Abstract
Background/purpose
The aim of this study was to explore the presence and variability of oral Candida in adolescents before and during treatment with fixed orthodontic appliances.
Materials and methods
A total of 50 patients aged 10–18 years old were randomly selected for this study. Microorganism samples were obtained prior to and after orthodontic treatment and identified by culture methods. Molecular biology techniques were used to investigate the samples further and the effect of the orthodontic appliance on oral pathogenic yeasts was studied longitudinally.
Results
The percentage of patients with candidiasis and the total number of colony-forming units significantly increased 2 months after orthodontic treatment. Changes in the type of oral candidiasis prior to and after treatment were significant.
Conclusion
Fixed orthodontic appliances can influence the growth of oral pathogenic yeasts among adolescents.
Keywords: adolescents, fixed orthodontic appliances, oral Candida
Introduction
Candida is a pathogenic fungus. The pathogenicity of Candida isolated from human mouths can be classified into eight strains: Candida albicans, Candida tropicalis, Candida glabrata, Candida parapsilosis, Candida krusei, Candida kefyr, Candida stellatoidea, and Candida dubliniensis. C. albicans accounts for 45–75% of the total incidence of candidiasis, whereas C. tropicalis and C. parapsilosis account for about 7% of all cases.1, 2, 3, 4, 5 Many internal and external factors, such as systemic disease and impaired immune function, can result in environmental changes in the oral cavity. These changes affect the kinds of microorganisms found in the oral cavity as well as their metabolic and pathogenic activities.6 Candida is often detected in the oral cavity of patients with denture stomatitis, especially middle-aged and elderly people with false teeth.7
Fixed orthodontic appliances (FOAs) are artificial devices in the mouth that can greatly affect oral health and allow plaque and food scraps to accumulate. FOAs can also bring about an increased number of microorganisms and amalgamated infections in the mouth,8 including caries of the teeth, lips, buccal surfaces, and tongue. FOAs can also cause an increase in the number of Gram-positive bacteria in the mouth.9, 10 Increased levels of dental plaque are related to the development of gingivitis.11 Patients with gingivitis are prone to periodontal disease12 and loss of periodontal support.13
Few studies have been published about fungal colonization in patients with FOAs. Among 60 patients treated with an FOA, oral Candida flora were found in 15 (25%) patients, 14 of whom were aged between 16 years and 18 years. Removable orthodontic appliances can temporarily affect Candida colonization.14, 15, 16 No study on the type, number, and pathogenic changes in oral Candida caused by orthodontic appliances has yet been published. The aim of this study was to explore changes in oral Candida strains among healthy adolescents prior to and after treatment with FOAs.
Materials and methods
Patients and samples
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Lanzhou University, Lanzhou, China. Written informed consent was obtained from all participants. Fifty patients with FOAs were randomly selected. Of these patients, 23 were male adolescents and 27 were female adolescents. The average age was 13.6 years. No patient had a systemic, oral mucosal, or periodontal disease. The administration of antibiotic drugs to patients was stopped 2 weeks prior to sampling. The participants carefully brushed their teeth after breakfast and samples were taken 2 hours after food consumption. Microbiological samples were obtained via the gargle method 1 month, 2 months, 3 months, and 6 months prior to and after installation of the FOAs. The patients were required to gargle with 10 mL of sterile Phosphate Buffered Saline (PBS) for 1 minute. The resulting gargle was sent to the laboratory within 2 hours.
Identification and culture
Microbiological samples were centrifuged and 50 μL of the supernatant were cultured in CHROMagar Candida identification Petri dishes (CHROMagar, Paris, France) at 37°C for 36–48 hours.
Different Candida strains were identified based on the color of the colonies. C. albicans exhibits green coloration, smooth Candida is purple, tropical Candida is blue, C. krusei is pink, and other unidentified fungi in the culture medium are white. Colonies that did not grow within 7 days were not included in the count.
Polymerase chain reaction identification
DNA was extracted according to the instructions provided in the kit used (Tiangen Biotech, Beijing, China). A specific sequence of a wild strain of phage M13 microsatellites was used as a single primer for polymerase chain reaction (PCR) amplification. The primer sequence was 5′-GAGGGTGGCGGTTCT-3′.16 For the PCR reaction, 1 μL of DNA, 2 μL of the primer, and 10 μL of 2 × PCR Master Mix (Tiangen Biotech, Beijing, China) were added to 20 μL of double-distilled H2O. The reaction conditions were as follows: 95°C denaturation for 1 minute, 95°C denaturation for 30 seconds, 60°C annealing for 30 seconds, extension at 68°C for 90 seconds, 25 cycles, and finally extension at 68°C for 10 minutes. PCR products were detected using 1.5% agarose gel electrophoresis and scanned by a gel imaging camera. International standards for C. albicans ATCC90028 were used as positive controls and sterilized PBS buffer was used as a negative control.
Results
Identification and culture results
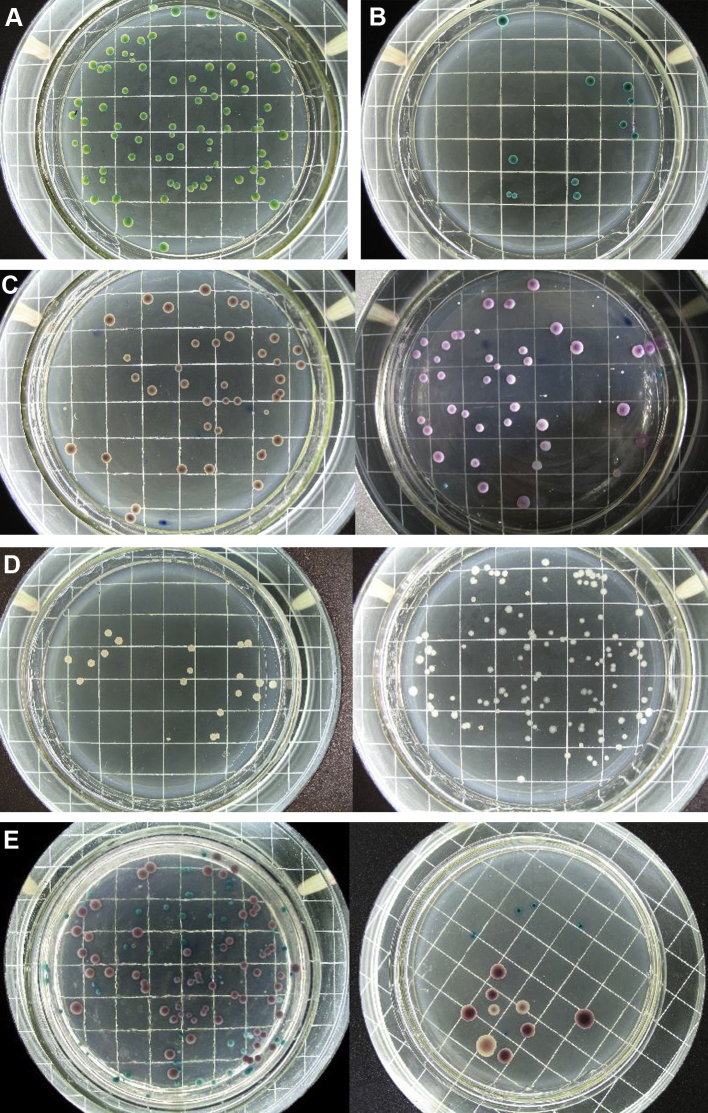
As shown in Fig. 1, several patients were carriers of pure strains of bacteria, which appeared as monochromatic colonies. The strains could be identified by colony color. Other patients were carriers of mixed bacteria, where the colonies had two or three different colors.
Figure 1.
Results obtained after 48 hours of culture of clinical samples. Different kinds of Candida appear as different colors. Scale 10 mm/square. (A) Candida albicans (green, light green). (B) Candida troplicalis (green, dark green). (C) Candida glabrata (purple; left, lamplight; right, natural light). (D) Unknown yeasts (white). (E) Fixed yeasts: C. glabrata (purple), C. albicans (green), Candida krusei (pink).
PCR results
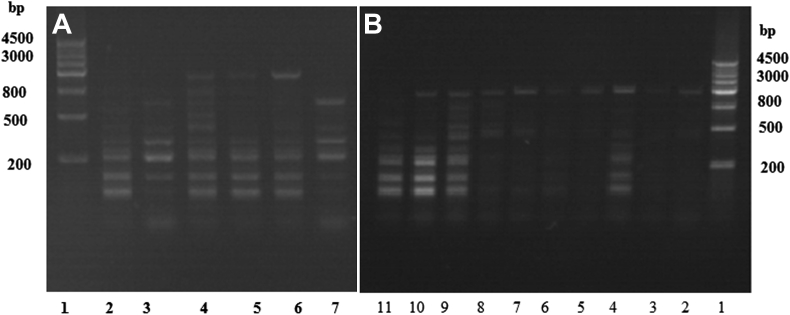
The PCR results showed that green colonies have bands similar to those of C. albicans ATCC90028, thus these colonies may be identified as C. albicans. This result further confirmed the accuracy of the CHROMagar Candida color culture. C. albicans was also detected by PCR using the international standard for C. dubliniensis ATCC6258 as the control. The results confirmed that the green colonies in the clinical samples were C. dubliniensis (Fig. 2).
Figure 2.
Identification of Candida albicans by polymerase chain reaction. Compared with ATCC90028 C. albicans, the clinical culture samples which appeared green are all C. albicans. At the same time, ATCC6258 Candida dubliniensis, which has a similar gene type to C. albicans had also been detected by polymerase chain reaction, the results confirmed that there are no C. dubliniensis in the clinical samples. (A) 1 = Marker; 2 = ATCC90028 C. albicans; 3, 7 = ATCC6258 C. dubliniensis; and 4–6 = clinical samples. (B) 1 = Marker; 2–4 = three colonies of patient A; 5–7 = three colonies of patient B; 8–9 = two colonies of patient C; 10 = one colony of patient D; and 11 = ATCC90028 C. albicans.
Rate of carrying bacteria and strain analyses
Table 1, Table 2 show that the number of total and mixed carriers was higher 2–3 months after treatment than prior to treatment. After 6 months these changes were comparable with the levels prior to treatment, suggesting that the most significant changes in the number and type of Candida strains in the mouth may be found 2–3 months after fitting an FOA.
Table 1.
Incidence of yeasts in different periods in orthodontic treatment.
| Before treatment | Months after treatment |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | ||
| Pure carrier | 7 (14) | 7 (14) | 9 (18) | 8 (16) | 7 (14) |
| Mixed carrier | 0 (0) | 2 (4) | 6 (12)* | 5 (10)** | 3 (6) |
| Total carrier | 7 (14) | 9 (18) | 15 (30) | 13 (26) | 10 (20) |
Data are presented as n (%); α = 0.05.
*χ2 = 6.3830, P < 0.05.
**χ2 = 5.2632, P < 0.05.
Table 2.
Occurrence of various kinds of yeasts in patients at different treatment periods.
| Before treatment | Months after treatment |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | ||
| Candida albicans | 6 (85.7) | 4 (44.4) | 5 (33.3) | 4 (30.8) | 6 (60) |
| Candida parapsilosis | 0 (0) | 2 (22.2) | 1 (6.67) | 2 (15.4) | 0 (0) |
| Candida tropicalis | 0 (0) | 0 (0) | 1 (6.67) | 1 (7.69) | 0 (0) |
| Candida krusei | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| X | 1 (14.3) | 1 (11.1) | 2 (13.3) | 1 (7.69) | 1 (10) |
| C. albicans + C. parapsilosis | 0 (0) | 0 (0) | 3 (20) | 1 (7.69) | 0 (0) |
| C. albicans + C. tropicalis | 0 (0) | 0 (0) | 0 (0) | 1 (7.69) | 0 (0) |
| C. albicans + C. krusei | 0 (0) | 0 (0) | 1 (6.67) | 0 (0) | 0 (0) |
| C. albicans + X | 0 (0) | 1 (11.1) | 1 (6.67) | 1 (7.69) | 1 (10) |
| C. parapsilosis + C. tropicalis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10) |
| C. albicans + C. parapsilosis + C. krusei | 0 (0) | 1 (11.1) | 0 (0) | 1 (7.69) | 1 (10) |
| C. albicans + C. parapsilosis + X | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| C. albicans + C. krusei + X | 0 (0) | 0 (0) | 1 (6.67) | 1 (7.69) | 0 (0) |
| Total | 7 (100) | 9 (100) | 15 (100) | 13 (10) | 10 (100) |
Data are presented as n (%).
X = unidentified by CHROM agar medium; + = two or three colonies.
Total number of colonies
The results were analyzed using two-factor variance analysis, which revealed that the total number of colonies in different Petri dishes were not significantly different (P = 0.928). This finding suggests that the cultivation conditions in the Petri dishes were exactly the same. However, the total number of colonies at different stages was significantly different (P = 0.003). This number increased during the next three periods: 2 months prior to treatment and during treatment (P = 0.000785); 3 months prior to treatment and during treatment (P = 0.046811); and between 1 month and 2 months after treatment (P = 0.002619). The total number of colonies significantly decreased (P = 0.009289) 2 months and 6 months after treatment (P = 0.009289). The number of colonies was highest 2 months after treatment. The results are shown in Table 3, Table 4.
Table 3.
Number of colony-forming units at different time periods.
| Prior to treatment | Months after treatment |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 6 | |
| 13.05 ± 11.294 | 17.30 ± 21.352 | 36.98 ± 37.857 | 27.38 ± 25.667 | 20.57 ± 14.258 |
Data are presented as mean ± SD.
Table 4.
Total number of colonies at different stages.
| Stage I | Stage J | Mean difference (I – J) | Standard error | P | 95% confidence interval |
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Prior to treatment (mo) | 1 | −4.24868 | 7.690483 | 0.58143 | −16.9745 | 8.477102 |
| 2 | −23.9302 | 6.985228 | 0.000785* | −35.4889 | −12.3714 | |
| 3 | −14.337 | 7.154153 | 0.046811* | −26.1753 | −2.49871 | |
| 6 | −7.51905 | 7.520372 | 0.318954 | −19.9633 | 4.925242 | |
| After 1 mo | After 2 mo | −19.6815 | 6.43432 | 0.002619* | −30.3286 | −9.03433 |
| After 3 mo | −10.0883 | 6.617323 | 0.129414 | −21.0383 | 0.861654 | |
| After 6 mo | −3.27037 | 7.011637 | 0.64157 | −14.8728 | 8.332093 | |
| After 2 mo | After 3 mo | 9.593162 | 5.782633 | 0.099146 | 0.024387 | 19.16194 |
| After 6 mo | 16.41111 | 6.230003 | 0.009289* | 6.102052 | 26.72017 | |
| After 3 mo | After 6 mo | 6.817949 | 6.418834 | 0.289807 | −3.80358 | 17.43947 |
P < 0.05 indicates significant difference.
Discussion
In this study, the incidence of oral Candida among normal adolescents prior to the application of FOAs was 14%, lower than the reported rate of 24–40%.17 This may be attributed to the fact that the patients brushed their teeth prior to sampling, thus reducing the number of microbes in the oral cavity. Regional differences may also contribute to the variations observed.
Within 3 months of the installation of FOAs, the rate of pathogenesis and number of colonies of oral Candida significantly increased compared with those prior to treatment, particularly at 2 months after FOA installation; these values then gradually decreased over time. These findings may be due to the FOAs resulting in a lowering of the local defense mechanism of oral mucosal cells. Oral mucosal cells, which act as mechanical barriers, and metabolism play important roles in increasing the resistance of the mouth to infection. Thus Candida can easily adhere to any damage in the oral epithelia.18 Lip buccal mucosal damage was observed in many patients shortly after the application of FOAs. Varying degrees of oral ulcers or gum inflammation, both of which can decrease local defense mechanisms, were also observed.
The interaction of Candida and other oral bacteria, including adhesion between C. albicans and other microorganisms in the host cells, is an important factor in maintaining the commensalism of bacteria in the human body. Escherichia coli, Streptococcus, Pseudomonas aeruginosa, and Staphylococcus aureus can restrain the pathogenicity of Candida. Obligate anaerobic bacteria can inhibit the proliferation and adhesion of C. albicans to mucosa. S. aureus and Candida have synergetic pathogenic characteristics.19 Grimaudo and Neabitt20 showed that the aggregation of C. albicans and oral Fusobacterium may be an important factor in colony formation in the mouth. According to oral microecological theory, an ecological balance exists between the microorganisms in the mouth and the host. When the host conditions change, the type, number, and proportion of mouth microbes also change. An FOA can change the original ecological balance of the oral cavity and oral microbial plexus. Studies have shown that the oral cavity of patients with FOAs have more Bacteroides melaninogenicus and intermediate Bacillus. Moreover, the proportion of anaerobic bacteria to facultative anaerobic bacteria also increased.21 The increase in the proportion of Bacillus in patients with FOAs can increase the number of Candida colonies in the oral cavity.
Adhesion of Candida to parts of the FOA, during which Candida adheres to different dental metal material surfaces, may also affect colony formation. The extent of adhesion is dependent on the surface roughness and type of material used. The adhesion of Candida to the surface of metal or ceramic orthodontic appliances should be studied further.
There is also a relationship between Candida and the resistance of patients, where an increase in the Candida population may cause a temporary weakening of the resistance of the body during the adaptive phase after the application of the FOA. The amount of bacteria gradually declined 3 months after treatment, which may be due to the gradual adaptation of the patients to the new oral environment. Moreover, full recoveries of systemic and local resistance were also observed. The increase in the number of Candida colonies may be associated with an increase in the amount of plaque and microbes in the oral cavity of patients shortly after FOAs start to be worn.
The increase in the population of other strains and in mixed bacteria, except C. albicans, may be related to differences in adhesion and interaction between bacteria and fungi.
The ratio of Candida to mixed bacteria is 10%; this value is about 11.3% in normal people. The mixed carrying bacterial rate of Candida carriers is 23.8%, higher than that of normal people. Our results reveal that the rate of carrying bacteria is significantly high. However, increases in the possibility of infection due to FOAs require further study.
The increase in the number of Candida colonies aside from C. albicans in patients may be due to differences in the living conditions of other Candida strains. After the application of FOAs, the pH of plaque and the strain and number of microorganisms in the oral cavity changed. In addition to C. albicans, other Candida strains aggregate or adhere more easily to FOAs. The adhesive force of C. krusei to the surface of nonliving substrates is very strong. C. krusei can also attach to and reproduce on the surface of nonliving substrates via cell surface hydrophobicity.22 Similar features of this behavior in FOAs require further research.
White colonies that were not identified by the CHROMagar Candida medium may be Candida strains other than C. albicans, C. parapsilosis, C. tropicalis, C. krusei, or yeast strains.
FOA can change the carrying rates of Candida in the oral cavities of adolescents. The carrying rates, total number of bacterial colonies, and strains of Candida all changed prior to and after the application of FOAs.
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- 1.Delgado W., Aguirre J.M. Las micosis orales en la era del sida. Rev Iberoam Micol. 1997;14:14–22. [In Spanish] [PubMed] [Google Scholar]
- 2.Samaranayake L.P. Churchill Livingston; London: 1996. Essential Microbiology for Dentistry; pp. 147–153. [Google Scholar]
- 3.Garber G.E. Treatment of oral Candida mucositis infections. Drugs. 1994;47:734–740. doi: 10.2165/00003495-199447050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Samaranayake Y.H., Wu P.C., Samaranayake L.P., So M., Yuen K.Y. Adhesion and colonization of Candida krusei on host surfaces. J Med Microbiol. 1994;41:250–258. doi: 10.1099/00222615-41-4-250. [DOI] [PubMed] [Google Scholar]
- 5.Scully C., el-Kabir M., Samaranayake L.P. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994;5:125–157. doi: 10.1177/10454411940050020101. [DOI] [PubMed] [Google Scholar]
- 6.Samaranayake L.P. (2nd ed.) Churchill Livingstone; Edinburgh: 2002. Essential Microbiology for Dentistry. [Google Scholar]
- 7.Olsen I. Denture stomatitis. Occurrence and distribution of fungi. Acta Odontol Scand. 1974;32:329–333. doi: 10.3109/00016357409002556. [DOI] [PubMed] [Google Scholar]
- 8.Atack N.E., Sandy J.R., Addy M. Periodontal and microbiological changes associated with the placement of orthodontic appliances. A review. J Periodontol. 1996;67:78–85. doi: 10.1902/jop.1996.67.2.78. [DOI] [PubMed] [Google Scholar]
- 9.Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons five years after treatment. Am J Orthod Dentofac Orthop. 1989;96:423–427. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbloom R.G., Tinanoff N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am J Orthod Dentofac Orthop. 1991;100:35–37. doi: 10.1016/0889-5406(91)70046-Y. [DOI] [PubMed] [Google Scholar]
- 11.Truchot G. Do multi-bracket orthodontic appliances favor the development of parasites and fungi in the oral environment? Pathological and therapeutic consequences. Orthod Fr. 1991;62:1019–1024. [In French, English abstract] [PubMed] [Google Scholar]
- 12.Petti S., Barbato E., Simonetti D'Arca A. Effect of orthodontic therapy with fixed and removable appliances on oral microbiota: a six-month longitudinal study. N Microbiol. 1997;20:55–62. [PubMed] [Google Scholar]
- 13.Hamp S.E., Lundström F., Nyman S. Periodontal conditions in adolescents subjected to multiband orthodontic treatment with controlled oral hygiene. Eur J Orthod. 1982;4:77–86. doi: 10.1093/ejo/4.2.77. [DOI] [PubMed] [Google Scholar]
- 14.Gokdal I., Kalkanci A., Pacal G., Altug Z. Candida colonization on the surface of orthodontic brackets and the adhesion of these strains to buccal epithelial cells. Mikrobiyol Bul. 2002;36:65–69. [In Turkish, English abstract] [PubMed] [Google Scholar]
- 15.Arendorf T., Addy M. Candidal carriage and plaque distribution before, during and after removable orthodontic appliance therapy. J Clin Periodontol. 1985;12:360–368. doi: 10.1111/j.1600-051x.1985.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 16.Meyer M., Maszewska K., Sorrell T.C. PCR fingerprinting: a convenient molecular tool to distinguish between Candida dubliniensis and Candida albicans. Med Mycol. 2001;39:185–193. doi: 10.1080/mmy.39.2.185.193. [DOI] [PubMed] [Google Scholar]
- 17.Kleinegger C.L., Lockhard S.R., Vargas K., Soll D.R. Frequency, intensity species, and strains of oral Candida vary as a function of host age. J Clin Microbiol. 1996;30:2246–2254. doi: 10.1128/jcm.34.9.2246-2254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odds F.C. Balliere Tindall; London: 1988. Candida and Candidosis: a Review and Bibliography; pp. 93–104. [Google Scholar]
- 19.Samaranayake L.P., MacFarlane T.W. Butterworth; London: 1990. Oral Candidosis; pp. 10–103. [Google Scholar]
- 20.Grimaudo N.J., Neabitt W.E. Coaggregation of Candida albicans with oral Fusobacterium species. Oral Microbiol Immunol. 1997;12:168–173. doi: 10.1111/j.1399-302x.1997.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 21.Diamanti-Kipiti A. Clinical and microbiological effects of fixed orthodontic appliance. J Clin Periodontol. 1987;14:326–333. doi: 10.1111/j.1600-051x.1987.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 22.Klotz S.A., Drutz D.J., Zajic J.E. Factors governing adherence of Candida species to plastic surfaces. J. Infect Immun. 1985;50:97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]