–73. [DOI] [PubMed] [Google Scholar]
- 25.Wang XC, Gong PT, Zhang X, Wang JL, Tai LX, Wang X, et al. NLRP3 inflammasome activation in murine macrophages caused by Neospora caninum infection. Parasite Vector. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black R, Kronheim S, Sleath P, Greenstreet T, Virca GD, March C, et al. The Proteolytic Activation of Interleukin-1-Beta. Agent Action Suppl. 1991;35:85–9. [PubMed] [Google Scholar]
- 27.Melehani JH, James DB, DuMont AL, Torres VJ, and Duncan JA. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. Plos Pathog. 2015;11(6):e1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakele M, Joos M, Burdi S, Allgaier N, Poschel S, Fehrenbacher B, et al. Localization and functionality of the inflammasome in neutrophils. J Biol Chem. 2014;289(8):5320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kettritz R Neutral serine proteases of neutrophils. Immunol Rev. 2016;273(1):232–48. [DOI] [PubMed] [Google Scholar]
- 30.Sadatomo A, Inoue Y, Ito H, Karasawa T, Kimura H, Watanabe S, et al. Interaction of Neutrophils with Macrophages Promotes IL-1beta Maturation and Contributes to Hepatic Ischemia-Reperfusion Injury. J Immunol. 2017;199(9):3306–15. [DOI] [PubMed] [Google Scholar]
- 31.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, and Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60(12):3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiber A, Pham CT, Hu Y, Schneider W, Luft FC, and Kettritz R. Neutrophil serine proteases promote IL-1beta generation and injury in necrotizing crescentic glomerulonephritis. J Am Soc Nephrol. 2012;23(3):470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60(12):3651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, et al. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. Plos Pathog. 2012;8(11):e1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson KM, Choi SM, McHugh KJ, Mandalapu S, Enelow RI, Kolls JK, et al. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1beta production in mice. J Immunol. 2013;191(10):5153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamkanfi M, Kalai M, Saelens X, Declercq W, and Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004;279(23):24785–93. [DOI] [PubMed] [Google Scholar]
- 37.Andreakos E, Salagianni M, Galani IE, and Koltsida O. Interferon-lambdas: Front-Line Guardians of Immunity and Homeostasis in the Respiratory Tract. Front Immunol. 2017;8:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broggi A, Tan Y, Granucci F, and Zanoni I. IFN-lambda suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat Immunol. 2017;18(10):1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swamydas M, and Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp. 2013(77):e50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, and Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker D CD80/CD86 signaling contributes to the proinflammatory response of Staphylococcus aureus in the airway. Cytokine. 2018;107:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.