Abstract
Background/purpose
The co-operative effect of exogenous dextranase (Dex) and sodium fluoride (NaF) on Streptococcus mutans monospecies biofilms is impressive. Here we investigated the effects of the combination on a mature cariogenic multispecies biofilm and analyzed the potential mechanism.
Materials and methods
A multispecies biofilm of S. mutans, Lactobacillus acidophilus, and Actinomyces viscosus was established in vitro. Dex and NaF were added separately or together. The effects of the agents on the biomass were measured. The exopolysaccharide production was determined with the scintillation counting method. The viability and morphology were evaluated using colony forming unit and confocal laser scanning microscopy, respectively.
Results
In general, biofilms treated with Dex and a little concentration of NaF exhibited a lower biomass, exopolysaccharide production, and viability compared with the control group (P < 0.05). Confocal laser scanning microscopy using a vital fluorescence technique showed the combination treated biofilms appeared to be loose relatively and single cells could be observed. Furthermore, the thickness and viability were also lower than either of the separate agent groups (P < 0.05).
Conclusion
Overall, these findings reveal that a combination of 1 U/mL Dex and 80 μg/mL NaF is a promising candidate for disrupting complex cariogenic multispecies biofilms. This feature may be in that Dex loses the structure of biofilms, thereby facilitating NaF penetration and enhancing its antibacterial effects.
Keywords: confocal laser scanning microscopy, dextranase, multispecies cariogenic biofilm, sodium fluoride, viability value
Introduction
Dental caries is one of the most prevalent oral infectious diseases, and they result from the interactions between dietary components and specific bacteria within a biofilm formed on the surfaces of teeth.1 Dental biofilms have complex structures that resemble the tissues of higher organisms,2 and they harbor cariogenic bacteria.3 At present, cariostatic agents with biofilm-killing and -disrupting effects are receiving increasing attention. In our previous study, we showed that a combined application of exogenous dextranase (Dex) and sodium fluoride (NaF) was effective against Streptococcus mutans monospecies biofilms.4 This combined agent decreased the amount of extracellular polysaccharide and loosened the tree-like biofilms, but the specific mechanism that mediates this anticaries action is unknown.
Confocal laser scanning microscopy (CLSM) has been used widely to study the biofilm structure, composition, and biomass in several different microorganisms because it facilitates the in-depth analysis of biological structures without damaging them.5, 6, 7, 8 Live/dead staining is also used as an indicator of cell viability, which is determined by the integrity of the cell wall membrane in many bacterial biofilm models.9, 10, 11 In the present study, we elucidated the anticaries properties of the combined Dex and NaF treatment by using live/dead staining and CLSM to determine the mechanism that mediates the killing and disruption of biofilms by this agent, thereby supporting the putative mechanism mentioned by Yang et al.4
Given its biofilm-killing and -disruption potential, the combined Dex and NaF treatment may be a possible advanced method for preventing caries. However, this agent needs to be assessed using relevant biofilm models that simulate the in vivo environment as closely as possible. The use of laboratory models to simulate the microbial conditions that lead to tooth decay has a long history, and many dental biofilm models have been used to study the effects and modes of action of caries-prevention agents.12, 13, 14, 15, 16, 17 Investigations have shown that organisms such as Streptococci, Lactobacilli, and Actinomyces have important roles in dental biofilm accumulation and maturation18, 19; they form multispecies cariogenic biofilms in the laboratory, which can be used to study antibacterial effects. For example, Mei et al20 used these bacteria to produce a mature multispecies biofilm to study the antibacterial effects of silver diamine fluoride.
Despite the promising antimicrobial effect of the combined treatment with Dex and NaF, little is known about its mode of action. Previously, we showed that the biofilm matrix composition and distribution of S. mutans monospecies biofilms were affected by Dex and NaF.4 In the present study, we conducted an in-depth analysis of the changes in the biofilm structure, viability, and biomass after treatment with Dex and NaF using mature multispecies cariogenic biofilms.
Material and methods
Test agents and bacterial strain
Dex was obtained from Penicillium sp. (Sigma-Aldrich Co., St. Louis, MO, USA), dissolved in 20mM phosphate buffer (pH 6.0), sterilized using filtration (0.22 μm membrane filter, Millipore Filter Corp., Bedford, MA, USA), and stored at 4°C. NaF was purchased from Sigma-Aldrich Co. The concentrations of the agents used in this study were based on data obtained from our previous study.
S. mutans ATCC 25175, Actinomyces viscosus ATCC 15987, and Lactobacillus acidophilus ATCC 4356 were provided by the Microbiology Division of the State Key Laboratory of Oral Diseases (Chengdu, China). Each bacterial strain was grown in brain heart infusion broth (BHI; Oxoid, Basingstoke, England) containing 1.0% sucrose in an atmosphere of 80% N2 and 20% CO2 at 37°C for 18 hours. After centrifugation at 750g for 15 minutes at 4°C, the precipitate was collected, washed twice with sterile saline, and then suspended in BHI. The bacterial concentrations of the suspensions were adjusted to a McFarland standard of 1.0, according to the method defined by the National Committee for Clinical Laboratory Standards.21 The same volume of each bacterial strain was mixed to produce the biofilm.
Biofilm preparation and treatment
Mixed bacterial biofilms were formed on standard glass microscope slides (1.0 × 1.0 cm2; Micro slides; VWR Scientific, Inc., West Chester, PA, USA) in batch cultures, and were placed into sterile eight-well plastic tissue culture plates, according to the method reported by Koo et al.22 The same volume of each three bacterial strains was mixed to produce the biofilm. In detail, each well contained a 0.6 mL mixed bacterial suspension and 5.4 mL sterile BHI with 1.0% sucrose in an atmosphere of 80% N2 and 20% CO2. During the first 24 hours, the organisms were cultured undisturbed to allow the initial biofilm formation. After 24 hours, the biofilms were treated twice daily (1 minute exposure at 10 am and 4 pm) until the 4th day of the experimental period (96 hours) with one of the following: (1) vehicle control (sterile saline solution as a negative control); (2) 1 U/mL Dex; (3) 80 μg/mL NaF; (4) 1 U/mL Dex + 80 μg/mL NaF; (5) 2 U/mL Dex; (6) 4 U/mL Dex; (7) 160 μg/mL NaF; or (8) 320 μg/mL NaF. Each biofilm was exposed to its respective treatment a total of six times. Biofilm assays were performed in triplicate in four different experiments described afterwards.
Biofilm analyses
At the end of the experimental period, the biofilms were dip-washed three times and then gently swirled in physiological saline to remove any loose adherent material. The biofilms were placed in 30 mL of sterile saline solution, and the glass surfaces were scraped gently with a sterile spatula to harvest adherent cells. The removed biofilms were subjected to sonication using a Branson Sonifier 450 (Branson, Danbury, CT, USA) applied for two treatments, each comprising three 10 second pulses at 50 W with 5 second intervals, as described previously.23 The homogenized suspensions were analyzed to determine: (1) the biomass (dry weight); (2) the number of viable cells, by counting the number of colony-forming units (CFU); and (3) the polysaccharide composition [water-soluble glucans (WSGs) and water-insoluble glucans (WIGs)], using scintillation counting method.22 All assays were performed in triplicate in at least three different experiments.
CLSM
After the treatment, the biofilms were dip-washed three times in physiological saline to remove any loose adherent material and then incubated with LIVE/DEAD BacLight Bacterial Viability Kits (L-13152, Molecular Probes Inc., Eugene, OR, USA) in a dark box for 15 minutes. The biofilms were washed three times with physiological saline and observed with a CLSM (type TSP SP2; Leica, Solms, Germany) using Ar (514/488 nm) and He–Ne (543 nm) lasers. Depth measurements were acquired at regular intervals across the width of the field. To determine the structure of the biofilms, we produced a series of horizontal (x–y) optical sections with a thickness of 1.5 μm separated by 0.5 μm intervals throughout the full depth of the biofilms. To determine the biofilm vitality per layer based on the digitized data, an automatic image analysis program (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA) was used to calculate the percentage of live bacteria (vitality, %) in each section, as well as the vitality values for the whole biofilm (mean vitality, %). To avoid overlaps, only every second section was used in the analysis.
Statistical analysis
Statistical analysis was performed with SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test demonstrated whether the data were normally distributed and Bartlett’s test was used to assess the homogeneity of variances. For parametric testing, Fisher’s exact tests and one-way analysis of variance were used to detect the significant effects of variables. The level of significance was set at P < 0.05 in both studies.
Results
Effects of test agents on the biofilm biomass
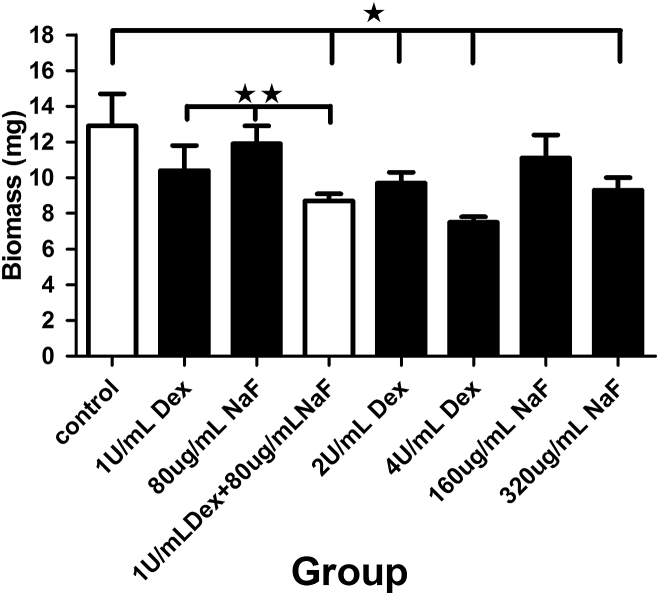
The dry weight (biomass) of the biofilm was analyzed after treatment with the test agents. With the exceptions of 80 μg/mL NaF and 160 μg/mL NaF, all of the test agents reduced the dry weight compared with the control (P < 0.05; Figure 1). The combination of 1 U/mL Dex and 80 μg/mL NaF was a more effective treatment than either of the separate test agents (P < 0.05; Figure 1): the combined effect was similar to the separate effects of 2 U/mL Dex and 320 μg/mL NaF.
Figure 1.
Effects of different treatments with dextranase and sodium fluoride on the biomass of multispecies biofilms. The data represent the means and standard errors of the means for individuals from three independent experiments (n = 12). ∗ Significant difference between the control group and other experiment groups (P < 0.05). ∗∗ Significant difference between the 1 U/mL dextranase + 80 μg/mL sodium fluoride group and either of the separate agent group (P < 0.05). Dex = dextranase; NaF = sodium fluoride.
Effects of test agents on biofilm viability
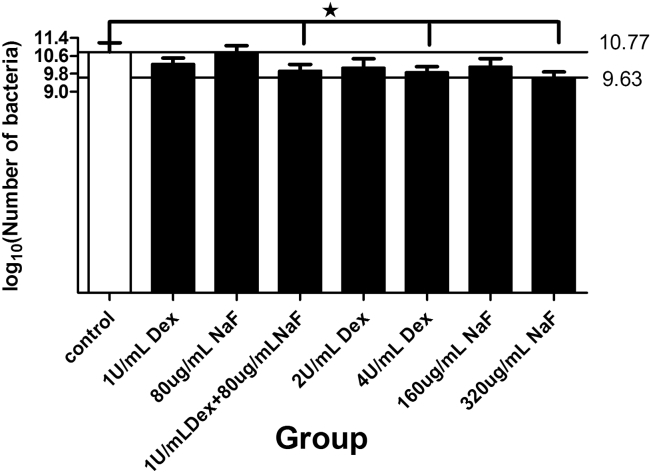
Figure 2 shows the viable cell population recovered from the biofilms after the different treatments. Among the test agents, 1 U/mL Dex and 80 μg/mL NaF, 4 U/mL Dex, and 320 μg/mL NaF yielded slightly lower numbers of recoverable viable cells compared with the control (ca. 1 log10 decrease in CFU/biofilm; P < 0.05), but none of the treatments appeared to have bactericidal effects on the biofilms. In addition, separate treatments with 1 U/mL Dex and 80 μg/mL NaF had no effects on the recoverable viable cells.
Figure 2.
The number of colony forming units (CFUs) in multispecies biofilms after different treatments with dextranase and sodium fluoride (NaF). The data represent the means and standard errors of the means for individuals from three independent experiments [n = 12, log10 (CFU/biofilm)]. The average CFU counts of 320 μg/mL NaF was the lowest in all experimental groups (9.63 ± 0.25). There were significant differences between the 1 U/mL dextranase + 80 μg/mL NaF group (9.93 ± 0.29) and the control group (10.77 ± 0.42; P < 0.05). Dex = dextranase; NaF = sodium fluoride.
Effects of test agents on the WIG and WSG levels in biofilms
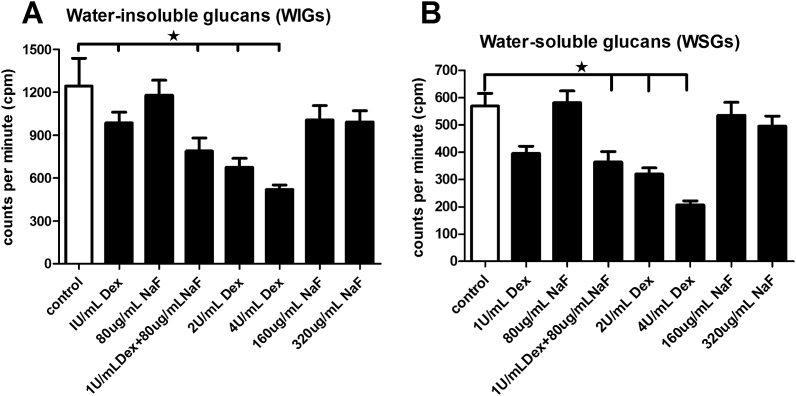
High concentrations of Dex (2 U/mL and 4 U/mL Dex) obviously reduced the levels of WIG and WSG in the biofilms (P < 0.05; Figure 3). However, NaF at 80 μg/mL had little effect on WIG and WSG production, whereas high concentrations of NaF (160 μg/mL and 320 μg/mL) slightly reduced the production of WIG and WSG production. The combined treatment of 80 μg/mL NaF and 1 U/mL Dex inhibited the synthesis of WIG and WSG more than the separate treatments did, and had a significant difference comparing with the control group (P < 0.05; Figure 3).
Figure 3.
Effects of different treatments with dextranase and sodium fluoride on (A) water-insoluble glucans and (B) water-soluble glucans of multispecies biofilms. The data represent the means and standard errors of the means for individuals from three independent experiments (n = 12). ∗ Significant difference between the control group and other experiment groups (P < 0.05). Dex = dextranase; NaF = sodium fluoride.
Architecture of biofilms according to CLSM
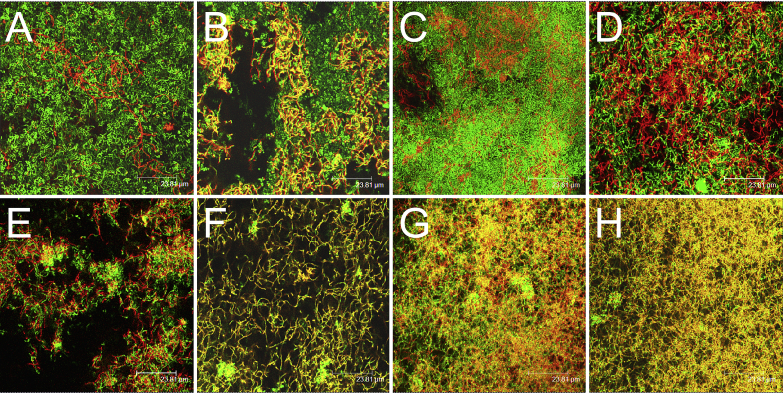
After staining with LIVE/DEAD fluorescent dye, the biofilms were observed using CLSM, and their depth and viability values were determined. Images of the BacLight LIVE/DEAD-stained biofilms were obtained, and examples are shown in Figure 4. Live cells with intact cell membranes were stained with SYTO9, and they emitted green fluorescence. Cells with damaged membranes were stained with propidium iodide, and they emitted red fluorescence. As shown in Figure 4A, the untreated biofilm was stained mostly green, but a few red cells were present. The biofilm was dense and coccobacteria and bacilli aggregated to form separated clumps with some dark channels.
Figure 4.
Confocal laser scanning microscopy images of mutispecies biofilms after different treatments with dextranase (Dex) and sodium fluoride (NaF). Biofilms were treated with: (A) sterile saline solution; (B) 1 U/mL Dex; (C) 80 μg/mL NaF; (D) 1 U/mL Dex + 80 μg/mL NaF; (E) 2 U/mL Dex; (F) 4 U/mL Dex; (G) 160 μg/mL NaF; and (H) 320 μg/mL NaF. Green indicates live bacteria (SYTO9), and red represents dead bacteria (propidium iodide). Scale bars, 23.81 μm.
The biofilm treated with 1 U/mL Dex appeared thinner, with larger channels among the bacterial clumps (Figure 4B), but its viability value did not differ from that of the control (P > 0.05; Figure 5B). After treatment with 2 U/mL and 4 U/mL Dex (Figures 4E and 4F), the biofilms appeared less dense and thinner, with diffuse microcolonies and single cells, but the viability values were similar to that of the control (P > 0.05; Figure 5B). The biofilm treated with 80 μg/mL NaF did not differ from the control in term of its depth and viability values (P > 0.05; Figures 5A and 5B), but its architecture appeared to be denser (Figure 4C). After treatment with 160 μg/mL and 320 μg/mL NaF, the architectures of the biofilms still appeared dense (Figures 4G and 4H), but the biofilm depths were slightly less than that of the control (P < 0.05; Figure 5A). After the combined treatment with 1 U/mL Dex and 80 μg/mL NaF, the biofilm structure appeared relatively loose compared with the control, and single cells or short streptococcal chains could be observed (Figure 4D). The thickness and viability values were also significantly lower compared with the separate Dex or NaF treatments (P < 0.05; Figures 5A and 5B).
Figure 5.
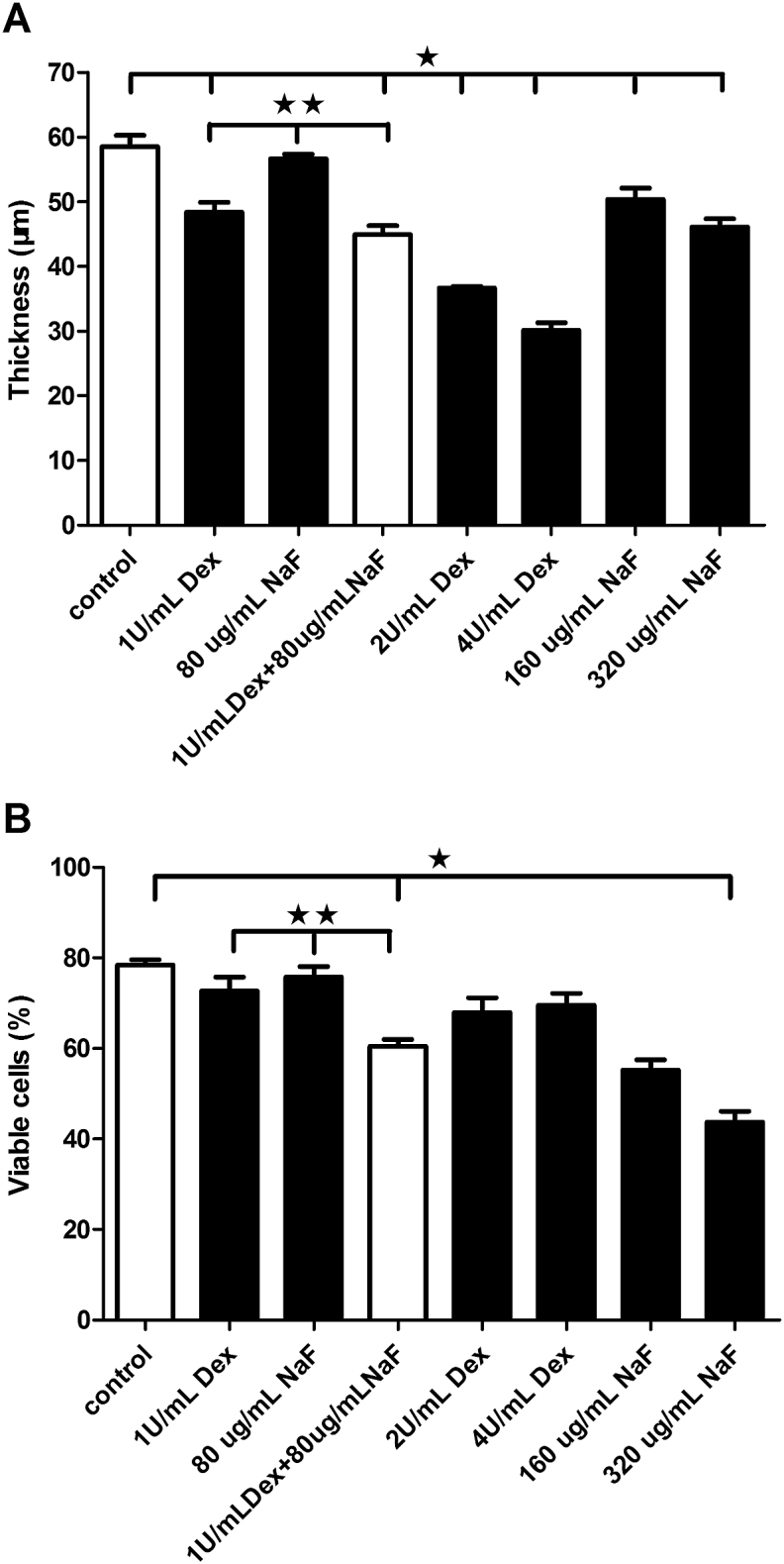
Antimicrobial effect of different treatments with dextranase (Dex) and sodium fluoride (NaF) on mutispecies biofilms. (A) Average thickness (μm) of multispecies biofilms after different treatments with Dex and NaF; (B) the percentage (%) of viable cells of multispecies biofilms after different treatments with Dex and NaF. The data represent the means and standard errors of the means for individuals (n = 10). ∗ Significant difference between the control group and other experiment groups (P < 0.05). ∗∗ Significant difference between the 1 U/mL Dex + 80 μg/mL NaF group and either of the separate agent groups (P < 0.05). Dex = dextranase; NaF = sodium fluoride.
Discussion
Using a vital fluorescence technique and CLSM, we determined the structure of intact biofilms and the spatial distributions of live and dead bacteria in different biofilm layers. We found that the biofilms treated with Dex had a porous structure with more channels among the cell clusters, and the biofilm thickness was reduced compared with the control, which agrees with previous reports that Dex loosens the biofilm structure.24, 25 The CLSM analysis showed that the combined treatment produced more clusters of dead bacteria (red) and less live bacteria (green) compared with treatment using the separate agents. The effects of antibacterial agents on biofilms vary according to their penetration extent.26 Thus, the loose structure benefited the penetration of NaF into biofilms to target cariogenic bacteria. It is well known that fluoride is one of the most effective anticaries agents because it disrupts bacterial metabolism.27, 28, 29
However, the results may have varied because the uneven distribution of the bacteria, which depended on the thickness of the biofilm. Furthermore, the image quality could have been affected by conditions such as brightness, white balance, and contrast. Therefore, the CFU test was used to support our conclusions. Figure 2 shows that the viability values declined when Dex and NaF were used in combination (P < 0.05). Bacterial activity of agents was evaluated with reduction factor that was calculated as the difference between logarithms of CFU/biofilm between the control group and other experiment groups. The sensitivity threshold of the method was considered as 1.0 × 103 CFU/biofilm.30 Interestingly, when treated with 1 U/mL Dex and 80 μg/mL NaF, the number of recoverable viable cells was slightly lower compared with the control (ca. 1 log10 decrease in CFU/biofilm), but there appeared to be no bactericidal effect, which agrees with a previous study.31 This type of combined treatment may be a new approach that fully exploits the use of low concentrations of soluble fluoride without any risk of toxicity, because exposure to high concentrations of fluoride can induce severe complications such as dental fluorosis and chronic fluoride poisoning.32
In nature, most biofilms comprise multiple species,33 and dental caries arise from a polymicrobial infection process that is largely attributable to the formation of dental biofilms.34 S. mutans is considered to be one of the most important odontopathogens involved in the initiation and maturation of dental biofilms.35 L. acidophilus is also known to be one of the most abundant species in dental biofilms,36 and it is connected to the production of extracellular homopolysaccharides and oligosaccharides, which are beneficial for biofilm accumulation and maturation.18 A. viscosus is the main bacterial colonizer during the early stage of biofilm formation,37 and it has distinct glucan adhesion properties, which are associated with its pathogenicity.19 Thus, these three major bacteria were selected to form a multispecies cariogenic biofilm in the present study.
Compared with monospecies biofilms, multispecies biofilm models are more useful for elucidating the antibacterial effects of different agents. Tests using multispecies models are essential to obtain a better understanding of the effects of potential agents for treating dental biofilms. Previously, we showed that Dex and NaF had a co-operative effect in inhibiting WIG synthesis and disrupting the biofilm structure in S. mutans monospecies biofilms.4 In the present study, we showed that the biomass, viability, and WIG production levels in biofilms were significantly reduced by the combined treatment compared with separate treatments (P < 0.05), thereby demonstrating the co-operative inhibition of mature multispecies biofilms using these two agents. Overall, our results suggest that the mechanism that underlies this combined effect is based on maximum biofilm disruption and minimum killing, where Dex loosens the structure of biofilms to facilitate their penetration by NaF, thereby enhancing its antibacterial effects.
In summary, improved antibacterial effects were achieved by combining low concentrations of NaF with Dex, which affected the virulence of cariogenic bacteria and disrupted the cariogenic biofilm. This may be a useful alternative approach to current chemotherapeutic strategies for preventing dental caries.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant number 81400507), Science and Technology of Sichuan Province, China (Grant number 2014SZ0024-1).
References
- 1.Koo H., Xiao J., Klein M.I., Jeon J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192:3024–3032. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D. Studying plaque biofilms on various dental surfaces. In: An Y.H., Friedman R.J., editors. Handbook of bacterial adhesion: Principles, methods, and applications. Humana Press; Torowa, NJ: 2000. pp. 353–370. [Google Scholar]
- 4.Yang Y.M., Jiang D., Qiu Y.X. Effects of combined exogenous dextranase and sodium fluoride on Streptococcus mutans 25175 monospecies biofilms. Am J Dent. 2013;26:239–243. [PubMed] [Google Scholar]
- 5.Almeida C., Azevedo N.F., Santos S., Keevil C.W., Vieira M.J. Discriminating multi-species populations in biofilms with peptide nucleic acid fluorescence in situ hybridization. PLoS One. 2011;6:e14786. doi: 10.1371/journal.pone.0014786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Q., Zhang G., Wood T.K. Escherichia coli BdcA controls biofilm dispersal in Pseudomonas aeruginosa and Rhizobium meliloti. BMC Res Notes. 2011;4:447. doi: 10.1186/1756-0500-4-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodor A.M., Jänsch L., Wissing J., Wagner-Döbler I. The luxS mutation causes loosely-bound biofilms in Shewanella oneidensis. BMC Res Notes. 2011;4:180. doi: 10.1186/1756-0500-4-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neu T.R., Lawrence J.R. Lectin-binding analysis in biofilm systems. Methods Enzymol. 1999;310:145–152. doi: 10.1016/s0076-6879(99)10012-0. [DOI] [PubMed] [Google Scholar]
- 9.Gomes F.I.A., Teixeira P., Azeredo J., Oliveira R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr Microbiol. 2009;59:118–122. doi: 10.1007/s00284-009-9408-9. [DOI] [PubMed] [Google Scholar]
- 10.Flemming K., Klingenberg C., Cavanagh J.P. High in vitro antimicrobial activity of synthetic antimicrobial peptidomimetics against staphylococcal biofilms. J Antimicrob Chemother. 2009;63:136–145. doi: 10.1093/jac/dkn464. [DOI] [PubMed] [Google Scholar]
- 11.Niazi S.A., Clark D., Do T. The effectiveness of enzymic irrigation in removing a nutrient-stressed endodontic multispecies biofilm. Int Endod J. 2014;47:756–768. doi: 10.1111/iej.12214. [DOI] [PubMed] [Google Scholar]
- 12.Sissons C.H. Artificial dental plaque biofilm model systems. Adv Dent Res. 1997;11:110–126. doi: 10.1177/08959374970110010201. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg D., Moldovan M., Molukandov D. Testing a degradable topical varnish of cetylpyridinium chloride in an experimental dental biofilm model. J Antimicrob Chemother. 2001;48:241–243. doi: 10.1093/jac/48.2.241. [DOI] [PubMed] [Google Scholar]
- 14.Bradshaw D.J., Marsh P.D., Hodgson R.J., Visser J.M. Effects of glucose and fluoride on competition and metabolism within in vitro dental bacterial communities and biofilms. Caries Res. 2002;36:81–86. doi: 10.1159/000057864. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro S., Giertsen E., Guggenheim B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 2002;36:93–100. doi: 10.1159/000057866. [DOI] [PubMed] [Google Scholar]
- 16.Lynch R.J., Ten Cate J.M. Effect of calcium glycerophosphate on demineralization in an in vitro biofilm model. Caries Res. 2006;40:142–147. doi: 10.1159/000091061. [DOI] [PubMed] [Google Scholar]
- 17.Exterkate R.A., Crielaard W., Ten Cate J.M. Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high throughput active attachment model. Caries Res. 2010;44:372–379. doi: 10.1159/000316541. [DOI] [PubMed] [Google Scholar]
- 18.Tieking M., Kaditzky S., Valcheva R., Korakli M., Vogel R.F., Gänzle M.G. Extracellular homopolysaccharides and oligosaccharides from intestinal lactobacilli. J Appl Microbiol. 2005;99:692–702. doi: 10.1111/j.1365-2672.2005.02638.x. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg D., Kopec L.K., Bowen W.H. Adhesion of actinomyces isolates to experimental pellicles. J Dent Res. 1993;72:1015–1020. doi: 10.1177/00220345930720060401. [DOI] [PubMed] [Google Scholar]
- 20.Mei M.L., Li Q.L., Chu C.H., Lo E.C., Samaranayake L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12:4. doi: 10.1186/1476-0711-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenblatt J.E., Gustafson D.R. Evaluation of the E test for susceptibility testing of anaerobic bacteria. Diagn Micr Infec Dis. 1995;22:279–284. doi: 10.1016/0732-8893(95)00049-g. [DOI] [PubMed] [Google Scholar]
- 22.Koo H., Hayacibara M.F., Schobel B.D. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 23.Koo H., Rosalen P.L., Cury J.A., Park Y.K., Bowen W.H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–1309. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamada S., Mizuno J., Murayama Y., Ooshima T., Masuda N., Sobue S. Effect of dextranase on the extracellular polysaccharide synthesis of Streptococcus mutans: chemical and scanning electron microscopy studies. Infect Immun. 1975;12:1415–1425. doi: 10.1128/iai.12.6.1415-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao Y.L., Wang S.J., Lv M.S. Characterization of a marine-derived dextranase and its application to the prevention of dental caries. J Ind Microbiol Biotechnol. 2014;41:17–26. doi: 10.1007/s10295-013-1369-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.H. Antimicrobial effects of herbal extracts on Streptococcus mutans and normal oral streptococci. J Microbiol. 2013;51:484–489. doi: 10.1007/s12275-013-3312-5. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson J.J., McLoughlin J. Role of fluoride in oral health promotion. Int Dent J. 2000;50:119–128. doi: 10.1111/j.1875-595x.2000.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 28.Buzalaf M.A., Pessan J.P., Honório H.M., ten Cate J.M. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97–114. doi: 10.1159/000325151. [DOI] [PubMed] [Google Scholar]
- 29.Maquis R.E., Clock S.A., Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;26:493–510. doi: 10.1111/j.1574-6976.2003.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 30.Vitt A., Sofrata A., Slizen V. Antimicrobial activity of polyhexamethylene guanidine phosphate in comparison to chlorhexidine using the quantitative suspension method. Ann Clin Microbiol Antimicrob. 2015;14:36. doi: 10.1186/s12941-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faller R.V., Casey K., Amburgey J. Anticaries potential of commercial fluoride rinses as determined by fluoridation and remineralization efficiency. J Clin Dent. 2011;22:29–35. [PubMed] [Google Scholar]
- 32.Whitford G.M. Intake and metabolism of fluoride. Adv Dent Res. 1994;8:5–14. doi: 10.1177/08959374940080011001. [DOI] [PubMed] [Google Scholar]
- 33.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 34.Zhi Q.H., Lo E.C., Lin H.C. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40:962–967. doi: 10.1016/j.jdent.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Loesche W.J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh P.D., Martin M.V., Lewis M.A., Williams D.W. Dental plaque. In: Marsh P.D., editor. Oral microbiology. Livingstone Churchill; London, UK: 2009. pp. 82–104. [Google Scholar]
- 37.Knight G.M., McIntyre J.M., Craig G.G., Mulyani, Zilm P.S. The inability of Streptococcus mutans and Lactobacillus acidophilus to form a biofilm in vitro on dentine pretreated with ozone. Aust Dent J. 2008;53:349–353. doi: 10.1111/j.1834-7819.2008.00077.x. [DOI] [PubMed] [Google Scholar]