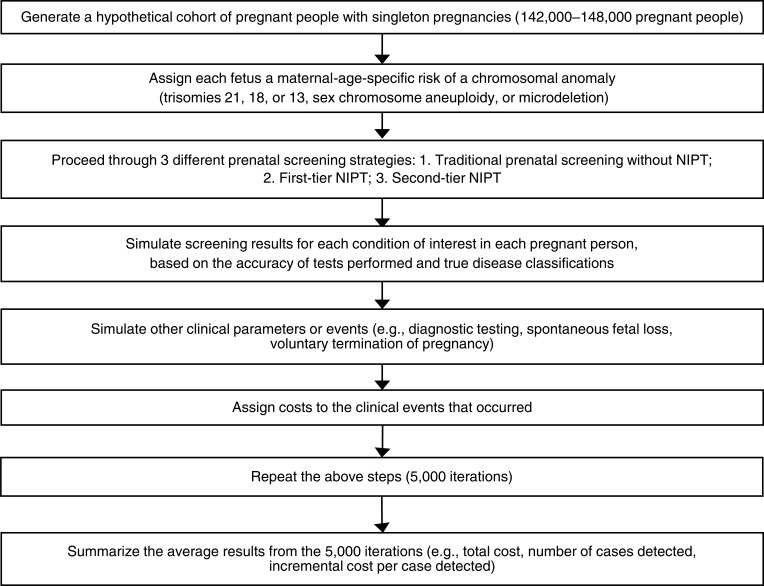
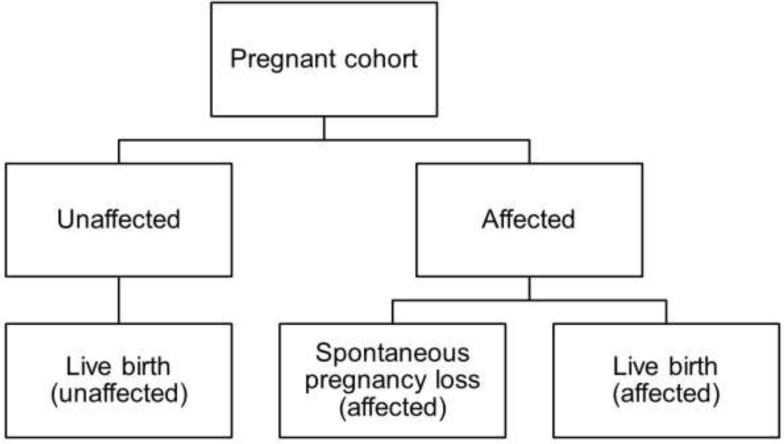
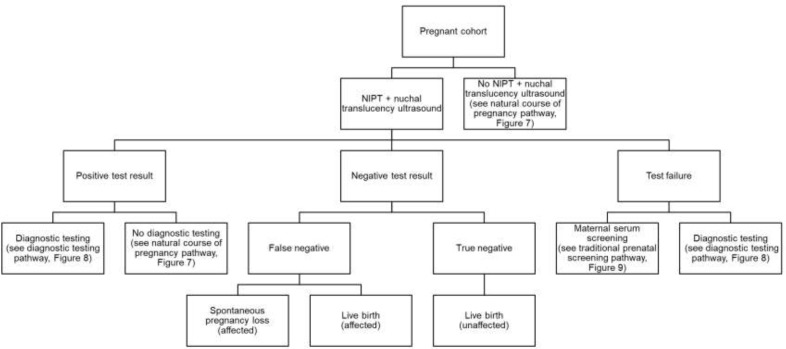
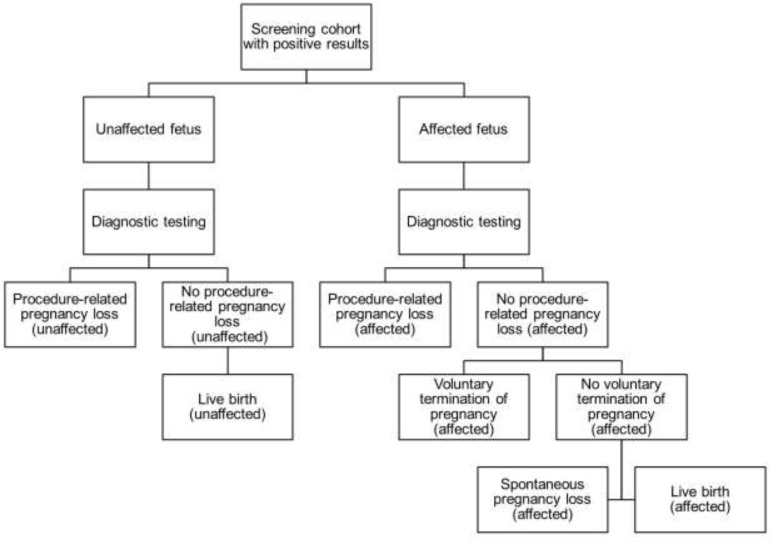
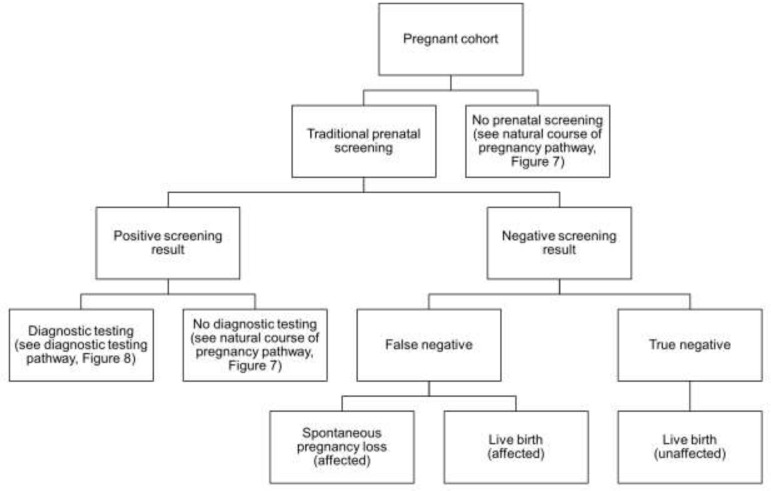
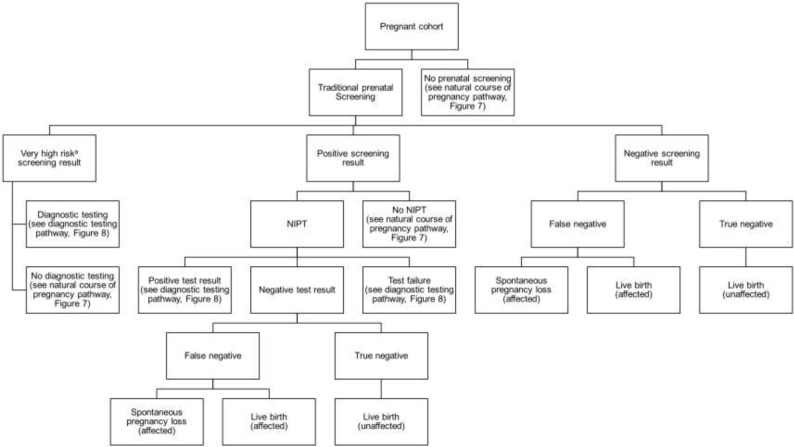
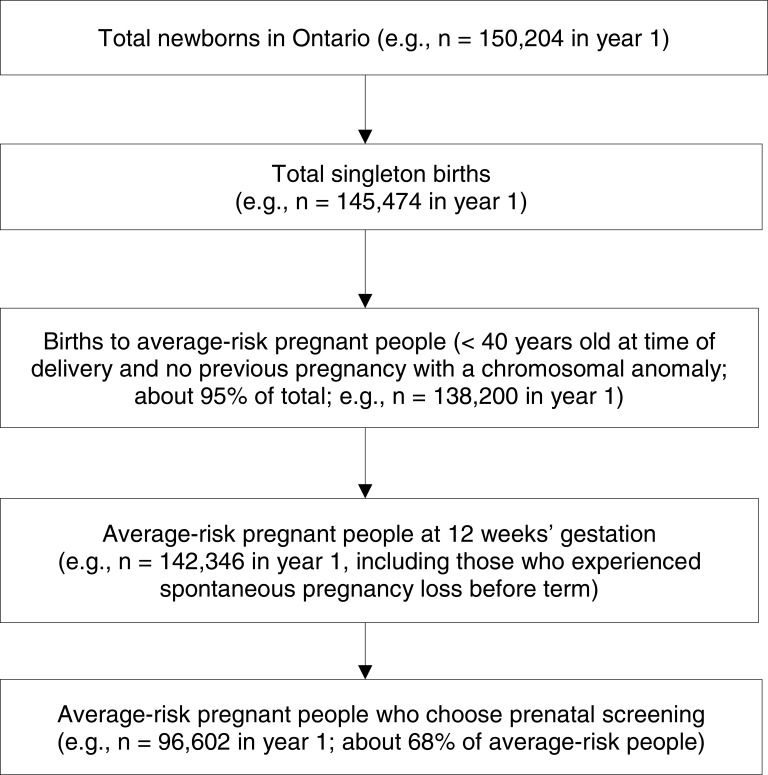
Abstract
Background
Pregnant people have a risk of carrying a fetus affected by a chromosomal anomaly. Prenatal screening is offered to pregnant people to assess their risk. Noninvasive prenatal testing (NIPT) has been introduced clinically, which uses the presence of circulating cell-free fetal DNA in the maternal blood to quantify the risk of a chromosomal anomaly. At the time of writing, NIPT is publicly funded in Ontario for pregnancies at high risk of a chromosomal anomaly.
Methods
We completed a health technology assessment, which included an evaluation of clinical benefits and harms, value for money, budget impact, and patient preferences related to NIPT. We performed a systematic literature search for studies on NIPT for trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions in the average-risk or general population. We evaluated the cost-effectiveness of traditional prenatal screening, NIPT as a second-tier test (performed after traditional prenatal screening), and NIPT as a first-tier test (performed instead of traditional prenatal screening). We also conducted a budget impact analysis to estimate the additional costs of funding first-tier NIPT. We interviewed people who had lived experience with NIPT and people living with the conditions NIPT screens for, or their families.
Results
The pooled clinical sensitivity of NIPT in the average-risk or general population was 99.5% (95% confidence interval [CI] 81.8%–99.9%) for trisomy 21, 93.1% (95% CI 75.9%–98.3%) for trisomy 18, and 92.7% (95% CI 81.6%–99.9%) for trisomy 13. The clinical specificity for any trisomy was 99.9% (95% CI 99.8%–99.9%). Compared with traditional prenatal screening, NIPT was more accurate in detecting trisomies 21, 18, and 13, and decreased the need for diagnostic testing. We found limited evidence on NIPT for sex chromosome aneuploidies or microdeletions in the average-risk or general population. Positive NIPT results should be confirmed by diagnostic testing.
Compared with traditional prenatal screening, second-tier NIPT detected more affected fetuses, substantially reduced the number of diagnostic tests performed, and slightly reduced the total cost of prenatal screening. Compared with second-tier NIPT, first-tier NIPT detected more affected cases, but also led to more diagnostic tests and additional budget of $35 million per year for average-risk pregnant people in Ontario.
People who had undergone NIPT were largely supportive of the test and the benefits of earlier, more accurate results. However, many discussed the need for improved pre- and post-test counselling and raised concerns about the quality of the information they received from health care providers about the conditions NIPT can screen for.
Conclusions
NIPT is an effective and safe prenatal screening method for trisomies 21, 18, and 13 in the average-risk or general population. Compared with traditional prenatal screening, second-tier NIPT improved the overall performance of prenatal screening and slightly decreased costs. Compared with second-tier NIPT, first-tier NIPT detected more chromosomal anomalies, but resulted in a considerable increase in the total budget. Interviewees were generally positive about NIPT, but they raised concerns about the lack of good informed-choice conversations with primary care providers and the quality of the information they received from health care providers about chromosomal anomalies.
OBJECTIVE
This health technology assessment looked at the test accuracy, clinical utility, cost-effectiveness, budget impact, and patient experiences of noninvasive prenatal testing (NIPT) for trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions.
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD42018086261), available at https://www.crd.york.ac.uk/PROSPERO.
BACKGROUND
Health Condition
Chromosomes consist of DNA and proteins and carry genetic information in the cells of living organisms. In humans, the correct number of chromosomes is 22 pairs of autosomes (chromosomes that are common to all humans, regardless of sex), plus a 23rd pair of chromosomes that determines biological sex (XX for females and XY for males).
Anyone who is pregnant has a small chance of having a baby with a chromosomal anomaly. Anomalies can include an incorrect number of chromosome copies (called chromosomal aneuploidies), and small missing pieces from chromosomes (called microdeletions). Table 1 lists the most common autosomal and sex chromosome aneuploidies and microdeletion syndromes.
Table 1:
Common Autosomal and Sex Chromosome Aneuploidies and Microdeletion Syndromes
| Condition | Common Name(s) | Estimated Prevalence in Newborns |
|---|---|---|
| Autosomal Chromosome Aneuploidies | ||
| Trisomy 21 | Down syndrome |
|
| Trisomy 18 | Edwards syndrome |
|
| Trisomy 13 | Patau syndrome |
|
| Sex Chromosome Aneuploidies | ||
| Monosomy X (45, X) | Turner syndrome |
|
| XXY syndrome (47, XXY) | Klinefelter syndrome |
|
| Triple X syndrome (47, XXX) | Trisomy X |
|
| XYY syndrome (47, XYY) | Jacob's syndrome |
|
| Microdeletion Syndromes | ||
| 15q11–q13 deletion (loss of function of active genes in regions on chromosome 15) | Prader-Willi syndrome |
|
| 15q11–q13 deletion (loss of function of gene UBE3A on chromosome 15) | Angelman syndrome |
|
| 22q11.2 deletion syndrome | DiGeorge syndrome |
|
| 5p deletion syndrome | Cri-du-chat syndrome |
|
| 1p36 deletion syndrome | Monosomy 1p36 |
|
Source: Adapted from the U.S. National Library of Medicine Genetics Home Reference.1
Chromosomal Aneuploidies
Chromosomal aneuploidies are errors in cell division that lead to trisomies (an extra, or third, copy of a chromosome) or monosomies (a lack of a copy of a chromosome).
Trisomies can occur as a result of:
Nondisjunction (chromosomes do not properly separate in the egg or sperm)
Translocation (part of a chromosome becomes attached to another during formation of the egg or sperm)
Mosaicism (an error in cell division during early development of the embryo, resulting in aneuploidy in some cells, but not all)
Inheritance from a carrier parent
Monosomies occur when a chromosome is partially or completely lost; they can also be mosaic.
The risk of autosomal aneuploidies increases with maternal age. These aneuploidies lead to genetic conditions that are associated with different levels of intellectual disability, developmental delay, dysmorphic features (i.e., differences in body structure), and impairments in body systems. In most cases, they also lead to a shorter life expectancy and increased risk of death. Affected fetuses may also have a higher risk of dying before they are born.
People typically have two sex chromosomes in each cell: females have XX and males have XY. Sex chromosome aneuploidies result from an incorrect number of X and/or Y chromosomes and result in phenotypic variability (i.e., differences in observable characteristics). Sex chromosome aneuploidies are not related to maternal age, except for X chromosome nondisjunction errors, which increase with maternal age. Some people with sex chromosome aneuploidies show few to no symptoms or signs and may never be diagnosed, leading to underdiagnosis and lower prevalence estimates for these conditions.
Microdeletions
Microdeletion syndromes occur when a small piece of a chromosome is deleted. Chromosomal deletions that involve more than 5 million base pairs (bases are the chemical building blocks of DNA; base pairs are two complementary bases that help connect two complementary strands of DNA) are usually visible under a microscope, but in the case of microdeletions, the changes in genetic material are too small (usually 1 to 3 million base pairs) to be seen without higher-resolution cytogenetic methods. The exact size and location of a clinically relevant microdeletion may vary, but usually a specific critical region is involved. Most phenotypic effects are due to the absence of a few critical genes, or in some cases, a single gene.
The risk of microdeletions is not related to maternal age. Microdeletions can be inherited, occur randomly during formation of the egg or sperm cells, or occur early in fetal development. Microdeletion syndromes are clinically recognized, and have distinct physical, behavioural, and mental characteristics. Similar to sex chromosome aneuploidies, microdeletion syndromes result in phenotypic variability depending on the location and length of the microdeletion.
Prenatal Screening Options
At the time of writing this report, screening for trisomy 21 and 18 is publicly funded in Ontario and available for all pregnant people through either enhanced first-trimester screening (eFTS) or maternal serum screening (MSS, also called quadruple screening; Table 2). The province has phased out previously offered screening options, including first-trimester screening (FTS), integrated prenatal screening (IPS), and serum integrated screening (SIPS).
Table 2:
Traditional Prenatal Screening and Diagnostic Options Available in Ontario
| Option | Components | Timing, weeks | Comments |
|---|---|---|---|
| Traditional Prenatal Screening Before 14 Weeks (First Trimester) | |||
| eFTS | One blood test Screens for trisomy 21 and trisomy 18, but not open neural tube defects NT ultrasound |
11–13 |
|
| Traditional Prenatal Screening After 14 Weeks (Second Trimester) | |||
| MSS (or quadruple screening) | One blood test Screens for trisomy 21, trisomy 18, and open neural tube defects NT ultrasound is integrated into the test, if available |
14–20 |
|
| Diagnostic Tests | |||
| CVS | — | 11–13 | — |
| Amniocentesis | — | Preferably 15–22 | — |
To generate an overall risk result for trisomy 21 or 18, eFTS and MSS may use a combination of maternal age, ultrasound measurements, and serum biomarkers. The sensitivity of eFTS for trisomy 21 is about 90% (specificity, about 95%).2 The sensitivity of MSS for trisomy 21 in the second trimester is 75% to 85% (specificity, 90% to 95%).3,4 The sensitivity and false-positive rates for trisomy 21 or 18 may differ slightly depending on the risk cutoff used (the threshold used to determine whether screening tests results are positive [high risk for a chromosomal anomaly] or negative [low risk for a chromosomal anomaly]).
Trisomy 13, sex chromosome aneuploidies, and microdeletion syndromes cannot be detected using the serum biomarkers that are part of traditional prenatal screening. However, because chromosomal anomalies usually lead to anatomical malformations, some may be detected during the 18- to 22-week fetal anatomical ultrasound that is part of standard obstetric care.
Results from prenatal screening cannot be used to diagnose a condition; they provide information about how likely a fetus is to have a chromosomal anomaly. To confirm that the fetus does have a chromosomal anomaly, diagnostic testing (chorionic villus sampling or amniocentesis) is needed. However, many pregnant people are uncomfortable with diagnostic testing because it is invasive, and because it is associated with physical discomfort and the risk (about 1% or less) of procedure-related pregnancy loss.6
Health Technology Under Review
In 2011, NIPT became clinically available in the United States and Hong Kong and changed the traditional prenatal screening paradigm. It offers earlier screening than traditional prenatal options, and if used early enough in pregnancy, can avoid the potential harms of invasive diagnostic testing.
Noninvasive prenatal testing is a DNA test of maternal blood that screens pregnancies for common chromosomal anomalies. It uses a blood sample to assess fragments of cell-free fetal DNA that are circulating in the maternal blood. The cell-free DNA in maternal blood consists of approximately 10% DNA from the placenta (so-called “fetal” DNA) and 90% DNA from maternal cells.7 Testing can be done as early as 9 to 10 weeks in pregnancy, but it can also be performed up to birth.8,9 The results of the test are usually available within 10 days (includes processing and shipment time).8,9
There are two main methods of NIPT. In massively parallel shotgun sequencing (MPSS), short stretches of randomly selected plasma DNA from both the mother and fetus are sequenced. Because the sequence of the entire genome is known, the chromosomal origin of each DNA fragment can be determined, and any small proportional increase in chromosomal fragments due to aneuploidies can be detected. In the targeted approach (either chromosome selective or single nucleotide polymorphism based), preselected DNA fragments from chromosomes of interest and a set of reference chromosomes are examined and quantified, instead of looking at all chromosomes.
An adequate amount of cell-free fetal DNA in the maternal blood (fetal fraction) is essential to obtain accurate test results. NIPT requires a minimum fetal fraction for a reportable result, commonly estimated to be around 3% to 4%.10 Fetal fraction typically increases with advancing gestational age. A failed test (i.e., a “no call” result) can also occur (1% to 8% chance), usually for one of three reasons: issues with blood collection or transportation, low fetal fraction, or assay (analytical test) failure.10 When results are not reported, indeterminate, or uninterpretable, it is recommended that the person repeat the test. If the test fails a second time, additional counselling and diagnostic testing are recommended because of an increased risk of aneuploidy.10
Different versions of NIPT may report cell-free DNA test results in different ways. Some report risk as “positive” or “negative,” while others use “>99%” as high risk and “<1/10,000” as low risk.10
Factors that influence NIPT accuracy and discordant or failed results include low fetal fraction, maternal body weight, fetal or maternal mosaicism, a vanishing twin (a fetus in a multiple-gestation pregnancy dies in utero and is partially or completely absorbed by the other fetus, placenta, or mother), or a maternal malignancy.11–13
Because NIPT is a maternal blood test, there is no risk of procedure-related pregnancy loss, similar to traditional prenatal screening. A negative NIPT result can allow pregnant people to avoid invasive diagnostic testing, which does carry a small risk of procedure-related pregnancy loss. As with any screening test, the potential disadvantages of NIPT include false-positive and false-negative results, although these rates are typically lower than with traditional prenatal screening (eFTS and MSS). Because NIPT is not a 100% accurate test, downstream harms can occur from subsequent diagnostic testing, which may either confirm or refute positive NIPT results. Negative NIPT results are typically not confirmed. According to clinical guidelines, a positive result from NIPT should be confirmed by diagnostic testing, such as amniocentesis or chorionic villus sampling, before any irrevocable action is taken.
Noninvasive prenatal testing can be used as a first-tier test (i.e., first screening test done) or as a second-tier test (i.e., test is done after positive results from traditional prenatal screening and before diagnostic testing). However, it is not a comprehensive prenatal testing option and cannot replace other aspects of prenatal screening. Ultrasound and other serum biomarkers that are part of traditional prenatal screening can detect conditions such as neural tube defects, other fetal structural abnormalities, and placental dysfunction.
Regulatory Information
At the time of writing, NIPT consists of laboratory-developed tests and are therefore outside the regulatory framework of Health Canada and the United States Food and Drug Administration. Test manufacturers can, however, voluntarily submit applications for approval consideration. In the United States, certification of the performing laboratory is required under Clinical Laboratory Improvement Amendments regulations to ensure the quality and validity of the test.14
Ontario, Canadian, and International Context
Ontario Context
In 2017, 14,217 NIPT tests were performed in Ontario, and 67.5% of those were publicly funded for high-risk pregnant people (9,593 tests; Better Outcomes Registry and Network [BORN] Ontario, March 22, 2018). Noninvasive prenatal testing is publicly funded for pregnant people at high risk for chromosome 21, 18, and 13, and sex chromosome aneuploidies in Ontario under two categories (testing for microdeletions is private-pay only). Any physician may order publicly funded NIPT for people in Category I. Category II is for situations in which additional specialist consultation is necessary to determine whether NIPT is warranted, and to provide appropriate pre- and post-test counselling. Testing for people in Category II must be ordered by a geneticist or maternal-fetal medicine specialist. People must meet any one of the following criteria in either category to be eligible to receive NIPT as a publicly funded test.15
-
Category I (can be ordered by any physician)
-
○
Maternal age ≥ 40 years at time of delivery
-
○
Positive maternal serum screen result
-
○
Nuchal translucency ≥ 3.5 mm on ultrasound
-
○
Previous pregnancy with a chromosome anomaly
-
○
-
Category II (must be ordered by a genetics or maternal-fetal medicine specialist)
-
○
Fetal congenital anomalies identified on ultrasound that are suggestive of trisomy 21, 18, or 13
-
○
Risk of aneuploidy for trisomies 21, 18, and 13 other than positive maternal multiple marker screen
-
▪
If maternal age is < 40 years at expected date of delivery, must have at least one other risk factor noted
-
▪
Risk of aneuploidy can be calculated by including any combination of risk indicators, including soft markers, biochemistry, maternal age, etc.
-
▪
-
○
NIPT for sex chromosome determination for at least one of the following:
-
▪
Risk of sex-limited disorder
-
▪
Ultrasound findings suggestive of a sex chromosome aneuploidy
-
▪
Ultrasound findings suggestive of a disorder of sex determination
-
▪
-
○
At the time of writing this report, only two tests are available for publicly funded NIPT in Ontario: Panorama and Harmony. LifeLabs Genetics offers Natera's Panorama Prenatal Test. Dynacare offers Ariosa Diagnostics’ (owned by Hoffmann-La Roche) Harmony test (Table 3).
Table 3:
Characteristics of the Panorama and Harmony Noninvasive Prenatal Tests
| Test Characteristic | Panorama Prenatal Test | Harmony Prenatal Test |
|---|---|---|
| Manufacturer | Natera | Ariosa Diagnostics (Roche) |
| Laboratory availability | LifeLabs | Dynacare |
| Pregnancy population | Can be performed in twin pregnancies, consanguineous couples, and singleton pregnancies conceived by in vitro fertilization, including egg-donor and surrogate pregnancies Should not be performed in people who have received a bone marrow transplant |
Can be performed in twin pregnancies, consanguineous couples, and singleton pregnancies conceived by in vitro fertilization, including egg-donor and surrogate pregnancies Should not be performed in people who have received a bone marrow or organ transplant, or in people who have metastatic cancer |
| Analysis method | Targeted approach Single nucleotide polymorphism |
Targeted approach Microarray-based digital analysis of selected regions (DANSR) |
| Sensitivity reported by manufacturer | Trisomy 21: >99.9% Trisomy 18: 98.2% Trisomy 13: >99% Monosomy X: 92.9% 22q11.2 deletion syndrome: 90% Microdeletion extended panel (1p36 deletion, 15q11–q13 deletions [Angelman syndrome and Prader-Willi syndrome], 5p deletion): 93.8% to >99% |
Trisomy 21: >99% Trisomy 18: 97.4% Trisomy 13: 93.8% Monosomy X: 94.3% |
| False-positive rate reported by manufacturera | Trisomy 21: 0% Trisomy 18: <0.1% Trisomy 13: 0% Monosomy X: <0.1% Each microdeletion syndrome: <1% |
Trisomy 21: <0.1% Trisomy 18: <0.1% Trisomy 13: <0.1% |
| Test timing | As early as 9 weeks of pregnancy | As early as 10 weeks of pregnancy |
| Time to result | Within 10 days | Within 10 business days |
| Out-of-pocket test costb | Trisomies 21, 18, and 13, and sex chromosome aneuploidies: $550 Plus 22q11.2 deletion: additional $195 Plus microdeletion extended panel: additional $245 |
Trisomies 21, 18, and 13, and sex chromosome aneuploidies: $495 Plus 22q11.2 deletion: additional $175 |
Systematic reviews suggest a higher false-positive rate than those reported by the manufacturers.16–19 The false-positive rate is also not equivalent to the positive predictive value, which is defined as the probability of a true positive given a positive test result.
Out-of-pocket test cost as of April 2018.
Each laboratory offers a special requisition form for ministry-funded NIPT, which must be completed by a physician to be eligible. Average-risk patients (patients who are not high risk and therefore do not meet the ministry's eligibility criteria) must pay out of pocket for either test. Both tests offer detection of fetal aneuploidies for chromosomes 21, 18, and 13, and the sex chromosomes (with sex determination as an option at no additional cost). Natera's Panorama test offers testing for a panel of five microdeletions (22q11.2 deletion, 1p36 deletion, 15q11–q13 deletions [Angelman syndrome and Prader-Willi syndrome], and 5p deletion). In June 2017, Roche announced the addition of 22q11.2 deletion testing as an option for the Harmony test.20 At the time of writing, Roche has also submitted an application for Health Canada approval of the Harmony test (Roche Diagnostics, November 12, 2018). Neither the Harmony nor the Panorama test is advisable for vanishing twin pregnancies.
Noninvasive prenatal testing is publicly funded only for pregnant people at high risk for fetal anomalies, so cost is one of the main barriers to accessing the test for people at average risk. Because NIPT can be performed earlier than any other traditional prenatal screening option (for which the earliest is currently 11 weeks), earlier access to results can allow parents more time to prepare (e.g., to look after an affected child) and to make other decisions about the pregnancy.21 People who pay out of pocket for NIPT may also make subsequent use of public health care resources such as physician visits, genetic counselling, confirmatory diagnostic testing, and other prenatal services, leading to preferential earlier access to related prenatal services.21
Canadian Context
In Canada, the following provinces and territories have some confirmed public funding for NIPT: British Columbia, Manitoba, Nova Scotia, Nunavut, Ontario, Prince Edward Island, and Yukon. Quebec announced the decision to fund NIPT for high-risk pregnant people in early 2018.22 Appendix 1 lists the funding status of NIPT in Canadian jurisdictions, according to a 2018 environmental scan.23
International Context
In the United States, most pregnant people at high risk for fetal aneuploidy are covered for NIPT by commercial and/or public insurance plans. Some insurance companies, including Blue Cross Blue Shield and Cigna, have expanded their coverage to all pregnant people, although the patient typically still bears a portion of the cost.24
In January 2016, the United Kingdom National Screening Committee recommended an evaluative implementation of NIPT to assess its impact on the existing National Health System Fetal Anomaly Screening Program.25 The proposed change was to offer NIPT to pregnant people at higher risk following traditional prenatal screening (risk score ≥ 1/150 for trisomy 21; combined risk score ≥ 1/150 for trisomies 18 and 13). The committee recommended screening with NIPT for high-risk pregnant people because of the high accuracy of NIPT and the potential to avoid diagnostic testing.
In Europe, a number of countries (Denmark, France, the Netherlands, and Switzerland) fund NIPT as a second-tier (contingent) test.17 At the time of writing, Belgium and the Netherlands are the only countries to publicly fund NIPT as a first-tier test (Roche Diagnostics, November 12, 2018). Any physician can order NIPT in Belgium, although it is reimbursed only from 12 weeks’ gestation onward.26,27
Values and Preferences
In general, pregnant people have supported NIPT as a positive development in prenatal care.28,29 In studies from the Netherlands, the United Kingdom, and the United States, pregnant people have said that they prefer NIPT over traditional prenatal screening or diagnostic testing because of its accuracy, early timing, ease of testing, safety, and comprehensive information.28,30–33 People who had NIPT expressed satisfaction with the test and low decisional regret.33,34
The values and preferences of pregnant people may be different from those of health care providers. For example, in Canada, pregnant people placed greater value on test safety and the comprehensiveness of information, while health care providers placed greater value on accuracy and the timing of the results.35
Concerns have also been raised about informed decision-making and consent. Because NIPT is a convenient blood test, its importance and impact may not be accurately conveyed to patients to allow for proper informed consent (i.e., “routinization” of NIPT).29 There is also concern that the ease of testing may lead to increased pressure to test, and to terminate affected pregnancies, possibly leading to stigma and discrimination against people with disabilities and their families, as well as the belief that fetuses with chromosomal anomalies should be screened out of the population (i.e., eugenic social attitudes).29,31,36 Another concern is using NIPT to screen for even more genetic conditions with variable phenotype without having adequate informed-choice conversations with pregnant people and their families.”
Guidelines
Numerous guidelines have provided recommendations on the use of NIPT from different disciplines, such as gynecology and obstetrics, medical genetics, and genetic counselling (Appendix 2). The Society of Obstetricians and Gynaecologists of Canada and the Canadian College of Medical Geneticists published an update in 2017 on prenatal screening for fetal aneuploidy, which notes that NIPT is a highly effective screening test for trisomies 21, 18, and 13, and should be offered as a possible screening option where available in Canada (or with the understanding that it may not be publicly funded).37
Some guidelines acknowledge that NIPT is an effective screening strategy as a second-tier test, but many have commented on the lack of data for NIPT as a first-tier test in the general population. None of the guidelines recommend NIPT as a first-tier screening test for sex chromosome aneuploidies or microdeletion syndromes. Other common themes in the guideline recommendations include the importance of patient choice for prenatal screening or testing, obtaining informed consent, and appropriate counselling on prenatal testing and the possible test results. A guideline on best ethical practices for clinicians who provide NIPT, and for manufacturers who offer NIPT, was published in 2013.38
Systematic Reviews
A number of systematic reviews and meta-analyses have been conducted on the accuracy of NIPT for trisomies 21, 18, and 13, and sex chromosome aneuploidies, but most have focused on the high-risk population, where much of the published literature exists.39–43 Only a few have performed analyses for the average-risk or general population (see Appendix 3 for a summary).16–19 These systematic reviews differed in their definitions of the general pregnancy population, and some focused only on trisomies 21, 18, and 13, while others also included sex chromosome aneuploidies.
We found no systematic reviews that included test accuracy for microdeletion syndromes, and none that specifically examined the clinical utility of NIPT. As well, we did not find a health technology assessment that addressed all of our research questions. Because the systematic reviews did not fit our specific purpose in this health technology assessment, and no other fully relevant health technology was found, we undertook our own review of primary studies.
Terminology
In this health technology assessment, average risk and high risk refer to pregnancies at average or high risk for a chromosomal anomaly (not risk of pregnancy complications).
CLINICAL EVIDENCE
Research Questions
-
1.
What is the test accuracy and clinical and personal utility of noninvasive prenatal testing (NIPT) for trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions in the average-risk or general population?
-
2.
What is the comparative accuracy between different NIPT methods in the average-risk or general population?
-
3.
What is health care providers’ understanding of NIPT?
Methods
We developed the research questions in consultation with health care providers, clinical experts, and other health system stakeholders.
Clinical Literature Search
We performed a literature search on September 11, 2017, to retrieve studies published from January 1, 2007, to the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, and National Health Service Economic Evaluation Database (NHS EED). We used the EBSCOhost interface to search the Cumulative Index to Nursing & Allied Health Literature (CINAHL).
Medical librarians developed the search strategy using controlled vocabulary (i.e., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.44 We created database auto-alerts in MEDLINE, Embase, and CINAHL and monitored them until April 2018.
We performed targeted grey literature searching of health technology assessment agency sites and clinical trial registries. See Appendix 4 for literature search strategies, including all search terms.
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using DistillerSR management software, and then obtained the full text of studies that appeared eligible for the review according to the inclusion criteria. The author then examined the full-text articles and selected studies that were eligible for inclusion. We also examined reference lists for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
Studies published between January 1, 2007, and September 11, 2017
Randomized controlled trials, cohort studies, or case-control studies
Comparative and noncomparative test accuracy studies and clinical utility studies on NIPT for the average-risk or general population
Comparative test accuracy studies of two different methods of NIPT in the average-risk or general population
Studies on health care providers’ understanding of NIPT
Exclusion Criteria
Studies including mixed-risk pregnant people (i.e., mixed average-risk and high-risk that were not representative of a general population)
Systematic reviews, meta-analyses, editorials, case reports, conference abstracts, or commentaries
Animal and in vitro studies
Studies where outcomes of interest could not be extracted (e.g., incomplete 2 × 2 table for test accuracy)
Outcomes of Interest
-
NIPT accuracy
-
○
Sensitivity (i.e., the true positive rate, or the probability of correctly identifying an affected fetus) and specificity (i.e., the true negative rate, or the probability of correctly identifying an unaffected fetus)
-
○
Positive predictive value (i.e., the probability that someone with a positive test result truly has an affected fetus), negative predictive value (i.e., the probability that someone with a negative test result truly does not have an affected fetus)
-
○
Test failures and inconclusive results, and associated true results
-
○
-
NIPT clinical utility
-
○
Reductions in diagnostic testing (diagnostic tests avoided)
-
○
Diagnostic-testing-related adverse events for the pregnant person or fetus
-
○
Differences in pregnancy outcomes
-
○
Test turnaround time
-
○
Uptake rate of NIPT (percentage of people who choose to receive NIPT)
-
○
Health care providers’ understanding of NIPT
-
○
-
NIPT personal utility
-
○
Differences in pregnant people's pregnancy decision-making
-
○
Maternal or parental psychological effects (e.g., depression, anxiety)
-
○
Maternal/parental education and satisfaction
-
○
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information about the following:
Study characteristics (e.g., study primary author, year, country, source of funding, study sponsors)
Methods (e.g., study design, inclusion/exclusion criteria, patient characteristics, length of follow-up; details on the index test, comparator, and reference standard)
Outcomes (e.g., outcomes measured, number of participants for each outcome, outcome definition, time points at which the outcome was assessed, covariates considered, loss to follow-up, and associated reasons)
We contacted authors of the studies to provide clarification as needed.
Statistical Analysis
We conducted statistical analyses for test accuracy using Stata version 13 (Stata Statistical Software, StataCorp, College Station, Texas). We used a bivariate random-effects model to pool study sensitivities and specificities and their corresponding 95% confidence intervals. If the model failed to converge, we used two univariate random-effects models, as recommended by Takwoingi et al.45 We created forest plots for NIPT accuracy using Review Manager version 5.3.46 Where meta-analysis was not appropriate because of clinical or statistical heterogeneity, we undertook a narrative synthesis of the results.
Critical Appraisal of Evidence
We assessed risk of bias for NIPT accuracy using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (Appendix 5).47 For studies that reported patient clinical utility outcomes, we used the Cochrane Risk of Bias tool48 for randomized controlled trials and the Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I) tool for nonrandomized studies.49
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.50 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality score reflects our assessment of the reliability of the evidence.
Expert Consultation
We solicited expert feedback on NIPT. The consultation included experts in medical genetics, fetal medicine, primary care, genetic counselling, prenatal health care services, laboratory medicine, methodology, and industry. The role of the expert advisers was to help define the scope and research question, contextualize the evidence, review the draft report, and provide advice on NIPT and its use in Ontario.
Results
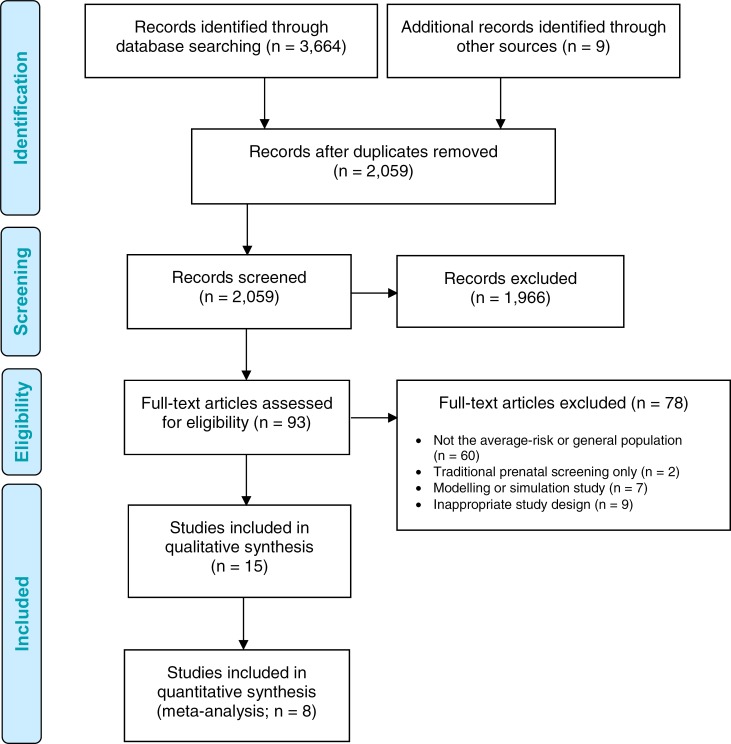
Literature Search
The literature search yielded 2,059 citations published between January 1, 2007, and September 11, 2017, after removing duplicates. We identified nine citations through other sources: eight from the grey literature during the literature search, and one from experts after the search date.
Eight studies on test accuracy and clinical utility met the inclusion criteria. Another seven survey studies on health care providers’ understanding of NIPT were found. We included the additional study identified after the search date.
We found no studies on the accuracy or utility of NIPT for microdeletions or on the comparative test accuracy of NIPT methods in the average-risk or general pregnant population. We found no studies that reported on clinical outcomes for affected infants for any of the chromosomal anomalies of interest, diagnostic-testing-related adverse events for the pregnant person or fetus, or differences in the psychological effects of NIPT for the average-risk or general population.
Figure 1 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.51
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Characteristics of Included Primary Studies
Tables 4 and 5 summarize the characteristics of the included primary studies. We found eight test accuracy studies,52–59 five52,54,56,58,59 of which were comparative and included a traditional prenatal screening option. However, the type of traditional prenatal screening varied across studies, as did the cutoffs to categorize results as high-risk or average-risk. Only one study assessed maternal satisfaction and education.57 Another seven explored health care providers’ understanding of NIPT.60–66
Table 4:
Characteristics of Included Studies on NIPT Accuracy or Clinical Utility
| Author, Year, Country | N | Study Design | Inclusion Criteria | Conditions Tested | NIPT Method, Index Test | Reference Standard(s) | Study Funding |
|---|---|---|---|---|---|---|---|
| Comparative Studies | |||||||
| Bianchi et al, 2014,52 United States | 2,052 | Prospective, comparative | Pregnant people ≥ 18 years old, ≥ 8 weeks’ gestation Exclusion: people who had diagnostic testing within 2 weeks prior to enrolment, or had only NT ultrasound |
Trisomies 21, 18, and 13 | Verifi test, MPSS | Fetal karyotype Neonate examination Birth medical record |
Illumina, Inc. |
| Langlois et al, 2017,54 Canada | 1,165 | Prospective, comparative | Pregnant people ≥ 19 years old, singleton pregnancy, < 14 weeks’ gestation | Trisomies 21, 18, and 13 | Harmony test, targeted approach (DANSR) | Fetal karyotype Neonate examination Birth medical record |
Genome Canada, Quebec, British Columbia, and Alberta Quebec ministry Ariosa Diagnostics (arms’ length) |
| Norton et al, 2015,56 International | 18,955 | Prospective, comparative | Pregnant people ≥ 18 years old, 10–14 weeks’ gestation Exclusion: people who had a pregnancy loss, or chose to terminate with confirmatory diagnostic testing |
Trisomies 21, 18, and 13, other aneuploidies | Harmony test, targeted approach (DANSR) | Fetal karyotype Neonate examination Birth medical record |
Ariosa Diagnostics Perinatal Quality Foundation |
| Quezada et al, 2015,58 United Kingdom | 2,905 | Prospective, comparative | Pregnant people 10–11 weeks’ gestation, singleton pregnancy, who had combined test | Trisomies 21, 18, and 13 | Harmony test, targeted approach (DANSR) | Fetal karyotype | Fetal Medicine Foundation |
| Song et al, 2013,59 China | 1,916 | Prospective, comparative | Pregnant people < 35 years old, singleton pregnancies No a priori risk of aneuploidy and undergoing routine prenatal screening |
Trisomies 21, 18, and 13, sex chromosome aneuploidies | Illumina HiSeq platform, MPSS | Fetal karyotype Cord blood Birth medical record |
Chinese National Key Technology Research and Development Program |
| Noncomparative Studies | |||||||
| del Mar Gil et al, 2014,53 United Kingdom | 207 | Retrospective, noncomparative | Pregnant people without a priori risk, undergoing first-trimester screening, twin pregnancies, 11–13 weeks’ gestation | Trisomies 21, 18, and 13 | Harmony test, targeted approach (DANSR) | Fetal karyotype | Fetal Medicine Foundation Ariosa Diagnostics (provided test and analysis only) |
| Nicolaides et al, 2012,55 United Kingdom | 2,230 | Retrospective, noncomparative | Pregnant people 11–13.9 weeks’ gestation, singleton pregnancy, archived sample ≥ 2 mL | Trisomies 21 and 18 | Harmony test, targeted approach (DANSR) | Fetal karyotype Neonate examination |
Fetal Medicine Foundation Ariosa Diagnostics (provided test and analysis only) |
| Palomaki et al, 2017,57 United States | 2,691 | Prospective, noncomparative | Pregnant people ≥ 10 weeks’ gestation, satisfy inclusion criteria for Panorama test | Trisomies 21, 18, and 13, monosomy X | Panorama test, targeted approach (single nucleotide polymorphism based) | Fetal karyotype Newborn karyotype and examination |
Natera, Inc. (no involvement) |
Abbreviations: DANSR, Digital Analysis of Selected Regions; MPSS, massively parallel shotgun sequencing; NIPT, noninvasive prenatal testing; NT, nuchal translucency.
Table 5:
Characteristics of Included Studies on Health Care Professionals’ Understanding of NIPT
| Author, Year, Country | N | Response Rate | Survey Method | Health Care Providers | Study Funding |
|---|---|---|---|---|---|
| Brewer et al, 2016,60 United States | 103 | 20.6% | Online survey sent to health care providers within a database for qualitative and quantitative research | Obstetricians | Ariosa Diagnostics |
| Filoche et al, 2017,61 New Zealand | 134 | 32% | Online survey sent to New Zealand–based Royal Australian and New Zealand Committee of Obstetricians and Gynaecologists members | Obstetricians and gynecologists | Lotteries Health Research–Te Tahua Rangahau Hauoratanga |
| Haymon et al, 2014,62 United States | 278 | 18.5% | Online survey sent to database of maternal–fetal medicine specialists | Maternal–fetal medicine specialists | Northwestern University Genetic Counseling Graduate Program, Center for Genetic Medicine of the Feinberg School of Medicine |
| Mayes et al, 2016,63 United States | 985 | 78% | Survey of physicians in the obstetrical department at University of Texas Graduate School of Biomedical Science | Obstetricians and gynecologists | Not reported |
| Musci et al, 2013,64 United States | 101 | 11.2% | Online survey sent to database of 900 obstetrician-gynecologists | Obstetricians and gynecologists | Ariosa Diagnostics |
| Sayres et al, 2011,65 United States | 62 | 34% | Paper survey distributed at continuing education course on obstetrics and gynecology | Obstetricians and gynecologists | Center for Integrating Ethics and Genetics Research grant |
| Swaney et al, 2016,66 United States | 160 | 42.3% | Online survey to database of United States maternal–fetal medicine fellows who are members of the Society of Maternal-Fetal Medicine | Maternal–fetal medicine fellows | None |
Abbreviation: NIPT, noninvasive prenatal testing.
The test accuracy studies included pregnant people of different gestational ages. Song et al59 followed pregnant people < 35 years of age. Six studies followed a general unselected population (without a priori risk).52–56,58 Palomaki et al57 included a general pregnancy primary screening population, but a small number of participants were considered to be high-risk. The authors noted that this composition would reflect a routinely screened general population.
Versions of NIPT varied between studies, with most using the Harmony test. Two studies included NIPT versions that are not available in Canada: the Verifi prenatal test and a version of the Illumina platform.52,59 The reference standard for all test accuracy studies included karyotyping from diagnostic testing (chorionic villus sampling or amniocentesis). Negative screening results were followed up by neonate examination, cord blood, or the birth medical record.
Two of the test accuracy studies were funded by industry.52,56 An additional four accuracy studies involved industry funds (in most cases for the cost of the NIPT testing and result analysis), but stated that industry was not involved in conducting the study.53–55,57
Among the test accuracy studies, risk of bias was often high because of concerns relating to patient selection and flow and timing. Studies were often unclear about the method of patient enrolment (e.g., whether enrolment was random or consecutive), and did not always include all patients in the analyses. The full risk-of-bias assessment can be found in Appendix 5.
We found seven studies evaluating health care providers’ knowledge of NIPT.60–66 All studies were cross-sectional surveys and were conducted in the United States, except for one study that was from New Zealand.61 All studies evaluated the knowledge of obstetricians, gynecologists, or maternal–fetal medicine specialists.
NIPT Accuracy
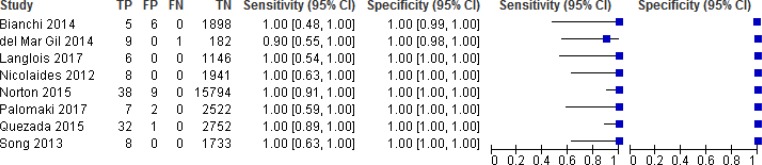
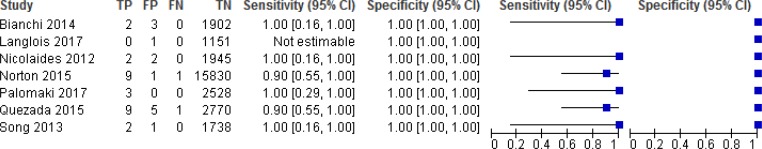
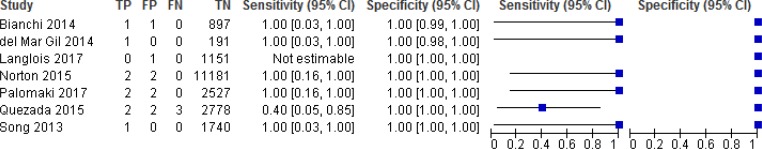
NIPT Accuracy for Trisomies 21, 18, and 13
NIPT accuracy was evaluated in 8 studies (Figures 2, 3, and 4).52–59 Sensitivity ranged from 90% to 100% for trisomy 21, 90% to 100% for trisomy 18, and 40% to 100% for trisomy 13. Sensitivity was most consistent for trisomy 21; ranges in sensitivity for trisomies 18 and 13 were more variable across studies. Because of the low prevalence of the three conditions, sensitivity was greatly influenced by the number of (rare) false negatives. Specificity for any of the three trisomies remained high at ≥ 99.9%, indicating the ability of NIPT to accurately identify pregnant people who have an unaffected fetus. The reference standards used in the studies included fetal karyotype, neonate examination, cord blood, and birth records.
Figure 2: NIPT Accuracy for Trisomy 21.
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; NIPT, noninvasive prenatal testing; TN, true negative; TP, true positive.
Figure 3: NIPT Accuracy for Trisomy 18.
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; NIPT, noninvasive prenatal testing; TN, true negative; TP, true positive.
Figure 4: NIPT Accuracy for Trisomy 13.
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; NIPT, noninvasive prenatal testing; TN, true negative; TP, true positive.
The pooled sensitivities for NIPT were 99.5% (95% confidence interval [CI], 81.8%–99.9%) for trisomy 21, 93.1% (95% CI, 75.9%–98.3%) for trisomy 18, and 92.7% (95% CI, 81.6%–99.9%) for trisomy 13. The pooled specificity for all three trisomies was 99.9% (95% CI, 99.8%–100%).
While test accuracy studies generally start at high GRADE, we downgraded the evidence in the risk of bias domain for trisomies 21, 18, and 13 because of concerns with patient selection and flow and timing in most studies. Many of the studies did not specify whether patients were consecutively or randomly enrolled or did not include all patients in the analysis. For trisomy 18 and 13, we further downgraded the GRADE because of imprecision.
The positive predictive value ranged from 45.5% to 100% for trisomy 21, 40% to 100% for trisomy 18, and 50% to 100% for trisomy 13 for the individual studies. The negative predictive value in all studies was 99.9% to 100% for all three trisomies.
NIPT Accuracy for Trisomies 21, 18, and 13 Versus Traditional Prenatal Screening
Five studies compared NIPT with traditional prenatal testing (Table 6).52,54,56,58,59 We did not pool the results because of differences in traditional prenatal testing options (e.g., different timing of screening and serum biomarkers) and thresholds used. Risk thresholds for positive results with traditional prenatal screening were variable in two studies, determined by either individual laboratories52 or provincial programs.54 The other two studies had predetermined thresholds: 1:270 for all conditions in Song et al59 and 1:270 for trisomy 21 and 1:150 for trisomies 18 and 13 in Norton et al.56 However, despite these differences in traditional prenatal screening, NIPT was more accurate (improved specificity, and in almost all studies, also higher sensitivity) than traditional prenatal screening in detecting trisomies 21, 18, and 13 in all studies. Song et al59 did not report separate accuracy results for trisomy 21, 18, of 13, instead reporting a combined result. The reference standards used were fetal karyotype, cord blood, or birth medical record.
Table 6:
NIPT Accuracy for Trisomies 21, 18, and 13 Versus Traditional Prenatal Screening
| Author, Year | Traditional Prenatal Screening Method | Traditional Trisomy 21 (95% CI) | NIPT Trisomy 21 (95% CI) | Traditional Trisomy 18 (95% CI) | NIPT Trisomy 18 (95% CI) | Traditional Trisomy 13 (95% CI) | NIPT Trisomy 13 (95% CI) |
|---|---|---|---|---|---|---|---|
| Bianchi et al, 201452 | First-trimester PAPP-A and total hCG or β-hCG Second-trimester α-fetoprotein, hCG, unconjugated estriol, inhibin-A, with or without NT |
Sensitivity: 100% (29.2%–100%) FPR: 3.6% (2.8%–4.6%) |
Sensitivity: 100% (47.8%–100%) FPR: 0.3% (0.1%–0.7%) |
Sensitivity: 100% (2.5%–100%) FPR: 0.6% (0.1%–0.3%) |
Sensitivity: 100% (15.8%–100%) FPR: 0.2% (0%–0.4%) |
Not reported | Sensitivity: 100% (20.7%–100%) FPR: 0.2% (0.1%–0.5%) |
| Langlois et al, 201754 | First-trimester PAPP-A, free β-hCG, NT First-trimester PAPP-A, second-trimester α-fetoprotein, hCG, unconjugated estriol, inhibin-A, with or without NT |
Sensitivity: 83% (36%–99%) FPR: 5.4% (4.2%–6.9%) |
Sensitivity: 100% (54%–100%) FPR: 0% (0%–0.3%) |
Not reported | Not reported | Not reported | Not reported |
| Norton et al, 201556 | PAPP-A, total hCG or β-hCG, NT | Sensitivity: 78.9% (62.7%–90.4%) FPR: 5.4% (5.1%–5.8%) |
Sensitivity: 100% (90.7%–100%) FPR: 0.06% (0.03%–0.11%) |
Sensitivity: 80.0% (44.4%–97.5%) FPR: 0% (0%–0.1%) |
Sensitivity: 90% (55.5%–99.7%) FPR: 0.3% (0.2%–0.4%) |
Sensitivity: 50% (1.2%–98.7%) FPR: 0.3% (0.2%–0.4%) |
Sensitivity: 100% (15.8%–100%) FPR: 0% (0%–0.1%) |
| Quezada et al, 201558 | PAPP-A, β-hCG, fetal crown-rump length, NT | Sensitivity: 100% (93%–100%) FPR: 4% (4%–5%) |
Sensitivity: 100% (89.3%–100%) FPR: 0.1% (0%–0.2%) |
Sensitivity: 100% (93%–100%) FPR: 4% (4%–5%) |
Sensitivity: 90% (59.6%–98.2%) FPR: 0.2% (0.1%–0.4%) |
Sensitivity: 100% (93%–100%) FPR: 4% (4%–5%) |
Sensitivity: 40% (11.8%–76.9%) FPR: 0.1% (0%–0.1%) |
| Song et al, 201359 | Second-trimester α-fetoprotein, free β-hCG, unconjugated estriol | Sensitivity: 55% (23%–83%)a FPR: 14% (12%–16%) |
Sensitivity: 100% (59.8%–100%) FPR: 0% (0%–0.3%) |
Sensitivity: 55% (23%–83%)a FPR: 14% (12%–16%) |
Sensitivity: 100% (19.8%–100%) FPR: 0.1% (0.1%–0.4%) |
Sensitivity: 55% (23%–83%)a FPR: 14% (12%–16%) |
Sensitivity: 100% (5.5%–100%) FPR: 0% (0%–0.3%) |
Abbreviations: CI, confidence interval; FPR, false-positive rate; hCG, human chorionic gonadotropin; NIPT, noninvasive prenatal testing; NT, nuchal translucency; PAPP-A, pregnancy-associated plasma protein A.
Combined test accuracy for trisomy 21, 18, and 13.
NIPT Accuracy for Sex Chromosome Aneuploidies
One study evaluated the accuracy of NIPT for monosomy X and XXY syndrome in addition to trisomies 21, 18, and 13.59 For monosomy X, sensitivity was 50% (95% CI, 9.2%–90.8%) and the false-positive rate was 0% (95% CI, 0%–0.1%).59 For XXY syndrome, the false-positive rate was 0.1% (95% CI, 0.1%–0.4%), but sensitivity could not be determined because no true XXY syndrome cases were detected in the study population.59
The authors also compared the combined performance of NIPT for trisomies 21, 18, and 13, and sex chromosome aneuploidies versus traditional prenatal screening. They found that NIPT performed substantially better than traditional prenatal testing, with a sensitivity of 86.7% (95% CI, 58.4%–97.7%) and a false-positive rate of 0.1% (95% CI, 0.1%–0.5%) for NIPT, compared with a sensitivity of 40.0% (95% CI, 17.5%–67.1%) and a false-positive rate of 14.1% (95% CI, 12.5%–15.8%) for traditional prenatal screening.59
NIPT Accuracy for Microdeletions
We found no studies that investigated the accuracy or utility of NIPT for the microdeletions of interest based on our inclusion criteria.
NIPT Failure Rate
Seven studies reported NIPT test failures (Table 7).52,54–59 Failures for the initial NIPT test performed ranged from 0.9% to 5.6% among the studies; low fetal fraction was one of the most common reasons for failure. Among studies that performed a second NIPT test, the success rate ranged from 45% to 77%.54,57,58 Palomaki et al57 noted higher failure rates among samples collected at 10 weeks compared to samples collected at 11 to 21 weeks (relative risk, 2.5%; 95% CI, 1.3%–4.5%; P = .007). Maternal weight was often associated with test failure in studies. As well, DNA failures were confirmed to be strongly associated with maternal weight ≥ 80 kg (relative risk, 11.4; 95% CI, 6.3–21; P < .001).57 Langlois et al54 similarly noted that maternal weight was ≥ 70 kg in 8 of 11 people who failed the initial NIPT test. In Norton et al,56 the median maternal weight in people with a low fetal fraction was 93.7 kg, compared with 65.8 kg in people with a successful NIPT result (P < .001).
Table 7:
NIPT Failure Rate
| Author, Year | Initial NIPT Test | Repeat NIPT Test | True Status Among Failures |
|---|---|---|---|
| Bianchi et al, 201452 | 0.9% (18/1,970)
|
None | Not reported |
| Langlois et al, 201754 | 0.9% (11/1,165)
|
Repeat NIPT test: 45.5% (5/11) success | 3/11 triploidy 3/11 normal |
| Nicolaides et al, 201255 | 4.9% (100/2,049)
|
None | Not reported |
| Norton et al, 201556 | 3.0% (488/16,329)
|
None | 13/488 aneuploidy |
| Palomaki et al, 201757 | 5.6% (150/2,681)
|
Repeat NIPT test: 76.5% (65/85) success | 0 trisomy 21, 18, or 13 |
| Quezada et al, 201558 | 4.2% (123/2,905)
|
Repeat NIPT test: 62.7% (69/110) success | 49/54 normal 2/54 trisomy 21 3/54 pregnancy loss with no karyotype |
| Song et al, 201359 | 3.8% (73/1,916)
|
None | Not reported |
Abbreviation: NIPT, noninvasive prenatal testing.
NIPT Clinical and Personal Utility
Reductions in Diagnostic Testing
Two studies reported on the potential for reductions in diagnostic testing if NIPT was used as a first-tier screening test.52,54 Bianchi et al52 noted a potential 89% reduction in the number of diagnostic tests performed if everyone with a false positive result underwent diagnostic testing (9 false positives in the NIPT group and 80 in the traditional prenatal screening group). Langlois et al54 noted that up to 62 diagnostic tests could have been avoided if NIPT had been used as a primary screen. The total diagnostic testing rate was 2% in the study cohort but could have been as high as 6.8% based on traditional prenatal screening and ultrasound examination.54
Test Turnaround Time
Three studies (United States, United Kingdom, and China) reported on the test turnaround time for NIPT.57–59 In Quezada et al,58 the median interval between blood sampling and receipt of results was 9 days (range 5–20 days); 98% of results were available within 14 days of sampling. In Song et al,59 the study design methods called for a test turnaround time of 10 working days. Palomaki et al57 reported a median turnaround time of 10 days; 95% of results were returned within 15 days.
Understanding of Health Care Providers
Seven studies evaluated health care providers’ understanding and knowledge of NIPT (Table 8).60–66
Table 8:
Health Care Providers’ Understanding of NIPT
| Author, Year, Country | Health Care Providers | Understanding of NIPT |
|---|---|---|
| Brewer et al, 2016,60 United States | Obstetricians |
|
| Filoche et al, 2017,61 New Zealand | Obstetricians |
|
| Haymon et al, 2014,{Haymon, 2014 #947} United States | Maternal–fetal medicine specialists |
|
| Mayes et al, 2016,63 United States | Obstetricians |
|
| Musci et al, 2013,64 United States | Obstetricians |
|
| Sayres et al, 2011,65 United States | Mainly obstetricians but also nurses and nurse–midwives |
|
| Swaney et al, 2016,66 United States | Maternal–fetal medicine fellows |
|
Abbreviation: NIPT, noninvasive prenatal testing.
The most common misconception among responses was that NIPT was a diagnostic test, not a screening test. Other gaps in knowledge were related the possible limitations of NIPT in twin pregnancies, and the availability of expanded NIPT testing for microdeletion syndromes. Health care providers’ confidence in offering NIPT to their patients was often related to their understanding and previous experience with the test.
Five of the seven surveys focused on the understanding of obstetricians and/or gynecologists.60,61,63–65 The varying study dates (from 2011 when NIPT was first introduced clinically, to 2017) and the geographic context (primarily the United States) made it difficult to generalize the results.
Maternal Education
Only one study57 addressed aspects of maternal education for NIPT. In a survey of the general pregnant population, 15% incorrectly thought NIPT identified all genetic anomalies—not only trisomy 21. Furthermore, 13% thought that a negative NIPT result ruled out trisomy 21, and 21% thought NIPT could confirm whether a fetus was affected with trisomy 21. Because NIPT is a screening test, false positives and negatives can still occur; confirmation must still be made with diagnostic testing.
Maternal Satisfaction
Maternal satisfaction was also evaluated in one study.57 Among a general population of pregnant people, 93% were satisfied with undergoing NIPT, and thought that their decision was “good” or “great” (rating average 4.2 out of 5).
Discussion
NIPT Accuracy
We conducted a systematic review of primary studies because we found no health technology assessment or systematic review that addressed all of our research questions. Two recently published systematic reviews relevant to our research questions16,17 were ongoing when we ran our literature search and were published only part way into our review process. However, our findings for test accuracy were similar to those of the recently and previously published systematic reviews for the average-risk or general unselected population.16–19 Our data showed that NIPT had high accuracy for trisomy 21 and lower accuracy for trisomies 18 and 13. However, NIPT accuracy was still higher than that of traditional prenatal screening for trisomies 21, 18, and 13.
One of the systematic reviews published during our review process was a Cochrane review on NIPT test accuracy.67 The review included 65 studies in total, but only five were conducted in a general unselected population, all of which we also included in our review. The Cochrane authors also found 18 studies in the mixed-risk group, but we excluded most of these from our review because results were reported for the entire mixed-risk group and did not focus solely on the average-risk or general population.
Our findings showed sensitivity and specificity for NIPT that were lower than those reported by the manufacturers.8,9 This could have been because the majority of evidence around test accuracy is for the high-risk population, which has higher test sensitivity for trisomies 21, 18, and 13.16–19 As well, although the specificity of NIPT was high for trisomies 21, 18, and 13, the positive predictive value was variable, ranging from as low as 40% to as high as 100% in the included studies. Positive predictive value is affected by the prevalence of a condition, and NIPT was sensitive to slight changes in the number of true positives and/or false positives, because the prevalence of the conditions screened for is low.
The literature on NIPT for sex chromosome aneuploidies in the average-risk or general population is limited, as noted by other authors.16 Previous systematic reviews in the high-risk population found that the accuracy of NIPT for sex chromosome aneuploidies was lower than that for trisomies 21, 18, and 13.39,41 Gil et al39 found a sensitivity of 95.8% (95% CI, 83.6%–100%) and a false-positive rate of 0.14% (95% CI, 0.05%–0.38%) for monosomy X in the high-risk population. Similarly, a systematic review by Mackie et al41 found a sensitivity of 92.9% (95% CI, 74.1%–98.4%) and a false-positive rate of 0.01% (95% CI, 0%–0.01%) for monosomy X in the high-risk population.
Because of the limited number of studies in the meta-analysis, it was not possible to adequately assess how NIPT method, gestational age, or fetal fraction affected test accuracy. However, most NIPT was performed using the targeted approach and the Harmony test. We included any NIPT test version in our review, but the accuracy results from other NIPT versions (e.g., Verifi or other lab-developed tests) may not be fully generalizable to Ontario, given that only the Harmony and Panorama tests are widely available.
Our review accepted different types of reference standards for test accuracy, and the included studies used a range of these. In addition to fetal karyotype from diagnostic testing, clinical phenotype was also accepted (e.g., neonate examination, birth record). The latter may not be as accurate, given the potential for phenotypic variability in affected fetuses. However, given that the focus of the review was the average-risk or general population, it would not have been appropriate for the only reference standard to be diagnostic testing, as it is for a high-risk population.
The true number of affected fetuses may also have been underreported. Fetuses affected by a chromosomal anomaly are likely to be at increased risk of spontaneous pregnancy loss,10 something that was not always captured in the included studies. As well, some studies excluded patients in the analysis if the initial NIPT test led to a failed result, and/or did not adequately report the true status of fetuses that failed NIPT to examine possible chromosomal reasons for test failure. However, the included studies found that the most common reason for test failure was low fetal fraction (i.e., below the minimum amount required to assess patient risk), a measure that was often strongly correlated with high maternal body weight.54,56,57
Clinical validation studies of test accuracy for microdeletions are difficult to perform because of the very low prevalence of microdeletions. We found no relevant studies for microdeletion test accuracy based on our inclusion criteria. There are accuracy studies, but they used archived samples or artificial mixtures to estimate test accuracy, and sensitivity ranged widely, from 60% to >99%.68 Test performance also depends on deletion size: 3 million base pairs is the approximate lower limit for detection.69 Because microdeletion syndromes can have deletions that are shorter in length, NIPT may not capture all syndromes that are clinically relevant. Similarly, NIPT may inadvertently capture “variants of unknown significance” (chromosomal anomalies without clear clinical manifestations).70 The relative rarity of microdeletion syndromes, combined with test performance, could mean that the actual false-positive rate of a microdeletion panel could exceed 1%.69 Combined with testing for other chromosomal aneuploidies, the false-positive rate would be even higher, because of the additive effect of false positives. Given that the literature on microdeletion testing is sparse, clinical guidelines do not support routine testing for microdeletions with NIPT.
NIPT Clinical and Personal Utility
Studies of clinical utility for the average-risk or general population are lacking. We identified only one study that focused on aspects of personal utility (i.e., maternal education and satisfaction).57 However, in high-risk populations, people have shown high satisfaction and low decisional regret with NIPT.33,34
We also assessed the published literature on health care providers’ understanding of NIPT. The studies we found were surveys, primarily in the United States obstetrician population. Because of the cross-sectional nature of the surveys and the varying publication dates, it was difficult to draw conclusions about health care providers’ current understanding of NIPT, particularly for the Canadian context. Nevertheless, a common gap in knowledge noted in the published studies was the misconception that NIPT is a diagnostic test rather than a screening test.60,62,63,66 Some health care providers were also unaware that NIPT is available for use in twin pregnancies and for testing certain microdeletion syndromes.
It has been noted in patient preferences literature that pregnant people want to understand the conditions being screened for, and often look to their health care provider for this information.71 Thus, ongoing education and training for health care professionals is essential to establish and maintain their understanding of NIPT, particularly as new indications emerge and additional conditions are tested for.
Ongoing Studies
We searched ClinicalTrials.gov for relevant ongoing studies of NIPT in the average-risk or general pregnant population. We also searched the World Health Organization International Clinical Trials Registry platform for ongoing studies.
We found seven potentially relevant ongoing studies (Appendix 6). Two recruited only participants from the average-risk population, three included both average-risk and high-risk participants, one recruited from the general population, and one did not specify risk. We found no studies on the accuracy of NIPT for microdeletions in the average-risk or general population, but one of the ongoing studies has set out to determine NIPT performance for 22q11.2 microdeletion syndrome in the general population.
We searched PROSPERO for ongoing systematic reviews but found no relevant reviews.
Conclusions
The pooled sensitivity of NIPT was 99.5% (95% CI, 81.8%–99.9%) for trisomy 21, 93.1% (95% CI, 75.9%–98.3%) for trisomy 18, and 92.7% (95% CI, 81.6%–99.9%) for trisomy 13 (GRADE: low to moderate). The specificity for any trisomy was 99.9% (95% CI, 99.8%–100%; GRADE: moderate).
The accuracy of NIPT was higher than that of traditional prenatal testing (GRADE: low to moderate) and decreased the number of diagnostic tests performed (GRADE: moderate).
Evidence for the use of NIPT in the average-risk or general population for sex chromosome aneuploidies was limited. We found no studies on the accuracy or clinical utility of NIPT for microdeletion syndromes in the average-risk or general population.
Although NIPT is a screening test, health care providers may misinterpret it to be a diagnostic test. Positive NIPT results should be confirmed by diagnostic testing.
ECONOMIC EVIDENCE
Research Question
What are the findings of the published evidence on the cost-effectiveness of first-tier or second-tier noninvasive prenatal testing (NIPT) for trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions in average-risk or general population?
Methods
Economic Literature Search
We performed an economic literature search on September 14, 2017, for studies published from January 1, 2007, to the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic filter applied.
We created database auto-alerts in MEDLINE, Embase, and CINAHL and monitored them for the duration of the health technology assessment. We performed a targeted grey literature search of health technology assessment agency websites, clinical trial registries, Tufts Cost-Effectiveness Analysis Registry and the Hospital for Sick Children Toronto's Paediatric Economic Database Evaluation (PEDE). See the Clinical Evidence literature search section above for further details on methods used. See Appendix 4 for the literature search strategies, including all search terms.
Literature Screening
A systematic review of NIPT economic evaluations by Nshimyumukiza et al72 captured relevant literature published between January 1, 2009, and December 31, 2015. We have summarized the findings of its included studies in this review. We then included studies published after January 1, 2016, in our review.72
A single reviewer reviewed titles and abstracts, and, for those studies likely to meet the eligibility criteria, we obtained full-text articles and performed further assessment for eligibility.
Inclusion Criteria
English-language full-text publications
Studies comparing traditional prenatal screening with NIPT for trisomies 21, 18, or 13, sex chromosome aneuploidies, or microdeletions in pregnant people at average risk for chromosomal anomalies
Studies published between January 1, 2016, and September 14, 2017
Cost–utility analyses, cost-effectiveness analyses, or cost–benefit analyses
Exclusion Criteria
Studies on cost analyses (e.g., no clinical outcomes)
Studies focusing on pregnancies at high risk for chromosomal anomalies
Studies from countries with economic levels (e.g., gross domestic product per capita) and health care systems (e.g., China, Turkey) considerably different from Canada's
Abstracts, letters, commentaries, and editorials
Outcomes of Interest
Total cost of different prenatal screening strategies
Number of affected cases identified (or detection rate)
Number of diagnostic tests (or number of diagnostic tests per diagnosis), including chorionic villus sampling or amniocentesis
False-positive rate
Incremental cost-effectiveness ratio (ICER; i.e., incremental cost per additional affected case identified)
Data Extraction
A single reviewer conducted the preliminary data extraction, applying the inclusion criteria. We extracted relevant data on the following:
Source (e.g., first author, country, year of publication)
Population, perspective, and time horizon
Interventions and comparators (e.g., different prenatal screening strategies)
Outcomes (e.g., health outcomes, costs, conclusions of economic evaluations)
We contacted authors of the studies to provide clarification as needed.
Study Applicability
We determined the usefulness of each identified study for decision-making by applying a modified applicability checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform development of clinical guidelines by NICE.73 We retained questions from the NICE checklist related to study applicability and modified the wording of the questions to remove references to guidelines and to make it Ontario-specific. A summary of the number of studies judged to be directly applicable, partially applicable, or not applicable to the research question is presented (Appendix 7).
Results
Literature Search
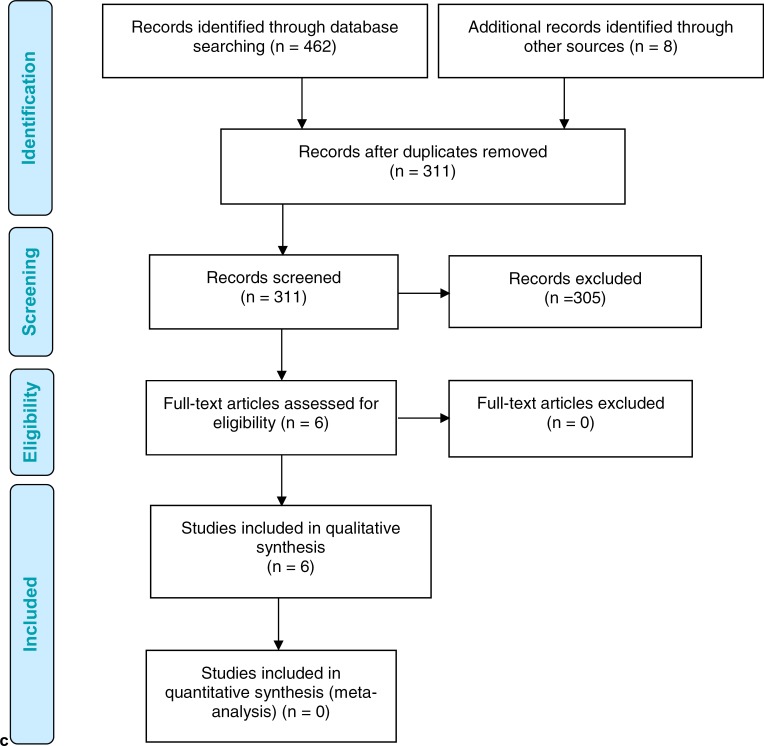
The literature search yielded 311 citations published between January 1, 2007, and September 14, 2017, after removing duplicates. Our literature search found a relevant economic systematic review that searched the literature up to 2016.72 For this reason, we limited studies to those published after January 1, 2016, to capture the updated economic evidence (i.e., studies published after the end search date of the published systematic review).72 We excluded a total of 305 articles based on information in the title and abstract, as well as the year of publication. We then obtained the full texts of six potentially relevant articles for further assessment. Figure 5 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).51
Figure 5: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.51
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Six economic evaluations published between January 1, 2016, and our search date met the inclusion criteria.74–79 We have also summarized the economic evidence before 2016 based on the systematic review by Nshimyumukiza et al.72
Review of Included Economic Studies
We included six studies, two of which were from Canada.74,75 The remaining four studies were from the United Kingdom,78 the United States,79 Italy,77 and Australia.76 Three studies74,75,77 included strategies for first-tier NIPT (i.e., first screening test done) and second-tier NIPT (i.e., test is done after positive results from traditional prenatal screening and before diagnostic testing); two studies76,78 included second-tier NIPT only. Only one study79 compared first-tier NIPT with traditional prenatal screening. In three of the included studies,75–77 the target population was pregnant people who had accepted prenatal screening; the other three studies74,78,79 included all pregnant people, with a screening uptake rate (percentage of people who choose to receive prenatal screening) of 66% to 70%.
In all six studies, the main economic outcome was the total cost required to implement each prenatal screening option, and the target cohort was all pregnant people. The total required budget included the costs of traditional prenatal screening, NIPT, and diagnostic testing. In five studies,74–78 the time horizon was the duration of pregnancy; one study79 used a lifetime horizon and reported direct medical costs and indirect costs for trisomy births. Most of the studies conducted various analyses by exploring different NIPT implementation strategies,74,75 risk cutoffs,76,78 traditional prenatal screening options,74,75,78 or prices of NIPT.75,79 We have reported the most relevant results (e.g., strategies including enhanced first-trimester screening and maternal serum screening, which are performed in Ontario) in Table 9. We have summarized the first-tier and second-tier NIPT findings for the six studies below.
Table 9:
Results of Economic Literature Review—Summary
| Results | ||||||
|---|---|---|---|---|---|---|
| Author, Year, Country | Study Designa and Perspective | Population/Target Fetal Aneuploidies | Intervention/Comparatorb | Health Outcomesc | Costsd | Conclusionse |
| Nshimyumukiza et al, 2018,74 Canada |
|
|
|
Cases identified, n (trisomy 21; trisomies 18 and 13):
Diagnostic tests, n:
|
CAD; fiscal year 2014/15 Cost of NIPT: $795
|
Compared with FTS, first-tier NIPT would cost $1.5 M to detect 1 additional case of trisomy 21 Compared with traditional prenatal screening, NIPT as a second-tier test for high-risk pregnant people was likely to be cost-effective |
| Huang et al, 2017,75 Canada |
|
|
|
Cases identified, n:
Diagnostic tests, n:
False-positive rate, %:
|
CAD; costing year not reported Cost of NIPT: $550
Cost of NIPT: $400
Cost of NIPT: $200
|
eFTS with second-tier NIPT provided performance similar to that of universal NIPT at a substantially lower cost |
| Maxwell et al, 2017,76 Australia |
|
|
|
Detection rate, %:
Diagnostic tests per diagnosis, n:
|
Australian dollars; 2014 Cost of NIPT: $400
|
Compared with FTS, second-tier NIPT models using more sensitive risk cutoffs improved the detection rate for trisomy 21, reduced procedure-related pregnancy loss and could be provided at a lower cost per diagnosis |
| Colosi et al, 2017,77 Italy |
|
|
|
Detection rate, %:
Diagnostic tests, n:
False-positive rate, %:
|
Euro; cost year not reported Cost of NIPT: 260 €
|
Second-tier NIPT could be a cost-efficient and feasible first-trimester screening test for aneuploidies in the public health system |
| Chitty et al, 2016,78 United Kingdom |
|
|
|
Cases identified, n:
Diagnostic tests, n:
|
GBP; 2012/13 Cost of NIPT: £250
|
Second-tier NIPT improved the overall performance of prenatal screening without increasing costs |
| Fairbrother et al, 2016,79 United States |
|
|
|
Total cases identified, n (trisomy 21; trisomy 18; trisomy 13):
Diagnostic tests, n:
|
USD; 2014
|
First-tier NIPT resulted in more trisomy cases detected than FTS, and was more economical at a NIPT unit cost of $453 |
Abbreviations: B, billion; eFTS, enhanced first-trimester screening; FTS, first-trimester screening; ICER, incremental cost-effectiveness ratio; IPS, integrated prenatal screening; MSS, maternal serum screening (also known as quadruple screening); M, million; NIPT, noninvasive prenatal testing; NT, nuchal translucency.
The included studies used different terms (e.g., cost-effectiveness analysis74 and cost analysis75,76) in their titles. Since all included studies reported both health outcomes and costs, we used the term “cost-effectiveness analysis” in this review. Moreover, some studies can be categorized as cost–consequence studies, since they did not report cost per case detected or incremental cost per case detected.
The intervention and comparator refer to NIPT strategies (i.e., first-tier or second-tier NIPT). When studies included numerous strategies, we reported the results from the strategies most relevant to this review:
- FTS, IPS, eFTS and MSS: traditional prenatal screening without NIPT
- FTS/IPS/eFTS/MSS + NIPT: second-tier NIPT
- Universal NIPT: first-tier NIPT
Published studies presented various health outcomes. We reported three health outcomes: the number of trisomy cases identified (or detection rate), the number of invasive diagnostic tests (or invasive diagnostic tests per diagnosis), and false-positive rate. We kept the original measures (e.g., the number of case identified or the detection rate) reported in these studies. Invasive diagnostic tests refers to amniocentesis or chorionic villus sampling.
The costs refer to the total costs of implementing each screening strategy for the entire hypothetical cohort, including the cost of traditional prenatal screening, NIPT, and diagnostic testing.
We summarized the conclusions from authors in each economic evaluation. We did not calculate the ICER (incremental cost per case detected or diagnostic test avoided) if it was not reported in the study.
Authors did not clearly state the perspective. Because this study included both direct medical costs and indirect costs for a given trisomy birth, we assumed that the study was conducted from a societal perspective with a lifetime horizon.
Five studies74–78 included second-tier NIPT. Compared to traditional prenatal screening, second-tier NIPT led to a substantial reduction in the number of subsequent confirmatory diagnostic tests. However, the performance of second-tier NIPT in identifying affected fetuses differed among studies. When using a lower risk cutoff for positive test results than that used for traditional prenatal screening (e.g., 1:1,000 for traditional prenatal screening for trisomy 21), second-tier NIPT had a higher detection rate.76–78 When using the same risk cutoff and the same acceptance rate for further testing (with NIPT or diagnostic testing) as for traditional screening, second-tier NIPT had a lower detection rate than traditional screening.74 However, when second-tier NIPT substantially increased the acceptance rate for further testing, it led to increased detection of affected fetuses.80 These studies also showed that second-tier NIPT and traditional prenatal screening incurred similar total costs. The cost and performance (e.g., number of affected cases identified and diagnostic tests avoided) of second-tier NIPT were affected by the unit price of NIPT and the risk cutoff for further testing.
Four studies74,75,77,79 evaluated first-tier NIPT. In one study, compared with traditional prenatal screening, first-tier NIPT identified more affected fetuses at a substantially increased cost when excluding the lifetime cost of trisomy births. Using a low-risk cutoff (e.g., 1:1,000) for second-tier NIPT, the performance of first-tier and second-tier NIPT was similar.75,77
The diagnostic pathway for people with a failed NIPT (e.g., an inconclusive test result) affected the number of diagnostic tests for first-tier NIPT.75,77 If people with inconclusive results did not receive diagnostic testing, the number of diagnostic tests performed would be substantially lower.
All studies reported clinical outcomes and costs for the entire cohort, and the cohort size varied between studies. Most studies did not report an ICER (i.e., incremental cost per additional affected case identified). To compare the clinical and economic outcomes of studies with different cohort sizes, we calculated the cost and main effectiveness measures for a standardized unit (per 10,000 pregnant people), as well as the ICER for first-tier and second-tier NIPT (see Table 10). These calculated results are based on reported study data and have not been verified by the study authors, so the findings should be interpreted with caution. In general, even after standardizing the cohort size, we still found considerable differences in cost, number of cases detected, number of diagnostic tests performed, and incremental cost per additional case identified, both for NIPT as a second-tier test versus traditional prenatal screening, and for first-tier NIPT versus second-tier NIPT.
Table 10:
Calculated Approximate Cost and Effectiveness Per 10,000 Pregnant People From Included Studies
| Approximated Cost and Effectiveness per 10,000 Pregnant People | ||||
|---|---|---|---|---|
| Name, Year, Location | Intervention/Comparator | Health Outcomes | Costs | ICER (Incremental Cost Per Additional Case Identified) |
| Nshimyumukiza et al, 2018,74 Canada |
|
Total trisomy 21, 18, and 13 cases identified, n:
Diagnostic tests, n:
|
CAD; fiscal year 2014/15 Cost of NIPT: $795 per test
|
Second-tier NIPT vs. traditional prenatal screening:
First-tier vs. second-tier NIPT:
|
| Huang et al, 2017,75 Canada |
|
Trisomy 21 cases identified, n:
Diagnostic tests, n:
|
CAD; costing year not reported Cost of NIPT: $400 per test
|
Second-tier NIPT vs. traditional prenatal screening:
First-tier vs. second-tier NIPT:
|
| Maxwell et al, 2017,76 Australia |
|
Trisomy 21 cases identified, na:
Diagnostic testsa:
|
Australian dollars; 2014 Cost of NIPT: $400 per test
|
NA |
| Colosi et al, 2017,77 Italy |
|
Trisomy 21 cases identified, na:
Diagnostic tests, n:
|
Euros; cost year not reported Cost of NIPT: €260 per test
|
NA |
| Chitty et al, 2016,78 United Kingdom |
|
Trisomy 21 cases identified, n:
Diagnostic tests, n:
|
GBP; 2012/13 Cost of NIPT: £250 per test
|
Second-tier NIPT
|
| Fairbrother et al 2016,79 United States |
|
Trisomy 21, 18, and 13 cases identified, n:
Diagnostic tests, n:
|
USD; 2014 No explicit cost for NIPT in analysis
|
NA |
Abbreviations: eFTS, enhanced first-trimester screening; FTS, first-trimester screening; ICER, incremental cost-effectiveness ratio (incremental cost per additional case detected); IPS, integrated prenatal screening; MSS, maternal serum screening (also known as quadruple screening); M, million; NA, not applicable; NIPT, noninvasive prenatal testing.
Note: These calculated results are based on the reported study data and have not been verified by study authors; the findings should be interpreted with caution.
It was not straightforward to obtain an accurate estimate of these parameters, so we did not calculate this value.
When both the incremental cost and incremental effectiveness have negative values, there is a positive ICER. However, its interpretation is opposite from a positive ICER because of a positive incremental cost and incremental effectiveness. To avoid confusion, we did not present these calculated ICERs.
To our knowledge, there are no thresholds to assess cost-effectiveness using incremental cost per case detected or number of diagnostic tests avoided. The main factors that affected the ICER included target population (entire pregnant population versus pregnant people who accepted prenatal screening), chromosomal aneuploidies (trisomies 21, 18, and 13, versus only trisomy 21), prenatal screening pathway (e.g., diagnostic testing or no diagnostic testing directly after NIPT test failure), diagnostic testing acceptance rate, test performance for traditional prenatal screening and NIPT, cost of NIPT, and other cost components included in the analyses.
Despite the differences between studies, the conclusions for second-tier NIPT were consistent. Compared with traditional prenatal screening, second-tier NIPT can improve the overall performance of prenatal screening without a significant increase in total budget.74–78 Compared with traditional prenatal screening, first-tier NIPT would improve overall performance, but with a substantial increase to the total budget.74,75,77
Economic Evidence Prior to January 1, 2016
The systematic review by Nshimyumukiza et al72 included 16 studies: eight from the United States, two from Canada, two from Australia, and one each from Belgium, the Netherlands, Sweden, and the United Kingdom. All studies were published between 2012 and 2015. The review authors concluded that the general quality of these studies was “fair.” Of the 16 studies, 10 focused on prenatal screening for trisomy 21; four included trisomies 21, 18, and 13; one included trisomies 21, 18, and 13, and monosomy X; and one included all detectable chromosomal abnormalities. The authors of these studies generally concluded that second-tier NIPT was cost-effective at the current NIPT test price (approximately $400 to $1,796 USD, purchasing power parity in 2015), but that first-tier NIPT was not cost-effective in most of the studies. Important factors affecting cost-effectiveness included the price of NIPT, the risk cutoff for positive test results for traditional prenatal screening, and the uptake rate of prenatal screening.
Applicability of the Included Studies
The results of the applicability checklist for the included studies are presented in Appendix 7. One study from the United States79 was not applicable, but the other five studies were partially applicable to the research question.74–78 Two studies conducted in Canada were relevant for the Ontario setting,74,75 but both studies included all pregnant people, while our target population was average-risk pregnant people. Thus, their results were not directly applicable to our research question.
Discussion
A number of economic evaluations of NIPT have been published over the past few years. The main economic results of these studies were the total budget required in the jurisdiction for each screening strategy, not the average cost per pregnant person. The studies also often reported the number of affected fetuses detected and the number of diagnostic tests performed as clinical outcomes; they did not report commonly used measures in health economic evaluations (e.g., quality-adjusted life-years). Because there are no explicit thresholds for assessing the cost-effectiveness or cost per additional affected fetus detected, the authors did not use ICERs to assess the cost-effectiveness of NIPT. However, the conclusions of these economic evaluations were consistent overall. Second-tier NIPT might be cost-effective, because it may improve performance (e.g., by using a lower risk cutoff and increasing the rate of further testing after positive results from traditional prenatal screening) compared with traditional prenatal screening and without increasing the total budget. In contrast, first-tier NIPT would increase the total screening budget substantially.
The two Canadian studies included pregnant people at various risk levels in Quebec74 and Ontario.75 However, our population of focus was average-risk pregnant people, because NIPT is already publicly funded for high-risk pregnant people in Ontario. In addition, some of the traditional prenatal screening options included (e.g., integrated prenatal screening and first-trimester screening) have been phased out and are no longer performed in Ontario. Enhanced first-trimester screening and maternal serum screening are now the only traditional prenatal screening options performed in Ontario. Because the previous Canadian economic evaluations did not focus on our target population, we decided to conduct a primary economic evaluation of NIPT for average-risk pregnant people.
Conclusions
The systematic review identified six studies published between January 1, 2016, and September 14, 2017, that evaluated the economic implications of first-tier and second-tier NIPT. The studies showed that compared with traditional prenatal screening, second-tier NIPT might improve the detection rate and reduce the number of diagnostic tests performed without a significant increase to the total prenatal screening budget. Compared with traditional prenatal screening, first-tier NIPT improved the overall performance of prenatal screening but at a substantial increase in total cost. Two Canadian studies were partially applicable to the Ontario context.
PRIMARY ECONOMIC EVALUATION
The clinical evidence review showed that in comparative test accuracy studies, noninvasive prenatal testing (NIPT) performed better than traditional prenatal screening and decreased the number of diagnostic tests required. However, the NIPT test is more expensive than traditional prenatal screening. The economic evidence review found a number of studies that evaluated the clinical and economic outcomes of implementing NIPT, including two recently published studies for trisomy 21 from Ontario and Quebec,74,75 but these two economic evaluations did not distinguish between average-risk and high-risk populations in their findings. This was important because in addition to risk differences for chromosomal anomalies, high-risk and average-risk pregnant people in Ontario follow different clinical pathways and have different reimbursement policies. In Ontario, NIPT is publicly funded as a first-tier test for people at high risk of having a chromosomal anomaly, including pregnant people who are 40 years of age or older at the time of delivery. For pregnant people at average risk, including people less than 40 years old, NIPT is funded as a second-tier test (i.e., only after positive results from traditional prenatal screening and before diagnostic testing). Finally, we were also interested in the economic implications of using NIPT for detecting chromosomal anomalies other than trisomy 21, including trisomy 13 and 18, sex chromosome aneuploidies, and microdeletion syndromes. For these reasons, we conducted a primary economic evaluation to investigate the cost-effectiveness of NIPT for chromosomal anomalies of interest for average-risk pregnant people in Ontario.
Research Question
What is the cost-effectiveness of three prenatal screening strategies for average-risk pregnant people in the context of the Ontario Ministry of Health and Long-Term Care: traditional prenatal screening (i.e., without NIPT); NIPT as a second-tier test (i.e., contingent NIPT, following positive results from traditional prenatal screening); and NIPT as a first-tier test (i.e., primary screening)?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.81
Type of Analysis
We conducted a cost-effectiveness analysis comparing three prenatal screening strategies (or screening pathways) in Ontario. We used the following clinical outcomes to measure clinical effectiveness:
Number of cases detected of the chromosomal anomalies of interest (i.e., identified by the screening test and confirmed by subsequent diagnostic testing)
Number of diagnostic tests performed (chorionic villus sampling or amniocentesis)
Number of pregnancy losses related to diagnostic testing
Number of live births with the chromosomal anomalies of interest
We considered the number of cases detected as the primary clinical outcome. Positive results from screening must have been confirmed by diagnostic testing to count as a detected case. If diagnostic testing was declined, we considered the case undetected.
Target Population
The study population was pregnant people less than 40 years of age at the time of delivery, with a singleton pregnancy of gestational age 10 to 20 weeks, and without a previous pregnancy that had a chromosomal anomaly. According to data from Ontario's Better Outcomes Registry and Network (BORN), 95.8% of pregnant people are less than 40 years old at the time of delivery.82 Assuming that a very small proportion of people have a previous pregnancy with a chromosomal anomaly, our target population would cover approximately 95% of pregnant people with a singleton pregnancy. We estimated the annual number of average-risk pregnant people to be 142,000 to 148,000 in the next 5 years. Details of the process for population estimation can be found in the budget impact analysis.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Interventions
Noninvasive prenatal testing can be used as a first-tier or second-tier test. We included three strategies in our reference case: traditional prenatal screening without NIPT, NIPT used as a second-tier test, and NIPT used as a first-tier test. It has been suggested that NIPT could be used as a third-tier test (i.e., as a replacement for diagnostic testing), but this is not a clinically recommended pathway and is not currently part of clinical practice in Ontario, so we excluded this strategy from our analysis.
The Ontario Ministry of Health and Long-Term Care has recently announced plans to reform prenatal screening. Laboratories have phased out first-trimester screening, integrated prenatal screening, and serum integrated prenatal screening, and switched to enhanced first-trimester screening (eFTS). Maternal serum screening (MSS, or quadruple screening) in the second trimester is still important for people who miss screening in the first trimester. Therefore, in this economic evaluation we focused on the traditional prenatal screening strategies of eFTS for people who present in the first trimester and MSS for people who present in the second trimester.
We based the proportions of pregnant people who undergo eFTS (90%) or MSS (10%) on recent data from BORN. We incorporated the proportion of pregnant people who undergo NIPT as a second-tier test into the eFTS and MSS pathways. As a first-tier test, NIPT may be performed along with nuchal translucency ultrasound when pregnant people are screened in the first trimester.
Overall, we investigated six screening options as part of three strategies. Table 11 describes the prenatal screening pathways.
Table 11:
Prenatal Screening Pathways for Pregnant People, Summary
| Strategy | Screening Test | Second Testa | Third Testb |
|---|---|---|---|
| Strategy 1: Traditional Prenatal Screening Without NIPT | |||
| First trimesterc | eFTSd | CVS (70%) or amniocentesis (30%) | NA |
| Second trimestere | MSSf | Amniocentesis (100%) | NA |
| Strategy 2: Second-Tier NIPT | |||
| First trimesterc | eFTSd | NIPT or amniocentesis for very high-risk pregnanciesg | Amniocentesish |
| Second trimestere | MSSf | NIPT or amniocentesis for very high-risk pregnanciesg | Amniocentesish |
| Strategy 3: First-Tier NIPT | |||
| First trimesterc | NIPT + NT ultrasound | CVS (70%) or amniocentesis (30%) | Not applicable |
| Second trimestere | NIPT | Amniocentesis (100%) | Not applicable |
Abbreviations: CVS, chorionic villus sampling; eFTS, enhanced first-trimester screening; MSS, maternal serum screening (also known as quadruple screening); NA, not applicable; NIPT, noninvasive prenatal testing; NT, nuchal translucency.
CVS and amniocentesis are diagnostic tests for confirming the results of a positive first test. Second-tier NIPT is still a screening test.
Amniocentesis is for confirming the results of a positive NIPT test.
90% of people who receive prenatal screening.
eFTS consists of a single blood test, which includes pregnancy-associated plasma protein A, free β-human chorionic gonadotrophin, placental growth factor, and α-fetoprotein. As well, eFTS includes NT ultrasound.
10% of people who receive prenatal screening.
MSS (quadruple screening) consists of a single blood test, which includes α-fetoprotein, unconjugated oestriol, free β-human chorionic gonadotrophin, and inhibin-A.
People who have a trisomy 21 risk > 1/10 or nuchal translucency ≥ 3.5 mm.
Only for people who had second-tier NIPT.
Discounting and Time Horizon
We did not include pregnancy-related outcomes or costs incurred beyond birth. Therefore, the time horizon was the duration of a full-term pregnancy (12 weeks’ gestation to term); discounting was not applicable.
Main Assumptions
To simplify the model, we assumed the following:
Pregnant people who declined prenatal screening followed the natural course of pregnancy. Fetuses affected by chromosomal anomalies were at a risk of spontaneous pregnancy loss because of these anomalies. Unaffected fetuses also had a small risk of spontaneous pregnancy loss (background risk unrelated to chromosomal anomalies).
Pregnant people who declined diagnostic testing (chorionic villus sampling or amniocentesis) after positive results from traditional prenatal screening or NIPT followed the natural course of pregnancy.
The sensitivity and specificity of confirmatory diagnostic testing (chorionic villus sampling or amniocentesis) were 100% for all chromosomal anomalies of interest. Diagnostic testing was associated with a small risk of procedure-related pregnancy loss.
Traditional prenatal serum screening (eFTS and MSS) screens for only trisomy 21 and 18, but some cases of trisomy 13 could be detected incidentally during diagnostic testing (i.e., some might undergo diagnostic testing because of false-positive results for trisomy 21 or 18 from traditional prenatal screening).
The fetal anatomical ultrasound at 18 to 20 weeks would be performed in all screening pathways but was not included because it is a part of standard obstetric care. The results of the ultrasound would not affect the risk score for the fetus.
Model Structure
The process for the cost-effectiveness analysis is outlined in Figure 6. We simulated 5,000 cohorts (i.e., outer loop to capture parameter uncertainty), each with 142,000 to 148,000 average-risk pregnant people with singleton pregnancies at 12 weeks’ gestation (i.e., inner loop to incorporate individual variability). We randomly assigned each fetus a risk level for the chromosomal anomaly of interest (trisomies 21, 18, and 13, sex chromosome aneuploidies, or microdeletions) using a distribution. Through microsimulation, each fetus obtained a latent (“true”) chromosomal anomaly status (i.e., had or did not have a chromosomal anomaly) based on maternal-age-specific risk. The hypothetical cohort then went through each of the three screening strategies. We simulated screening results (true positive, false negative, false positive, and true negative) for each condition of interest in each pregnant person, based on the accuracy of the tests performed (detection rate and false-positive rate) and true disease classifications. Then, we simulated other clinical parameters and events (e.g., diagnostic testing and spontaneous pregnancy loss). We assigned costs related to the clinical events. Finally, we summarized the average results from the 5,000 iterations (i.e., total cost, number of cases detected, incremental cost per case detected) and calculated 95% credible intervals for cost and effectiveness results. Additional details of each prenatal screening strategy can be found in Figures 7 to 11.
Figure 6: Cost-Effectiveness Analysis, Simplified Process.
Figure 7: Simplified Pathway, Natural Course of Pregnancy.
Figure 11: Simplified Pathway, First-Tier NIPT.
Abbreviation: NIPT, noninvasive prenatal testing
Two of the strategies (traditional prenatal screening without NIPT and second-tier NIPT) included two different initial prenatal screening options (eFTS and MSS). For simplicity, we did not differentiate between the clinical pathways for these initial prenatal screening options, but we did incorporate their different screening performance (i.e., detection rate and false-positive rate) into the model, along with the confirmatory diagnostic test that would be performed (chorionic villus sampling or amniocentesis).
The clinical pathways for each of the three screening strategies are briefly described below, along with the pathways for the natural course of pregnancy (e.g., for people who choose to decline prenatal screening) and diagnostic testing (to confirm positive test results).
Natural Course of Pregnancy
Pregnant people with an unaffected fetus will give birth to an unaffected newborn, but they have a small risk of spontaneous pregnancy loss (Figure 7). Pregnant people with an affected fetus will give birth to an affected newborn or experience spontaneous pregnancy loss. For simplicity, we did not present spontaneous pregnancy loss for unaffected fetuses in any of the models, because this pathway did not include our clinical and economic outcomes of interest, and the number of pregnancy losses for unaffected fetuses was similar for all pathways.
We also modelled the risk of spontaneous loss of affected and unaffected fetuses between 12 and 18 weeks’ gestation. Given the considerable risk of spontaneous pregnancy loss as a result of chromosomal anomalies, the prevalence of trisomies 21, 18, and 13 in the second trimester was lower than in the first trimester (see the clinical evidence review for details).
Diagnostic Testing
People who choose to undergo diagnostic testing with chorionic villus sampling or amniocentesis have a risk of procedure-related pregnancy loss, regardless of whether the fetus is affected or unaffected by a chromosomal anomaly (Figure 8). Pregnant people with an unaffected fetus may give birth to an unaffected newborn, while people with an affected fetus may choose voluntary termination of pregnancy, experience spontaneous pregnancy loss, or give birth to an affected newborn.
Figure 8: Simplified Pathway, Diagnostic Testing.
Traditional Prenatal Screening Without NIPT
Figure 9 shows the clinical pathway for traditional prenatal screening without NIPT. Pregnant people who choose to decline prenatal screening follow the natural course of pregnancy pathway (Figure 7). If they choose traditional prenatal screening and the results are positive, they can choose to undergo confirmatory diagnostic testing (Figure 8). If they choose to decline diagnostic testing, they will follow the natural course of pregnancy (similar to those who decline prenatal screening). If the results are negative, there is no further testing.
Figure 9: Simplified Pathway, Traditional Prenatal Screening Without NIPT.
Abbreviation: NIPT, noninvasive prenatal testing
Second-Tier NIPT
Figure 10 shows the clinical pathway for second-tier NIPT. In general, this screening strategy reflects current practice in Ontario for people at average risk for a chromosomal anomaly. Pregnant people who decline prenatal screening follow the natural course of pregnancy (Figure 7). A small group (about 0.6%; BORN data) of very high-risk people (defined as a risk of trisomy 21 greater than 1/10 from traditional prenatal screening or nuchal translucency ≥ 3.5 mm) undergo diagnostic testing directly without NIPT. For those remaining, second-tier NIPT is performed following positive results from traditional prenatal screening and before diagnostic testing. If NIPT results are positive, people can choose to proceed to diagnostic testing (Figure 8). If NIPT results are negative, no further testing is conducted. If second-tier NIPT fails (once or twice), people are offered diagnostic testing. Test failure can occur for different reasons, such as inconclusive test results or test assay issues.
Figure 10: Simplified Pathway, Second-Tier NIPT.
Abbreviation: NIPT, noninvasive prenatal testing
aPregnant people who have a trisomy 21 risk > 1/10 or nuchal translucency ≥ 3.5 mm.
First-Tier NIPT
Figure 11 shows the clinical pathway for first-tier NIPT. Pregnant people who decline prenatal screening follow the natural course of pregnancy (Figure 7). If NIPT test results are positive, people may choose diagnostic testing (Figure 8). If people decline diagnostic testing, they will follow the natural course of pregnancy (similar to those who decline prenatal screening). If NIPT results are negative, no further testing is conducted. If first-tier NIPT fails (either once or twice), pregnant people are offered MSS if screening was performed in the first trimester, or diagnostic testing if screening was performed in the second trimester.
For first-tier NIPT, we assumed that pregnant people would have a nuchal translucency ultrasound if they were screened in the first trimester. Therefore, this strategy would see the same proportion (i.e., 90%) of pregnant people undergoing nuchal translucency ultrasound as traditional prenatal screening and second-tier NIPT. Nuchal translucency ultrasound is not used only to detect chromosomal anomalies (e.g., it is also used to detect cardiac disease and gastrointestinal malformations), but we focused only on its use for chromosomal anomalies. For simplicity, we included the cost of nuchal translucency ultrasound for pregnant people who may receive it, but we did not incorporate the potential effect of the results on screening or diagnosis pathways.
Clinical Outcome Parameters
Ontario's Better Outcomes Registry and Network (BORN) collects data on pregnancy, prenatal screening, diagnostic testing, births, and newborns. We requested data from BORN for the key model inputs for pregnant people who were less than 40 years old at the estimated date of delivery, including screening uptake rate; test performance for eFTS, MSS, and NIPT for trisomy 21; uptake rate for further testing after positive screening results; and the proportion of pregnant people at very high risk for a chromosomal anomaly. We used BORN data from 2012 to 2016 to obtain the number of average-risk pregnant people who were less than 40 years of age at the estimated date of delivery, had singleton pregnancies, and did not have a previous pregnancy with a chromosomal anomaly.
We also used Ontario data from other sources when possible. When Ontario data were unavailable or inappropriate, we used data from published Canadian and international studies. The model inputs are provided below.
Prevalence of Chromosomal Anomalies and Risk of Pregnancy Loss
Based on the distribution of maternal age at the estimated date of delivery in Ontario,82 we assigned a maternal age to each simulated pregnant person. We did not use local data to estimate the prevalence of chromosomal anomalies, because local prenatal data linkages were incomplete and ongoing.83 Based on estimated maternal-age-specific live birth prevalence (in the absence of prenatal diagnosis) from the United Kingdom,84 and the spontaneous pregnancy loss rate, we calculated the prevalence of trisomies 21, 18, and 13 in viable fetuses at 12 weeks’ gestation (first trimester), with an adjustment for spontaneous pregnancy loss because of the chromosomal anomaly. Thus, live birth prevalence (excluding voluntary termination of pregnancy) was approximately equal to the prevalence of viable fetuses at a given time minus spontaneous pregnancy loss at the same time point:
Live birth prevalencea = prevalence of viable fetuses × (1 – spontaneous pregnancy loss rateb)
aLive birth prevalence of a chromosomal anomaly in the absence of prenatal diagnosis and voluntary termination of pregnancy.
bSpontaneous pregnancy loss rate from 12 weeks (first trimester) to term for a given chromosomal anomaly.
Then:
Prevalence of viable fetuses = live birth prevalence ÷ (1 – spontaneous pregnancy loss rate)
Studies from the United Kingdom have estimated spontaneous pregnancy loss rates from 12 weeks to term of 43% (95% confidence interval [CI], 31%–54%) for trisomy 21,85 72% (95% CI, 61%–81%) for trisomy 18,86 and 49% (95% CI, 29%–73%) for trisomy 1386 when estimating live birth prevalence.84 We used the same spontaneous pregnancy loss rates to estimate the prevalence of trisomies 21, 18, and 13 at 12 weeks’ gestation. We obtained the distribution of maternal age groups from 2014–2016 BORN data.82 Using the formula above, we calculated the maternal-age-specific prevalence of trisomies 21, 18, and 13 at 12 weeks’ gestation, and have summarized the results in Table 12.
Table 12:
Estimated Prevalence of Chromosomal Anomalies at 12 Weeks of Pregnancy
| Chromosomal Anomaly | N (95% CrI) per 10,000 Pregnant People, First Trimester (12 weeks) | N (95% CrI) per 10,000 Pregnant People, Second Trimester (18 weeks) | Sources |
|---|---|---|---|
| Autosomal Trisomies (Overall and by Maternal Age) | |||
| Trisomy 21 (overall)a | 23.9 (18.9–30.6) | 18.1 (14.2–23.1) | BORN, 2016; Savva et al, 2010; Morris et al, 199982,84,85 |
| < 20 years old | 11.8 (2.9–24.2) | 8.9 (2.2–18.3) | |
| 20–24 years old | 12.1 (7.1–18.6) | 9.1 (5.4–14.0) | |
| 25–29 years old | 13.4 (9.2–18.5) | 10.1 (6.9–13.9) | |
| 30–34 years old | 19.8 (14.7–25.8) | 15.0 (11.1–19.5) | |
| 35–39 years old | 55.2 (41.9–72.6) | 41.7 (31.6–54.8) | |
| Trisomy 18 (overall)a | 8.4 (5.4–12.7) | 6.9 (4.4–10.4) | BORN, 2016; Savva et al, 2010; Morris et al, 200882,84,86 |
| < 20 years old | 4.0 (0–12.2) | 3.2 (0–10.0) | |
| 20–24 years old | 4.1 (1.2–7.9) | 3.3 (1.0–6.5) | |
| 25–29 years old | 4.5 (2.2–7.7) | 3.7 (1.8–6.3) | |
| 30–34 years old | 6.5 (3.7–10.4) | 5.3 (3.1–8.5) | |
| 35–39 years old | 20.8 (12.7–32.0) | 17.0 (10.4–26.1) | |
| Trisomy 13 (overall)a | 3.0 (1.7–5.1) | 2.7 (1.5–4.6) | BORN, 2016; Savva et al, 2010; Morris et al, 200882,84,86 |
| < 20 years old | 1.5 (0–6.1) | 1.3 (0–5.5) | |
| 20–24 years old | 1.5 (0–4.1) | 1.3 (0–3.7) | |
| 25–29 years old | 1.7 (0.5–3.4) | 1.5 (0.4–3.1) | |
| 30–34 years old | 2.4 (1.1–4.5) | 2.2 (1.0–4.1) | |
| 35–39 years old | 7.1 (3.5–12.8) | 6.4 (3.1–11.5) | |
| Expected Sex Chromosome Aneuploidiesb | |||
| Monosomy X (45,X) | 13.0 per 10,000 female fetuses | NA | Viuff et al, 2015; Gravholt et al, 199687,88 |
| Triple X syndrome | 10.0 per 10,000 female fetuses | NA | Viuff et al, 2015; Tartaglia et al, 201087,89 |
| XXY syndrome (47,XXY) | 15.3 per 10,000 male fetuses | NA | Bojesen et al, 200390 |
| XYY syndrome | 10.0 per 10,000 male fetuses | NA | Viuff et al, 2015; Stochholm et al, 201287,91 |
| Detected Sex Chromosome Aneuploidiesb | |||
| Monosomy X (45,X) | 6.28 per 10,000 female fetuses | NA | European Registry92 |
| Triple X syndrome | 1.6 per 10,000 female fetuses | NA | Viuff et al, 2015; Tartaglia et al, 201087,89 |
| XXY syndrome (47,XXY) | 1.94 per 10,000 male fetuses | NA | European Registry92 |
| XYY syndrome | 0.5 per 10,000 male fetuses | NA | Viuff et al, 2015; Stochholm et al, 201287,91 |
| Microdeletion Syndromes | |||
| 22q11.2 deletion syndrome | 10.0 | NA | McDonald-McGinn et al, 201593 |
Abbreviations: BORN, Better Outcomes Registry and Network; CrI: credible interval; NA, not applicable.
Overall prevalence for pregnant people < 40 years old. Age distribution is based on BORN data from 2014 to 2016.82
The expected prevalence can be thought of as the “true” prevalence based on screening and subsequent confirmatory diagnostic testing. Because of the possible underdiagnosis and underreporting of sex chromosome aneuploidies, the expected prevalence is higher than the detected prevalence.
Because about 10% of pregnant people receive prenatal screening only in the second trimester, we also estimated the prevalence of chromosomal anomalies during the second trimester. We estimated the risk of spontaneous pregnancy loss between 12 and 18 weeks as a result of trisomy 21, 18, or 13 using the probabilities of spontaneous pregnancy loss from the first trimester to term, and from the second trimester to term. We also estimated the background risk of spontaneous pregnancy loss between 12 and 18 weeks for unaffected fetuses to be approximately 2%,94 much lower than for affected fetuses. Therefore, the prevalence of chromosomal anomalies in the second trimester would be lower than that in the first trimester. Furthermore, people may decide to voluntarily terminate a pregnancy if the fetus is confirmed to be affected. The risk of pregnancy loss is presented in Table 13.
Table 13:
Risk of Pregnancy Loss
| Chromosomal Anomaly | Mean; Distribution (alpha; beta) | Sources |
|---|---|---|
| Spontaneous Pregnancy Loss Between 12 Weeks (First Trimester) and Term | ||
| Trisomy 21 | 43%; beta (30.2; 40.0) | Morris et al, 199985 |
| Trisomy 18 | 72%; beta (55.0; 21.4) | Morris et al, 200886 |
| Trisomy 13 | 49%; beta (9.2; 9.6) | Morris et al, 200886 |
| Spontaneous Pregnancy Loss Between 18 Weeks (Second Trimester) and Term | ||
| Trisomy 21 | 23%; calculated | Morris et al, 199985 |
| Trisomy 18 | 65%; calculated | Morris et al, 200886 |
| Trisomy 13 | 42%; calculated | Morris et al, 200886 |
| Spontaneous Pregnancy Loss Between 12 Weeks (First Trimester) and 18 Weeks (Second Trimester) | ||
| Trisomy 21 | 26%; calculated | Morris et al, 199985 |
| Trisomy 18 | 20%; calculated | Morris et al, 200886 |
| Trisomy 13 | 12%; calculated | Morris et al, 200886 |
| Spontaneous Pregnancy Loss Between Diagnostic Testing (CVS or Amniocentesis) and Term | ||
| Monosomy X (45,X) | 40%; beta (8; 12) | Iyer et al, 201295 |
| Triple X syndrome | 3%a; fixed | Estimate based on Tartaglia et al, 2010; Hook, 198389,96 |
| XXY syndrome (47,XXY) | 4%; fixed | Bojesen et al, 2003; Hook, 198396 |
| XYY syndrome | 3%a; fixed | Hook, 198396 |
| 22q11.2 deletion syndrome | 62%; fixed | Estimate based on Hook et al, 198396 |
| Voluntary Termination of Pregnancy for a Confirmed Affected Fetus | ||
| Trisomy 21 | 74.6%; beta (2,033; 691) | Natoli et al, 201297 |
| Trisomy 18 | 74.6%; beta (2,033; 691) | Estimate |
| Trisomy 13 | 74.6%; beta (2,033; 691) | Estimate |
| Sex chromosome aneuploidy | 68%; beta (115; 54) | Christian et al, 200098 |
| 22q11.2 deletion syndrome | 68%; beta (115; 54) | Estimate |
| Diagnostic-Procedure-Related Pregnancy Loss | ||
| Amniocentesis | 0.11%; fixed | Akolekar et al, 20156 |
| Chorionic villus sampling | 0.22%; fixed | Akolekar et al, 20156 |
| Background Risk of Spontaneous Pregnancy Loss for Unaffected Fetuses | ||
| From 12 weeks (first trimester) to term | 3%; fixed | Ammon et al, 2012; Wyatt et al, 200594,99 |
| From 18 weeks (second trimester) to term | 1%; fixed | Ammon et al, 2012; Wyatt et al, 200594,99 |
| From 12 weeks (first trimester) to 18 weeks (second trimester) | 2%; fixed | Ammon et al, 2012; Wyatt et al, 200594,99 |
We assumed that the risk of spontaneous pregnancy loss for affected fetuses was greater than or equal to that of the unaffected fetus: 3%.
We also included sex chromosome aneuploidies and microdeletion syndromes in the scenario analysis (Tables 12 and 13). Prevalence data for sex chromosome aneuploidies and microdeletion syndromes are sparse. As well, because phenotypes vary widely for these conditions, they may be underdiagnosed. We included four sex chromosome aneuploidies (monosomy X, XXY syndrome, triple X syndrome, XYY syndrome), and the most prevalent microdeletion syndrome, 22q11.2 deletion syndrome. Unlike the autosomal trisomies, we accounted for the fact that sex chromosome aneuploidies and microdeletions occur independently of maternal age. We also assumed a sex ratio (male vs. female) of 1:1 for fetuses. Sex chromosome aneuploidies were conditional on fetal sex (i.e., monosomy X and triple X syndrome affected only female fetuses, and XXY syndrome and XYY syndrome affected only male fetuses). Because of the lack of literature, we could not distinguish between the prevalence of sex chromosome aneuploidies and microdeletions in the first and second trimester.
Accuracy of Prenatal Screening Tests
The accuracy of prenatal screening tests is presented in Table 14. Data from BORN showed a NIPT detection rate of 98.5% and a false-positive rate of 1.9% for trisomy 21 (note: BORN's data collection is ongoing and not all data for confirmatory diagnostic testing are available. The detection rate reflects confirmed affected cases). The false-positive rate for NIPT from preliminary local data was much higher than that found in the clinical evidence review and published meta-analyses (about 0.1%).19,67 A Cochrane review found that the combined NIPT false-positive rate for trisomies 21, 18, and 13 was approximately 0.1%.67 We used various NIPT false-positive rates in the sensitivity analyses. For first-tier NIPT, we estimated test performance for trisomies 18 and 13 based on our clinical evidence review of the average-risk or general population. For second-tier NIPT, we based NIPT test performance for trisomies 18 and 13 on a meta-analysis of select high-risk pregnant people from the Cochrane review (from studies that used the targeted massively parallel sequencing method).67
Table 14:
Accuracy of Prenatal Screening Tests, Reference Case
| Mean; Distribution (Parameter 1; Parameter 2) | |||
|---|---|---|---|
| Screening Option | Detection Rate | False-Positive Rate | Sources |
| NIPT | |||
| Trisomy 21 | 98.5%; beta (197; 3) in first-tier and second-tier NIPT | 2%; beta (160; 7,840)a | BORN data |
| Trisomy 18 | 93.1%; beta (27; 2) in first-tier NIPT | NA | Clinical evidence review |
| 98.2%; beta (110; 2) in second-tier NIPT | NA | Badeau et al, 201767 | |
| Trisomy 13 | 75%; beta (9; 3) in first-tier NIPT | NA | Clinical evidence review |
| 100%; fixed in second-tier NIPT | NA | Badeau et al, 201767 | |
| Failure rate at first and/or second blood draw | 2% uniform (0.01, 0.03) | NA | Clinical evidence review |
| eFTS | |||
| Trisomy 21 (risk cutoff: 1/400) | 91.2%; beta (125; 12) | 5.0%; beta (34; 650) | Huang et al, 20152 |
| Trisomy 18 | 72.9%; beta (35; 13) | Extra 0.2%b; fixed | Okun and Dougan, 2017; Huang et al 201883,103 |
| MSS (quadruple screening) | |||
| Trisomy 21 (risk cutoff: 1/200) | 65.4%; beta (39.2; 20.8) | 3.5%; beta (1,038; 28,962) | BORN data |
| Trisomy 18 | 52.8%; beta (66; 59) | 0.15%; beta (647, 423123) | Summers et al, 2003104 |
Abbreviation: BORN, Better Outcomes Registry and Network; eFTS, enhanced first-trimester screening; MSS, maternal serum screening (also known as quadruple screening); NA, not applicable; NIPT, noninvasive prenatal testing.
Note: The accuracy of traditional prenatal screening is based on multiple serum biomarker testing and may or may not include nuchal translucency ultrasound (depending on the prenatal screening option).
Combined false-positive rate: according to BORN data, the false-positive rate of NIPT for trisomy 21 was 1.9%. We estimated that the combined false-positive rate for trisomies 21, 18, and 13 was 2%. Note: BORN data collection is currently ongoing and data is incomplete. We addressed potential data issues by using a lower false-positive rate in the sensitivity analysis.
The combined false-positive rate for trisomies 21 and 18 was the false-positive rate of trisomy 21 plus 0.2% for trisomy 18.
We included sex chromosome aneuploidies and microdeletions in the scenario analysis. We based the performance of NIPT for sex chromosome aneuploidies on the Cochrane review, which focused on a high-risk population.67 Published data on NIPT test performance for microdeletion syndromes are sparse, but a case-control study showed a sensitivity of 90%, and nine out of 10 fetuses affected by the 22q11.2 deletion were identified.100 However, test accuracy studies with a case–control design may overestimate test performance, and may not represent an average-risk or general population.101 Negative NIPT results for microdeletions have seldom been further investigated in test accuracy studies, so potential false-negative results are often missed. For this reason, the sensitivity of NIPT for microdeletion syndromes in cohort studies has been rarely reported. However, most studies have shown a very low false-positive rate for NIPT for the 22q11.2 deletion.100,102 We used a fixed false-positive rate of 0.1%100,102 and varied the NIPT sensitivity for 22q11.2 deletion (90%, 75%, and 50%; see Table 17, below).
Table 17:
Additional Model Inputs Used in the Scenario Analyses
| Mean; Distribution (Alpha; Beta) | |||
|---|---|---|---|
| Screening Option | Detection Rate | False-Positive Rate | Sources |
| Inclusion of Sex Chromosome Aneuploidies and/or Microdeletions | |||
| Sex chromosome aneuploidies | 93.8%; beta (90; 6) | 0.4%; beta (4; 964) | Badeau et al, 201767 |
| 22q11.2 deletion | 90% or 75% or 50%; fixed | 0.1%; fixed | Ravi et al, 2018100; Martin et al, 2018102 |
| NIPT Performance Based on Cochrane Reviewa | |||
| Trisomy 21 | 99.2%; beta (87.3; 0.7) in first-tier NIPT | 0.1%; beta (20; 19,980)b in first-tier NIPT | Badeau et al, 201767 |
| 99.2%; beta (244; 2) in second-tier NIPT | 0.1%; beta (4; 3,996)a in second-tier NIPT | Badeau et al, 201767 | |
| Trisomy 18 | 90.9%; beta (20; 2) in first-tier NIPT | NA | Badeau et al, 201767 |
| 98.2%; beta (110; 2) in second-tier NIPT | NA | Badeau et al, 201767 | |
| Trisomy 13 | 62.5%; beta (5; 3) in first-tier NIPT | NA | Badeau et al, 201767 |
| 100%; fixed in second-tier NIPT | NA | Badeau et al, 201767 | |
| Lower Trisomy 21 Risk Cutoff for Second-Tier NIPT | |||
| eFTSb (risk cutoff: 1/1,000) | 94.8%; beta (129.9; 7.1) | 10.5%b; beta (71.8; 612.2) | Huang et al, 20152 |
| MSS (risk cutoff: 1/700) | 80.8%; beta (48.5; 11.5) | 10.1%; beta (3024; 26,976) | BORN data |
| Lower Trisomy 21 Risk Cutoff and Higher Acceptance of Further Testing for Second-Tier NIPT | |||
| Higher acceptance rate after positive results from traditional prenatal screening | 90% | — | Estimate |
Abbreviations: eFTS, enhanced first-trimester screening; MSS, maternal serum screening (also known as quadruple screening); NA, not applicable; NIPT, noninvasive prenatal testing.
Test failures (for any reason) were excluded in estimating the performance of NIPT.67
Combined false-positive rate for trisomies 21, 18, and 13.
We did not distinguish between the reasons for NIPT test failure at initial testing and repeat testing, because there is no additional cost to repeat the NIPT in Ontario.
Screening Uptake Rate
In Ontario from 2014 to 2016, the uptake of prenatal screening was approximately 68% in people who were less than 40 years of age at their estimated date of delivery (BORN data). In the reference scenario, we assumed that all three strategies had the same uptake rate of 68%. However, we assumed that for first-tier NIPT, the uptake rate may have been higher. Therefore, in the scenario analyses we kept the uptake rate of 68% for traditional prenatal screening and second-tier NIPT but increased it for first-tier NIPT to 80%, 90%, and 100% (Table 15).
Table 15:
Additional Clinical Parameters
| Parameter | Mean; Distribution (Parameter 1; Parameter 2) | Sources |
|---|---|---|
| Hypothetical cohort size of target population | 145,000; uniform (142,000; 148,000) | Calculated |
| Uptake rate for prenatal screening | ||
| Traditional prenatal screening | 68%; fixed | BORN data |
| Second-tier NIPT | 68%; fixed | BORN data |
| First-tier NIPT | 68%; fixed | BORN data |
| Pregnant people who accept prenatal screening | ||
| Screening in the first trimester | 90%; fixed | BORN data |
| Screening in the second trimester | 10%; fixed | BORN data |
| Accept further testing after a positive result | ||
| Traditional prenatal screening | 60%; fixed | Huang et al, 201775; Okun et al, 201483 |
| Second-tier NIPT | 76%; fixed | BORN data |
| Diagnostic testing after positive results from traditional prenatal screening and NIPT | 95%; fixed | Estimate |
| First-tier NIPT | 85%; fixed | Estimate |
| Very-high-risk populationa | 0.6%; fixed | BORN data |
Abbreviation: NIPT, noninvasive prenatal testing.
Very high risk was defined as pregnant people with a trisomy 21 risk > 1/10 or nuchal translucency ≥ 3.5 mm. In the second-tier NIPT strategy, these people would receive diagnostic testing directly instead of second-tier NIPT.
Further Testing After Positive Results From Traditional Prenatal Screening and/or NIPT
Based on an earlier Ontario study, we estimated that 60% of pregnant people with positive results from traditional prenatal screening would accept diagnostic testing.75,105 Based on BORN data, we estimated that 76% of pregnant people with positive results from traditional prenatal screening would accept second-tier NIPT. The rate of acceptance of further testing after receiving positive results from traditional prenatal screening substantially increased after the NIPT was implemented in Denmark,106 the United Kingdom,107 the Netherlands,108 and the United States109 (see Table A9). Therefore, we used a higher acceptance rate (i.e., 85% and 95%) for further testing for second-tier NIPT in a sensitivity analysis. We assumed that if both traditional prenatal screening and NIPT results were positive, 95% of people would choose diagnostic testing.
In the reference case, we estimated that 85% of pregnant people with positive results from first-tier NIPT would accept diagnostic testing. In the sensitivity analysis, we also explored diagnostic testing acceptance rates of 90% and 95% after first-tier NIPT.
Cost Parameters
Cost parameters are presented in Table 16. Based on the Ontario Schedule of Benefits,110 the Ontario Case Costing Initiative database,111 consultation with experts, and published Canadian economic studies, we identified cost parameters related to each screening strategy. Costs are reported in 2017 Canadian dollars.
Table 16:
Cost Parameters
| Parameter | Cost, $ | Sources |
|---|---|---|
| Prenatal screening | ||
| eFTSa | 124.50 | Data from an Ontario hospital, 2018 |
| MSSb | 186.75 | Data from an Ontario hospital, 2018 |
| NIPTc | ||
| Trisomies 21, 18, and 13, and sex chromosome aneuploidies | 390 | Ministry of Health and Long-Term Care |
| Inclusion of 22q11.2 deletion | Extra 195 (Panorama) | LifeLabs Genetics112 |
| NT ultrasound (for 90% people for first-tier NIPT) | 59.85 | Ontario Schedule of Benefits113 |
| General assessment physician visit (e.g., to introduce prenatal screening) | 77.20 | Ontario Schedule of Benefits113 |
| Genetic counselling for trisomies 21, 18, and 13 | ||
| Positive NIPT result | 74.70 | Ontario Schedule of Benefits113 |
| Negative NIPT result | 37.65 | Ontario Schedule of Benefits113 |
| Additional counselling for sex chromosome aneuploidies and/or 22q11.2 deletion | 74.70 | Ontario Schedule of Benefits113 |
| Amniocentesis (total) | 586 | |
| Physician | 102 | Ontario Schedule of Benefits113 |
| Laboratory expenses, labour, and reagents | 484 | Lilley et al, 2017114 |
| Chorionic villus sampling (total) | 1,003 | |
| Physician | 153 | Ontario Schedule of Benefits113 |
| Laboratory expenses, labour, and reagents | 850 | Lilley et al, 2017114 |
| Post-test physician visit for pregnant people with positive screening test results (any strategy) | 74.70 | Ontario Schedule of Benefits113 |
| Physician visit for pregnant people with a confirmed affected fetus | 161.15 | Ontario Schedule of Benefits113 |
| Voluntary termination of pregnancy (total) | 1,308 | |
| Surgeon and anesthesiologist | 204 | Ontario Schedule of Benefits113 |
| Termination of pregnancy | 1,104 | Ontario Case Costing Initiative115 |
| Spontaneous pregnancy loss or diagnostic testing procedure-related pregnancy loss (total) | 658 | |
| Surgeon and anesthesiologist | 204 | Ontario Schedule of Benefits113 |
| Ambulatory care | 454 | Ontario Case Costing Initiative115 |
Abbreviations: eFTS, enhanced first-trimester screening; MSS, maternal serum screening (also known as quadruple screening); NIPT, noninvasive prenatal testing; NT, nuchal translucency.
eFTS includes pregnancy-associated plasma protein A, free β-human chorionic gonadotrophin, placental growth factor, α-fetoprotein, and NT ultrasound.
MSS (quadruple screening) includes α-fetoprotein, unconjugated estriol, free β-human chorionic gonadotrophin. and inhibin-A.
If NIPT fails for any reason, there are no additional costs for a repeat test. If the repeat (second) NIPT test also fails, there are no refunds for unsuccessful tests.
We included an initial general assessment cost for all pregnant people in all three strategies. For the proportion of pregnant people who accepted prenatal screening, we included the costs of screening. We assumed that pregnant people with positive results from traditional prenatal screening or NIPT would have a post-test counselling visit, regardless of their decision to continue with diagnostic testing. If they decided to undergo diagnostic testing, there were associated physician fees. Those with a confirmed affected fetus would have another physician visit to discuss next steps.
The ministry-funded cost of NIPT is $390 per test. For pregnant people who choose NIPT (first-tier or second-tier), we included the cost of genetic counselling for trisomies 21, 18, and 13: $74.70 for a positive NIPT result and $37.65 for a negative NIPT result. We also assumed that about 90% of people would have the nuchal translucency ultrasound. Therefore, the total screening cost for first-tier NIPT was approximately $480 per person. We assumed an additional counselling unit for sex chromosome aneuploidies and 22q11.2 deletion, because genetic counselling may take longer for these conditions.
Most cost parameters (e.g., from the Schedule of Benefits) are either fixed in Ontario, or have a small standard error because of large sample sizes, such as the cost of termination of pregnancy from the Ontario Case Costing Initiative database.111 We assumed that costs followed normal distributions, and assigned a standard error of 10% of the mean in the probabilistic analyses to capture potential uncertainty in the estimates (e.g., varying the future cost of NIPT).
We included costs related to spontaneous pregnancy loss for affected fetuses or because of diagnostic testing but did not include the cost of spontaneous pregnancy loss for unaffected fetuses.
Analysis
Using the models and parameters above, we estimated the total annual health care costs and effectiveness of traditional prenatal screening without NIPT, second-tier NIPT, and first-tier NIPT for a hypothetical cohort of pregnant people with singleton pregnancies in Ontario. In the reference case, we presented the cost-effectiveness results in a standardized unit, per 10,000 pregnant people. We conducted a reference case analysis using probabilistic analysis by assigning probability distributions to model parameters. The probabilistic analysis can capture parameter uncertainty and individual variability.
We also conducted the following scenario analyses to examine the cost-effectiveness of adopting NIPT (see Table 17 for the additional parameter estimates used in the analyses):
Inclusion of sex chromosome aneuploidies (i.e., trisomies 21, 18, and 13 + sex chromosome aneuploidies) in first-tier NIPT
Inclusion of both sex chromosome aneuploidies and microdeletion syndromes (i.e., trisomies 21, 18, and 13 + sex chromosome aneuploidies + microdeletions) in first-tier NIPT
Increased uptake of first-tier NIPT (80%, 90%, and 100%)
Results from a recent Cochrane systematic review67 on NIPT test accuracy
A lower trisomy 21 risk cutoff (of positive results for traditional prenatal screening) in second-tier NIPT; and increasing the acceptance rate of further testing (after a positive result from traditional prenatal screening) from 76% to 90% for second-tier NIPT
A subgroup of pregnant people 35 to 39 years old at time of delivery (pregnant people at higher risk of chromosomal anomalies, but who do not meet the currently defined threshold of high-risk based on age [i.e., ≥ 40 years old])
We also conducted sensitivity analyses (e.g., changing from the mean to other fixed values for the input variables) to assess the impact of key variables on the total budget and effectiveness of each screening strategy, and the incremental cost-effectiveness ratio.
We ran 5,000 iterations for the reference case and 1,000 iterations for each of the scenario and sensitivity analyses. We reported the total cost and effectiveness of the target cohort, rather than the cost and effectiveness per target individual. For the reference case, we calculated the cost-effectiveness results in a standardized unit, per 10,000 people, to make the results comparable with earlier publications. The results are expressed as means (95% credible interval [CrI], i.e., 2.5th and 97.5th percentiles from the Monte Carlo simulation). We conducted all analyses using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Reference Case Analysis
The main cost and effectiveness outcomes of the three reference case strategies are summarized in Table 18. We have also reported costs and effectiveness for a standardized cohort size of 10,000 people in Table 19. We have presented the incremental cost, incremental effectiveness, and incremental cost per additional affected case detected for second-tier NIPT versus traditional prenatal screening, and for first-tier versus second-tier NIPT in Table 20.
Table 18:
Reference Case Analysis Results, Costs and Effectiveness for Three Prenatal Screening Strategiesa
| Strategy | Total Costs (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Affected Live Births (95% CrI), n |
|---|---|---|---|---|---|
| Traditional prenatal screening | 27,487,017 (24,140,385–30,815,491) | All: 158 (123–202) Trisomy 21: 123 (92–161) Trisomy 18: 35 (19–55) Trisomy 13: 1 (0–3) |
3,110 (2,297–4,040) | 6 (1–12) | All: 189 (162–216) Trisomy 21: 142 (119–166) Trisomy 18: 26 (17–36) Trisomy 13: 21 (12–30) |
| eFTS | 21,120,295 (18,338,516–23,895,779) | 149 (116–190) | 2,893 (2,078–3,820) | 6 (1–11) | 97 (78–116) |
| MSS | 2,719,663 (2,326,295–3,111,197) | 9 (3–16) | 218 (186–250) | 0 (0–1) | 12 (6–20) |
| No screening | 3,647,059 (2,950,378–4,341,750) | 0 | 0 | 0 | 80 (63–98) |
| Second-tier NIPT | 26,656,979 (23,423,892–29,934,127) | All: 188 (148–238) Trisomy 21: 147 (112–193) Trisomy 18: 41 (23–65) Trisomy 13: 0 (0–1) |
718 (634–814) | 1 (0–3) | All: 177 (151–204) Trisomy 21: 132 (109–155) Trisomy 18: 24 (15–35) Trisomy 13: 21 (12–30) |
| eFTS + NIPT | 20,283,588 (17,612,370–22,963,994) | 178 (139–226) | 678 (596–769) | 1 (0–3) | 86 (68–105) |
| MSS + NIPT | 2,726,332 (2,330,961–3,116,509) | 11 (4–18) | 40 (28–54) | 0 (0–1) | 12 (5–19) |
| No screening | 3,647,059 (2,950,378–4,341,750) | 0 | 0 | 0 | 80 (63–98) |
| First-tier NIPT | 61,292,985 (53,532,496–69,178,382) | All: 273 (219–337) Trisomy 21: 191 (148–245) Trisomy 18: 63 (38–97) Trisomy 13: 18 (7–35) |
2,125 (1,837–2,427) | 4 (0–8) | All: 147 (123–171) Trisomy 21: 112 (91–134) Trisomy 18: 20 (11–29) Trisomy 13: 14 (7–23) |
| First trimester | 52,509,033 (45,522,346–59,513,228) | 251 (202–313) | 1,780 (1,537–2,035) | 3 (0–7) | 60 (46–77) |
| Second trimester | 5,136,893 (4,375,162–5,895,052) | 22 (12–32) | 346 (253–442) | 0 (0–2) | 7 (2–12) |
| No screening | 3,647,059 (2,950,378–4,341,750) | 0 | 0 | 0 | 80 (63–98) |
Abbreviations: CrI, credible interval; eFTS, enhanced first-trimester screening; MSS, maternal serum screening (also known as quadruple screening); NIPT, noninvasive prenatal testing.
Note: Numbers may appear inexact due to rounding. The prevalence of chromosomal anomalies in the second trimester was lower than in the first trimester because of a risk of spontaneous pregnancy loss between the first and second trimester.
Mean cohort size: 145,022 pregnant people.
Table 19:
Reference Case Analysis Results, Cost and Effectiveness Per 10,000 Average-Risk Pregnant People
| Strategy | Total Costs, $ | Cases Detected, n | Diagnostic Tests Performed, n | Diagnostic-Procedure-Related Pregnancy Losses, n | Affected Live Births, n |
|---|---|---|---|---|---|
| Traditional prenatal screening | 1,895,372 | 10.9 | 214.5 | 0.4 | 13.0 |
| Second-tier NIPT | 1,838,137 | 13.0 | 49.5 | 0.1 | 12.2 |
| First-tier NIPT | 4,226,468 | 18.8 | 146.6 | 0.3 | 10.1 |
Abbreviation: NIPT, noninvasive prenatal testing.
Table 20:
Reference Case Analysis Results, Incremental Cost, Incremental Effectiveness, and Incremental Cost per Case Detected
| Comparison | Incremental Costs (95% CrI), $ | Additional Cases Detected (95% CrI), n | Difference in Diagnostic Tests (95% CrI), n | Difference in Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Difference in Affected Live Births (95% CrI), n | Incremental Cost Per Case Detected, $ |
|---|---|---|---|---|---|---|
| Second-tier NIPT vs. traditional prenatal screening | −830,038 (−1,502,076 to –266,284) | 30 (6–56) | −2,392 (−3,275 to –1,612) | −5 (−11 to 0) | −11 (−34 to 12) | Dominant |
| First-tier vs. second-tier NIPT | 34,636,006 (27,131,648– 42,272,116) | 84 (54–117) | 1,407 (1,128–1,696) | 3 (−1 to 7) | −31 (−53 to –8) | 411,274 |
Abbreviations: CrI, credible interval; NIPT, noninvasive prenatal testing.
Note: Numbers may appear inexact due to rounding.
The mean cohort size of the target population was 145,022 (95% CrI, 142,160–147,856) in 5,000 simulations. This reflected the approximate number of annual singleton pregnancies in Ontario over next 5 years. Of these, 88,754 (95% CrI, 86,972–90,541) and 9,657 (95% CrI, 9,373–9,944) people received prenatal screening in the first and second trimester, respectively. The remaining 46,611 (95% CrI, 45,586–47,627) had no prenatal screening. The total number of fetuses with any trisomy was 512 (95% CrI, 424–623). The total numbers of fetuses with trisomy 21, 18, or 13 were 347 (95% CrI, 275–440), 121 (95% CrI, 80–181), and 44 (95% CrI, 24–75), respectively.
Second-tier NIPT dominated traditional prenatal screening. Compared with traditional prenatal screening, second-tier NIPT detected more affected fetuses, substantially reduced the number of diagnostic tests performed, and slightly reduced the total screening budget.
Compared with second-tier NIPT, first-tier NIPT was associated with 84 (95% CrI, 54–117) additional affected fetuses detected, 1,407 (95% CrI, 1,128–1,696) additional diagnostic tests performed, and an additional cost of $35 million (95% CrI, $27 million–$42 million).
Scenario Analyses
Inclusion of Sex Chromosome Aneuploidies and 22q11.2 Deletion in First-Tier NIPT
The results for first-tier NIPT including screening for sex chromosome aneuploidies, with or without the 22q11.2 deletion, are presented in Table 21.
Table 21:
Scenario Analysis Results, Inclusion of Sex Chromosome Aneuploidies and 22q11.2 Deletion in First-Tier NIPT
| Scenario | Total Cost (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Affected Live Births (95% CrI), n | Incremental Cost Per Case Detected, vs. Second-Tier NIPT,a $ |
|---|---|---|---|---|---|---|
| Trisomies and Sex Chromosome Aneuploidies | ||||||
| Trisomies 21, 18, and 13, and sex chromosome aneuploidies (expected prevalence) | 69,137,967 (60,606,739–77,198,825) | All: 456 (396–530) Trisomies 21, 18, 13: 272 (218–335) SCA: 185 (156–215) |
2,696 (2,290–3,203) |
5 (1–10) | All: 339 (302–382) Trisomies 21, 18, and 13: 147 (123–171) SCA: 192 (163–227) |
158,623 |
| Trisomies 21, 18, and 13, and sex chromosome aneuploidies (detected prevalence) | 68,994,511 (60,901,341–76,637,397) | All: 313 (258–378) Trisomies 21, 18, 13: 273 (219–340) SCA: 40 (27–52) |
2,501 (2,098–2,957) |
4 (1–9) | All: 181 (154–211) Trisomies 21, 18, and 13: 146 (122–172) SCA: 35 (22–49) |
339,908 |
| Trisomies and Sex Chromosome Aneuploidies and 22q11.2 Deletion | ||||||
| Trisomies 21, 18, and 13, sex chromosome aneuploidies (expected prevalence) and 22q11.2 deletion (90% detection rate) | 88,748,952 (77,081,835–100,742,353) | All: 531 (468–603) Trisomies 21, 18, 13: 273 (220–334) SCA: 185 (156–215) 22Q: 74 (57–93) |
2,801 (2,392–3,307) |
5 (1–10) | All: 374 (329–420) Trisomies 21, 18, 13: 146 (121–173) SCA: 193 (159–227) 22Q: 36 (24–48) |
181,381 |
| Trisomies 21, 18, and 13, sex chromosome aneuploidies (expected prevalence) and 22q11.2 deletion (75% detection rate) | 88,806,163 (76,749,356–100,783,584) | All: 520 (456–596) Trisomies 21, 18, 13: 273 (225–337) SCA: 186 (157–216) 22Q: 62 (46–77) |
2,791 (2,381–3,303) |
5 (1–9) | All: 378 (333–424) Trisomies 21, 18, 13: 147 (124–170) SCA: 193 (158–227) 22Q: 39 (27–51) |
187,534 |
| Trisomies 21, 18, and 13, sex chromosome aneuploidies (expected prevalence) and 22q11.2 deletion (50% detection rate) | 88,711,674 (76,501,667–100,834,662) | All: 499 (432–572) Trisomies 21, 18, 13: 273 (218–339) SCA: 185 (157–213) 22Q: 42 (30–55) |
2,769 (2,379–3,273) |
5 (1–9) | All: 383 (340–427) Trisomies 21, 18, 13: 146 (123–171) SCA: 192 (161–224) 22Q: 44 (32–58) |
199,667 |
Abbreviations: 22Q, 22q11.2 deletion; CrI, credible interval; DR, detection rate; NIPT, noninvasive prenatal testing; SCA, sex chromosome aneuploidies.
Note: Numbers may appear inexact due to rounding.
We compared first-tier NIPT (including sex chromosome aneuploidies and 22q11.2 deletion) with second-tier NIPT. The cost and effectiveness of first-tier NIPT (including sex chromosome aneuploidies and 22q11.2 deletion) were based on simulations from the scenario analysis, but the cost and effectiveness of second-tier NIPT were based on the results of the reference case analysis. See Table 18 (reference case) for cost and effectiveness outcomes for second-tier NIPT.
When first-tier NIPT included trisomies 21, 18, and 13, and sex chromosome aneuploidies, the total cost increased to $69 million (95% CrI, $61 million–$77 million) using either the expected or detected sex chromosome aneuploidy prevalence (the detected prevalence is much lower than the expected prevalence). Although there are no additional costs associated with testing for sex chromosome aneuploidies in NIPT, more genetic counselling would be needed (additional $74.70). On average, 185 cases of sex chromosome aneuploidies would be detected using expected detected sex chromosome aneuploidy prevalence, and 40 cases would be detected using the expected prevalence.
When first-tier NIPT included trisomies 21, 18, and 13, sex chromosome aneuploidies, and 1 microdeletion syndrome (22q11.2 deletion), the total cost increased to $89 million (95% CrI, $77 million–$101 million). Including the 22q11.2 deletion would involve an additional $195 for the test and an additional $74.70 for more genetic counselling. First-tier NIPT would detect 74, 62, and 42 cases of 22q11.2 deletion at detection rates of 90%, 75%, and 50%, respectively.
Increased Uptake of First-Tier NIPT
Increasing the uptake of first-tier NIPT led to increased total costs and a greater number of affected fetuses detected (Table 22) compared to the reference case. The incremental cost-effectiveness of first-tier NIPT improved with an increase in uptake rate.
Table 22:
Scenario Analysis Results, Increased Uptake of First-Tier NIPT
| Uptake of First-Tier NIPT | Total Costs (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Affected Live Births (95% CrI), n | Incremental Cost Per Case Detected, vs. Second-Tier NIPT,a $ |
|---|---|---|---|---|---|---|
| 80% | 70,132,717 (60,705,461–79,300,267) | All: 321 (263–400) Trisomy 21: 224 (176–286) Trisomy 18: 75 (45–116) Trisomy 13: 22 (9–42) |
2,497 (2,154–2,859) | 4 (1–9) | All: 129 (105–155) Trisomy 21: 98 (78–120) Trisomy 18: 18 (10–26) Trisomy 13: 13 (6–21) |
327,247 |
| 90% | 77,488,657 (67,296,999–87,898,000) | All: 362 (296–451) Trisomy 21: 253 (197–321) Trisomy 18: 84 (53–129) Trisomy 13: 24 (10–46) |
2,807 (2,443–3,187) | 5 (1–9) | All: 114 (92–136) Trisomy 21: 86 (68–104) Trisomy 18: 15 (8–24) Trisomy 13: 12 (5–20) |
293,134 |
| 100% | 84,473,692 (72,615,687–96,107,514) | All: 400 (326–495) Trisomy 21: 280 (220–360) Trisomy 18: 93 (58–141) Trisomy 13: 27 (12–52) |
3,124 (2,708–3,563) | 6 (2–11) | All: 98 (79–120) Trisomy 21: 74 (57–93) Trisomy 18:14 (7–21) Trisomy 13: 11 (5–19) |
273,187 |
Abbreviations: CrI, credible interval; NIPT, noninvasive prenatal screening.
Note: Numbers may appear inexact due to rounding.
See Table 18 (reference case) for cost and effectiveness outcomes for second-tier NIPT.
Results From a Cochrane Systematic Review on NIPT Test Accuracy
In general, the detection rates of trisomy 21, 18, and 13 from BORN data and our clinical evidence review were similar to those found in the Cochrane review.67 However, the number of NIPT false positives from the BORN data was much higher than that from the Cochrane review (2% versus 0.1%). When we used the test accuracy results from the Cochrane review in our model, the number of diagnostic tests performed for first-tier NIPT was substantially reduced, from 2,125 in the reference case to 572 (95% CrI, 442–710). As a result, the total cost of first-tier NIPT also decreased. In contrast, using the Cochrane review results had only a marginal effect on the second-tier NIPT results (Table 23).
Table 23:
Scenario Analysis Results, Results From a Cochrane Systematic Review on NIPT Test Accuracy
| Strategy | Total Costs (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Affected Live Births (95% CrI), n | Incremental Cost Per Case Detected, vs. Second-Tier NIPT, $ |
|---|---|---|---|---|---|---|
| Second-tier NIPT | 26,649,544 (23,187,889–29,795,150) | All: 189 (148–236) Trisomy 21: 148 (112–195) Trisomy 18: 41 (23–64) Trisomy 13: 0 (0–1) |
659 (584–742) | 1 (0–3) | All: 177 (150–204) Trisomy 21: 132 (109–155) Trisomy 18: 25 (15–35) Trisomy 13: 21 (12–31) |
— |
| First-tier NIPT | 59,674,614 (51,919,853–67,932,570) | All: 269 (215–331) Trisomy 21: 192 (150–246) Trisomy 18: 62 (37–94) Trisomy 13: 15 (5–31) |
572 (442–710) | 1 (0–3) | All: 148 (124–173) Trisomy 21: 112 (91–134) Trisomy 18: 20 (12–30) Trisomy 13: 15 (8–24) |
412,010 |
Abbreviations: CrI, credible interval; NIPT, noninvasive prenatal testing.
Note: Numbers may appear inexact due to rounding.
A Lower Trisomy 21 Risk Cutoff for Traditional Prenatal Screening in Second-Tier NIPT
We used a trisomy 21 risk cutoff of 1:1,000 for eFTS and 1:700 for MSS (compared with 1:400 for eFTS and 1:200 for MSS in the reference case) to determine positive results for traditional prenatal screening (i.e., false-positive rate around 10% for trisomy 21) in second-tier NIPT. A risk cutoff is a threshold used to determine whether screening results are positive (high risk for a chromosomal anomaly) or negative (low risk for a chromosomal anomaly). A risk cutoff of 1:1,000 means that the screening test results are described as “positive” if they show that a fetus's chance of having a chromosomal anomaly is 1 in 1,000 or higher. With these lower cutoffs, the detection rate increased compared with the reference case (eFTS, 94.8% versus 91.2%; MSS, 80.8% versus 65.4%), and an additional seven cases of trisomy 21 were detected (Table 24). Compared with second-tier NIPT in the reference case, the volumes of NIPT and diagnostic testing, as well as the total screening cost, also increased. We did not consider a potential increase in detection of trisomies 18 and 13 when using a lower cutoff for trisomy 21.
Table 24:
Scenario Analysis Results, A Lower Trisomy 21 Risk Cutoff for Traditional Prenatal Screening in Second-Tier NIPT
| Scenario | Total Costs (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Affected Live Births (95% CrI), n | Incremental Cost Per Case Detected, First-Tier vs. Second-Tier NIPT, $ |
|---|---|---|---|---|---|---|
| Acceptance of further testing after a positive result from traditional prenatal screening: 76% (same as reference case) | 28,949,494 (25,455,144–32,548,127) | All: 195 (153–247) Trisomy 21: 154 (117–202) Trisomy 18: 41 (23–65) Trisomy 13: 0 (0–2) |
908 (782–1,047) | 1 (0–3) | All: 174 (148–201) Trisomy 21: 129 (106–152) Trisomy 18: 24 (15–35) Trisomy 13: 21 (13–31) |
415,702 |
| Acceptance of further testing after a positive result from traditional prenatal screening: 90% | 29,488,926 (25,937,229–32,769,117) | All: 231 (181–288) Trisomy 21: 182 (140–234) Trisomy 18: 49 (27–76) Trisomy 13: 0 (0–2) |
1,055 (920–1,210) | 1 (0–4) | All: 161 (135–188) Trisomy 21: 117 (94–140) Trisomy 18: 23 (13–34) Trisomy 13: 21 (12–31) |
758,384 |
Abbreviations: CrI, credible interval; N, number; NIPT, noninvasive prenatal screening.
Note: Numbers may appear inexact due to rounding.
Incremental cost per case detected for first-tier NIPT (see Table 18 for reference case results) versus second-tier NIPT (the present scenario).
We also conducted analyses for second-tier NIPT using a lower trisomy 21 risk cutoff in combination with a higher acceptance rate for further testing (90%) after positive results from traditional prenatal screening. Compared with second-tier NIPT in the reference case, this scenario detected an additional 43 affected fetuses. However, the detection of affected fetuses using second-tier NIPT was still less than that using first-tier NIPT.
A Subgroup of Pregnant People 35 to 39 Years Old
Results for the scenario analysis assessing a subgroup of pregnant people aged 35 to 39 years are presented in Table 25. According to our simulations, this subgroup accounted for approximately 20% of average-risk pregnant people who were less than 40 years old. This subgroup had 237 (95% CrI, 190–295) affected fetuses, accounting for about 46% of the affected fetuses in the entire average-risk cohort. Compared with second-tier NIPT, first-tier NIPT led to an additional cost of $6.8 million (95% CrI, $5.4 million–$8.5 million) for this cohort of 28,563 pregnant people. The incremental cost per affected case detected of first-tier versus second-tier NIPT was $173,680, lower than that for the entire reference case cohort ($411,274).
Table 25:
Scenario Analysis Results, A Subgroup of Pregnant People 35 to 39 Years Olda
| Strategy | Total Costs (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Diagnostic-Procedure-Related Pregnancy Losses (95% CrI), n | Affected Live Births (95% CrI), n | Incremental Cost Per Case Detected, vs. Second-Tier NIPT, $ |
|---|---|---|---|---|---|---|
| Traditional prenatal screening | 5,540,080 (4,888,764–6,164,005) | All: 73 (52–98) Trisomy 21: 56 (38–77) Trisomy 18: 17 (7–29) Trisomy 13: 0 (0–2) |
653 (482–841) | 1 (0–4) | All: 87 (69–107) Trisomy 21: 65 (49–82) Trisomy 18: 12 (6–20) Trisomy 13: 10 (4–16) |
— |
| Second-tier NIPT | 5,398,917 (4,744,893–6,045,225) | All: 87 (64–114) Trisomy 21: 67 (46–91) Trisomy 18:20 (10–33) Trisomy 13: 0 (0–1) |
191 (159–225) | 0 (0–1) | All: 82 (65–100) Trisomy 21: 60 (46–76) Trisomy 18: 12 (6–19) Trisomy 13: 10 (4–17) |
— |
| First-tier NIPT | 12,257,869 (10,619,425–13,982,271) | All: 126 (98–162) Trisomy 21: 87 (64–117) Trisomy 18:31 (17–50) Trisomy 13: 9 (2–18) |
490 (423–564) | 1 (0–3) | All: 68 (52–85) Trisomy 21: 51 (38–67) Trisomy 18: 10 (4–16) Trisomy 13:7 (2–13) |
173,680 |
Abbreviations: CrI, credible interval; NIPT, noninvasive prenatal testing.
Note: Numbers may appear inexact due to rounding.
Average cohort size: 28,563 pregnant people.
Sensitivity Analysis
Table 26 shows the results of the sensitivity analyses. We examined several factors that could affect the costs and effectiveness of different screening strategies: NIPT price, NIPT false-positive rate, higher trisomy prevalence, acceptance rate for further testing, and higher NIPT failure rate.
Table 26:
Sensitivity Analysis Results, Prenatal Screening Strategies
| Variable | Strategy | Total Costs (95% CrI), $ | Cases Detected (95% CrI), n | Diagnostic Tests Performed (95% CrI), n | Incremental Cost Per Case Detected, First-Tier vs. Second-Tier NIPT, $ |
|---|---|---|---|---|---|
| NIPT Price | |||||
| $200 | Second-tier NIPT | 26,084,204 (22,798,602–29,280,686) | NAa | NAa | — |
| First-tier NIPT | 42,750,076 (38,190,790–47,735,425) | NAa | NAa | 198,236 | |
| $300 | Second-tier NIPT | 26,412,508 (22,909,454–29,548,072) | NAa | NAa | — |
| First-tier NIPT | 52,333,813 (45,555,192–58,487,874) | NAa | NAa | 309,106 | |
| $500 | Second-tier NIPT | 27,119,609 (23,728,181–30,263,419) | NAa | NAa | — |
| First-tier NIPT | 72,141,215 (62,179,210–82,628,259) | NAa | NAa | 535,404 | |
| $600 | Second-tier NIPT | 27,460,478 (24,118,240–30,805,287) | NAa | NAa | — |
| First-tier NIPT | 81,849,048 (70,303,593–93,705,152) | NAa | NAa | 647,421 | |
| NIPT False-Positive Rate | |||||
| 0.1% | Second-tier NIPT | 26,654,498 (23,246,365–29,967,176) | NAa | 659 (582–746) | — |
| First-tier NIPT | 59,789,622 (51,478,080–68,306,686) | NAa | 571 (444–699) | 394,997 | |
| 0.5% | Second-tier NIPT | 26,715,288 (23,418,883–29,980,976) | NAa | 672 (599–757) | — |
| First-tier NIPT | 59,991,375 (51,792,152–68,305,006) | NAa | 901 (766–1,036) | 396,309 | |
| 1% | Second-tier NIPT | 26,725,446 (23,407,759–29,910,452) | NAa | 687 (608–773) | — |
| First-tier NIPT | 60,395,928 (52,277,838–68,913,184) | NAa | 1,308 (1,178–1,445) | 403,157 | |
| Trisomy Prevalence | |||||
| 10% lower prevalence of trisomies 21, 18 and 13 than the reference case | Second-tier NIPT | 26,637,545 (23,471,426–30,144,412) | 169 (130–211) | 702 (623–796) | — |
| First-tier NIPT | 61,228,599 (53,246,695–69,691,743) | 245 (194–301) | 2,108 (1,821–2,406) | 456,702 | |
| 20% higher prevalence of trisomies 21, 18, and 13 than the reference case | Second-tier NIPT | 26,757,004 (23,432,884–29,950,239) | 225 (179–282) | 754 (671–857) | — |
| First-tier NIPT | 61,468,969 (53,499,592–69,764,090) | 326 (260–399) | 2,177 (1,900–2,492) | 342,973 | |
| Acceptance Rate for Further Testing | |||||
| After positive NIPT results, first-tier NIPT: 90% | First-tier NIPT | 61,461,986 (53,728,139–69,621,998) | 288 (232–358) | 2,242 (1,936–2,554) | 347,947b |
| After positive NIPT results, first-tier NIPT: 95% | First-tier NIPT | 61,526,572 (53,679,534–69,414,722) | 305 (244–374) | 2,368 (2,049–2,719) | 299,771b |
| After positive results from traditional prenatal screening, second-tier NIPT: 85% | Second-tier NIPT | 26,910,157 (23,520,315–30,207,872) | 210 (166–267) | 804 (711–916) | 546,804c |
| After positive results from traditional prenatal screening, second-tier NIPT: 95% | Second-tier NIPT | 27,163,740 (23,753,183–30,751,347) | 234 (188–298) | 898 (800–1,008) | 891,252c |
| NIPT Failure Rate | |||||
| 4%d | Second-tier NIPT | 26,774,569 (23,364,183–30,097,831) | 188 (147–236) | 783 (687–880) | — |
| First-tier NIPT | 61,567,714 (53,390,895–70,139,842) | 270 (216–330) | 2,310 (2,046–2,588) | 426,967 | |
| Cost of Post-Test Physician Visits for Pregnant People With Positive Screening Results for All Strategies | |||||
| $33.7 | Second-tier NIPT | 26,475,773 (23,201,201–29,875,843) | NAa | NAa | — |
| First-tier NIPT | 61,197,204 (52,937,184–69,348,009) | NAa | NAa | 413,414 | |
Abbreviations: CrI, credible interval, NIPT, noninvasive prenatal testing.
Note: Numbers may appear inexact due to rounding.
This analysis did not affect the results of this outcome, which was same as that reported in Table 18 (reference case).
The cost and effectiveness of second-tier NIPT was based on the reference case results.
The cost and effectiveness of first-tier NIPT was based on the reference case results.
There was no additional cost for a repeat NIPT test.
The price of NIPT affected the total screening costs of first-tier NIPT, but it had minimal impact on the costs for second-tier NIPT. When the NIPT false-positive rate was lower (e.g., 0.5% or 1%), the number of diagnostic tests for first-tier NIPT decreased substantially, and the total cost of screening for this strategy was also reduced. When the prevalence of trisomies increased (e.g., due to the increasing maternal age at date of delivery), the number of affected cases detected also increased. When a greater proportion of people with positive results from initial screening accepted further testing (for first-tier or second-tier NIPT), the number of affected cases detected also increased.
Discussion
Our economic evaluation showed that second-tier NIPT performed better than traditional screening—increasing the number of detected fetuses and decreasing the number of diagnostic tests performed. The main reason for the higher detection rate was increased acceptance of further testing after positive screening results with second-tier NIPT. The total cost of second-tier NIPT was slightly lower than that of traditional prenatal screening, mainly because of the reduced volume of diagnostic testing. In general, these findings were consistent with a recently published study by Huang et al75 using Ontario data and Ontario-specific costs.
Compared with second-tier NIPT, first-tier NIPT detected more affected fetuses, but at a significantly increased total cost. According to the study by Huang et al, detection rates for trisomy 21 with first-tier or second-tier NIPT could be very close if a lower risk cutoff was used to define positive results in traditional prenatal screening for second-tier NIPT.75
There were some other differences between our evaluation and the study by Huang et al. We included trisomies 21, 18, and 13, and used different acceptance rates for further testing after a positive NIPT result (85% for first-tier NIPT and 76% for second-tier NIPT).56 Therefore, some of the additional affected cases identified by first-tier NIPT occurred not only because of the higher detection rate of NIPT, but also because of expected higher uptake of diagnostic testing. In contrast, the study by Huang et al. focused only on trisomy 21 and assumed that 100% of pregnant people with positive screening results would accept further testing for both first-tier and second-tier NIPT.75 Furthermore, our economic evaluation included all average-risk pregnant people who accepted (68%) or declined (32%) prenatal screening. The study by Huang et al included only pregnant people who accepted prenatal screening.75
First-tier NIPT has not been adopted in most developed countries, including Canada. Therefore, the exact diagnostic pathway of first-tier NIPT is difficult to determine. Debates exist as to whether the costs of nuchal translucency ultrasound and genetic counselling for negative NIPT results should be included in the cost of first-tier NIPT; we chose to include these costs in the reference case analyses. However, if these costs are excluded, the cost of first-tier NIPT would be reduced by around $8.9 million annually ($5.3 million for the nuchal translucency ultrasound and $3.6 million for genetic counselling for negative NIPT results). If two additional factors favoring first-tier NIPT are considered (i.e., using the Cochrane review for the accuracy data of NIPT and excluding the cost of unsuccessful NIPT tests), the total cost of first-tier NIPT would be reduced by about $11.2 million annually. Then, the annual budget increase of first-tier NIPT compared with second-tier NIPT would be reduced from $34.6 million to around $23.4 million in the reference case, and the incremental cost to detect an affected case would be reduced to $280,000.
In this study, the main factors affecting the costs and effectiveness of the different prenatal screening strategies were the uptake rate of prenatal screening, the acceptance rate of further testing after positive screening results, test accuracy, and the prices of both traditional prenatal screening and NIPT.
We discuss other findings from our economic evaluation below.
Measure of Effectiveness
Canadian economic evaluation guidelines recommend using quality-adjusted life-years (QALYs) as the primary measure of effectiveness whenever possible.116 However, QALYs were not a feasible measure for this evaluation. Detection of an affected fetus may lead a pregnant person to terminate the affected pregnancy. The “effectiveness” of these two outcomes (i.e., newborn affected with a chromosomal anomaly versus termination of pregnancy) cannot be readily compared using a measure such as the life-year or QALY. There is also no consensus in the literature about whether to include only pregnant people in economic evaluations, or to include pregnant people, their partners, and their newborns. Furthermore, because of a paucity of published studies assessing health utilities related to terminating a pregnancy, health utilities related to having (or not having) a child with a chromosomal anomaly, and the duration of psychological effects related to clinical outcomes, it is difficult to reliably estimate QALYs for pregnant people and newborns. For this reason, we did not conduct a cost-utility analysis.
As well, we did not include psychological outcomes in our economic evaluation. The two most relevant psychological outcomes are anxiety and depression related to either a positive test result or a pregnancy loss (i.e., spontaneous pregnancy loss, procedure-related pregnancy loss, or voluntary termination). A Canadian study concluded that prenatal screening did not cause serious psychological harm to pregnant people in Ontario.117 However, there are psychological outcomes associated with the termination of pregnancy, and likely psychological effects for pregnant people who choose to continue with an affected pregnancy. It was therefore difficult to justify how to appropriately incorporate psychological outcomes in our model.
Sex Chromosome Aneuploidies and Microdeletion Syndromes
In addition to trisomies 21, 18, and 13, we included four common sex chromosome aneuploidies and 22q11.2 deletion syndrome in the scenario analyses. At present, reliable prevalence data for these conditions are sparse. In addition, the accuracy of NIPT for these conditions in the average-risk or general population has not been well investigated. Therefore, the results of these analyses were less robust and should be interpreted with caution.
Real-World Performance of Screening Tests
We based detection rates and false-positive rates for NIPT and MSS on local Ontario data. Data collection is still ongoing, so findings on test performance are still preliminary. We addressed these data-quality issues in the scenario analyses. Clinical trials often recruit homogeneous populations by setting rigid inclusion and exclusion criteria; real-world data likely includes more heterogeneous cohorts that better reflect the actual population. In addition, the chromosomal anomalies of interest are relatively rare, so one or two affected cases can greatly affect detection rates; it is not surprising that greater variation in test performance may be observed in real-world data.
Prenatal Screening in Twin Pregnancies
In Ontario, approximately 3% of infants are from a multiple-gestation pregnancy (4,730 from 147,244 pregnancies in 2015–2016),82 and the majority of multiple-gestation pregnancies involve twins. We did not include twin pregnancies in our analyses because the published data on eFTS and MSS performance for twins is sparse. One review19 found lower detection rates with NIPT in twins compared with singleton pregnancies (9%, 28%, and 22% lower for trisomies 21, 18, and 13, respectively), but another systematic review118 reported very high pooled NIPT accuracy for twin pregnancies (99% [95% CI, 92%–100%] for trisomy 21 and 85% [95% CI, 55%–98%] for trisomy 18). Furthermore, the clinical pathway for screening twin pregnancies is more complex. Because of these uncertainties in the model inputs, we did not conduct an analysis for twins.
Cost of Live Births Affected by Chromosomal Anomalies
We did estimate the number of live births affected by the chromosomal anomalies of interest. However, we did not estimate the long-term costs of managing these conditions because of ethical considerations related to valuing the lives of people with these chromosomal anomalies, and to comparing those costs to the consequences of pregnancy termination.
Health Costs Not Included in the Economic Analysis
We did not include the following costs in the economic analysis, because it was difficult to accurately estimate them, and because they would have had a minimal impact on our analyses:
We did not include additional follow-up costs for those who received a positive screening result but declined confirmatory diagnostic testing; still, this population may receive additional follow-up visits or interventions based on their positive screening result
We did not consider the additional use of health care resources for a second blood draw for NIPT. We assumed that there would be no additional test cost because a second draw for NIPT is currently covered for a pregnant person if the first draw fails
In the case of first-tier NIPT, MSS would be performed only for pregnant people with a failed NIPT test result. In this situation, the cost of MSS would likely be higher because of the low volume of tests performed
Study Strengths
Our study had the following strengths:
Our key parameters, main model assumptions, and screening pathways reflected the current prenatal screening context in Ontario and were verified by clinical experts
We obtained local Ontario data to estimate several important parameters in the model, including NIPT test accuracy for trisomy 21 and the uptake of prenatal screening
We evaluated the effect of including NIPT for additional chromosomal anomalies (i.e., sex chromosome aneuploidies and microdeletions). Previous economic evaluations have focused on the use of NIPT for only trisomies 21, 18, and 13
Study Limitations
The following limitations should be noted when interpreting the findings of this analysis:
First-tier NIPT has not been adopted in most developed countries; therefore, we did not find high-quality evidence for input parameters related to this screening strategy
The reality of prenatal screening is more complex than our current model. However, our model includes the most common screening pathways that affect most pregnant people
We found no high-quality evidence for input parameters related to sex chromosome aneuploidies and microdeletions; results should be interpreted with caution
Data collection by BORN is ongoing; results using this preliminary data should be interpreted with caution
Conclusions
Compared with traditional prenatal screening, NIPT as a second-tier test improved overall prenatal screening performance by detecting more affected fetuses and substantially reducing the number of diagnostic tests performed, with decreased costs. Compared with second-tier NIPT, first-tier NIPT could detect more affected fetuses, but would increase total prenatal screening costs considerably.
BUDGET IMPACT ANALYSIS
Research Question
What is the potential budget impact for the Ontario Ministry of Health and Long-Term Care of publicly funding first-tier noninvasive prenatal testing (NIPT) for average-risk pregnant people with singleton pregnancies?
Methods
Analytic Framework
We estimated the budget impact of NIPT as the cost difference between two scenarios: second-tier NIPT (the current scenario) and first-tier NIPT (the new scenario). We focused on average-risk pregnant people with singleton pregnancies in our analysis.
When risk classification is determined at the initial stage of screening (i.e., before prenatal screening), pregnant people aged 40 years or older at date of delivery and pregnant people who have had a previous pregnancy with a chromosomal anomaly are considered high-risk; NIPT is already publicly funded for this population, according to the current Ontario funding policy. Thus, we have limited our scope to the average-risk population: pregnant people less than 40 years old and who have had no previous pregnancy with a chromosomal anomaly. Because the published literature on the performance of NIPT and traditional prenatal screening in twin pregnancies is limited, we did not include twins in our analysis. The clinical pathway for twin pregnancies is also more complex, and the funding policy for twin pregnancies is not the same as for singleton pregnancies.
We conducted a reference case analysis and scenario analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our scenario analyses explored how the results are affected by varying input parameters and model assumptions.
Target Population
The target population was average-risk pregnant people with a singleton pregnancy who chose prenatal screening. The process for estimating the target population is outlined in Figure 12, and expected target populations for years 1 to 5 (i.e., 2017/18 to 2021/22) are presented in Table 27. The total number of newborns has increased slightly in recent years, from 140,999 in 2011/12 to 147,244 in 2015/16 (Table 28).119 Assuming that this trend continues (1% increase per year), the projected number of newborns would be 150,204 in year 1 (2017/18) and 156,303 in year 5 (2021/22; Table 27). In addition, the number of newborns from multiple-gestation pregnancies decreased slightly from 2012/13 to 2014/15, but was stable in 2014/15 and 2015/16 (Table 28).82 We assumed that the total newborns per year from multiple-gestation pregnancies over the next 5 years would be same as that in 2015/16. We then approximated the total number of people with singleton pregnancies.
Figure 12: Estimating the Target Population.
Table 27:
Expected Target Population for Prenatal Screening in Ontario
| Population | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Total newborns | 150,204 | 151,706 | 153,223 | 154,755 | 156,303 |
| Newborns from a multiple-gestation pregnancy | 4,730 | 4,730 | 4,730 | 4,730 | 4,730 |
| Total singleton births | 145,474 | 146,976 | 148,493 | 150,025 | 151,573 |
| Births to average-risk pregnant peoplea | 138,200 | 139,627 | 141,068 | 142,524 | 143,994 |
| Average-risk pregnant people at 12 weeks’ gestationb | 142,346 | 143,816 | 145,300 | 146,800 | 148,314 |
| Average-risk pregnant people who choose prenatal screeningc | 96,602 | 97,600 | 98,607 | 99,625 | 100,653 |
| First trimester (90%) | 87,116 | 88,016 | 88,924 | 89,842 | 90,769 |
| Second trimester (10%) | 9,486 | 9,584 | 9,683 | 9,783 | 9,884 |
Note: Some numbers may appear inexact due to rounding.
According to data for 2014–2016 from the Better Outcomes Registry and Network (BORN), 4.2% of people were ≥ 40 years old at time they delivered.82 Assuming that a very small proportion of pregnant people had a previous pregnancy with a chromosomal anomaly, approximately 95% of people would fall into in the average-risk category.
Assuming a spontaneous pregnancy loss background risk of 3% between 12 weeks (first trimester) and term, 94,99 we estimated that the number of average-risk people with a singleton pregnancy during the first trimester would be approximately equal to the estimated number of average-risk people multiplied by 1.03 (e.g., 138,200 × 1.03 = 142,346 in year 1).
The uptake rate for prenatal screening is approximately 68% among average-risk pregnant people in Ontario (BORN data).
Table 28:
Total Newborns and Multiple-Gestation Pregnancies in Ontario, 2011/12–2015/16
| 2011/12 | 2012/13 | 2013/14 | 2014/15 | 2015/16 | Sources | |
|---|---|---|---|---|---|---|
| Total newborns | 140,999 | 142,367 | 144,051 | 145,513 | 147,244 | Statistics Canada119 |
| Newborns from a multiple-gestation pregnancy | NA | 5,263 | 5,185 | 4,784 | 4,730 | BORN82 (2014–2016) |
Abbreviations: BORN, Better Outcomes Registry and Network.
According to data for 2014–2016 from the Better Outcomes Registry and Network (BORN), 4.2% of people were aged 40 years or older at time they delivered.82 Assuming that a very small proportion of pregnant people had a previous pregnancy with a chromosomal anomaly, approximately 95% of people would fall into in the average-risk category. Including a background risk of 3% of spontaneous pregnancy loss between 12 weeks (first trimester) and full term,94,99 we estimated that the number of average-risk people with a singleton pregnancy in the first trimester would be approximately equal to the estimated number of average-risk people multiplied by 1.03 (e.g., 138,200 × 1.03 = 142,346 in year 1).
Prenatal screening is voluntary, and pregnant people may choose to accept or decline it. The uptake rate is approximately 68% among average-risk pregnant people in Ontario (BORN data). Assuming that uptake is constant over the next 5 years, we estimated that about 96,602 and 100,653 pregnant people would accept prenatal screening in years 1 and 5, respectively.
Current and New Screening Scenarios
In Ontario, NIPT is publicly funded only as a second-tier test for our target population (i.e., pregnant people less than 40 years old at time of delivery, with a singleton pregnancy, without a previous pregnancy with a chromosomal anomaly) for trisomies 21, 18, and 13, and for sex chromosome aneuploidies (the current scenario). The new screening scenario is first-tier NIPT for trisomies 21, 18, and 13.
Resource and Costs
Based on results from the primary economic evaluation, we estimated the health care costs of first-tier and second-tier NIPT for average-risk pregnant people, using uniform uptake rates and including additional chromosomal anomalies. We translated the health care costs for each strategy in the reference case and various scenario analyses into a standardized cohort size (per 10,000 pregnant people; Table 29). The scenario analyses are described in the primary economic evaluation. Costs included physician fees, prenatal screening, genetic counselling, diagnostic testing, and the costs related to termination of pregnancy. All costs are reported in 2017 Canadian dollars. Additional details on the included cost components can be found in the primary economic analysis.
Table 29:
Average Costs for First-Tier and Second-Tier NIPT Per 10,000 Pregnant People
| Analysis | First-Tier NIPT, $ | Second-Tier NIPT, $ |
|---|---|---|
| Reference casea | 4,226,461 | 1,838,133 |
| Scenario Analyses | ||
| Increased uptake of first-tier NIPT | ||
| 80% | 4,836,005 | 1,838,133 |
| 90% | 5,343,235 | 1,838,133 |
| 100% | 5,824,888 | 1,838,133 |
| Inclusion of sex chromosome aneuploidies in first-tier NIPT | 4,767,412a | 1,838,133 |
| Inclusion of sex chromosome aneuploidies and 22q11.2 deletion in first-tier NIPT | 6,117,118b | 1,838,133 |
| Varying price of NIPT | ||
| $200 | 2,947,834 | 1,798,638 |
| $300 | 3,608,681 | 1,821,276 |
| $500 | 4,974,501 | 1,870,034 |
| Funding first-tier NIPT for pregnant people 35–39 years old | 4,291,520 | 1,890,179 |
Abbreviation: NIPT, noninvasive prenatal testing.
Note: Some numbers may appear inexact due to rounding. All costs are in 2017 Canadian dollars.
Price of NIPT is $390.
Costs were based on the expected prevalence of sex chromosome aneuploidies and trisomies 21, 18, and 13.
Costs were based on an NIPT detection rate of 50% for 22q11.2 deletion.
Analysis
We calculated the budget impact as the cost difference between the new scenario (first-tier NIPT) and the current scenario (second-tier NIPT) for average-risk pregnant people. We calculated the total cost for each scenario using the average cost per pregnant person multiplied by the number of pregnant people per year. We also calculated the total net budget impact over 5 years.
In addition to the reference case, we conducted the following scenario analyses (Table 29):
Increased uptake of first-tier NIPT
Inclusion of sex chromosome aneuploidies in first-tier NIPT
Inclusion of sex chromosome aneuploidies and 22q11.2 deletion in first-tier NIPT
Varying price of NIPT
Funding first-tier NIPT for pregnant people 35 to 39 years old
We conducted the budget impact analysis using Excel 2013 (Microsoft, Redmond, Washington).
Expert Consultation
We solicited expert feedback on NIPT. The consultation included experts in medical genetics, fetal medicine, primary care, genetic counselling, prenatal health care services, laboratory medicine, methodology, and industry. The role of the expert advisers was to help define the scope and research question, contextualize the evidence, review the draft report, and provide advice on NIPT and its use in Ontario.
Results
Reference Case
Table 30 presents the projected total costs of first-tier and second-tier NIPT, as well as the expected net budget impact. In the reference case analysis, first-tier NIPT would lead to an annual budget increase of $34 million to $35 million per year over the next 5 years.
Table 30:
Budget Impact Analysis Results, Reference Case
| Cost, $ | |||||
|---|---|---|---|---|---|
| Strategy | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| Second-tier NIPT | 26,165,094 | 26,435,300 | 26,708,079 | 26,983,799 | 27,262,093 |
| First-tier NIPT | 60,161,984 | 60,783,274 | 61,410,481 | 62,044,450 | 62,684,336 |
| Net budget impact | 33,996,890 | 34,347,974 | 34,702,402 | 35,060,651 | 35,422,243 |
Abbreviation: NIPT, noninvasive prenatal testing.
Note: Some numbers may appear inexact due to rounding. All costs are in 2017 Canadian dollars.
Scenario Analysis
Table 31 presents the results of the scenario analyses. If uptake of first-tier NIPT increased, it would lead to a greater budget impact. If first-tier NIPT screening included trisomies 21, 18, and 13, and sex chromosome aneuploidies, the annual budget increase would be $42 million to $43 million per year. If the price of NIPT decreased, the net budget impact would decrease accordingly. If first-tier NIPT were funded for pregnant people 35 to 39 years old, the annual budget increase would be $7 million per year.
Table 31:
Budget Impact Analysis Results, Scenario Analyses
| Cost, $ | ||||||
|---|---|---|---|---|---|---|
| Scenario | Strategy | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| Increased Uptake of First-Tier NIPTa | ||||||
| 80% | First-tier NIPT | 68,838,602 | 69,549,495 | 70,267,158 | 70,992,559 | 71,724,730 |
| Net budget impact | — | 42,673,507 | 43,114,195 | 43,559,079 | 44,008,760 | 44,462,637 |
| 90% | First-tier NIPT | 76,058,807 | 76,844,263 | 77,637,199 | 78,438,684 | 79,247,650 |
| Net budget impact | — | 49,893,713 | 50,408,963 | 50,929,120 | 51,454,885 | 51,985,557 |
| 100% | First-tier NIPT | 82,914,952 | 83,771,210 | 84,635,624 | 85,509,357 | 86,391,245 |
| Net budget impact | — | 56,749,857 | 57,335,910 | 57,927,545 | 58,525,558 | 59,129,153 |
| Inclusion of Sex Chromosome Aneuploidies in First-Tier NIPTa | ||||||
| Inclusion of sex chromosome aneuploidies | First-tier NIPT | 67,862,207 | 68,563,017 | 69,270,501 | 69,985,613 | 70,707,399 |
| Net budget impact | — | 41,697,113 | 42,127,717 | 42,562,422 | 43,001,814 | 43,445,307 |
| Inclusion of Sex Chromosome Aneuploidies and 22q11.2 Deletion in First-Tier NIPTa | ||||||
| Inclusion of sex chromosome aneuploidies and 22q11.2 deletion | First-tier NIPT | 87,074,733 | 87,973,950 | 88,881,730 | 89,799,298 | 90,725,429 |
| Net budget impact | — | 60,909,639 | 61,538,649 | 62,173,651 | 62,815,499 | 63,463,337 |
| Varying Price of NIPT | ||||||
| $200 | Second-tier NIPT | 25,602,889 | 25,867,288 | 26,134,206 | 26,404,002 | 26,676,316 |
| First-tier NIPT | 41,961,236 | 42,394,567 | 42,832,026 | 43,274,201 | 43,720,503 | |
| Net budget impact | — | 16,358,347 | 16,527,279 | 16,697,820 | 16,870,199 | 17,044,187 |
| $300 | Second-tier NIPT | 25,925,135 | 26,192,862 | 26,463,139 | 26,736,331 | 27,012,072 |
| First-tier NIPT | 51,368,130 | 51,898,606 | 52,434,134 | 52,975,436 | 53,521,791 | |
| Net budget impact | — | 25,442,995 | 25,705,744 | 25,970,995 | 26,239,106 | 26,509,719 |
| $500 | Second-tier NIPT | 26,619,188 | 26,894,083 | 27,171,596 | 27,452,101 | 27,735,224 |
| First-tier NIPT | 70,810,038 | 71,541,290 | 72,279,506 | 73,025,681 | 73,778,821 | |
| Net budget impact | — | 44,190,851 | 44,647,207 | 45,107,910 | 45,573,580 | 46,043,597 |
| Funding First-Tier NIPT for Pregnant People 35–39 Years Oldb | ||||||
| Funding for pregnant people 35–39 years old | Second-tier NIPT | 5,299,305 | 5,353,931 | 5,409,313 | 5,465,073 | 5,521,401 |
| First-tier NIPT | 12,031,706 | 12,155,731 | 12,281,472 | 12,408,072 | 12,535,960 | |
| Net budget impact | — | 6,732,401 | 6,801,800 | 6,872,159 | 6,942,999 | 7,014,559 |
Abbreviation: NIPT, noninvasive prenatal testing.
Note: Some numbers may appear inexact due to rounding. All costs are in 2017 Canadian dollars.
This scenario did not affect the cost of second-tier NIPT. We estimated the net budget impact using the cost of first-tier NIPT in this scenario minus the total cost of second-tier NIPT in the reference case.
This subgroup accounted for about 20% of average-risk pregnant people < 40 years old. Assuming that the proportion of pregnant people in this age group would be constant over the next 5 years, we estimated that the target population in this subgroup would be 28,036 in year 1, 28,325 in year 2, 28,618 in year 3, 28,913 in year 4, and 29,211 in year 5.
Discussion
Funding first-tier NIPT could lead to a budget increase of approximately $35 million per year, based on the current price of NIPT ($390 per test). When we included the costs for genetic counselling and nuchal translucency ultrasound, approximately $90 in additional costs were associated with first-tier NIPT. This total cost for NIPT (approximately $480 [$390 + $90] per person) is much higher than that of eFTS ($124.50) or maternal serum screening ($186.75). If the price of NIPT decreased to $200 per test, the budget impact for first-tier NIPT would decrease to approximately $17 million per year.
It is likely that the uptake rate of prenatal screening would increase if NIPT were used as a first-tier test. If the uptake rate increased, first-tier NIPT would lead to a higher budget increase (e.g., an additional $57 million to $59 million at an uptake rate of 100%). Finally, if screening for sex chromosome aneuploidies and/or microdeletion syndromes were included in first-tier NIPT, the net budget impact would also increase considerably.
Conclusions
Noninvasive prenatal testing is currently used as a second-tier test in average-risk pregnancies in Ontario. If it were used as a first-tier test instead, it would lead to an annual budget increase of approximately $35 million per year.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, preferences, and priorities of those who have lived experience with noninvasive prenatal testing (NIPT).
Background
The patient preferences and values section explores the lived experience of a person with a health condition, including the impact the condition and its treatment has on the patient, the patient's family or other caregivers, and the patient's personal environment. Patient, Caregiver, and Public Engagement intends to increase awareness and build appreciation for the needs, priorities, and preferences of the individual at the centre of a treatment program. The insights provide an in-depth picture of lived experience through an intimate look at the values that underpin the experience.
Lived experience is a unique source of evidence about the personal impact of a health condition and how that condition is managed, including what it is like to navigate the health care system with that condition, and how technologies may or may not make a difference in people's lives. Information shared from lived experience can also identify gaps or limitations in published research (for example, outcome measures that do not reflect what is important to those with lived experience).120–122 Additionally, lived experience can provide information or perspectives on the ethical and social values implications of technologies and treatments.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored by published literature, Health Quality Ontario makes an effort to reach out to, and directly speak with, people who are affected by this technology, including those who may have experience with the intervention in question.
This health technology assessment will affect two distinct populations: pregnant people who would like to access prenatal screening, and people living with the conditions that NIPT screens for. Noninvasive prenatal testing may be important to people who would like access to less invasive prenatal screening earlier in their pregnancy. It may also be important to help alleviate anxieties about a pregnancy or help people make decisions about the next steps of testing for a definitive diagnosis.
Recognizing this, we looked to engage with people who represented a variety of perspectives and experiences. The potential effects of implementing or not implementing NIPT more broadly in Ontario may affect people with a diverse range of lived experiences.
Methods
Engagement Plan
Engagement as a concept captures a range of efforts used to involve the public and patients in various domains and stages of health technology assessment decision-making.123 Rowe and Frewer outline three types of engagement: communication, consultation, and participation.124 Communication describes a one-way transfer of information from the sponsor to the individual, while participation involves the sponsor and individual collaborating through real-time dialogue. The engagement approach for this health technology assessment was consultation, defined as the seeking out and soliciting of information (for example, experiential input) by the public, patients, and caregivers who are affected by the technology or intervention in question.125 Within this typology, the engagement design focused on interview methodology to examine the lived experience of patients, caregivers, and families, including those who have undergone the intervention.
We selected the qualitative interview as an appropriate consultation methodology because it allowed Health Quality Ontario staff to explore the meaning of central themes in the lived experience of the participants. The main task in interviewing is to understand the meaning of what participants say.126 Interviews are particularly useful for getting the story behind a participant's experiences, which was the aim of this portion of the health technology assessment. The sensitive nature of exploring quality-of-life issues supports the use of interviews for this project.
Outreach Methods
For this project we connected with the Ontario Prenatal Screening Program, the Better Outcomes Registry and Network (BORN), the Provincial Council for Child and Maternal Health, the Association of Ontario Midwives, support groups such as Pregnancy and Infant Loss, the Down Syndrome Association of Ontario (local chapters and the provincial organization), the Foundation for Prader-Willi Research Canada, Support Organization for Trisomy 18, 13 and Related Disorders (an international organization; there is no related Canada- or Ontario-specific organization), the Turner Syndrome Society and the Klinefelter Syndrome Association of Canada. We also recruited through social media on a dozen Facebook parent groups representing communities across the province.
Inclusion Criteria
We sought to speak with Ontario residents who had had a pregnancy affected by one of the conditions screened for and who may or may not have used NIPT. We also sought to speak with people living with the conditions NIPT screens for, or with their parents or family members. Participants were not required to have experience with NIPT. We sought broad geographic, cultural, and socioeconomic representations to identify possible equity issues in accessing NIPT.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
From the population of pregnant people potentially affected by NIPT, we interviewed:
7 people who had had false-positive results with traditional prenatal screening and went on to have NIPT
2 people who experienced barriers to accessing NIPT
5 people whose pregnancies were affected by Down syndrome, Edwards syndrome, Patau syndrome, or Turner syndrome, and who experienced pregnancy loss or infant loss, or who terminated the pregnancy.
From the population living with conditions that NIPT screens for, we interviewed:
16 parents of children with Down syndrome
2 parents of children with sex chromosome disorders
5 adult women with Turner syndrome
2 parents of children with Prader-Willi syndrome
Overall, 18 of 39 participants had used NIPT: 13 had accessed it as a second-tier screening test, and 5 had accessed it as a first-tier screening test, either out of pocket or covered by the Ontario Health Insurance Plan because they met the funding criteria.
Approach
At the outset of the interview, we explained the purpose of the health technology assessment, the risks from participation, and how we would protect participants’ personal health information. We explained this context verbally and through a call for participation (see Appendix 9). Interviews were recorded and transcribed with participants’ consent.
Interviews lasted approximately 20 to 60 minutes. They were semi-structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.127 Questions focused on the impact of living with one of the conditions screened for, or of parenting a child with one of the conditions screened for, as applicable; the impact of having access or not having access to NIPT; the impact of the timing of access to NIPT; the experience of going through both traditional prenatal screening and/or NIPT as applicable; and participant perceptions of the benefits or limitations of NIPT. See Appendix 10 for our patient interview guide.
Data Extraction and Analysis
We used a modified version of grounded theory methodology to analyze transcripts of participant interviews. Grounded theory follows an iterative process of eliciting, documenting, and analyzing responses while simultaneously collecting and analyzing data using a constant comparative approach.128,129 This allowed us to organize and compare information on experiences across participants. Staff coded transcripts and compared themes using NVivo (QSR International, Doncaster, Victoria, Australia), a qualitative software program that enabled identification and interpretation of patterns in the interview data. The themes we identified helped us highlight the impact of NIPT, as well as different aspects of the prenatal screening experience.
Results
Lived Experience With NIPT
Access
The review of the qualitative literature on NIPT130 that is a companion to this report revealed that access to NIPT and timing of NIPT were important considerations for people, and this rang true for the people we spoke with as well. People articulated their frustration that the test can be used as early as 9 to 10 weeks of pregnancy, but that their pathway to the screen was not as straightforward as that. Many participants noted that people who would like to access NIPT currently must pay out of pocket if they do not qualify as “high risk” under the Ontario Health Insurance Plan.
Awareness of Self-Pay Option
Several participants said that they had not known that NIPT was an option for them, even if they had to pay for it:
NIPT has never been presented to me—ever—in any of my previous pregnancies as an option that I could have paid for. I now know that there is an option that I can pay—I believe it is $500, or maybe $750.
One parent of a child with Down syndrome said that not enough people knew about NIPT as an option:
I think more knowledge of it [NIPT], definitely. People don't understand that it's out there. And I think a lot of people are blindsided by a diagnosis of Down syndrome at delivery, because they're not really aware of how beneficial it is to have this information ahead of time. I'm a big believer that having that information ahead of time really made a world of difference for my family.
Second-Tier Screen
Participants who received false-positive results from traditional screening expressed their frustration and stress knowing that if NIPT had been the first prenatal screening test they underwent, they would likely have gone through less emotional turmoil:
Having this other test available that has these fairly high chances of … false positives, it does put a lot of people through a lot of stress. If there's a test that's more accurate that is also noninvasive, that it's just blood work, why wouldn't they have the more accurate test available?
If the NIPT test had been available for me from the beginning. I wouldn't have to go through this gruelling process that took weeks and weeks.
Timing of NIPT
Many participants talked about the anxiety and emotional distress of getting a positive result from traditional prenatal screening and having to decide between invasive testing and NIPT, based on the timing of the screening and the accuracy of the results. An example of how this pathway can play out for people is highlighted in the following example: a participant shared the many stages of screening she went through to finally confirm a diagnosis of trisomy 18, a condition in which her baby was unlikely to survive after birth. She had traditional prenatal screening, ultrasounds, NIPT and an amniocentesis. The entire process took many weeks, and by the time she was able to access a medical termination, she was 22 weeks’ pregnant.
I think that they should actually do that [NIPT] at 10 weeks, to be completely honest, because you're not at that point where you're so attached. I had already sent out invitations for my baby shower for her. At 10 weeks, it's still obviously hard, but I think it's not as traumatic. I was 5 months pregnant, and the baby was moving, and I was forced to sit there and just wait.
Cost to the System
Delays in accessing publicly funded screening was very frustrating for many people we spoke with, and is well-documented in Ontario-specific research.131 Several participants who went through traditional screening, followed by NIPT and invasive testing, remarked on the stress they experienced, as well as the cost to the system:
Just taking my personal feelings out of it and looking at it from a system perspective, it would have saved the system a lot of money, in my case, if we had done the NIPT screening at 11 weeks. Because what ended up happening was that I went under a full anaesthetic for a D&C [dilation and curettage] and it was in an emergency operating room—not to mention the CVS [chorionic villus sampling] is expensive; I'd imagine more expensive than the NIPT. There were numerous OB [obstetrician] appointments, there were ultrasounds with specialists … I required a lot of treatment that could have been avoided with a simple blood test. Not to mention the risk of miscarriage with CVS. When you're in the position I was in, you're already uncomfortable that your baby's not healthy, and to have to make a choice to put them at increased risk [with invasive testing] is really difficult.
Perceived Benefits of NIPT
The majority of participants, representing a wide range of perspectives and experiences, said that NIPT was a great option because it was more accurate than traditional screening, but because it is noninvasive they were less concerned about safety and potential miscarriage than with CVS or amniocentesis. This was articulated by participants who would opt to end a pregnancy and by participants who knew that they would continue a pregnancy regardless of the diagnosis.
Accurate and Noninvasive
As has been noted in the qualitative companion to this report,130 the fact that NIPT is noninvasive, can be done early in pregnancy, and is more accurate than traditional prenatal screening makes it very appealing to people who would choose prenatal screening. Participants said that the lower frequency of false positives made it a more desirable screening tool. Many participants preferred being able to access information to support pregnancy decision-making without having to move straight to invasive diagnostic testing. In particular, people who would not have opted to end a pregnancy for any reason could confidently prepare for the birth of a child with a disability without putting their pregnancy at risk.
Many parents of children with Down syndrome who received their positive result from NIPT felt grateful for the information. They appreciated that NIPT wasn't a risk to the pregnancy, and that they could have more accurate information than with traditional prenatal screening. A mother who chose to continue a pregnancy after a positive result for trisomy 18 felt grateful that she and her husband could have the information from NIPT without putting an already at-risk pregnancy at further risk.
We both knew we didn't want to terminate, which is why I turned down the [amniocentesis], because I didn't want to risk terminating the pregnancy even through a miscarriage; I've been through those, and they were horrible. So for me, the NIPT was strictly to confirm the diagnosis, to be able to prepare for what was coming. I think NIPT is a good tool and a good confirmation for those diagnoses, so that you know and you can research what's coming rather than being surprised at birth.
Access to Information
People with children who had one of the conditions screened for noted that having the information allowed them time to prepare for the birth and educate themselves about their baby's condition. This information also allowed them to access extra medical care for their baby, such as additional ultrasounds and a fetal echocardiogram. They also appreciated having the information so that they could prepare for a baby with a disability by reaching out to support groups, finding other parents in their community who had experience with the condition, and deciding about where to give birth. They were able to read about their baby's condition, talk to clinicians, and share information with family and friends.
Because we knew we weren't going to terminate, we liked the idea of the NIPT, because it still gave us 99% positive without doing anything that we felt was too invasive or too risky. Because we could prepare ourselves, that was the greatest thing for us. We were so OK with everything by the time our daughter arrived. I mean, her birth was pretty typical, because everybody knew she had Down syndrome, nobody had to come in and give us bad news. Finding out at birth would be very difficult because we had basically 6 months of time knowing that she was going to be born with Down syndrome to research Down syndrome, to research what supports were available, to get all that in place as much as we could before she was born, whereas if you find out at birth not only are you a new parent with a new baby, you all of a sudden have all these other things to consider at once, and it's hard enough caring for an infant.
Some participants who had children with Down syndrome were offered the test with subsequent pregnancies and said that it was beneficial to have the information ahead of time so they could prepare for another child with special needs.
A mother of an adult daughter with Down syndrome said that as much information as possible should be provided in terms of screening, testing, and diagnosis. Based on her experience and because it is often women who take on a disproportionate load of caregiving she felt that expectant parents, should be given the opportunity to make informed decisions about an affected pregnancy.
I would want them to have as many facts about everything as they could possibly have. I think it would be helpful for people. My experience taught me that the mother, no matter what kind of situation you're in always is the person who bears the greatest load for caregiving. I don't care how wonderful your family is, in our society it's the woman who does the most and is responsible ultimately even into old age. So first of all, I would like to say if they're making this decision, it's the mother who should call all the shots, because it's the mother who is going to be getting all of the results of her decisions. She is going to mostly bear the brunt. Government promises I have found are not reliable.
For parents expecting a child with a sex chromosome condition, access to this information during pregnancy can lead to earlier intervention with recommended treatments and lead to better health outcomes.
From a growth-hormone standpoint, it's starting to be given earlier and earlier, so I think that that's helping patients with Turner syndrome gain a higher average height. That's one of the benefits of earlier diagnosis. Also, being able to test for other complications and monitor for heart issues is probably one of the biggest advantages [of NIPT].
Preparation for Potential Pregnancy Loss
Another benefit of using NIPT for information is that it can let pregnant people understand if their pregnancy is at risk of miscarriage and can help them prepare for potential loss. A participant with Turner syndrome said:
Because there's such a high miscarriage rate in Turner syndrome, it [NIPT] helps to track the reason why the baby was miscarried. So, from a medical standpoint, I see why this would be very important. I don't know, at a patient level, how that affects mothers emotionally. I guess they get a diagnosis and they can prepare for the worst, should the worst happen.
Concerns About NIPT
Routinization of Screening
A number of participants said that it was often taken for granted by health care providers that people want screening, thereby limiting informed choice and decision-making.
I am somebody who does respect choice, but choice that is informed, right? And for me, this is not an area where people are being fully informed before being asked to make a choice.
Many participants who had received traditional prenatal screening noted that in hindsight it felt routine, just something you did as part of pregnancy care, without being fully aware of the potential consequences.
Well, we didn't really think about it. The prenatal screening was just something you did because everybody did it. We didn't even really think about it until we had the positive screen come back.
As screening such as NIPT becomes more widespread, early in pregnancy and part of the standard of care for pregnant people, there may be increasing expectations of “opt-out” rather than “opt-in” screening.
I was never asked if I wanted to do screening; they just did it. They did not give me a choice. And it was my first pregnancy. I didn't know. I thought this was what everybody gets.
Honestly, it was one of those things where they hand you the requisition, you go and do it, and I never really thought anything of it. People mentioned nuchal fold and Down syndrome and that, but I didn't really pay any attention, because you never really think, “That's going to be me.”
It is important to note that not everyone decides to do screening, and several participants noted that they wanted this choice to be respected. A parent of a toddler with Down syndrome said that she didn't want to do testing or screening of any kind in pregnancy, because of how it would shape her view of her child and her experience of pregnancy.
A lot of people say the advantage [of NIPT] is so that they can prepare, but I really fall into the camp of, I want my hopes and dreams for my child to not be consumed during my pregnancy with what they're not going to be able to do. Disadvantages [of NIPT] are people not having the right information before they take the test, and then potentially just spending their whole pregnancy learning about all the things their child is not going to do when that's not the whole story, and that's not at all what the life of it is.
Another participant shared that she had declined prenatal screening, and she wasn't aware she was being screened when she presented for a nuchal translucency ultrasound, having been told by her doctor, “It's an opportunity for you to see the baby.” We heard from participants who initially opted out of screening but then something came up during the pregnancy that led them to do testing, even if they didn't want that information at first.
I did not want to do any testing. You know—the triple screen or whatever is offered early on in your pregnancy—so I didn't do it, and it was at my 21-week scan, the anatomical scan, that they noticed some soft markers for Down syndrome, and that's when I kind of was led down the path of genetic testing … leading ultimately to an amniocentesis. Certainly, what was being portrayed to me was: “You've got to find this out, because this could be a bad thing,” right?
There were also concerns—particularly from parents of children with the disabilities screened for—that the routinization of prenatal screening would extend to NIPT if it were implemented more broadly across the province, and that people would not feel that they could opt out, or would not understand that this simple blood test early in pregnancy could have a significant effect on their pregnancy experience.
It's almost like parents are being faced with two decisions: one to have the testing done, and then second, what does this then mean for your pregnancy?
Understanding Options: Pre- and Post-screening Counselling
At times it was evident that some participants did not always have all the information they needed about what NIPT can screen for or about the accuracy and limitations of NIPT. Some common misperceptions were shared by a couple of participants who incorrectly perceived that NIPT could screen for autism and that NIPT is more accurate than diagnostic testing.
I was told that Down syndrome and autism were the primary sort of catches that NIPT was responsible for.
It's more accurate than the CVS. That was how they described it to me at that prenatal screening clinic. Like its 99.9% where the CVS was—it was a little bit less, actually.
Some participants were concerned that NIPT could falsely reassure people, leading them to believe that their baby had no health issues, even though the screening looks for only a few possible conditions.
I feel like people take the test and they're like, “If it comes back negative, then all of my fears about my child are clear.” They're not focusing enough on the fact that it doesn't test for everything.
Participants weren't always given the opportunity for an informed-choice discussion with their health care provider about whether they wanted to do screening and what they would do with the information. Some of the stories we heard pointed to a lack of awareness and education about the conditions screened for, as well as how people might get on a path of screening and testing, perhaps without enough information, and this demonstrated how the implementation of prenatal screening and testing affects people's choices. When people do not understand the full implications of screening and diagnostic testing, they may make choices they would not otherwise make.
We should've sat down before having the CVS, looking back. We should've sat down and we should've talked about, “OK. Well, what do we want to do if this baby does have Turner syndrome? And what is Turner syndrome?” because we didn't even know at that point, before doing the CVS. And if it was, “We want to carry on with the pregnancy,” well, maybe we don't want to risk having the CVS. I think when you're in this situation, you go to the hospital, they say there might be something wrong with your baby. You have NIPT, and they kind of brush over some of the things that it might be, but you don't go into any detail, because there's too many options, you know? So then you get your result back from the NIPT and it's like, “OK. We're looking at Turner syndrome. And if you want a CVS, then we need to do it tomorrow, because we're running out of time.” And as a pregnant lady, you feel this panic, like, “Oh, my goodness. I need to do things now. I don't want to wait another two or three weeks till I can have an amnio, because what happens if I do decide to terminate the pregnancy? This baby's going to be growing bigger …”
The impact of the experience of screening can change people's experience of pregnancy, from an exciting time to a time of anxiety and concern. One participant said that after she went through traditional screening and NIPT only to find out that her pregnancy was unaffected by any of the conditions screened for, the experience stayed with her and her partner, to the point that it was affecting their decision-making about where to give birth; they were no longer considering home birth:
After this experience, he's still really freaking out for the chances of me having any issues, so he wants me to give birth at the hospital. So, it does change your view of your pregnancy and your labour, because you're so scared.
The need for information—about screening and testing, and about what information NIPT can provide—was highlighted in many of the stories people shared with us. Participants sometimes felt the urgency of continuing down a pathway for invasive testing because their pregnancy was progressing. Sometimes they felt pressured to seek a definitive diagnosis before they had all the information, even about what condition they might be presented with. One mother said that the notion of screening itself was something people have a negative association with—the idea that if they are screening for it, it must be bad.
You're going to screen for it, then this is something bad, just like we screen for all kinds of things: prostate cancer, breast cancer, and all kinds of stuff. So if you're going to screen for these three [trisomies 13, 18, 21] or anything else, then obviously this is bad for me. And that's my sticking point. This is a philosophical thing, I guess. You know, I have three children. I know exactly who their parents are, and they are three very different individuals. You don't know ahead of time what the future may bring. You have your parenting skills, you have all kinds of things, but there are issues that people come with that you have no idea.
Another shared that in our culture “if something is different, then it's wrong and it's not good for us.” Other parents worried about people being persuaded to do invasive testing.
I was in the genetic clinic. They have this system where they cattle you in with like 10 or 20 other families in a really small room. And then they explain to you the different stages of the testing. Afterwards, I reflected on it. This is social coercion. Because they put you in a room with all these other families, and they minimize the risk for amniocentesis. They don't talk a lot about the false positives, and they really minimize, just brushed over, the risks for amniocentesis. I'm imagining somebody with English as a second language or isn't as educated or wants to follow the doctor. So here you are sitting in a room, 10 or 20 other people, and this nurse or whoever it was, sitting there saying, “OK, so this is the next step. You do this. And then this is the next step if it comes positive.” And everybody was like, “OK, OK, OK.” And I'm saying, “Whoa, whoa, whoa, wait a minute. Like, where are your other options here?”
Quality of Information: Support for Informed Decision-Making
For many parents of children with the conditions screened for, as well as women with Turner syndrome, up-to-date information presented in a balanced and empathetic way was something they saw as essential to accompany prenatal screening and diagnosis. If there was one thing that nearly all participants were unanimous about, it was the importance of access to good information to support decision-making. Many parents of children with the conditions screened for articulated negative experiences with physicians, nurses and genetic counsellors. They talked about being given one-sided or out-of-date information about their child's condition.
There's the testing and all that, but it's how doctors or genetic counsellors give you the results, and then what kind of information prospective parents are given about a chromosomal condition. That's what makes it. It's not the test itself, it's the information you're getting, and that's where we're trying to help in our community is to try to make sure that new parents get accurate information and they can make a decision that's really well-informed. Access to accurate—not outdated, not stereotypical—information and doctors who deliver the diagnosis in a way that isn't leading is crucial. Especially if it's going to be available widespread, the testing. I think the test is great. The information delivery needs to be worked on.
So, I'm fully in support of early testing, better testing. I think it really does give you all of your options. I hope that along with that comes more education on things like Down syndrome, because a lot of people just aren't aware of how not scary it is in the end. I'm a big believer that you should have all the information at the ready. That's in today's age your right as a parent.
One point that several participants made was the importance of being supported in making decisions that were right for them. The mother who received a diagnosis of trisomy 18 but decided to continue the pregnancy shared this experience:
The genetic counsellor, to be totally honest, really pushed for us to terminate. She kept telling us that we didn't understand how bad this was going to be. She told us that, you know, babies with trisomy 18 are considered incompatible with life. And she showed us pictures of babies with trisomy 18 and prepared us for a completely different lifestyle. We were told numerous times that we weren't listening to them, we weren't taking in what they were telling us, we weren't prepared for what was coming. We knew life expectancy was 48 hours to 2 weeks, and that was if he was full-term. So we had many doctors try to suggest we terminate. Many doctors hinted that we didn't understand the depth, the severity. I work as an educational assistant with children with all kinds of disabilities, so I kept saying, “I do get it,” and they kept saying, “No, you're not listening.” And I said, “I am listening, but you know, this is our choice.” We saw numerous doctors who felt we were making the wrong decision, but, in the end, it was our decision to make, and so we did.
In conditions such as Turner syndrome, participants who had lived experience with the condition said that early diagnosis prenatally or in infancy is important for long-term outcomes, but that it is equally important that accurate and up-to-date information is shared with expectant parents.
In terms of things that wouldn't be beneficial, it's not necessarily with the testing, I would say, but with how it is disclosed and handled after it's done. I know with a lot of those disorders there still tends to be some misinformation within the medical community, especially with Turner syndrome. Physicians tend to either not know much about it, or present the worst-case scenario in some cases, and there still are some physicians who will recommend termination if they find out that there's a fetus diagnosed with Turner syndrome.
Another parent said that she fully supported NIPT and thought that the information it could provide was important.
I'm for it. I absolutely am. The more knowledge the better, and like I said, the most important thing is not the test, it's the information, and how it's delivered, and making sure that parents have access to accurate, up-to-date, current information, because I think we all have our understanding of what Down syndrome is in our minds, and I have to say our understanding before our daughter was completely based on old information and biases, and what you see on TV etc., and it's not reality. I don't know how you break through that. It's a long process, and we're still working on it, but it's something that needs to change, and I think that this testing available widespread is cause for a great dialogue, but I just hope that the government sees that having this test open also indicates the need for educating and giving doctors the tools they need to deliver the diagnosis in the right way. I think that's really important to make sure they go hand in hand.
Ethical Concerns About NIPT
A number of participants, particularly parents of children with the conditions screened for, brought up some of the larger ethical and societal issues around prenatal screening, which at a macro level can be seen as screening out genetic conditions or disabilities in society—a devaluing of certain kinds of lives—but at a micro level is about a pregnant person's right to make choices that are best for their family. Many parents of children with Down syndrome wanted to bring up these issues, and their fears about how their children are viewed in a society in which many choose to end an affected pregnancy. As certain conditions are screened for and some pregnancies terminated, some participants expressed concern that there will be fewer and fewer people with those conditions in society, and that those pregnancies would be expected to be terminated and fewer supportive resources made available.
I think the testing itself is great, but I might not be the majority of opinions, because I know a lot of people in the community worry that if this screening is more readily available, it means there will be less people born with Down syndrome, and for them that's not a good thing, or it makes you feel kind of sad, because it's like people saying that your child shouldn't be born, or something like that.
A participant whose son was born before NIPT was available noted:
This kind of noninvasive testing wasn't available when I was pregnant. What caught my attention for this, although I appreciate the benefits of testing, it also worries me in the way that I don't feel people are always given a very balanced view of what it's like to have a child who has a disability, and that really, really worries me when it comes to making this testing a lot easier.
This was why many wanted to ensure that people had access to good information in pregnancy, and to share their stories about the joys of having a child with Down syndrome in their family. Participants wanted pregnant people to have as much information as possible to make truly informed decisions about their pregnancies, as well as access to support for their choices. One mother of a child with Down syndrome noted that the government should offer choices to pregnant people in terms of screening and access to termination but should also provide support and options to people who continue an affected pregnancy—to not judge that family as a burden on the system, but to support a society that truly considers people with disabilities to be citizens. Parents of children with Down syndrome also expressed fears that their children would grow up without other children like them. They spoke of news stories about countries like Iceland, which have virtually eliminated Down syndrome:
I'm OK if more funding goes into it. I'm not OK if it's not hand in hand with more appropriate education for the people that are going to deliver the news. I just really want there to be more positive conversations around what a life with Down syndrome actually looks like, or what a life for anybody with a disability looks like … There just needs to be a larger story about it, or a larger conversation about what it actually means.
Parents of children with Down syndrome shared their concerns about the potential negative effects of widespread screening on the social treatment of their children and others with the conditions screened for. They expressed concern that the rapid uptake of NIPT into the provincial health care system without public discourse and a deeper exploration of societal values will negatively affect people living with disabilities and their families. This was a big concern for some participants: is good information enough in a society that doesn't always value and support people with disabilities and their families?
This is the thing I wonder. If you offer the screening, but at the same time you offer good information, is that enough for people to make truly informed choices when they're living in a world that still thinks of diversity the way that it does?
Lived Experience With the Conditions Screened For
Parents of children born with one of the conditions screened for with NIPT wanted this report to reflect the meaning and significance of their children's lives. There was no doubt that parenting a child with a disability could be challenging:
The other thing I guess I would want people to know is that my life with my daughter with Down syndrome has been wonderful in many ways. It was crushingly difficult, but it's been stunningly beneficial to me as well, in ways that I hadn't anticipated. So, my experience—although very taxing and still is—has made me a happier person, ultimately. I would want people to understand that.
At the same time, it could be challenging for some parents to express the negative aspects of their experience, because they were so often advocating for their children and trying to represent a positive view of disability to their friends, family, and the greater public.
You know what? No, life is not always rosy, it totally sucks sometimes, but that's OK, too. I don't understand why people think that a life is always rosy, right? There is going to be hardship, and there are going to be things that aren't the best, but that's part of life. I have to hesitate on what I share on social media or to people that are not very close to me, because, you know, I don't want there to be an unfavourable view of her or her life.
A mother of an adult daughter with Down syndrome said:
I paid a heavy price financially to get her to the stage where she was able to go off to school and just be OK, and go home, and then go off to work. It was a huge, huge investment of time. I took 12 years off, not working, to support her, and then when it became time for my retirement, I had to work longer than most of my peers to get my full pension, because I hadn't been able to work full-time. And those last few years were very hard on me. So it was a sacrifice that I made at the time, which you have to think about. You don't think about what your life is going to be like when you retire when you're in your 20s, but the decisions I made then directly impacted everything that happened to me after. I guess that's the most important thing I think I've learned.
Participants also expressed their desire to share that “a diagnosis in utero cannot predict a life.” Many parents of children with Down syndrome wanted to say that their children with Down syndrome brought joy and challenges, just as their other children did.
And you know we're only like 3 years into this journey—our little girl turns 3 next month—but it has been like an amazing gift that we would never have chosen for ourselves. And it's so funny—I remember when I was pregnant I was reading this about other people who had like kids with Down syndrome, and they're like, “I wouldn't change it if I could,” and I'm like, “Really? Seriously?” And I still don't know that I could say that, but I'm getting to the point where I understand why, I really do, and it's almost like you have to live that experience to realize the benefits that having someone with Down syndrome in your life brings, and it's really hard to explain that to someone who hasn't been there. But you don't even get there if you don't have somebody in your life.
Sex chromosome conditions such Turner syndrome and Klinefelter syndrome can have relatively minor effects on people's lives. What is the effect of screening on people with these conditions and on the way they are viewed by society? We spoke to five women with Turner syndrome who ranged in age from their early twenties to their mid-sixties. Some were diagnosed soon after birth, while others weren't diagnosed until later in childhood. We also spoke with 2 mothers of children with Turner syndrome. Participants with Turner syndrome shared what they wanted people to understand:
Turner's girls can live a healthy, happy, productive life, and there's no reason to end a pregnancy. I mean I know that a lot of parents have a lot of questions when they get a diagnosis with that, and I don't blame them, and that's where the medical world needs to be informed enough to help them understand what exactly Turner's means.
One parent commented on the unpredictability of life, and that a better understanding of the ways disability can affect everyone is important:
I think we forget that at one time or another we'll all be disabled. You know, as we get older … we will rely on other people to do different things. We should truly consider that disability can affect us at any time. I want people to understand that disability is a sector in life we can all become members of, as we age, or without having been born into it.
Discussion
Patient engagement surrounding the topic of noninvasive prenatal screening was robust. We were able to interview many people with a wide range of experiences related to prenatal screening.
People who had accessed NIPT were able to discuss their experience with the screening and were often able to compare it to previous pregnancies with traditional screening. Those interviewed were, for the most part, supportive of NIPT and the benefits of the earlier, more accurate results it can provide. However, while most people interviewed were positively inclined toward NIPT, many—particularly from the disability community—identified concerns or challenges related to the screening. These concerns were largely about pre- and post-test counselling, and the information offered by health care providers. They were concerned that some health care providers offer biased, out-of-date information. Another concern for some participants was the potential for inaccurate results with the rarer conditions screened for.
We did not discuss the particular benefits of specific NIPT tests as part of patient engagement for this topic.
Conclusions
Participants were largely positive about NIPT. They perceived that it could provide important information about pregnancies—earlier and with more accuracy. Many participants highlighted that NIPT is currently only offered as a second-tier screen after traditional prenatal screening for people considered average-risk. They felt that this process caused them undue stress and anxiety and led to a definitive diagnosis too late in pregnancy.
A notable concern for many participants was the lack of good informed-choice conversations with their primary care providers about the benefits of screening, the limitations of screening, and what the pregnant person might do with the information provided. Many participants brought up ethical concerns with NIPT and broadened access to more accurate prenatal screening, the quality of information provided when people received a positive result, the lack of support in society for raising children with disabilities and the potential stigma associated with knowingly choosing to raise a child with one of the conditions screened for.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
The clinical sensitivity of NIPT was 99.5% (95% confidence interval [CI] 81.8%–99.9%) for trisomy 21, 93.1% (95% CI 75.9%–98.3%) for trisomy 18, and 92.7% (95% CI 81.6%–99.9%) for trisomy 13 (GRADE: Low to Moderate). The specificity for any trisomy was 99.9% (GRADE: Moderate). The accuracy of NIPT was higher than that of traditional prenatal testing (GRADE: Low to Moderate) and decreased the number of diagnostic tests performed (GRADE: Moderate). However, evidence for the use of NIPT in the average-risk or general population for sex chromosome aneuploidies was limited. We found no studies on the accuracy or clinical utility of NIPT for microdeletion syndromes in the average-risk or general population. As well, NIPT should not be considered a diagnostic test; positive results should be confirmed by diagnostic testing.
Compared with traditional prenatal screening, NIPT as a second-tier test detected more affected fetuses and substantially reduced the number of diagnostic tests performed, with decreased costs. Compared with second-tier NIPT, first-tier NIPT could detect an additional 84 fetuses with chromosomal anomalies, but it would lead to an annual budget increase of approximately $35 million per year.
Participants in this health technology assessment were largely positive about NIPT. They perceived that screening could provide important information about their pregnancy—earlier and with more accuracy. However, many participants noted that because NIPT is currently offered only as a second-tier screen, they experienced undue stress and anxiety and felt they received a definitive diagnosis too late in pregnancy. A notable concern for many participants was the lack of informed-choice conversations with their primary care providers about the benefits of screening, the limitations of screening, and what pregnant people might do with the information provided. Participants spoke of the complexities of the larger ethical and societal issues around prenatal screening, which at a macro level can be seen as screening out genetic conditions or disabilities in society—a devaluing of certain kinds of lives—but at a micro level is about a pregnant person's right to make choices that are best for their family.
Acknowledgments
The medical editor was Jeanne McKane. Others involved in the development and production of this report were Harrison Heft, Claude Soulodre, Kara Cowan, Ana Laing, Kellee Kaulback, Sarah McDowell, Vivian Ng, Andrée Mitchell, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are grateful to June Carroll (Sinai Health System), Shelley Dougan (Better Outcomes Registry and Network [BORN] Ontario), Elaine Goh (Trillium Health Partners), Tianhua Huang (BORN Ontario), Raymond Kim (University of Toronto), Julian Little (University of Ottawa), Wendy Meschino (North York General Hospital), Nan Okun (Sinai Health System), François Rousseau (CHU de Québec—Laval University), Wendy Ungar (Hospital for Sick Children), and Meredith Vanstone (McMaster University) for their clinical and methodological expertise and input.
We thank all our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those consulted.
ABBREVIATIONS
- BORN
Better Outcomes Registry and Network
- CI
Confidence interval
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- CrI
Credible interval
- eFTS
Enhanced first-trimester screening
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- MPSS
Massively parallel shotgun sequencing
- MSS
Maternal serum screening (also known as quadruple screening)
- NICE
National Institute for Health and Care Excellence
- NIPT
Noninvasive prenatal testing
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
GLOSSARY
- Amniocentesis
A procedure performed during pregnancy in which a needle is used to withdraw a small amount of fluid from the sac around the fetus. It is used to diagnose certain genetic anomalies.
- Aneuploidy
An incorrect number of chromosomes.
- Chorionic villus sampling
A procedure performed during pregnancy in which a small number of cells is taken from the placenta and tested. It is used to diagnose certain genetic anomalies.
- Chromosomal anomaly
Having an incorrect number of chromosomes, or a change in the structure of a chromosome.
- Fetal fraction
The percentage of cell-free DNA present in a pregnant person's blood that is from the fetus.
- Incremental cost-effectiveness ratio (ICER)
Determines a “unit of benefit” for an intervention by dividing the incremental cost by the incremental effectiveness. The incremental cost is the difference between the cost of the treatment under study and an alternative treatment. The effectiveness is usually measured as years of life or as “quality-adjusted life-years.”
- Microdeletion
Loss of a tiny piece of a chromosome that is too small to be easily seen by a microscope.
- Monosomy
The presence of one copy of a chromosome rather than two.
- Nuchal translucency
A collection of fluid under the skin at the back of a fetus's neck early in pregnancy. Nuchal translucency ultrasound screening is done during pregnancy to see if a fetus is likely to have Down syndrome (trisomy 21) or another anomaly, since many babies with Down syndrome or other anomalies have more of this fluid than babies without genetic anomalies.
- Phenotype
The observable characteristics (e.g., appearance, development, behaviour) resulting from a person's genetic profile (their genotype).
- Trisomy
The presence of three copies of a chromosome rather than two; trisomy is the most common aneuploidy.
APPENDICES
Appendix 1: Funding Status of Noninvasive Prenatal Testing in Canada
Table A1:
Funding Status of Noninvasive Prenatal Testing in Canada
| Province or Territory | Public Fundinga | Eligibility Criteria/Notes |
|---|---|---|
| Nova Scotia | Yes | Funded based on the following criteriaa:
|
| Prince Edward Island | Yes | Nova Scotia provides medical genetics services to people from Prince Edward Island |
| New Brunswick | No | None |
| Newfoundland and Labrador | No | None |
| Quebec | Yes | Announced funding for high-risk pregnant people in early 2018. Previously, Harmony, Panorama, or MaterniT21 PLUS were available at private clinics |
| Ontario | Yes | Funded for people who meet one of the following criteria:
Funding is available for NIPT analysis for trisomies 21, 18, and 13 and sex chromosome aneuploidies. Microdeletion testing is not funded |
| Manitoba | Yes | Funded for people who meet one of the following criteriaa:
Manitoba also serves some Ontario patients and counsels them according to Ontario criteria |
| Saskatchewan | No | None |
| Alberta | No | None |
| British Columbia | Yes | Funded for people who meet one of the following criteria:
Funding is available for NIPT analysis for trisomies 21, 18, and 13, and sex chromosome aneuploidy. Microdeletion testing is not funded The Harmony test is the only publicly funded test available for NIPT (i.e., Panorama is not publicly funded) |
| Yukon | Yes | Funded for people who meet one of the following criteria:
|
| Northwest Territories | Yes | A proposal to fund NIPT for pregnancies at increased risk of trisomy 21, 18, or 13 has been accepted |
| Nunavut | No | Funded for people who meet one of the following criteria:
|
Abbreviations: FTS, first trimester screening; HIV, human immunodeficiency virus; IPS, integrated prenatal screening; NIPT, noninvasive prenatal testing; NT, nuchal translucency; SIPS, serum integrated prenatal screening.
According to a 2018 Canadian environmental scan on NIPT.23
Appendix 2: Guideline Recommendations
Table A2:
Guideline Recommendations for Noninvasive Prenatal Testing
| Author, Year | Recommendation Excerpts |
|---|---|
| Canadian Guideline | |
| Society of Obstetricians and Gynaecologists of Canada, Canadian College of Medical Geneticists, 201737 |
Women who are considering undergoing maternal plasma cell-free DNA (cfDNA) screening should be informed that:
|
| International Guidelines | |
| American College of Medical Genetics and Genomics, 2016132 | For implementation of NIPS into practice, ACMG recommends:
For average- and low-risk women, ACMG recommends:
For autosomal aneuploidies other than Patau, Edwards, and Down syndrome, ACMG does not recommend:
For sex chromosome aneuploidies, ACMG recommends:
For CNV, ACMG recommends:
For CNV, ACMG does not recommend:
|
| American College of Obstetricians and Gynecologists, Society for Maternal–Fetal Medicine, 2016133 | The following recommendations and conclusions are based on good and consistent scientific evidence (Level A):
The following recommendations and conclusions are based on limited or inconsistent scientific evidence (Level B):
The following recommendations and conclusions are based primarily on consensus and expert opinion (Level C):
|
| American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine, 2015134 |
|
| Society for Maternal–Fetal Medicine, 2015135 |
After a failed cfDNA test, genetic counseling should be performed that includes offering diagnostic testing (chorionic villous sampling or amniocentesis) and repeat cfDNA screening [Best practice recommendation] |
| Society for Maternal–Fetal Medicine, 2017136 |
|
| National Society of Genetic Counselors, 2013137 |
|
| Human Genetics Society of Australia, Royal Australian and New Zealand College of Obstetricians and Gynaecologists, 2016138 |
|
| European Society of Human Genetics, American Society of Human Genetics, 2015139 |
|
| Schmid et al, 2015140 (Supported by: Austrian Society of Obstetrics and Gynecology, Austrian Society of Ultrasound in Medicine, Austrian Society of Pre- and Perinatal Medicine, Austrian Society of Human Genetics, German Society of Ultrasound in Medicine, Fetal Medicine Foundation of Germany, Swiss Society of Ultrasound in Medicine) |
|
| Polish Gynecological Society, Polish Human Genetics Society, 2017141 |
|
| International Society for Prenatal Diagnosis, 2015142 |
|
| International Society of Ultrasound in Obstetrics and Gynecology, 2017143 |
|
| Allyse et al, 201338 |
Best ethical practices for clinicians Medical providers offering noninvasive prenatal testing should:
Best ethical practices for commercial test providers Companies offering noninvasive prenatal testing should:
|
Abbreviations: ACMG, American College of Medical Genetics and Genomics; cfDNA, cell-free DNA; cFTS, combined first-trimester testing; CNV, copy-number variant; CVS, chorionic villus sampling; DR, detection rate; DANSR, Digital Analysis for Selected Regions; FPR, false-positive rate; ISUOG; International Society of Ultrasound in Obstetrics and Gynecology; MPS, massively parallel sequencing; NIPS, noninvasive prenatal screening; NIPT, noninvasive prenatal testing; NSGC, National Society of Genetic Counselors; NPV, negative predictive value; PPV, positive predictive value; NT, nuchal translucency; SPEC, specificity.
Note: Guideline statements and recommendations are verbatim.
Appendix 3: Systematic Reviews
Table A3:
Systematic Reviews on NIPT in the Average-Risk or General Population
| Author, Year | Search Period | Databases | Included Studies | Results |
|---|---|---|---|---|
| Taylor-Phillips et al, 201619 | 1997 to Feb 9, 2015 (auto-alerts until April 1, 2015) | PubMed, Medline, Embase, Cochrane Library Trial registries: ClinicalTrials.gov, WHO ICTRP |
6 studies in general population |
|
| Iwarsson et al, 201718 | To Apr 2, 2015 | PubMed, Embase, Cochrane Library | 6 studies for average risk of trisomy 21 5 studies for average risk of trisomy 18 5 studies for average risk of trisomy 13 |
|
| Badeau et al, 201716 | January 2007 to July 12, 2016 | Medline, Embase, Web of Science, Cochrane Register of Diagnostic Test Accuracy Studies, Cochrane Library, ClinicalTrials.gov, European Clinical Trials Register, WHO ICTRP, National Technical Information Service, OpenGrey, National Guideline Clearing House | 5 studies in general unselected pregnant population |
|
| Varela-Lema et al, 201717 | Inception to February/March 2017 | Centre for Reviews and Dissemination, Cochrane Library Plus, Medline, Embase, Web of Science | 8 studies in general population | Trisomy 21: sensitivity 99.3% (95% CI, 97.8%–99.8%), specificity 99.9% (95% CI, 99.8%–99.9%) Trisomy 18: sensitivity 97.4% (95% CI, 94.4%–98.8%), specificity 99.9% (95% CI, 99.87%–99.97%) Trisomy 13: sensitivity 98.8% (95% CI, 1.41%–100%), specificity 99.9% (95% CI, 99.94%–99.97%) |
Abbreviations: CI, confidence interval; NIPT, noninvasive prenatal testing; PPV, positive predictive value; TMPS, targeted massively parallel sequencing; WHO ICTRP, World Health Organization International Clinical Trials Registry Platform.
Appendix 4: Literature Search Strategies
Clinical Evidence Search
Search date: September 11, 2017
Databases searched: Ovid MEDLINE, Embase, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <August 2017>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to September 7, 2017>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2017 Week 37>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1.
exp Aneuploidy/ (72175)
Annotation: Sub-terms under Aneuploidy/ include Trisomy/ and Chromosome Deletion/
-
2.
aneuploid*.ti,ab,kf. (43746)
-
3.
(trisom* or chromosom* triplicat*).ti,ab,kf. (41658)
-
4.
Chromosome Disorders/ (24985)
-
5.
((chromosom* or subchromosom* or sub-chromosom*) adj (disorder* or anomal* or abnormal*)).ti,ab,kf. (46333)
-
6.
exp Chromosome Duplication/ (17855)
Annotation: Scope note:An aberration in which an extra chromosome or a chromosomal segment is made
Subterms:
Tetrasomy/ (The possession of four chromosomes of any one type in an otherwise diploid cell.)
Trisomy/
-
7.
Chromosomes, Human, Pair 13/ (8168)
-
8.
(chromosome* 13 or chromosome* thirteen or patau* or bartholin-patau* or T13).ti,ab,kf. (9488)
-
9.
Chromosomes, Human, Pair 18/ (8817)
-
10.
(chromosome* 18 or chromosome* eighteen or edward* syndrome* or T18).ti,ab,kf. (6226)
-
11.
Chromosomes, Human, Pair 21/ (9778)
-
12.
Down Syndrome/ (53600)
-
13.
(chromosome* 21 or chromosome* twenty-one or chromosome* twentyone or down* syndrome* or T21).ti,ab,kf. (50915)
-
14.
Chromosomes, Human, X/ (23292)
-
15.
Chromosomes, Human, Y/ (17885)
-
16.
exp Sex Chromosome Disorders/ (69290)
-
17.
(((x or y) adj chromosom*) or male sex chromosom* or female sex chromosom*).ti,ab,kf. (61635)
-
18.
Turner syndrome/ (17279)
-
19.
(45 x or turner* syndrome* or bonnevie-ullrich or monosom* x or ullrich-turner*).ti,ab,kf. (21326)
-
20.
Klinefelter syndrome/ (8401)
-
21.
(xxy or klinefelter* syndrome*).ti,ab,kf. (8330)
-
22.
XYY Karyotype/ (884)
-
23.
(xyy or jacob* syndrome* or yy syndrome*).ti,ab,kf. (2427)
-
24.
(xxx or triple-x or triplo-x*).ti,ab,kf. (3572)
-
25.
(micro-deletion* or microdeletion* or (copy number adj variant*) or (chromosom* adj deletion*) or (partial adj monosom*)).ti,ab,kf. (22337)
-
26.
1p36*.ti,ab,kf. (3788)
-
27.
DiGeorge Syndrome/ (4568)
-
28.
(digeorge* or di george* or CATCH22 or 22q11* or velocardiofacial or velo-cardio-facial or VCFS or cayler cardiofacial syndrome* or conotruncal anomaly face syndrome* or CTAF or sedlackova syndrome* or shprintzen syndrome* or takao syndrome*).ti,ab,kf. (11931)
-
29.
Prader-Willi Syndrome/ (7648)
-
30.
(prader-willi* or labhart-willi* or prader-labhart-willi* or prader* syndrome* or royer* syndrome* or PWS or PLW or PLWS).ti,ab,kf. (9960)
-
31.
Angelman Syndrome/ (2915)
-
32.
(angelman* or happy puppet* or 15q11*).ti,ab,kf. (5712)
-
33.
Williams Syndrome/ (3656)
-
34.
(((supravalvar or hypercalcemia-supravalvar) adj aortic stenosis) or williams* syndrome* or beuren* syndrome* or 7q11*).ti,ab,kf. (5631)
-
35.
22q11 Deletion Syndrome/ (742)
-
36.
22q11*.ti,ab,kf. (8233)
-
37.
Cri-du-Chat Syndrome/ (1372)
-
38.
(cri-du-chat or crying cat or cat cry or ((5p or 5q) adj2 (syndrome* or monosom*))).ti,ab,kf. (2593)
-
39.
or/1-38 (382292)
-
40.
Sequence Analysis, DNA/ (305481)
-
41.
((DNA or parallel or next-generation or shotgun or target*) adj sequenc*).ti,ab,kf. (255174)
-
42.
(MPSS or NGS or CSS or TMPS).ti,ab,kf. (36772)
-
43.
High-Throughput Nucleotide Sequencing/ (28228)
-
44.
((high throughput adj2 (analys#s or sequenc*)) or single nucleotide polymorphism* or SNP or SNPs).ti,ab,kf. (255638)
-
45.
or/40-44 (745097)
-
46.
Genetic Testing/ (61686)
-
47.
((genetic* or gene*1 or genome*1 or genomic*) adj2 (test or tests or testing or diagnos#s or screen*)).ti,ab,kf. (134466)
-
48.
or/46-47 (173089)
-
49.
(noninvasive* or non-invasive*).ti,ab,kf. (394139)
-
50.
48 and 49 (2231)
-
51.
45 or 50 (746958)
-
52.
Prenatal Diagnosis/ (88789)
-
53.
((antenatal or ante-natal or intrauterine or intra-uterine or prenatal or pre-natal) adj2 (test or tests or testing or diagnos#s or detect* or screen*)).ti,ab,kf. (75086)
-
54.
(maternal adj2 (plasm* or blood)).ti,ab,kf. (28418)
-
55.
or/52-54 (145986)
-
56.
51 and 55 (4879)
-
57.
(((f?etal or f?etus* or free-f?etal or placenta*) adj2 dna) or cell-free dna).ti,ab,kf. (9774)
-
58.
(cff DNA or cffDNA or cf DNA or cfDNA or f DNA or fDNA or ff DNA or ffDNA).ti,ab,kf. (3746)
-
59.
((noninvasive* or non-invasive*) adj5 (prenatal or f?etal or f?etus*) adj (test or tests or testing or diagnos#s or detect* or screen*)).ti,ab,kf. (3888)
-
60.
(NIPT or NIPD or NIDT or gNIPT or NIPS).ti,ab,kf. (2880)
-
61.
or/56-60 (17100)
-
62.
39 and 61 (4887)
-
63.
exp Animals/ not Humans/ (14739671)
-
64.
62 not 63 (3258)
-
65.
Case Reports/ or Comment.pt. or Editorial.pt. or Letter.pt. or Congresses.pt. (4954736)
-
66.
64 not 65 (2964)
-
67.
limit 66 to english language [Limit not valid in CDSR; records were retained] (2713)
-
68.
67 use ppez,coch,cctr,clhta,cleed (1498)
-
69.
exp aneuploidy/ (72175)
-
70.
aneuploid*.tw,kw. (44692)
-
71.
trisomy/ (21627)
-
72.
(trisom* or chromosom* triplicat*).tw,kw. (42442)
-
73.
chromosome disorder/ (28876)
-
74.
((chromosom* or subchromosom* or sub-chromosom*) adj (disorder* or anomal* or abnormal*)).tw,kw. (46385)
-
75.
chromosome duplication/ (5471)
-
76.
trisomy 13/ (2267)
-
77.
(chromosome* 13 or chromosome* thirteen or patau* or bartholin-patau* or T13).tw,kw. (9592)
-
78.
trisomy 18/ (3144)
-
79.
Edwards syndrome/ (463)
-
80.
(chromosome* 18 or chromosome* eighteen or edward* syndrome* or T18).tw,kw. (6334)
-
81.
trisomy 21/ (30940)
-
82.
Down syndrome/ (53600)
-
83.
(chromosome* 21 or chromosome* twenty-one or chromosome* twentyone or down* syndrome* or T21).tw,kw. (51741)
-
84.
X chromosome/ (38452)
-
85.
Y chromosome/ (21567)
-
86.
exp sex chromosome aberration/ (15516)
-
87.
(((x or y) adj chromosom*) or male sex chromosom* or female sex chromosom*).tw,kw. (62294)
-
88.
Turner syndrome/ (17279)
-
89.
(45 x or turner* syndrome* or bonnevie-ullrich or monosom* x or ullrich-turner*).tw,kw. (21502)
-
90.
exp Klinefelter syndrome/ (8508)
-
91.
(xxy or klinefelter* syndrome*).tw,kw. (8405)
-
92.
karyotype 47,XYY/ (529)
-
93.
(xyy or jacob* syndrome* or yy syndrome*).tw,kw. (2455)
-
94.
47,XXX syndrome/ (54)
-
95.
(xxx or triple-x or triplo-x*).tw,kw. (3598)
-
96.
chromosome deletion/ (40585)
-
97.
(micro-deletion* or microdeletion* or (copy number adj variant*) or (chromosom* adj deletion*) or (partial adj monosom*)).tw,kw. (22975)
-
98.
1p36*.tw,kw. (3799)
-
99.
DiGeorge syndrome/ (4568)
-
100.
(digeorge* or di george* or CATCH22 or 22q11* or velocardiofacial or velo-cardio-facial or VCFS or cayler cardiofacial syndrome* or conotruncal anomaly face syndrome* or CTAF or sedlackova syndrome* or shprintzen syndrome* or takao syndrome*).tw,kw. (12043)
-
101.
Prader Willi syndrome/ (7648)
-
102.
(prader-willi* or labhart-willi* or prader-labhart-willi* or prader* syndrome* or royer* syndrome* or PWS or PLW or PLWS).tw,kw. (10100)
-
103.
happy puppet syndrome/ (3489)
-
104.
(angelman* or happy puppet* or 15q11*).tw,kw. (5816)
-
105.
Williams Beuren syndrome/ (4505)
-
106.
(((supravalvar or hypercalcemia-supravalvar) adj aortic stenosis) or williams* syndrome* or beuren* syndrome* or 7q11*).tw,kw. (5693)
-
107.
chromosome deletion 22q11/ (1205)
-
108.
22q11*.tw,kw. (8320)
-
109.
cat cry syndrome/ (1420)
-
110.
(cri-du-chat or crying cat or cat cry or ((5p or 5q) adj2 (syndrome* or monosom*))).tw,kw. (2634)
-
111.
or/69-110 (392560)
-
112.
dna sequence/ (623128)
-
113.
next generation sequencing/ (23023)
-
114.
((DNA or parallel or next-generation or shotgun or target*) adj sequenc*).tw,kw,dv. (257559)
-
115.
(MPSS or NGS or CSS or TMPS).tw,kw,dv. (37286)
-
116.
high throughput sequencing/ (15438)
-
117.
((high throughput adj2 (analys#s or sequenc*)) or single nucleotide polymorphism* or SNP or SNPs).tw,kw,dv. (261185)
-
118.
or/112-117 (1044893)
-
119.
genetic screening/ (99427)
-
120.
((genetic* or gene*1 or genome*1 or genomic*) adj2 (test or tests or testing or diagnos#s or screen*)).tw,kw,dv. (137097)
-
121.
or/119-120 (195072)
-
122.
non invasive procedure/ (22579)
-
123.
(noninvasive* or non-invasive*).tw,kw,dv. (397409)
-
124.
or/122-123 (401063)
-
125.
121 and 124 (2555)
-
126.
118 or 125 (1047024)
-
127.
prenatal diagnosis/ (88789)
-
128.
((antenatal or ante-natal or intrauterine or intra-uterine or prenatal or pre-natal) adj2 (test or tests or testing or diagnos#s or detect* or screen*)).tw,kw,dv. (78643)
-
129.
maternal plasma/ (3085)
-
130.
(maternal adj2 (plasm* or blood)).tw,kw,dv. (28664)
-
131.
or/127-130 (147990)
-
132.
126 and 131 (5826)
-
133.
(((f?etal or f?etus* or free-f?etal or placenta*) adj2 dna) or cell-free dna).tw,kw,dv. (9994)
-
134.
(cff DNA or cffDNA or cf DNA or cfDNA or f DNA or fDNA or ff DNA or ffDNA).tw,kw,dv. (3786)
-
135.
((noninvasive* or non-invasive*) adj5 (prenatal or pre-natal or f?etal or f?etus*) adj (test or tests or testing or diagnos#s or detect* or screen*)).tw,kw,dv. (4002)
-
136.
(NIPT or NIPD or NIDT or gNIPT or NIPS).tw,kw,dv. (2948)
-
137.
or/132-136 (18162)
-
138.
111 and 137 (5130)
-
139.
(exp animal/ or nonhuman/) not exp human/ (10421029)
-
140.
138 not 139 (5084)
-
141.
Case Report/ or Comment/ or Editorial/ or Letter/ or conference abstract.pt. (9439143)
-
142.
140 not 141 (3647)
-
143.
limit 142 to english language [Limit not valid in CDSR; records were retained] (3309)
-
144.
143 use emez (1775)
-
145.
68 or 144 (3273)
-
146.
limit 145 to yr=“2007 -Current” (2613)
-
147.
146 use ppez (1148)
-
148.
146 use emez (1424)
-
149.
146 use cctr (25)
-
150.
146 use coch (1)
-
151.
146 use clhta (6)
-
152.
146 use cleed (9)
-
153.
remove duplicates from 146 (1605)
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Chromosome Aberrations+”) | 1,656 |
| S2 | aneuploid* | 1,492 |
| S3 | (trisom* or chromosom* triplicat*) | 1,480 |
| S4 | (MH “Chromosome Disorders”) | 3,823 |
| S5 | ((chromosom* or subchromosom* or sub-chromosom*) N1 (disorder* or anomal* or abnormal*)) | 4,891 |
| S6 | (MH “Trisomy 13”) | 62 |
| S7 | (chromosome* 13 or chromosome* thirteen or patau* or bartholinpatau* or T13) | 459 |
| S8 | (MH “Trisomy 18”) | 75 |
| S9 | (chromosome* 18 or chromosome* eighteen or edward* syndrome* or T18) | 311 |
| S10 | (MH “Down Syndrome”) | 5,566 |
| S11 | (chromosome* 21 or chromosome* twenty-one or chromosome* twentyone or down* syndrome* or T21) | 7,008 |
| S12 | (((x or y) N1 chromosom*) or male sex chromosom* or female sex chromosom*) | 673 |
| S13 | (MH “Turner's Syndrome”) | 483 |
| S14 | (45 x or turner* syndrome* or bonnevie-ullrich or monosom* x or ullrich-turner*) | 868 |
| S15 | (MH “Klinefelter's Syndrome”) | 236 |
| S16 | (xxy or klinefelter* syndrome*) | 332 |
| S17 | (MH “XYY Syndrome”) | 5 |
| S18 | (xyy or jacob* syndrome* or yy syndrome*) | 66 |
| S19 | (xxx or triple-x or triplo-x*) | 183,845 |
| S20 | (micro-deletion* or microdeletion* or (copy number N1 variant*) or (chromosom* N1 deletion*) or (partial N1 monosom*)) | 729 |
| S21 | 1p36* | 693 |
| S22 | (MH “DiGeorge Syndrome”) | 237 |
| S23 | (digeorge* or di george* or CATCH22 or 22q11* or velocardiofacial or velo-cardio-facial or VCFS or cayler cardiofacial syndrome* or conotruncal anomaly face syndrome* or CTAF or sedlackova syndrome* or shprintzen syndrome* or takao syndrome*) | 863 |
| S24 | (MH “Prader-Willi Syndrome”) | 539 |
| S25 | (prader-willi* or labhart-willi* or prader-labhart-willi* or prader* syndrome* or royer* syndrome* or PWS or PLW or PLWS) | 823 |
| S26 | (MH “Angelman Syndrome”) | 154 |
| S27 | (angelman* or happy puppet* or 15q11*) | 264 |
| S28 | (MH “Williams Syndrome”) | 424 |
| S29 | (((supravalvar or hypercalcemia-supravalvar) N1 aortic stenosis) or williams* syndrome* or beuren* syndrome* or 7q11*) | 558 |
| S30 | (MH “22q11 Deletion Syndrome+”) | 297 |
| S31 | 22q11* | 438 |
| S32 | (MH “Cri-Du-Chat Syndrome”) | 84 |
| S33 | (cri-du-chat or crying cat or cat cry or ((5p or 5q) N2 (syndrome* or monosom*))) | 858 |
| S34 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 201,252 |
| S35 | (MH “Sequence Analysis+”) | 12,935 |
| S36 | ((DNA or parallel or next-generation or shotgun or target*) N1 sequenc*) | 3,714 |
| S37 | (MPSS or NGS or CSS or TMPS) | 1,678 |
| S38 | ((high throughput N2 (analys#s or sequenc*)) or single nucleotide polymorphism* or SNP or SNPs) | 8,017 |
| S39 | S35 OR S36 OR S37 OR S38 | 23,341 |
| S40 | (MH “Genetic Screening”) | 9,414 |
| S41 | ((genetic* or gene or genes or genome* or genomic*) N2 (test or tests or testing or diagnos#s or screen*)) | 14,372 |
| S42 | S40 OR S41 | 14,372 |
| S43 | (MH “Noninvasive Procedures”) | 1,777 |
| S44 | (noninvasive* or non-invasive*) | 22,509 |
| S45 | S43 OR S44 | 22,509 |
| S46 | S42 AND S45 | 294 |
| S47 | S39 OR S46 | 23,575 |
| S48 | (MH “Prenatal Diagnosis”) | 6,277 |
| S49 | ((antenatal or ante-natal or intrauterine or intra-uterine or prenatal or pre-natal) N2 (test or tests or testing or diagnos#s or detect* or screen*)) | 9,738 |
| S50 | (maternal N2 (plasm* or blood)) | 1,823 |
| S51 | S48 OR S49 OR S50 | 11,233 |
| S52 | S47 AND S51 | 546 |
| S53 | (((f?etal or f?etus* or free-f?etal or placenta*) N2 dna) or cell-free dna) | 5,146 |
| S54 | (cff DNA or cffDNA or cf DNA or cfDNA or f DNA or fDNA or ff DNA or ffDNA) | 311 |
| S55 | ((noninvasive* or non-invasive*) N5 (prenatal or pre-natal or f?etal or f?etus*) N1 (test or tests or testing or diagnos#s or detect* or screen*)) | 1,084 |
| S56 | (NIPT or NIPD or NIDT or gNIPT or NIPS) | 409 |
| S57 | S52 OR S53 OR S54 OR S55 OR S56 | 6,493 |
| S58 | S34 AND S57 | 1,247 |
| S59 | PT Case Study or Commentary or Editorial or Letter or Proceedings | 403,814 |
| S60 | S58 not S59 | 1,181 |
| S61 | Limiters - Published Date: 20070101-20171231 | 1,055 |
| S62 | Narrow by Language: - english | 1,051 |
Economic Evidence Search
Search date: September 14, 2017
Databases searched: Ovid MEDLINE, Embase, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <August 2017>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to September 13, 2017>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2017 Week 37>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1.
exp Aneuploidy/ (72195)
Annotation: Sub-terms under Aneuploidy/ include Trisomy/ and Chromosome Deletion/
-
2.
aneuploid*.ti,ab,kf. (43738)
-
3.
(trisom* or chromosom* triplicat*).ti,ab,kf. (41658)
-
4.
Chromosome Disorders/ (24987)
-
5.
((chromosom* or subchromosom* or sub-chromosom*) adj (disorder* or anomal* or abnormal*)).ti,ab,kf. (46325)
-
6.
exp Chromosome Duplication/ (17858)
Annotation: Scope note:An aberration in which an extra chromosome or a chromosomal segment is made
Subterms:
Tetrasomy/ (The possession of four chromosomes of any one type in an otherwise diploid cell.) Trisomy/
-
7.
Chromosomes, Human, Pair 13/ (8171)
-
8.
(chromosome* 13 or chromosome* thirteen or patau* or bartholin-patau* or T13).ti,ab,kf. (9486)
-
9.
Chromosomes, Human, Pair 18/ (8819)
-
10.
(chromosome* 18 or chromosome* eighteen or edward* syndrome* or T18).ti,ab,kf. (6226)
-
11.
Chromosomes, Human, Pair 21/ (9778)
-
12.
Down Syndrome/ (53611)
-
13.
(chromosome* 21 or chromosome* twenty-one or chromosome* twentyone or down* syndrome* or T21).ti,ab,kf. (50915)
-
14.
Chromosomes, Human, X/ (23294)
-
15.
Chromosomes, Human, Y/ (17887)
-
16.
exp Sex Chromosome Disorders/ (69295)
-
17.
(((x or y) adj chromosom*) or male sex chromosom* or female sex chromosom*).ti,ab,kf. (61627)
-
18.
Turner syndrome/ (17283)
-
19.
(45 x or turner* syndrome* or bonnevie-ullrich or monosom* x or ullrich-turner*).ti,ab,kf. (21325)
-
20.
Klinefelter syndrome/ (8401)
-
21.
(xxy or klinefelter* syndrome*).ti,ab,kf. (8332)
-
22.
XYY Karyotype/ (884)
-
23.
(xyy or jacob* syndrome* or yy syndrome*).ti,ab,kf. (2428)
-
24.
(xxx or triple-x or triplo-x*).ti,ab,kf. (3572)
-
25.
(micro-deletion* or microdeletion* or (copy number adj variant*) or (chromosom* adj deletion*) or (partial adj monosom*)).ti,ab,kf. (22332)
-
26.
1p36*.ti,ab,kf. (3789)
-
27.
DiGeorge Syndrome/ (4568)
-
28.
(digeorge* or di george* or CATCH22 or 22q11* or velocardiofacial or velo-cardio-facial or VCFS or cayler cardiofacial syndrome* or conotruncal anomaly face syndrome* or CTAF or sedlackova syndrome* or shprintzen syndrome* or takao syndrome*).ti,ab,kf. (11932)
-
29.
Prader-Willi Syndrome/ (7649)
-
30.
(prader-willi* or labhart-willi* or prader-labhart-willi* or prader* syndrome* or royer* syndrome* or PWS or PLW or PLWS).ti,ab,kf. (9960)
-
31.
Angelman Syndrome/ (2915)
-
32.
(angelman* or happy puppet* or 15q11*).ti,ab,kf. (5715)
-
33.
Williams Syndrome/ (3656)
-
34.
(((supravalvar or hypercalcemia-supravalvar) adj aortic stenosis) or williams* syndrome* or beuren* syndrome* or 7q11*).ti,ab,kf. (5628)
-
35.
22q11 Deletion Syndrome/ (742)
-
36.
22q11*.ti,ab,kf. (8233)
-
37.
Cri-du-Chat Syndrome/ (1372)
-
38.
(cri-du-chat or crying cat or cat cry or ((5p or 5q) adj2 (syndrome* or monosom*))).ti,ab,kf. (2593)
-
39.
or/1-38 (382280)
-
40.
Sequence Analysis, DNA/ (305684)
-
41.
((DNA or parallel or next-generation or shotgun or target*) adj sequenc*).ti,ab,kf. (255120)
-
42.
(MPSS or NGS or CSS or TMPS).ti,ab,kf. (36771)
-
43.
High-Throughput Nucleotide Sequencing/ (28412)
-
44.
((high throughput adj2 (analys#s or sequenc*)) or single nucleotide polymorphism* or SNP or SNPs).ti,ab,kf. (255606)
-
45.
or/40-44 (745279)
-
46.
Genetic Testing/ (61722)
-
47.
((genetic* or gene*1 or genome*1 or genomic*) adj2 (test or tests or testing or diagnos#s or screen*)).ti,ab,kf. (134472)
-
48.
or/46-47 (173107)
-
49.
(noninvasive* or non-invasive*).ti,ab,kf. (394165)
-
50.
48 and 49 (2232)
-
51.
45 or 50 (747141)
-
52.
Prenatal Diagnosis/ (88801)
-
53.
((antenatal or ante-natal or intrauterine or intra-uterine or prenatal or pre-natal) adj2 (test or tests or testing or diagnos#s or detect* or screen*)).ti,ab,kf. (75088)
-
54.
(maternal adj2 (plasm* or blood)).ti,ab,kf. (28413)
-
55.
or/52-54 (145987)
-
56.
51 and 55 (4879)
-
57.
(((f?etal or f?etus* or free-f?etal or placenta*) adj2 dna) or cell-free dna).ti,ab,kf. (9771)
-
58.
(cff DNA or cffDNA or cf DNA or cfDNA or f DNA or fDNA or ff DNA or ffDNA).ti,ab,kf. (3747)
-
59.
((noninvasive* or non-invasive*) adj5 (prenatal or f?etal or f?etus*) adj (test or tests or testing or diagnos#s or detect* or screen*)).ti,ab,kf. (3885)
-
60.
(NIPT or NIPD or NIDT or gNIPT or NIPS).ti,ab,kf. (2878)
-
61.
or/56-60 (17096)
-
62.
39 and 61 (4885)
-
63.
economics/ (254063)
-
64.
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (784912)
-
65.
economics.fs. (408693)
-
66.
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (775112)
-
67.
exp “costs and cost analysis”/ (549308)
-
68.
(cost or costs or costing or costly).ti. (238182)
-
69.
cost effective*.ti,ab,kf. (277495)
-
70.
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (180176)
-
71.
models, economic/ (11024)
-
72.
markov chains/ or monte carlo method/ (72818)
-
73.
(decision adj1 (tree* or analy* or model*)).ti,ab,kf. (35829)
-
74.
(markov or markow or monte carlo).ti,ab,kf. (114677)
-
75.
quality-adjusted life years/ (33915)
-
76.
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (58350)
-
77.
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (94568)
-
78.
or/63-77 (2320493)
-
79.
62 and 78 (434)
-
80.
79 use ppez,coch,cctr,clhta (142)
-
81.
62 use cleed (10)
-
82.
or/80-81 (152)
-
83.
limit 82 to english language [Limit not valid in CDSR; records were retained] (140)
-
84.
exp aneuploidy/ (72195)
-
85.
aneuploid*.tw,kw. (44685)
-
86.
trisomy/ (21629)
-
87.
(trisom* or chromosom* triplicat*).tw,kw. (42441)
-
88.
chromosome disorder/ (28878)
-
89.
((chromosom* or subchromosom* or sub-chromosom*) adj (disorder* or anomal* or abnormal*)).tw,kw. (46380)
-
90.
chromosome duplication/ (5472)
-
91.
trisomy 13/ (2267)
-
92.
(chromosome* 13 or chromosome* thirteen or patau* or bartholin-patau* or T13).tw,kw. (9590)
-
93.
trisomy 18/ (3144)
-
94.
Edwards syndrome/ (463)
-
95.
(chromosome* 18 or chromosome* eighteen or edward* syndrome* or T18).tw,kw. (6334)
-
96.
trisomy 21/ (30951)
-
97.
Down syndrome/ (53611)
-
98.
(chromosome* 21 or chromosome* twenty-one or chromosome* twentyone or down* syndrome* or T21).tw,kw. (51741)
-
99.
X chromosome/ (38453)
-
100.
Y chromosome/ (21567)
-
101.
exp sex chromosome aberration/ (15517)
-
102.
(((x or y) adj chromosom*) or male sex chromosom* or female sex chromosom*).tw,kw. (62286)
-
103.
Turner syndrome/ (17283)
-
104.
(45 x or turner* syndrome* or bonnevie-ullrich or monosom* x or ullrich-turner*).tw,kw. (21501)
-
105.
exp Klinefelter syndrome/ (8508)
-
106.
(xxy or klinefelter* syndrome*).tw,kw. (8407)
-
107.
karyotype 47,XYY/ (529)
-
108.
(xyy or jacob* syndrome* or yy syndrome*).tw,kw. (2456)
-
109.
47,XXX syndrome/ (54)
-
110.
(xxx or triple-x or triplo-x*).tw,kw. (3598)
-
111.
chromosome deletion/ (40591)
-
112.
(micro-deletion* or microdeletion* or (copy number adj variant*) or (chromosom* adj deletion*) or (partial adj monosom*)).tw,kw. (22970)
-
113.
1p36*.tw,kw. (3800)
-
114.
DiGeorge syndrome/ (4568)
-
115.
(digeorge* or di george* or CATCH22 or 22q11* or velocardiofacial or velo-cardio-facial or VCFS or cayler cardiofacial syndrome* or conotruncal anomaly face syndrome* or CTAF or sedlackova syndrome* or shprintzen syndrome* or takao syndrome*).tw,kw. (12044)
-
116.
Prader Willi syndrome/ (7649)
-
117.
(prader-willi* or labhart-willi* or prader-labhart-willi* or prader* syndrome* or royer* syndrome* or PWS or PLW or PLWS).tw,kw. (10100)
-
118.
happy puppet syndrome/ (3489)
-
119.
(angelman* or happy puppet* or 15q11*).tw,kw. (5819)
-
120.
Williams Beuren syndrome/ (4505)
-
121.
(((supravalvar or hypercalcemia-supravalvar) adj aortic stenosis) or williams* syndrome* or beuren* syndrome* or 7q11*).tw,kw. (5690)
-
122.
chromosome deletion 22q11/ (1205)
-
123.
22q11*.tw,kw. (8320)
-
124.
cat cry syndrome/ (1420)
-
125.
(cri-du-chat or crying cat or cat cry or ((5p or 5q) adj2 (syndrome* or monosom*))).tw,kw. (2634)
-
126.
or/84-125 (392546)
-
127.
dna sequence/ (623222)
-
128.
next generation sequencing/ (23023)
-
129.
((DNA or parallel or next-generation or shotgun or target*) adj sequenc*).tw,kw,dv. (257498)
-
130.
(MPSS or NGS or CSS or TMPS).tw,kw,dv. (37282)
-
131.
high throughput sequencing/ (15438)
-
132.
((high throughput adj2 (analys#s or sequenc*)) or single nucleotide polymorphism* or SNP or SNPs).tw,kw,dv. (261153)
-
133.
or/127-132 (1044877)
-
134.
genetic screening/ (99463)
-
135.
((genetic* or gene*1 or genome*1 or genomic*) adj2 (test or tests or testing or diagnos#s or screen*)).tw,kw,dv. (137099)
-
136.
or/134-135 (195087)
-
137.
non invasive procedure/ (22579)
-
138.
(noninvasive* or non-invasive*).tw,kw,dv. (397434)
-
139.
or/137-138 (401088)
-
140.
136 and 139 (2555)
-
141.
133 or 140 (1047008)
-
142.
prenatal diagnosis/ (88801)
-
143.
((antenatal or ante-natal or intrauterine or intra-uterine or prenatal or pre-natal) adj2 (test or tests or testing or diagnos#s or detect* or screen*)).tw,kw,dv. (78640)
-
144.
maternal plasma/ (3085)
-
145.
(maternal adj2 (plasm* or blood)).tw,kw,dv. (28659)
-
146.
or/142-145 (147987)
-
147.
141 and 146 (5825)
-
148.
(((f?etal or f?etus* or free-f?etal or placenta*) adj2 dna) or cell-free dna).tw,kw,dv. (9991)
-
149.
(cff DNA or cffDNA or cf DNA or cfDNA or f DNA or fDNA or ff DNA or ffDNA).tw,kw,dv. (3787)
-
150.
((noninvasive* or non-invasive*) adj5 (prenatal or pre-natal or f?etal or f?etus*) adj (test or tests or testing or diagnos#s or detect* or screen*)).tw,kw,dv. (3998)
-
151.
(NIPT or NIPD or NIDT or gNIPT or NIPS).tw,kw,dv. (2946)
-
152.
or/147-151 (18157)
-
153.
126 and 152 (5127)
-
154.
Economics/ (254063)
-
155.
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (128271)
-
156.
Economic Aspect/ or exp Economic Evaluation/ (421883)
-
157.
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw. (799420)
-
158.
exp “Cost”/ (549308)
-
159.
(cost or costs or costing or costly).ti. (238182)
-
160.
cost effective*.tw,kw. (288324)
-
161.
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (181289)
-
162.
Monte Carlo Method/ (58993)
-
163.
(decision adj1 (tree* or analy* or model*)).tw,kw. (39527)
-
164.
(markov or markow or monte carlo).tw,kw. (119639)
-
165.
Quality-Adjusted Life Years/ (33915)
-
166.
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (62103)
-
167.
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw. (113787)
-
168.
or/154-167 (1964482)
-
169.
153 and 168 (412)
-
170.
169 use emez (274)
-
171.
limit 170 to english language [Limit not valid in CDSR; records were retained] (265)
-
172.
83 or 171 (405)
-
173.
limit 172 to yr=“2007 -Current” (384)
-
174.
173 use ppez (118)
-
175.
173 use emez (253)
-
176.
173 use coch (0)
-
177.
173 use cctr (3)
-
178.
173 use clhta (1)
-
179.
173 use cleed (9)
-
180.
remove duplicates from 173 (281)
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Chromosome Aberrations+”) | 1,658 |
| S2 | aneuploid* | 1,492 |
| S3 | (trisom* or chromosom* triplicat*) | 1,480 |
| S4 | (MH “Chromosome Disorders”) | 3,823 |
| S5 | ((chromosom* or subchromosom* or sub-chromosom*) N1 (disorder* or anomal* or abnormal*)) | 4,890 |
| S6 | (MH “Trisomy 13”) | 62 |
| S7 | (chromosome* 13 or chromosome* thirteen or patau* or bartholinpatau* or T13) | 460 |
| S8 | (MH “Trisomy 18”) | 75 |
| S9 | (chromosome* 18 or chromosome* eighteen or edward* syndrome* or T18) | 312 |
| S10 | (MH “Down Syndrome”) | 5,567 |
| S11 | (chromosome* 21 or chromosome* twenty-one or chromosome* twentyone or down* syndrome* or T21) | 7,008 |
| S12 | (((x or y) N1 chromosom*) or male sex chromosom* or female sex chromosom*) | 674 |
| S13 | (MH “Turner's Syndrome”) | 484 |
| S14 | (45 x or turner* syndrome* or bonnevie-ullrich or monosom* x or ullrich-turner*) | 868 |
| S15 | (MH “Klinefelter's Syndrome”) | 236 |
| S16 | (xxy or klinefelter* syndrome*) | 333 |
| S17 | (MH “XYY Syndrome”) | 5 |
| S18 | (xyy or jacob* syndrome* or yy syndrome*) | 66 |
| S19 | (xxx or triple-x or triplo-x*) | 183,921 |
| S20 | (micro-deletion* or microdeletion* or (copy number N1 variant*) or (chromosom* N1 deletion*) or (partial N1 monosom*)) | 729 |
| S21 | 1p36* | 693 |
| S22 | (MH “DiGeorge Syndrome”) | 237 |
| S23 | (digeorge* or di george* or CATCH22 or 22q11* or velocardiofacial or velo-cardio-facial or VCFS or cayler cardiofacial syndrome* or conotruncal anomaly face syndrome* or CTAF or sedlackova syndrome* or shprintzen syndrome* or takao syndrome*) | 863 |
| S24 | (MH “Prader-Willi Syndrome”) | 539 |
| S25 | (prader-willi* or labhart-willi* or prader-labhart-willi* or prader* syndrome* or royer* syndrome* or PWS or PLW or PLWS) | 823 |
| S26 | (MH “Angelman Syndrome”) | 154 |
| S27 | (angelman* or happy puppet* or 15q11*) | 264 |
| S28 | (MH “Williams Syndrome”) | 424 |
| S29 | (((supravalvar or hypercalcemia-supravalvar) N1 aortic stenosis) or williams* syndrome* or beuren* syndrome* or 7q11*) | 558 |
| S30 | (MH “22q11 Deletion Syndrome+”) | 297 |
| S31 | 22q11* | 438 |
| S32 | (MH “Cri-Du-Chat Syndrome”) | 84 |
| S33 | (cri-du-chat or crying cat or cat cry or ((5p or 5q) N2 (syndrome* or monosom*))) | 858 |
| S34 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 201,329 |
| S35 | (MH “Sequence Analysis+”) | 12,938 |
| S36 | ((DNA or parallel or next-generation or shotgun or target*) N1 sequenc*) | 3,716 |
| S37 | (MPSS or NGS or CSS or TMPS) | 1,679 |
| S38 | ((high throughput N2 (analys#s or sequenc*)) or single nucleotide polymorphism* or SNP or SNPs) | 8,019 |
| S39 | S35 OR S36 OR S37 OR S38 | 23,347 |
| S40 | (MH “Genetic Screening”) | 9,418 |
| S41 | ((genetic* or gene or genes or genome* or genomic*) N2 (test or tests or testing or diagnos#s or screen*)) | 14,379 |
| S42 | S40 OR S41 | 14,379 |
| S43 | (MH “Noninvasive Procedures”) | 1,779 |
| S44 | (noninvasive* or non-invasive*) | 22,526 |
| S45 | S43 OR S44 | 22,526 |
| S46 | S42 AND S45 | 294 |
| S47 | S39 OR S46 | 23,581 |
| S48 | (MH “Prenatal Diagnosis”) | 6,277 |
| S49 | ((antenatal or ante-natal or intrauterine or intra-uterine or prenatal or pre-natal) N2 (test or tests or testing or diagnos#s or detect* or screen*)) | 9,737 |
| S50 | (maternal N2 (plasm* or blood)) | 1,823 |
| S51 | S48 OR S49 OR S50 | 11,232 |
| S52 | S47 AND S51 | 546 |
| S53 | (((f?etal or f?etus* or free-f?etal or placenta*) N2 dna) or cell-free dna) | 5,148 |
| S54 | (cff DNA or cffDNA or cf DNA or cfDNA or f DNA or fDNA or ff DNA or ffDNA) | 311 |
| S55 | ((noninvasive* or non-invasive*) N5 (prenatal or pre-natal or f?etal or f?etus*) N1 (test or tests or testing or diagnos#s or detect* or screen*)) | 1,084 |
| S56 | (NIPT or NIPD or NIDT or gNIPT or NIPS) | 409 |
| S57 | S52 OR S53 OR S54 OR S55 OR S56 | 6,495 |
| S58 | S34 AND S57 | 1,248 |
| S59 | (MH “Economics”) | 11,409 |
| S60 | (MH “Economic Aspects of Illness”) | 6,976 |
| S61 | (MH “Economic Value of Life”) | 524 |
| S62 | MH “Economics, Dental” | 110 |
| S63 | MH “Economics, Pharmaceutical” | 1,801 |
| S64 | MW “ec” | 145,411 |
| S65 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmacoeconomic*) | 224,154 |
| S66 | (MH “Costs and Cost Analysis+”) | 87,576 |
| S67 | TI cost* | 41,334 |
| S68 | (cost effective*) | 30,213 |
| S69 | AB (cost* N2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)) | 20,956 |
| S70 | (decision N1 (tree* or analy* or model*)) | 5,470 |
| S71 | (markov or markow or monte carlo) | 3,627 |
| S72 | (MH “Quality-Adjusted Life Years”) | 2,874 |
| S73 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs) | 6,879 |
| S74 | ((adjusted N1 (quality or life)) or (willing* N2 pay) or sensitivity analys?s) | 12,813 |
| S75 | S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 | 300,110 |
| S76 | S58 AND S75 | 84 |
| S77 | Limiters - Published Date: 20070101-20171231 | 78 |
| S78 | Narrow by Language: - english | 78 |
Grey Literature Search
Search dates: August 25–30, 2017
Websites searched: HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, clinicaltrails.gov, Tufts Cost Effectiveness Analysis Registry, Sick Kids’ Paediatric Economic Database Evaluation (PEDE)
Keywords: noninvasive, non-invasive, prenatal, fetus, fetal, faetus, faetal, cell-free DNA, aneuploidy, aneuploidies, trisomy, trisomies, chromosome triplication, chromosomal triplication, micro-deletion, microdeletion, copy number variant, NIPT, NIPD, NIDT, gNIPT, NIPS
Results
HTA = 8
Trials = 41 (not counted in PRISMA flow diagram)
Appendix 5: Critical Appraisal of Clinical Evidence
Table A4:
Risk of Biasa Among Test Accuracy Studies (QUADAS-2 Tool)
| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| Bianchi et al, 201452 | Highb | Low | Low | Highc | Highd | Low | Low |
| del Mar Gil et al, 201453 | Uncleare | Low | Low | Highc | Low | Low | Low |
| Langlois et al, 201754 | Highb | Low | Low | Low | Low | Low | Low |
| Nicolaides et al, 201255 | Highb | Low | Low | Highc | Low | Low | Low |
| Norton et al, 201556 | Highb | Low | Low | Highc | Low | Low | Low |
| Palomaki et al, 201757 | Highb | Low | Low | Highc | Low | Low | Low |
| Quezada et al, 201558 | Highb | Low | Unclearf | Highc | Low | Low | Low |
| Song et al, 201359 | Highb | Low | Low | Highc | Low | Low | Low |
Abbreviation: NIPT, noninvasive prenatal testing; QUADAS-2; Quality Assessment of Diagnostic Accuracy Studies.
Possible risk of bias levels: low, high, unclear.
Did not avoid inappropriate exclusions or had unclear consecutive or random enrolment of patients.
Inappropriate interval between NIPT and reference standard. Some studies missing or excluded patients in analysis.
Applicability concerns because of inappropriate patient exclusions.
Unclear patient enrolment and study exclusions.
Unclear whether reference standard was interpreted without knowledge of NIPT results.
Table A5:
Risk of Biasa Among Nonrandomized Trials (ROBINS-I Tool)
| Preintervention | At Intervention | Postintervention | |||||
|---|---|---|---|---|---|---|---|
| Author, Year | Confounding | Study Participation Selection | Classification of Interventions | Deviations From Intended Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Results |
| Bianchi et al, 201452 | Low | Moderatea | Low | Low | Moderateb | Low | Low |
| Langlois et al, 201754 | Low | Moderatea | Low | Low | Low | Low | Low |
| Palomaki et al, 201757 | Low | Moderatea | Low | Low | Moderateb | Seriousc | Moderated |
| Quezada et al, 201558 | Low | Moderatea | Low | Low | Moderateb | Moderatee | Low |
| Song et al, 201359 | Low | Moderatea | Low | Low | Low | Moderatee | Low |
Abbreviation: ROBINS-I, Risk of Bias in Non-randomized Studies–of Interventions.
Possible risk of bias levels: low, moderate, serious, critical, and no information.
Did not avoid inappropriate exclusions or had unclear consecutive or random enrolment of patients.
Missing data for some patients who did not have a successful noninvasive prenatal testing result.
Limited information regarding how clinical utility was measured among patients. Did not use a validated measurement tool.
Not all patients were included in the analysis.
Limited information regarding how clinical utility was measured among patients.
Table A6:
GRADE Evidence Profile for Noninvasive Prenatal Testing
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| NIPT Accuracy | |||||||
| Trisomy 21, sensitivity (8 test accuracy studiesa) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ ⊕ Moderate |
| Trisomy 21, specificity (8 test accuracy studiesa) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ ⊕ Moderate |
| Trisomy 18, sensitivity (7 test accuracy studiesa) | Serious limitations (−1)b | No serious limitations | No serious limitations | Serious limitations (−1)c | Undetected | None | ⊕⊕ Low |
| Trisomy 18, specificity (7 test accuracy studiesa) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ ⊕ Moderate |
| Trisomy 13, sensitivity (7 test accuracy studiesa) | Serious limitations (−1)b | No serious limitations | No serious limitations | Serious limitations (−1)c | Undetected | None | ⊕⊕ Low |
| Trisomy 13, specificity (7 test accuracy studiesa) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ ⊕ Moderate |
| Test failure rate (7 observational studies) | Serious limitations (−1)b | Serious limitations (−1)d | No serious limitations | No serious limitations | Undetected | None | ⊕ Very Low |
| NIPT Clinical Utility | |||||||
| Reduction in diagnostic testing (2 observational studies) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | +2 for large magnitude of effect | ⊕⊕ ⊕ Moderate |
| Test turnaround time (3 observational studies) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very Low |
| Understanding of health care providers (7 observational studies) | Serious limitations (−1)e | Very serious limitations (−2)f | No serious limitations | Very serious limitations (−2)f | Undetected | None | ⊕ Very Low |
| NIPT Personal Utility | |||||||
| Maternal education (1 observational study) | Serious limitations (−1)b | No serious limitations | No serious limitations | Very serious limitations (−2)g | Undetected | None | ⊕ Very Low |
| Maternal satisfaction (1 observational study) | Serious limitations (−1)b | No serious limitations | No serious limitations | Very serious limitations (−2)g | Undetected | None | ⊕ Very Low |
Abbreviation: Grading of Recommendations, Assessment, Development and Evaluation.
Note: Publication bias cannot be adequately assessed for test accuracy, unless there are known studies that cannot be accessed.
Test accuracy studies start at high GRADE.
Risk of bias concerns regarding patient selection and flow and timing among studies. Not all patients were included in analyses, some of which were likely at higher risk of chromosomal anomalies.
Wide confidence intervals because of low prevalence of conditions and very low number of false negatives that highly influenced test sensitivity.
Differences in NIPT testing platform and algorithms may have affected the cutoff for low fetal fraction, affecting the failure rate.
Not a validated method of assessing satisfaction or education.
Low response rate among some studies. Convenience sampling of health care providers. Response bias.
Differences in geographical location, education, and regional practice patterns impact providers’ education. Different survey design and questions (non-validated) used to assess provider understanding. Broad range of understanding is covered within studies.
Appendix 6: Ongoing Studies of Noninvasive Prenatal Testing
Table A7:
Ongoing Studies of Noninvasive Prenatal Testing
| Registry | ID | Population | Country | Official Title | Condition(s) | Sponsor |
|---|---|---|---|---|---|---|
| ClinicalTrials.gov | NCT02424474 | Average-risk | France | Fetal aneuploidies screening (21, 18, and 13) by cell-free fetal DNA analysis | Trisomies 21, 18, 13 | Assistance Publique—Hôpitaux de Paris |
| Australian New Zealand Clinical Trials Registry | ACTRN12617001587392 | Average-risk | Australia | Prenatal screening for aneuploidy in the Australian public hospital system: a noninvasive prenatal screening test (NIPT) feasibility study | Trisomies 21, 18, 13 Monosomy X |
Nepean Hospital |
| ClinicalTrials.gov | NCT02787486 | Average-risk and high-risk | United States | A clinical study to evaluate the relative clinical sensitivity, specificity, and performance of a laboratory-developed test as a screening test for fetal chromosomal aneuploidy, infectious and other diseases, and RhD genotyping in the general population of pregnant people | Trisomies 21, 18, 13 Sex chromosome aneuploidies |
Progenity, Inc. |
| ClinicalTrials.gov | NCT03200041 | Average-risk and high-risk | United Kingdom | Clinical evaluation of the IONA test for noninvasive prenatal screening in twin pregnancies | Trisomies 21, 18, 13 | Premaitha Health |
| Chinese Clinical Trial Registry | ChiCTR-DDD-17013213 | Average-risk and high-risk | China | The value of noninvasive prenatal testing in pregnancies of Hebei province: a prospective multi-center study | Trisomies 21, 18, 13 | Second Hospital of Hebei Medical University |
| ClinicalTrials.gov | NCT02381457 | General pregnant population | United States | SNP-based microdeletion and aneuploidy registry | Trisomies 21, 18, 13 Sex chromosome aneuploidies Microdeletion syndromes |
Natera, Inc. |
| UMIN Clinical Trials Registry | UMIN000023935 | Pregnant people (risk not specified) | Japan | Analysis of state-trait anxiety in clients of noninvasive prenatal testing: an investigation to improve the quality of genetic counselling | Not specified | Niigata University Hospital |
Abbreviation: RhD, rhesus D antigen; SNP, single nucleotide polymorphism.
Appendix 7: Results of Applicability Checklists for Studies Included in the Economic Literature Review
Table A8:
Assessment of the Applicability of Studies on the Cost-Effectiveness of NIPT
| Objective: To assess the cost-effectiveness of NIPT | |||||
|---|---|---|---|---|---|
| Author, Year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Were the perspectives clearly stated and what were they? | Are estimates of relative treatment effect from the best available source? |
| Nshimyumukiza et al, 201874 | Partially | Partially | Yes (Quebec, Canada) | Yes; public payer | Yes |
| Huang et al, 201775 | Partially | Yes | Yes (Ontario, Canada) | Yes; public payer | Yes |
| Maxwell et al, 201776 | Partially | Partially | Yes (Australia) | Yes; public payer | Yes |
| Colosi et al 201777 | Partially | Partially | Yes (Italy) | Yes; health care perspective | NA |
| Chitty et al, 201678 | Partially | Partially | Yes (United Kingdom) | Yes; United Kingdom National Screening Committee | Yes |
| Fairbrother et al 201679 | Partially | Partially | No (United States) | No | Yes |
| Author, Year | Are all future costs and outcomes discounted? (If yes, at what rate?) | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgment (directly applicable/partially applicable/not applicable) |
|---|---|---|---|---|
| Nshimyumukiza et al, 201874 | NA | No | No | Partially applicable |
| Huang et al, 201775 | NA | No | No | Partially applicable |
| Maxwell et al, 201776 | NA | No | No | Partially applicable |
| Colosi et al 201777 | NA | No | No | Partially applicable |
| Chitty et al, 201678 | NA | No | No | Partially applicable |
| Fairbrother et al 201679 | Unclear | No | Unclear | Not applicable |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Appendix 8: Acceptance Rate for Further Testing After a Positive Screening Result
Table A9:
Acceptance Rate for Further Testing After a Positive Screening Result
| Countrya | Risk Cutoff | Before Implementation of NIPT | After Implementation of NIPTb |
|---|---|---|---|
| Canada | Combined risk of trisomy 21, ≥ 1:200 | 60%75 | 76% (BORN data) |
| Denmark106 | Combined risk of trisomy 21, ≥ 1:300 | 74% | 97% |
| United Kingdom107 | Risk of trisomy 21 or trisomies 18/13, ≥ 1:100 | 66% | 98% |
| Netherlands108 | Combined risk of trisomies 21, 18, or 13, ≥ 1:200 | 50% | ≥ 86%c |
| United States109 | NA | 53% | 79% |
Abbreviations: NA, not available; NIPT, noninvasive prenatal testing.
Not based on national statistics, but regional studies.
Any further testing, NIPT and/or chorionic villus sampling or amniocentesis.
This study showed that 86% of people (1,211 out of 1,413) with a risk of trisomy 21 ≥ 1:200 received NIPT, but the status of further testing for the remaining 202 people was unclear.
Appendix 9: Letter of Information
CALL FOR PARTICIPATION: REVIEW OF NON-INVASIVE PRENATAL TESTING
WHAT IS THE OPPORTUNITY?
Health Quality Ontario is currently reviewing Noninvasive Prenatal Testing (NIPT). The purpose is to understand whether this screening test should be more broadly funded in Ontario. An important part of this review is to make sure a variety of perspectives and experiences are taken into account.
WHO ARE WE LOOKING FOR?
We are looking to speak to people with any of the following perspectives and experiences:
People who accessed NIPT during a pregnancy and received a positive screening result
People who received a false-positive or false-negative result from NIPT
People who didn't access NIPT in pregnancy but would have liked to
People with other personal experience relevant to NIPT who have views to share
At this time we are also seeking to speak with parents of children who have or had a condition screened for with NIPT—you do not need to have experience with NIPT to participate. We will ask you about your experience with an affected pregnancy and/or having an infant or child with this condition and your thoughts on public funding of a new prenatal test.
Trisomy 13, 18 and 21
Prader Willi syndrome, Angelman syndrome, 1p36 deletion syndrome, Cri-du-chat syndrome or Jacob's syndrome
Klinefelter syndrome or triple X syndrome
WHY GET INVOLVED?
This review will result in a recommendation to the Ministry of Health and Long-Term Care about the public funding of Non-Invasive Prenatal Testing. The views, values, and experiences of people affected by this technology are a really important source of information that will help with the development of a recommendation.
If you are interested in participating, please click here: [survey link]
WHAT DO WE NEED FROM YOU?
20-60 minutes of your time for a phone interview (or online survey if that is your preference)
Willingness to share your story
We are hoping to conduct interviews through the end of February 2018. If you are interested in sharing your story or have any questions about this opportunity, please don't hesitate to reach out.
Appendix 10: Interview Guide
Intro
Explain HQO purpose, HTA process, privacy and confidentiality
Background context of prenatal screening in Ontario
Why are you interested in participating? (to guide direction of conversation)
Prenatal testing/screening decision-making
Can you tell me about your experience with prenatal screening?
What options are/were available to you?
Cost/inconveniences?
Were there any issues related to access to NIPT or other prenatal screening?
Was it difficult to weigh up potential benefits and risks when deciding to do prenatal screening or not? For NIPT?
What factors influenced those decisions?
Role of family in decision-making?
Role of Physician? Midwife? Genetics counsellor?
If you were given information about screening, how easy or difficult was it to understand?
Previous pregnancies?
Other sources of information (Internet)?
Lived experience
Emotional impact: Anxiety? Experience of waiting for results? Waiting to do invasive testing?
Receiving a diagnosis? Termination of pregnancy?
Lived experience (for people with a condition screened for or parents of children with a condition screened for)
As policy makers are considering making available a new test which will allow pregnant people to find out whether their pregnancy is affected by certain conditions, what would you want them to know about what it's like to parent a child with _____?
Impact on parent/family if child has one of the conditions screened for (or impact on individual if it is an adult with the condition answering the questions).
For everyone
What you think are the advantages and disadvantages of using NIPT for prenatal screening?
What do you think about increasing access to NIPT for any pregnant person in Ontario who wants it?
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The clinical epidemiologist was Myra Wang, the health economists were Xuanqian Xie and Hong Anh Tu, the Patient, Caregiver, and Public Engagement analyst was Jenny Gilbert, and the medical librarians were Caroline Higgins and Corinne Holubowich.
KEY MESSAGES
What Is This Health Technology Assessment About?
Anyone who is pregnant has a small chance of having a baby with a chromosomal anomaly. A chromosomal anomaly is a change in the usual structure or number of chromosomes that carry genetic information. Prenatal screening tests check whether a fetus (an unborn baby) has a chromosomal anomaly. Traditional prenatal screening uses a blood test and ultrasound to determine a fetus's risk of certain chromosomal anomalies. More recently, a new screening method called noninvasive prenatal testing has been introduced. It is a blood test that checks the fetus's DNA found in the mother's blood.
At the time of writing this report, noninvasive prenatal testing is publicly funded for people whose pregnancy is at high risk for a chromosomal anomaly (for example, pregnant people aged 40 years or older, or those who have had a previous pregnancy with a chromosomal anomaly). Pregnant people at average risk must pay out of pocket if they want the test.
This health technology assessment evaluates how accurate and useful noninvasive prenatal testing is for detecting several chromosomal anomalies in the average-risk or general population, and whether it is good value for money. It also explores the preferences and values of pregnant people, their families, and the parents of children affected by the conditions that noninvasive prenatal testing screens for.
What Did This Health Technology Assessment Find?
Noninvasive prenatal testing was effective for screening in average-risk pregnant people. It was more accurate than traditional prenatal screening, and it decreased the number of diagnostic tests (for example, amniocentesis) performed.
When used as a follow-up screening test after traditional prenatal screening, noninvasive prenatal testing detected more affected fetuses and slightly lowered the overall costs of prenatal screening compared with not using it. But when it was used as the first screening test (that is, instead of traditional prenatal screening), it increased costs substantially.
People who had undergone noninvasive prenatal testing talked about its benefits (more accurate results, no risk to the fetus). However, they raised concerns about the quality of the information people are given for decision-making when the result of testing is positive for a chromosomal anomaly. They also raised ethical concerns about prenatal screening more generally.
Contributor Information
Health Quality Ontario:
Myra Wang, Xuanqian Xie, Hong Anh Tu, Jenny Gilbert, Caroline Higgins, and Corinne Holubowich
About Health Quality Ontario
Health Quality Ontario is the provincial lead on the quality of health care. We help nurses, doctors and other health care professionals working hard on the frontlines be more effective in what they do – by providing objective advice and data, and by supporting them and government in improving health care for the people of Ontario.
We focus on making health care more effective, efficient and affordable through a legislative mandate of:
Reporting to the public, organizations, government and health care providers on how the health system is performing,
Finding the best evidence of what works, and
Translating this evidence into clinical standards; recommendations to health care professionals and funders; and tools that health care providers can easily put into practice to make improvements.
Health Quality Ontario is governed by a 12-member Board of Directors with a broad range of expertise – doctors, nurses, patients and from other segments of health care – and appointed by the Minister of Health and Long-Term Care.
In everything it does, Health Quality Ontario brings together those with first-hand experience to hear their experiences and views of how to make them better. We partner with patients, residents, families and caregivers to be full participants in designing our programs and services, to ensure they are aligned to their needs and priorities. We work collaboratively with organizations across the province to encourage the spread of innovative and proven programs to support high quality care, while also saving money and eliminating redundancy. And, we work with clinicians on the frontlines to use their collective wisdom and experience to bring about positive change in areas important to Ontario – such as addressing the challenges of hallway health care and mental health.
For example, 29 Ontario hospitals participated in a pilot program last year that reduced infections due to surgery by 18% – which in turn reduces the number of patients returning to hospital after surgery and alleviating some of the challenges faced in hallway health care. This program enabled surgeons to see their surgical data and how they perform in relation to each other and to 700 other hospitals worldwide. We then helped them identify and action improvements to care. Forty-six hospitals across Ontario are now part of this program, covering 80% of hospital surgeries.
Health Quality Ontario also develops quality standards for health conditions that demonstrate unnecessary gaps and variations in care across the province, such as in major depression or schizophrenia. Quality standards are based on the best evidence and provide recommendations to government, organizations and clinicians. They also include a guide for patients to help them ask informed questions about their care.
In addition, Health Quality Ontario's health technology assessments use evidence to assess the effectiveness and value for money of new technologies and procedures, and incorporate the views and preferences of patients, to make recommendations to government on whether they should be funded.
Each year, we also help hospitals, long-term care homes, home care and primary care organizations across the system create and report on the progress of their annual Quality Improvement Plans, which is their public commitment on their priorities to improve health care quality.
Health Quality Ontario is committed to supporting the development of a quality health care system based on six fundamental dimensions: efficient, timely, safe, effective, patient-centred and equitable.
Our goal is to challenge the status quo and to focus on long-lasting pragmatic solutions that improve the health of Ontarians, enhance their experience of care, reduce health care costs, and support the well-being of health care providers. A quality health system results in Ontarians leading healthier and more productive lives, and a vibrant society in which everyone benefits.
REFERENCES
- (1).U.S. National Library of Medicine. Genetics home reference [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2017. [cited 2017 Jun 5]. Available from: https://ghr.nlm.nih.gov [Google Scholar]
- (2).Huang T, Dennis A, Meschino WS, Rashid S, Mak-Tam E, Cuckle H. First trimester screening for Down syndrome using nuchal translucency, maternal serum pregnancy-associated plasma protein A, free-beta human chorionic gonadotrophin, placental growth factor, and alpha-fetoprotein. Prenat Diagn. 2015;35(7):709–16. [DOI] [PubMed] [Google Scholar]
- (3).Prenatal Screening Ontario. Screening options [Internet]. Toronto (ON): Ontario Ministry of Health and Long-Term Care; 2017. [cited 2017 Apr 10]. Available from: http://prenatalscreeningontario.ca/for-health-care-providers/screening-options [Google Scholar]
- (4).Prenatal Screening Ontario. Screening choices [Internet]. Toronto (ON): Ontario Ministry of Health and Long-Term Care; 2017. [cited 2017 Apr 10]. Available from: http://prenatalscreeningontario.ca/for-parents/screening-choices [Google Scholar]
- (5).Trillium Health Partners. Enhanced FTS information FAQs [Internet]. Toronto (ON): Trillium Health Partners; 2017. [cited 2017 Jun 5]. Available from: https://trilliumhealthpartners.ca/patientservices/genetics/Pages/Enhanced-FTS-FAQs.aspx [Google Scholar]
- (6).Akolekar R, Beta J, Picciarelli G, Ogilvie C, D'Antonio F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(1):16–26. [DOI] [PubMed] [Google Scholar]
- (7).Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33(7):662–6. [DOI] [PubMed] [Google Scholar]
- (8).Natera Inc. About Panorama [Internet]. San Carlos (CA): Natera Inc.; 2017. [cited 2017 Apr 5]. Available from: https://www.natera.com/panorama-test/clinical-information [Google Scholar]
- (9).Ariosa Diagnostics. Harmony prenatal test [Internet]. San Jose (CA): F. Hoffmann-La Roche Ltd; 2017. [cited 2017 Apr 5]. Available from: http://www.ariosadx.com/healthcare-professionals [Google Scholar]
- (10).Skrzypek H, Hui L. Noninvasive prenatal testing for fetal aneuploidy and single gene disorders. Best Pract Res Clin Obstet Gynaecol. 2017;42:26–38. [DOI] [PubMed] [Google Scholar]
- (11).Bianchi DW, Chudova D, Sehnert AJ, Bhatt S, Murray K, Prosen TL, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314(2):162–9. [DOI] [PubMed] [Google Scholar]
- (12).Kinnings SL, Geis JA, Almasri E, Wang H, Guan X, McCullough RM, et al. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat Diagn. 2015;35(8):816–22. [DOI] [PubMed] [Google Scholar]
- (13).Song Y, Huang S, Zhou X, Jiang Y, Qi Q, Bian X, et al. Non-invasive prenatal testing for fetal aneuploidies in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2015;45(1):55–60. [DOI] [PubMed] [Google Scholar]
- (14).United States Food and Drug Administration. Clinical Laboratory Improvement Amendments (CLIA) [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2018. [cited 2017 May 10]. Available from: https://www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm124105.htm [Google Scholar]
- (15).LifeLabs. Panorama funded by MOHLTC [Internet]. Toronto (ON): LifeLabs; 2017. [cited 2017 Jun 2]. Available from: http://www.lifelabsgenetics.com/wp-content/uploads/2015/10/Panorama-ON-MOH-01Oct2015.pdf [Google Scholar]
- (16).Badeau M, Lindsay C, Blais J, Takwoingi Y, Langlois S, Legare F, et al. Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database Syst Rev. 2017;11:CD011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Varela-Lema L, Puñal-Riobóo J, Ballini L. Screening of fetal trisomies 21, 18 and 13 by noninvasive prenatal testing [Internet]. Europe: EUnetHTA; 2017. [cited 2018 Mar 21]. Available from: https://www.eunethta.eu/wp-content/uploads/2018/03/OTCA03_Screening-of-fetal-trisomies-21-18-and-13-by-noninvasive-prenatal-testing_V1.5.pdf [Google Scholar]
- (18).Iwarsson E, Jacobsson B, Dagerhamn J, Davidson T, Bernabe E, Heibert Arnlind M. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population – a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(1):7–18. [DOI] [PubMed] [Google Scholar]
- (19).Taylor-Phillips S, Freeman K, Geppert J, Agbebiyi A, Uthman OA, Madan J, et al. Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: a systematic review and meta-analysis. BMJ Open. 2016;6(1):e010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Roche Sequencing. Roche expands Harmony prenatal test to include 22q11.2 deletion testing [Internet]. Pleasanton (CA): Roche Sequencing; 2017. [cited 2017 Sep 3]. Available from: https://sequencing.roche.com/en/news-overview/2017/roche-expands-harmony-prenatal-test-to-include-22q112-deletion-testing.html [Google Scholar]
- (21).Vanstone M, Giacomini M, Nisker J. Non-invasive prenatal testing [Internet]. Ottawa (ON): Royal College of Physicians and Surgeons of Canada; 2017. [cited 2017 Apr 10]. Available from: http://www.royalcollege.ca/rcsite/bioethics/cases/section-4/non-invasive-prenatal-testing-e [Google Scholar]
- (22).CBC/Radio-Canada. Trisomie: premier financement public à grande échelle pour un test d'ADN [Internet]. Ottawa (ON): CBC/Radio-Canada; 2018. [cited 2018 Apr 24]. Available from: https://ici.radio-canada.ca/nouvelle/1096429/trisomie-test-adn-financement-public-quebec [Google Scholar]
- (23).Ryan S. Non-invasive prenatal testing: Canadian environmental scan 2018. Toronto (ON): RYAN Policy Research Consulting for Yukon Government; 2018. [Google Scholar]
- (24).Illumina Inc. NIPT – accurate information for your patients [Internet]. San Diego (CA): Illumina Inc.; 2017. [cited 2017 May 4]. Available from: https://www.illumina.com/clinical/reproductive-genetic-health/nipt/healthcare-providers.html [Google Scholar]
- (25).UK National Screening Programme. The UK NSC recommendation on fetal anomaly screening in pregnancy [Internet]. London: UK National Screening Programme; 2016. [cited 2017 May 4]. Available from: https://legacyscreening.phe.org.uk/fetalanomalies [Google Scholar]
- (26).Centrum Medische Genetica. Prenatal examinations [Internet]. Brussels: Centrum Medische Genetica; 2017. [cited 2017 Jul 24]. Available from: http://www.brusselsgenetics.be/nipt-en [Google Scholar]
- (27).Institut national d'assurance maladie-invalidité (INAMI). Remboursement du test prénatal non invasif (test DPNI/NIPT) [Internet]. Brussels: Institut national d'assurance maladie-invalidité (INAMI); 2017. [cited 2017 Jul 24]. Available from: http://www.inami.fgov.be/fr/nouvelles/Pages/remboursement-test-prenatal-non-invasif.aspx [Google Scholar]
- (28).Lewis C, Hill M, Silcock C, Daley R, Chitty LS. Non-invasive prenatal testing for trisomy 21: a cross-sectional survey of service users’ views and likely uptake. BJOG. 2014;121(5):582–94. [DOI] [PubMed] [Google Scholar]
- (29).van Schendel RV, Dondorp WJ, Timmermans DR, van Hugte EJ, de Boer A, Pajkrt E, et al. NIPT-based screening for Down syndrome and beyond: what do pregnant women think? Prenat Diagn. 2015;35(6):598–604. [DOI] [PubMed] [Google Scholar]
- (30).Allyse M, Sayres LC, Goodspeed TA, Cho MK. Attitudes towards non-invasive prenatal testing for aneuploidy among US adults of reproductive age. J Perinatol. 2014;34(6):429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Farrell RM, Agatisa PK, Nutter B. What women want: lead considerations for current and future applications of noninvasive prenatal testing in prenatal care. Birth. 2014;41(3):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tiller GE, Kershberg HB, Goff J, Coffeen C, Liao W, Sehnert AJ. Women's views and the impact of noninvasive prenatal testing on procedures in a managed care setting. Prenat Diagn. 2015;35(5):428–33. [DOI] [PubMed] [Google Scholar]
- (33).Lewis C, Hill M, Chitty LS. Women's experiences and preferences for service delivery of non-invasive prenatal testing for aneuploidy in a public health setting: a mixed methods study. PLoS One. 2016;11(4):e0153147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).van Schendel RV, Page-Christiaens GCML, Beulen L, Bilardo CM, de Boer MA, Coumans ABC, et al. Women's experience with non-invasive prenatal testing and emotional well-being and satisfaction after test-results. J Genet Couns. 2017;26(6):1348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hill M, Johnson JA, Langlois S, Lee H, Winsor S, Dineley B, et al. Preferences for prenatal tests for Down syndrome: an international comparison of the views of pregnant women and health professionals. Eur J Hum Genet. 2016;24(7):968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kellogg G, Slattery L, Hudgins L, Ormond K. Attitudes of mothers of children with Down syndrome towards noninvasive prenatal testing. J Genet Couns. 2014;23(5):805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Audibert F, De Bie I, Johnson JA, Okun N, Wilson RD, Armour C, et al. No. 348-Joint SOGC-CCMG guideline: update on prenatal screening for fetal aneuploidy, fetal anomalies, and adverse pregnancy outcomes. J Obstet Gynaecol Can. 2017;39(9):805–17. [DOI] [PubMed] [Google Scholar]
- (38).Allyse MA, Sayres LC, Havard M, King JS, Greely HT, Hudgins L, et al. Best ethical practices for clinicians and laboratories in the provision of noninvasive prenatal testing. Prenat Diagn. 2013;33(7):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017. [DOI] [PubMed] [Google Scholar]
- (40).Mersy E, Smits LJ, van Winden LA, de Die-Smulders CE, South-East Netherlands NC, Paulussen AD, et al. Noninvasive detection of fetal trisomy 21: systematic review and report of quality and outcomes of diagnostic accuracy studies performed between 1997 and 2012. Hum Reprod Update. 2013;19(4):318–29. [DOI] [PubMed] [Google Scholar]
- (41).Mackie FL, Hemming K, Allen S, Morris RK, Kilby MD. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124(1):32–46. [DOI] [PubMed] [Google Scholar]
- (42).Yang H, Xu HB, Liu TT, He XL. Systematic review of noninvasive prenatal diagnosis for abnormal chromosome genetic diseases using free fetal DNA in maternal plasma. Genet Mol Res. 2015;14(3):10603–8. [DOI] [PubMed] [Google Scholar]
- (43).Jin J, Yang J, Chen Y, Huang J. Systematic review and meta-analysis of non-invasive prenatal DNA testing for trisomy 21: implications for implementation in China. Prenat Diagn. 2017;07:07. [DOI] [PubMed] [Google Scholar]
- (44).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (45).Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res. 2017;26(4):1896–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Nordic Cochrane Centre. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen (Denmark): The Cochrane Collaboration; 2014. [Google Scholar]
- (47).Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. [DOI] [PubMed] [Google Scholar]
- (48).Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (51).Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808. [DOI] [PubMed] [Google Scholar]
- (53).del Mar Gil M, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204–11. [DOI] [PubMed] [Google Scholar]
- (54).Langlois S, Johnson J, Audibert F, Gekas J, Forest JC, Caron A, et al. Comparison of first-tier cell-free DNA screening for common aneuploidies with conventional publically funded screening. Prenat Diagn. 2017;37(12):1238–44. [DOI] [PubMed] [Google Scholar]
- (55).Nicolaides KH, Syngelaki A, Ashoor G, Birdir C, Touzet G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obstet Gynecol. 2012;207(5):374.e1–6. [DOI] [PubMed] [Google Scholar]
- (56).Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–97. [DOI] [PubMed] [Google Scholar]
- (57).Palomaki GE, Kloza EM, O'Brien BM, Eklund EE, Lambert-Messerlian GM. The clinical utility of DNA-based screening for fetal aneuploidy by primary obstetrical care providers in the general pregnancy population. Genet Med. 2017;19(7):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Quezada MS, Gil MM, Francisco C, Orosz G, Nicolaides KH. Screening for trisomies 21, 18 and 13 by cell-free DNA analysis of maternal blood at 10–11 weeks’ gestation and the combined test at 11–13 weeks. Ultrasound Obstet Gynecol. 2015;45(1):36–41. [DOI] [PubMed] [Google Scholar]
- (59).Song Y, Liu C, Qi H, Zhang Y, Bian X, Liu J. Noninvasive prenatal testing of fetal aneuploidies by massively parallel sequencing in a prospective Chinese population. Prenat Diagn. 2013;33(7):700–6. [DOI] [PubMed] [Google Scholar]
- (60).Brewer J, Demers L, Musci T. Survey of US obstetrician opinions regarding NIPT use in general practice: implementation and barriers. J Matern Fetal Neonatal Med. 2016:1–4. [DOI] [PubMed] [Google Scholar]
- (61).Filoche SK, Lawton B, Beard A, Stone P. Views of the obstetric profession on noninvasive prenatal testing in Aotearoa New Zealand: a national survey. Aust N Z J Obstet Gynaecol. 2017;57(6):617–23. [DOI] [PubMed] [Google Scholar]
- (62).Haymon L, Simi E, Moyer K, Aufox S, Ouyang DW. Clinical implementation of noninvasive prenatal testing among maternal fetal medicine specialists. Prenat Diagn. 2014;34(5):416–23. [DOI] [PubMed] [Google Scholar]
- (63).Mayes S, Hashmi S, Turrentine MA, Darilek S, Friel LA, Czerwinski J. Obstetrician and gynecologist utilization of the noninvasive prenatal testing expanded option. AJP Rep. 2016;6(1):e18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Musci TJ, Fairbrother G, Batey A, Bruursema J, Struble C, Song K. Non-invasive prenatal testing with cell-free DNA: US physician attitudes toward implementation in clinical practice. Prenat Diagn. 2013;33(5):424–8. [DOI] [PubMed] [Google Scholar]
- (65).Sayres LC, Allyse M, Norton ME, Cho MK. Cell-free fetal DNA testing: a pilot study of obstetric healthcare provider attitudes toward clinical implementation. Prenat Diagn. 2011;31(11):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Swaney P, Hardisty E, Sayres L, Wiegand S, Vora N. Attitudes and knowledge of maternal-fetal medicine fellows regarding noninvasive prenatal testing. J Genet Couns. 2016;25(1):73–8. [DOI] [PubMed] [Google Scholar]
- (67).Badeau M, Lindsay C, Blais J, Nshimyumukiza L, Takwoingi Y, Langlois S, et al. Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database Syst Rev. 2017;11:CD011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Wapner RJ, Babiarz JE, Levy B, Stosic M, Zimmermann B, Sigurjonsson S, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212(3):332.e1–9. [DOI] [PubMed] [Google Scholar]
- (69).Yaron Y, Jani J, Schmid M, Oepkes D. Current status of testing for microdeletion syndromes and rare autosomal trisomies using cell-free DNA technology. Obstet Gynecol. 2015;126(5):1095–9. [DOI] [PubMed] [Google Scholar]
- (70).Hall MP. Panorama non-invasive prenatal screening for microdeletion syndromes [Internet]. San Carlos (CA): Natera, Inc.; 2017. [cited 2017 Jan 24]. Available from: http://www.lifelabsgenetics.com/wp-content/uploads/2015/07/whitepaper-microdeletion.pdf [Google Scholar]
- (71).Agatisa PK, Mercer MB, Leek AC, Smith MB, Philipson E, Farrell RM. A first look at women's perspectives on noninvasive prenatal testing to detect sex chromosome aneuploidies and microdeletion syndromes. Prenat Diagn. 2015;35(7):692–8. [DOI] [PubMed] [Google Scholar]
- (72).Nshimyumukiza L, Menon S, Hina H, Rousseau F, Reinharz D. Cell-free DNA noninvasive prenatal screening for aneuploidy versus conventional screening: a systematic review of economic evaluations. Clin Genet. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- (73).Vestergaard EM, Singh R, Schelde P, Hatt L, Ravn K, Christensen R, et al. On the road to replacing invasive testing with cell-based NIPT: five clinical cases with aneuploidies, microduplication, unbalanced structural rearrangement or mosaicism. Prenat Diagn. 2017;07. [DOI] [PubMed] [Google Scholar]
- (74).Nshimyumukiza L, Beaumont JA, Duplantie J, Langlois S, Little J, Audibert F, et al. Cell-free DNA-based non-invasive prenatal screening for common aneuploidies in a Canadian province: a cost-effectiveness analysis. J Obstet Gynaecol Can. 2018;40(1):48–60. [DOI] [PubMed] [Google Scholar]
- (75).Huang T, Meschino WS, Teitelbaum M, Dougan S, Okun N. Enhanced first trimester screening for trisomy 21 with contingent cell-free fetal DNA: a comparative performance and cost analysis. J Obstet Gynaecol Can. 2017;39(9):742–9. [DOI] [PubMed] [Google Scholar]
- (76).Maxwell S, O'Leary P, Dickinson JE, Suthers GK. Diagnostic performance and costs of contingent screening models for trisomy 21 incorporating non-invasive prenatal testing. Aust N Z J Obstet Gynaecol. 2017;57(4):432–9. [DOI] [PubMed] [Google Scholar]
- (77).Colosi E, D'Ambrosio V, Periti E. First trimester contingent screening for trisomies 21, 18, 13: is this model cost efficient and feasible in public health system? J Matern Fetal Neonatal Med. 2017;30(24):2905–10. [DOI] [PubMed] [Google Scholar]
- (78).Chitty LS, Wright D, Hill M, Verhoef TI, Daley R, Lewis C, et al. Uptake, outcomes, and costs of implementing non-invasive prenatal testing for Down's syndrome into NHS maternity care: prospective cohort study in eight diverse maternity units. BMJ. 2016;354:i3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Fairbrother G, Burigo J, Sharon T, Song K. Prenatal screening for fetal aneuploidies with cell-free DNA in the general pregnancy population: a cost-effectiveness analysis. J Matern Fetal Neonatal Med. 2016;29(7):1160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Huang T, Owolabi T, Summers AM, Meier C, Wyatt PR. The identification of risk of spontaneous fetal loss through second-trimester maternal serum screening. Am J Obstet Gynecol. 2005;193(2):395–403. [DOI] [PubMed] [Google Scholar]
- (81).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. [DOI] [PubMed] [Google Scholar]
- (82).Better Outcomes Registry and Network. Data analysis for annual report 2014–2016 [Internet]. Toronto (ON): BORN; 2016. [cited 2017 Sep 25]. Available from: https://www.bornontario.ca/assets/documents/Annual%20report%202014-2016%20-%20Data%20Slides.pdf [Google Scholar]
- (83).Okun N, Dougan S, editors. Prenatal screening in Ontario: past, present and future using data to inform policy. Toronto (ON): BORN; 2017. [Google Scholar]
- (84).Savva GM, Walker K, Morris JK. The maternal age-specific live birth prevalence of trisomies 13 and 18 compared to trisomy 21 (Down syndrome). Prenat Diagn. 2010;30(1):57–64. [DOI] [PubMed] [Google Scholar]
- (85).Morris JK, Wald NJ, Watt HC. Fetal loss in Down syndrome pregnancies. Prenat Diagn. 1999;19(2):142–5. [PubMed] [Google Scholar]
- (86).Morris JK, Savva GM. The risk of fetal loss following a prenatal diagnosis of trisomy 13 or trisomy 18. Am J Med Genet A. 2008;146A(7):827–32. [DOI] [PubMed] [Google Scholar]
- (87).Viuff MH, Stochholm K, Uldbjerg N, Nielsen BB, Danish Fetal Medicine Study G, Gravholt CH. Only a minority of sex chromosome abnormalities are detected by a national prenatal screening program for Down syndrome. Hum Reprod. 2015;30(10):2419–26. [DOI] [PubMed] [Google Scholar]
- (88).Gravholt CH, Juul S, Naeraa RW, Hansen J. Prenatal and postnatal prevalence of Turner's syndrome: a registry study. BMJ. 1996;312(7022):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet J Rare Dis. 2010;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622–6. [DOI] [PubMed] [Google Scholar]
- (91).Stochholm K, Juul S, Gravholt CH. Socio-economic factors affect mortality in 47,XYY syndrome: a comparison with the background population and Klinefelter syndrome. Am J Med Genet A. 2012;158A(10):2421–9. [DOI] [PubMed] [Google Scholar]
- (92).European Registry of Congenital Anomalies and Twins. Cases and prevalence (per 10,000 births) of anomalies in 2015 in the United Kingdom [Internet]. Ispra, Italy: EUROCAT; 2018. [cited 2018. Mar 27]. Available from: http://www.eurocat-network.eu/accessprevalencedata/prevalencetables [Google Scholar]
- (93).McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1:15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94(6):417–23. [DOI] [PubMed] [Google Scholar]
- (95).Iyer NP, Tucker DF, Roberts SH, Moselhi M, Morgan M, Matthes JW. Outcome of fetuses with Turner syndrome: a 10-year congenital anomaly register based study. J Matern Fetal Neonatal Med. 2012;25(1):68–73. [DOI] [PubMed] [Google Scholar]
- (96).Hook EB. Chromosome abnormalities and spontaneous fetal death following amniocentesis: further data and associations with maternal age. Am J Hum Genet. 1983;35(1):110–6. [PMC free article] [PubMed] [Google Scholar]
- (97).Natoli JL, Ackerman DL, McDermott S, Edwards JG. Prenatal diagnosis of Down syndrome: a systematic review of termination rates (1995–2011). Prenat Diagn. 2012;32(2):142–53. [DOI] [PubMed] [Google Scholar]
- (98).Christian SM, Koehn D, Pillay R, MacDougall A, Wilson RD. Parental decisions following prenatal diagnosis of sex chromosome aneuploidy: a trend over time. Prenat Diagn. 2000;20(1):37–40. [PubMed] [Google Scholar]
- (99).Wyatt PR, Owolabi T, Meier C, Huang T. Age-specific risk of fetal loss observed in a second trimester serum screening population. Am J Obstet Gynecol. 2005;192(1):240–6. [DOI] [PubMed] [Google Scholar]
- (100).Ravi H, McNeill G, Goel S, Meltzer SD, Hunkapiller N, Ryan A, et al. Validation of a SNP-based non-invasive prenatal test to detect the fetal 22q11.2 deletion in maternal plasma samples. PLoS One. 2018;13(2):e0193476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ. 2006;174(4):469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Martin K, Iyengar S, Kalyan A, Lan C, Simon AL, Stosic M, et al. Clinical experience with a single-nucleotide polymorphism-based non-invasive prenatal test for five clinically significant microdeletions. Clin Genet. 2018;93(2):293–300. [DOI] [PubMed] [Google Scholar]
- (103).Huang T, Meschino WS, Rashid S, Dennis A, Mak-Tam E, Cuckle H. Enhanced first trimester aneuploidy screening with placental growth factor and alpha feto-protein: detection of trisomies 18 and 13. J Obstet Gynaecol Can. Forthcoming 2018. [DOI] [PubMed] [Google Scholar]
- (104).Summers AM, Farrell SA, Huang T, Meier C, Wyatt PR. Maternal serum screening in Ontario using the triple marker test. J Med Screen. 2003;10(3):107–11. [DOI] [PubMed] [Google Scholar]
- (105).Okun N, Teitelbaum M, Huang T, Dewa CS, Hoch JS. The price of performance: a cost and performance analysis of the implementation of cell-free fetal DNA testing for Down syndrome in Ontario, Canada. Prenat Diagn. 2014;34(4):350–6. [DOI] [PubMed] [Google Scholar]
- (106).Bjerregaard L, Stenbakken AB, Andersen CS, Kristensen L, Jensen CV, Skovbo P, et al. The rate of invasive testing for trisomy 21 is reduced after implementation of NIPT. Dan Med J. 2017;64(4). [PubMed] [Google Scholar]
- (107).Gil MM, Revello R, Poon LC, Akolekar R, Nicolaides KH. Clinical implementation of routine screening for fetal trisomies in the UK NHS: cell-free DNA test contingent on results from first-trimester combined test. Ultrasound Obstet Gynecol. 2016;47(1):45–52. [DOI] [PubMed] [Google Scholar]
- (108).Oepkes D, Page-Christiaens GC, Bax CJ, Bekker MN, Bilardo CM, Boon EM, et al. Trial by Dutch laboratories for evaluation of non-invasive prenatal testing. Part I-clinical impact. Prenat Diagn. 2016;36(12):1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Chetty S, Garabedian MJ, Norton ME. Uptake of noninvasive prenatal testing (NIPT) in women following positive aneuploidy screening. Prenat Diagn. 2013;33(6):542–6. [DOI] [PubMed] [Google Scholar]
- (110).Livergood MC, LeChien KA, Trudell AS. Obesity and cell-free DNA “no calls”: is there an optimal gestational age at time of sampling? Am J Obstet Gynecol. 2017;216(4):413.e1–e9. [DOI] [PubMed] [Google Scholar]
- (111).Peng XL, Jiang P. Bioinformatics approaches for fetal DNA fraction estimation in noninvasive prenatal testing. Int J Mol Sci. 2017;18(2):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).LifeLabs Genetics. Cost of prenatal test options [Internet]. Toronto (ON): LifeLabs; 2018. [cited 2018 May 23]. Available from: https://www.lifelabsgenetics.com/product/22q112-microdeletion/ [Google Scholar]
- (113).Ministry of Health and Long-Term Care. Schedule of benefits for physician services under the Health Insurance Act [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2018 Feb 28]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/ [Google Scholar]
- (114).Lilley M, Hume S, Karpoff N, Maire G, Taylor S, Tomaszewski R, et al. Assessing the cost of implementing the 2011 Society of Obstetricians and Gynecologists of Canada and Canadian College of Medical Genetics practice guidelines on the detection of fetal aneuploidies. Prenat Diagn. 2017;37(9):916–23. [DOI] [PubMed] [Google Scholar]
- (115).Ministry of Health and Long-Term Care. Ontario case costing initiative for termination of pregnancy in 2015–16 [Internet]. Toronto (ON): Queen's Printer for Ontario; 2018. [cited 2018 Feb 28]. Available from: https://hsim.health.gov.on.ca/hdbportal/ [Google Scholar]
- (116).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. Ottawa (ON): CADTH; 2017. [Google Scholar]
- (117).Goel V, Glazier R, Summers A, Holzapfel S. Psychological outcomes following maternal serum screening: a cohort study. CMAJ. 1998;159(6):651–6. [PMC free article] [PubMed] [Google Scholar]
- (118).Liao H, Liu S, Wang H. Performance of non-invasive prenatal screening for fetal aneuploidy in twin pregnancies: a meta-analysis. Prenat Diagn. 2017;37(9):874–82. [DOI] [PubMed] [Google Scholar]
- (119).Statistics Canada. Births, estimates, by province and territory [Internet]. Ottawa (ON): Statistics Canada; 2016. [cited 2017 Sep 25]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo04a-eng.htm [Google Scholar]
- (120).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. The patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (121).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. The patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (122).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (123).Tjornhoj-Thomsen T, Hansen HP. Knowledge in health technology assessment: who, what, how? Int J Technol Assess Health Care. 2011;27(4):324–9. [DOI] [PubMed] [Google Scholar]
- (124).Rowe G, Frewer LJ. A typology of public engagement mechanisms. Sci Technol Hum Val. 2005;30(2):251–90. [Google Scholar]
- (125).Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients’ experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2013;13(15):1–33. [PMC free article] [PubMed] [Google Scholar]
- (126).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (127).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (128).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (129).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]
- (130).Vanstone M, Cernat A, Majid U, Trivedi F, De Freitas C. Perspectives of pregnant people and clinicians on noninvasive prenatal testing: a systematic review and qualitative meta-synthesis. Ontario Health Technology Assessment Series [Internet]. 2018. [cited 2018 Jun 15]; 18(TBD):[1-TBD pp.]. Available from: http://www.hqontario.ca/evidence-to-improve-care/journal-ontario-health-techology-assessment-series [PMC free article] [PubMed] [Google Scholar]
- (131).Vanstone M, Yacoub K, Giacomini M, Hulan D, McDonald S. Women's experiences of publicly funded non-invasive prenatal testing in Ontario, Canada: considerations for health technology policy-making. Qual Health Res. 2015;25(8):1069–84. [DOI] [PubMed] [Google Scholar]
- (132).Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18(10):1056–65. [DOI] [PubMed] [Google Scholar]
- (133).American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Practice bulletin no. 163: screening for fetal aneuploidy. Obstet Gynecol. 2016;127(5):e123–37. [DOI] [PubMed] [Google Scholar]
- (134).American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Committee opinion no. 640: cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126(3):e31–7. [DOI] [PubMed] [Google Scholar]
- (135).Society for Maternal-Fetal Medicine. #36: Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–6. [DOI] [PubMed] [Google Scholar]
- (136).Society for Maternal-Fetal Medicine, Norton ME, Biggio JR, Kuller JA, Blackwell SC. The role of ultrasound in women who undergo cell-free DNA screening. Am J Obstet Gynecol. 2017;216(3):B2–B7. [DOI] [PubMed] [Google Scholar]
- (137).Devers PL, Cronister A, Ormond KE, Facio F, Brasington CK, Flodman P. Noninvasive prenatal testing/noninvasive prenatal diagnosis: the position of the National Society of Genetic Counselors. J Genet Couns. 2013;22(3):291–5. [DOI] [PubMed] [Google Scholar]
- (138).Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Prenatal screening and diagnosis of chromosomal and genetic conditions in the fetus in pregnancy [Internet]. Melbourne: Royal Australian and New Zealand College of Obstetricians and Gynaecologists; 2016. [cited 2017 Apr 10]. Available from: https://www.ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Prenatal-screening-and-diagnosis-of-chromosomal-and-genetic-conditions-(C-Obs-59)-Amended-May-2016.pdf?ext=.pdf [Google Scholar]
- (139).Dondorp W, de Wert G, Bombard Y, Bianchi DW, Bergmann C, Borry P, et al. Noninvasive prenatal testing for aneuploidy and beyond: challenges of responsible innovation in prenatal screening. Eur J Hum Genet. 2015;23(11):1438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Schmid M, Klaritsch P, Arzt W, Burkhardt T, Duba HC, Hausler M, et al. Cell-free DNA testing for fetal chromosomal anomalies in clinical practice: Austrian-German-Swiss recommendations for non-invasive prenatal tests (NIPT). Ultraschall Med. 2015;36(5):507–10. [DOI] [PubMed] [Google Scholar]
- (141).Sieroszewski P, Wielgos M, Radowicki S, Sasiadek M, Borowiec M, Borowski D, et al. Cell-free fetal DNA testing in prenatal diagnosis: recommendations of the Polish Gynecological Society and the Polish Human Genetics Society. Eur J Obstet Gynecol Reprod Biol. 2017;214:190–1. [DOI] [PubMed] [Google Scholar]
- (142).Benn P, Borrell A, Chiu RW, Cuckle H, Dugoff L, Faas B, et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2015;35(8):725–34. [DOI] [PubMed] [Google Scholar]
- (143).Salomon LJ, Alfirevic Z, Audibert F, Kagan KO, Paladini D, Yeo G, et al. ISUOG updated consensus statement on the impact of cfDNA aneuploidy testing on screening policies and prenatal ultrasound practice. Ultrasound Obstet Gynecol. 2017;49(6):815–6. [DOI] [PubMed] [Google Scholar]