Abstract
We have described a novel cytokine encoded by a gene called Meteorin-like (Metrnl). Metrnl is a small (~27kDa) secreted protein expressed by activated macrophages and barrier tissues (mucosa, skin). Metrnl production by bone marrow macrophages is induced by several cytokines including TNFα, IL-17α, IL-12 and IL-4 and inhibited by IFNγ and TGFβ. Metrnl expression in macrophages is also induced by lipopolysaccharide (LPS) and its levels in circulation are associated with inflammatory responses in vivo. Furthermore, Metrnl regulates the production of several cytokines and chemokines in macrophages. We have produced a Metrnl−/− mouse which is viable and shows normal development. However, it exhibits dysregulated cytokine production, alterations in IgG production, and is highly susceptible to LPS in a sepsis model. Furthermore, older Metrnl−/− mice develop inflammatory lesions, suggesting that Metrnl participates in the control of inflammatory responses. Taken together, these observations indicate that Metrnl encodes a novel immunoregulatory cytokine associated with inflammatory responses that we have designated Meteorin Beta.
Introduction
Cytokines are small secreted proteins that play key roles in many biological processes including hematopoiesis, embryonic development, and immune responses. Approximately 10% of the human genome is estimated to encode secreted proteins. Given the importance of cytokines in the immune system, we asked whether there were secreted proteins linked to the immune system that remained to be described. To address this question, we screened a comprehensive database of gene expression (Body Index of Gene Expression: 4BIGE) (1) looking for genes encoding secreted proteins expressed by cells or organs of the immune system. This screen yielded several novel secreted proteins expressed by various cells of the immune system. One of these genes (C17ORF99) encodes a small secreted protein that we have recently identified as a novel B-cell associated cytokine which we have called Interleukin 40 (2). The same screen identified another gene with cytokine-like characteristics including another gene encoding a small secreted protein (~28kDa) annotated as Meteorin-like (Metrnl). This name reflects the fact that Metrnl is evolutionarily related to a gene that encodes a known neurotrophic factor called Meteorin (Metrn) that is expressed in the Central Nervous System (3). Metrnl has recently been described to be a hormone (4) or adipokine (5) involved in metabolic responses. We have reported that Metrnl is produced by activated macrophages, and that its expression is associated with several human autoimmune diseases including psoriasis (3). Furthermore, Metrnl, under homeostatic conditions, is mainly expressed in human ‘barrier’ tissues including skin and mucosal sites of the digestive and respiratory tract (3). We, therefore, hypothesized that Metrnl encodes a novel cytokine whose functions likely include important roles in inflammation, and in innate and acquired immunity (3). In order to confirm this hypothesis, we sought to continue the functional characterization of Metrnl by producing and characterizing a Metrnl−/− mouse.
In the present study, we report that the production of Metrnl in bone marrow-derived macrophages (BMM) is regulated by many cytokines (including TNFα, IL17a, IL12, IL4, IL-1β, IFNγ, and TGFβ). Conversely, Metrnl can also regulate the expression of several cytokines by macrophages. Importantly, the expression of Metrnl is strongly induced in macrophages by TNFα and by lipopolysaccharide (LPS) and Metrnl levels parallel the development and resolution of inflammatory responses in vivo. We have produced a Metrnl−/− mouse that lacks the capacity to express Metrnl in all tissues. Metrnl−/− mice develop and reproduce normally, but exhibit multiple immune system abnormalities. They have lower levels of IgG in plasma, and show dysregulation of cytokine and chemokine production. When tested in a model of sepsis induced by LPS, they show enhanced susceptibility. Furthermore, a significant proportion of older Metrnl−/− mice developed inflammatory lesions. Taken together, these observations support and expand our previous findings (3), and confirm that Metrnl encodes a novel immunoregulatory cytokine associated with inflammation. Metrnl−/− mice do not show metabolic abnormalities when fed either a normal or high-fat diet, further suggesting that the main functions of Metrnl are in the immune system. The last novel cytokines described include a new member of the IL-12 family that has been called IL-39 (6, 7) and our own recent description of IL-40/C17Orf99 (2). The name Meteorin-like only reflects its evolutionary relationship to Meteorin, but does not describe that it is a cytokine, or that it plays an important role in immunity, inflammation and likely other systems (4, 5, 8). We therefore propose that Meteorin should be renamed Meteorin alpha (Metrnα) and that Meteorin-like should be renamed meteorin beta (Metrnβ) (9), a designation that we will use in the present report.
Materials and Methods:
Generation of Metrnl/Metrnβ−/− mice
In order to produce a Metrnl/Metrnβ−/− mouse, we obtained Mouse Embryonic Stem (ES) cells from the KOMP (Knockout mouse project: www.KOMP.org) containing a selection cassette that was inserted into the mouse Metrnl locus by homologous recombination (Supplementary Figure 1). These ES clones were microinjected into C57BL/6 blastocysts, which were transferred into pseudo-pregnant female mice. Resulting chimeras were crossed with WT C57BL/6 mice, and MetrnβNeo/+ heterozygotes were subsequently bred with FLPeR mice. The MetrnβNeo-/loxP+ mice were then bred to CRE mice to delete the loxP-flanked target region. Finally, heterozygote Metnrβ+/− mice were intercrossed to generate homozygote Metrnβ−/− mice in the C57Bl/6 background. (Supplementary Figure 1).
Flow Cytometry and Imaging Flow Cytometry
Single-cell suspensions were stained with the following antibodies: CD11b (M1/70), Ly6G (1A8), CCR3 (J073E5), F4/80, (BM8), MHC II (M5/114.15.2) (Biolegend, San Diego, CA); SiglecF (eBioscience, Carlsbad, CA). Samples were processed in a NovoCyte (Acea) and analyzed with FlowJo software (Tree Star). Imaging was performed using an imaging flow cytometer (ImageStream X Mark II, Amnis).
Mice
C57BL/6, FLPeR and Cre mice were obtained from Jackson Laboratories (Bar Harbor, ME). All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine. Wild type (WT) mice used as controls in experiments using Metrnβ−/− mice were age and sex matched littermates of Metrnβ−/− mice het-het crossings identified through genotyping.
Macrophages
Bone-marrow was isolated from murine femurs and cultured in DMEM with 50ng/mL M-CSF (BioLegend). After 3 days, non-adherent cells were removed, and fresh medium was added. Bone Marrow-Derived Macrophages (BMM) were used after 7 days in culture. For stimulation, BMM were cultured in the presence of TNFα, IL-4, IFNγ, TGFß, IL-17A, IL-6, IL-10 or IL-12 (BioLegend). All cytokines were used at 50ng/ml. Peritoneal macrophages were obtained from the mouse peritoneal cavity following lavage with 4 ml balanced salt solution. For some experiments, macrophages were also incubated with recombinant Metrnl/Metrnβ (100ng/mL, Biotechne, Minneapolis, MN).
Cytokine Assays.
For cytokine measurements, samples were analyzed using LegendPlex mouse 13plex kits (BioLegend). Plates were read using a NovoCyte (Acea) instrument. The cytokines assessed by LegendPlex included: CCL5, CCL20, CCL11, CCL17, CXCL1, CCL2, CXCL9, CXCL10, CL3, CCL4, CXCL13, CXCL5, CCL22, IL-23, IL-1α, IFNγ, TNFα, IL-12p70, IL-1ß, IL-10, IL-6, IL-27, IL-17A, IFNß, and GM-CSF.
ELISA
Mouse Metrnl ELISA was purchased from R&D systems (Minneapolis, MN). IL-6 and IL-10 ELISAs were obtained from BioLegend. All ELISAs were used according to each manufacturer’s protocol.
Quantitative PCR
RNA was isolated from tissues using the QIAGEN RNeasy Kit, according to the manufacturer’s instructions (QIAGEN, Valencia, CA). cDNA reactions were performed using QuantiTect Reverse Transcription (QIAGEN). Quantitative PCR (qPCR) was performed using the Roche LightCycler 480 Real-Time PCR system with probes designed to detect TNFα (B cell marker), and GAPDH (housekeeping gene) (Roche, Pleasanton, CA).
Thioglycollate peritonitis model
Mice were injected intraperitoneally with 3 mL 3% Brewer thioglycollate (DIFCO, Detroit, MI) in phosphate-buffered saline (PBS). Serum and peritoneal exudate samples were collected at different time point post-injection at days 0, 2, 4, 6 and 9. Metrnβ levels in these samples were measured by ELISA (Biotechne, Minneapolis, MN) per the manufacturer’s instructions.
Sepsis Assay
This model has been characterized previously (10). Briefly, 8-wk-old female mice were injected intraperitoneally (i.p.) with LPS derived from Escherichia coli Serotype 0111:B4 (Sigma, St. Louis) dissolved in PBS (10 mg/kg body weight). Survival of the mice was monitored every 6 h during the day and until 9:00 pm for 72 h.
Metrnβ−/− mouse inflammatory lesions
20 female mice were followed and monitored for the development of inflammatory lesions. Two groups were euthanatized at months 4 or 8. Tissues and where applicable cell infiltrates were characterized by flow cytometry. Expression of TNFα was determined by qPCR as previously described.
Database Analyses
Comparative expression analyses of Metrnα and Metrnβ in Brain, Cervix, Colon and Esophagus tissues were performed using data generated or provided by the Genotype Tissue Expression (GTEx) project database, The Human Protein Atlas (HPA) project, Body Index of Gene Expression (BIGE) database (1) and the Expression Atlas (GXA) compilation.
https://www.proteinatlas.org/ENSG00000176845-METRNL/tissue
Statistical analyses
The significance of differences between two groups was determined by Student’s t-test (two-tailed) or Gehan-Breslow-Wilcoxon Test which were performed using Prism software (GraphPad, La Jolla, CA). A p-value <0.05 was considered significant.
Results
The production of Metrnβ by macrophages is regulated by multiple cytokines.
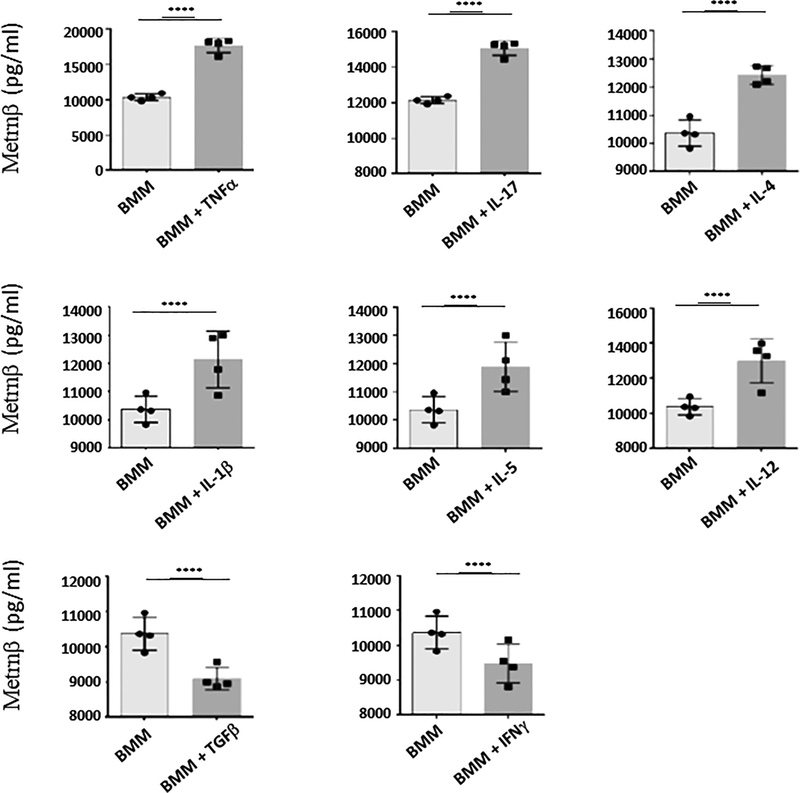
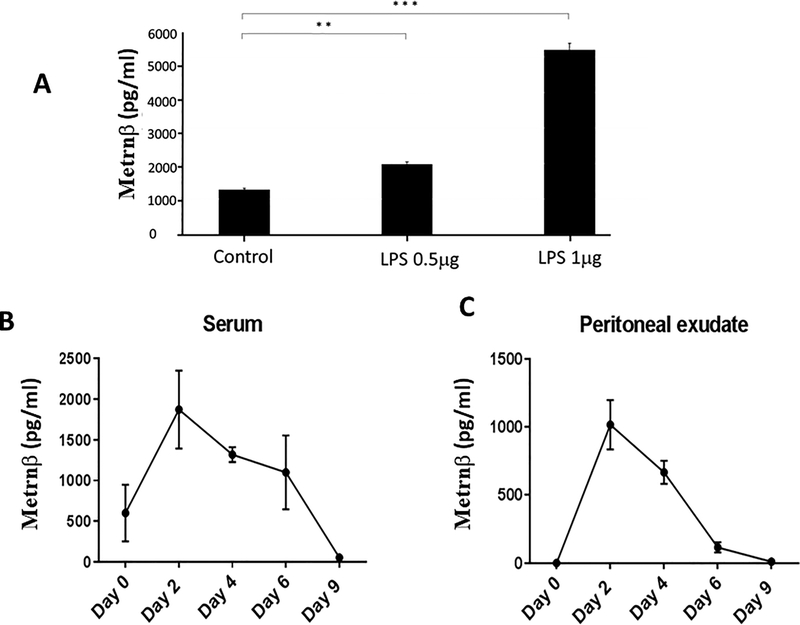
We have reported the identification of a new cytokine, Metrnl (or Metrnβ) which is highly expressed in barrier tissues (skin, mucosa) and activated macrophages (3). Depending on the stimuli present in their microenvironment, macrophages can assume distinct activation phenotypes (11) and have been classified into M1 (IFNγ-activated macrophages) or M2 (IL-4- and/or IL-13- activated macrophages) states (12). However, these initially described states have been expanded into a more accurate ‘spectrum’ model of macrophage activation in response to diverse stimuli (13). We initially reported that Metrnβ is produced by alternatively activated macrophages because its expression was up-regulated in peritoneal macrophages cultured with IL-4 and inhibited by IFNγ (3). However, we have now expanded these studies to test other cytokines (Fig 1). We cultured mouse BMM with various cytokines and measured Metrnβ in the supernatant by ELISA. Interestingly, in BMM, TNFα is the most potent inducer of Metrnβ production by macrophages while, conversely, IFNγ or TGFß suppress its expression (Fig 1). In addition, other cytokines, including IL-17A, IL12, IL4, and IL1β also induced Metrnβ expression in BMM (Fig 1). These observations indicate that Metrnβ production by macrophages is under complex regulation, consistent with the hypothesis that it likely plays multiple functional roles in different immune responses. We should note that TNFα is the most powerful inducer of Metrnβ expression in macrophages. This observation suggested that Metrnβ is produced during (and likely participates in) inflammatory responses. These results also suggested that Metrnβ is produced in response to inflammatory stimuli. We therefore investigated whether it is induced by lipopolysaccharide (LPS) in macrophages. As shown in Fig 2A, LPS induces strong Metrnβ expression in RAW 264.7macrophages in a dose-dependent manner. We observed similar responses in peritoneal and BMM macrophages (data not shown). We then measured Metrnβ levels in circulation during an inflammatory response induced by thioglycollate injection into the peritoneal cavity of mice. As shown in Figures 2B/C, the levels of Metrnβ markedly increased in both serum and in the peritoneal exudate following thioglycollate challenge and subsided gradually, mirroring the resolution of inflammation. These observations indicate that Metrnβ is associated with inflammation in vivo.
Figure 1. Metrnβ production by bone marrow macrophages (BMM) is regulated by cytokines.
Levels of Metrnβ production by BMM in response to various cytokines. TNFα induced the highest expression of Metrnβ in BMM, but IL-17α, IL-4, IL-1β, IL-5, and IL-12 also induced significant increases in Metrnβ levels. IFNγ and TGFβ reduced baseline production of Metrnβ by BMM. Bars represent mean+/− SEM. *p<0.05; **p<0.02; ***p<0.01; ****p<0.005. Results are representative of 3 experiments.
Figure 2. Metrnβ levels are associated with inflammation.
(A) Metrnβ production is induced in macrophages by Lipopolysaccharide (LPS). Levels of Metrnβ production by RAW 264.7 macrophages were measured by ELISA in response to 0.5 or 1 mg of LPS stimulus for 48h. Metrnβ levels correlate with inflammation in vivo. Levels of Metrnβ in serum (B) and peritoneal exudate (C) were measured by ELISA over the course of sterile inflammation induced by intraperitoneal thioglycollate injection. Bars represent mean+/− SEM. **: p<0.02; ***: p<0.01. Results are representative of at least 3 experiments.
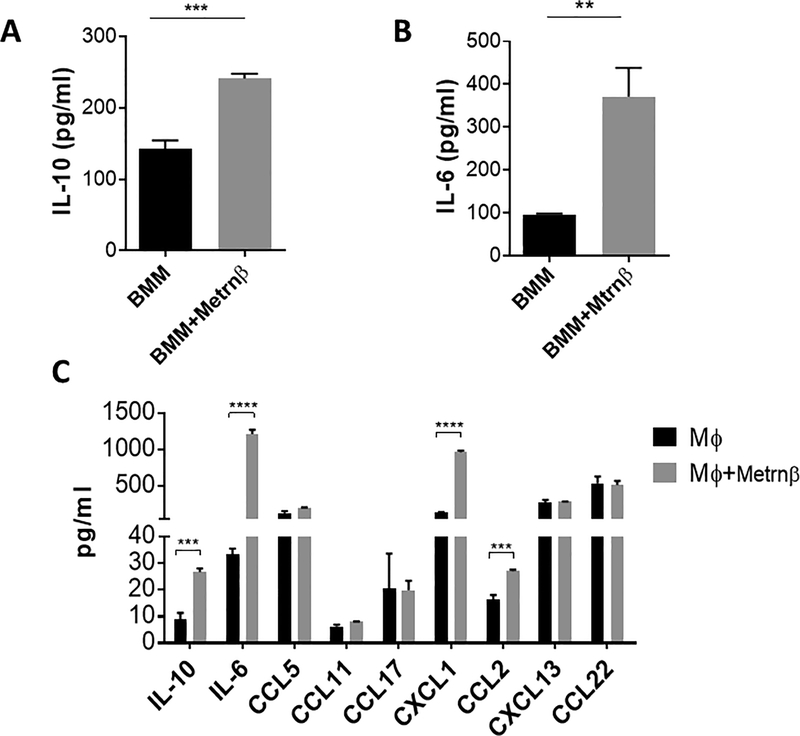
We also explored whether Metrnβ can regulate cytokine production in macrophages. To this end, we incubated BMM with Metrnβ and measured several cytokines. As shown in Figure 3A/B, Metrnβ induces the expression of IL-6 and IL-10 in BMM, and it also increases the expression of CXCL1 and CCL2 in peritoneal macrophages (Figure 3C). We conclude that Metrnβ expression is associated with inflammation, that its production is regulated by several cytokines, and that it can also regulate the expression of other cytokines and chemokines. Taken together, these results indicate that Metrnβ is a new major player in inflammatory responses and is likely to play a role in the pathogenesis of human inflammatory diseases. In support of the latter hypothesis, we have already reported that Metrnβ is up-regulated in several human autoimmune diseases including psoriasis and rheumatoid arthritis (3).
Figure 3. Metrnβ induces production of several cytokines and chemokines in macrophages.
Induction of (A) IL-6 and (B) IL-10 in bone marrow macrophages (BMM) by Metrnβ as measured by ELISA. BMM were incubated in control medium or 100 ng/ml of Metrnβ for 24h and their supernatants were measured for IL-6 or IL-10 by ELISA. (C) Metrnβ induces expression of CXCL1 and CCL2 in peritoneal macrophages. Peritoneal cavity macrophages were incubated in the presence of Metrnβ (100 ng/ml) for 24 h prior to measuring levels of different cytokines and chemokines in supernatants using Legendplex (BioLegend). Bars represent the mean+/− SEM. **: p<0.02; ***: p<0.01. Results are representative of at least 3 experiments.
Metrnβ deficient mice exhibit immune system defects
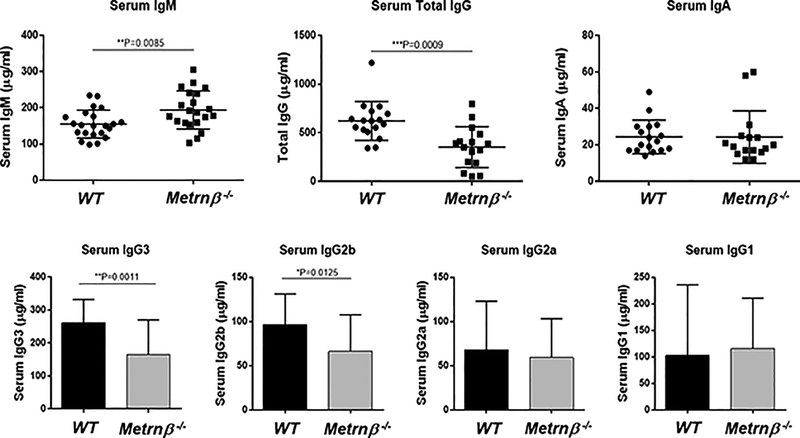
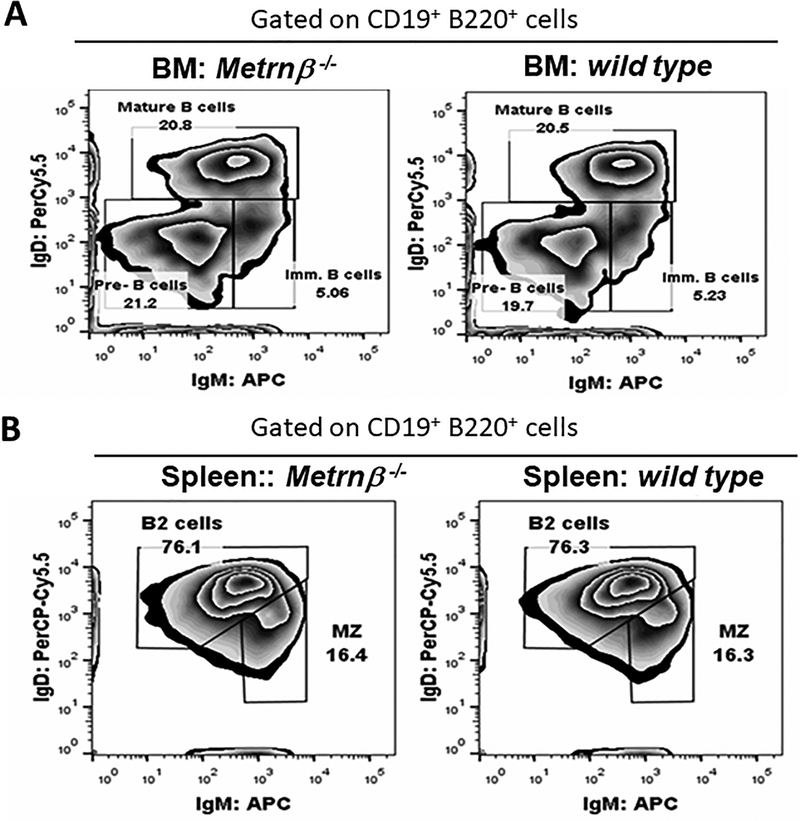
The results shown in Figure 1–3 strongly suggested that Metrnβ represents a novel cytokine associated with immune responses and inflammation. To learn more about its possible role in the immune system, we generated a full body Metrnβ−/− mouse (Supplementary Figure 1). The resulting Metrnβ−/− mouse exhibits normal development, gains weight normally (data not shown), breeds well, and is otherwise healthy. These results are similar to information reported by the KOMP (Knockout mouse project) for a knockout mouse of this gene http://www.mousephenotype.org/data/experiments?geneAccession=MGI:2384806. Metrnβ has been reported to be involved in metabolic regulation of energy expenditure and control of glucose tolerance (4), but we have not observed abnormalities in weight gain in Metrnβ −/− mice (either under a normal diet or when fed a high fat diet) (data not shown). These results confirm data from Li et al who performed similar studies with an adipocyte-specific Metrnβ−/− mouse (14, 15). Analyses of the lymphoid populations of immune organs (spleen, lymph nodes, thymus and bone marrow) of Metrnβ−/− mice (compared to Wild Type (WT) littermates) failed to reveal abnormalities in major lymphoid populations in these organs. It has been reported that Metrnβ overexpression results in eosinophil recruitment (4). The Metrnβ−/− mouse, however, shows normal numbers of eosinophils in blood, spleen, peritoneal cavity and bone marrow, and the numbers of white blood cells in the blood of Metrnβ−/− mice are also normal (data not shown). However, we did detect higher levels of Immunoglobulin M (IgM) and lower levels of IgG in serum of Metrnβ−/− mice. Further analyses indicate that the IgG isotypes affected were IgG3 and IgG2b (Fig 4). We analyzed the spleen B cell populations in Metrnβ−/− mice, but did not detect any abnormalities in these populations (Figure 5). Since immunoglobulin class switching is largely determined by cytokines (16), we hypothesized that the defect in IgG production may be due to cytokine production abnormalities in Metrnβ−/− mice.
Figure 4. Metrnβ−/− mice have altered levels of serum immunoglobulins.
(A) The level of IgM is elevated in Metrnβ−/− mice when compared to WT littermate controls while levels of total IgG increased. (B) Among four different IgG subtypes, levels of IgG3 and IgG2b are decreased in Metrnβ−/− mice whereas levels of IgG2a and IgG1 are comparable between WT and Metrnβ−/− mice. Bars represent the mean+/− SEM. **: p<0.02; ***: p<0.01.
Figure 5. Normal B cell population frequencies in Metrnβ−/− mice.
(A) Staining shows frequencies of B cells in Metrnβ−/− mice or WT littermate mice. The plot of B cell populations indicates the percentage of pre-B, mature, or immature (imm.) B cells in bone marrow (BM). (B) Percentage of marginal zone (MZ) B cells and B2 cells in the spleen. Data are representative of three independent experiments.
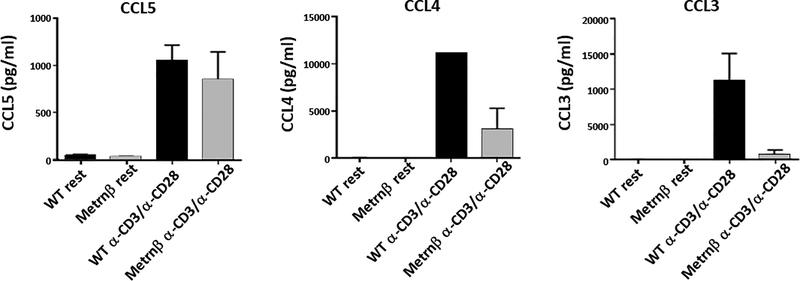
Indeed, a Legendplex analysis of the cytokine and chemokine production by splenocytes from the Metrnβ−/− mice revealed multiple abnormalities. Some of the most affected were CCL3 and CCL4. As shown in Figure 6, we confirmed this result by measuring the ability of spleen cells to produce CCL3 and CCL4 by ELISA. The results indicate that the ability of spleen cells (most likely T cells because the activating stimulus was anti-CD3 and anti-CD28) to produce these chemokines is strongly inhibited in Metrnβ−/− mice (Figure 6).
Figure 6. Splenocytes from Metrnβ−/− mice produce altered levels of CCL3 and CCL4.
Splenocytes isolated from Metrnβ−/− or WT littermate mice were activated with α-CD3/α-CD28 for 24hrs prior to measuring the production of different chemokines (CCL5, CCL4, and CCL3) by ELISA. Bars represent the mean +/− SEM. **: p<0.02. Results are representative of at least 3 experiments.
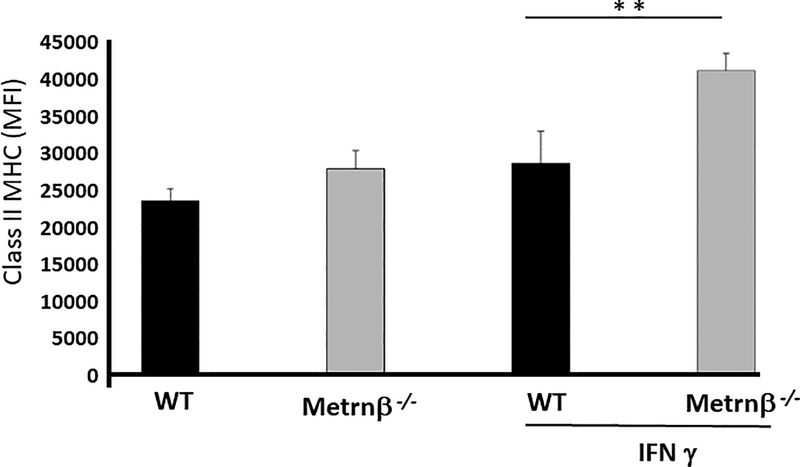
IFNγ induces higher levels of class II MHC in Peritoneal Macrophages in Metrnβ−/− mice.
Interestingly, IFNγ inhibits the expression of Metrnβ by macrophages (Fig 1) (3). However, IFNγ is known to induce expression of class II MHC in macrophages (17). We therefore investigated whether Metrnβ had an effect on the expression of class II MHC in macrophages. To this end, we analyzed the levels of class II MHC in peritoneal macrophages from WT or Metrnβ−/− mice in the presence or absence of IFNγ. As shown in Figure 7, IFNγ induced a significantly higher level of class II MHC expression in macrophages from Metrnβ−/− mice, suggesting that Metrnβ normally has a dampening effect on the expression of class II MHC by macrophages during immune responses. We confirmed this hypothesis by testing the capacity of recombinant Metrnβ to inhibit IFNγ-induced expression of class II MHC in peritoneal macrophages. As shown in Supplementary Figure 2, the presence of Metrnβ during IFNγ induction of class II MHC in peritoneal macrophages from Metrnβ−/− mice inhibited the expression of class II MHC.
Figure 7. IFNγ induces higher levels of class II MHC in Metrnβ−/− peritoneal macrophages.
Levels of class II MHC in peritoneal macrophages from Metrnβ−/− or WT littermate mice in the presence or absence of IFNγ. Macrophages were stained for class II MHC upon harvest (left) or following stimulation with IFNγ (250 ng/mL) for 24 h and analyzed by flow cytometry. MFI: Mean fluorescence index. Bars represent the mean +/− SEM. **p<0.02; Results are representative of at least 3 experiments.
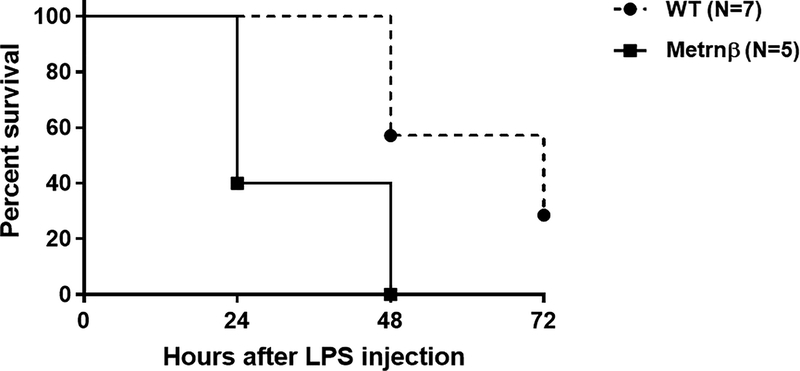
Metrnβ−/− mice are highly susceptible to endotoxin shock.
The data presented so far indicate that Metrnβ is associated with inflammation, is produced by macrophages exposed to LPS and is also an immunoregulatory cytokine. We therefore hypothesized that Metrnβ is produced and plays a role during sepsis. To test this hypothesis, we tested Metrnβ or WT mice in a model of endotoxemia by injecting them with LPS and monitoring their responses. As shown in Figure 8, Metrnβ−/− mice are more susceptible to endotoxic shock than WT mice and started to die more than 24 h before WT mice. This result strongly suggests that Metrnβ normally plays an anti-inflammatory role during sepsis.
Figure 8. Metrnβ−/− mice are highly susceptible to endotoxemia.
Survival curve of WT littermate (dashed line) or Metrnβ−/− (solid line) mice following an LPS challenge (10 mg/kg body weight) (n=7 for WT mice and n=5 KO mice). Differences in survival curves (between WT and Metrnβ−/− mice) were statistically significant P≤0.01. One out of two experiments with similar results is shown.
Metrnβ−/− mice develop inflammatory lesions.
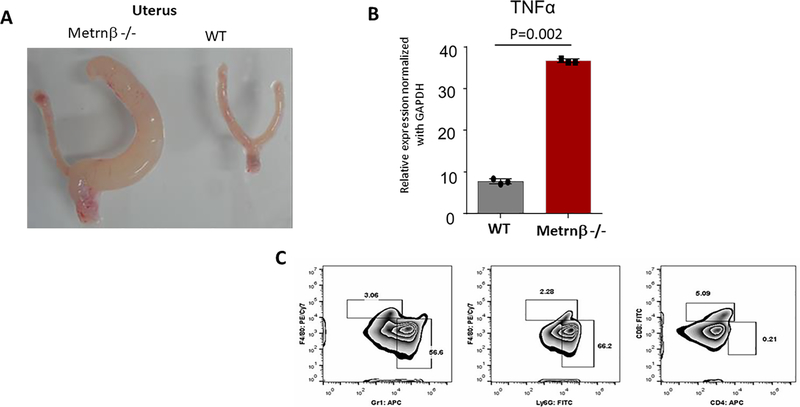
As we developed our colony of Metrnβ−/− mice, we noticed that several Metrnβ−/− mice developed inflammatory lesions. The most common one was unilateral inflammatory lesions of one horn of the uterus, but we also observed inflammatory lesions in kidneys and liver. As shown in Figure 9, these lesions typically contained large numbers neutrophils and high levels of TNFα consistent with inflammatory responses. To quantify this effect, we selected a cohort of 20 Metrnβ−/− mice that were followed for several months and monitored for the development of inflammatory lesions. At 4 months 12 mice were sacrificed and 3 of them (25%) showed large inflammatory lesions in the uterus; the rest of the mice (8) were sacrificed at 8 months, and again 2 of them (25%) showed inflammatory lesions. In contrast, none of their WT littermates developed lesions. These observations indicate that the lack of Metrnβ favors the development of these inflammatory lesions. Given these observations, we hypothesized whether Metrnβ could affect the production of TNFα by activated macrophages. However, as shown in Supplemental Figure 3, the addition of Metrnβ to macrophages following activation with LPS failed to modify TNFα production.
Figure 9. Recurring lesions observed in uterus of Metrnβ−/− mice show inflammation.
(A) Pathology observed in one of the horns of a uterus from an Metrnβ−/− mouse compared with WT littermate uterus. (B) TNFα upregulation in Metrnβ−/− mouse uterus was measured by qPCR. (C) Flow cytometry analysis revealed leukocytic infiltrate, including neutrophils (Gr1; Ly6G) and macrophages (F4/80). Bars represent mean +/− SEM. ****p<0.005; Results are representative of at least 3 experiments.
These data indicate that Metrnβ is an important novel immunoregulatory cytokine that is likely involved in various types of immune responses, through its ability to regulate the production of cytokines and chemokines. Other cytokines exhibit anti-and pro-inflammatory effects. For example, IL-10 can inhibit T cell activation by inhibiting the ability of macrophages to present antigen (18), but it also enhances development of cytotoxic T cells (19). Our data indicates that multiple cytokines are able to regulate the production of Metrnβ in macrophages, and conversely, that Metrnβ is able to regulate the production of other cytokines and chemokines by macrophages. Taken together, we conclude that Metrnβ is an important novel immunoregulatory cytokine involved in inflammation.
Discussion
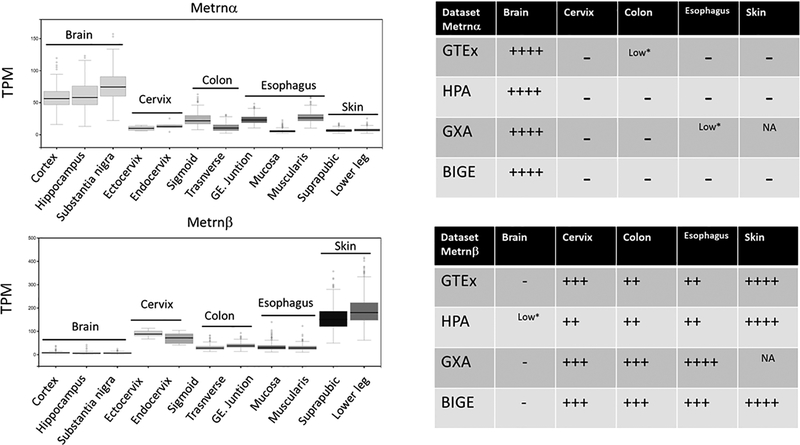
Meteorin-like is a poorly characterized small (~27kDa) secreted protein produced by activated macrophages, and homeostatically expressed by barrier tissues. In the latter, the main cellular sources are epithelial cells in the mucosa (20) and fibroblasts in the skin (3). This expression pattern is consistent with a homeostatic function in innate immunity. Moreover, we show here that Metrnβ expression is also associated with inflammatory responses. The latter results strongly suggest that it must have functions during both homeostasis and inflammatory responses. Metrnβ has also been reported to be a hormone that regulates thermogenesis and adipocyte metabolism (4). However, we have not observed significant differences in the development of Metrnl−/− mice. Overall, the main phenotype of Metrnβ−/− mice is associated with the immune system, supporting the view that the gene currently annotated as Meteorin-like (Metrnl) encodes a novel cytokine that we recommend should be renamed Meteorin β, to distinguish it from the original neurotrophic factor Meteorin, which we suggest should be renamed Meterorin α. Metrnα is expressed in the Central Nervous System, while Metrnβ is expressed mainly in skin and mucosal tissues, as we have previously reported (3). We confirmed their expression patterns by analyzing their expression in several tissue databases (Figure 10). Based on these results, we conclude that Metrnα and Metrnβ represent two cytokines that likely represent the evolutionary offspring of an ancestral gene that duplicated to give rise to these cytokines, a phenomenon that has previously been described in other cytokine families (21). These observations suggest that Metrnα and Metrnβ may have similar functions except that Metrnα is primarily acting in the CNS while Metrnβ may be part of innate immunity. In support of this conclusion, Wen et al. have described the disulfide structure of Meteorin α, and observed high conservation of the ten Cys residues between Meteorin α and Meteorin β, suggesting that the disulfide linkages in these proteins are highly conserved (22). A corollary to these observations is that structures and their functions may be similar, although Metrnα will likely exert these effects in the Central Nervous System where it is predominantly expressed while Metrnβ may mediate similar functions in skin, mucosa and inflammatory responses. These observations also predict that their receptor(s) should be evolutionary linked as well.
Figure 10.
Comparative expression of Metrnα and Metrnβ in Brain, Cervix, Colon and Esophagus using the Genotype Tissue Expression (GTEx) project database (left) and its correlation with data generated by The Human Protein Atlas (HPA) project, Body Index of Gene Expression (BIGE) database and Expression Atlas (GXA) compilation. GE (Gastroesophageal) TPM (Transcripts Per Kilobase Million) (right). There is general agreement between these databases on the expression of Metrnα and Metrnβ in these tissues, with Metrnα expressed mainly in the Brain while Metrnβ is expressed mainly in mucosal tissues and skin.
Taken together, our results strongly suggest that the main cell associated with Metrnβ inflammatory functions is the macrophage. In support of this, Gong et al have reported that Metrnl affects bone development likely through effects on osteoblasts (23), which are related to macrophages by lineage. Our data indicate that many cytokines regulate Metrnβ expression in macrophages and conversely, Metrnβ can also regulate the production of several cytokines and chemokines in macrophages (Fig 3). Importantly, the fact that TNFα is the most potent inducer of Metrnβ production in macrophages (Fig 1) strongly supports an important role for Metrnβ in inflammation. Our data suggest that Metrnβ plays an anti-inflammatory role during normal homeostasis, for example, by modulating cytokine and chemokine production and expression of class II MHC. This potential role would explain its expression in the normal mucosa and skin, important sites for innate immunity that receive a large number of antigenic stimuli. Interestingly, other anti-inflammatory molecules, such as IL-1 receptor antagonist (IL1RN), shows an expression pattern in the human body similar to Metrnβ. Its highest expression is in the oral mucosa and esophagus, and is also strongly expressed by activated macrophages (supplementary Figure 4). These are tissues where Metrnβ is also highly expressed. These data suggest that the tissues from the oral cavity to the esophagus are strongly anti-inflammatory environments. This makes sense because of the large number of substances that mammals place in their mouths. Likely their function is to regulate the extent of potential inflammatory responses in barrier tissues. This hypothesis is further supported by the effectiveness that IL1RN shows in certain autoimmune/inflammatory diseases such as Schnitzler syndrome (24, 25). However, Metrnβ is also strongly expressed in the skin whereas IL1RN is not, suggesting that it may exhibit other functions in the skin. The ability of Metrnβ to regulate cytokine and chemokine expression by macrophages further supports this hypothesis. Several results from our study, including the high sensitivity of Metrnβ−/− mice to LPS (Figure 8) and the development of inflammatory lesions in older Metrnβ−/− mice (Figure 9) strongly support an anti-inflammatory function for Metrnβ. In this sense, it is noteworthy that we have also observed that it is capable of enhancing IL-10 production by macrophages (Figure 3B), which also points to an anti-inflammatory cytokine.
Our results also suggest that Metrnβ is likely to play important roles in acquired immunity. For example, the ability of IL-4 and IL17A to induce Metrnβ production in macrophages, along with the inhibitory effect of IFNγ on Metrnβ production strongly suggests that Metrnβ may play specific roles in the development of Th1, Th2, and Th17 responses. We also detected abnormalities in cytokine production in the Metrnβ−/− mouse, suggesting abnormalities in acquired immunity. For example, as shown in Figure 6, the production of CCL3 and CCL4 by Metrnβ−/− mouse splenocytes activated with anti-CD3 and anti-CD28 is strongly inhibited. This suggests abnormalities in the T cell compartment as well. Interestingly, the Immgen database (https://www.immgen.org/ )indicates that Metrnβ is produced by thymic medullary epithelial cells. These observations suggest that Metrnβ may also have effects on T cell development.
Overall, the main phenotype detected in Metrnβ−/− mice includes immune system abnormalities as well as abnormal inflammatory responses. We should note that Metrnβ does not inhibit TNFα production by macrophages (Supplementary Figure 3), suggesting that the inflammatory lesions observed in older Metrnβ−/− mice are not due to dysregulation of TNFα production. Instead, we hypothesize that many immune and inflammatory control mechanisms are likely altered in this mouse resulting in the failure to control inflammatory lesions, a phenotype that may become more accentuated with age. Taken together, we hypothesize that Metrnβ has both homeostatic and inflammatory functions. Its homeostatic functions, however, are likely to include the prevention of development of both immune responses and inflammation.
We therefore conclude that Metrnβ encodes a novel cytokine that we suggest should be called Metrnβ that is involved in immunity and inflammation. We hope that our results will encourage other studies that should expand and clarify the potential functional roles of Metrnβ in both innate and acquired immune responses.
Supplementary Material
Acknowledgments
This work was supported by a NIAID grant R21AI096278–01, and NIH NIAID AI120846 (to AZ).
Abbreviations used in this paper:
- BIGE
body index of gene expression
- Metrnβ
Meteorin-Beta
- Metrnl
Meteorin-like
- BMM
bone marrow derived macrophages
- LPS
lipopolysaccharide
- IP
intraperitoneally
- IL1RN
IL-1 receptor antagonist
References
- 1.Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, and Zlotnik A 2006. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7: 67–80. [DOI] [PubMed] [Google Scholar]
- 2.Catalan-Dibene J, Vazquez MI, Luu VP, Nuccio SP, Karimzadeh A, Kastenschmidt JM, Villalta SA, Ushach I, Pone EJ, Casali P, Raffatellu M, Burkhardt AM, Hernandez-Ruiz M, Heller G, Hevezi PA, and Zlotnik A 2017. Identification of IL-40, a Novel B Cell-Associated Cytokine. J Immunol 199: 3326–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, Schrumpf H, Valle-Rios R, Vazquez MI, Homey B, and Zlotnik A 2015. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol 156: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, and Spiegelman BM 2014. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li ZY, Zheng SL, Wang P, Xu TY, Guan YF, Zhang YJ, and Miao CY 2014. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther 20: 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Liu X, Zhang Y, Wang Z, Zhu G, Han G, Chen G, Hou C, Wang T, Ma N, Shen B, Li Y, Xiao H, and Wang R 2016. Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol 186: 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, Chen G, Hou C, Ma N, Shen B, Li Y, Egwuagu CE, and Wang R 2016. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol 46: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe K, Akimoto Y, Yugi K, Uda S, Chung J, Nakamuta S, Kaibuchi K, and Kuroda S 2012. Latent process genes for cell differentiation are common decoders of neurite extension length. J Cell Sci 125: 2198–2211. [DOI] [PubMed] [Google Scholar]
- 9.1997. Nomenclature for secreted regulatory proteins of the immune system (interleukins): update. IUIS/WHO Standing Committee on Interleukin Designation. Bull World Health Organ 75: 175. [PMC free article] [PubMed] [Google Scholar]
- 10.Howard M, Muchamuel T, Andrade S, and Menon S 1993. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med 177: 1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ushach I, and Zlotnik A 2016. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Italiani P, and Boraschi D 2014. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol 5: 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, and Schultze JL 2014. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40: 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li ZY, Song J, Zheng SL, Fan MB, Guan YF, Qu Y, Xu J, Wang P, and Miao CY 2015. Adipocyte Metrnl Antagonizes Insulin Resistance Through PPARgamma Signaling. Diabetes 64: 4011–4022. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SL, Li ZY, Song J, Liu JM, and Miao CY 2016. Metrnl: a secreted protein with new emerging functions. Acta Pharmacol Sin 37: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffman RL, Savelkoul HF, and Lebman DA 1989. Cytokine regulation of immunoglobulin isotype switching and expression. Semin Immunol 1: 55–63. [PubMed] [Google Scholar]
- 17.Zlotnik A, Shimonkevitz RP, Gefter ML, Kappler J, and Marrack P 1983. Characterization of the gamma-interferon-mediated induction of antigen-presenting ability in P388D1 cells. J Immunol 131: 2814–2820. [PubMed] [Google Scholar]
- 18.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, and O’Garra A 2016. Pillars Article: IL-10 Inhibits Cytokine Production by Activated Macrophages. J. Immunol. 1991. 147: 3815–3822. [PubMed] [Google Scholar]; J Immunol 197: 1539–1546. [Google Scholar]
- 19.Chen WF, and Zlotnik A 1991. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol 147: 528–534. [PubMed] [Google Scholar]
- 20.Li ZY, Fan MB, Zhang SL, Qu Y, Zheng SL, Song J, and Miao CY 2016. Intestinal Metrnl released into the gut lumen acts as a local regulator for gut antimicrobial peptides. Acta Pharmacol Sin 37: 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zlotnik A, and Yoshie O 2012. The chemokine superfamily revisited. Immunity 36: 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen D, Xiao Y, Vecchi MM, Gong BJ, Dolnikova J, and Pepinsky RB 2017. Determination of the Disulfide Structure of Murine Meteorin, a Neurotrophic Factor, by LC-MS and Electron Transfer Dissociation-High-Energy Collisional Dissociation Analysis of Proteolytic Fragments. Anal Chem 89: 4021–4030. [DOI] [PubMed] [Google Scholar]
- 23.Gong W, Liu Y, Wu Z, Wang S, Qiu G, and Lin S 2016. Meteorin-Like Shows Unique Expression Pattern in Bone and Its Overexpression Inhibits Osteoblast Differentiation. PLoS One 11: e0164446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouveia AI, Micaelo M, Pierdomenico F, and Freitas JP 2016. Schnitzler Syndrome: A Dramatic Response to Anakinra. Dermatol Ther (Heidelb) 6: 299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Launay D, Dutoit-Lefevre V, Faure E, Robineau O, Hauspie C, Sobanski V, Hachulla E, Labalette M, Hatron PY, and Dubucquoi S 2013. Effect of in vitro and in vivo anakinra on cytokines production in Schnitzler syndrome. PLoS One 8: e59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.