Abstract
An aortic mural thrombus (AMT) on a non-atherosclerotic wall is a rare but important cause of arterial thromboembolism. We herein report two cases of AMT in the thoracic aorta. Both showed multiple hypercoagulable factors (case 1: protein S deficiency and positive finding of anti-cardiolipin antibody; case 2: protein C deficiency, gastric cancer, and cisplatin-based chemotherapy) and were successfully treated with anticoagulation. Hypercoagulable states, including malignancy, can influence the formation of AMT; therefore, the accurate assessment of a hypercoagulable condition is necessary when we encounter patients with AMT.
Keywords: aortic thrombus, arterial embolism, hypercoagulable disorder, anticoagulant therapy
Introduction
Systemic arterial thromboembolism, which can result in cerebral infarction, myocardial infarction, visceral ischemia, or limb ischemia, is a serious and sometimes life-threatening problem. Thrombi, in most cases of arterial thromboembolism, are considered to have cardiac origins, specifically from complications of atrial fibrillation and myocardial infarction (1). However, there are a few cases without such sources, often called “cryptogenic embolism” (2,3). With recent developments in imaging modalities, especially transesophageal echocardiography (TEE), the aorta has been considered a potential embolic source. When the aorta is suspected as the embolic source, complex atherosclerotic lesions are usually involved (3,4); however, mural thrombus on a normal or minimally atherosclerotic aorta has also been regarded as an unusual but possible cause of arterial thromboembolism (2,5). Furthermore, some hypercoagulable disorders have frequently been detected in cases of aortic mural thrombus (AMT) (5-9).
As its clinical features are not well-known, we herein report two cases of AMT in patients with multiple hypercoagulable factors.
Case Reports
Case 1
A 50-year-old man who was a smoker with dyslipidemia presented to the emergency department due to sudden dysarthria and hand paralysis on the right side. There was asymmetry in the upper limb blood pressures: 135/87 mmHg on the left, unmeasurable on the right. He had sinus rhythm on his electrocardiogram, a normal left ventricular function, and no abnormal findings in the aortic or mitral valve on transthoracic echocardiography (TTE). Magnetic resonance imaging revealed acute cerebellar infarction, and contrast-enhanced computed tomography (CT) showed right subclavian artery occlusion and a low-density lesion on the thoracic aorta with minimal atherosclerosis (Fig. 1). We were unable to image the lesions detected by CT with TEE and TTE.
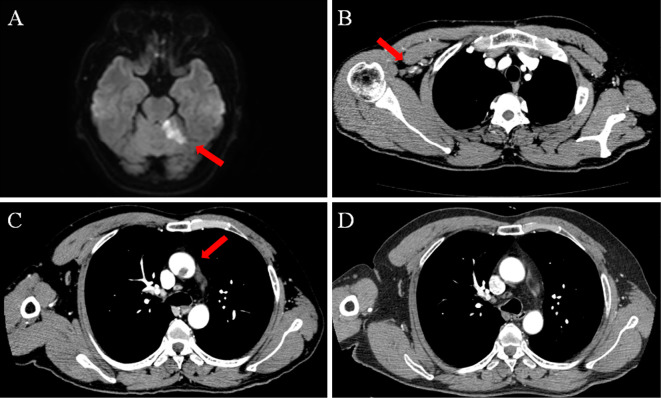
Figure 1.
Imaging findings of a 50-year-old man with sudden dysarthria and right-hand paralysis (Case 1). On admission, brain magnetic resonance imaging showed acute left cerebellar infarction on diffusion-weighted imaging (A, arrow). Contrast-enhanced computed tomography revealed an occluded right subclavian artery (B, arrow) and low-density lesion on the non-atherosclerotic aorta (C, arrow). Two weeks after anticoagulant therapy, the aortic lesion had disappeared (D).
We considered the lesion on the thoracic aorta to be a thrombus and started anticoagulant therapy (ACT) with intravenous unfractionated heparin. A laboratory examination on thrombophilia prior to ACT demonstrated low proteins S free antigen levels at <11% (normal range: 65-135%) and an activity of 21% (normal range: 60-150%). Furthermore, positive anti-cardiolipin antibody (IgG), a known antiphospholipid antibody, was detected at 20.9 units/mL (normal range: <10.0 units/mL), which was confirmed again 10 months later. Other coagulation parameters were within the normal ranges (Table 1, Case 1). Duplex ultrasonography showed no findings of deep venous thrombosis in the lower limbs.
Table 1.
Laboratory Data on Coagulation-fibrinolysis System.
| Variables | Reference | Case 1 | Case 2 | |||
|---|---|---|---|---|---|---|
| PT (%) | 70-130 | 93 | 68 | |||
| PT (INR) | 0.9-1.3 | 1.03 | 1.21 | |||
| APTT (s) | 25-37 | 36 | 25 | |||
| Platelet (×104/mm3) | 12.0-38.0 | 13.5 | 6.4 | |||
| D-dimer (μg/mL) | 0-1.0 | 0.6 | 10.1 | |||
| Antithrombin III (mg/dL) | 23-34 | 29.9 | 29.6 | |||
| Antithrombin III (%) | 80-130 | 105 | 104 | |||
| Protein S free antigen (%) | 65-135 | <11 | 69 | |||
| Protein S activity (%) | 60-150 | 21 | 71 | |||
| Protein C antigen (%) | 62-131 | 125 | 42 | |||
| Protein C activity (%) | 64-135 | N/A | 48 | |||
| Antiphospholipid antibody (unit/mL) | <10.0 | 20.9 | <8.0 | |||
| Lupus anticoagulant (normalized ratio) | <1.3 | 1.14 | 1.05 |
PT: prothrombin time, INR: international normalized ratio, APTT: activated partial thromboplastin time
Two weeks after the initiation of ACT and following careful observation, contrast-enhanced CT confirmed the disappearance of the lesion in the aortic arch without any further embolic events. The laterality of the blood pressure was no longer noted. Eight months later, a follow-up imaging study showed no further embolic findings, and he claimed no further symptoms following oral ACT with warfarin.
Case 2
A 70-year-old man with gastric cancer (stage III) was undergoing chemotherapy prior to cancer surgery. After one course of chemotherapy with cisplatin, contrast-enhanced CT performed for a response evaluation revealed a low-density lesion on the thoracic aorta (Fig. 2A), which was not seen prior to the chemotherapy. There were no findings or symptoms that implied systemic embolism. Upon testing for thrombophilia, it was observed that the patient had low protein C antigen levels at 42% (normal range: 62-131%) and an activity of 48% (normal range: 64-135%). Other coagulation parameters were within the normal ranges. (Table 1, Case 2). With additional TEE, TTE, and duplex ultrasonography of the lower limbs, we were unable to confirm the findings detected by CT in the thoracic aorta, abnormal observations on the aortic valve, on the mitral valve, and in the deep veins of the legs. We also considered the lesion to be a thrombus and started ACT with intravenous unfractionated heparin.
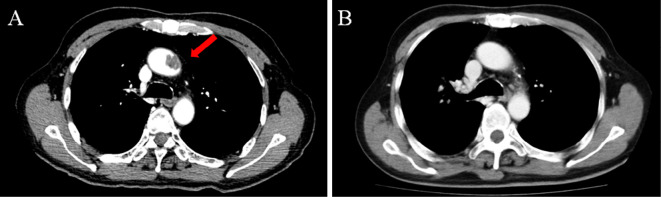
Figure 2.
Imaging findings of a 70-year-old man with gastric cancer receiving cisplatin-based chemotherapy (Case 2). A low-density lesion on the aorta that had not been detected on previous imaging tests was noted on contrast-enhanced computed tomography after a course of chemotherapy (A, arrow). The lesion disappeared two months after anticoagulant therapy (B).
On follow-up CT two months later, we noted the disappearance of the filling defect on the thoracic aorta without any embolic clinical event (Fig. 2B). As the planned courses of chemotherapy with cisplatin were discontinued, he underwent gastric surgery. After successful surgery, we started oral chemotherapy with tegafur/gimeracil/oteracil and decided to discontinue the administration of warfarin due to concerns of anticoagulant instability during the chemotherapy. On follow-up after eight months without ACT, no recurrence of the thrombus was found on imaging tests.
Discussion
Aortic plaque instability is an important etiology of systemic embolism from non-cardiac sources and is usually caused by thrombus or cholesterol crystals, both of which arise from severe aortic atherosclerotic plaques (3,4). There is likely to be some overlap, but embolism by the former etiology tends to occur in single small or medium arteries, while the latter tends to cause damage to multiple tissues and organs by the occlusion of small arteries.
AMT on a non-atherosclerotic wall, such as in our cases, is relatively rare. In a report of 10,671 consecutive autopsies, AMT in a normal aorta was found in 48 cases (0.45%); of them, 38 cases were found in the abdominal aorta, 1 in the thoracic aorta, and 9 in both the abdominal and thoracic aortas. Of the 48 cases, evidence of arterial embolism was found in 8 (17%) (2). In another report of 27,855 examinations of TEE for patients with recent arterial embolic events, mobile aortic thrombi in the aortic arch without obvious diffuse aortic atherosclerosis were detected in 23 cases (0.08%). Their mean age was younger (45 years) than that of patients with stroke from aortic atherosclerotic plaques or debris (>70 years) (5). Thus, AMT and thromboembolism are likely to form in relatively young patients. The former study showed the diagnostic value of TEE; however, in our cases, we were unable to acquire any images of the aortic thrombus by TEE. It should be noted that examinations with TEE are unable to provide useful evidence of the existence of AMT. Furthermore, the plasma D-dimer level, a useful marker for the diagnosis of venous thrombus, was not elevated in Case 1, suggesting that the plasma D-dimer level may provide little information on thrombus formation in the aorta.
Several reports have stated that AMT is frequently associated with some hypercoagulable states, as shown in Table 2 (5-9). In a systematic review of 200 AMT patients from 98 articles, hypercoagulable disorders were detected in 49 patients (25%) (8). In another report of prospectively collected data from a single center, routine work-up on hypercoagulable states, including malignant diseases, in patients with AMT showed a high prevalence of 60% (6). The same report showed that malignant diseases were found less frequently than hypercoagulable disorders. Vascular thrombus and malignancy have been called “Trousseau's syndrome" and has been well-known for some time (10). Recently, it has been reported that the risk of arterial thromboembolism in patients with cancer is quite high (11). Furthermore, with recent developments in chemotherapeutic agents for malignant diseases, cancer treatment has been discussed in relation to the occurrence of thrombosis (12). There have been several reports of AMT suspected to be related to chemotherapy, particularly in cisplatin-based chemotherapy, such as in Case 2 of this report (13,14). Regarding hypercoagulable states in our AMT cases, we confirmed protein S deficiency and positive anti-cardiolipin antibody in Case 1 and protein C deficiency, cancer, and a history of cisplatin-based chemotherapy in Case 2. Whether or not multiple thrombotic predispositions are related to the acceleration of AMT formation on a non-atherosclerotic aortic wall is unclear because we did not conduct specific tests to assess the coagulation activity or platelet function and no reports have described direct evidence of hypercoagulability in patients with AMT. We must cautiously assess the comorbidities associated with prothrombotic conditions, as various hypercoagulable states, including malignancy and chemotherapy, are often found in patients with AMT.
Table 2.
Reported Underlying Pathologies in Relation to Aortic Mural Thrombus.
| Hypercoagulable factor | Case 1 | Case 2 | ||
|---|---|---|---|---|
| Essential thrombocythemia | ||||
| Protein C deficiency | ● | |||
| Protein S deficiency | ● | |||
| Antithrombin III deficiency | ||||
| Antiphospholipid syndrome | ● | |||
| Heparin-induced thrombocytopenia | ||||
| Hyperhomocysteinemia | ||||
| Cancer | ● | |||
| Chemotherapy | ● | |||
| Iatrogenic (e.g., IABP) | ||||
| Aortic wall tumor | ||||
| Blunt aortic trauma | ||||
| Drug abuse |
IABP: intra-aortic balloon pump
ACT, surgical treatment, and endovascular treatment have been reported as effective strategies in the treatment for AMT; however, which strategy is the most appropriate remains unclear. As a primary treatment, ACT for patients without contraindications should be considered, as this approach has proven effective in eliminating aortic thrombi (9,15). Indeed, both patients in our report were treated with ACT alone and showed favorable outcomes. However, the appropriate duration of ACT is unclear. In Case 1, as two persistent hypercoagulable disorders were confirmed and the patient was able to tolerate ACT, we continued to treat him with anticoagulation therapy. In contrast, in Case 2, as two of three hypercoagulable factors were resolved (cancer and cisplatin-based chemotherapy) and the combination of oral chemotherapy and warfarin might have resulted in harmful drug interactions, inducing anticoagulant instability, we decided to discontinue ACT. As there is no consensus on how long ACT should be administered, we must consider the duration on a case-by-case basis.
Surgical treatment, including thrombectomy and segmental aortic resection, is a very invasive strategy but is supported by a systematic review, which showed fewer recurrent embolic events in the surgically treated group than in the ACT group (8). In that study, the ACT group showed a significantly higher persistence or recurrence rates and higher major limb amputation rates than the surgical group. In addition, 25% of patients initially treated with ACT eventually required subsequent aortic surgery because of the recurrence of embolism or persistence of the thrombus. However, as surgical procedures on the aorta are associated with a high perioperative morbidity and mortality, ACT tends to be favored as the primary treatment in many cases. In a report, the authors recommend surgery for patients not responding to ACT (with thrombus resolution) after two weeks of therapeutic anticoagulation (15). Recently, several cases of AMT treated successfully with endovascular treatment have been reported (16,17). Although reports of treatment with stent grafts for AMT have been limited, favorable results may be reported in the future.
Conclusion
AMT is a rare but important embolic source. In cases of mural thrombosis on a non-atherosclerotic aortic wall, we should consider underlying hypercoagulable disorders and concurrent malignancy and carefully select proper treatment depending on each situation.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Abbott WM, Maloney RD, McCabe CC, Lee CE, Wirthlin LS. Arterial embolism: a 44 year perspective. Am J Surg 143: 460-464, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Machleder HI, Takiff H, Lois JF, Holburt E. Aortic mural thrombus: An occult source of arterial thromboembolism. J Vasc Surg 4: 473-478, 1986. [PubMed] [Google Scholar]
- 3.Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical therapeutic update. J Am Coll Cardiol 35: 545-554, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cassella CR, Jagoda A. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med 334: 1216-1221, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Laperche T, Laurian C, Roudaut R, Steg PG. Mobile thromboses of the aortic arch without aortic debris. Circulation 96: 288-294, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Verma H, Meda N, Vora S, George RK, Tripathi RK. Contemporary management of symptomatic primary aortic mural thrombus. J Vasc Surg 60: 1524-1534, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Tsilimparis N, Hanack U, Pisimisis G, Yousefi S, Wintzer C, Ruckert RI. Thrombus in the non-aneurysmal, non-atherosclerotic descending thoracic aorta - an unusual source of arterial embolism. Eur J Vasc Endovasc Surg 41: 450-457, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Fayad ZY, Semaan E, Fahoum B, Briggs M, Tortolani A, D'Ayala M. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg 27: 282-290, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Bowdish ME, Weaver FA, Liebman HA, Rowe VL, Hood DB. Anticoagulation is an effective treatment for aortic mural thrombi. J Vasc Surg 36: 713-719, 2002. [PubMed] [Google Scholar]
- 10.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood 110: 1723-1729, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 70: 926-938, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falanga A, Marchetti M. Anticancer treatment and thrombosis. Thromb Res 129: 353-359, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol 29: 3466-3473, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y-G, Lee E, Kim I, et al. Cisplatin-based chemotherapy is a strong risk factor for thromboembolic events in small-cell lung cancer. Cancer Res Treat 47: 670-675, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choukroun EM, Labrousse LM, Madonna FP, Deville C. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg 16: 714-722, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fueglistaler P, Wolff T, Guerke L, Stierli P, Eugster T. Endovascular stent graft for symptomatic mobile thrombus of the thoracic aorta. J Vasc Surg 42: 781-783, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Luebke T, Aleksic M, Brunkwall J. Endovascular therapy of a symptomatic mobile thrombus of the thoracic aorta. Eur J Vasc Endovasc Surg 36: 550-552, 2008. [DOI] [PubMed] [Google Scholar]