Abstract
Background/purpose
Salvia miltiorrhiza (SM) Bunge (Labiatae/Lamiaceae; common name danshen) is a Chinese medicine that improves blood circulation and inhibits inflammatory response. Thus, it is used for the treatment of cardiac diseases and inflammation. In this study, we aimed to evaluate the effect of an ethanolic extract of SM (SME) on the dental alveolar bone resorption induced by bacterial lipopolysaccharide (LPS) in rats.
Materials and methods
An ethanolic extract was prepared from roots of SM. The major constituents of this extract were determined by high-performance liquid chromatography. The activity of the extract was evaluated in a rat model in which the dental alveolar bone resorption was induced by injection of bacterial LPS into the palatal gingiva around the maxillary molar teeth. The effect of SME on the bone resorption was studied by histologic and histomorphometric analysis.
Results
The number of osteoclasts and the percentage of osteoclasts covering the alveolar bone surfaces were significantly increased in the LPS group compared with those in the phosphate-buffered saline (PBS) group. The number and percentage of the osteoclasts on the bony surfaces were significantly reduced in the SME group in comparison with the LPS group, although it was still higher than the numbers observed in the PBS group.
Conclusion
Because SME reduced bone resorption caused by the injections of bacterial LPS in rats, we suggest that SME might have a protective effect on dental alveolar bone resorption in periodontitis.
Keywords: inflammation, lipopolysaccharide, periodontitis, Salvia miltiorrhiza
Introduction
Periodontitis, a disease of the periodontium characterized by destruction of tooth-supporting tissues, is caused by a host-mediated inflammatory response to bacterial plaque residing in gingival pockets and can eventually lead to tooth loss.1 Several species of Gram-negative bacteria are known to be key etiologic pathogens of periodontitis.2 These bacteria, as well as their biological exudates, can induce inflammatory cell infiltration, edema, and vascular dilatation in inflamed tissues.3 Although the detailed pathogenesis of the disease is still being investigated, significant effort is now focused on finding pharmacological agents that can treat periodontitis by either acting against the causative bacteria or reducing the host inflammatory response.4
Dried roots of Salvia miltiorrhiza (SM) Bunge (Labiatae/Lamiaceae; common name danshen) are listed in the Chinese Pharmacopoeia as a treatment for cardiovascular disease and inflammation.5 A water decoction of SM is reported to be effective for improving reperfusion of ischemic myocardium and enhancing blood circulation.6 Similarly, an ethanolic extract of SM (SME) inhibits the inflammatory response associated with myocardial infarction and atherosclerosis.7, 8 The potential anti-inflammatory effects of SME were evaluated both in vitro and in vivo. The anti-inflammatory effects of SME on lipopolysaccharide (LPS)-induced nitric oxide production and inducible nitric oxide synthase expression in RAW 264.7 cells were demonstrated previously. In addition, SME was shown to significantly reduce inflammation on carrageenan- or dextran-induced acute arthritis in rats.9 Results of antimicrobial studies indicated that the major portion of its activity was due to the presence of tanshinones and phenolic acids in its hairy roots.10 The mechanism of antimicrobial activity of the hexane fraction of SM Bunge against Staphylococcus aureus and methicillin-resistant S. aureus was evidenced by its ability to inhibit the expression of the resistant genes, mecA, mecR1, and femA, in messenger RNA.11 Additional bioactivities of the SM have also been reported, including antineoplasmic and antiosteoporotic effects.12, 13 Recently, it has been reported that SME can ameliorate the effect of periodontal damage induced by silk ligation around the tooth neck area in a rat model.14 We herein evaluated whether the ethanolic SME could inhibit bone resorption in rats with the experimental periodontitis induced by LPS injection; the antimicrobial effect of ethanolic SME was not considered in this study.14
Materials and methods
Preparation of the ethanolic extract of SM root
SME was prepared as described previously.15 In brief, 1 kg of dried SM roots were pulverized and soaked in 10 L of 95% ethanol for 72 hours at room temperature. The extract was filtered and concentrated by evaporation under vacuum. The resulting solid residue was soaked in 95% ethanol (10 L) for 48 hours, filtered, and then concentrated again. This soak–filter–evaporation process was repeated two more times, and the filtrates were evaporated to dryness, yielding 100.3 g of crude SME (10% yield). A quantitative analysis of SME was carried out by reverse-phase high-performance liquid chromatography (Waters XBridge Shield RP18, Hitachi, Tokyo, Japan, 5 μm, 4.6 mm × 250 mm) using a mobile phase of 75% acetonitrile/25% H2O at a flow rate of 1.0 mL/min with an injection volume of 10 μL and a UV detection wavelength of 254 nm. The extract was then dissolved in 10% Tween 80 for the in vivo experiment.
LPS-induced osteoclast resorption from the dental alveolus of rats
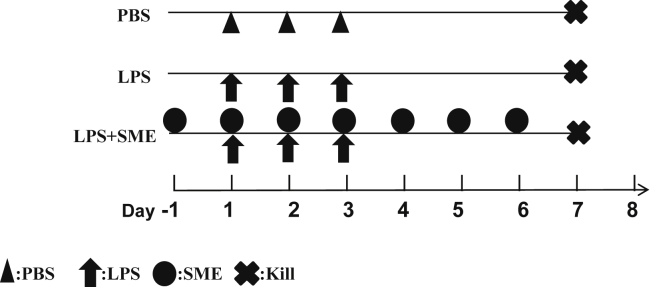
The effect of SME on LPS-induced osteoclast resorption from the dental alveolus was evaluated after administering injections of Escherichia coli LPS [dissolved in phosphate-buffered saline (PBS) solution, 5 mg/mL, E. coli serotype 055:B5; Sigma Co., St. Louis, MO, USA] into the palatal gingiva of rats as described previously.16, 17, 18 In brief, 15 rats were randomly divided into the following three treatment groups based on the treatment they received: PBS, LPS, and SME treatment groups. Rats in the PBS treatment group received a 10-μL PBS injection daily for 3 days; rats in the LPS treatment group received a 10-μL LPS (5 mg/mL) injection for 3 days; and rats in the SME group received the same LPS injections for 3 days and a daily 300 mg/kg intraperitoneal dose of SME for 7 days, beginning 1 day prior to the 1st day of LPS injection. At the end of the 7-day treatment period, all animals in the three groups were killed and palatal specimens were taken and prepared for histologic analysis (Figure 1). In this study, all animals were housed in a dedicated, pathogen-free facility and were handled in accordance with protocols approved by the Institutional Animal Care and Use Committee, National Defense Medical Center, Taipei, Taiwan (IACUC-11-033).
Figure 1.
Experimental design for the lipopolysaccharide-induced dental alveolar bone resorption in rats. LPS = lipopolysaccharide; PBS = phosphate-buffered saline; SME = Salvia miltiorrhiza extract.
Histology and histometric analysis
After fixation, the specimens were decalcified with 14% EDTA and embedded in paraffin. The maxillary specimens were then cut into 4-μm-thick sections in the bucco-palatal direction. All sections were routinely stained with hematoxylin and eosin (H&E). Tartrate-resistant acid phosphatase (TRAP) staining was further performed on the each section to determine the level of active osteoclasts on the bony surface. The TRAP solution was prepared as follows: 9.6 mg naphthol AS-BI phosphate substrate (Sigma) was dissolved in 0.6 mL N,N-dimethylformamide (Sigma) with 60 mL of 0.2M sodium acetate buffer (pH 5.0, Sigma) containing 84 mg of Fast red-violet LB diazonium salt (Sigma), 58.2 mg of tartaric acid (Sigma), and 240 μL of 10% MgCl2. The mixture was then filtered through a 0.22-μm pore-size filter. Slides were incubated for 5 minutes in the staining solution at 37°C in the dark. Afterward, the slides were washed with water for 30 minutes, and then counterstained with H&E for 6 minutes.19
A total of eight slides were selected from the 40 slides of each specimen (three sections on one slide) for histologic and histometric analysis. The number of TRAP-positive osteoclasts with three or more nuclei on the palatal bony lacunae identified in the area between the bone crest and the great palatine vessels were recorded.16, 20 The bone resorption was quantified statistically in terms of the number of osteoclasts (number of osteoclasts/alveolar bone surface, no./mm) and the percentage of the bone surface covered with osteoclasts (osteoclast surface/alveolar bone surface, %).21, 22, 23
Statistical analysis
All data were analyzed using a statistical software package (SPSS version 15.0, IBM, Chicago, IL, USA). The effect of SME on the number of osteoclasts measured on surfaces of dental alveolar bones in the three study groups was examined by one-way analysis of variance and analyzed with a Duncan test (post hoc). A P value less than 0.05 was considered to indicate a significant difference.
Results
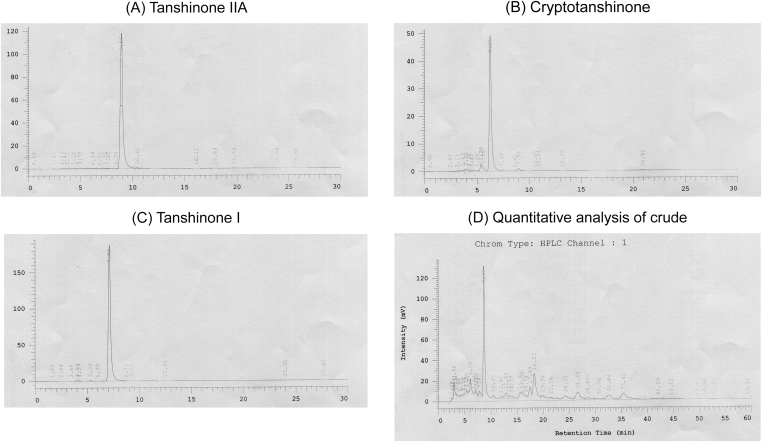
The major components of 95% ethanolic SME were determined by comparison with authentic standards. In this study, the major components were 30% tanshinone IIA, 5.8% cryptotanshinone, and 2.8% tanshinone I (Figure 2).
Figure 2.
Quantitative analysis of crude Salvia miltiorrhiza extract (SME) and authentic standards for retention time of each substituent. The quantitative analysis of SME was performed by reverse-phase high-performance liquid chromatography with a UV detection wavelength of 254 nm. Upon comparison with authentic standards (A–C), the major SME components were identified as 30% tanshinone IIA, 5.8% cryptotanshinone, and 2.8% tanshinone I (D).
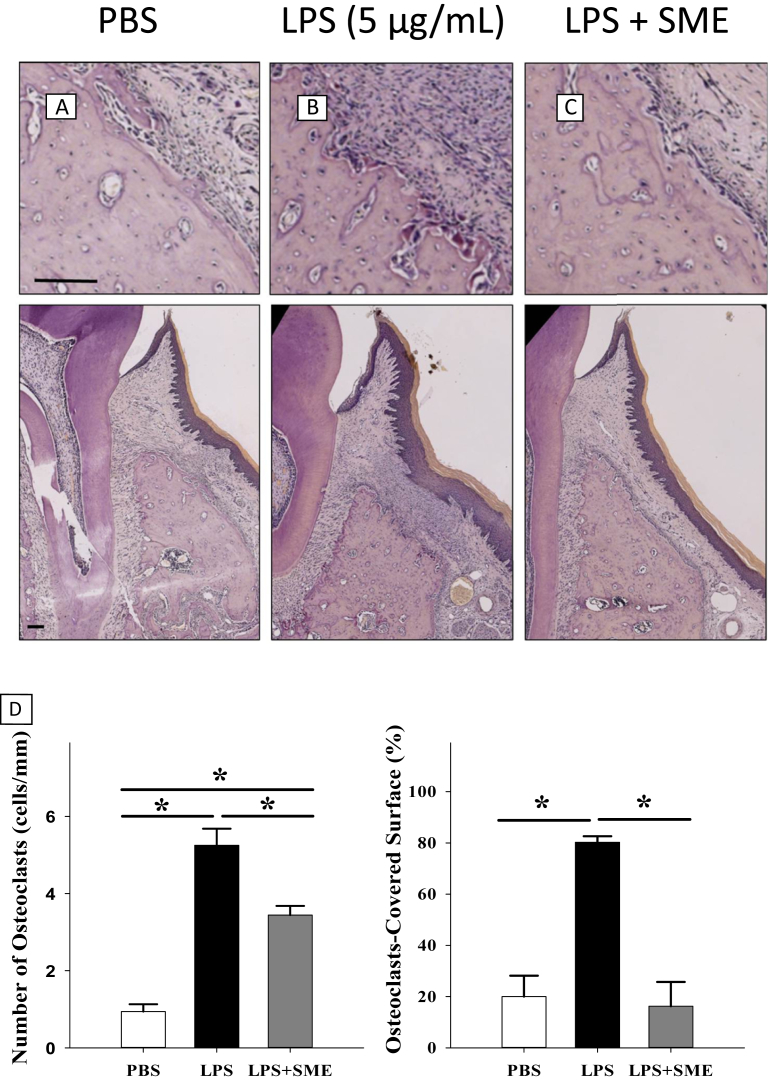
On the palatal bony surface in specimens from rats in the PBS control group, we observed a small number of osteoblasts with osteoid underneath (Figure 3A). By contrast, many TRAP-positive-stained osteoclasts with resorptive lacunae were observed in specimens from rats in the LPS group (Figure 3B). Importantly, the number of osteoclasts was significantly reduced by SME treatment compared with the LPS group, although it was still higher than that observed in the PBS group (Figures 3C and 3D).
Figure 3.
Effect of Salvia miltiorrhiza extract (SME) on lipopolysaccharide (LPS)-induced osteoclast resorption on the dental alveolar bony surface. (A–C) The palatal histology of rats in the phosphate-buffered saline (PBS), LPS (5 μg/mL), and LPS + SME groups. Sections are tartrate-resistant acid phosphatase stained and shown at 200× and 40× magnifications in the upper and lower rows, respectively (scale bar = 50 μm). (D) The number of osteoclasts (number of cells/mm) and the percentage of osteoclasts-covered surfaces (%) measured in the three animal groups (* significant difference at P < 0.05).
Discussion
SME, especially tanshinone IIA as an active component of the ethanolic extracts, is a very popular medicinal formulation that has been extensively used to treat various diseases, such as myocardial infarction and atherosclerosis.7, 8 However, in other extraction processes using a ratio of water to raw SM, polysaccharides were reported as the main antioxidant component.24 In two previous studies, higher amounts of tanshinones were obtained with a higher percentage of ethanol extract, and this component was used as an ingredient in health-care foods to improve the effectiveness of chemotherapeutic agents of cancer25 and drugs for cardiovascular protection.26 In our study, about 30% tanshinone IIA, 5.8% cryptotanshinone, and 2.8% tanshinone I were obtained by using 95% ethanol extract, which demonstrated the inhibitory effect of SME on LPS-induced alveolar bone resorption. These data indicate that using the ethanol fraction of SM increases the amounts of tanshinones obtained, which exhibits significant anti-inflammatory and bone resorption inhibition effects.
The SME also inhibited dental alveolar bone resorption induced by the trans-gingival injection of bacterial LPS in our study. Several previous studies have also shown that periodontitis and associated tissue destruction can be induced in animals by dietary manipulation,27 injection of bacterial toxins,14 placement of peri-dental silk ligatures or orthodontic elastics for bacterial colonization, or surgical removal of alveolar bone.28 Each type of induced periodontitis has certain experimental limitations. The ligation-induced periodontitis, for instance, is thought to cause abnormally exaggerated plaque retention and potential trauma to the local gingival tissue. Although LPS-induced periodontitis model is utilized herein, it does not completely replicate the chronic characteristics of the bacteria-associated periodontitis. However, the presence of the foreign body in the ligation model could prevent the attenuation of the inflammatory process after the treatment agent is tested for use.18 Recently, it has been reported that SME can ameliorate the effect of periodontal damage induced by silk ligation around the tooth neck area in a rat model. To further exclude the antimicrobial activities of SMEs reported in literature,11 the trans-gingival delivery of bacterial LPS was chosen to be used in this study.
Several species of Gram-negative bacteria, including Porphyromonas gingivalis, are known to be the etiologic pathogens of periodontitis.2, 29 However, the LPS of the bacteria is the key inflammatory mediator, which causes a highly unusual host response. Some studies have suggested that P. gingivalis LPS has an ability to stimulate cytokine production comparable to that of E. coli, whereas others suggest a much lower potency, including a lower activity in stimulating some cytokines [interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and prostaglandin E2] but a comparable activity in stimulating others (IL-6, IL-1ra). In addition, LPS from P. gingivalis binds lipopolysaccharide-binding protein 10–100 times less efficiently than that from E. coli; additionally, P. gingivalis LPS does not interact with toll-like receptors in the same manner as E. coli LPS does.30 Nevertheless, the effect of P. gingivalis, rather than E. coli, on the bone resorption of this model needs further evaluation.
Our result indicated that treatment with SME significantly reduced bone resorption compared with the control group. This observation is consistent with previous studies, in which SME suppressed bone loss caused by ovariectomy.31, 32 SME was also found to stimulate bone formation within a collagen matrix surrounding surgically created parietal bone defects in New Zealand white rabbits.33 The authors of that study suggested that SME could be useful for bone grafting, especially in cases with a compromised vascular response. These previous reports are consistent with our finding that SME has an ameliorative effect on dental alveolar bone resorption associated with periodontitis. Although the detailed mechanism for this effect was not explored in this study, other studies have documented the bioactivity of SME toward osteoblasts, osteoclasts, and macrophages. For example, purified tanshinone IIA, a major lipophilic ingredient of SME, inhibits the production of bacterial LPS-induced inflammatory mediators of prostaglandin E2, inducible nitric oxide, and cyclooxygenase-2, as well as inflammatory cytokines in osteoblasts and macrophages (e.g., IL-1β, IL-6, and TNF-α).34, 35, 36 SME has also been shown to inhibit LPS-induced nuclear factor-κB activation in osteoclasts.37 Finally, several other lipophilic tanshinones are known to inhibit osteoclast differentiation38 or LPS-induced inflammatory cytokine production.39 All of these activities suggest that the mechanism by which SME exerts anti-inflammatory and antiresorptive activity could involve repression of inflammatory signaling. In our study, the antiresorptive effect may have partially resulted from components in SME, particularly tanshinones IIA, which constituted 30% of the major SME components; however, this component is lipophilic and has poor oral bioavailability owing to its low water solubility.40 Therefore, it is necessary to identify hydrophilic substances in SME, so that the extract could be administered orally (per os).
Our results show a statistically significant inhibitory effect of SME on LPS-induced alveolar bone resorption. We therefore suggest that SME merits further evaluation as an inhibitor of alveolar bone loss caused by bacteria-associated periodontal disease.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was partially supported by a research grant from Tri-Service General Hospital (Grant No. TSCH-C104-035 & TSGH-C103-034), Taiwan, R.O.C.
References
- 1.Feng Z., Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Page R.C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 4.Leszczyńska A., Buczko P., Buczko W., Pietruska M. Periodontal pharmacotherapy—an updated review. Adv Med Sci. 2011;56:123–131. doi: 10.2478/v10039-011-0044-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng T.O. Cardiovascular effects of danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Shang Q., Xu H., Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med. 2012;2012:716459. doi: 10.1155/2012/716459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren Z.H., Tong Y.H., Xu W., Ma J., Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. 2010;17:212–218. doi: 10.1016/j.phymed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Fang Z.Y., Lin R., Yuan B.X., Liu Y., Zhang H. Tanshinone IIA inhibits atherosclerotic plaque formation by down-regulating MMP-2 and MMP-9 expression in rabbits fed a high-fat diet. Life Sci. 2007;81:1339–1345. doi: 10.1016/j.lfs.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W.Y., Jeon B.H., Kim Y.C., Lee S.H., Sohn D.H., Seo G.S. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int Immunopharmacol. 2013;16:160–164. doi: 10.1016/j.intimp.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J., Lou J., Mou Y., Li P., Wu J., Zhou L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules. 2011;16:2259–2267. doi: 10.3390/molecules16032259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.W., Ji Y.J., Lee S.O., Lee I.S. Effect of Salvia miltiorrhiza Bunge on antimicrobial activity and resistant gene regulation against methicillin-resistant Staphylococcus aureus (MRSA) J Microbiol. 2007;45:350–357. [PubMed] [Google Scholar]
- 12.Cui Y., Bhandary B., Marahatta A. Characterization of Salvia miltiorrhiza ethanol extract as an anti-osteoporotic agent. BMC Complement Altern Med. 2011;11:120. doi: 10.1186/1472-6882-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Morris-Natschke S.L., Lee K.H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 14.Dumitrescu A.L., Abd-El-Aleem S., Morales-Aza B., Donaldson L.F. A model of periodontitis in the rat: effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J Clin Periodontol. 2004;31:596–603. doi: 10.1111/j.1600-051X.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 15.Park E.J., Zhao Y.Z., Kim Y.C., Sohn D.H. Preventive effects of a purified extract isolated from Salvia miltiorrhiza enriched with tanshinone I, tanshinone IIA and cryptotanshinone on hepatocyte injury in vitro and in vivo. Food Chem Toxicol. 2009;47:2742–2748. doi: 10.1016/j.fct.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Cheng W.C., Huang R.Y., Chiang C.Y. Ameliorative effect of quercetin on the destruction caused by experimental periodontitis in rats. J Periodontal Res. 2010;45:788–795. doi: 10.1111/j.1600-0765.2010.01301.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee B.A., Lee H.S., Jung Y.S. The effects of a novel botanical agent on lipopolysaccharide-induced alveolar bone loss in rats. J Periodontol. 2013;84:1221–1229. doi: 10.1902/jop.2012.120460. [DOI] [PubMed] [Google Scholar]
- 18.Ossola C.A., Surkin P.N., Pugnaloni A., Mohn C.E., Elverdin J.C., Fernandez-Solari J. Long-term treatment with methanandamide attenuates LPS-induced periodontitis in rats. Inflamm Res. 2012;61:941–948. doi: 10.1007/s00011-012-0485-z. [DOI] [PubMed] [Google Scholar]
- 19.Chiang C.Y., Fu E., Shen E.C., Chiu H.C. Effects of CD14 receptors on tissue reactions induced by local injection of two gram-negative bacterial lipopolysaccharides. J Periodontal Res. 2003;38:36–43. doi: 10.1034/j.1600-0765.2003.01617.x. [DOI] [PubMed] [Google Scholar]
- 20.Rogers J.E., Li F., Coatney D.D. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007;78:550–558. doi: 10.1902/jop.2007.060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.S., Kang S.J., Kim J.W. Effects of polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J Periodontal Res. 2012;47:800–810. doi: 10.1111/j.1600-0765.2012.01502.x. [DOI] [PubMed] [Google Scholar]
- 22.Pacios S., Andriankaja O., Kang J. Bacterial infection increases periodontal bone loss in diabetic rats through enhanced apoptosis. Am J Pathol. 2013;183:1928–1935. doi: 10.1016/j.ajpath.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misawa Y., Kageyama T., Moriyama K. Effect of age on alveolar bone turnover adjacent to maxillary molar roots in male rats: a histomorphometric study. Arch Oral Biol. 2007;52:44–50. doi: 10.1016/j.archoralbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y., Wang L., Zhang L. Optimization of extraction and antioxidant activity of polysaccharides from Salvia miltiorrhiza Bunge residue. Int J Biol Macromol. 2015;79:533–541. doi: 10.1016/j.ijbiomac.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Sung B., Chung H.S., Kim M. Cytotoxic effects of solvent-extracted active components of Salvia miltiorrhiza Bunge on human cancer cell lines. Exp Ther Med. 2015;9:1421–1428. doi: 10.3892/etm.2015.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M., Hao H., Jiang L. In vitro inhibitory effects of ethanol extract of danshen (Salvia miltiorrhiza) and its components on the catalytic activity of soluble epoxide hydrolase. Phytomedicine. 2015;22:444–451. doi: 10.1016/j.phymed.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Robinson M., Hart D., Pigott G.H. The effects of diet on the incidence of periodontitis in rats. Lab Anim. 1991;25:247–253. doi: 10.1258/002367791780808374. [DOI] [PubMed] [Google Scholar]
- 28.Schou S., Holmstrup P., Kornman K.S. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993;64:497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- 29.Slots J., Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 30.Bainbridge B.W., Darveau R.P. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol Scand. 2001;59:131–138. doi: 10.1080/000163501750266710. [DOI] [PubMed] [Google Scholar]
- 31.Chae H.J., Chae S.W., Yun D.H., Keum K.S., Yoo S.K., Kim H.R. Prevention of bone loss in ovariectomized rats: the effect of Salvia miltiorrhiza extracts. Immunopharmacol Immunotoxicol. 2004;26:135–144. doi: 10.1081/iph-120029951. [DOI] [PubMed] [Google Scholar]
- 32.Cui L., Wu T., Liu Y.Y., Deng Y.F., Ai C.M., Chen H.Q. Tanshinone prevents cancellous bone loss induced by ovariectomy in rats. Acta Pharmacol Sin. 2004;25:678–684. [PubMed] [Google Scholar]
- 33.Wong R.W., Rabie A.B. Effect of Salvia miltiorrhiza extract on bone formation. J Biomed Mater Res A. 2008;85:506–512. doi: 10.1002/jbm.a.31577. [DOI] [PubMed] [Google Scholar]
- 34.Kwak H.B., Sun H.M., Ha H. Tanshinone IIA suppresses inflammatory bone loss by inhibiting the synthesis of prostaglandin E2 in osteoblasts. Eur J Pharmacol. 2008;601:30–37. doi: 10.1016/j.ejphar.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Chen T.H., Hsu Y.T., Chen C.H., Kao S.H., Lee H.M. Tanshinone IIA from Salvia miltiorrhiza induces heme oxygenase-1 expression and inhibits lipopolysaccharide-induced nitric oxide expression in RAW 264.7 cells. Mitochondrion. 2007;7:101–105. doi: 10.1016/j.mito.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Fan G.W., Gao X.M., Wang H. The anti-inflammatory activities of tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 2009;113:275–280. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Jang S.I., Kim H.J., Kim Y.J., Jeong S.I., You Y.O. Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. 2006;542:1–7. doi: 10.1016/j.ejphar.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.Y., Choi D.Y., Woo E.R. Inhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza bunge. Arch Pharm Res. 2005;28:909–913. doi: 10.1007/BF02973876. [DOI] [PubMed] [Google Scholar]
- 39.Choi H.S., Cho D.I., Choi H.K., Im S.Y., Ryu S.Y., Kim K.M. Molecular mechanisms of inhibitory activities of tanshinones on lipopolysaccharide-induced nitric oxide generation in RAW 264.7 cells. Arch Pharm Res. 2004;27:1233–1237. doi: 10.1007/BF02975887. [DOI] [PubMed] [Google Scholar]
- 40.Yu X.Y., Lin S.G., Zhou Z.W. Role of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza bunge. Curr Drug Metab. 2007;8:325–340. doi: 10.2174/138920007780655450. [DOI] [PubMed] [Google Scholar]