Abstract
Objective
The aim of this study was to assess the relationship between hypercholesterolemia (HC) and clinical events through a percutaneous coronary intervention (PCI) registry. HC is a well-known independent risk factor for long-term cardiovascular events after PCI. However, it has been reported to be associated with a lower risk of adverse events in patients with cancer or acute coronary syndrome.
Methods
We analyzed the relationship between HC and adverse events in patients treated with everolimus-eluting stents (EESs) through the Tokyo-MD PCI study (an all-comer, multicenter, observational registry). The propensity score method was applied to select two groups with similar baseline characteristics.
Results
The unadjusted population included 1,536 HC patients and 330 non-HC patients. Propensity score matching yielded 314 matched pairs. After baseline adjustment, the outcomes of HC patients were significantly better than those of the non-HC patients with respect to the primary endpoint, which was a combination of mortality from all causes, nonfatal myocardial infarction (MI), nonfatal neurological events, and major bleeding [hazard ratio (HR) 0.56, 95% confidence interval (CI) 0.39-0.81; p=0.002], and the secondary endpoints, which included a combination of mortality from all causes, nonfatal MI, and nonfatal neurological events (HR 0.59, 95% CI 0.39-0.88; p=0.01), and major bleeding (HR 0.42, 95% CI 0.20-0.88; p=0.02). A subgroup analysis showed age as an interaction factor for the primary endpoint (interaction p=0.035).
Conclusion
HC was associated with better outcomes in patients who underwent EES implantation, even after baseline adjustment.
Keywords: percutaneous coronary intervention, hypercholesterolemia, drug eluting stent, mortality, major adverse cardiac or cerebrovascular events
Introduction
Hypercholesterolemia (HC) is recognized as an independent risk factor for the development of coronary heart disease (1-3). Statin treatment is strongly recommended for patients with cardiovascular disease, while no recommendations have been made for patients with hypocholesterolemia (4,5). On the other hand, a J- or U-shaped relationship between the serum cholesterol level and all-cause mortality has been reported in patients with life-threatening diseases such as cancer (6-8) or chronic obstructive pulmonary disease (9). Such a relationship has been referred to as the “lipid paradox” in studies on rheumatoid arthritis (10,11) and acute coronary disease (12-14).
In addition, a low serum cholesterol level is reported to increase the risk of intraparenchymal hemorrhage (15,16). The choice of standard medical therapy after percutaneous coronary intervention (PCI) has been controversial, especially with regard to the types of medicines and duration of treatment in patients receiving antithrombotic therapy (17-19). The situation is more complicated if the patient is on anticoagulant therapy, or surgical intervention is planned. Knowledge of the relationship between HC and therapy outcomes would provide information that is valuable for selecting appropriate medical therapy.
It is well known that the serum cholesterol level depends on a patient's comorbidities, physical status, nutritional condition, and use of medications, particularly statins. The main questions in this study were as follows: 1) will the lipid paradox occur in the PCI registry, which mostly includes patients with stable angina, after adjustment for confounding factors; 2) if a relationship is present, which outcomes are related to HC; and 3) are HC and statin use related to the outcomes?
Materials and Methods
Study population
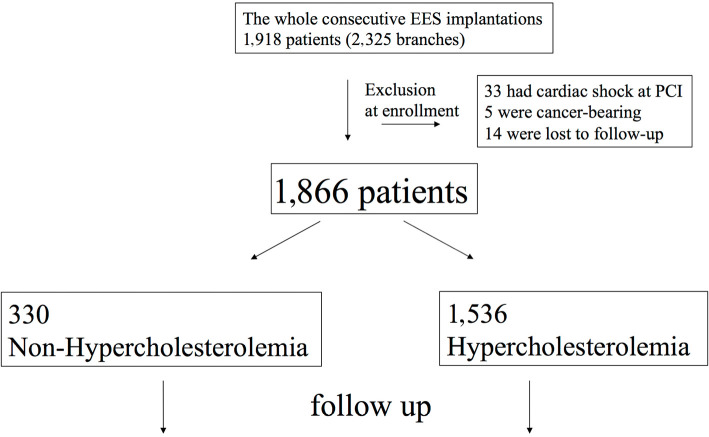
This study was performed as a sub-study of the Tokyo-MD PCI registry, a physician-initiated, multi-center, observational registry in Japan. The registry has been described previously (20-22). In brief, the registry included consecutive patients who underwent everolimus-eluting stent (EES) (Xience V, Abbott, Abbott Park, USA; Promus, Boston Scientific, Natick, USA) implantation in 22 centers in Japan from January 2010 to December 2011. The purpose of the present study was to compare HC and non-HC patients on the day of PCI. Patients with cardiac shock at hospitalization, cancer, and those lost to follow-up were excluded from this study. A total of 1,866 patients of 1,918 patients who underwent EES implantation were included in this study (Fig. 1).
Figure 1.
Flowchart of the methodology. EES: everolimus-eluting stent, PCI: percutaneous coronary intervention
Data definitions
As all the patients included in the present study underwent PCI, we defined the criteria for HC according to the target cholesterol level for secondary prevention indicated by the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. The criteria were as follows: 1) treatment with a lipid-lowering agent prior to hospital admission (documented in the patient's medical record), or 2) a total cholesterol level of ≥180 mg/dL or a low-density lipoprotein cholesterol level ≥100 mg/dL (among patients without a history of taking lipid-lowering agents). In all cases, lipid measurements had been evaluated previously or within 48 hours after hospitalization. The primary endpoint was the combination of mortality from all causes, nonfatal myocardial infarction (MI), nonfatal neurological events, and major bleeding (GUSTO definition: moderate or higher) during the entire follow-up period. Details of the endpoint criteria have been described previously (20-22). Key secondary endpoints for the entire follow-up period were as follows: 1) the combined incidence of mortality from all causes, nonfatal MI, and nonfatal neurological events, and 2) major bleeding.
Statistical analysis
Normally distributed variables were represented as the mean with standard deviation and compared using Student's t-test. Non-normally distributed variables were represented as the median with the 25th and 75th percentile values and were compared using the Mann-Whitney U test. Categorical variables were summarized as percentages and compared using the chi-squared test. Propensity score matching was performed to adjust for significant differences in baseline covariates and potential confounders that may lead to the biased estimation of the treatment outcome (23). Possible confounders (except the rate of statin use, which is related to the rate of HC) were chosen based on clinical knowledge of their potential association with the outcome of interest. The predicted probability score of HC was calculated by fitting a logistic regression model using confounding factors with p values of <0.5 in the baseline characteristic analyses. Using the probability score, we performed rigorous adjustment for significant differences in the baseline characteristics of patients with propensity score matching using the algorithm 1:1 optimal match with a ±0.01 caliper and no replacement. The cumulative incidence of the endpoints was represented as Kaplan-Meier estimates with a log-rank analysis. The HR of each endpoint was calculated using Cox's proportional hazards model. All p values were 2-sided, and p values of <0.05 were considered to indicate statistical significance. All of the statistical analyses were performed using the “R” software program (version 3.1.2 for Windows) using the “tableone”, “Matching”, and “RcmdrPlugin. EZR” packages (24).
Results
Study patients and HC
A total of 330 patients were included in the non-HC group while 1,536 patients were included in the HC group. The mean follow-up periods for the entire cohort were 983.4 days for the non-HC group and 952.0 days for the HC group (p=0.26). The follow-up rates were 88.0% (n=1,643) for one year, 74.2% (n=1,385) for two years, and 54.6% (n=1,019) for three years. The baseline characteristics of the entire cohort and propensity score-matched patients are shown in Table 1 (details in Supplementary material 1). Propensity score matching yielded 314 pairs (628 patients in total). The rate of statin use was excluded from confounding factors used to calculate the propensity score; thus, a significant difference in statin use existed even after score matching.
Table 1.
Unadjusted and Adjusted Baseline Characteristics according to Hypercholesterolemia.
| Full cohort | Propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Non-hypercholesterolemia | Hypercholesterolemia | p | Non-hypercholesterolemia | Hypercholesterolemia | p | |
| Total number | n=330 | n=1,536 | n=314 | n=314 | |||
| Age, mean y.o | 71.7 | 69.5 | <0.001*** | 71.6 | 71.0 | 0.45 | |
| Gender male, no. (%) | 246 (74.5) | 1,138 (74.1) | 0.89 | 236 (75.2) | 224 (71.3) | 0.32 | |
| Smoker, no. (%) | 115 (34.8) | 703 (45.8) | <0.001*** | 115 (36.6) | 109 (34.7) | 0.68 | |
| BMI>25 kg/m2, no. (%) | 61 (20.9) | 405 (29.1) | <0.01** | 61 (22.0) | 75 (26.1) | 0.28 | |
| <Comorbidity> | |||||||
| Hypertension, no. (%) | 225 (68.2) | 1,140 (74.2) | 0.03 | 220 (70.1) | 225 (71.7) | 0.73 | |
| Chronic heart failure, no. (%) | 36 (10.9) | 120 (7.8) | 0.08 | 31 (9.9) | 29 (9.2) | 0.89 | |
| Peripheral arterial disease, no. (%) | 44 (13.3) | 144 (9.4) | 0.03* | 40 (12.7) | 47 (15.0) | 0.49 | |
| Chronic kidney disease, no. (%) | 102 (30.9) | 340 (22.1) | <0.001*** | 93 (29.6) | 98 (31.2) | 0.73 | |
| Hemodialysis, no. (%) | 49 (14.8) | 76 (4.9) | <0.001*** | 42 (13.4) | 36 (11.5) | 0.55 | |
| Cerebral vascular disease, no. (%) | 42 (12.7) | 141 (9.2) | 0.05 | 40 (12.7) | 40 (12.7) | 1.00 | |
| Diabetes mellitus, no. (%) | 130 (39.4) | 646 (42.1) | 0.39 | 126 (40.1) | 115 (36.6) | 0.41 | |
| Previous CABG, no. (%) | 21 (6.4) | 96 (6.2) | 0.90 | 20 (6.4) | 31 (9.9) | 0.14 | |
| Previous PCI, no. (%) | 106 (32.1) | 569 (37.0) | 0.10 | 101 (32.2) | 102 (32.5) | 1.00 | |
| Previous MI, no. (%) | 89 (27.0) | 477 (31.1) | 0.15 | 87 (27.7) | 87 (27.7) | 1.00 | |
| <Medication at discharge> | |||||||
| Statin, no. (%) | 49 (14.8) | 1,433 (93.3) | <0.001*** | 48 (15.3) | 281 (89.5) | <0.001*** | |
| βblocker, no. (%) | 102 (30.9) | 797 (51.9) | <0.001*** | 102 (32.5) | 104 (33.1) | 0.93 | |
| Anticoagulant, no. (%) | 44 (13.3) | 166 (10.8) | 0.21 | 42 (13.4) | 44 (14.0) | 0.91 | |
| ARB or ACE-inhibitor, no. (%) | 130 (39.4) | 965 (62.8) | <0.001*** | 130 (41.4) | 136 (43.3) | 0.69 | |
| DAPT duration, mean days | 856.8 | 834.1 | 0.43 | 846.8 | 858.5 | 0.77 | |
| Proton pump inhibitor, no. (%) | 155 (47.0) | 953 (62.0) | <0.001*** | 154 (49.0) | 155 (49.4) | 1.00 | |
| <Procedural characteristics> | |||||||
| PCI for MI, no. (%) | 44 (13.3) | 332 (21.6) | <0.001*** | 44 (14.0) | 39 (12.4) | 0.64 | |
| PCI for angina, no. (%) | 286 (86.7) | 1,204 (78.4) | <0.001*** | 270 (86.0) | 275 (87.6) | 0.64 | |
| Mean stent size, median mm | 3.00 | 3.00 | 0.67 | 3.00 | 3.00 | 0.96 | |
| Total stent length, median mm | 23.0 | 23.0 | 0.01* | 23.0 | 23.0 | 0.40 | |
| PCI with imaging device, no. (%) | 324 (98.2) | 1,454 (94.7) | <0.01** | 308 (98.1) | 309 (98.4) | 1.00 | |
(The details of baseline characteristics is available in Supplementary material 1.)
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
no.: number, BMI: body mass index, CABG: coronary artery bypass graft, PCI: percutaneous coronary intervention, MI: myocardial infarction, ARB: angiotensin 2 receptor blocker, ACE: angiotensin converting enzyme, DAPT: dual antiplatelet therapy, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, LMT: main coronary trunk
Periprocedural outcomes
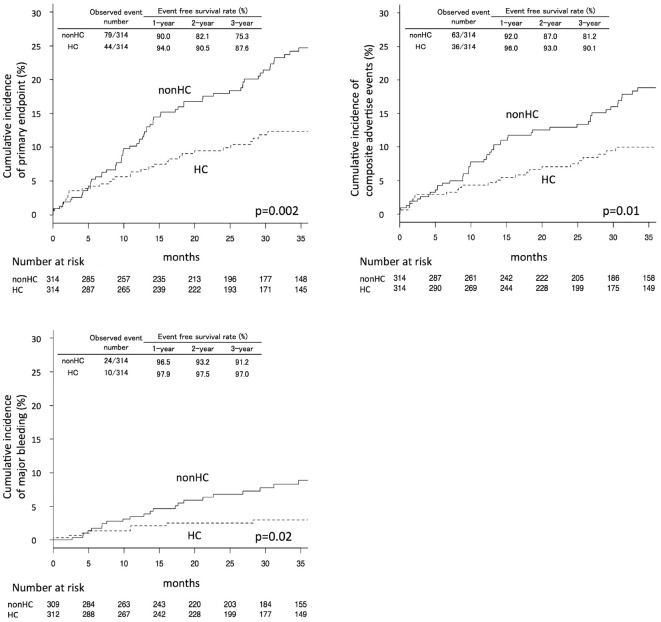
The Kaplan-Meier curves of the primary and secondary outcomes in the propensity score-matched patients are shown in Fig. 2 with yearly event-free survival rates were calculated based on Kaplan-Meier estimates. p values were calculated by a log-rank analysis. For all primary and secondary endpoints, the HC group had significantly lower rates of events. The trends were similar in the analyses of the entire cohort (Supplementary material 2a-c). Event numbers during the entire follow-up period, and Cox's univariate hazard ratios (HRs) are shown in Table 2. In analyses of the propensity score-matched patients, the HR for the HC group was 0.56 (95% CI 0.39-0.81; p=0.002) for the primary endpoint, 0.59 (95% CI 0.39-0.88; p=0.01) for mortality from all causes, MI, or neurological events, and 0.42 (95% CI 0.20-0.88; p=0.02) for major bleeding. In the analyses of the other endpoints, all-cause mortality showed a significant difference with an HR of 0.57 (95% CI 0.35-0.95, p=0.03); no significant difference was observed in the other cardiovascular endpoints.
Figure 2.
Cumulative Kaplan-Meier estimates of the rates of key study end points. Panel A shows the data for the primary endpoint-mortality from all causes, myocardial infarction, neurological events, or major bleeding. Panel B shows data for the secondary endpoint-mortality from all causes, myocardial infarction, or neurological events. Panel C shows data for the secondary endpoint of major bleeding. p values were calculated using the log-rank test. Event-free survival rates were calculated based on Kaplan-Meier estimates. HC: hypercholesterolemia
Table 2.
Major Endpoints according to Hypercholesterolemia.
| End point | Non-Hypercholesterolemia | Hypercholesterolemia | HR for hypercholesterolemia group | p |
|---|---|---|---|---|
| Full cohort, event no. | n=330 | n=1,536 | ||
| Primary endpoint | ||||
| Mortality from all cause, MI, neurological events, or major bleeding | 82 | 199 | 0.53 (0.41-0.69) | <0.001*** |
| Secondary endpoints | ||||
| Mortality from all cause, MI, neurological events | 66 | 163 | 0.55 (0.42-0.74) | <0.001*** |
| Major Bleeding | 25 | 47 | 0.41 (0.25-0.66) | <0.001*** |
| Other endpoints | ||||
| Mortality from all cause | 46 | 96 | 0.47 (0.33-0.67) | <0.001*** |
| Mortality from cardiovascular cause | 17 | 47 | 0.61 (0.35-1.07) | 0.09 |
| Neurological events | 9 | 28 | 0.71 (0.33-1.50) | 0.37 |
| MI | 11 | 39 | 0.78 (0.40-1.52) | 0.46 |
| Stent thrombosis | 4 | 16 | 0.85 (0.29-2.55) | 0.78 |
| Propensity score matching, event no. | n=314 | n=314 | ||
| Primary endpoint | ||||
| Mortality from all cause, MI, neurological events, or major bleeding | 79 | 44 | 0.56 (0.39-0.81) | 0.002** |
| Secondary endpoints | ||||
| Mortality from all cause, MI, or neurological events | 63 | 36 | 0.59 (0.39-0.88) | 0.01* |
| Major Bleeding | 24 | 10 | 0.42 (0.20-0.88) | 0.02* |
| Other endpoints | ||||
| Mortality from all cause | 43 | 24 | 0.57 (0.35-0.95) | 0.03* |
| Mortality from cardiovascular cause | 16 | 11 | 0.71 (0.33-1.52) | 0.37 |
| Neurological events | 9 | 5 | 0.59 (0.20-1.77) | 0.35 |
| MI | 11 | 8 | 0.73 (0.29-1.82) | 0.50 |
| Stent thrombosis | 3 | 2 | 0.66 (0.11-3.94) | 0.65 |
Event numbers are cumulative event numbers of whole follow up period. Patients could have had more than one type of end point. For example mortality and major bleeding or neurological events are not mutually exclusive; hence if patients went through major bleeding before mortality, the events were included in both endpoints. Stent thrombosis was defined as definite or probable thrombosis, according to the Academic Research Consortium. p values were calculated by means of Cox regression analysis. HR: hazard ratio, MI: myocardial infarction
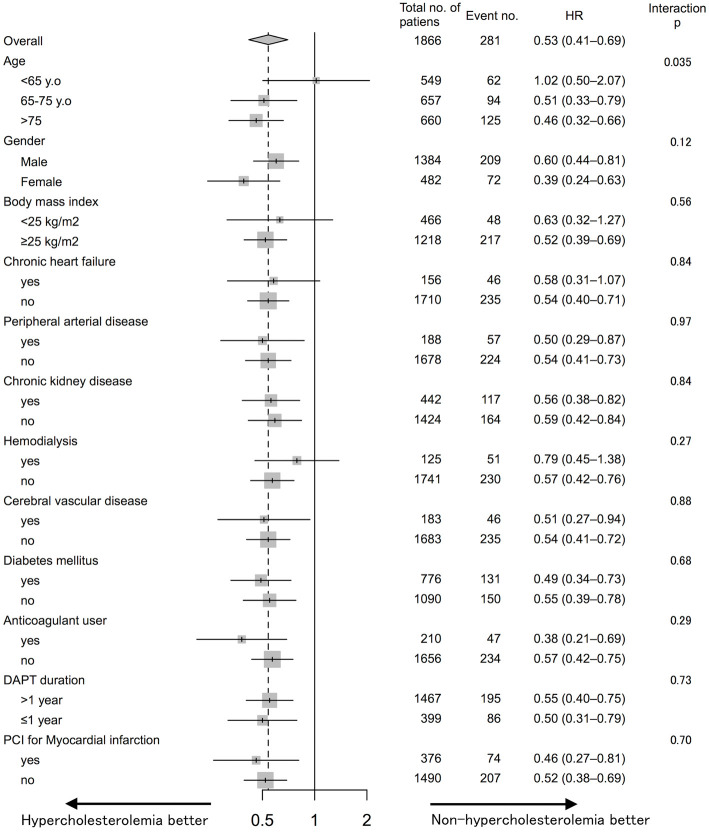
Subgroup analysis
Fig. 3 shows a summary of the subgroup analysis for the primary endpoint. Overall, HC tended to be associated with a lower risk of events; nonetheless, HC and non-HC patients of <65 years of age showed equivalent risk. The interaction between age and HC was significant while the interactions between other factors and HC were not.
Figure 3.
Hazard ratios (HRs) and event numbers for the primary endpoint-mortality from all causes, myocardial infarction, neurological events, or major bleeding in the subgroups. The HRs were calculated using a univariate Cox regression analysis. p values for the interaction are shown next to the respective event rates for each designated subgroup. HC: hypercholesterolemia
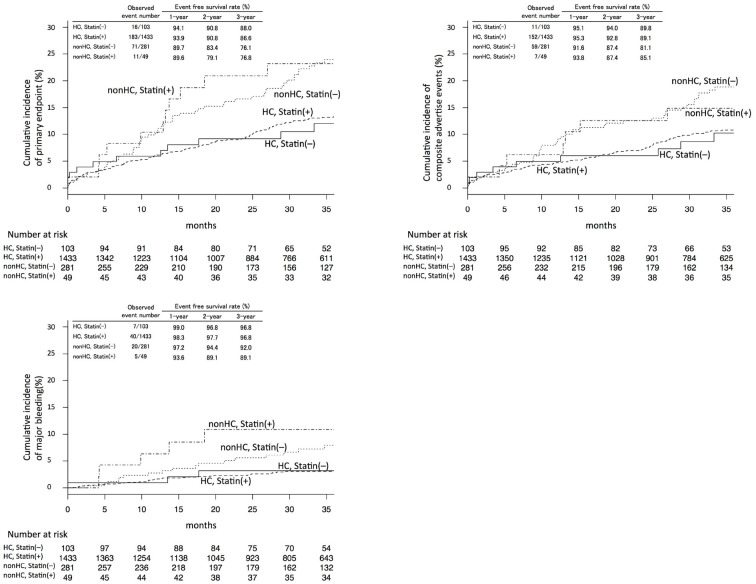
Statin use and HC
The Kaplan-Meier curves of the primary and secondary endpoints in the entire cohort according to HC and statin use are shown in Fig. 4. The cumulative incidence seemed to be less influenced by statin use. We performed a baseline adjustment between statin users vs. non-users using propensity score matching in HC patients and non-HC patients separately (Supplementary material 3, and 4, respectively). In patients with HC, the comparison between statin users and non-users did not show a significant difference after baseline adjustment. In non-HC patients, statin use seemed to be associated with a higher incidence of events; however, the result was not statistically significant was observed (Supplementary material 5a-c and 6a-c).
Figure 4.
Cumulative Kaplan-Meier estimates of the rates of key study endpoints according to hypercholesterolemia (HC) and statin use. Panel A shows data for the primary endpoint-mortality from all causes, myocardial infarction, neurological events, or major bleeding. Panel B shows the data for the secondary endpoint-mortality from all causes, myocardial infarction, or neurological events. Panel C shows data for the secondary endpoint of major bleeding. p values were calculated using the log-rank test. Event-free survival rates were calculated based on Kaplan-Meier estimates.
Discussion
This study supports the occurrence of the lipid paradox in patients who undergo PCI; HC patients had a lower rates of mortality and the combination of cardiovascular adverse events and bleeding in comparison to non-HC patients. HC did not show a significant difference according to any endpoint, including mortality from cardiovascular causes, neurological events, MI, or stent thrombosis. In the subgroup analysis, HC showed interaction with patient age.
In accordance with previous studies on the lipid paradox, three interpretations of this association should be discussed (25,26). The first is that HC plays an etiological role; HC is directly associated with the reduced rate of adverse events. The second is that a hypocholesterolemic effect of preclinical comorbidities or latent diseases results in a high rate of events. Finally, the association between HC and the rate of events may be due to other unknown factors that are related to both low total cholesterol (TC) and disease. Iribarren et al. analyzed the TC change over a 6-year period in relation to subsequent 16-year mortality in a cohort registry (27). They reported that a falling total cholesterol level was accompanied by a subsequent increased risk of death due to some types of cancer, non-cardiovascular non-cancer causes (particularly liver disease), and all causes. In addition, there was no significant increase in all-cause mortality risk among a cohort of men with stable low total cholesterol levels. They concluded that their finding supported the hypothesis that low TC, independent of the risk factors considered in this study, appears to be a manifestation of tumor activity or a consequence of chronic liver disease. In our study, the following results seemed to support the existence of an etiological role of HC in adverse events. First, the lower rates of events in patients with HC were significant, even after adjustment. Second, in the subgroup analysis, HC showed significance in healthier patients such as those without chronic heart failure or hemodialysis. Third, in the patient group with a body mass index of ≥25 kg/m2, the significance was unchanged; the result was not due to the existence of extreme leanness or emaciation alone. On the other hand, the similar findings - in terms of the primary endpoint - in HC and non-HC patients of <65 years of age, and the fact that HC was associated with adverse events, especially from non-cardiovascular causes, support the interpretation that low cholesterol is a consequence of other diseases. In our opinion, an acceptable explanation is that life-threatening diseases cause a decrease in cholesterol before the physical manifestation of the disease. Assuming the existence of a phase in which a decrease in cholesterol precedes the physical manifestation, it would be reasonable that non-HC patients who would suffer from life-threatening diseases later had had a similar risk profile (with the exception of their cholesterol level) to the healthy HC patients. Frailty should also be considered. Frailty is a health state related to aging in which physical and mental activity and physical appearance gradually decline (28), It is a strong predictor of mortality in patients with cardiovascular disease, independent of age, disease severity, comorbidity and disability (29). In fact, some studies have reported a relationship between serum cholesterol and frailty (30,31). Non-HC might represent these non-physical vulnerabilities.
The lipid paradox has been reported among patients with acute coronary disease. Most of these reports suggested that the higher rate of adverse events in non-HC patients was due to severe secondary liver failure and/or inflammation (12-14). Changes in the liver function, including lipoprotein breakdown and excretion, may alter the low-density lipoprotein cholesterol levels in patients with inflammation (32,33). The subgroup analysis in the present study revealed that non-HC patients with MI had worse outcomes. However, no significant interaction between MI and the influence of HC was observed. Moreover, the HRs of MI and non-MI patients were similar. The above explanation, that severe myocardial damage causes severe secondary liver failure with an inflammatory response contributing to a lower cholesterol level (12,14), is not sufficient to explain the findings. The finding that HC influenced all cause mortality but not cardiac mortality would support that the lipid paradox is due to non-cardiac causes.
In accordance with several reports that suggested a relationship between the cholesterol level and bleeding, our study showed that non-HC patients had approximately twice the risk of bleeding in comparison to HC patients. However, no well-known scoring scales for evaluating the bleeding risk in patients receiving antithrombotic therapy (34,35) have included the cholesterol level as a confounding factor. Although no interactions between the primary endpoint and anticoagulant use/dual antiplatelet therapy duration were observed, a reduction in the dose and duration of antithrombotic drug treatment could be recommended for non-HC patients.
The Kaplan-Meier curves of the groups divided according to HC/non-HC status and statin use revealed that statin use showed less influence or appeared harmful for non-HC patients, especially with respect to major bleeding. In patients with HC, the comparison between statin users and non-users did not show a significant difference after adjustment for baseline factors. In the non-HC patients, statin use appeared harmful but no statistical significance was observed, although there was a baseline difference between statin users and non-users despite the very small number of matched patients and the fact that the comparison was made after baseline adjustment.
The present study was associated with several limitations. First, it was an observational registry trial and all medical and procedural strategies depended on the physicians; the HC analysis was performed retrospectively and was not previously included in the registry protocol. Second, our registry only included patients who underwent EES placement; we need to consider the validity of expanding the results to other stent users. However, EES are among the most widely used stents and the clinical features of the outcomes after stent implantation are similar among all modern stents. In our opinion, the results have general applicability to patients undergoing PCI. Third, the medical therapies used differed from current trends; thienopyridine was limited to clopidogrel or ticlopidine, and the duration of dual antiplatelet therapy was longer. Fourth, this study lacked information regarding the serum cholesterol level and its alterations, as well as detailed information about mortality. Previous statin treatment could be a general marker for patients with more hospital visits prior to PCI, which could be related to a better outcome. Finally, our analysis of the relationship between HC and statin use had less power because 79.6% of the patients in this study were treated with statins. The statistical power to prove a medium effect (Cohen's w=0.3) in the comparison between the HC and non-HC subgroups was 55.6% in statin users and 86.1% in statin non-users (calculated with smaller groups: n=49 and n=103, respectively, assuming a type I error of 0.05 and a type II error of 0.8).
In conclusion, among patients who underwent PCI with EES, HC was associated with a lower rate of adverse events, even after baseline adjustment. In other words, patients who underwent PCI were at high risk for adverse events and mortality, even if they did not have HC. Statin use seemed to be ineffective for these patients; special care, medication, and/or nutrition using the cholesterol level as a scale of effectiveness should be considered in such cases.
The authors state that they have no Conflict of Interest (COI).
Supplementary Materials
Details of unadjusted and adjusted baseline characteristics.
Cumulative Kaplan-Meier estimates of the key endpoints among full cohort.
Baseline characteristics in patients of hypercholesterolemia, according to statin use.
Baseline characteristics in patients of non-hypercholesterolemia, according to statin use.
Cumulative Kaplan-Meier estimates of the key endpoints in patients of hypercholesterolemia after propensity score matching.
Cumulative Kaplan-Meier estimates of the key endpoints in patients of non-hypercholesterolemia after propensity score matching.
Cumulative Kaplan-Meier estimates of the key endpoints in patients of non-hypercholesterolemia after propensity score matching.
References
- 1. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350: 1495-1504, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285: 1711-1718, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372: 2387-2397, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129 (Suppl): S76-S99, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 33: 1635-1701, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Sorlie PD, Feinleib M. The serum cholesterol-cancer relationship: an analysis of time trends in the Framingham Study. J Natl Cancer Inst 69: 989-996, 1982. [PubMed] [Google Scholar]
- 7. Sherwin RW, Wentworth DN, Cutler JA, Hulley SB, Kuller LH, Stamler J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the multiple risk factor intervention trial. JAMA 257: 943-948, 1987. [PubMed] [Google Scholar]
- 8. Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol 50: 409-418, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Fruchter O, Yigla M, Kramer MR. Lipid profile and statin use: the paradox of survival after acute exacerbation of chronic obstructive pulmonary disease. Am J Med Sci 349: 338-343, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 70: 482-487, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perez-Baos S, Barrasa JI, Gratal P, et al. Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. Br J Pharmacol 174: 3018-3031, 2017(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy VS, Bui QT, Jacobs JR, et al. Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am J Cardiol 115: 557-562, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Wang TY, Newby LK, Chen AY, et al. Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome. Clin Cardiol 32: E22-E28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng KH, Chu CS, Lin TH, Lee KT, Sheu SH, Lai WT. Lipid paradox in acute myocardial infarction-the association with 30-day in-hospital mortality. Crit Care Med 43: 1255-1264, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Noda H, Iso H, Irie F, et al. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki prefectural health study. Circulation 119: 2136-2145, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Iso H, Jacobs DR Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 320: 904-910, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 371: 2155-2166, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 381: 1107-1115, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation 127: 634-640, 2013. [DOI] [PubMed] [Google Scholar]
- 20. Konishi Y, Ashikaga T, Sasaoka T, Kurihara K, Yoshikawa S, Isobe M. Comparison of outcomes after everolimus-eluting stent implantation in diabetic versus non-diabetic patients in the Tokyo-MD PCI study. J Cardiol 67: 241-247, 2016. [DOI] [PubMed] [Google Scholar]
- 21. Ueshima D, Ashikaga T, Yoshikawa S, et al. Effect of over-2-year dual antiplatelet therapy on the rate of major adverse cardiac and cerebral events for everolimus-eluting stent implantation: the landmark analysis from Tokyo-MD PCI registry. J Cardiol 69: 815-822, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Kurihara K, Ashikaga T, Sasaoka T, Yoshikawa S, Isobe M; Tokyo MDPCISI.. Incidence and predictors of early and late target lesion revascularization after everolimus-eluting stent implantation in unselected patients in japan. Catheter Cardiovasc Interv 90: 78-86, 2017. [DOI] [PubMed] [Google Scholar]
- 23. D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17: 2265-2281, 1998. [DOI] [PubMed] [Google Scholar]
- 24. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ 308: 373-379, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iribarren C, Reed DM, Burchfiel CM, Dwyer JH. Serum total cholesterol and mortality. Confounding factors and risk modification in Japanese-American men. JAMA 273: 1926-1932, 1995. [PubMed] [Google Scholar]
- 27. Iribarren C, Reed DM, Chen R, Yano K, Dwyer JH. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation 92: 2396-2403, 1995. [DOI] [PubMed] [Google Scholar]
- 28. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146-M156, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 103: 1616-1621, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Ramsay SE, Arianayagam DS, Whincup PH, et al. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart 101: 616-622, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranieri P, Rozzini R, Franzoni S, Barbisoni P, Trabucchi M. Serum cholesterol levels as a measure of frailty in elderly patients. Exp Aging Res 24: 169-179, 1998. [DOI] [PubMed] [Google Scholar]
- 32. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. J Am Coll Cardiol 51: 1440-1445, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol 22: 933-940, 1993. [DOI] [PubMed] [Google Scholar]
- 34. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138: 1093-1100, 2010. [DOI] [PubMed] [Google Scholar]
- 35. Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 315: 1735-1749, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of unadjusted and adjusted baseline characteristics.
Cumulative Kaplan-Meier estimates of the key endpoints among full cohort.
Baseline characteristics in patients of hypercholesterolemia, according to statin use.
Baseline characteristics in patients of non-hypercholesterolemia, according to statin use.
Cumulative Kaplan-Meier estimates of the key endpoints in patients of hypercholesterolemia after propensity score matching.
Cumulative Kaplan-Meier estimates of the key endpoints in patients of non-hypercholesterolemia after propensity score matching.
Cumulative Kaplan-Meier estimates of the key endpoints in patients of non-hypercholesterolemia after propensity score matching.