Abstract
Objective:
To determine if radical plakophilin-2 (PKP2) variants might underlie some cases of clinically diagnosed catecholaminergic polymorphic ventricular tachycardia (CPVT) and exercise-associated, autopsy negative sudden unexplained death in the young (SUDY).
Background:
Pathogenic variants in PKP2 cause arrhythmogenic right ventricular cardiomyopathy (ARVC). Recently, a cardiomyocyte-specific PKP2 knockout mouse model revealed that loss of PKP2 markedly reduced expression of genes critical in intracellular calcium handling. The mice, with structurally normal hearts, exhibited isoproterenol-triggered polymorphic ventricular arrhythmias that mimicked CPVT.
Methods:
A PKP2 gene mutational analysis was performed on DNA from 18 unrelated patients (9 males, average age at diagnosis, 19.6 ± 12.8 years) clinically diagnosed with CPVT but who were RYR2-, CASQ2-, KCNJ2-, and TRDN-negative and 19 decedents with SUDY during exercise (13 males, average age at death, 14 ± 3 years). Only radical (i.e. frame-shift, canonical splice-site, or nonsense) variants with a minor allele frequency of ≤ 0.00005 in the genome aggregation database (gnomAD) were considered pathogenic.
Results:
Radical PKP2 variants were identified in 5/18 (27.7%) CPVT patients and 1/19 (5.3%) exercise-related SUDY cases compared to 96/138,632 (0.069%) individuals in gnomAD (p=3.1 ×10−13). Cardiac imaging or autopsy demonstrated a structurally normal heart in all patients at the time of their CPVT diagnosis or sudden death.
Conclusions:
Our data suggests that the progression of the PKP2-dependent electropathy can be independent of structural perturbations and can precipitate exercise-associated sudden cardiac arrest or sudden cardiac death prior to the presence of an overt cardiomyopathy, clinically mimicking CPVT akin to the PKP2 knockout mouse model. Thus, CPVT and SUDY genetic test panels should now include PKP2.
Keywords: arrhythmogenic right ventricular cardiomyopathy, catecholaminergic polymorphic ventricular tachycardia, genetic testing, plakophilin-2, sudden unexplained death in the young
CONDENSED ABSTRACT
Pathogenic plakophilin-2 (PKP2) variants cause arrhythmogenic right ventricular cardiomyopathy. Cardiomyocyte-specific PKP2 knockout mice, with structurally normal hearts, exhibited isoproterenol-triggered polymorphic ventricular arrhythmias that mimicked catecholaminergic polymorphic ventricular tachycardia (CPVT). Radical PKP2 variants were identified in 5/18 patients with CPVT and 1/19 exercise-associated autopsy negative sudden unexplained death in the young (SUDY) cases. Cardiac imaging/autopsy demonstrated a structurally normal heart in all patients at the time of their CPVT diagnosis/sudden death. The PKP2-dependent electropathy can precipitate exercise-associated sudden cardiac arrest/sudden cardiac death prior to the presence of an overt cardiomyopathy clinically mimicking CPVT. Thus, CPVT and SUDY genetic test panels should include PKP2.
INTRODUCTION
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a potentially lethal heritable arrhythmia syndrome that classically manifests with exercise-induced syncope/seizures, sudden cardiac arrest (SCA), or sudden cardiac death (SCD) in the setting of a structurally normal heart.(1) CPVT is associated with a normal resting electrocardiogram (ECG). CPVT is suspected clinically based on the patient’s personal and/or family history and documentation of the emergence of significant and progressive ventricular ectopy during either treadmill or catecholamine stress testing which can culminate in its specific but insensitive trademark arrhythmia of exercise-associated bidirectional ventricular tachycardia.(2) Once thought to manifest only during childhood, more recent studies have suggested that the age of first presentation can range from infancy to 40 years of age.(2,3) CPVT’s potential lethality is evident by mortality rates of 30% to 50% by age 35 years and the presence of a positive family history of young (< 40 years) SCD for more than a third of CPVT individuals.(4) In addition, 15% of autopsy negative sudden unexplained death in the young (SUDY) cases may be caused by CPVT especially when the SCD occurred during exercise.(5,6)
The pathogenic substrates for CPVT involve key components of intracellular calcium-induced calcium-release from the sarcoplasmic reticulum. The most common subtype of CPVT, CPVT1, stems from mutations in the RYR2-encoded cardiac ryanodine receptor/calcium release channel and accounts for ~60% of CPVT.(7-9) Mutations in RYR2 confer a so-called “gain-of-function” phenotype to the calcium release channel resulting in increased calcium leak during sympathetic stimulation, particularly in diastole. In contrast to the autosomal dominant/sporadic CPVT1, very rarely, CPVT is autosomal recessive, due to mutations in CASQ2-encoded calsequestrin or TRDN-encoded triadin.(10-12) In addition, bidirectional ventricular tachycardia can be seen in patients with KCNJ2-mediated Andersen-Tawil syndrome (ATS1) which can have some overlap features with CPVT (CPVT3) although it can be distinguished typically by a very high burden of ambulatory ectopy which is absent generally in CPVT1.(13)
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease characterized by a fibrous or fibro-fatty infiltration of the heart muscle and an increased risk for SCD in the young(14). ARVC-triggered SCD is often associated with exercise and can occur during the subclinical (or “concealed”) phase of the disease when overt cardiomyopathy is not yet detectable by cardiac imaging (echocardiography or cardiac MRI).(15,16) ARVC is associated primarily with dysfunctional desmosomal proteins and is caused most commonly by loss-of-function mutations in the PKP2-encoded plakophilin-2 protein (PKP2).(15-18)
Recently, Delmar and colleagues demonstrated in a cardiomyocyte-specific PKP2 knockout mouse model that the loss of PKP2 markedly reduced the transcriptional expression of genes critical to intracellular calcium handling, including RYR2 and TRDN, and reduced Casq2 protein levels as well.(19) While still manifesting a structurally normal heart, the PKP2-knockout mice exhibited isoproterenol-triggered polymorphic ventricular arrhythmias that mimicked CPVT. These data further support loss-of-function PKP2 variants as a potential cause of life-threatening ventricular arrhythmias even in the absence of overt structural heart disease in humans. Therefore, we speculated that radical PKP2 variants might underlie some patients who had been diagnosed clinically with genetically elusive CPVT as well as some decedents classified as exercise-associated, autopsy negative SUDY.
MATERIALS AND METHODS
Population
Briefly, 18 (9 males, average age at diagnosis = 19.6 ± 12.8 years) unrelated patients with clinically diagnosed but RYR2/CASQ2/KCNJ2/TRDN-negative (i.e. genotype-negative) CPVT and 19 (13 males, average age at death = 14 ± 3 years) medical/coroner cases of exercise-associated, autopsy negative SUDY were referred to the Windland Smith Rice Sudden Death Genomics Laboratory at the Mayo Clinic in Rochester, Minnesota, for exploratory genetic testing (Table 1). All CPVT patients and the decedents’ next-of-kin signed a Mayo Clinic IRB-approved written consent form prior to genetic analysis.
Table 1.
Demographics summary of the CPVT- and exercise-associated autopsy negative SUDY cohorts.
| Overall | PKP2 Negative | PKP2 Positive | |
|---|---|---|---|
| Patients Diagnosed with CPVT (N) | 18 | 13 | 5 |
| Males/Females | 9/9 | 7/6 | 2/3 |
| Average Age (years) | 19.6 ± 12.8 | 19.7 ± 14.7 | 19.4 ± 6.3 |
| Age Range (years) | 6 to 58 | 6 to 58 | 12 to 28 |
| Average QTc (ms) | 417 ± 26 | 416 ± 23.4 | 419 ± 35 |
| Ventricular Ectopy | 13 (72%) | 8 (61.5%) | 5 (100%) |
| Symptomatic | 15 (83%) | 10 (77%) | 5 (100%) |
| Syncope | 13 (72%) | 10 (77%) | 3 (60%) |
| Cardiac Arrest | 2 (11%) | 0 (0%) | 2 (40%) |
| Exertional Trigger | 13 (72%) | 8 (61.5%) | 5 (100%) |
| Positive Family History of Cardiac Events (syncope, seizures, cardiac arrest, etc.) | 5 (28%) | 4 (31%) | 1 (20%) |
| Family History of SCD in the Young (< 40 years of age) | 2 (11%) | 1 (8%) | 1 (20%) |
| Cases of Exercise-Associated SUDY (N) | 19 | 18 | 1 |
| Males/Females | 13/6 | 12/6 | 1/0 |
| Average Age (years) | 13.9 ± 3.2 | 13.8 ± 3.2 | 16 |
| Age Range (years) | 6 to 19 | 6 to 19 | 16 |
| Exertion Related Death | 19 (100%) | 18 (100%) | 1 (100%) |
| Previous Personal History of Cardiac Event | 4 (21%) | 4 (22%) | 0 (0%) |
| Positive Family History of Cardiac Events (syncope, seizures, cardiac arrest, etc.) | 4 (21%) | 4 (22%) | 0 (0%) |
| Family History of SCD in the Young (< 40 years of age) | 1 (5%) | 1 (5.5%) | 0 (0%) |
SUDY= sudden death in the young; CPVT = catecholaminergic polymorphic ventricular tachycardia; SCD=sudden cardiac death
PKP2 Mutation Analysis
Comprehensive mutational analysis of PKP2’s (NM_004572) 14 coding region exons was performed on genomic DNA from these 37 cases using DNA Sanger sequencing or next-generation whole exome sequencing (WES). Consistent with the American College of Medical Genetics and Genomics and Association of Molecular Pathology variant interpretation guidelines,(20) only rare, non-synonymous radical (i.e. frame-shift, nonsense, or splice-site altering) variants with a minor allele frequency (MAF) of ≤ 0.00005 in the genome aggregation database (gnomAD)(21) were considered to be likely pathogenic. PKP2 missense variants were excluded in this study because of their relatively high frequency in gnomAD.
RESULTS
Overall, radical (i.e. nonsense, frame-shift, splice-error) variants in PKP2 were identified in 5/18 (27.7%) patients who had been diagnosed clinically with CPVT but were genotype-negative and 1/19 (5.3%) exercise-related autopsy negative SUDY cases compared to 96/138,632 (0.069%) individuals in gnomAD (p=3.1 ×10−13, Figure 1, Table 1, Supplemental Table 1). The only additional PKP2 non-synonymous variant identified was a S70I-PKP2 variant identified in a 16-year-old male diagnosed with CPVT. This variant has a minor allele frequency of 0.021 in GnomAD. Additionally, the variant has been seen as a homozygote in 65/108,411 individuals in GnomAD.
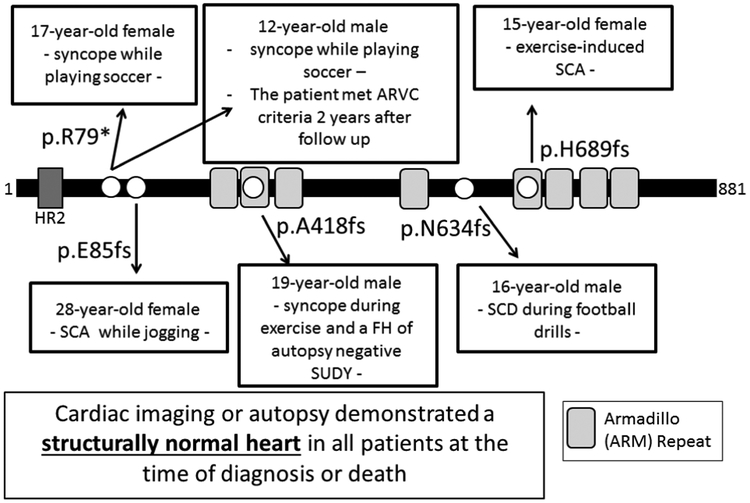
Figure 1.
Likely Pathogenic PKP2 Truncation Variants in Patients with CPVT and Decedents with Autopsy Negative SUDY – Shown is a linear topology figure of the PKP2 protein with localization of truncation variants (white circles) identified in CPVT patients or Autopsy Negative SUDY cases. The 12 year-old boy with p.R79X-PKP2 met the 2010 task force criteria for ARVC after 2 years of follow-up.
A p.R79X-PKP2 nonsense variant was identified in two unrelated cases (17-year-old female and 12-year-old male) diagnosed clinically with CPVT (Figure 1). Both patients had experienced syncope while playing soccer. One of the two p.R79X-PKP2-positive CPVT patients has satisfied subsequently (two years after sentinel presentation and CPVT diagnosis) the 2010 task force criteria for ARVC and required a cardiac transplant at 16 years of age. However, the second p.R79X-PKP2 positive patient reportedly still exhibits a structurally normal heart.
A p.E85fs-PKP2 frame-shift variant was identified in a 28-year-old female who experienced SCA while jogging (Figure 1). A p.A418fs-PKP2 variant was identified in a 19-year-old male who experienced syncope during exercise and had a family history of autopsy negative SUDY. A p.H689fs-PKP2 variant was identified in a 15-year-old female with exercise-induced ventricular fibrillation (Figure 1). A p.N634fs*22-PKP2 variant was identified in a 16-year-old white male who died suddenly while participating in non-contact football drills (Figure 1). His autopsy examination failed to reveal any primary congenital anomaly, primary pathological disease or abnormality, or any definitive traumatic injury to account for death. There was no evidence of fibrous or fibro-fatty infiltration of the heart muscle. His heart valves, chamber sizes, and myocardial wall thicknesses were within normal limits. Microscopic analysis of the heart was normal. The toxicology report was negative. The medical examiner concluded that the cause of death was “primary cardiac electrical dysfunction.”
DISCUSSION
Here, using a combination of Sanger DNA sequencing and/or WES and a PKP2 gene-specific analysis, we identified an ultra-rare, radical (i.e. frame-shift or nonsense) PKP2 pathogenic variant in ~28% (5/18) of our cohort of unrelated patients who had been diagnosed clinically with CPVT by at least one pediatric/adult heart rhythm specialist but remained genetically elusive following standard CPVT genetic testing and 5% (1/19) of our exercise-related autopsy negative SUDY cases.
These observations are consistent with previous reports indicating that during the course of first degree relative evaluations that occurred following a reportedly autopsy negative SUDY, clinical evidence for ARVC has emerged.(22) In addition, PKP2 truncation variants have been reported previously in autopsy negative SUDY victims.(22) In 2012, Skinner and colleagues reported on a 16-year-old male who died suddenly during exercise. Despite a negative autopsy, the decedent’s father and sibling both had cardiac magnetic resonance imaging that was suggestive for ARVC.(22) In 2016, Bagnall and colleagues identified PKP2 frame-shift variants in 2 of their 113 exertional and non-exertional SCD in the young cases (1.8%); one of which was a 17-year-old male autopsy negative SUDY victim that hosted the same E85fs-PKP2 variant that was identified in one of our patients, a 28-year-old female diagnosed with CPVT after a sentinel event of SCA while jogging.(23) In 2017, Lahrouchi and colleagues identified radical PKP2 variants in one of 30 autopsy negative SUDY cases (3.3%) with either exercise- or extreme emotion-related sudden death, a R609fs-PKP2 variant in a 22-year-old male who died during exercise.(24)
A recent study described a cardiomyocyte-specific, tamoxifen-activated, PKP2 conditional knockout (PKP2-cKO) murine model that allowed control of the onset of PKP2 loss of expression.(19) The data showed that PKP2 deficiency in adult ventricular myocytes was sufficient to cause ARVC. However, shortly after loss of PKP2 expression mice mimicked the “concealed stage” of ARVC, showing a high propensity to ventricular arrhythmias, most apparent during a catecholaminergic challenge. In fact, polymorphic ventricular arrhythmias were seen frequently in the PKP2-cKO mice. Interestingly, lethal arrhythmias were only observed in the early stage after loss of PKP2, when the mice did not yet show evidence of overt structural disease. In fact, a third of the PKP2-cKO mice in the “concealed stage” died during the isoproterenol (ISO) challenge. Of note, echocardiographic images of these PKP2-cKO mice did not show structural heart disease. Importantly, the ISO-induced polymorphic ventricular ectopy, couplets, triplets, and runs of polymorphic non-sustained ventricular tachycardia along with the ISO-induced ventricular fibrillation documented lethal arrhythmias in PKP2-cKO mice with structurally normal hearts, mimicked the profile of CPVT. In fact, at this early stage of PKP2 loss, the mice fully satisfied the diagnostic criterion for CPVT. Moreover, the ISO-induced arrhythmias were prevented by flecainide treatment.
Given that previous studies have indicated that flecainide can limit the outflow of calcium through RyR2 channels, these results support the notion that ventricular arrhythmias in the PKP2-cKO mice occurring during the concealed stage of PKP2-mediated ARVC may result from dysregulation of intracellular calcium cycling by increased RyR2-dependent calcium release, akin to RYR2-mediated CPVT. Considering the phenotypic overlap between ARVC and CPVT, it is noteworthy that about 9% of patients with an overt ARVC phenotype may have RYR2 mutations as their predominant pathogenic substrate.(25)
Although flecainide is widely viewed as the first addition to beta-blockers when breakthrough cardiac events occur in patients with RYR2-mediated CPVT (class IIa recommendation; Priori et al, Heart Rhythm, 2013 and Priori et al, EHJ, 2015), there is currently insufficient evidence to comment on the safety or efficacy of flecainide in PKP2-mediated CPVT/ARVC. Anecdotally, a ~9 month trial of nadolol plus flecainide in one of the patients included in this study resulted in a marked reduction in the burden of ventricular ectopy and non-sustained ventricular tachycardia. However, flecainide was discontinued subsequently when a diagnosis of p.R79X-PKP2-ARVC was rendered out of concern that accentuation of an underlying PKP2-mediated reduction in INa by flecainide could be pro-arrhythmic.
Nevertheless, the potentially beneficial use of flecainide does appears to fit with i) the prevention of ISO-induced arrhythmias in PKP2 null mice by flecainide and ii) the suggestion that flecainide plus sotalol/metoprolol may be an effective antiarrhythmic strategy for the control of refractory ventricular arrhythmias in patients with ARVC, including two patients with unspecific PKP2 variants (Ermakov, Heart Rhythm 2017; 14:564-569). However, the long-term safety of flecainide in patients with PKP2-mediated CPVT/ARVC, particularly in regards to the potential for synergistic and potentially pro-arrhythmic effect on the cardiac sodium channel that may worsen with progression to ARVC (desmosome destabilization) or with age, remains undefined. As such, there is insufficient evidence to recommend the use of flecainide in individuals with PKP2-mediated CPVT/electrical disease at this time.
PKP2-Mediated CPVT or PKP2-Mediated Electrical Phase (Concealed) ARVC
Despite the PKP2-cKO mouse model which completely mimics CPVT, do these human patients with truncating mutations in PKP2-encoded plakophilin-2 merely have classic PKP2-mediated ARVC, albeit in the concealed phase of the disease’s natural history? It is well known that ARVC may present with exercise-associated potentially lethal arrhythmias during the phase of disease progression when structural abnormalities are undetectable. Such exercise-triggered events, occurring in the young patient in the setting of a structurally normal heart, may compel the heart rhythm specialist to diagnose the patient with CPVT, especially if the patient’s stress test shows normal sinus rhythm at rest with exercise-associated ventricular ectopy, when in fact the patient may have so-called concealed ARVC.
Indeed, for the 12-year-old male with a p.R79X-PKP2 variant, only two years after his sentinel presentation and initial diagnosis of 'CPVT', he satisfied subsequently the 2010 task force criteria for ARVC. However, the remaining PKP2-positive patients have not yet converted to an overt cardiomyopathy and apart from their genetic test result; they still do not satisfy 2010 task force criteria for ARVC. In addition, their electrocardiographic evaluation lacks T wave inversions, epsilon waves, and late potentials making it difficult/impossible to suspect PKP2-mediated electrical phase ARVC. In contrast, they were all diagnosed by at least one pediatric/adult heart rhythm specialist as having CPVT secondary to exercise-associated cardiac events in the setting of a structurally normal heart (and all but the aforementioned individual still do), a normal resting 12-lead electrocardiogram, and a treadmill stress test showing exercise-associated ventricular ectopy.
Given that pathogenic PKP2 variants may result in a “CPVT-like” phenotype during an early pre-cardiomyopathic phase of the disorder and the natural progression of the disease towards a cardiomyopathic condition, one might expect to see segregation of both a “CPVT” and “ARVC” phenotype within a single pedigree. However, there are no examples, in the literature or in our current study, of CPVT and ARVC phenotypes tracing through the same pedigree. Our limited familial cascade genetic testing and clinical evaluation for three of our index cases with pathogenic PKP2 variants have not illustrated this. For the 12-year-old male hosting the R79X-PKP2 nonsense variant, neither his variant positive brother nor mother has any phenotypic expression of CPVT or ARVC, including normal ECG, stress test, and echocardiogram. For the 28-year-old female patient hosting the E85fs-PKP2 frameshift variant, there has been no phenotypic expression of ARVC in her family. Several of her first-degree relatives have been genetically tested for this disease causative variant. The patient’s older brother, younger sister, and her mother are E85fs-PKP2 variant positive; however they do not express any apparent cardiomyopathic or arrhythmic phenotype. For the 19-year-old male hosting the A418Pfs*2-PKP2 variant, his variant positive 22-year-old brother who died suddenly had a negative autopsy. The patient’s 56-year-old variant positive father has no ARVC phenotypic expression. There is a paternal history of sudden death in the young. The patient’s paternal uncle died at age 50 years while running. In addition, there have been fifth and sixth degree relatives coming from the paternal family line who have died at age 31 during basketball and another one in the 50s. However, it is currently unknown if these relatives possessed the A418Pfs*2-PKP2 variant or if there was any cardiomyopathic expression of ARVC.
Importantly, in contrast to the characteristically progressive ventricular ectopy seen in patients with CPVT1 (i.e. RYR2-mediated CPVT), the stress-test associated ectopy was less predictable. As such, we suspect that all of the patients in this present study that were diagnosed with CPVT, yet host a PKP2 truncation variant, are likely to be concealed ARVC. However, among many heart rhythm specialists, without the genetic test result, we suspect that these patients will continue to be diagnosed with CPVT. Here, the difference in clinical diagnosis is not trivial, since patients with syncope due to ARVC may warrant an ICD whereas patients with syncope/seizures stemming from CPVT may be treated with beta blockers, flecainide, and/or left cardiac sympathetic denervation as the first line of defense. Hence, a wrong diagnosis might lead to the wrong treatment. Therefore, we suggest that PKP2 should be added to the CPVT genetic test panel.
As seen in the public compendia of exomic databases like gnomAD, radical variants in PKP2 that result in premature truncation of plakophilin-2 and anticipated haploinsufficiency have a very low, but non zero, background rate of about 0.7 per 1,000 individuals. Although this is far lower than the 2-3% background rate of radical, truncating variants in TTN-encoded titin,(29) the patient’s phenotype must still be considered carefully before reflexively viewing any such PKP2 variant as probable cause. While PKP2 mutations may represent genetic disease-susceptibility/modifiers with phenotypes ranging from none to severe and dependent on age, environment, and other disease states, when obtained as merely an incidental finding, a radical PKP2 variant is most likely just part of his/her background genetic noise (especially if buttressed by a normal stress test and normal imaging studies). In fact, before such a cardiologic evaluation is conducted, a purely incidental finding of a radical PKP2 variant, in an asymptomatic person with no family history, would be associated with only an estimated 1-2% chance of having stumbled into PKP2-mediated CPVT/ARVC. However, in the setting of an individual clinically diagnosed with CPVT previously and having a negative CPVT genetic test, the identification of a radical PKP2 variant would have an estimated signal-to-noise ratio of nearly 400:1. In contrast, when the phenotype is that of a decedent with exercise-associated SCD but a normal conventional autopsy, the signal-to-noise ratio decreases to an estimated 75:1. In such a decedent-centered context, there would be about a 1-2% chance that the PKP2 finding is irrelevant.
LIMITATIONS
Limitations of this study include the small sample size and the lack of genetic evaluation of PKP2 copy number variations (CNV). Not including a PKP2 CNV analysis may have resulted in an under-estimate of the prevalence of pathogenic loss-of-function PKP2 variants in our genotype-negative CPVT and exercise-related autopsy-negative sudden death in the young cohorts.
CONCLUSIONS
Approximately 28% of our genotype-negative CPVT cohort and 5% of our exercise-related autopsy-negative sudden death in the young cases hosted a likely pathogenic PKP2 truncation variant. Based on our observations as well as others, (22) (23) (24) we would estimate that PKP2 pathogenic variants may be responsible for 2-5% of autopsy-negative SUDY. Our data suggest that the progression of the PKP2-mediated electropathy can be independent of structural perturbations and can precipitate exercise-induced sudden cardiac arrest prior to the presence of an overt cardiomyopathy akin to the PKP2-knockout mouse model that mimics CPVT. Consequently, both the CPVT genetic test panel and the molecular autopsy panel for autopsy-negative sudden unexplained death in the young cases should now include PKP2.
Supplementary Material
PERSPECTIVES:
Competency in Patient Care:
Progression of the PKP2-mediated electropathy can be independent of structural perturbations and can precipitate exercise-induced sudden cardiac arrest/sudden cardiac death prior to the presence of an overt cardiomyopathy akin to the PKP2-knockout mouse model that mimics catecholaminergic polymorphic ventricular tachycardia (CPVT). Both the CPVT genetic test panel and the molecular autopsy panel for autopsy-negative sudden unexplained death in the young cases should now include PKP2.
Translational Outlook:
Further studies are needed to distinguish clinically PKP2-mediated concealed arrhythmogenic right ventricular cardiomyopathy (ARVC) from the possibility of PKP2-mediated catecholaminergic polymorphic ventricular tachycardia (CPVT) or to determine that prior to manifestation of an overt cardiomyopathy, these two designations reflect the same underlying disease phenotype for patients with plakophilin-2 truncations.
ACKNOWLEDGMENTS:
The authors would like to thank the Genome Aggregation Database (gnomAD) and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at http://gnomad.broadinstitute.org/about.
FUNDING: This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (MJA), and by grants RO1HL134328 and RO1HL136179 from the National Institutes of Health (MD) and AHA14SDG18580014 from the American Heart Association (MC).
ABBREVIATIONS:
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- gnomAD
genome aggregation database
- MAF
minor allele frequency
- PKP2
plakophilin-2
- SCA
sudden cardiac arrest
- SCD
sudden cardiac death
- SUDY
sudden unexplained death in the young
- WES
whole exome sequencing
Footnotes
DISCLOSURES: MJA is a consultant for Audentes Therapeutics, Boston Scientific, Gilead Sciences, Invitae, Medtronic, MyoKardia, and St. Jude Medical. MJA and Mayo Clinic have an equity/royalty relationship (without remuneration so far) with AliveCor, Blue Ox Health Corporation, and StemoniX. However, none of these entities were involved in this study in any way. DJT, JPA, JRG, NCA, MC, and MD have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Leenhardt A, Lucet V, Denjoy I, al. e. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation 1995;91:1512–1519. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69–74. [DOI] [PubMed] [Google Scholar]
- 3.Tester DJ, Dura M, Carturan E, et al. A mechanism for sudden infant death syndrome (SIDS): Stress-induced leak via ryanodine receptors. Heart Rhythm 2007;4:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu N, Ruan Y, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Prog Cardiovasc Dis 2008;51:23–30. [DOI] [PubMed] [Google Scholar]
- 5.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young.[see comment]. J Am Coll Cardiol 2007;49:240–6. [DOI] [PubMed] [Google Scholar]
- 6.Tester DJ, Ackerman MJ. The role of molecular autopsy in unexplained sudden cardiac death. Curr Opin Cardiol 21(3):166–72, 2006. May. 2006. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001;103:485–490. [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001;103:196–200. [DOI] [PubMed] [Google Scholar]
- 9.Ackerman M, Priori S, Willems S, et al. HRS/EHRA Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies: This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011;8:1308–1339. [DOI] [PubMed] [Google Scholar]
- 10.Lahat H, Eldar M, Levy-Nissenbaum E, al. e. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13-21. Circulation 2001;103:2822–2827. [DOI] [PubMed] [Google Scholar]
- 11.Postma AV, Denjoy I, Hoorntje TM, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res 2002;91:E21–E26. [DOI] [PubMed] [Google Scholar]
- 12.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet 2012;21:2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tester DJ, Arya P, Will M, et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing.[see comment]. Heart Rhythm 2006;3:800–5. [DOI] [PubMed] [Google Scholar]
- 14.Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 15.Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016;31:46–56. [DOI] [PubMed] [Google Scholar]
- 16.Groeneweg JA, Bhonsale A, James CA, et al. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet 2015;8:437–46. [DOI] [PubMed] [Google Scholar]
- 17.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009;373:1289–300. [DOI] [PubMed] [Google Scholar]
- 18.Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res 2010;107:700–14. [DOI] [PubMed] [Google Scholar]
- 19.Cerrone M, Montnach J, Lin X, et al. Plakophilin-2 is required for transcription of genes that control calcium cycling and cardiac rhythm. Nat Commun 2017;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Exome Aggregation Consortium (ExAC), Cambridge, MA: (URL: http://exac.broadinstitute.org) [(July, 2018) date accessed]. [Google Scholar]
- 22.Skinner J, Crawford J, Smith W, et al. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm 2011;8:412–9. [DOI] [PubMed] [Google Scholar]
- 23.Bagnall RD, Weintraub RG, Ingles J, et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N Engl J Med 2016;374:2441–2452. [DOI] [PubMed] [Google Scholar]
- 24.Lahrouchi N, Raju H, Lodder EM, et al. Utility of Post-Mortem Genetic Testing in Cases of Sudden Arrhythmic Death Syndrome. J Am Coll Cardiol 2017;69:2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roux-Buisson N, Gandjbakhch E, Donal E, et al. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: Results of a systematic screening. Heart Rhythm 2014;11:1999–2009. [DOI] [PubMed] [Google Scholar]
- 26.Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 27.Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac deathThe Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European Heart Journal 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 28.Ermakov S, Gerstenfeld EP, Svetlichnaya Y, Scheinman MM. Use of flecainide in combination antiarrhythmic therapy in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2017;14:564–569. [DOI] [PubMed] [Google Scholar]
- 29.Herman DS, Lam L, Taylor MRG, et al. Truncations of Titin Causing Dilated Cardiomyopathy. N Engl J Med 2012;366:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.