Abstract
We herein report a 44-year-old man suffering from systemic edema due to protein-losing enteropathy (PLE) with superior mesenteric vein (SMV) obstruction and development of collateral veins, which subsequently proved to be a chronic result of thrombosis and a complication of Crohn's disease (CD). PLE was supposedly induced by both intestinal erosion and thrombosis-related lymphangiectasia, which was histologically proven in his surgically-resected ileal stenosis. Elemental diet and anti-TNFα agent improved his hypoalbuminemia after surgery. The rarity of the simultaneous coexistence of SMV obstruction and PLE and the precedence of these complications over typical abdominal symptoms of CD made the clinical course complex.
Keywords: Crohn's disease (CD), mesenteric vein thrombosis, protein-losing enteropathy (PLE)
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease (CD), is an independent, disease-specific risk factor for venous thromboembolism (VTE) (1-3). Approximately half of IBD patients develop VTE without any identifying reason (4). The incidence of VTE in IBD patients varies from 0.7% to 7% in clinical studies (5), and the odds ratio is between 1.48 and 3.6 compared with non-IBD patients (6). Although the mechanisms remain incompletely elucidated, smoking and active fistulizing diseases are reported to be specific risk factors of VTE in CD in addition to traditional risk factors, including immobility, older age, surgery, pregnancy or oral contraceptives, corticosteroids and central venous lines (6-9). Among VTE cases in IBD, the mesenteric vein is a rare location for thromboembolism, and mesenteric vein thrombosis (MVT) occurs in about 4% of these cases, according to a previous report [superior mesenteric vein (SMV): 1.3%, splenic vein: 0.6%, portal vein: 2.5%] (10).
Mesenteric vein thrombosis is an uncommon cause of mesenteric ischemia, and its incidence is estimated to be 2.7 per 100,000 (11). Predisposing conditions for MVT have been classified as idiopathic or heritable, such as thrombophilias, acquired thrombophilias and systemic hypercoagulable states (11). Chronic MVT, which is differentiated from acute MVT based on the presence of collaterals and cavernoma, accounts for 20% to 40% of cases (12). The prognosis of chronic MVT ranges from 78% to 83% during 1-5 years, with the main causes of death being variceal hemorrhaging, underlying malignance, sepsis, decompensated liver disease and ischemic bowel disease (12).
We herein report a 44-year-old man who presented with systemic edema due to PLE with SMV obstruction and development of collateral veins, which was subsequently found to be a result of chronic thrombosis and a complication of CD. Although thromboembolism is a major complication of CD, the simultaneous coexistence of SMV obstruction and PLE is very rare; therefore, the initial symptoms derived from these rare complications made the differential diagnosis of CD difficult.
Case Report
A 44-year-old man suffering from systemic edema and body weight gain (6 kg gain from 72 kg in 2 weeks) visited a general hospital in December 2015 after he had been detained in a prison for several months. He had been regularly defecating soft or diarrheal stool, with about 5 bowel movements per day, since he was approximately about 20 years old but had never undergone a physical examination due to a lack of other symptoms. He also had no symptoms of irritable bowel syndrome and no other psychological comorbidities. His history included asthma and atopic dermatitis with no medication.
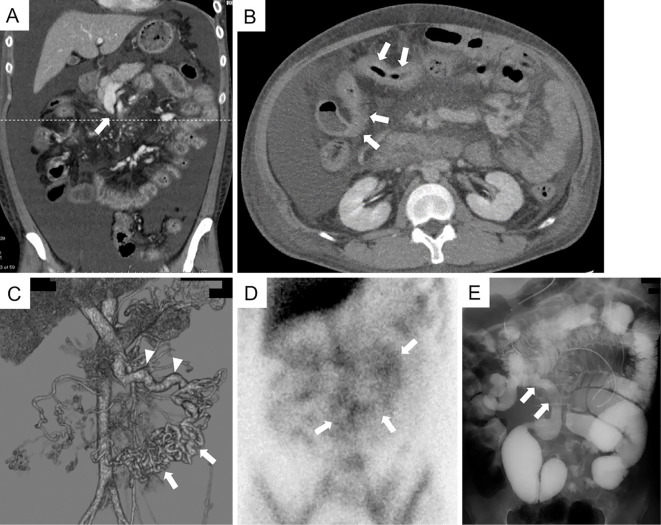
Laboratory examinations revealed severe hypoalbuminemia (0.8 g/dL; normal range: 3.8-5.1 g/dL). Abdominal computed tomography (CT) showed severe ascites and SMV obstruction (Fig. 1A). CT also showed a stenotic lumen of the small intestine without any evident cause at this point (Fig. 1B). Three-dimensional reconstruction of the abdominal mesenteric vein from enhanced CT showed complete SMV obstruction and development of numerous collateral veins flowing into the viable mesenteric veins (Fig. 1C). The status of his congealing fibrinogenolysis system is as follows (normal values or range shown in parentheses): PT-international normalized ratio: 1.06 (0.8-1.2), AT-III: 72% (80-130%), FDP: 8.29 μg/mL (2.0-8.0 μg/mL), D-dimer: 6.71 μg/mL (<1.0 μg/mL), protein S: 105.4% (60-150%), protein C: 100% (70-140%). Lupus anticoagulant and anti-cardiolipin β2-glycoprotein I complex antibody were negative. A urinalysis showed only a slightly elevated urinary protein level (0.05 g/dL). Albumin scintigraphy revealed protein leakage from a wide region of the small intestine (Fig. 1D). Deformed Bauhin's valve was observed by colonoscopy, but no specific findings were detected histologically from the specimen taken from the terminal ileum and colon. Although no specific diagnosis was made, symptomatic treatments, including the administration of human serum albumin (HSA) preparation and diuretics, gradually relieved his symptoms for a while. However, in May 2016, he began to complain of abdominal distention, anorexia and frequent diarrhea (>10 bowel movements per day), so he was hospitalized and treated by fasting with total parenteral nutrition. CT and small-bowel follow-through showed small intestinal dilatation with anal-side stenosis about 20 cm upward from the terminal ileum (Fig. 1E). Predonisolone (starting dose of 60 mg/day) was administered, but his condition did not improve. He was subsequently transferred to our hospital in June 2016 for more detailed examinations (Fig. 2: day 0).
Figure 1.
Imaging studies performed in the first hospital. (A) The coronal view on abdominal CT showed severe ascites and SMV obstruction (“rat tail” appearance, indicated by an arrow). (B) The axial view on CT cut along the dashed line in (A) showed a stenotic lumen of the small intestine (arrows). (C) Three-dimensional reconstruction of the abdominal mesenteric vein from enhanced CT showed the complete obstruction of the SMV and development of numerous collateral veins (arrows) flowing into the viable mesenteric veins (arrowheads). (D) Albumin scintigraphy showed protein leakage from a wide region of the small intestine (arrows, 6 h after injection). (E) Small-bowel follow-through showed small intestinal dilatation with anal-side stenosis about 20 cm upward from the terminal ileum (arrows). CT: computed tomography, SMV: superior mesenteric vein
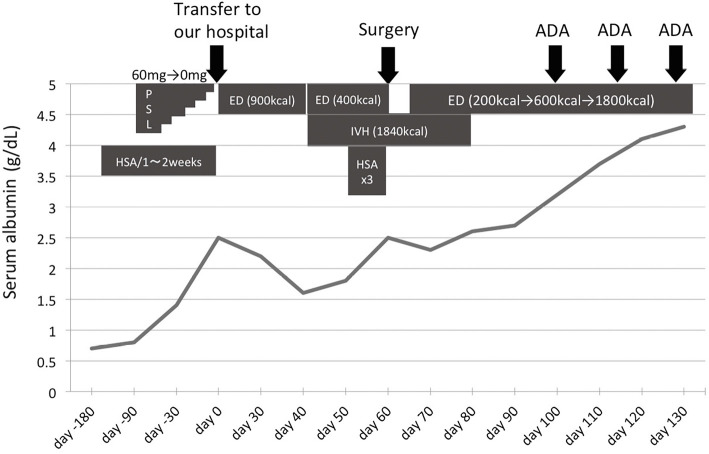
Figure 2.
Clinical course along with the serum albumin levels. ADA: adalimumab, ED: elemental diet, HSA: human serum albumin, IVH: intravenous hyperalimentation, PSL: prednisolone
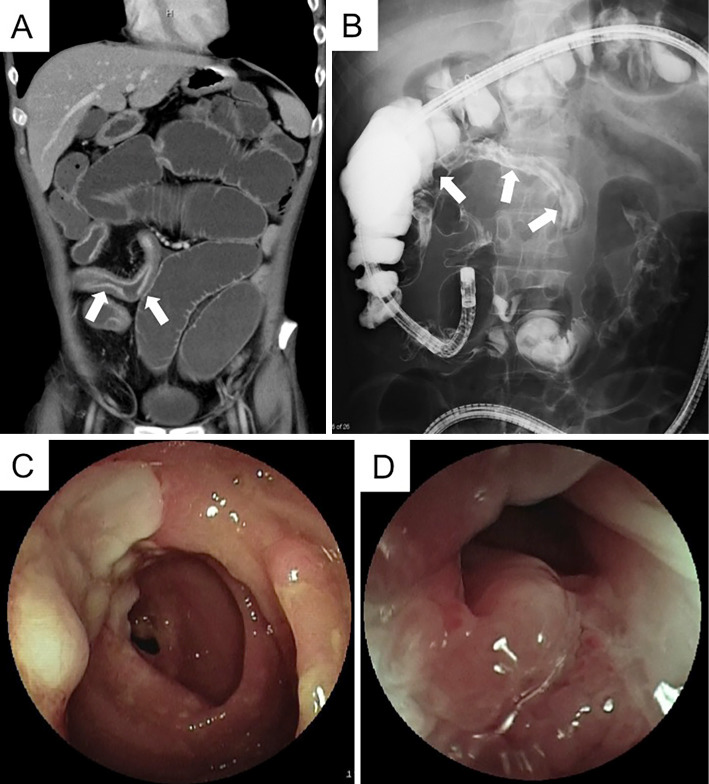
CT and contrast inspection of the small intestine combined with an enteroscope inserted from the anus showed ileal stenosis with dilated lumen of the oral side (Fig. 3A and B). Enteroscopy showed long segmental stenosis (about 30 cm) of the ileum with a longitudinal ulcer, through which the enteroscope could not pass (Fig. 3C and D). Although a histological examination showed no evidence, the above findings were suggestive of CD, and he began to take mesalazine (3,000 mg/day). In addition, elemental diet (ED) was attempted when possible (maximum dose of 900 kcal), but his distention worsened along with his decreasing serum albumin level. Since his ileus did not seem to improve prior to small intestine removal, we attempted to ameliorate his hypoalbuminemia before surgery by increasing the intake of calories through intravenous hyperalimentation (IVH) and the administration of HSA preparation (Fig. 2).
Figure 3.
Imaging studies performed in our hospital. (A and B) CT and contrast inspection of the small intestine combined with an enteroscope inserted from the anus showed ileal stenosis with a dilated lumen at the oral side (arrows). (C and D) Enteroscopy showed long segmental stenosis (about 30 cm) of the ileum with a longitudinal ulcer, through which the enteroscope could not pass.
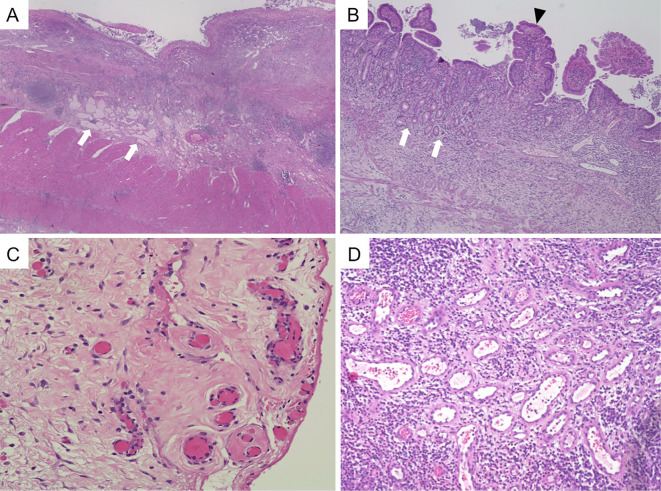
His serum albumin level was barely elevated to 2.5 g/dL right before surgery around day 60. The long segmental stenosis was surgically resected, and a temporary stoma was constructed. The proximal short segmental stenosis was left unresected to avoid the risk of short bowel syndrome. The resected specimen showed an extended ulcer with transmural inflammation, some granulomas, atrophic villus, jejunization of the ileum, and pyloric gland metaplasia (Fig. 4A and B), all of which were compatible with CD. In addition, some dilated submucosal lymphatics were observed (Fig. 4A), which seemed to be a form of lymphangiectasia that is characteristic of PLE. The findings of an organized thrombus around the subserosa and numerous dilated veins around the submucosa (Fig. 4C and D) appeared to be due to the obstruction of the SMV and the development of collateral veins.
Figure 4.
A histological examination of the resected stricturing small intestine. (A, B) The resected specimen showed an extended ulcer with transmural inflammation, some granulomas (not shown), severe atrophic villus, jejunalization of the ileum (arrowhead in B) and pyloric gland metaplasia (arrows in B), all of which were compatible with CD. In addition, some dilated submucosal lymphatics were observed (arrows in A). (C, D) An organized thrombus around the subserosa and numerous dilated veins around the submucosa were observed. Each magnification: ×40 in A, ×100 in B, ×200 in C and D.
The patient started taking ED six days after surgery, and the dose was gradually increased along with IVH. His hypoalbuminemia was gradually improved but did not fully recover (Fig. 2). After we confirmed that there was an active ulcer on the remaining stenosis using an enteroscope inserted via the temporary stoma, the anti-TNFα agent adalimumab (ADA) was administered, and his albumin level recovered to the normal range, although SMV obstruction showed no change. After three administrations of ADA, he was transferred to his former hospital and scheduled to undergo enteroscopy later to confirm the therapeutic effect on the small intestinal lesion. Balloon dilatation for a residual short stricturing lesion was also planned after healing of the ulcer and before the closure of the stoma.
Discussion
We herein report a rare case in which CD was diagnosed following an initial presentation of systemic edema due to PLE with SMV obstruction and development of collateral veins, which are chronic changes of thrombosis and complications of CD.
According to previous reports, acute and chronic MVT, including SMV thrombosis, occur in 0.6-1.3% of CD patients (13,14). Among them, chronic MVT cases can be differentiated from acute cases based on the presence of collateral veins (12). According to our research, five cases of CD with MVT were accompanied by the presence of collateral veins (13-16), and none of them were described as having PLE as a comorbidity, suggesting that the coexistence of chronic MVT and PLE in our case is very rare. Of further note, the primary symptoms of this case did not include the typical abdominal symptoms of CD but instead consisted of ascites or edema derived from PLE.
CT in the present patient showed a “rat tail” appearance, identified by Kopylov et al. as a sign of chronic change of the SMV thrombosis (14), which was supposedly a complication of CD with small intestinal lesions, according to their report and others (14,16). The assumed mechanism is that incessant mesenteric inflammation derived from stricturing small intestine gradually encroaches and obliterates the MV lumen (14). This phenomenon likely applies to our case. The chronic morphological changes of MVT described in the above referenced report also indicate that the SMV obliteration in our case resulted from chronic thrombosis and occurred prior to the weight gain induced by systemic edema and ascites that we observed initially. Since prolonged immobilization is a risk factor of VTE in IBD (7), the patient's detention in prison for several months was another likely risk factor for his SMV thrombosis. He had no smoking habit or fistulizing lesion, which are specific risk factors for VTE in CD patients (8,9). Hyperhomocysteinemia induced by malabsorption of dietary folate, cobalamin and pyridoxine is another possible risk factor for the VTE in our case (17,18), although we did not examine the value of homocysteine.
In our case, hypoalbuminemia may have been induced by a decreased oral intake due to the stenosed lumen and protein loss from the active lesion of the small intestine, as shown in the scintigraphy. After the resection of the stenosed lumen, hypoalbuminemia was partially improved with ED. However, it was not completely resolved before the administration of ADA, suggesting that the lesion responsible for PLE was not localized only in the resected intestine but was also located in the residual small intestine. PLE can be classified into three categories: 1) diseases with increased lymphatic pressure (e.g., lymphangiectasia), 2) diseases with mucosal erosion (e.g., CD) and 3) diseases without mucosal erosion (e.g., celiac disease) (19). In our case, active lesions with mucosal erosions in the small intestine, which were confirmed by enteroscopy before and after surgery, seem to be the only factor for PLE. However, the degree of injury was small and could not be solely responsible for the patient's protein loss. The increased pressure of the lymphatics due to MVT and erosions together may account for the patient's refractory hypoalbuminemia.
According to the European Crohn's and Colitis Organization (ECCO) statement 13I (20), “Antithrombotic prophylaxis should be considered in all hospitalized patients with CD,” and anticoagulant prophylaxis including low-molecular-weight heparin is recommended; prophylactic anticoagulation is safe in cases of IBD, despite the presence of rectal bleeding on admission (21). In addition, outpatients with active disease and patients after discharge from hospitalization require particularly close attention. Preventing VTE is a vital part of the management to reduce the burden of IBD (22). Since MVT was retrospectively diagnosed upon a review of the imaging studies in some patients (14), the therapeutic benefit of anticoagulation in IBD patients with incidentally discovered MVT remains to be determined, and the true prevalence of MVT may be higher.
Inflammation may drive a hypercoagulable state through activation of the coagulation cascade, coupled with the promotion of platelet aggregation and impairment of anticoagulant or fibrinolytic mechanisms (7,9). Other mechanisms potentially underlying the hypercoagulable state in IBD patients have also been proposed, including elevated levels of soluble CD40 ligand and P-selectin produced by activated platelets (23,24). We previously revealed that the suppression of P-selectin derived from platelets by inhibiting phosphodiesterase-3 led to the amelioration of murine ileitis (25). Furthermore, existing therapy methods, 5-aminosalicylic acid, and anti-TNFα agents, have been reported to down-regulate circulating activated platelets and potentially suppress gut inflammation (26,27). Based on these findings, activated platelets may be a useful therapeutic target not just for preventing VTE but also for preventing IBD itself in the near future.
Conclusion
We herein report a case showing an atypical clinical presentation of SMV occlusion accompanied by PLE, which subsequently turned out to be a thrombotic complication of CD. Although thromboembolism is a major complication of CD, the simultaneous coexistence of SMV obstruction and PLE is very rare; it is therefore important to recognize that these complications can cause initial atypical symptoms of CD before finalizing the diagnosis.
The authors state that they have no Conflict of Interest (COI).
Suguru Ito and Masaaki Higashiyama contributed equally to this work.
References
- 1.Grainge MJ, West J, Card TR, Mill K. Venous thromboembolism during active disease and remission in infl ammatory bowel disease: a cohort study. Lancet 375: 657-663, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Yuhara H, Steinmaus C, Corley D, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther 37: 953-962, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Miehsler W, Reinisch W, Valic E, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut 53: 542-548, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson LM, O'Gorman PJ, O'Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM 90: 183-188, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Irving PM, Pasi KJ, Rampton DS. Thrombosis and inflammatory bowel disease. Clin Gastroenterol Hepatol 3: 617-628, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol 106: 713-718, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol 102: 174-186, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev 3: 394-400, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol 103: 2272-2280, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Papay P, Miehsler W, Tilg H, et al. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis 7: 723-729, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med 15: 407-418, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol 4: 257-263, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatoum OA, Spinelli KS, Abu-Hajir M, et al. Mesenteric venous thrombosis in inflammatory bowel disease. J Clin Gastroenterol 39: 27-31, 2005. [PubMed] [Google Scholar]
- 14.Kopylov U, Amitai MM, Lubetsky A, Eliakim R. Clinical and radiographic presentation of superior mesenteric vein thrombosis in Crohn's disease: a single center experience. J Crohns Colitis 6: 543-549, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson RS, Jackson JE, Walters JRF. Superior mesenteric vein stenosis complicating Crohn's disease. Gut 45: 459-462, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson CS, Fryer J, Danese S, Vanagunas A, Polensky S, Buchman AL. Mesenteric vascular thromboembolism in inflammatory bowel disease: a single center experience. J Gastrointest Surg 15: 97-100, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Cobalamin CW, Concentrations F. Hyperhomocysteinemia in inflammatory bowel disease patients without past intestinal resections. J Clin Gastroenterol 42: 481-486, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Danese S, Sgambato A, Papa A, et al. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol 100: 886-895, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Levitt DG, Levitt MD. Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states. Clin Exp Gastroenterol 10: 147-168, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assche G Van, Dignass A, Reinisch W, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: special situations. J Crohns Colitis 4: 63-101, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Ra G, Thanabalan R, Ratneswaran S, Nguyen GC. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis 7: e479-e485, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Bryant RV, Jairath V, Curry N, Travis SPL. Thrombosis in inflammatory bowel disease: Are we tailoring prophylaxis to those most at risk? J Crohns Colitis 8: 166-171, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Danese S, Katz J, Saibeni S, et al. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut 52: 1435-1442, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andoh A, Tsujikawa T, Hata K, et al. Elevated circulating platelet-derived microparticles in patients with active inflammatory bowel disease. Am J Gastroenterol 100: 2042-2048, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga H, Hokari R, Higashiyama M, et al. Cilostazol, a specific PDE-3 inhibitor, ameliorates chronic ileitis via suppression of interaction of platelets with monocytes. Am J Physiol Gastrointest Liver Physiol 297: G1077-G1084, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Rty ECA, Macey M, Rampton DS. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment Pharmacol Ther 14: 1169-1179, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Danese S, Sans M, Scaldaferri F, et al. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease. J Immunol 176: 2617-2624, 2006. [DOI] [PubMed] [Google Scholar]