Abstract
CD4+ T cells play critical roles in defending against poxviruses, both by potentiating cellular and humoral responses and by directly killing infected cells. Despite this central role, the basis for pox-specific CD4+ T cell activation, specifically the origin of the poxvirus-derived peptides (epitopes) that activate CD4+ T cells, remains poorly understood. In addition, since the current licensed poxvirus vaccines can cause serious adverse events and even death, elucidating the requirements for MHC-II processing and presentation of poxviral antigens could be of great utility. In order to address these questions, we explored the CD4+ T cell immunogenicity of ectromelia (ECTV), the causative agent of mousepox. Having identified a large panel of novel epitopes via a screen of algorithm-selected synthetic peptides we observed that immunization of mice with inactivated poxvirus primes a virtually undetectable CD4+ T cell response, even when adjuvanted, and is unable to provide protection against disease after a secondary challenge. We postulated that an important contributor to this outcome is the poor processability of whole virions for MHC-II-restricted presentation. In line with this hypothesis we observed that whole poxvirions are very inefficiently converted into MHC-II binding peptides by the antigen-presenting cell as compared to subviral material. Thus, stability of the virion structure is a critical consideration in the rational design of a safe alternative to the existing live smallpox vaccine.
INTRODUCTION
CD4+ T cells have a diverse set of functions, which make them a crucial immune cell type for protection against a diverse array of infectious diseases. Key roles include coordination of B cell and CD8+ T cell responses, production of inflammatory cytokines and, in some cases, direct killing of infected cells. For these reasons CD4+ T cell engagement is a critical consideration in rational vaccine design.
CD4+ T cell activation is initiated by interactions at the cell surface with major histocompatibility class II (MHC-II) molecules in complex with antigenic peptides (epitopes). A large body of work with stable globular proteins has suggested that the majority of MHC-II-restricted epitopes are derived from exogenous antigens digested in the endosomal compartment. However, these model antigens do not predict the processing of more complex structures, such as viral particles. Our work with influenza has shown that a more complicated network of MHC-II antigen processing and presentation is at play, in many cases utilizing as processing substrates proteins synthesized within the antigen-presenting cell [1,2]. This requirement derives both from the ability of nascent viral proteins to be engaged by a diverse network of cellular components capable of producing a wide array of peptides and from the poor processability of whole virions [1]. However, it is unclear whether these observations reflect general principles in viral immunity or are specific to influenza.
Like influenza, orthopoxviruses continue to be a public health concern and the current vaccination strategy, while effective, can cause severe adverse reactions. Greater mechanistic understanding of how poxviruses engage the adaptive immune system could be of considerable benefit. Various orthopoxviruses can cause severe disease in people, most notably smallpox, one of the most lethal diseases in human history [3] and an ongoing threat as a bioterrorism agent. More recently, zoonotic poxviruses such as monkeypox have emerged as pathogenic in humans and evidence with monkeypox suggests that the virus is rapidly evolving to more efficiently counteract the human immune system resulting in more severe disease [4]. Despite promising results with subunit vaccines [5–7], the gold standard of immunization against poxviruses remains replication competent vaccinia (VACV), which has been used for centuries as a live vaccine against smallpox [8]. While VACV immunization is efficacious, cases of severe pathogenesis, secondary infection and even death have been reported, with symptoms ranging from mild, such as fatigue and headaches, to severe, such as progressive vaccinia, eczema vaccinatum and, rarely, fatal encephalitis [9–16]. During the era of smallpox eradication there were attempts at an inactivated whole virus vaccine, and while there was limited success under boosting conditions, no efficacy in producing neutralizing antibody titers in previously naïve individuals was observed [17–19]. Thus, efforts in this regard have largely ceased.
The production of neutralizing antibodies is considered a requirement for protection against secondary poxvirus infections, and many of the vaccination studies have focused primarily on the antibody response. Thus far, the CD4+ T cell response generated in response to inactivated versus live poxvirus agents has not been characterized, despite CD4+ T cell help being crucial for a strong antibody response as well as direct CD4+ T cell-mediated killing of pox-infected cells [20,21]. While we have previously characterized the poor antigen processing of whole virions as a key determinant in the poor CD4+ T cell response to inactivated influenza, the influenza virion is relatively fragile, surviving only minutes in solution [22]. In contrast, orthopoxvirus virions are remarkably durable, owing to large size with an extremely dense protein content [23,24]. Remarkably, these resilient particles can be freeze-dried and subsequently reconstituted with complete viability [25]. Because of these properties, we speculated that orthopoxvirus-specific CD4+ T cell responses are dependent upon infectivity, and the production of more processable forms of antigen, even more so than what we observed for influenza-specific responses.
In order to characterize the CD4+ T cell response to inactivated poxvirions we turned to a murine model of smallpox, ectromelia (ECTV), colloquially known as mousepox and a close relative to VACV as well as smallpox [26]. ECTV naturally infects mice, displays a restricted host range to that species, and closely mimics the disease progression of smallpox and monkeypox in humans [27,28]. Compared to VACV, ECTV is better able to infect immune cells, produce a systemic infection, and cause fatal disease at low infectious doses in certain inbred strains [29,30]. Furthermore, immunization of mice with live VACV can protect even susceptible mouse strains from a secondary infection with ECTV [31], thereby mimicking the current human immunization protocols.
Studies focusing on CD4+ T cell immunogenicity have been limited because of the difficulties in predicting MHC-II binding epitopes as well as the focus on B and CD8+ T cell responses. Thus, by using a panel of algorithm-selected synthetic peptides and CD4+ from ECTV-primed C57Bl/6 mice, we first identified a large number of novel ECTV-derived I-Ab-restricted epitopes. Assaying for responses to these epitopes under various conditions, we observed that poxvirion particles are exceptionally poor processing substrates for the MHC-II antigen processing machinery, even when an adjuvant was included, providing an important guiding principle in rational poxvirus vaccine design.
MATERIALS AND METHODS
Cells:
Bone Marrow Dendritic Cells (BMDCs) were derived from the bone marrow of pooled female C57Bl/6 mice (Jackson Laboratories, 00064) and cultured for 7 days in RPMI (10%FBS, antibiotics, L-glutamine and 2-me) with GM-CSF (Gemini Bio-products).
ELISpot assay:
Female C57Bl/6 mice 6–8 weeks of age were inoculated with appropriate virus (3 mice per group). ELISpot plates were coated with α-IFN γ at 1:200 in PBS and incubated overnight at 4°C. Ten days post infection spleens from each group were harvested. CD4+ T cells were purified from bulk splenocytes using negative bead isolation (Invitrogen dynabead untouched mouse CD4+ isolation kit) and incubated with BMDCs and 15mer peptides, virions or subviral material. After an overnight incubation, the plates were developed (BD bioscience ELISpot IFNγ antibody pair and AEC substrate kit) and IFNγ spots produced by activated T cells were counted (Immunospot reader).
Peptide screen:
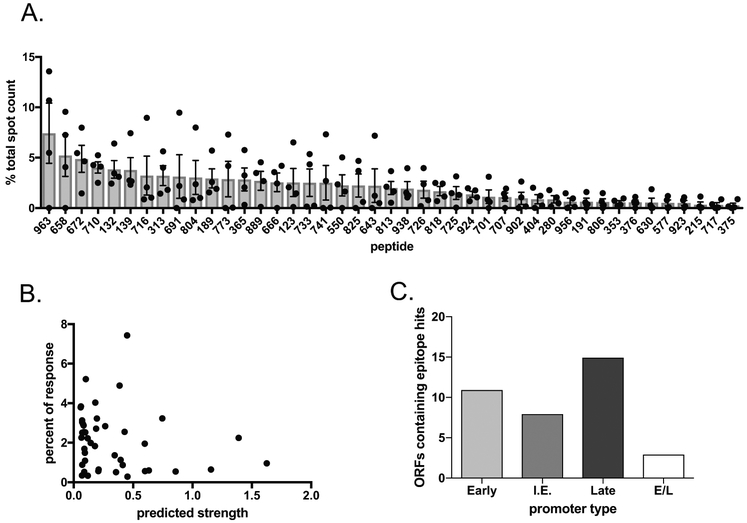
All protein sequences for the ECTV genome (NC_004105.1) were collected from GenBank (173 open reading frames) and broken down in silico into 15-mer peptides, overlapping by 10 residues, starting at position 1, and including a peptide covering the C-terminus. For example, for a 27-residue protein peptides 1–15, 6–20, 11–25 and 13–27 would be selected (Total = 10,984 15mers). After removing duplicate peptides (due to sequence homology between the inverted terminal repeat regions), binding predictions for the remaining 10,721 15-mers were performed for the MHC allele I-Ab using the consensus method available at IEDB MHC II binding prediction tool [35]. The 1000 peptides predicted for highest binding to I-Ab were synthesized (Pepscan Systems). Female C57BL/6 mice were immunized with 3×103 pfu ECTV (Moscow strain) in 30uL of saline via the footpad route. After 10 days, CD4+ T cells were purified from pooled spleens and peptides were assessed in an ELISpot assay for CD4+ T cell recognition. The 1000 peptides were screened individually via a matrix approach, and then the top responders were assayed in triplicate. Spots represent IFNγ-producing cells per 100,000 purified CD4 cells. Four independent experiments were performed and for each experiment the percent of the total response was calculated for all individual peptides. The mean and SEM of the percent of the responses across all four independent experiments was calculated for each peptide that appeared in 3 or more independent experiments and is shown in Figure 1.
Figure 1.
Analysis of novel epitopes identified from 1000 peptide library. A. 3 Female C57Bl/6 mice were infected via footpad with 3×103 pfu ECTV. 10 days later spleens were pooled and CD4+ T cells were isolated by negative bead selection and mixed with peptide pulsed BMDCs and analyzed for IFNγ production by ELISpot. For each of 4 independent experiments, the percent of the total response (total spot count) was calculated for each peptide. The average percent of the total response and SEM of these 4 independent experiments is shown for each peptide that had a positive spot count for 3 or more independent experiments. B. The 1000 peptide library was identified using an algorithm predicting strength of binding to I-Ab. For each novel epitope identified from this larger library the experimental percent of the response was graphed against the predicted strength of binding.. C. ECTV open reading frames were correlated to the VACV homolog. The promoter type for each open reading frame was analyzed through use of a previously published VACV data set [36]. For ECTV open reading frames that contained more than one unique epitope hit, the open reading frame was counted only once. I.E. stands for intermediate early promoter type and E/L stands for early/late promotor type.
Viral purification:
TK- cells infected with ECTV (Moscow) and VACV (Western Reserve) for 72 or 48 hours respectively were lysed by repeated freeze, thaw, sonication cycles. The cell lysate was then purified through a 36% sucrose cushion and resuspended in 1mM Tris-HCL for use in animal and in vitro experiments.
Live and inactivated ECTV comparison:
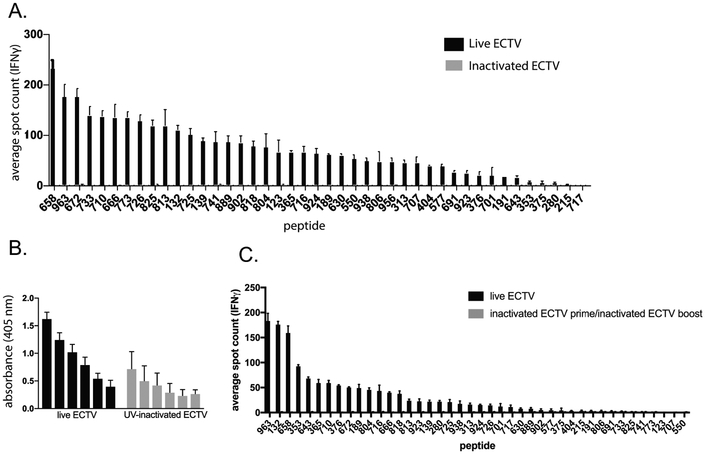
Female C57BL/6 mice were immunized with either live or inactivated ECTV. Live ECTV was inoculated in the footpad with 3×103 pfu of virus in 30uL of saline, a commonly used infection route and dose for this virus; alternately, 3×106 pfu of virus was inactivated with UV radiation/psoralen and injected i.p. Ten days post injection CD4+ T cells from pooled spleens were tested in an ELISpot assay for their ability to recognize the 42 consistent peptide hits. Spots represent IFNγ-producing cells per 100,000 purified CD4 cells.
ECTV and VACV comparison:
Female C57Bl/6 mice were inoculated with ECTV in the footpad with 3×103 pfu of virus in 30uL of saline; alternatively, mice were inoculated i.p. with 3×105pfu of VACV (WR strain). Ten days post injection CD4+ T cells from the pooled spleens from each set of mice were tested in an ELISpot assay for their ability to recognize the 42 consistent peptide hits. Spots represent IFNγ-producing cells per 100,000 purified CD4 cells. In order to normalize the two conditions, each peptide is shown as the percent of the total response (total spot count).
Adjuvant study:
Female C57BL/6 mice were immunized with live ECTV, inactivated ECTV or inactivated ECTV plus adjuvant. Adjuvant consisted of 10% aluminum hydroxide gel (Rehydragel LV, Chemtrade Chemicals), 1mg/mL saponin (Sigma Aldrich), as previously published by [32]. Live ECTV was inoculated in the footpad with 3×103 pfu of virus in 30uL of saline; alternately, 3×106 pfu of virus was inactivated with UV radiation/psoralen and injected s.c. in 500uL of either saline or adjuvant. Ten days post injection CD4+ T cells from individual mice were tested in an ELISpot assay for their ability to recognize 5 consistently strong peptide hits. Spots represent IFNγ-producing cells per 100,000 purified CD4 cells.
Vaccination study:
Female C57BL/6 mice were infected with either 3×103 pfu live VACV, 3×106 pfu VACV inactivated with UV radiation/psoralen or a saline control injected i.p. Four weeks later serum was collected via cheek bleed prior to inoculation with 3×103 pfu live ECTV via footpad scarification. Five days post-challenge mice were sacrificed; the dorso-plantar thickness of the infected foot was measured for swelling by digital calipers and spleens and livers were collected for organ titering. Harvested organs were processed using a gentleMACS dissociator and live virus was titered by plaque assay on BSC-1 cells from homogenates.
Serum IgG titers:
Serum was collected from the heart of primed mice immediately following death and analyzed for IgG titer by ELISA. Briefly, serum was serially diluted in PBS supplemented with 1% low-IgG bovine serum albumin (BSA; Gemini Bio-Products), ranging from 1:500 to 1:16,000, and incubated in high binding EIA/RIA plates (Corning) pre-coated with 6.25×104 pfu purified ECTV. Plates were then washed with PBS + 0.01% Tween (PBST) and incubated with peroxidase labeled anti-mouse IgG (H+L) (Vector Laboratories, catalog no. PI-2000) at 1:1500 dilution in PBS/BSA (1%). Plates were developed using BST Peroxidase Substrate (KPL) and read at detection wavelength of 405 nm.
Neutralizing antibody assay:
Serum from infected mice was subjected to a previously published protocol for assessing neutralizing antibodies to poxviruses [33]. Briefly, serum was incubated for 1 hour with a β-galactosidase expressing VACV prior to dispensing on a monolayer of HeLa cells for overnight infection (18–20 hours). β-galactosidase activity was measured using a plate reader at 405nm and compared to a condition prepared without serum (100% infectivity).
In vitro processing assay fraction generation:
TK- cells were infected with ECTV for 3 days until significant cytopathic effect was observed. Cells were harvested and subjected to 3 consecutive cycles of freezing, thawing and vortexing. Whole virions were separated from free proteins via a previously published procedure [34], namely ultracentrifugation at 36,000xg for 30 minutes at 4° C. The pellet (virions) was resuspended in PBS and both pellet and supernatant were assayed for live virus by plaque assay on BSC-1 cells.
Western blot:
Samples were boiled in non-reducing conditions and loaded onto a pre-cast NuPage 4–12% Bis-Tris gel (Thermofisher). Following semi-dry transfer onto a nitrocellulose membrane and subsequent blocking (Licor blocking solution), presence of A27L (Santa Cruz Biotechnology sc-69950) and A33R (BEI Resources NR-49231) structural proteins were probed and detected using Licor suitable secondary reagents.
RESULTS
Novel Set of ECTV CD4 activating peptides
Our previous work has illustrated on numerous occasions the heterogeneous MHC-II processing properties of individual epitopes within a complex pathogen [1, 35–37], and the inaccurate generalizations that can result from studying a limited number of epitopes or bulk responses. We therefore set out to obtain a large panel of individual epitopes that we could utilize to probe CD4+ T cell responses to ECTV. This was accomplished by measuring reactivity of CD4+ T cells from ECTV-infected mice to a panel of synthetic peptides derived from the ECTV proteome. Orthopoxiruses have extremely large genomes, encoding on the order of 200 distinct proteins. Rather than creating a comprehensive overlapping peptide screen covering the entire proteome, we utilized in silico epitope prediction software in order to focus our screening efforts. All protein sequences for the ECTV genome (NC_004105.1) were collected from GenBank (173 open reading frames) and broken down in silico into 10,984 15-mer peptides, overlapping by 10 residues, starting at position 1, and including a peptide covering the C-terminus. For example, for a 27-residue protein peptides 1–15, 6–20, 11–25 and 13–27 would be selected. After removing duplicate peptides (due to sequence homology between the inverted terminal repeat regions), binding predictions for the remaining 10,721 15-mers were performed for the MHC allele I-Ab using the consensus method available at IEDB MHC II binding prediction tool [38]. Based on the IEDB consensus percentile score (peptides with lower percentile score being better binders), the 1000 15-mer peptides with strongest predicted binding affinity to the I-Ab MHC-II molecule expressed by C57Bl/6 mice, excluding those already identified in previous screens [39,40], were synthesized. An additional stipulation was that each of the 173 ECTV ORFs be represented by at least 2 peptides, resulting in a range of 2 – 36 peptides per ORF.
The peptides were tested individually in ELISpot assays using purified CD4+ T cells from ECTV-infected mice and the numbers of IFNγ-producing cells were recorded. Over four independent experiments we identified a group of 42 novel peptides that reproducibly induced responses above background (present in 3 or more experiments) and 17 novel peptides that appeared sporadically (Table S1 and S2). The set displays a reproducible hierarchy of activation, which is depicted in Figure 1A. Relevant to the heterologous prime/challenge experiments detailed below, immunization with VACV displayed a broadly similar repertoire of epitopes, as expected from the high degree of homology between ECTV and VACV, with only two peptides not showing responses against VACV (both of which had amino acid changes between ECTV and VACV) (Supplemental Fig. 1).
Relationship between binding affinity and immunogenicity
MHC-II binding predictions have historically been more challenging than for MHC-I because MHC-II molecules have less stringent and predictable binding requirements and can therefore accept a greater range of potential peptides [41]. In general, it has been found that binding affinity is a necessary but not sufficient condition for T cell immunogenicity [42,43]. In this light, we assessed the relationship of predicted binding strength to observed CD4+ T cell stimulation. While we saw only a modest correlation between predicted rank and actual activation of T cells (Fig. 1B), 7 out of the top 10 peptides were predicted to bind I-Ab quite strongly, with predicted binding strengths of greater than 0.2. This suggests effective discrimination in the predictive algorithm’s performance, and confirms that prediction of MHC binding can be used to identify candidates for immunogenicity screen, but MHC binding alone is an incomplete predictor of T cell immunogenicity, presumably reflective of other factors, such as the available T cell repertoire and the varying efficiency of the antigen processing machinery for the production of individual epitopes [42,43].
Expression profiles of peptide sources
Since our screen was designed for broad representation of the ECTV genome, we were able to determine whether there are trends among the parent proteins of the novel epitopes that were identified. Poxviruses have well-defined promoter sequences that segregate protein expression into early, intermediate and late phases during an infectious cycle; a minority of promoters allow for expression at both early and late timepoints and some genes appear to have immediate-early kinetics [44,45]. Work with a previous set of CD4 T cell activating VACV-derived peptides revealed a bias toward late stage genes [39], which was attributed to the greater expression levels of the proteins in this category. The data from our larger screen suggest that proteins from all phases of infection can be presented by MHC-II, in response to both ECTV and VACV (Fig 1C, Fig S1 and Table S1). Here, the expression profile of our group of activating peptides more closely mirrors the overall distribution of poxvirus genes [39]. Importantly, our data set also shows that proteins with structural, regulatory and virulence roles can also be presented by MHC-II (Table S1).
Inactivated virus does not provoke a detectable CD4+ T cell response
Having identified a large panel of MHC-II-restricted ECTV-derived epitopes, we were positioned to analyze the CD4+ T cell response to immunization with inactivated virus. We infected C57Bl/6 mice with live ECTV via footpad injection, the standard infection route for this virus, or immunized with UV-inactivated ECTV at 100x the input dose to account for the inability of this virus to proliferate. In addition, for inactivated virus we immunized intraperitoneally rather than via footpad to allow immune cells more direct exposure to the inactivated virus particles. We then utilized our large panel of epitopes to probe the CD4+ T cells resulting from these challenges using IFNγ ELISpots. In line with our prediction that whole virions would be poor processing substrates for MHC-II, the inactivated virus induced exceedingly weak CD4+ T cell responses to a very small number of peptides, despite eliciting a detectable antibody response (Fig. 2, A and B). Indeed, only a single ECTV peptide (741) demonstrated a consistent response above background and the magnitude of the response to this epitope was substantially lower than that elicited by live virus (Fig. 2A). Three other peptides were sporadically detectable (1 out of 3 independent experiments), with all of these decidedly low in magnitude when at all detectable. As observed with influenza [1], screens of the entire 1000-peptide library did not uncover any novel hits with inactivated virus (data not shown), arguing against immunodominance effects. Notably, there was a low level of virus specific IgG antibodies in the serum of mice infected with UV-inactivated ECTV, suggesting some class-switching in the absence of detectable CD4-mediated “help”. In comparison with our previous work with influenza, where several epitopes of a much smaller peptide panel displayed relatively robust responses against inactivated virus [1], CD4+ T cell responses against inactivated ECTV are markedly worse. What is more, whereas boosting with inactivated influenza produced an appreciable and expanded secondary response [1], boosting with UV-inactivated ECTV did not detectably enhance the response (Fig. 2C).
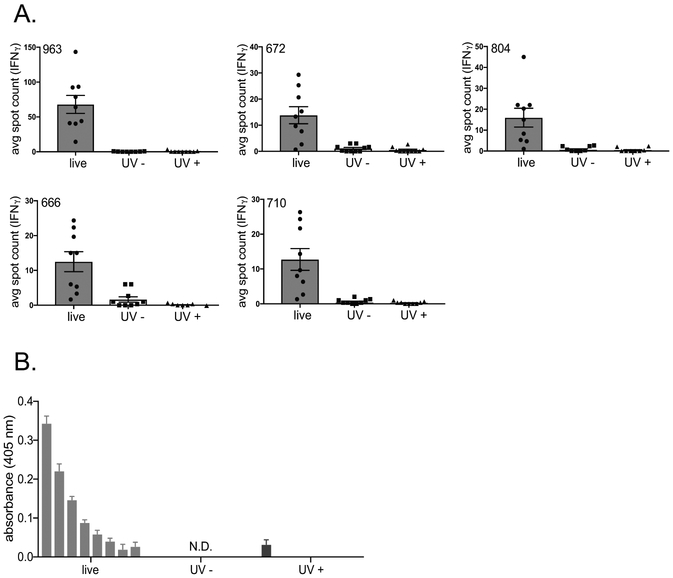
Figure 2.
Analysis of CD4+ T cell reactivity to inactivated ECTV. A. 3 Female C57Bl/6 mice were infected with either 3×103 pfu ECTV via footpad injection or 3×106 pfu UV-inactivated ECTV via i.p. injection. UV-inactivated ECTV was confirmed to be replication incompetent via plaque assay prior to injection. 10 days later mice were sacrificed. A. Spleens were pooled and CD4+ T cells were isolated by negative bead selection and mixed with peptide pulsed BMDCs and analyzed for IFNγ production by ELISpot. Representative of 3 independent experiments. B. Serum was analyzed for levels of virus-specific IgG antibodies by ELISA and background subtracted from pooled naïve serum. C. 3 Female C57Bl/6 mice were immunized with either PBS or 3×106pfu UV-inactivated ECTV via i.p. injection. 28 days later the mice were infected with either 3×103 pfu ECTV via footpad injection (live prime group) or 3×106pfu UV-inactivated ECTV via i.p. injection (inactivated ECTV prime/boost group) respectively. 10 days later spleens were pooled and CD4+ T cells were isolated by negative bead selection and mixed with peptide pulsed BMDCs and analyzed for IFNγ production by ELISpot. Representative of 3 independent experiments.
Adjuvant does not restore CD4+ T cell response to inactivated virus
In order to determine whether an inflammatory milieu could help boost CD4+ T cell responses to inactivated poxvirus, we employed a well-established adjuvant, namely aluminum hydroxide + saponin [32]. Importantly, this adjuvant mixture has recently been shown to increase antibody responses to inactivated ECTV in a prime/boost vaccination setting via subcutaneous immunization [32]. After confirming that immunizing with inactivated virus via intraperitoneal vs subcutaneous routes did not alter the CD4+ T cell response (data not shown), we infected C57Bl/6 mice with live ECTV via footpad or inactivated ECTV subcutaneously with or without adjuvant, using the same dosages as previously discussed. When the serum IgG titers were analyzed, in contrast to the intraperitoneal route we did not observe any titers in mice immunized with inactivated virus without adjuvant (Figure 3B). However, we did observe a low level of serum IgG titers following immunization with inactivated virus in the presence of adjuvant, though this was several orders of magnitude lower than when mice were infected with live virus (Figure 3B). Irrespective of any boost to antibody responses when adjuvant was included, when we assessed CD4+ T cell responses in individual mice for a representative subset of our 42 peptides (Figure 3A) we did not observe any enhancement of CD4+ T cell responses to inactivated virus. These data suggest that the lack of inflammatory environment was not a causative explanation for the lack of CD4+ T cell responses to inactivated poxvirus.
Figure 3.
Impact of adjuvanting inactivated virus. A. 5 Female C57Bl/6 mice were infected with either 3×103 pfu ECTV via footpad injection, 3×106 pfu UV-inactivated ECTV via s.c. injection or 3×106 pfu UV-inactivated ECTV in adjuvant via s.c. injection. UV-inactivated ECTV was confirmed to be replication incompetent via plaque assay prior to injection. 10 days later mice were sacrificed. A. spleens were individually harvested and CD4+ T cells were isolated by negative bead selection and mixed with peptide pulsed BMDCs and analyzed for IFNγ production by ELISpot. Individual mice from 2 independent experiments are represented here. B. Serum collected was analyzed for levels of virus-specific IgG antibodies by ELISA and background subtracted from pooled naïve serum.
Inactivated Poxvirus provides insufficient protection from challenge
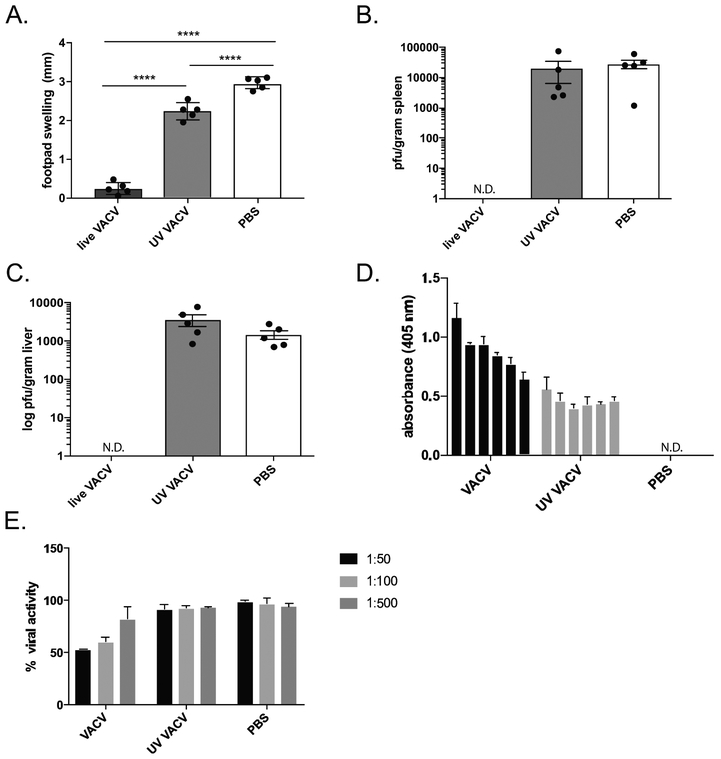
We predicted that the low level of CD4+ T cell activation we observed following inactivated ECTV immunization, and the resultant poor antibody response, would not be protective against a secondary challenge, correlating with the empirical observations in vaccine trials with inactivated smallpox [17–19]. We utilized VACV as the immunization agent, as it is the smallpox vaccine virus and displays limited pathology in mice while still generating an immune response. Importantly, UV-inactivated VACV showed a similar dearth of epitope-specific CD4+ T cell activation as inactivated ECTV (data not shown). We immunized C57Bl/6 mice via the intraperitoneal route with live VACV, inactivated VACV or a saline control and four weeks later, assessed protection by challenging with ECTV via footpad scarification. As C57Bl/6 mice will invariably survive this challenge [31], we utilized footpad inflammation as well as viral titers in the spleen and liver as correlates of protection. As expected, the mice immunized with live VACV were completely protected from secondary challenge, while the mice immunized with the saline control displayed significant footpad inflammation as well as ECTV titers in both the spleen and the liver (Fig. 4 A-C). In line with our hypothesis, we observed that the cohort of mice immunized with inactivated VACV displayed footpad inflammation as well as organ titers similar to the saline control mice. In addition, we observed low levels of serum IgG in response to the inactivated virus condition at four weeks post vaccination, consistent with our previous observations at ten days (Fig. 4 D). Utilizing a well-established viral neutralization assay, it was apparent that this low level of virus-specific antibodies did not display neutralizing capabilities (Fig. 4 E).
Figure 4.
Protection from secondary ECTV challenge following immunization with inactivated VACV. Female C57Bl/6 mice were vaccinated with either 3×103 pfu live VACV, 3×106 pfu VACV inactivated by UV radiation/psoralen or a saline control injected i.p.. 4 weeks later mice were challenged with 3×103 pfu live ECTV via footpad scarification. 5 days post challenge mice were analyzed for: A. Footpad inflammation B-C. spleen and liver organ titers of ECTV, and serum titers (D and E). D. Serum collected 2 days pre-challenge was analyzed for levels of virus-specific IgG antibodies, and background subtracted from pooled naïve serum. E. Serum collected 2 days pre-challenge was analyzed for levels of VACV neutralizing antibodies using a β-gal reporter VACV as previously described [53]. Representative of 3 independent experiments.
Viral proteins robustly activate CD4+ T cells in comparison to whole virions
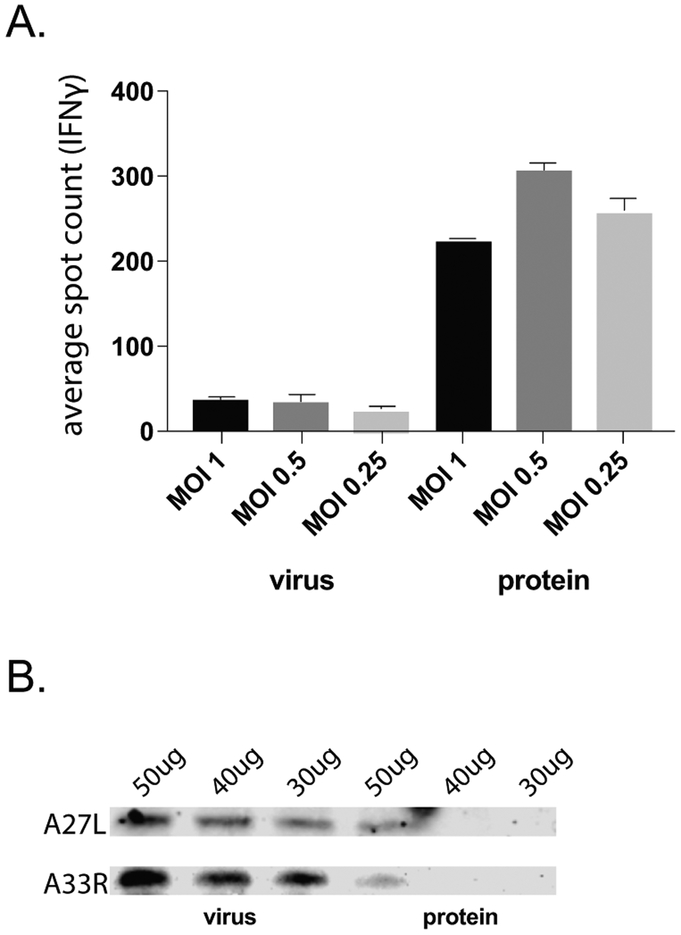
Thus far, using a large panel of epitopes, we observed that CD4+ T cells are poorly primed in response to immunization with inactivated poxvirions and that this low level of CD4+ T cell activation is not protective. We hypothesized that poor processing of whole virions by the cellular machinery provides an explanation for these results. In contrast, live virus infection creates a pool of viral proteins that are not incorporated into virions and could be more efficiently converted to epitopes by the MHC-II processing machinery. To test this notion, we infected mouse fibroblasts with ECTV and separated the lysate into whole virions and free viral proteins using a previously published centrifugation technique [34], validating the procedure by observing the presence of infectious virus in the ‘virus’ but not ‘protein’ fraction (data not shown). We then incubated bone marrow-derived dendritic cells with dilutions of the ‘virus’ fraction or the equivalent volumes of the ‘protein’ fraction and probed the ability of these preparations to re-activate ECTV-experienced CD4+ T cells via ELISpot (Figure 5A). As we had incubated BMDCs with the same volumes of the ‘protein’ and ‘virus’ fractions, we were able to normalize the number of re-activated CD4+ T cells based on the total protein in each fraction as assessed by a standard protein quantification assay. As shown in Figure 5A, the free protein fraction stimulated a substantially more robust CD4+ T cell response than the whole virus fraction. Furthermore, we confirmed via western blot the greater individual protein content in the whole virus fraction, reflecting the substantially higher stimulatory capacity of poxvirus proteins unencumbered by the dense structure (Figure 5B).
Figure 5.
Impact of virion processability on CD4+ T cell activation. Following infection of mouse fibroblast cells in cell culture with ECTV, whole virions were separated from a subviral protein fraction using a previously published protocol [36]. A. BMDCs were incubated overnight with virus or protein fractions prior to co-culture with primary splenic CD4+ T cells isolated from female C57Bl/6 mice infected with ECTV for 10 days. CD4+ T cell activation was analyzed via IFNγ production by ELISpot. Spot count was normalized to the total protein content of fractions provided to BMDCs, as assessed by a BCA assay. B. Virus and protein fractions were assessed for protein concentration and various protein amounts were assessed via western blot for the indicated poxvirus structural proteins. Representative of 3 independent experiments.
DISCUSSION
CD4+ T cells are central to coordinating many aspects of the adaptive immune response, including the production of protective neutralizing antibody responses, the elaboration of pro-inflammatory cytokines and in some cases, direct cytolytic killing of infected cells. For all these reasons, the optimization of CD4+ T cell function and activation is a key consideration in rational vaccine design. However, the factors necessary for the development of effective CD4+ T cell responses remain poorly understood. While epitope-MHC-II complex recognition by a cognate T cell receptor is established as the critical initiating step of CD4+ T cell activation, the steps preceding complex formation, processing of viral proteins into MHC-II-binding epitopes, is increasingly understood to entail a complex network of host proteins functioning in many cellular compartments. In addition, specific aspects of the viral replication cycle are expected to play a large role in which cellular factors are required for processing and presenting antigenic material. Therefore, a careful analysis of epitope-specific CD4+ T cell responses against a given virus could greatly inform how vaccination strategies could be modified to enhance protection.
Vaccination has provided a particularly effective means of protecting against poxvirus infection, as neutralizing antibodies can cross-react with many pathogenic poxviruses, including both smallpox and emerging zoonotic poxviruses such as monkeypox [46]. Rational vaccine design against poxviruses, both smallpox and emerging zoonotic poxviruses, remains a high priority as the only licensed vaccine is live VACV, which can cause severe pathogenesis in vaccinees, even death in some cases [9–16]. However, the basis for the superior immunogenicity of live virus, motivating continued use of a vaccine that has significant risks, is poorly understood. Here we carried out an extensive analysis of epitope-specific CD4+ T cell responses to a pathogenic poxvirus, providing an opportunity to both analyze CD4+ T cell responses to inform vaccination strategies as well as add new information to our growing understanding of the determinants necessary to drive a strong CD4+ T cell response.
In order to carry out these studies the first step was to establish a panel of I-Ab-restricted ECTV epitopes that could be leveraged in subsequent studies. It was critical that we examine CD4+ T cell responses to many epitopes since not all epitopes have the same properties. For this reason, bulk responses to the whole virus would also be of limited utility; detection of a polyclonal CD4+ T cell response to virus does not indicate whether the response is targeted largely to a single epitope or is distributed across many epitopes. Prior to our studies, there had been a dearth of published MHC-II restricted epitopes derived from the ECTV proteome. A previous study performed a large scale epitope analysis of the vaccine strain VACV-Western Reserve and identified 14 I-Ab-restricted epitopes, the majority of which (12/14) bound with intermediate/high affinity to I-Ab [39]. In designing our screen, we consciously excluded these previously identified epitopes, in order to focus on identification of novel epitopes. Our peptide screen, selected from a virtual overlapping peptide library combined with predictive binding affinity, yielded 42 novel I-Ab-restricted epitopes that consistently and, in many cases, robustly re-activated CD4+ T cells from ECTV-infected mice. As the binding algorithms continue to undergo refinement [8], a screen of the entire ECTV peptidome would likely have yielded additional epitopes, both previously published and novel.
CD4+ T cell epitopes are potentially skewed toward certain types of source proteins, such as abundantly produced late phase proteins. The parental proteins that produced our epitopes are from every phase of the poxvirus lifecycle, as was previously observed with a large scale epitope mapping study of VACV [39]. In contrast to this previous study where the epitope-bearing early gene products were not virulence factors, several of our epitopes deriving from early proteins are predicted to be encoded by virulence genes. This most likely reflects the larger set of epitopes identified in our study, as indeed, we too found a higher number of epitopes deriving from early-expressed proteins involved in gene regulation as compared to virulence.
Our first objective was to utilize this panel of novel ECTV epitopes to determine the potency of the CD4+ T cell response to inactivated poxvirus. The adverse reactions to immunization with live VACV are considerable [9–16], but the live vaccine has remained the standard since early attempts to immunize with inactivated virus failed to elicit strong antibody responses and protection [17–19]. Based on our previous work in other viral systems, we hypothesized that one reason for the failure of inactivated poxviruses to provide protection is the absence of a strong CD4+ T cell response. The basis for CD4+ T cell activation, recognition of peptide/MHC-II complexes, has been deduced mainly through use of stable globular proteins. This has perhaps led to the assumption that CD4+ T cell responses to virus infections entails internalization of whole virions followed by antigen processing and MHC-II loading via the classical, endosomal pathway. However, whole virions are far more complex structures than monomeric globular proteins that may not be so readily converted to MHC-II-binding peptides. Furthermore, viruses that infect antigen presenting cells such as influenza and ECTV can interact with host processing machinery beyond the endocytic compartments. Indeed, our previous studies with influenza demonstrated that, in fact, inactivated virions were poor drivers of a CD4+ T cell response compared to live virus [1]. By extension, if processability of whole virions is an important factor in the strength of the CD4+ T cell response, the more stable and refractory to processing the virion in question, the poorer the CD4+ T cell response should be. While influenza is a fragile RNA virus surviving only minutes outside the host [22] poxvirus virions are far more durable structures which can even be lyophilized without loss of infectivity, a property that was instrumental in the eradication of natural smallpox [25].
When we assessed the recall response of CD4+ T cells isolated from mice immunized with inactivated ECTV, no consistent specificities were detected against any of our 42 novel epitopes; one epitope sporadically induced a vastly reduced response compared to what is elicited by live virus. In addition, unlike our previous work with influenza, the poor epitope specific CD4+ T cell response was not amplified in a prime/boost scenario. The results further support our prediction that poxvirions are poor MHC-II antigen processing substrates. Furthermore, we did not observe responses to the epitopes derived from late structural proteins present in the mature virion, suggesting limited efficacy of endosomal proteases in the digestion of whole virions.
Despite the absence of a detectable CD4+ T cell response, we did observe a low level of serum IgG against inactivated ECTV. Considering the lack of a strong response to any peptide we tested, it seems unlikely that class-switching was driven by CD4+ T cells whose specificities were not analyzed. Rather, we favor the notion of T-independent class-switching, as has been observed in other viral infections [48–55]. An alternative is the presence of antigen presenting cell populations in the peritoneum that are distinct from those in the skin since subcutaneous immunization did not produce detectable serum anti-ECTV IgG. Regardless of the mechanism at play, this class- switched antibody response was approximately eight-fold lower than that induced by a much lower input dose of live ECTV and was not sufficient to protect mice from a secondary challenge.
There are several factors, in addition to poor virion processability, that could contribute to the poor CD4+ T cell responses generated against inactivated virus. One is reduced viral load due to the absence of replication. We aimed to address this factor by immunizing with a much higher dose of inactivated virus compared to live virus, and also by altering the immunization route to one that would result in more direct exposure of viral particles to immune cells. It is also possible that lack of inflammatory signals raised against inactivated virus could contribute to poor CD4+ T cell activation. This was a possibility that we ruled out in our previous influenza studies; co-immunization with an infectious non-cross-reactive strain of influenza which provided inflammatory signals had no impact on response to inactivated virus [1]. Recently published work corroborates our finding that inactivated VACV does not prime a protective antibody response, however the inclusion of particular adjuvants were reported to confer protection [32,56]. Therefore, we utilized the same adjuvant as was previously published preparation in conjunction with our inactivated virus. While we did observe induction of serum anti-ECTV IgG at low levels with inclusion of adjuvant, we did not observe enhancement of CD4+ T cell responses. This suggests that lack of inflammatory signals against inactivated poxvirus is not a causative explanation for our results. It should be noted, however, that more dramatic antibody production might have been observed had adjuvantation been implemented in a prime/boost immunization as in [32].
Having determined that CD4+ T cell responses are compromised following inactivated poxvirus immunization, we sought to probe directly whether poor processability of whole virions was in fact a mechanistic explanation. Therefore, after growing ECTV in cell culture we separated whole virions from a fraction containing poxviral proteins produced during replication but not incorporated into virions. Despite the presence of more poxvirus-derived proteins in the ‘virus’ fraction than in the ‘protein’ fraction, we observed that the protein fraction facilitated considerably more robust CD4+ T cell activation than the virus fraction. Thus, virion-free protein is far more efficiently processed, resulting in stronger CD4+ T cell responses, perhaps providing a basis for the promising results observed with subunit vaccines [5–7]. This data is consistent with published literature suggesting that individual proteins rather than whole virions drive CD4+T cell-B cell collaboration during the response to VACV infection [57].
Here we have demonstrated that inactivated poxvirus elicits poor CD4+ T cell responses and minimal protection to a secondary challenge. Further, we provided a mechanistic explanation in that whole virions are poor substrates for the MHC-II processing machinery, which leads to poor CD4+ T cell activation. This work adds to the growing evidence in the field that MHC-II processing and presentation is much more complex than generally envisioned. In addition, it points to principles that may be critical considerations in the rational design of vaccines intended to provoke strong CD4+ T cell responses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stuart N. Isaacs for critical reading of the manuscript.
FUNDING SOURCE
This work was supported by National Institutes of Health grants R01AI110542 (LCE), F31CA206338 (KSF) and HHSN272201400045C (AS).
REFERENCES
- 1.Miller MA, Ganesan AP, Luckashenak N, Mendonca M, and Eisenlohr LC. 2015. Endogenous antigen processing drives the primary CD4+ T cell response to influenza. Nat. Med. 21: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller MA, Ganesan AP, and Eisenlohr LC. 2013. Toward a Network Model of MHC Class II-Restricted Antigen Processing. Front. Immunol 4: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its Eradication. 1988. Geneva: World Health Organization [Google Scholar]
- 4.Shchelkunov SN 2013. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog 9: e1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman GW, Cohen ME, Xiao Y, Richardson-Harman N, Silvera P, DeTolla LJ, Davis HL, Eisenberg RJ, Cohen GH, and Isaacs SN. 2010. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine 28: 6627–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heraud JM, Edghill-Smith Y, Ayala V, Kalisz I, Parrino J, Kalyanaraman VS, Manischewitz J, King LR, Hryniewicz A, Trindade CJ, Hassett M, Tsai WP, Venzon D, Nalca A, Vaccari M, Silvera P, Bray M, Graham BS, Golding H, Hooper JW, and Franchini G. 2006. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 177: 2552–2564. [DOI] [PubMed] [Google Scholar]
- 7.Hirao LA, Draghia-Akli R, Prigge JT, Yang M, Satishchandran A, Wu L, Hammarlund E, Khan AS, Babas T, Rhodes L, Silvera P, Slifka M, Sardesai NY, and Weiner DB. 2011. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 203: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reidel S 2005. Edward Jenner and the history of smallpox and vaccination. Proceedings (Baylor University Medical Center) 18: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen AA 1966. A severe complication of smallpox vaccination. Can. Med. Assoc. J 94: 1316–1317. [PMC free article] [PubMed] [Google Scholar]
- 10.Cono J, Casey CG, Bell DM, and Centers for Disease Control and Prevention. 2003. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm Rep. 52: 1–28. [PubMed] [Google Scholar]
- 11.Maurer DM, Harrington B, and Lane JM. 2003. Smallpox vaccine: contraindications, administration, and adverse reactions. Am. Fam. Physician 68: 889–896. [PubMed] [Google Scholar]
- 12.Hughes CM, Blythe D, Li Y, Reddy R, Jordan C, Edwards C, Adams C, Conners H, Rasa C, Wilby S, Russell J, Russo KS, Somsel P, Wiedbrauk DL, Dougherty C, Allen C, Frace M, Emerson G, Olson VA, Smith SK, Braden Z, Abel J, Davidson W, Reynolds M, and Damon IK. 2011. Vaccinia virus infections in martial arts gym, Maryland, USA, 2008. Emerg. Infect. Dis. 17: 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Said MA, Haile C, Palabindala V, Barker N, Myers R, Thompson R, Wilson L, Allan-Martinez F, Montgomery J, Monroe B, Tack D, Reynolds M, Damon I, and Blythe D. 2013. Transmission of vaccinia virus, possibly through sexual contact, to a woman at high risk for adverse complications. Mil. Med. 178: 1375. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery JR, Carroll RB, and McCollum AM. 2011. Ocular vaccinia: a consequence of unrecognized contact transmission. Mil. Med. 176: 699–701. [DOI] [PubMed] [Google Scholar]
- 15.Auckland C, Cowlishaw A, Morgan D, and Miller E. 2005. Reactions to small pox vaccine in naive and previously-vaccinated individuals. Vaccine 23: 4185–4187. [DOI] [PubMed] [Google Scholar]
- 16.Belongia EA and Naleway AL. 2003. Smallpox vaccine: the good, the bad, and the ugly. Clin. Med. Res. 1: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giurca A, Topciu VL, Voiculescu D, Moldovan E, and Plavosin L. 1976. Investigations on allergic and serological reactions following inoculation of inactivated smallpox vaccines by cutaneous scarification. Virologie 27: 173–177. [PubMed] [Google Scholar]
- 18.Turner GS and Squires EJ. 1971. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J. Gen. Virol. 13: 19–25. [DOI] [PubMed] [Google Scholar]
- 19.Marennikova SS and Macevic GR. 1975. Experimental study of the role of inactivated vaccine in two-step vaccination against smallpox. Bull. World Health Organ. 52: 51–56. [PMC free article] [PubMed] [Google Scholar]
- 20.Fang M, Siciliano NA, Hersperger AR, Roscoe F, Hu A, Ma X, Shamsedeen AR, Eisenlohr LC, and Sigal LJ. 2012. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc. Natl. Acad. Sci. U. S. A. 109: 9983–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang M and Sigal LJ. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175: 6829–6836. [DOI] [PubMed] [Google Scholar]
- 22.Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, and Balfour HH. 1982. Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 146: 47–51. [DOI] [PubMed] [Google Scholar]
- 23.Resch W, Hixson KK, Moore RJ, Lipton MS, and Moss B. 2007. Protein composition of the vaccinia virus mature virion. Virology 358: 233–247. [DOI] [PubMed] [Google Scholar]
- 24.Ngo T, Mirzakhanyan Y, Moussatche N, and Gershon PD. 2016. Protein Primary Structure of the Vaccinia Virion at Increased Resolution. J. Virol. 90: 9905–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke CJ, Hsu TA, and Volkin DB. 1999. Formulation, stability, and delivery of live attenuated vaccines for human use. Crit. Rev. Ther. Drug Carrier Syst. 16: 1–83. [PubMed] [Google Scholar]
- 26.Gubser C, Hue S, Kellam P, Smith GL. Poxvirus genomes: a phylogenetic analysis. 2004. J Gen Virol 85(Pt 1):105–117. [DOI] [PubMed] [Google Scholar]
- 27.Esteban DJ and Buller RM. 2005. Ectromelia virus: the causative agent of mousepox. J. Gen. Virol. 86: 2645–2659. [DOI] [PubMed] [Google Scholar]
- 28.McCollum AM and Damon IK. 2014. Human monkeypox. Clin. Infect. Dis. 58: 260–267. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt PN and Jacoby RO. 1987. Mousepox in inbred mice innately resistant or susceptible to lethal infection with ectromelia virus. III. Experimental transmission of infection and derivation of virus-free progeny from previously infected dams. Lab. Anim. Sci. 37: 23–27. [PubMed] [Google Scholar]
- 30.Brownstein D, Bhatt PN, and Jacoby RO. 1989. Mousepox in inbred mice innately resistant or susceptible to lethal infection with ectromelia virus. V. Genetics of resistance to the Moscow strain. Arch. Virol. 107: 35–41. [DOI] [PubMed] [Google Scholar]
- 31.Sigal LJ 2016. The Pathogenesis and Immunobiology of Mousepox. Adv. Immunol. 129: 251–276. [DOI] [PubMed] [Google Scholar]
- 32.Eisenlohr LC and Hackett CJ. 1989. Class II major histocompatibility complex-restricted T cells specific for a virion structural protein that do not recognize exogenous influenza virus. Evidence that presentation of labile T cell determinants is favored by endogenous antigen synthesis. J. Exp. Med. 169: 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tewari MK, Sinnathamby G, Rajagopal D, and Eisenlohr LC. 2005. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat. Immunol. 6: 287–294. [DOI] [PubMed] [Google Scholar]
- 34.Sinnathamby G and Eisenlohr LC. 2003. Presentation by recycling MHC class II molecules of an influenza hemagglutinin-derived epitope that is revealed in the early endosome by acidification. J. Immunol. 170: 3504–3513. [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, and Peters B. 2010. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 11: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moutaftsi M, Tscharke DC, Vaughan K, Koelle DM, Stern L, Calvo-Calle M, Ennis F, Terajima M, Sutter G, Crotty S, Drexler I, Franchini G, Yewdell JW, Head SR, Blum J, Peters B, and Sette A. 2010. Uncovering the interplay between CD8, CD4 and antibody responses to complex pathogens. Future Microbiol. 5: 221–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siciliano NA, Hersperger AR, Lacuanan AM, Xu RH, Sidney J, Sette A, Sigal LJ, and Eisenlohr LC. 2014. Impact of distinct poxvirus infections on the specificities and functionalities of CD4+ T cell responses. J. Virol. 88: 10078–10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleri W, Paul S, Dhanda SK, Mahajan S, Xu X, Peters B, and Sette A. 2017. The Immune Epitope Database and Analysis Resource in Epitope Discovery and Synthetic Vaccine Design. Front. Immunol. 8: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotturi MF, Peters B, Buendia-Laysa F, Sidney J, Oseroff C, Botten J, Grey H, Buchmeier MJ, and Sette A. 2007. The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. J. Virol. 81: 4928–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui HH, Frahm N, Brander C, Peters B, Grey H, and Sette A. 2007. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J. Immunol. 178: 7890–7901. [DOI] [PubMed] [Google Scholar]
- 41.Broyles SS 2003. Vaccinia virus transcription. J. Gen. Virol. 84: 2293–2303. [DOI] [PubMed] [Google Scholar]
- 42.Assarsson E, Greenbaum JA, Sundstrom M, Schaffer L, Hammond JA, Pasquetto V, Oseroff C, Hendrickson RC, Lefkowitz EJ, Tscharke DC, Sidney J, Grey HM, Head SR, Peters B, and Sette A. 2008. Kinetic analysis of a complete poxvirus transcriptome reveals an immediate-early class of genes. Proc. Natl. Acad. Sci. U. S. A. 105: 2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwartouw HT, Westwood JC, Appleyard G. 1962. Purification of pox viruses by density gradient centrifugation. J. Gen. Microbiol. 29: 523–529. [DOI] [PubMed] [Google Scholar]
- 44.Gilchuk I, Gilchuk P, Sapparapu G, Lampley R, Singh V, Kose N, Blum DL, Hughes LJ, Satheshkumar PS, Townsend MB, Kondas AV, Reed Z, Weiner Z, Olson VA, Hammarlund E, Raue HP, Slifka MK, Slaughter JC, Graham BS, Edwards KM, Eisenberg RJ, Cohen GH, Joyce S, and Crowe JE. 2016. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 167: 694.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, Sette A, Peters B, and Nielsen M. 2018. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sha Z and Compans RW. 2000. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 74: 4999–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szomolanyi-Tsuda E and Welsh RM. 1996. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J. Exp. Med. 183: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fehr T, Bachmann MF, Bluethmann H, Kikutani H, Hengartner H, and Zinkernagel RM. 1996. T-independent activation of B cells by vesicular stomatitis virus: no evidence for the need of a second signal. Cell. Immunol. 168: 184–192. [DOI] [PubMed] [Google Scholar]
- 49.Borca MV, Fernandez FM, Sadir AM, Braun M, and Schudel AA. 1986. Immune response to foot-and-mouth disease virus in a murine experimental model: effective thymus-independent primary and secondary reaction. Immunology 59: 261–267. [PMC free article] [PubMed] [Google Scholar]
- 50.Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, and McGettigan JP. 2012. Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection. J. Virol. 86: 11533–11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snapper CM, Yamaguchi H, Moorman MA, and Mond JJ. 1994. An in vitro model for T cell-independent induction of humoral immunity. A requirement for NK cells. J. Immunol. 152: 4884–4892. [PubMed] [Google Scholar]
- 52.Raval FM, Mishra R, Garcea RL, Welsh RM, and Szomolanyi-Tsuda E. 2013. Long-lasting T cell-independent IgG responses require MyD88-mediated pathways and are maintained by high levels of virus persistence. MBio 4: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kataoka K, Fujihashi K, Terao Y, Gilbert RS, Sekine S, Kobayashi R, Fukuyama Y, Kawabata S, and Fujihashi K. 2011. Oral-nasopharyngeal dendritic cells mediate T cell-independent IgA class switching on B-1 B cells. PLoS One 6: e25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielinska AU, Chepurnov AA, Landers JJ, Janczak KW, Chepurnova TS, Luker GD, and Baker JR. 2008. A novel, killed-virus nasal vaccinia virus vaccine. Clin. Vaccine Immunol. 15: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matos ACD, Guedes MIMC, Rehfeld IS, Costa EA, Costa AG, Silva NLD, Lage AP, and Lobato ZIP. 2017. Bovine vaccinia: Inactivated Vaccinia virus vaccine induces protection in murine model. Vet. Microbiol. 204: 84–89. [DOI] [PubMed] [Google Scholar]
- 56.Sette A, Grey H, Oseroff C, Peters B, Moutaftsi M, Crotty S, Assarsson E, Greenbaum J, Kim Y, Kolla R, Tscharke D, Koelle D, Johnson RP, Blum J, Head S, and Sidney J. 2009. Definition of epitopes and antigens recognized by vaccinia specific immune responses: their conservation in variola virus sequences, and use as a model system to study complex pathogens. Vaccine 27 Suppl 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrop R, Ryan MG, Golding H, Redchenko I, Carroll MW. 2004. Monitoring of human immunological responses to vaccinia virus. Methods Mol Biol 269:243–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.