Abstract
Hydropericardium syndrome (HPS) is one of the important emerging diseases causing huge losses to the poultry industry. It affects mainly 3- to 6-week-old broiler chickens and rarely occurs in breeding and laying flocks. Recently, an HPS case was recorded with a sudden heavy mortality in a 100-day-old laying flock. A fowl adenovirus serotype 4 (FAdV-4), named as GDMZ strain, was isolated and identified using polymerase chain reaction coupled with electron microscopy. The animal experiment showed that a mortality of 100% was recorded with hydropericardium as a conspicuous lesion throughout the course of infection. Microscopically, vacuolar changes and intranuclear inclusion bodies were observed in the liver and vacuolar changes were observed in the heart. The complete genome sequence of GDMZ strain was determined to investigate the molecular properties of GDMZ strain. The comparative analysis revealed that the novel Chinese FAdV-4 isolate contained open reading frame (ORF) 19, ORF27, and ORF48 genomic deletions. The phylogenetic analysis revealed that FAdV-4 could be divided into two major clades, of which Chinese FAdV-4 were located at a distinct clade.
Keywords: fowl adenovirus serotype 4, hydropericardium syndrome, replacement pullet
Hydropericardium syndrome (HPS) is an important emerging disease causing huge economic losses to the poultry industry. HPS affects mainly 3- to 6-week-old broilers and rarely occurs in breeding and laying flocks [4, 6]. The major gross pathologic lesion associated with HPS is hydropericardium characterized by pericardial effusion with a clear accumulation of straw-colored pericardial fluid. Other lesions include enlargement and discoloration of the liver with hemorrhagic or necrotic foci [2, 14, 17, 18, 25]. The first recorded outbreak of HPS was observed in 1987 at Angara Goth, Pakistan. Since then, similar cases have subsequently been reported in several other parts of the world [1, 2, 4, 5, 13, 17, 32]. Until 2015, the prevalence of HPS was relatively low and only sporadic in China. However, since 2015, a significant increase in the HPS outbreak began in the various production areas of broilers in China [20, 28, 34]. Epidemiological studies on HPS outbreaks have shown that the HPS is mainly caused by a fowl adenovirus serotype 4 (FAdV-4) of FAdV-C [2, 3, 7, 13, 22], although other agents may also be involved [29,30,31].
Fowl adenovirus belongs to the family Adenoviridae, which can be divided into five genera: Mastadenovirus, Aviadenovirus, Siadenovirus, Atadenovirus, and Ichtadenovirus [4, 6]. Within the genus Aviadenovirus, FAdVs are divided into five species, designated as A–E, based on the restriction endonuclease cleavage patterns and sequence data, and then subdivided further into serotypes [11, 12]. FAdVs have been associated with a series of infections including inclusion body hepatitis, HPS, and gizzard erosion in chickens and other birds [4, 24, 26]. A previous study revealed that 2015 Chinese serotype 4 FAdVs had 33-nt or 66-nt deletions in open reading frame (ORF) 29 compared with 2013 Chinese serotype 4 FAdV [34].
The present study reported an HPS case that occurred in a 100-day-old replacement pullet flock with 60% mortality, which was characterized by the accumulation of a clear, straw-colored fluid in the pericardial sac. The causative agent was isolated, and its genetic properties were analyzed. This study might provide novel insights into the epidemic and pathogenic characteristics of FAdVs causing HPS in replacement pullets.
MATERIALS AND METHODS
Sample collection and virus isolation
An HPS case was occurred in a 100-day-old replacement pullet flock in Guangdong province, with a high mortality of up to 60%. Sick chickens showed diarrhea, ataxia with the gross lesions of the accumulation of a clear, straw-colored fluid in the pericardial sac. The pathogen isolation and identification were carried out according a study described previously with some modifications [8]. Briefly, tissue samples were collected from livers and the bacterial isolation was carried out. The livers and hearts of the affected replacement pullets and homogenized in Dulbecco’s modified Eagle’s medium. The tissue suspensions were subjected to three rounds of freeze-thawing before centrifugation at 5,000 rpm for 15 min at 4°C. Then, the supernatants were filtered through a 220-nm membrane and used for polymerase chain reaction (PCR) assays to detect FAdV-4. The PCR assays were conducted to detect Hexon of FAdV-4 using specific primers (5′-GCC GTC CTT CAA GCC CTA CTG C-3′ and 5′-GAC TTG GCG AAG CGA CCG A-3′). The PCR/RT-PCR for the detection of Avian influenza virus, Newcastle disease virus, Chicken infectious bronchitis virus, Avian cerebrospinal virus, Chicken leukemia virus, Infectious bursal virus, Avian metapneumovirus and Inclusion body hepatitis were also carried out. FAdV-positive supernatants were inoculated into 9-day-old specific-pathogen-free (SPF) chicken embryos via the choriollantoic membrane route, and the presence of FAdV-4 was confirmed using PCR and sequencing. The isolate was designated as GDMZ strain.
For observation using electronic microscopy, virus in the allantoic fluid of GDMZ-infected embryos was clarified by centrifugation at 8,000 rpm for 30 min using a Ti19 rotor (Beckman coulter, Brea, CA, U.S.A.) at 4°C. The supernatant was collected, overlaid onto a 20% sucrose solution, and then centrifuged at 50,000 rpm for 3 hr at 4°C in a Ti70 rotor (Beckman coulter). The pellet was resuspended in TE buffer (10 mM Tris, pH 8.0, 1 mM EDTA). The virus preparations were stained with phosphotungstic acid and observed under an electron microscope.
Animal experiment
In order to detect the viral pathogenic properties in chickens, groups of ten 14-week-old white SPF chickens was inoculated intramuscularly with 100 EID50 (50% egg infectious dose) of virus or mocked infected with phosphate-buffered saline and monitored daily for two weeks.
Another animal experiment was carried out with the isolated strain through the same route described above (ten birds per group). For the viral replication kinetics in chickens, a total of five chickens from each group were euthanized on day 3 after inoculation and their hearts, livers, spleens, lungs, kidneys, brains, and pancreases were sampled for virus titration using the EID50 assay. For a histological examination, tissue samples (heart, liver, spleen, lung, kidney, brain, and pancreas) from affected birds were fixed in 10% neutral buffered formalin, embedded in paraffin wax, and cut into sections. The sections were stained with hematoxylin and eosin and examined for lesions using light microscopy. The remaining birds were monitored daily for a week. All animal experiments were approved by animal welfare committee of Fujian Academy of Agricultural Sciences (No.1306129FAAS).
Sequencing
Viral DNAs were extracted using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, U.S.A.) according to the manufacturer’s protocol. Finally, DNA was eluted in 50 µl of elution buffer and stored at −80°C until use. A total of 45 pairs of specific primers were designed according to the FAdV-4 CH/JSXZ/2015 strain (accession number: KU569296) to amplify the GDMZ genome. Each pair of the neighbored primer was overlapped, and the amplified fragments were within 1,500 bp. PCR was performed using 2 µl of template DNA in a total volume of 50 µl containing 25 µl of 2 × EasyTaq PCR SuperMix (TransGen Biotech, Beijing, China), 2 µl of upstream primers (20 µM), 2 µl of downstream primers (20 µM), and ddH2O up to 50 µl. The thermal cycling conditions were as follows: 95°C for 5 min (1 cycle); 94°C for 45 sec, 53–62°C for 45 sec, 72°C for 90–150 sec (35 cycles); 72°C extension for 10 min (1 cycle). The PCR fragments were purified and sequenced in both directions using an ABI 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, U.S.A.) by BioSune Biotech Co., Shanghai, China. The viral genomic sequence of the FAdV-4 GDMZ isolate was assembled using the Lasergene sequence analysis software package (DNASTAR Inc., Madison, WI, U.S.A.) and deposited in GenBank.
Sequence analysis
The nucleotide and deduced amino acid sequence of the FAdV-4 GDMZ isolate were analyzed using the MegAlign program of the Lasergene software package. Phylogenetic trees for each major structural gene segment were constructed using the neighbor-joining method with 1,000 bootstrap replicates using the MEGA 4 software.
Statistical analysis
Comparisons of experimental groups were estimated by Student’s t-test with two-tailed analysis to determine significant differences. If a P-value was found to be less than 0.05, then the result would be considered statistically significant.
RESULTS
Virus isolation
Heart and liver samples from affected replacement pullets were processed for virus isolation using embryonated SPF chicken eggs, and the embryos died 5–6 days after inoculation. The allantoic fluids were then harvested for passaging. At the fifth passage, the allantoic fluid had an infectivity titer of 103.0 EID50/ml. The isolated virus was named as GDMZ strain.
Using specific primers for the conserved hexon gene of FAdV-4, a band of ~300 bp was amplified from GDMZ, indicating that the GDMZ was positive for FAdV-4. The PCR products were sequenced and BLAST search ( https://blast.ncbi.nlm.nih.gov/Blast.cgi) were performed. The result showed that GDMZ shared high homologies with FAdV-4 counterparts. The virus preparations were stained with phosphotungstic acid, and the presence of spherical particles ranging between 60 and 80 nm was observed under an electron microscope (Fig. 1). This data, along with the sequencing results of the PCR products, suggested that the causative agent has been isolated and identified as an FAdV-4 isolate.
Fig. 1.
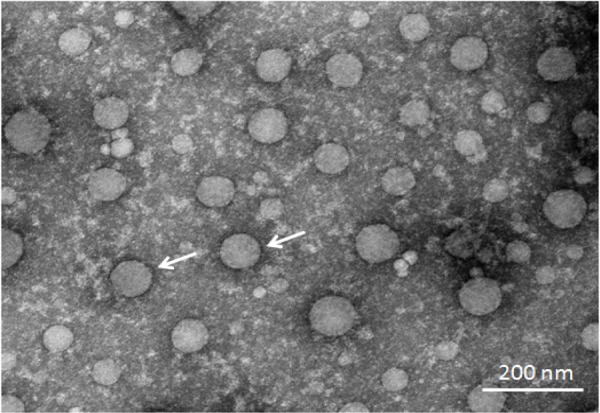
Electron microphotograph showing the characteristic morphology of fowl adenovirus serotype 4 GDMZ strain (bar=200 nm).
Mortality and viral load in SPF chickens infected with FAdV-4 GDMZ
The animal experiment showed that FAdV-4 GDMZ was highly virulent for chickens with a mortality rate of 100%, which started on day 3 after inoculation, peaked, and ended on 4 days post-inoculation. Chickens infected with FAdV-4 GDMZ showed waterly diarrhea, ataxia and acute death within a few min. To detect the viral replication kinetics in chickens, SPF chickens were inoculated intramuscularly with 100 EID50 viruses. On day 3 after inoculation, five chickens from each group were euthanized for post-mortem examinations. The typical HPS gross lesions characterized by an accumulation of straw-colored clear fluid in the pericardial sac were observed (Fig. 2).
Fig. 2.
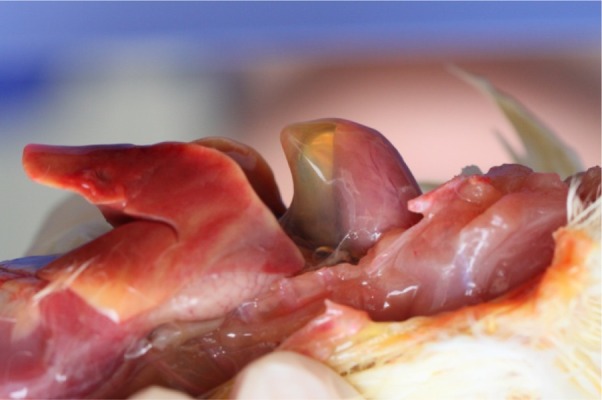
Postmortem examinations of the heart from affected birds with hydropericardium syndrome (HPS). The accumulation of clear, straw-colored fluid in the pericardial sac.
The viral load of FAdV-4 in different tissues of SPF chickens was determined using the EID50 assay. The viral loads in all tissues were higher than 102 EID50/g, and the viral titers in livers were significantly higher (P<0.01) compared with those in any other tissues (Fig. 3). All chickens in the control group remained clinically healthy and negative for the virus.
Fig. 3.
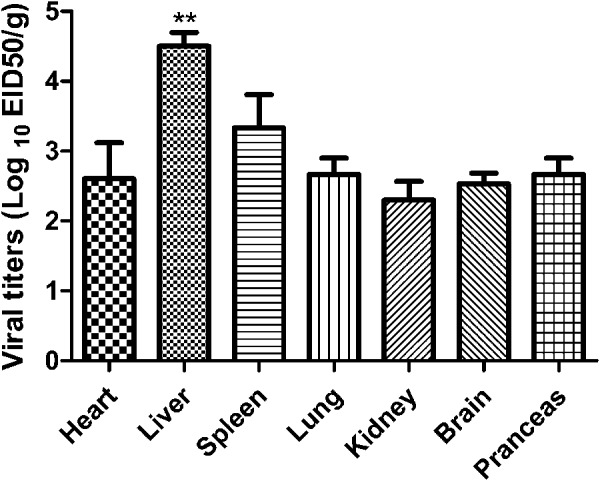
Dynamic distribution of the novel FAdV-4 in experimentally infected SPF chickens. On days 3 post-inoculation, affected chickens were euthanized and their hearts, livers, spleens, lungs, kidneys, brains, and pancreases were sampled for virus titration through EID50 assay (P<0.01).
Histopathology in different tissues
Tissues of the heart, liver, spleen, lung, kidney, brain, and pancreas of chickens in different groups were collected 3 days after challenge, fixed, cut into sections, and stained with hematoxylin and eosin (Fig. 4). Various histopathological changes were observed in different tissues of chickens in the infection group: degeneration, vacuolar necrosis, and basophilic inclusion bodies in liver cells; vacuolar necrosis in kidney cells; vacuolar changes in heart cells; and severe reduction and necrosis of lymphocytes evident in the spleen. No significant histopathological damage was found in chickens of the control group.
Fig. 4.
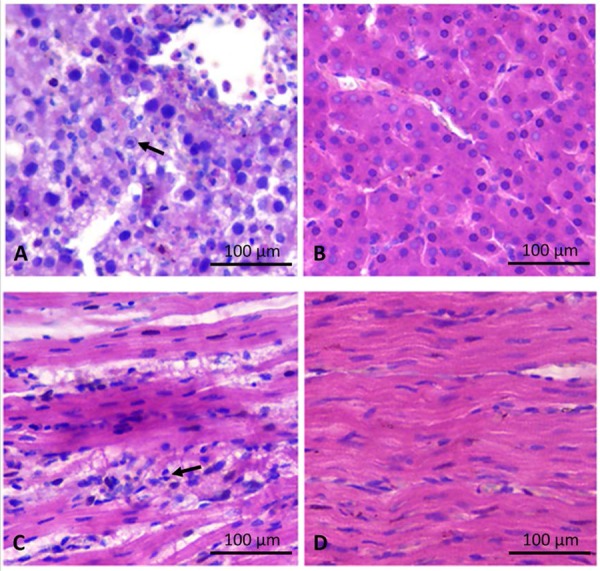
Histological examinations of the liver and heart from affected birds with hydropericardium syndrome (HPS). (A) Small multifocal areas of necrosis and basophilic intranuclear inclusion bodies in hepatocytes (H&E stain, original magnification × 400, scale bar=100 µm). (B) Histological examinations of the liver from control birds (H&E stain, original magnification × 400, scale bar=100 µm). (C) Lymphocytic infiltrates in association with myocarditis (H&E stain, original magnification × 400, scale bar=100 µm). (D) Histological examinations of the heart from control birds (H&E stain, original magnification × 400, scale bar=100 µm).
Genome sequencing and sequence analysis
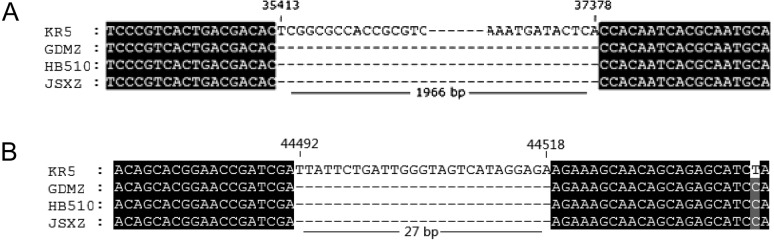
The complete genomic sequence of FAdV-4 GDMZ isolate was sequenced and deposited in GenBank with accession number MG856954. The full-length genome of GDMZ was 43,723 bp. The G + C content of GDMZ was 54.87%, which was similar to those of other reported FAdV-4 strains. The predicted ORFs were consistent with those of FAdV-4 KR5, except for the deletions of ORF19, ORF27, and ORF48 in the genome of GDMZ [10, 15, 21, 33, 35]. A 1,966-bp deletion was located at 35,413–37,378 bp compared with the nucleotide positions within the KR5 genome. The deletion was in the right-end region of the genome of FAdV-4 GDMZ compared with FAdV-4 KR5, resulting in the absence of ORF19, ORF27, and ORF48 [21]. In addition, a deletion was located at 44,492–44,518 bp compared with the nucleotide positions within the KR5 genome contributed a deletion in ORF19A (Fig. 5).
Fig. 5.
Comparison of the complete genome of FAdV-4 GDMZ and other FAdV-4 counterparts. (A) A 1,966-bp deletion located at 35,413–37,378 bp compared with the nucleotide positions within the KR5 genome, resulting in the absence of ORF19, ORF27, and ORF48. (B) A deletion located at 44,492–44,518 bp compared with the nucleotide positions within the KR5 genome contributed a deletion in ORF19A.
The phylogenetic tree of FAdV-4 hexon protein gene was constructed employing a neighbor-joining method based on already available hexon protein sequences in GenBank. The results of the phylogenetic analysis showed that FAdV-4 GDMZ and other Chinese FAdV-4 isolates clustered into a distinct group within FAdV-C. However, other FAdV-4 isolates did not show a strict clustering. A phylogenetic analysis based on the available fiber 1 and fiber 2 gene sequences also showed that all Chinese FAdV-4 isolates clustered together (Fig. 6).
Fig. 6.
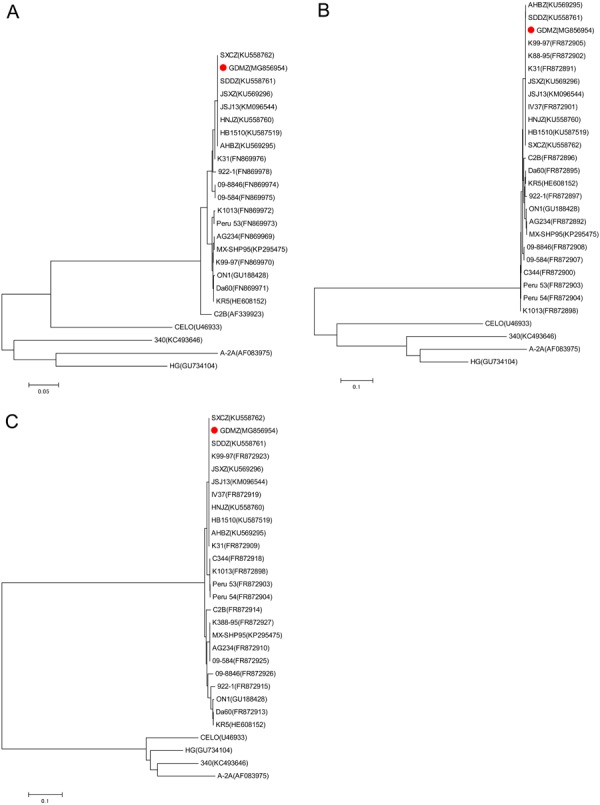
The results of phylogenetic analyses based on the nucleotide sequences of the currently available hexon (A), fiber 1 (B), fiber 2 (C) gene sequences of FAdV-4 isolates and other FAdV species. The trees were generated by the neighbor-joining method with bootstrap tests of 1,000 replicates using the MEGA 4 software. Bootstrap values are presented at key nodes. The scale bars indicate the nucleotide substitutions per site. Filled circles indicate HPS-inducing FAdV-4 strain GDMZ.
DISCUSSION
In recent years, severe outbreaks of HPS have increasingly emerged in chicken flocks in several Chinese provinces. Most cases were observed in broilers aged 3–5 weeks. Mortality reported during these outbreaks varied from a slight increase to more than 30% in severe cases, and FAdVs were isolated from all the cases [35]. The present study reported an HPS case that emerged in a replacement pullet flock aged 100 days. An FAdV-4 named as GDMZ was isolated, which was consistent with a previous study demonstrating that HPS occasionally occurred in layers and breeder pullets aged 10–20 weeks [35]. However, clinical cases and animal experiment showed that FAdV-4 GDMZ was highly virulent for replacement pullets, which was not consistent with prior knowledge describing that HPS also rarely occurred in breeding and laying flocks with lower mortality rates [20]. Hence, the findings needed further investigation.
The factors determining the infection are still unclear due to the limited information available on the pathogenic and genetic properties of FAdVs. To date, the complete genome sequences available from FAdVs that infected replacement pullets were limited. Recently, FAdV-4 isolates were reported in China, providing limited information [20, 34, 35]. No previous studies have reported the whole genome of FAdVs infecting the replacement pullets in China. Consequently, the findings of the present study might enrich the knowledge regarding the FAdV genomes and their pathogenesis.
Similar to the recent Chinese FAdV-4 isolates, the genome of the FAdV-4 GDMZ differed in size from the previously reported FAdV-4 strains despite its pathogenic characteristics [10, 21, 33]. Alignment of the FAdV-4 GDMZ genome sequence with that of FAdV-4 strains available in GenBank showed various nucleotide sequence deletions, which was consistent with FAdV-4 recently prevailing in China. The influence of these deletions and sequence differences in various regions of the genomes of Chinese isolates on viral replication and pathogenicity remains to be explored further. However, the fact that strain FAdV MX-SHP95 with its truncated ORF19 is highly pathogenic implied that the deletion of ORF19 and/or ORF27 might influence the virulence of the novel FAdV-4 GDMZ isolate, which was consistent with the results of Ye et al [34] and Liu et al [20]. ORF19 is considered as a lipase homologue [9]. It is demonstrated that the lipase might be important in replication and pathogenicity of virus because mutantion in v-Lip of the Marek’s disease viruses causes a significantly lower incidence of Marek’s disease in chickens and resulted in enhanced survival relative to two independently produced vLIP revertants or parental virus [16, 19]. It would be interesting to know if the lipase ORFs from the analyzed FAdV genomes are also important in virus replication in vivo and in pathogenesis. Hexon is one of the major structural proteins of FAdV. It contains group-, type-, and subtype-specific antigenic determinants to raise different antibodies [23]. A phylogenetic analysis of the hexon gene showed that all Chinese FAdV-4 isolates clustered into a distinct group within FAdV-C. The fiber protein is associated with virus neutralization, cellular receptor binding, tissue tropism, and variations in virulence [27]. The fiber gene can also be used to differentiate the HPS-inducing FAdV-4 isolates from other FAdV-4 strains [22]. The phylogenetic analysis based on the fiber 1 and fiber 2 gene sequences also revealed that all Chinese FAdV-4 isolates clustered together.
In summary, the present study reported an HPS case in replacement pullets infected with FAdV-4 in China, which was different from other reports on broil infection. The findings of this study might enrich the knowledge regarding the FAdV genomes and their pathogenesis.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Project No. 2017YFD0500802021), the National Natural Science Foundation of China (No. 31472222), Fund for Modern Agro-industry Technology Research System (CARS-42) from the Ministry of Agriculture of P. R. China, the Natural Science Foundation of Fujian Province (No. 2017J01060) and the Basic Scientific Research Funds of Public Welfare Scientific Research Institutes of Fujian Province (2016R1022-1, 2016R1022-9) and Financial special of Fujian Province (FJFS-2017).
REFERENCES
- 1.abdul-Aziz T. A., al-Attar M. A.1991. New syndrome in Iraqi chicks. Vet. Rec. 129: 272. doi: 10.1136/vr.129.12.272 [DOI] [PubMed] [Google Scholar]
- 2.Abe T., Nakamura K., Tojo H., Mase M., Shibahara T., Yamaguchi S., Yuasa N.1998. Histology, immunohistochemistry, and ultrastructure of hydropericardium syndrome in adult broiler breeders and broiler chicks. Avian Dis. 42: 606–612. doi: 10.2307/1592690 [DOI] [PubMed] [Google Scholar]
- 3.Afzal M., Muneer R., Stein G.1991. Studies on the aetiology of hydropericardium syndrome (Angara disease) in broilers. Vet. Rec. 128: 591–593. doi: 10.1136/vr.128.25.591 [DOI] [PubMed] [Google Scholar]
- 4.Anjum A. D., Sabri M. A., Iqbal Z.1989. Hydropericarditis syndrome in broiler chickens in Pakistan. Vet. Rec. 124: 247–248. doi: 10.1136/vr.124.10.247 [DOI] [PubMed] [Google Scholar]
- 5.Asrani R. K., Gupta V. K., Sharma S. K., Singh S. P., Katoch R. C.1997. Hydropericardium-hepatopathy syndrome in Asian poultry. Vet. Rec. 141: 271–273. doi: 10.1136/vr.141.11.271 [DOI] [PubMed] [Google Scholar]
- 6.Balamurugan V., Kataria J. M.2004. The hydropericardium syndrome in poultry—a current scenario. Vet. Res. Commun. 28: 127–148. doi: 10.1023/B:VERC.0000012115.86894.1e [DOI] [PubMed] [Google Scholar]
- 7.Balamurugan V., Kataria J. M., Kataria R. S., Verma K. C., Nanthakumar T.2002. Characterization of fowl adenovirus serotype-4 associated with hydropericardium syndrome in chicken. Comp. Immunol. Microbiol. Infect. Dis. 25: 139–147. doi: 10.1016/S0147-9571(01)00032-7 [DOI] [PubMed] [Google Scholar]
- 8.Chen Z., Shi S. H., Huang Y., Han Y. S., Chen C. T., Liu R. C., Cai G. Z., Zhu C. H., Liu B. Q., Lin Y.2016. Isolation and preliminary identification of avian adenovirus serotype 4. Fujian Agri. Sci. 31: 1020–1023. [Google Scholar]
- 9.Corredor J. C., Garceac A., Krell P. J., Nagy E.2008. Sequence comparison of the right end of fowl adenovirus genomes. Virus Genes 36: 331–344. doi: 10.1007/s11262-007-0194-9 [DOI] [PubMed] [Google Scholar]
- 10.Griffin B. D., Nagy E.2011. Coding potential and transcript analysis of fowl adenovirus 4: insight into upstream ORFs as common sequence features in adenoviral transcripts. J. Gen. Virol. 92: 1260–1272. doi: 10.1099/vir.0.030064-0 [DOI] [PubMed] [Google Scholar]
- 11.Hess M.2000. Detection and differentiation of avian adenoviruses: a review. Avian Pathol. 29: 195–206. doi: 10.1080/03079450050045440 [DOI] [PubMed] [Google Scholar]
- 12.Hess M.2013. Adenovirus infections. pp. 289−300. In: Diseases of Poultry (Swayne, D. E. ed.), John Wiley & Sons, Hoboken. [Google Scholar]
- 13.Hess M., Raue R., Prusas C.1999. Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol. 28: 433–439. doi: 10.1080/03079459994443 [DOI] [PubMed] [Google Scholar]
- 14.Ivanics E., Palya V., Markos B., Dán A., Ursu K., Harrach B., Kaján G., Glávits R.2010. Hepatitis and hydropericardium syndrome associated with adenovirus infection in goslings. Acta Vet. Hung. 58: 47–58. doi: 10.1556/AVet.58.2010.1.5 [DOI] [PubMed] [Google Scholar]
- 15.Kaján G. L., Davison A. J., Palya V., Harrach B., Benko M.2012. Genome sequence of a waterfowl aviadenovirus, goose adenovirus 4. J. Gen. Virol. 93: 2457–2465. doi: 10.1099/vir.0.042028-0 [DOI] [PubMed] [Google Scholar]
- 16.Kamil J. P., Tischer B. K., Trapp S., Nair V. K., Osterrieder N., Kung H. J.2005. vLIP, a viral lipase homologue, is a virulence factor of Marek’s disease virus. J. Virol. 79: 6984–6996. doi: 10.1128/JVI.79.11.6984-6996.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J. N., Byun S. H., Kim M. J., Kim J., Sung H. W., Mo I. P.2008. Outbreaks of hydropericardium syndrome and molecular characterization of Korean fowl adenoviral isolates. Avian Dis. 52: 526–530. doi: 10.1637/8178-112207-Case [DOI] [PubMed] [Google Scholar]
- 18.Kumar R., Chandra R., Kumar V., Bhatt P., Shukla S.K., Dhama K.2013. Hydropericardium syndrome (HPS) Virus: Immunofluorescence studies on aspects of pathogenesis in chickens. Adv. Anim. Vet. Sci. 1: 25–29. [Google Scholar]
- 19.Lee L. F., Wu P., Sui D., Ren D., Kamil J., Kung H. J., Witter R. L.2000. The complete unique long sequence and the overall genomic organization of the GA strain of Marek’s disease virus. Proc. Natl. Acad. Sci. U.S.A. 97: 6091–6096. doi: 10.1073/pnas.97.11.6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Wan W., Gao D., Li Y., Yang X., Liu H., Yao H., Chen L., Wang C., Zhao J.2016. Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg. Microbes Infect. 5: e117. doi: 10.1038/emi.2016.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marek A., Nolte V., Schachner A., Berger E., Schlötterer C., Hess M.2012. Two fiber genes of nearly equal lengths are a common and distinctive feature of Fowl adenovirus C members. Vet. Microbiol. 156: 411–417. doi: 10.1016/j.vetmic.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 22.Mase M., Nakamura K., Imada T.2010. Characterization of Fowl adenovirus serotype 4 isolated from chickens with hydropericardium syndrome based on analysis of the short fiber protein gene. J. Vet. Diagn. Invest. 22: 218–223. doi: 10.1177/104063871002200207 [DOI] [PubMed] [Google Scholar]
- 23.McFerran J. B., Adair B. M.1977. Avian adenoviruses—a review. Avian Pathol. 6: 189–217. doi: 10.1080/03079457708418228 [DOI] [PubMed] [Google Scholar]
- 24.McFerran J. B., McCracken R. M., Connor T. J., Evans R. T.1976. Isolation of viruses from clinical outbreaks of inclusion body hepatitis. Avian Pathol. 5: 315–324. doi: 10.1080/03079457608418201 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura K., Mase M., Yamaguchi S., Shibahara T., Yuasa N.1999. Pathologic study of specific-pathogen-free chicks and hens inoculated with adenovirus isolated from hydropericardium syndrome. Avian Dis. 43: 414–423. doi: 10.2307/1592638 [DOI] [PubMed] [Google Scholar]
- 26.Ono M., Okuda Y., Yazawa S., Shibata I., Sato S., Okada K.2003. Outbreaks of adenoviral gizzard erosion in slaughtered broiler chickens in Japan. Vet. Rec. 153: 775–779. [PubMed] [Google Scholar]
- 27.Pallister J., Wright P. J., Sheppard M.1996. A single gene encoding the fiber is responsible for variations in virulence in the fowl adenoviruses. J. Virol. 70: 5115–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Q., Yang Y., Shi Z., Liu L., Gao Y., Qi X., Liu C., Zhang Y., Cui H., Wang X.2017. Different dynamic distribution in chickens and ducks of the hypervirulent, novel genotype fowl adenovirus serotype 4 recently emerged in China. Front. Microbiol. 8: 1005. doi: 10.3389/fmicb.2017.01005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahul S., Kataria J. M., Senthilkumar N., Dhama K., Sylvester S. A., Uma R.2005. Association of fowl adenovirus serotype 12 with hydropericardium syndrome of poultry in India. Acta Virol. 49: 139–143. [PubMed] [Google Scholar]
- 30.Shivachandra S. B., Sah R. L., Singh S. D., Kataria J. M., Manimaran K.2003. Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Vet. Res. Commun. 27: 39–51. doi: 10.1023/A:1022058623634 [DOI] [PubMed] [Google Scholar]
- 31.Toro H., Gonzalez C., Cerda L., Hess M., Reyes E., Geissea C.2000. Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/ hydropericardium syndrome. Avian Dis. 44: 51–58. doi: 10.2307/1592507 [DOI] [PubMed] [Google Scholar]
- 32.Toro H., Prusas C., Raue R., Cerda L., Geisse C., González C., Hess M.1999. Characterization of fowl adenoviruses from outbreaks of inclusion body hepatitis/hydropericardium syndrome in Chile. Avian Dis. 43: 262–270. doi: 10.2307/1592616 [DOI] [PubMed] [Google Scholar]
- 33.Vera-Hernández P. F., Morales-Garzón A., Cortés-Espinosa D. V., Galiote-Flores A., García-Barrera L. J., Rodríguez-Galindo E. T., Toscano-Contreras A., Lucio-Decanini E., Absalón A. E.2016. Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol. 45: 73–81. doi: 10.1080/03079457.2015.1125443 [DOI] [PubMed] [Google Scholar]
- 34.Ye J., Liang G., Zhang J., Wang W., Song N., Wang P., Zheng W., Xie Q., Shao H., Wan Z., Wang C., Chen H., Gao W., Qin A.2016. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg. Microbes Infect. 5: e50. doi: 10.1038/emi.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J., Zhong Q., Zhao Y., Hu Y. X., Zhang G. Z.2015. Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One 10: e0133073. doi: 10.1371/journal.pone.0133073 [DOI] [PMC free article] [PubMed] [Google Scholar]