Abstract
Objective
Depression, apathy, and gait instability are cardinal symptoms in patients with Parkinson’s disease (PD). Selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) are used for treating the psychiatric symptoms of PD. This is the first prospective randomized study to compare the efficacy of an SNRI (duloxetine) with SSRIs (paroxetine, escitalopram) in improving depressive symptoms and apathy (primary) and freezing of gait (FOG; secondary) in patients with PD.
Methods
In this prospective, multicenter, open-label, randomized study, Japanese PD patients with a Quick Inventory of Depressive Symptomatology-Japanese (QIDS-J) score ≥6 were randomly assigned to receive an SSRI (27 enrolled, 25 analyzed) or duloxetine (28 enrolled, 27 analyzed) and were assessed at 6 and 10 weeks.
Results
The mean change (SD) in the QIDS J [SSRI -2.4 (3.6), p=0.015; SNRI -2.3 (3.9), p=0.029] and FOG-Questionnaire [SSRI -2.9 (4.2), p=0.012; SNRI -3.4 (4.7), p=0.010] scores (from baseline) at 10 weeks was statistically significant, while the mean change in the Apathy Scale scores was not [SSRI -2.7 (5.4), p=0.054; SNRI -1.5 (3.7), p=0.109]. No significant differences were observed between the SSRI and SNRI groups. The treatments were well-tolerated; however, gastrointestinal events were more common with SSRIs. Two SNRI-treated patients reported an exacerbation of tremor.
Conclusion
SSRIs and SNRIs improve the depressive symptoms and FOG in PD patients with mild to severe depressive symptoms. However, their effectiveness in treating apathy remains to be elucidated.
Keywords: apathy, depression, gait instability, Parkinson’s disease, serotonin and noradrenaline reuptake inhibitors, serotonin uptake inhibitors
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by the marked deterioration of motor and non-motor functions. Gait instability, which most commonly occurs in patients with advanced PD, and depression and apathy are critical symptoms of PD that greatly reduce activities of daily living and affect the quality of life (QOL) (1,2). Depression, which is common in patients with PD (3), is the main psychiatric factor related to the reduced QOL in patients with PD.
Depressed mood, apathy, and anhedonia are core characteristics for a clinical diagnosis of depression (4) and are closely related to the low levels of norepinephrine, dopamine, and serotonin in patients with PD. Recent studies suggest that these psychiatric symptoms are related but distinct in patients with PD and that they may also be associated with increased motor symptom severity (5). Thus, the treatment of psychiatric symptoms-including depression-as well as motor dysfunction, is a key to improving the QOL of patients with PD.
To date, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) have been considered to be the standard treatment for depression in patients with PD (6). Nevertheless, several meta-analyses suggest that the current evidence to support the efficacy of TCAs and SSRIs in the treatment of psychiatric symptoms is inconclusive (7-9). Moreover, TCAs do not appear to have an effect on the motor symptoms of patients with PD (10) and the use of SSRIs is reported to be associated with greater apathy in comparison to other antidepressants (11).
Serotonin and norepinephrine reuptake inhibitors (SNRIs) are a newer class of antidepressant that may improve depressive symptoms in patients with PD (12-14). In addition, one SNRI, duloxetine, may be effective in the treatment of several motor symptoms, including freezing of gait (FOG) (15). FOG is sometimes intractable, despite treatment with dopaminergic agents including levodopa, and it is problematic for many patients with PD, due to its impact on activities of daily living and because it is associated with a higher risk of falls (16). Thus, FOG should be improved. Given these findings and the fact that SNRIs inhibit both serotonin and norepinephrine reuptake (17), we hypothesized that SNRIs may be more effective for treating apathy and FOG than SSRIs, and that any improvement in apathy and depression may have synergistic effects on FOG.
This randomized comparative study aimed to compare the efficacy of duloxetine with SSRIs (paroxetine or escitalopram) in reducing depressive symptoms and apathy in patients with PD. The secondary aims included the comparison of the effects of both types of drugs on FOG, the Clinical Global Impression of Severity (CGI-S) score, and safety.
Materials and Methods
Study design
The compaRativE study Between SSRI and SNRI treatment On depRessive patients with ParkinsoN’s disease (REBORN) was a prospective, multicenter, open-label, randomized, active-controlled study in Japanese patients with PD (UMIN-CTR; UMIN000015559). The study was conducted at 5 hospitals in Osaka, Japan, between January 2014 and June 2016. The study was approved by each site’s institutional ethical review board and was conducted in compliance with the Japanese Ethical Guideline for Clinical Studies and the Declaration of Helsinki. All of the patients who were enrolled in the study provided their written informed consent.
Study population
Male and female outpatients who met the following criteria were enrolled: ≥40 years of age, diagnosed with PD, and a Quick Inventory of Depressive Symptomatology Japanese version (QIDS-J) score of ≥6. The main exclusion criteria were a history of hypersensitivity to study drugs, treatment with a monoamine oxidase inhibitor within 2 weeks of study entry, pimozide treatment, QT prolongation, severe hepatic or renal dysfunction, uncontrolled narrow-angle glaucoma, suicidal ideation or attempt, psychiatric disease requiring medication within 1 year (excluding insomnia), and pregnant or breastfeeding women.
Treatment protocol
Patients were randomized (central registration with an electronic data capture system) to receive either an SSRI or an SNRI (specifically duloxetine) using a minimization procedure with the baseline QIDS-J, Unified Parkinson's Disease Rating Scale (UPDRS) II, and UPDRS III scores used as allocation factors. Patients randomized to the SSRI group were automatically allocated to receive paroxetine or escitalopram using the same central system and minimization method. The oral paroxetine doses were increased from 10 mg/day (2 weeks) to 20 mg/day (8 weeks) for tablets (Paxil® Tablets, GlaxoSmithKline, London, UK), and from 12.5 mg/day (2 weeks) to 25 mg/day (8 weeks) for controlled release (CR) tablets (Paxil® CR Tablets, GlaxoSmithKline), once daily after dinner. Oral escitalopram (Lexapro®, Mochida Pharmaceutical, Tokyo, Japan) was administered once daily at a dose of 10 mg/day (10 weeks). Patients randomized to the SNRI group received duloxetine. The oral duloxetine (Cymbalta®, Shionogi, Osaka, Japan) dose was increased from 20 mg/day (2 weeks), once daily to 40 mg/day (8 weeks), once daily. The treatment regimen for PD was not changed during the course of the study.
Outcome measures
Clinical symptoms were evaluated using the UPDRS II and III (for the severity of PD; baseline only), the QIDS-J (for depressive symptoms; baseline, 6 and 10 weeks), the Apathy Scale (baseline, 6 and 10 weeks), the FOG-Questionnaire (FOG-Q; baseline, 6 and 10 weeks), and the CGI-S (6 and 10 weeks; completed for depression and psychiatric symptoms).
The UPDRS Part II mainly assesses motor experience in daily living, and Part III assesses the motor function. In the QIDS-J (16 item), depressive symptoms are scored from 0 to 3 (total score 0 to 27); patients with a score of ≥6 are considered to have depression. In the Apathy Scale (14 items), patients (or caregivers) rate apathy symptoms from 0 to 3 (total score 0 to 42); scores of ≥14 indicate apathy (18). In the FOG-Q (6 items), patients rate each item from 0, which indicates the absence of a symptom, to 4, which indicates the highest degree of severity (total score 0 to 24) (19). In the CGI-S (7 point scale), patients are rated from 1 (“normal, not at all ill”) to 7 (“among the most extremely ill patients”) (20).
The primary endpoints were the change in the QIDS-J and Apathy Scale scores from baseline to 10 weeks. The secondary endpoints were the change in FOG-Q from baseline to 10 weeks and the proportion of patients with CGI-S scores of 1 (normal) or 2 (“borderline mentally ill”) at 6 and 10 weeks. Safety was assessed by the incidence and type of adverse events (AEs) using the Medical Dictionary for Regulatory Activities (version 19.0).
Statistical analysis
The final sample size was determined based on an interim analysis (target of 20 patients per group), which indicated no differences between the treatment groups.
The safety analysis included all randomized patients who received a study drug [full analysis set (FAS)]. The efficacy analysis included patients who had data available at baseline and at 6 or 10 weeks. The efficacy data are presented as the mean value and 95% confidence interval (CI). The efficacy scores at baseline and each time point (6 weeks, 10 weeks) were compared using a paired t-test.
Changes in efficacy scores within and between groups were compared using an unpaired t test (Welch’s test). The treatment effects were tested with a 2-sided significance level of 0.05.
All statistical analyses were performed using the SAS software program (version 9.3; SAS Institute, Cary, USA).
Results
Patient disposition
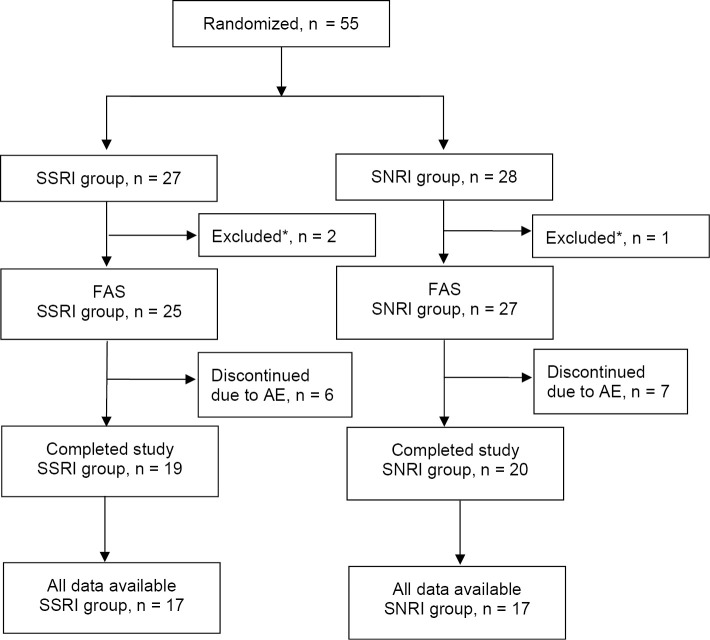
Fifty-five patients were enrolled and randomized to the SSRI (n=27) or SNRI (n=28) groups; 52 patients were included in the FAS (SSRI group, n=25; SNRI group, n=27) (Fig. 1). Three patients (SSRI group, n=2; SNRI group, n=1) had no data after starting the study treatment and were excluded from the FAS. Six patients in the SSRI group and seven patients in the SNRI group discontinued treatment due to an AE.
Figure 1.
The study flow. Fifty-five patients with PD and depression who were randomly assigned to the SSRI (n=27) and SNRI (n=28) groups. *Two patients in the SSRI group and one patient in the SNRI group had no data after starting the study treatment and were excluded from the FAS. AE: adverse event, FAS: full analysis set, SNRI: serotonin norepinephrine reuptake inhibitor, SSRI: selective serotonin reuptake inhibitor
Baseline characteristics
The baseline characteristics of the patients in each treatment group were similar (Table). Approximately 52% of patients were male, most patients were >60 years of age, and the mean duration of PD was approximately 4.5 years. Most patients were treated with levodopa; other PD medications included zonisamide, pramipexole, and ropinirole. Approximately 54% of the patients had mild depressive symptoms (QIDS-J ≥6), 27% had moderate symptoms, and 19% had severe symptoms; none had very severe depressive symptoms. Most patients displayed signs of apathy (Apathy Scale score ≥10). The mean baseline FOG-Q scores of the SSRI and SNRI groups were 9.5 and 9.8, respectively.
Table.
The Baseline Patient Characteristics.
| Variable | SNRI n=27 |
SSRI n=25 |
||
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 14 (51.9) | 13 (52.0) | ||
| Female | 13 (48.1) | 12 (48.0) | ||
| Age (y) | ||||
| Mean (SD) | 70.3 (8.4) | 72.4 (8.4) | ||
| Median (range) | 70.0 (49-82) | 72.0 (57-86) | ||
| Age category, n (%) | ||||
| ≥ 40 years,<50 years | 1 (3.7) | 0 | ||
| ≥ 50 years,<60 years | 1 (3.7) | 1 (4.0) | ||
| ≥ 60 years | 25 (92.6) | 24 (96.0) | ||
| Duration of Parkinson’s disease (y) | ||||
| Mean (SD) | 4.6 (4.7) | 4.3 (3.7) | ||
| Median (range) | 3.0 (0-20) | 3.0 (1-14) | ||
| Treatment for Parkinson’s disease, n (%)a | 27 (100) | 25 (100) | ||
| Levodopa | 26 (96.3) | 20 (80.0) | ||
| Zonisamide | 8 (29.6) | 6 (24.0) | ||
| Pramipexole | 5 (18.5) | 4 (16.0) | ||
| Ropinirole | 4 (14.8) | 6 (24.0) | ||
| UPDRS II | ||||
| Mean (SD) | 13.5 (10.9) | 11.7 (5.3) | ||
| Median (range) | 12.0 (4-62) | 11.0 (3-24) | ||
| UPDRS III | ||||
| Mean (SD) | 26.0 (11.6) | 28.0 (14.4) | ||
| Median (range) | 22.0 (11-55) | 26.0 (2-68) | ||
| QIDS-J | ||||
| Mean (SD) | 11.3 (4.0) | 10.9 (4.1) | ||
| Median (range) | 11.0 (6-20) | 10.0 (6-20) | ||
| QIDS-J severity category, n (%) | ||||
| Mild (≥ 6, ≤ 10) | 13 (48.1) | 15 (60.0) | ||
| Moderate (≥ 11, ≤ 15) | 9 (33.3) | 5 (20.0) | ||
| Severe (≥ 16, ≤ 20) | 5 (18.5) | 5 (20.0) | ||
| Very severe (≥ 16, ≤ 20) | 0 | 0 | ||
| Apathy Scale score | ||||
| Mean (SD) | 19.3 (5.8) | 18.8 (7.9) | ||
| Median (range) | 21.0 (3-28) | 19.0 (6-41) | ||
| Apathy Scale score category, n (%) | ||||
| <10 | 2 (7.4) | 3 (12.0) | ||
| ≥ 10,<20 | 8 (29.6) | 10 (40.0) | ||
| ≥ 20,<30 | 17 (63.0) | 10 (40.0) | ||
| ≥ 30,<40 | 0 | 1 (4.0) | ||
| ≥ 40 | 0 | 1 (4.0) | ||
| FOG-Q score | ||||
| Mean (SD) | 9.8 (5.8) | 9.5 (4.6) | ||
| Median (range) | 10.0 (0-20) | 9.0 (0-20) |
FOG-Q: Freezing of Gait Questionnaire, QIDS-J: Quick Inventory of Depressive Symptomatology Japanese version, SD: standard deviation, SNRI: serotonin norepinephrine reuptake inhibitor, SSRI: selective serotonin reuptake inhibitor, UPDRS: Unified Parkinson’s Disease Rating Scale
aPatients could receive more than 1 treatment for Parkinson’s disease.
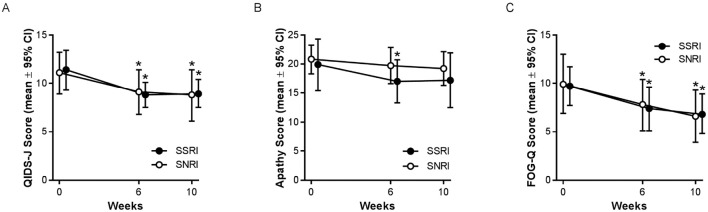
Efficacy
Significant improvements in depressive symptoms from baseline were observed in both groups (Fig. 2A). The mean change [standard deviation (SD)] in the QIDS-J scores (from baseline) at 6 and 10 weeks was statistically significant in both the SSRI [6 weeks, -2.7 (4.6), p=0.024; 10 weeks, -2.4 (3.6), p=0.015] and SNRI [6 weeks, -1.9 (2.8), p=0.012; 10 weeks, -2.3 (3.9), p=0.029] groups. However, the mean (95% CI) change in the QIDS-J scores (from baseline) at 10 weeks did not differ between the groups to a statistically significant extent [SSRI, -2.4 (-4.3, -0.5); SNRI, -2.3 (-4.3, -0.3); p=0.928].
Figure 2.
(a) SSRIs and the SNRI duloxetine were equally effective in reducing depressive symptoms in patients with PD. The mean (95% CI) QIDS-J scores at baseline, 6 weeks, and 10 weeks. n=17 in each group. (b) SSRIs and the SNRI duloxetine were not effective in reducing apathy in patients with PD. The mean (95% CI) Apathy Scale scores at baseline, 6 weeks, and 10 weeks. n=17 in each group. (c) SSRIs and the SNRI duloxetine were equally effective in reducing gait instability in patients with PD. The mean (95% CI) FOG-Q scores at baseline, 6 weeks, and 10 weeks. n=17 in each group. *p<0.05 in comparison to baseline. CI: confidence interval, FOG-Q: Freezing of Gait Questionnaire, PD: Parkinson’s disease, QIDS-J: Quick Inventory of Depressive Symptomatology Japanese version, SNRI: serotonin norepinephrine reuptake inhibitor, SSRI: selective serotonin reuptake inhibitor
Although the symptoms of apathy tended to be reduced from baseline in both groups, most changes did not reach statistical significance (Fig. 2B). For the SSRI group, the mean (SD) change in the Apathy Scale score (from baseline) was statistically significant at 6 weeks [-3.1 (5.2), p=0.023], but not at 10 weeks [-2.7 (5.4), p=0.054]. In the SNRI group, the mean (SD) change in the Apathy Scale scores (from baseline) was not statistically significant at either time point [6 weeks, -1.0 (3.5), p=0.244; 10 weeks, -1.5 (3.7), p=0.109]. Although the reduction in the Apathy Scale score was numerically greater in the SSRI group, the mean (95% CI) change (from baseline) at 10 weeks was not statistically significant [SSRI, -2.7 (-5.5, 0.0); SNRI, -1.5 (-3.4, 0.4); p=0.463].
Significant reductions in the frequency and severity of FOG from baseline were observed in both groups (Fig. 2C). The mean (SD) changes (from baseline) in the FOG-Q scores at 6 and 10 weeks were statistically significant in both the SSRI [6 weeks, -2.2 (3.6), p=0.018; 10 weeks, -2.9 (4.2), p=0.012] and SNRI [6 weeks, -2.1 (4.2), p=0.047; 10 weeks, -3.4 (4.7), p=0.010] groups. The mean (95% CI) change in FOG-Q score (from baseline) at 10 weeks did not differ between the groups to a statistically significant extent [SSRI, -2.9 (-5.0, -0.7); SNRI, -3.4 (-5.8, -0.9); p=0.761].
The CGI-S suggested a general improvement in disease severity in both groups. At 6 and 10 weeks, 8 of 18 patients (44.4%) and 11 of 17 patients (64.7%), respectively, had CGI-S scores of 1 or 2 in both the SSRI and SNRI groups.
Safety
Both treatments were well tolerated. The percentage of patients with AEs in the SSRI and SNRI groups was 36.0% (9 of 25) and 37.0% (10 of 27), respectively. In the SSRI group, 6 of 25 (24.0%) patients discontinued because of AEs (nausea, vomiting, headache, and dizziness in 1 patient, nausea and vomiting in 1 patient, and nausea, somnolence, diarrhea, and tremor in 1 patient each; all AEs, with the exception of somnolence and diarrhea, were considered to be probably or clearly related to the study drug). In the SNRI group, 7 of 27 (25.9%) patients discontinued because of AEs [somnolence and malaise in 1 patient, hearing hypersensitivity and tremor in 1 patient, and tremor, rash, dyskinesia, urinary retention, and parkinsonism in 1 patient each; among these, tremor (by itself), urinary retention, somnolence, and malaise were considered to be probably or clearly related to the study drug].
In the SSRI group, the most frequent AEs were nausea (5 patients), vomiting (2 patients), and somnolence (2 patients); other reported AEs (1 patient each; some patients had ≥1 event) included headache, dizziness, diarrhea, abdominal discomfort, and tremor. In the SNRI group, tremor (2 patients) was most frequently reported; other reported AEs (1 patient each; some patients had ≥1 event) included headache, dyskinesia, parkinsonism, shoulder joint arthritis, rash, hearing hypersensitivity, urticaria, vomiting, urinary retention, somnolence, general malaise, and memory disturbance. Notably, no falls were reported as AEs during the study.
No deaths were reported during the study. One patient (SNRI group) reported a serious AE (dyskinesia) that led to discontinuation; this AE was considered to have resulted from an overdose of levodopa, which was used concomitantly during the study period.
Discussion
In spite of its open-label, non-placebo-controlled design, this study is the first multicenter, randomized prospective study to demonstrate that the efficacy of the SNRI duloxetine in reducing the non-motor symptoms of depression and apathy, and the motor symptoms of gait instability in patients with PD does not differ from that of SSRIs to a statistically significant extent. Similar and significant improvements (from baseline) in depressive symptoms (assessed by QIDS-J scores) and the frequency and severity of FOG were observed at 10 weeks in both treatment groups. Although there was a tendency for reduced apathy (as assessed by the Apathy Scale score) in both the SNRI and SSRI groups, the change (from baseline) at 10 weeks was not statistically significant in either group. Because of the limited sample size, we could not find any significant difference between duloxetine (an SNRI) and SSRIs regarding the improvement of the apathy score. Both treatments were well-tolerated, with no unexpected differences in the type or frequency of AEs that were reported. These findings suggest that SNRIs and SSRIs can be considered for the treatment of non-motor symptoms, including depression and gait instability, in patients with PD.
Our finding, that duloxetine and SSRIs are effective in reducing depressive symptoms in patients with PD, is consistent with previous studies comparing SNRIs and SSRIs (14,21). In a randomized study by Richard et al., 12-week treatment with an SNRI (venlafaxine) or an SSRI (paroxetine) significantly reduced Hamilton Rating Scale for Depression (HAM-D) scores relative to a placebo in patients with PD and clinically diagnosed depression-with no significant difference between the two groups (14). The mean HAM-D score in the venlafaxine and paroxetine groups decreased by 11 and 13 points, from baseline scores of 21.2 and 22.2, respectively, indicating that patients’ depressive symptoms improved from moderate (HAM-D scores of 17 to 23) to mild (HAM-D scores of 8 to 16) (22). Although most patients in the REBORN study had milder depression (QIDS-J score ≥6; Table), depressive symptoms were significantly improved by both duloxetine and the SSRIs. This is consistent with the results of a small randomized study that reported the proportion of patients whose depression improved with treatment with an SNRI (venlafaxine) or SSRI (sertraline) did not differ to a statistically significant extent (21).
Several meta-analyses have examined the efficacy of antidepressants in the treatment of depression in patients with PD (7-9,23,24).Although two meta-analyses concluded that SSRIs were effective (23,24), others indicated that there was insufficient evidence to confirm an effect (8,9). Two network meta-analyses have considered SNRIs separately (7,24), with both including data from the same studies [the study by Richard et al. (14) and a study of atomoxetine (25), a norepinephrine reuptake inhibitor that may also inhibit serotonin reuptake (26)]. Similar to SSRIs, one of the analyses found SNRIs to be effective (although less effective than SSRIs) (24), and one of the analyses concluded that there was insufficient evidence to confirm an effect (7). Although methodological differences might have contributed to these various conclusions, it is obvious that more evidence is needed to confirm the relative efficacy of SSRIs and SNRIs in the treatment of depression in patients with PD.
Cummings (27) advocated a “behavioral and psychological symptoms of dementia” model, whereby apathy, depression, and movement disorders are associated with frontal lobe dysfunction. On the basis of this theory, we hypothesized that SNRIs might be more effective for treating these symptoms than SSRIs because of the upregulation of norepinephrine at the frontal lobe (17). However, the effects of SSRIs and SNRIs in improving these symptoms did not differ to a statistically significant extent. Moreover, the apathy score was only significantly different (from baseline) in the SSRI group at 6 weeks, while the FOG significantly improved (from baseline) in both groups. This lack of effect on apathy is in accordance with one study showing that there were no significant differences between atomoxetine (a norepinephrine reuptake inhibitor) and a placebo in patients with PD (25).
Monoamine oxidase B (MAO-B) inhibitors (selegiline, rasagiline), which upregulate monoamines including dopamine, serotonin, and norepinephrine, have been shown to improve apathy (11) and gait instability (28) in patients with PD. Regarding gait instability, this effect is thought to be promising because of the noradrenergic effects of MAO-B inhibitors on the frontal lobe and pons, rather than their effects on other monoamines (16). Moreover, Morgante et al. reported the case of a 58-year-old man who was diagnosed with primary progressive freezing gait without other parkinsonian symptoms and who showed sustained a improvement of FOG with duloxetine, but not with levodopa or an SSRI (escitalopram) (15). This case suggests that the benefit of duloxetine on FOG may result from the enhancement of both noradrenergic and serotonergic transmission, especially in the frontal lobe. However, the pathological basis of FOG is complex and may be influenced by the patient’s current state (i.e., on vs. off state), anti-PD medication, and other factors, including the level of depression and anxiety (16). Future studies of the effect of SSRIs or SNRIs on FOG should ideally include objective measures of gait instability as well as actively monitor any effects on the risk of falls.
The type and frequency of AEs and the rate of study discontinuation were similar in both groups, suggesting that SSRIs and SNRIs were equally well-tolerated. Although the frequency of AEs in both groups was lower than in the study of Richard et al. (85% to 86%) (14), many events led to study discontinuation. However, the rate of discontinuation due to AEs was similar in both groups (SSRI group, 24%; SNRI group, 26%). Gastrointestinal AEs, which are associated with SSRI treatment (29), were among the most common events-especially in the SSRI group-but most cases resolved within a few weeks (30). In our study, several patients in both treatment groups experienced tremor, dyskinesia, or parkinsonism, but there was no clear relationship between SSRIs or SNRIs and the worsening of their motor symptoms. The incidence of motor AEs in this study (9.6% of all patients) was similar to or less than the incidence of tremor and dyskinesia in the study by Richard et al., including the incidence in the placebo group (7.7% for both tremor and dyskinesia) (14).
This randomized study is the first to compare the effectiveness of SSRIs and SNRIs in treating depression, apathy, and FOG in patients with PD. Because placebo effects are common in studies of depression and PD (7,14,25), we included an active comparator (SSRIs) with demonstrated effectiveness versus placebo (14,24), rather than a placebo group. However, we could not observe a difference in effect between the groups; this might have been due to a true lack of difference or because the statistical power of the study was not sufficient to demonstrate significant inter-group differences. In addition, most patients had mild to moderate depressive symptoms, which might have contributed to the inability to demonstrate differences between the groups. We acknowledge that the QIDS-J, although fully validated and convenient, contains some items that overlap with physical symptoms of PD (e.g., sleep, weight loss), and that improvements in the QIDS-J scores might have resulted from the better management of PD symptoms. However, at the time of their enrolment, the patients were on a stable anti-PD medication regimen, which did not change during the study; thus, we believe that the observed effects on depressive symptoms can be attributed to the addition of an SSRI or SNRI. Finally, patients in the SNRI group were only prescribed duloxetine, whereas patients in the SSRI group were randomly prescribed escitalopram, paroxetine, or paroxetine CR. Thus, because of the limited sample size, we could not investigate differences in the treatment effects of the individual SSRIs.
In conclusion, our observation that both SSRIs and SNRIs significantly reduced the symptoms of depression and FOG to a similar extent suggests that these antidepressants may improve motor and non-motor symptoms that are often coincident in patients with PD. Furthermore, SNRIs may represent an alternative treatment for patients who do not respond to SSRIs or who experience unwanted side effects with SSRIs. However, the effectiveness of SSRIs and SNRIs in the treatment of apathy in patients with PD remains to be elucidated.
Author's disclosure of potential Conflicts of Interest (COI).
Makio Takahashi: Research funding, Shionogi. Hayato Tabu: Research funding, Shionogi. Akihiko Ozaki: Research funding, Shionogi. Toshiaki Hamano: Research funding, Shionogi. Takao Takeshima: Honoraria, Eisai and Pfizer; Research funding, Shionogi.
Financial Support
The data management, data analysis, publication support, and insurance of this study were supported by funding from Shionogi & Co., Ltd. The company had no role in determining how the study was conducted.
Acknowledgments
The authors would like to thank all study participants.
References
- 1.Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Dibble LE. Which measures of physical function and motor impairment best predict quality of life in Parkinson's disease? Parkinsonism Relat Disord 17: 693-697, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry 69: 308-312, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reijnders JSAM, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 23: 183-189, 2008. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association.. Diagnostic and Statistical Manual of Mental Disorders (5th Ed). American Psychiatric Association, Washington, DC, 2013. [Google Scholar]
- 5.Nagayama H, Maeda T, Uchiyama T, et al. . Anhedonia and its correlation with clinical aspects in Parkinson's disease. J Neurol Sci 372: 403-407, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Kelberman MA, Vazey EM. New pharmacological approaches to treating non-motor symptoms of Parkinson's disease. Curr Pharmacol Rep 2: 253-261, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Dong J, Wang L, Su Y, Yan P, Sun S. Comparative efficacy and acceptability of antidepressants in Parkinson's disease: a network meta-analysis. PLoS ONE 8: e76651, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha FL, Murad MG, Stumpf BP, Hara C, Fuzikawa C. Antidepressants for depression in Parkinson's disease: systematic review and meta-analysis. J Psychopharmacol 27: 417-423, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Troeung L, Egan SJ, Gasson N. A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson's disease. PLoS ONE 8: e79510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen J, Aabro E, Gulmann N, Hjelmsted A, Pedersen HE. Anti-depressive treatment in Parkinson's disease. A controlled trial of the effect of nortriptyline in patients with Parkinson's disease treated with L-DOPA. Acta Neurol Scand 62: 210-219, 1980. [DOI] [PubMed] [Google Scholar]
- 11.Zahodne LB, Bernal-Pacheco O, Bowers D, et al. . Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson’s disease? J Neuropsychiatry Clin Neurosci 24: 326-330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonuccelli U, Meco G, Fabbrini G, et al. . A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson's disease. Expert Opin Pharmacother 13: 2269-2280, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Nishijima H, Ueno T, Kon T, et al. . Effects of duloxetine on motor and mood symptoms in Parkinson's disease: an open-label clinical experience. J Neurol Sci 375: 186-189, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Richard IH, McDermott MP, Kurlan R, et al. . A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 78: 1229-1236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgante F, Fasano A. Improvement with duloxetine in primary progressive freezing gait. Neurology 75: 2130-2132, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Giladi N. Medical treatment of freezing of gait. Mov Disord 23: S482-S488, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Kihara T, Ikeda M. Effects of duloxetine, a new serotonin and norepinephrine uptake inhibitor, on extracellular monoamine levels in rat frontal cortex. J Pharmacol Exp Ther 272: 177-183, 1995. [PubMed] [Google Scholar]
- 18.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 4: 134-139, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Giladi N, Tal J, Azulay T, et al. . Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord 24: 655-661, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 4: 28-37, 2007. [PMC free article] [PubMed] [Google Scholar]
- 21.Akca A, Mehmet AK, Sutcugil L, Ozsahin A, Kutukcu Y. Comparison of sertraline and venlafaxine treatments for depression in Parkinson's disease. Noropsikiyatri Arsivi (Archives of Neuropsychiatry) 48: 201-206, 2011. [Google Scholar]
- 22.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 150: 384-388, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 21: 833-842, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Zhuo C, Xue R, Luo L, et al. . Efficacy of antidepressive medication for depression in Parkinson disease: a network meta-analysis. Medicine 96: e6698, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weintraub D, Mavandadi S, Mamikonyan E, et al. . Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 75: 448-455, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding YS, Naganawa M, Gallezot JD, et al. . Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: implications on treatment of depression and ADHD. NeuroImage 86: 164-171, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol 50: 873-880, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Giladi N, McDermott MP, Fahn S, et al. . Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology 56: 1712-1721, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry 3: 22-27, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moret C, Isaac M, Briley M. Review: problems associated with long-term treatment with selective serotonin reuptake inhibitors. J Psychopharmacol 23: 967-974, 2008. [DOI] [PubMed] [Google Scholar]