Abstract
Currently, several commercially available biochemical kits are validated for their use in human but not in animals. The purpose of this work is to demonstrate the applicability of human kits for alanine-aminotransferase, aspartato-aminotransferase, albumin, total protein, total cholesterol, and triglycerides in ovine plasma. Assays were validated according to international guidelines and stability was explored. Accuracy values were between 67 and 100%, and intra and interday precisions (%RSD) were <15% for all studied parameters. These results confirm the suitability of the studied human kits for their use in ovine plasma and they were used in plasma collected from pregnant ewes.
Keywords: biochemical parameter, plasma, sheep, spectrophotometric kit, validation
Blood metabolic profile can be determined using a set of diagnostic procedures that are based on determining various indicators of health in animals and humans [12]. Actually, several commercial kits are available for their use in human, but there is a lack of specific kits for animals. The determination of biochemical blood parameters can be used also to evaluate information related to nutrition, sex, age, health, and physiological status (pregnancy and lactation) in animals [3]. Several studies describe the correlation between the different phases of the reproduction cycle and biochemical parameters in livestock animal species. It is shown [4,5,6] that, in some sheep’s and goats’ physiological status (as estrus cycle, pregnancy and lactation) there is a modification of these parameters. For instance, liver dysfunction can constitute a pregnancy-related disorder [10]. Liver has a central role in carbohydrate, protein, and fat metabolism; several processes of detoxification, as amino acid deamination and the subsequent urea production, are carried out in this site. Its integrity is essential for the organism well-being, so it is important to monitor the main indicators of hepatic lesions and functions. Between these are the enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total proteins (TP), total cholesterol (TC), triglycerides (TG) and bilirubin. The effect of pregnancy in ALT and AST activity levels are somewhat controversial, however in the majority of published studies carried out in humans, ALT and AST do not normally change during pregnancy or remain within the normal limits [1]. Albumin levels are known to decrease during the first trimester of pregnancy, and this trend can be explained by the hemodilution phenomenon.
All available commercial kits to evaluate biochemical parameters are validated for their use in human blood, even if they are commonly used also for other animal species [2, 9]. Given the importance of these dosages in animals, the purpose of this work is to verify if commercially available human kits are suitable for their use in animals. In particular, we focused on the assays of ALT, AST, Alb, TP, TC and TG in ovine plasma. All procedures involving animals in this study were approved by the Local Animal Care and Use Committee (authorization code: 2899 of 17/01/2018). For this study, five Sarda ewes aged 4–5 years were used. The ewes were penned outdoor with access to a sheltered area, at the experimental facilities of the Department of Veterinary Medicine at the University of Sassari, Italy, IT (40°43′40.33″N, 8°33′1.33″E). Blood samples were collected at fasting (07:00 a.m.) from the jugular vein from using 10 ml vacuum collection tubes containing EDTA K2 (Vacutainer Systems Europe; Becton Dickinson, MeylanCedex, France). Blood was centrifuged (2,000 rpm, 10 min, +4°C) and plasma was collected and pooled. Spectrophotometric kits from Real Time Hagen Diagnostic Systems were used to assay ALT, AST, Alb, TP, TC and TG (cod: 001-10122; 001-10132; 001-0005R; 001-0053; 001-0088; 001-0089 respectively) in sheep plasma using a BS-200 Mindray clinical chemistry analyzer. Quality controls provided with the kits were used to verify the performance of the measurement procedures. Then, two lyophilized multi calibrators (Biocal H, Real Time Hagen Diagnostic Systems, Firenze, Italia, IT), containing known amounts of all the assayed parameters, were dissolved properly in order to obtain control samples both in water (control water, CW) and in pooled ovine plasma (control plasma, CP) at two different concentrations (CW1, CW2, CP1, CP2). Given that all the studied parameters are endogenous, they were analyzed also in the plasma itself (blank plasma, BP) and the obtained values were subtracted from the fortified plasma (CP) to obtain the basal value (CP-BP). The precision of the method, evaluated at two concentrations for each parameter in plasma matrix, was expressed as the percent relative standard deviation (RSD%). The sample standard deviation (SD) was calculated for five replicates for each concentration for the intra-day repeatability and over three consecutive days for the inter-day repeatability. The intra-day and inter-day repeatability were far below under 15% as reported by the guidelines for method validation [8, 11]. Accuracy, which represents the closeness of the test results to the true values, was determined for five replicates for each parameter, using the following formula:
All used kits showed good accuracy values comprises between 67 and 100%.
The freeze-and-thaw stability of the analytes in the sample matrix was determined after performing three freeze-and-thaw cycles on CP samples at the two concentrations listed in Table1. CP samples (n=5), fortified with the multicalibrator, were first analysed as fresh, and then frozen for 24 hr and thawed for 1 hr at room temperature. This freeze-and-thaw cycle was performed three times. The long-term stability was determined on the same samples after 30 days of freezing. The average result of the stability samples was within 90–110% corresponding average result of the fresh samples for all tested parameters in accordance to international guidelines for method validation [8, 11]. All results are shown in Table 1. All the studied spectrophotometric kits, resulted to be adequate for the determination of the analysed biochemical parameters also in ovine plasma.
Table 1. Repeatability, accuracy and stability of the methods in ovine plasma.
| Biochemical Parameters |
Concentration | Repeatability (RSD %) | Accuracy % ± SD | Stability (%) | |||
|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | Freeze-thaw (3 cycles −20°C) |
Long term (30 days −20°C) |
||||
| ALT | 63.30 U/l | 1.38 | 5.12 | 97.52 ± 2.18 | 93.36 ± 2.69 | 95.59 ± 1.95 | |
| 38.00 U/l | 1.97 | 2.56 | 103.13 ± 3.97 | 96.45 ± 1.80 | 91.00 ± 0.14 | ||
| AST | 65.00 U/l | 1.35 | 4.56 | 86.34 ± 1.05 | 97.48 ± 1.29 | 91.70 ± 0.69 | |
| 39.00 U/l | 1.38 | 2.76 | 94.97 ± 3.25 | 95.62 ± 0.70 | 92.21 ± 0.49 | ||
| Alb | 7.35 g/dl | 0.83 | 2.12 | 66.56 ± 2.34 | 96.39 ± 0.20 | 92.16 ± 0.08 | |
| 4.41 g/dl | 0.70 | 4.73 | 76.60 ± 3.01 | 92.03 ± 0.76 | 91.88 ± 0.05 | ||
| TP | 10.17 g/dl | 0.50 | 8.00 | 68.94 ± 2.40 | 94.17 ± 0.61 | 96.32 ± 0.13 | |
| 6.10 g/dl | 0.49 | 13.44 | 76.76 ± 1.11 | 95.34 ± 1.09 | 93.30 ± 0.27 | ||
| TC | 250.00 mg/dl | 3.00 | 3.87 | 93.28 ± 0.95 | 102.15 ± 2.82 | 98.74 ± 4.48 | |
| 150.00 mg/dl | 2.20 | 3.98 | 87.84 ± 4.03 | 101.41 ± 2.19 | 102.15 ± 0.83 | ||
| TG | 168.33 mg/dl | 0.28 | 2.02 | 102.20 ± 1.27 | 96.50 ± 0.83 | 94.68 ± 0.53 | |
| 101.00 mg/dl | 0.68 | 1.35 | 96.53 ± 1.87 | 98.80 ± 0.96 | 98.06 ± 0.68 | ||
ALT=alanine aminotransferase; AST=aspartate aminotransferase; Alb=albumin; TP=total proteins; TC=total cholesterol; TG=triglycerides.
Finally, the studied kits were used to quantify ALT, AST, Alb, TP, TC and TG in 30 pregnant ewes during their five months of pregnancy. Blood was drawn from the jugular vein every 30 days starting from the day of mating (time 0) to 30 days post-parturition (time 6). Differences among biochemical parameters during pregnancy were analyzed by a mono-factorial ANOVA (Minitab® 18.1). The method used to discriminate between the means was Fisher’s least-significant-difference (l.s.d.) procedure. All results were expressed as mean ± S.E. and a probability of P<0.05 was considered to be significant. Data are shown in Fig. 1. All parameters are in accordance with those reported in literature [7]. To our knowledge, this is the first report that demonstrates the applicability of the human kits studied to the analysis of a matrix belonging to ovine species. Given that it is internationally recognized that validation is necessary in analytical laboratories this study can be useful for researchers and clinicians working in the veterinary field.
Fig. 1.
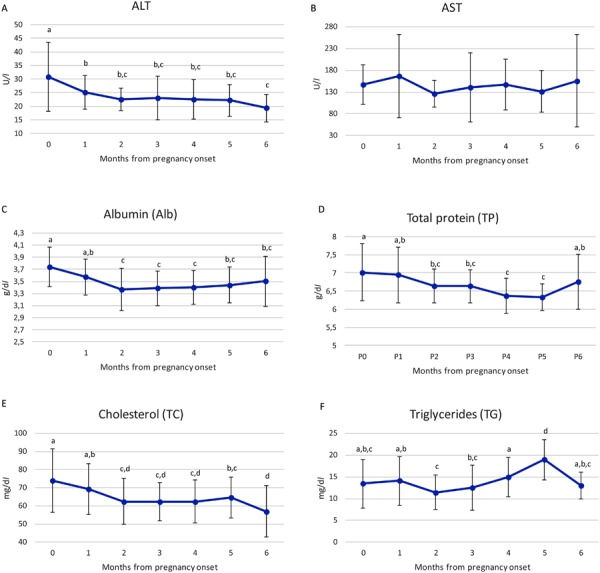
Trend in ALT, AST, Alb, TP, TC and TG values during sheep pregnancy. Values are expressed as mean ± S.E. Different letters indicate a significant statistical difference (P<0.05) analyzed by a mono-factorial ANOVA.
REFERENCES
- 1.Carter J.1990. Liver function in normal pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 30: 296–302. doi: 10.1111/j.1479-828X.1990.tb02014.x [DOI] [PubMed] [Google Scholar]
- 2.González F. D., Muiño R., Pereira V., Campos R., Benedito J. L.2011. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet. Sci. 12: 251–255. doi: 10.4142/jvs.2011.12.3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herdt T. H., Rumbeiha W., Braselton W. E.2000. The use of blood analyses to evaluate mineral status in livestock. Vet. Clin. North Am. Food Anim. Pract. 16: 423–444. doi: 10.1016/S0749-0720(15)30078-5 [DOI] [PubMed] [Google Scholar]
- 4.Iriadam M.2007. Variation in certain hematological and biochemical parameters during the peri-partum period in Kilis does. Small Rumin. Res. 73: 54–57. doi: 10.1016/j.smallrumres.2006.11.001 [DOI] [Google Scholar]
- 5.Krajnicakova M., Bekeova E., Hendrichovsky V., Maracek I.1993. [Concentrations of total lipids, cholesterol and progesterone in the period of estrus synchronization and during gravidity of sheep]. Vet. Med. (Praha) 38: 349–357 (in Slovak). [PubMed] [Google Scholar]
- 6.Krajnicakova M., Kovac G., Kostecky M., Valocky I., Maracek I., Sutiakova I., Lenhardt L.2003. Selected clinico-biochemical parameters in the puerperal period of goats. Bull. Vet. Inst. Pulawy 47: 177–182. [Google Scholar]
- 7.Morgante M., Gianesella M., Versace E., Contalbrigo L., Casella S., Cannizzo C., Piccione G., Stelletta C.2010. Preliminary study on metabolic profile of pregnant and non-pregnant ewes with high or low degree of behavioral lateralization. Anim. Sci. J. 81: 722–730. doi: 10.1111/j.1740-0929.2010.00786.x [DOI] [PubMed] [Google Scholar]
- 8.Peters F. T., Drummer O. H., Musshoff F.2007. Validation of new methods. Forensic Sci. Int. 165: 216–224. doi: 10.1016/j.forsciint.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 9.Piccione G., Caola G., Giannetto C., Grasso F., Runzo S. C., Zumbo A., Pennisi P.2009. Selected biochemical serum parameters in ewes during pregnancy, post-parturition, lactation and dry period. Anim. Sci. Pap. Rep. 27: 321–330. [Google Scholar]
- 10.Shekhar S., Diddi G.2015. Liver disease in pregnancy. Taiwan. J. Obstet. Gynecol. 54: 475–482. doi: 10.1016/j.tjog.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 11.Taverniers I., De Loose M., Van Bockstaele E.2004. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends in Analytical Chemistry 23: 535–552. doi: 10.1016/j.trac.2004.04.001 [DOI] [Google Scholar]
- 12.Van Saun R.2000. Blood profiles as indicators of nutritional status. Adv. Dairy Technol. 12: 401–410. [Google Scholar]


